CHAPTER 279 Posterior Approach to Cervical Degenerative Disease
Surgical approaches for the management of degenerative disorders of the cervical spine, including herniated disk and spondylosis, may be broadly segregated into anterior and posterior approaches. Recent evidence-based guidelines have reported that there is insufficient evidence to recommend one surgical approach over another for the treatment of cervical spondylotic myelopathy (CSM).1 Although in many cases a clearly superior approach may not be evident, understanding the specific techniques and advantages and disadvantages of each will allow the surgeon to make informed, rational decisions.
On the negative side, however, posterior approaches are generally associated with greater postoperative neck pain than is the case with anterior surgery because of the more extensive muscular dissection required. Restoration of lordosis, particularly in an osteopenic patient, can be difficult from a solely posterior approach; anterior interbody support plus correction is often a more powerful technique if hypolordosis is a significant concern. The infection rate with posterior approaches is higher than that with anterior approaches.2 This is more reflective of an extremely low rate of infection with anterior cervical surgery than of a high rate with the former.
Imaging
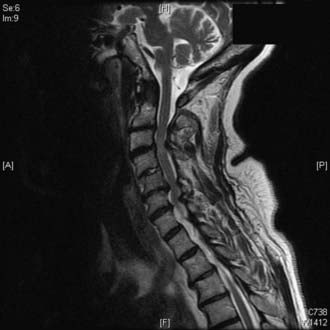
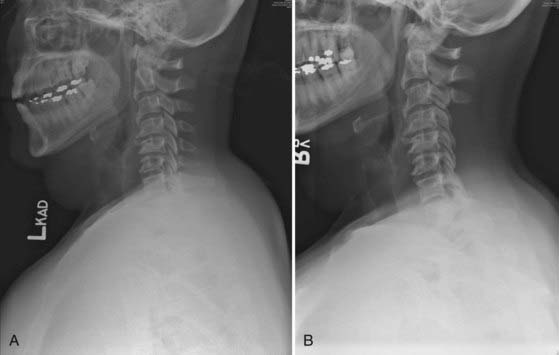
The three most important factors in determining whether a posterior (dorsal) approach to cervical degenerative disease is appropriate or feasible are the location of the disease (dorsal or ventral), the extent of disease (number of spinal segments involved), and the alignment of the cervical spine (lordotic, straight, or kyphotic). Compression that is purely dorsal or dorsolateral is generally best relieved with surgery from a posterior approach. A common scenario involves a patient in the seventh or eighth decade with a thick, buckling ligamentum flavum causing multisegmental spinal cord compression. Occasionally, such a patient is initially seen after a fall with an acute neurological deficit (Fig. 279-1).
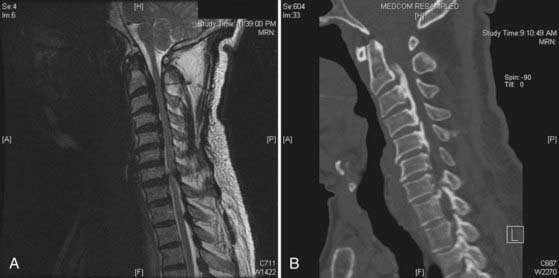
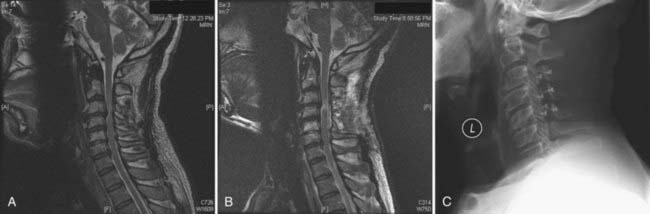
Pathology that extends over many cervical segments, particularly if it crosses into the thoracic spine, is often treated preferably via a posterior approach. Degenerative spinal disease superimposed on a congenitally narrow canal (<12 mm, identified on a lateral radiograph by near-superimposition of the posterior cortex of the lateral masses and the spinolaminar line) is one such situation. Another condition that often involves multiple segments at initial evaluation is ossification of the posterior longitudinal ligament (OPLL) (Fig. 279-2). The typically extensive, confluent disease seen with OPLL ossification is often optimally approached posteriorly, particularly because the ventral dura is frequently annealed to the ossified ligament and a spinal fluid leak is unavoidable if an anterior approach is used.
