Pneumonia
Considerations for the Critically Ill Patient
Colonization of the Upper Respiratory System and Digestive Tract
The Role of Respiratory Therapy Equipment and Endotracheal Tubes
Severe pneumonia with respiratory failure and sepsis is one of the commonest indications necessitating intensive care unit (ICU) admission. Pneumonia along with influenza was the eighth leading cause of death in 2009 alone. Pneumonia patients are managed in the ICU when severe forms of community-acquired pneumonia (CAP) are present or when a hospitalized patient develops life-threatening nosocomial pneumonia. Pneumonia patients admitted to the ICU usually have more severe disease, other comorbid medical conditions, and much higher mortality rate compared to the patients admitted to the wards.1
The incidence of pneumonia in hospitalized patients is directly related to the degree of underlying systemic illness and number of days on the ventilator.2 The elderly account for a disproportionate number of critically ill patients with all forms of pneumonia, often because they commonly have comorbid illness, which predisposes them to more severe forms of infection, and their short- and long-term mortality rates are higher than those of younger patients.3 Also, hospitalized patients receive therapeutic interventions that predispose them to pneumonia such as endotracheal intubation, nasogastric feeding, antibiotic therapy, and use of immunomodulatory medications. In 2005, there were more than 60,000 deaths in persons aged 15 years or older related to CAP in the United States.4 The mortality rate from severe CAP is over 30% in patients admitted to the ICU.5 Critically ill patients who develop ventilator-associated pneumonia (VAP) have a higher mortality rate compared with similar patients without VAP and incur USD $10,019 or more in additional hospital costs.6
With newer guidelines, there has been an emphasis on identifying patients with severe pneumonia early in the clinical course, facilitating early ICU admission, because this has been shown to decrease the mortality rate.7 Different scoring systems such as the ATS/IDSA guidelines (developed by American Thoracic Society and Infectious Diseases Society of America), PIRO (Predisposition, Insult, Response and Organ dysfunction) scores for CAP, and Acute Physiology and Chronic Health Evaluation score (APACHE II) and VAP PIRO score for VAP are used for assessing the severity of illness in pneumonia patients. It is still uncertain whether the use of any scoring system can lead to decreased mortality rates and more favorable outcomes in the management of patients with pneumonia. There is also interest in using biomarkers, such as procalcitonin (PCT), to diagnose the severity and guide the use and duration of antimicrobial therapy in patients with severe CAP and VAP.8–10
The emergence of multidrug-resistant (MDR) microorganisms is an alarming problem in the ICU and considerable forethought is necessary in choosing the right antibiotic to be used in this setting. In the ICU, almost 90% of episodes of nosocomial pneumonia occur in patients who are being mechanically ventilated for other reasons. Recent directives by the Centers for Medicare and Medicaid Services (CMS) and the Institute for Healthcare Improvement have led to the belief that VAP is preventable and should be a “never event” during the hospital stay. This may have led to underreporting in many instances.11 Many ICUs have incorporated ventilator bundles as a preventive strategy to minimize VAP, which may be an effective strategy, even if not being able to completely eliminate the problem.
The incidence of pneumonia in nursing homes varies from 0.3 to 2.3 episodes per 1000 resident care days with a 30-day mortality rate of 14.7%.12 In the 2005 ATS guidelines on nosocomial pneumonia, patients with exposure to MDR organisms by virtue of being in contact with the health care environment prior to admission were defined as having health care–associated pneumonia (HCAP). This group included residents of a nursing home or long-term care facility; recipients of recent intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days; and chronic hemodialysis patients.2 The focus in managing these patients was to empirically treat for MDR pathogens. However, recent studies have shown that the HCAP population has significant heterogeneity, and that treatment of all patients does not always need to include coverage for methicillin-resistant Staphylococcus aureus (MRSA) and drug-resistant gram-negative organisms.13
Definitions and Risk Factors
Patients who are unable to mount an effective immune response are at increased risk of developing serious pneumonia. If the host defense mechanism is overwhelmed by the size of the inoculum, the virulence of the infecting microorganisms, or an excessive inflammatory response to infection, the patient can develop severe respiratory failure or sepsis. Depending on the time of onset and setting in which the infection developed, pneumonia is classified as community-acquired or nosocomial pneumonia, with specific definitions for VAP and HCAP among patients with nosocomial pneumonia (Box 42.1).
Severe Community-Acquired Pneumonia
Several prognostic scoring systems were developed to predict the mortality risk and guide the site of care decision. The pneumonia severity index (PSI) and the British Thoracic Society’s CURB-65 score help with determining mortality risk for patients with CAP.14 The PSI was developed to identify patients with a low risk of dying, placing all patients into five classes, based on the demographic characteristics, coexisting illnesses, physical examination findings, laboratory measurements, and radiographic findings. The ICU is most often used for patients in classes IV and V, who have a 30-day mortality risk of 4% to 10% and 27%, respectively.15 However, the PSI score may overestimate severity of illness in patients with older age, while underestimating severity in young patients without comorbid conditions.16 The complexity of the PSI contrasts with the simpler bedside scoring system, CURB-65. With this tool, 1 point is assigned each for the presence of confusion, blood urea nitrogen (BUN) greater than 7.0 mol/L (19.6 mg/dL), respiratory rate of 30 or more breaths/minute, systolic blood pressure 90 mm Hg or less, or diastolic blood pressure 60 mm Hg or less, and age 65 years or more.17 Mortality rate increases proportionately with scores higher than 3 (score 3, 17%; score 4, 41.5%; and score 5, 57%).
Severe pneumonia and the host inflammatory response to infection are determined by several variables, including the identity of the pathogen, the timeliness and appropriateness of therapy, and patient variables. The latter include the genetic makeup of the individual, age, nutritional status, sex, and comorbid medical conditions. Although the PSI and CURB-65 score performed well to determine mortality risk, there is still a need to identify patients who will need ICU level of care at the earliest possible time point.18,19 According to the 2007 ATS/IDSA guidelines, severe CAP is present if a patient requires invasive mechanical ventilation or vasopressors or has any three of the nine minor criteria (Box 42.2).19 The PIRO score has also been used and is calculated within 24 hours of ICU admission with 1 point assigned for each of comorbid conditions (chronic obstructive pulmonary disease [COPD], immunosuppressed state), age older than 70 years, multilobar opacities on chest radiograph, shock, severe hypoxemia, acute renal failure, bacteremia, and acute respiratory distress syndrome (ARDS). Patients can be stratified into four classes: (1) low, 0 to 2 points; (2) mild, 3 points; (3) high, 4 points; (4) very high, 5 to 8 points. In patients admitted to the ICU, the PIRO score had a better performance than APACHE II and ATS/IDSA criteria to predict 28-day mortality rate.18
Severe pneumonia is a dynamic inflammatory process, and predicting which stable-appearing patients will require ICU care later is difficult. Hence, critically ill patients who do not have obvious need for ICU care, such as invasive respiratory/vasopressor support (IRVS), must satisfy certain minor criteria in order to be recognized. However, the predictive value of using just three minor criteria alone for making a decision to admit to the ICU is uncertain, and in one study, the use of four minor criteria improved the accuracy of predicting subsequent need for ICU care.20,21 A recent prospective study from Scotland excluded patients with the two major ATS/IDSA criteria present on admission, and demonstrated that each of the nine minor criteria was associated with an increased risk of need for mechanical ventilation, need for vasopressor support, and 30-day mortality.22
Another system developed in Australia—SMART-COP—can assess the need for intensive respiratory or vasopressor support.23 The SMART-COP was developed primarily to identify the need for IRVS. Different point scores were assigned to various parameters: low Systolic blood pressure less than 90 mm Hg (2 points), Multilobar pneumonia (1 point), low Albumin level less than 3.5 g/dL (1 point), high Respiratory rate 25 to 30 breaths/minute (1 point), Tachycardia higher than 125 beats/minute (1 point), Confusion (1 point), poor Oxygenation (2 points), and low arterial pH less than 7.35 (2 points). A score of more than 3 points identified 92% of patients requiring IRVS, with a specificity of 62.3%, outperforming PSI and CURB-65 scores to identify this specific end point.23 The SMART-COP as well as the ATS/IDSA 2007 guidelines are the most accurate for predicting need for ICU care.24
Renaud and coworkers examined risk factors for early admission to the ICU among 6560 patients, who presented to the emergency department and did not require immediate respiratory or circulatory support.25 They identified 11 criteria independently associated with ICU admission: male gender, age younger than 80 years, comorbid conditions, respiratory rate of 30 breaths/minute or higher, heart rate of 125 beats/minute or higher, multilobar infiltrate or pleural effusion, white blood cell count less than 3 or more than 20 g/L, hypoxemia (oxygen saturation <90% or arterial partial pressure of oxygen [PaO2] <60 mm Hg), BUN of 11 mmol/L or higher, pH less than 7.35, and sodium less than 130 mEq/L. They used these criteria to develop the risk of early admission to ICU index (REA-ICU index), which stratified patients into four risk classes, with the risk of ICU admission on days 1 to 3 ranging from 0.7% to 31%.25