Indications for Surgical Treatment
Surgical treatment of cervical degenerative disorders, such as CSM or radiculopathy, may be indicated in patients with progressive signs of myelopathy, progressive or severe weakness in a cervical myotome, or intractable radicular pain and correlative imaging. The natural history of CSM is variable and unpredictable for any particular patient.3 Recommendations may be tailored to the patient based on age, symptoms, and findings on electromyography (EMG) or radiography, among other factors.4 Once progression has been demonstrated, however, it is unlikely that the process will halt completely or reverse spontaneously.5 In these circumstances, a frank, clear discussion should be held with the patient and family regarding the potential risks associated with surgical intervention versus the risks associated with continued observation.
Anesthesia and Positioning
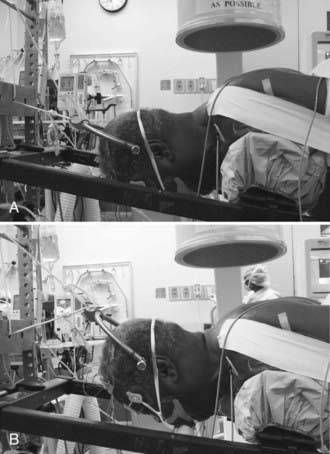
We use the prone position exclusively for posterior cervical surgery. Based on surgeon preference, either an electric operating table with padded bolsters and a rigid Mayfield head holder or a Jackson spine table with Gardner-Wells traction is used. With the Jackson table, a bivector traction setup with two ropes is used to permit the patient to be positioned initially in relative cervical flexion to facilitate the decompressive portion of the procedure and placement of the fixation points. The weight is moved to the upper rope to enhance cervical lordosis before placement of the rods and performing the arthrodesis (Fig. 279-3). A horseshoe head holder is placed 2 to 3 cm away from the patient’s face to serve as a backup should a problem occur with the traction.
Spinal Cord Monitoring
Although we routinely monitor SSEPs and MEPs in these patients, the utility of this strategy and the appropriate response to changes in signal quality, amplitude, or latency in different clinical scenarios are sometimes unclear. We have, on occasion, identified problems with positioning of the arms and shoulders based on monitoring changes. During decompression of a stenotic foramen it is not unusual to see transient nerve root irritation on free-running EMG that has no apparent clinical postoperative correlation. An evidence-based review found conflicting data regarding the use of intraoperative improvements in electrophysiologic data for clinical prognostication.6
Foraminotomy/Diskectomy
The posterior keyhole foraminotomy as first described by Scoville has a role in the treatment of isolated foraminal stenosis or lateral disk herniation.7 Patients with symptoms referable to a single cervical nerve root are the best candidates for this procedure. Bone removal is tailored to the nature of the pathology. In patients with spondylosis and osteophytic foraminal stenosis, a keyhole-shaped decompression extending from the lateral thecal sac to the lateral border of the pedicle is performed to achieve complete decompression of the exiting nerve root. For the removal of lateral soft herniated disks, a smaller amount of bone can be removed to preserve the majority of the facet joint.
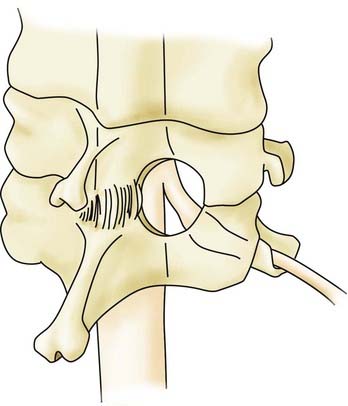
A herniated cervical disk impinges on the exiting nerve root that corresponds to the pedicle caudal to the disk space (e.g., a C5-6 herniated disk causes C6 impingement). The foraminotomy is performed at the appropriate interspace just proximal to the pedicle of the level caudal to the disk herniation (Fig. 279-4). The first part of the foraminotomy procedure consists of a small laminotomy and flavectomy to expose the lateral dura and the origin of the exiting nerve root. The foraminotomy is extended laterally by using a high-speed drill to thin the ventromedial superior facet. Bone removal is completed with small straight and up-going curets. A minimal amount of the inferior facet is removed. After the axilla of the exiting nerve root is identified and a portion of the root approximately 2 to 3 mm in length is exposed, a micro–nerve hook is used to dissect parallel with the nerve root along its caudal edge. With removal of less than half the facet joint, up to 5 mm of the nerve root may be exposed.8 The pedicle is palpated, the nerve hook is then rotated ventral to the root, and the disk herniation is identified. A microdissector or micro–nerve hook may be used to place gentle retraction on the nerve root to expose the disk fragment, which is removed with micro–pituitary rongeurs. Complete decompression of the nerve root is confirmed with passage of the micro–nerve hook through the foramen dorsal and ventral to the nerve root. During the dissection or after the decompression, hemostasis of the epidural veins is accomplished with thrombin-soaked collagen in either a patty (Gelfoam, Pharmacia & Upjohn, New York) or semiliquid (FloSeal, Baxter, Deerfield, IL) formulation.
Laminectomy and Laminaplasty
Both procedures are most effective in relieving compression caused by dorsally located pathology. Ventral compression may also be relieved by posterior decompression under certain circumstances. First, a sufficient rostral-caudal extent of the canal must be decompressed to ensure that the spinal cord is not compressed against the edge of the decompression. Second, the cervical spine should be lordotic, or at least straight, in the region of the pathology so that the spinal cord will tend to lift away from the compression after release or removal of the dorsal elements. A laminectomy or laminaplasty will not effectively decompress the spinal cord in a kyphotic region without realignment of the spine. The dorsal decompression may, in fact, lead to progression of the kyphosis secondary to disruption of the posterior tension band. With proper patient selection and care in avoiding disruption of the facet joints, however, good long-term results may be achieved with laminectomy alone (Fig. 279-5).
There are several ways to maintain the laminaplasty opening. Small titanium plates may be affixed to the lateral mass and the lamina with small screws. There are plates made especially for this purpose. Alternatively, instrumentation from a maxillofacial fracture repair set may be adapted for use. The plate is anchored to the center of the lateral mass to avoid impingement on the adjacent facet joints. An alternative or supplemental method to maintain the laminaplasty is to place a spacer, either bone or synthetic, into the opening and suture or wire it in place (Fig. 279-6).
Cervical Posterior Segmental Instrumentation
C2 Instrumentation
The starting point for the pars screw is 2 to 3 mm rostral and a similar distance lateral to the junction of the caudal lamina and the inferior facet. It can be helpful to dissect along the lateral aspect of the rostral edge of the lamina onto the medial pars to confirm proper screw trajectory. Lateral fluoroscopy may be used to determine the sagittal trajectory, which is optimally parallel to the rostral edge of the lamina and pars. The overall trajectory is similar to that of a C1-2 transarticular screw except that it is slightly less rostrally directed to avoid violation of the C1-2 joint. Based on preoperative imaging, a 16-mm-long screw is typically selected so that it stops short of the transverse foramen.9
The starting point for the pedicle screw is more rostral and lateral than that for the pars screw. Useful landmarks are the rostral edge of the lamina and a point just lateral to the midpoint of the pars. Again, dissection along the medial pars lateral to the thecal sac can facilitate determination of the lateral to medial trajectory. The rostrocaudal angle is approximately 20 degrees, and the screw is directed 15 to 25 degrees medially.9 Screw lengths of 20 to 24 mm may be placed safely with this trajectory.
An alternative technique for achieving fixation at C2 that does not place the vertebral artery at risk was first described by Wright in 2004.10 Bilateral crossing translaminar screws may be placed safely in many patients in whom placement of axial pars or pedicle screws is not possible or thought to be dangerous. The CT or MRI scans are examined preoperatively to ensure that the lamina is sufficiently thick to contain a 3.5- or 4-mm-diameter screw. The entry point is at the junction of the spinous process and the lamina, and the screw is directed contralaterally toward the C2 inferior facet. Screws up to 30 mm in length may be placed. One obvious disadvantage of this technique versus pars or pedicle fixation is that concomitant or subsequent C2 laminectomy is not possible.
Lateral Mass Screw Fixation
Several techniques for the insertion of lateral mass screws have been described. Jeannert and Magerl described a technique of insertion that maximizes the length of the screw while directing it away from the nerve root and vertebral artery. They described a starting point slightly medial and cranial to the geometric center of the lateral mass; using a point slightly caudal to that can help optimize the length of the screw and minimize interference with the cranially adjacent facet joint.10a This location is marked with a small bur. A twist drill with a depth stop is then used to drill the pilot hole. The angle is approximately 20 to 30 degrees medial to lateral and parallel to the facet joints. The hole is drilled until the ventral cortex is breached. This may occur at a depth between 12 and 16 mm or greater, depending on the patient’s size, the presence of osteophytes, and the precise trajectory taken. The depth is measured directly, the hole is tapped, and the screw is placed.