Recent data from the German Competence Network for the Study of CAP (CAPNETZ) Study Group demonstrated that serum biomarkers (PCT) can be used as good predictors for short- and long-term all-cause mortality rates in patients admitted with CAP.26 A low level of PCT value in patients classified as high risk by PSI and CURB-65 scores predicted a low risk of dying, and probably a low need for ICU admission.27 Ramirez and colleagues showed that the ATS/IDSA 2007 minor criteria had comparable predictive value with other scores, such as CURB-65 and SMART-COP, but that the use of biomarkers like PCT and C-reactive protein (CRP), along with minor criteria, helped in predicting the need for delayed ICU admission.28 Pathophysiologic severity markers of severe sepsis, like soluble receptor for advanced glycation end products (SRAGE) along with severity scores have been examined in patients with CAP and are encouraging but need further studies for validation.29
Risk Factors for Severe Forms of Community-Acquired Pneumonia
Most patients with severe CAP (45-65%) have coexisting illnesses, and patients who are chronically ill have an increased likelihood of developing a complicated pneumonary illness (Box 42.3).30 The most common chronic illnesses in these patients are respiratory disease such as COPD, cardiovascular disease, and diabetes mellitus. In addition, certain habits such as cigarette smoking and alcohol abuse are also quite common in those with severe CAP, and cigarette smoking has been identified as a risk factor for bacteremic pneumococcal infection.31 Other common illnesses in those with CAP include malignancy and neurologic illness (including seizures). Milder forms of pneumonia may be more severe on presentation, if patients have not received antibiotic therapy prior to hospital admission. In addition, genetic differences in the immune response may predispose certain individuals to more severe forms of infection and adverse outcomes and may be reflected by a family history of severe pneumonia or adverse outcomes from infection.32 Also, genetic variability of the pulmonary surfactant proteins A and D may affect clearance of microorganisms and the extent of the inflammatory response, influencing the severity and outcomes for patients with CAP, notably missense single nucleotide polymorphisms and haplotypes of SFTPA1, SFTPA2, and SFTPD.33 Recent evidence also provides insights on the risk of developing pneumonia in patients taking inhaled corticosteroids. In a meta analysis of 18 randomized controlled trials with a total of 16,996 patients, inhaled corticosteroids were associated with a significantly increased risk of serious pneumonia when compared with placebo (relative risk [RR] 1.81; P < 0.001) or when the combination of inhaled corticosteroids and long-acting β-agonists was compared with long-acting β-agonists (RR 1.68; P < 0.002).34 However, the mortality rate of patients developing CAP while on inhaled corticosteroids may actually be lower than in patients not receiving this therapy.
Mortality Risk from Community-Acquired Pneumonia
In a meta analysis of 33,148 patients with CAP, the overall mortality rate was 13.7%, but those admitted to the ICU had a mortality rate of 36.5%.5 Eleven prognostic factors were significantly associated with different odds ratios (OR) for mortality rate: male sex (OR = 1.3), pleuritic chest pain (OR = 0.5), hypothermia (OR = 5.0), systolic hypotension (OR = 4.8), tachypnea (OR = 2.9), diabetes mellitus (OR = 1.3), neoplastic disease (OR = 2.8), neurologic disease (OR = 4.6), bacteremia (OR = 2.8), leukopenia (OR = 2.5), and multilobar infiltrates (OR = 3.1). In other studies, the clinical features that predict a poor outcome (Box 42.4) include advanced age (>65 years), preexisting chronic illness of any type, the absence of fever on admission, respiratory rate more than 30 breaths/minute, diastolic or systolic hypotension, elevated BUN (>19.6 mg/dL), profound leukopenia or leukocytosis, inadequate antibiotic therapy, need for mechanical ventilation, hypoalbuminemia, and the presence of certain “high-risk” organisms (type III pneumococcus, S. aureus, gram-negative bacilli, aspiration organisms, or postobstructive pneumonia). Timing of initial appropriate antibiotics is also important, with a delay in the initiation of appropriate antibiotic therapy of more than 4 hours being associated with increased mortality risk.35–37 Other clinical features associated with an increased mortality risk include rapid radiographic progression during therapy, nonrespiratory clinical presenting symptoms, and the presence of HCAP risk factors.