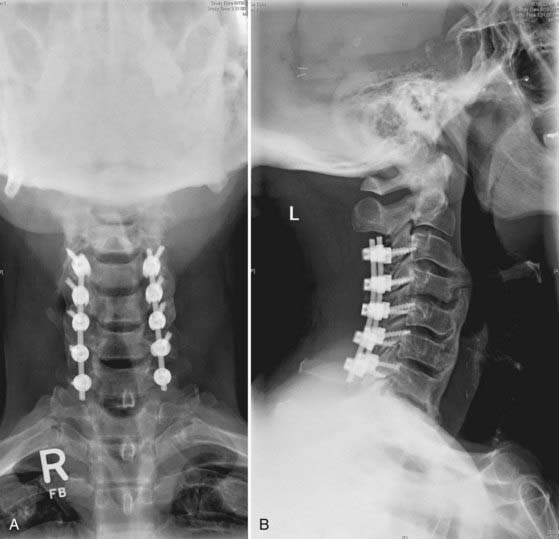
The rods are contoured to match the sagittal alignment and secured to the screws (Fig. 279-7). Current multiaxial lateral mass screws can accommodate minor variations in sagittal and coronal alignment of the screws. It is important, however, to minimize the force exerted on any individual screw to avoid the possibility of fixation failure. Cross-linkages to increase construct rigidity are available for most instrumentation systems.
Complications and Avoidance
Postoperative dissociated motor loss has been reported to occur in 5% to 8% of patients after cervical laminaplasty.11,12 The most commonly involved root is C5; the next most common is C6. It is manifested as painless deltoid or biceps weakness and may be more common in patients with more severe foraminal or central stenosis preoperatively. Monitoring of free-running EMG signals from the biceps and deltoids may demonstrate signs of nerve root irritation, but there are no definitive data that such monitoring reduces the incidence of this complication. Ensuring complete decompression of the nerve root may also help reduce the risk.
Outcomes
Although there are many historical reports of the results of posterior surgery for cervical degenerative disease, the literature suffers from a paucity of studies reporting outcomes with validated outcomes instruments.6 There are also few comparative studies of surgical techniques, and those that do exist are generally of low evidential quality and thus preclude meaningful conclusions.13–19 Outcomes may be expected to depend, in large part, on the type of pathology present rather than on the specific procedure, assuming that the operation effectively addresses the pathology.
Retrospective studies report good to excellent outcomes on mostly nonvalidated outcome measures for 70% to 98% of patients treated with laminoforaminotomy for cervical radiculopathy.20–26 The proportion of patients in whom improvement is achieved may depend to some degree on the specific symptoms present; one study has reported that pain resolves more reliably than weakness, which improves in more patients better than sensory abnormalities do.20
There are mixed data regarding prognostic factors for patients undergoing surgery for CSM. A longer duration of symptoms and the presence of more severe symptoms do seem to correlate with a lower likelihood of significant improvement or a lesser degree of improvement than seen in patients with a shorter or less severe symptomatic period preoperatively; the effect may vary with patient age.27–29
Multiple uncontrolled studies of cervical laminectomy, laminectomy and fusion, and laminaplasty have reported significant clinical improvement in patients with myelopathy.30–37 All these studies suffer from the methodologic limitations of noncomparative study designs. Comparisons of outcomes of specific techniques are likewise fraught with potential bias, and absolute statements about the relative effectiveness of one procedure versus another are not warranted based on current evidence.38–40
Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95:51-57.
Anderson PA, Matz PG, Groff MW, et al. Laminectomy and fusion for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:150-156.
Bishara SN. The posterior operation in treatment of cervical spondylosis with myelopathy: a long-term follow-up study. J Neurol Neurosurg Psychiatry. 1971;34:393-398.
Carol MP, Ducker TB. Cervical spondylitic myelopathies: surgical treatment. J Spinal Disord. 1988;1:59-65.
Casotto A, Buoncristiani P. Posterior approach in cervical spondylotic myelopathy. Acta Neurochir (Wien). 1981;57:275-285.
Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine. 2002;27:2108-2115.
Clark E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
Fager CA. Results of adequate posterior decompression in the relief of spondylotic cervical myelopathy. J Neurosurg. 1973;38:684-692.
Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51:S37-S45.
Gonzalez-Feria L, Peraita-Peraita P. Cervical spondylotic myelopathy: a cooperative study. Clin Neurol Neurosurg. 1975;78:19-33.
Gorter K. Influence of laminectomy on the course of cervical myelopathy. Acta Neurochir (Wien). 1976;33:265-281.
Grieve JP, Kitchen ND, Moore AJ, et al. Results of posterior cervical foraminotomy for treatment of cervical spondylitic radiculopathy. Br J Neurosurg. 2000;14:40-43.
Hamanishi C, Tanaka S. Bilateral multilevel laminectomy with or without posterolateral fusion for cervical spondylotic myelopathy: relationship to type of onset and time until operation. J Neurosurg. 1996;85:447-451.
Handa Y, Kubota T, Ishii H, et al. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96:173-179.
Heary RF, Ryken TC, Matz PG, et al. Cervical laminoforaminotomy for the treatment of cervical degenerative radiculopathy. J Neurosurg Spine. 2009;11:198-202.
Heller JG, Edwards CCI, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001;26:1330-1336.
Henderson CM, Hennessy RG, Shuey HMJ, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081-1088.
Huang RC, Girardi FP, Poynton AR, et al. Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech. 2003;16:123-129.
Kaminsky SB, Clark CR, Traynelis VC. Operative treatment of cervical spondylotic myelopathy and radiculopathy. A comparison of laminectomy and laminoplasty at five year average follow-up. Iowa Orthop J. 2004;24:95-105.
Kihara S, Umebayashi T, Hoshimaru M. Technical improvements and results of open-door expansive laminoplasty with hydroxyapatite implants for cervical myelopathy. Neurosurgery. 2005;57:348-356.
Korinth MC, Kruger A, Oertel MF, et al. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for soft disc disease: results in 292 patients with monoradiculopathy. Spine. 2006;31:1207-1216.
Kumar GR, Maurice-Williams RS, Bradford R. Cervical foraminotomy: an effective treatment for cervical spondylotic radiculopathy. Br J Neurosurg. 1998;12:563-568.
Kumar VG, Rea GL, Mervis LJ, et al. Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery. 1999;44:771-777.
LaRocca H. Cervical spondylotic myelopathy: natural history. Spine. 1988;13:854-855.
Matsunaga S, Sakou T, Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37:20-24.
Matz PG, Anderson PA, Groff MW, et al. Cervical laminoplasty for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:157-169.
Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104-111.
Mummaneni PV, Haid RW. Atlantoaxial fixation: overview of all techniques. Neurol India. 2005;53:408-415.
Mummaneni PV, Kaiser MG, Matz PG, et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98:230-238.
Raynor RB. Anterior or posterior approach to the cervical spine: an anatomical and radiographic evaluation and comparison. Neurosurgery. 1983;12:7-13.
Resnick DK, Anderson PA, Kaiser MG, et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245-252.
Ryken TC, Heary RF, Matz PG, et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:142-149.
Shamji MF, Cook C, Tackett S, et al. Impact of preoperative neurological status on perioperative morbidity associated with anterior and posterior cervical fusion. J Neurosurg Spine. 2008;9:10-16.
Uematsu Y, Tokuhashi Y, Matsuzaki H. Radiculopathy after laminoplasty of the cervical spine. Spine. 1998;23:1443-1448.
Wright NM. Posterior C2 fixation using bilateral, crossing C2 laminar screws. Case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
Yamazaki T, Yanaka K, Sato H, et al. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122-126.
Yonenobu K, Fuji T, Ono K, et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine. 1985;10:710-716.
Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33:356-362.
1 Mummaneni PV, Kaiser MG, Matz PG, et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
2 Shamji MF, Cook C, Tackett S, et al. Impact of preoperative neurological status on perioperative morbidity associated with anterior and posterior cervical fusion. J Neurosurg Spine. 2008;9:10-16.
3 LaRocca H. Cervical spondylotic myelopathy: natural history. Spine. 1988;13:854-855.
4 Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104-111.
5 Clark E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
6 Resnick DK, Anderson PA, Kaiser MG, et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245-252.
7 Heary RF, Ryken TC, Matz PG, et al. Cervical laminoforaminotomy for the treatment of cervical degenerative radiculopathy. J Neurosurg Spine. 2009;11:198-202.
8 Raynor RB. Anterior or posterior approach to the cervical spine: an anatomical and radiographic evaluation and comparison. Neurosurgery. 1983;12:7-13.
9 Mummaneni PV, Haid RW. Atlantoaxial fixation: overview of all techniques. Neurol India. 2005;53:408-415.
10 Wright NM. Posterior C2 fixation using bilateral, crossing C2 laminar screws. Case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
10a Jeannert B, Magerl F, Ward E, et al. Posterior stabilization of the cervical spine with hook plates. Spine. 1991;165:S56-S63.
11 Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine. 2002;27:2108-2115.
12 Uematsu Y, Tokuhashi Y, Matsuzaki H. Radiculopathy after laminoplasty of the cervical spine. Spine. 1998;23:1443-1448.
13 Carol MP, Ducker TB. Cervical spondylitic myelopathies: surgical treatment. J Spinal Disord. 1988;1:59-65.
14 Gonzalez-Feria L, Peraita-Peraita P. Cervical spondylotic myelopathy: a cooperative study. Clin Neurol Neurosurg. 1975;78:19-33.
15 Hamanishi C, Tanaka S. Bilateral multilevel laminectomy with or without posterolateral fusion for cervical spondylotic myelopathy: relationship to type of onset and time until operation. J Neurosurg. 1996;85:447-451.
16 Heller JG, Edwards CCI, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001;26:1330-1336.
17 Kaminsky SB, Clark CR, Traynelis VC. Operative treatment of cervical spondylotic myelopathy and radiculopathy. A comparison of laminectomy and laminoplasty at five year average follow-up. Iowa Orthop J. 2004;24:95-105.
18 Matsunaga S, Sakou T, Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37:20-24.
19 Yonenobu K, Fuji T, Ono K, et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine. 1985;10:710-716.
20 Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95:51-57.
21 Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51:S37-S45.
22 Grieve JP, Kitchen ND, Moore AJ, et al. Results of posterior cervical foraminotomy for treatment of cervical spondylitic radiculopathy. Br J Neurosurg. 2000;14:40-43.
23 Henderson CM, Hennessy RG, Shuey HMJ, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
24 Korinth MC, Kruger A, Oertel MF, et al. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for soft disc disease: results in 292 patients with monoradiculopathy. Spine. 2006;31:1207-1216.
25 Kumar GR, Maurice-Williams RS, Bradford R. Cervical foraminotomy: an effective treatment for cervical spondylotic radiculopathy. Br J Neurosurg. 1998;12:563-568.
26 Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33:356-362.
27 Casotto A, Buoncristiani P. Posterior approach in cervical spondylotic myelopathy. Acta Neurochir (Wien). 1981;57:275-285.
28 Handa Y, Kubota T, Ishii H, et al. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96:173-179.
29 Yamazaki T, Yanaka K, Sato H, et al. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122-126.
30 Bishara SN. The posterior operation in treatment of cervical spondylosis with myelopathy: a long-term follow-up study. J Neurol Neurosurg Psychiatry. 1971;34:393-398.
31 Fager CA. Results of adequate posterior decompression in the relief of spondylotic cervical myelopathy. J Neurosurg. 1973;38:684-692.
32 Gorter K. Influence of laminectomy on the course of cervical myelopathy. Acta Neurochir (Wien). 1976;33:265-281.
33 Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081-1088.
34 Huang RC, Girardi FP, Poynton AR, et al. Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech. 2003;16:123-129.
35 Kihara S, Umebayashi T, Hoshimaru M. Technical improvements and results of open-door expansive laminoplasty with hydroxyapatite implants for cervical myelopathy. Neurosurgery. 2005;57:348-356.
36 Kumar VG, Rea GL, Mervis LJ, et al. Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery. 1999;44:771-777.
37 Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98:230-238.
38 Anderson PA, Matz PG, Groff MW, et al. Laminectomy and fusion for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:150-156.
39 Matz PG, Anderson PA, Groff MW, et al. Cervical laminoplasty for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:157-169.
40 Ryken TC, Heary RF, Matz PG, et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:142-149.