Many studies suggest that early ICU admission confers a better outcome in patients with severe CAP than delayed admission. In a study comparing patients directly admitted to the ICU from the emergency department to those moved to the ICU within 3 days after admission, delayed-transfer patients had a higher 28-day mortality rate (23.4% vs. 11.7%; p = 0.02) and a longer median hospital length of stay (13 days vs. 7 days; p < 0.001).38 In a study of 17,869 cases of CAP in the United Kingdom, only 5.9% needed ICU care, but early admission (within 2 days of hospitalization) appeared to be preferable and was associated with a lower mortality rate (46.3%) than was late admission (>7 days in the hospital, 57.6% mortality rate).7 In patients with severe CAP, the expected mortality rate for those admitted to the ICU is 35% to 40%, but higher rates have been observed if the percentage of patients who are mechanically ventilated is higher than 60%, implying that the prognosis is worse if ICU care is first provided late in the course of illness, after the onset of overt respiratory failure.7 Restrepo and colleagues noted that late admission to the ICU, more than 24 hours after presentation in patients with severe CAP, resulted in a higher mortality rate than did early admission (47.4% vs. 23.2%, p = 0.02; hazard ratio 2.6).39
Pneumonia Acquired in the Hospital
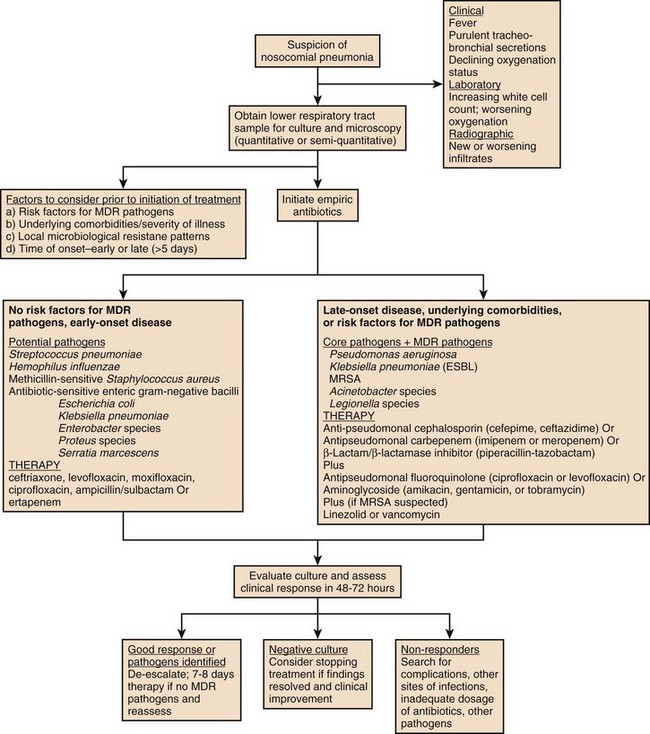
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection in the United States,40 and current guidelines have emphasized the importance of the time of onset of the disease and the presence of risk factors for infection due to MDR pathogens in defining the approach to therapy. The major goals are early, appropriate antibiotic therapy in adequate doses and appropriate de-escalation of initial antibiotic therapy, based on microbiologic cultures and the clinical response of the patient.2
Unlike CAP, there are no well-studied scoring systems to assess disease severity in patients with HAP and VAP. Clinical studies have used surrogate markers such as the APACHE II, or the Sequential Organ Failure Assessment (SOFA) score as a measure of organ dysfunction and severity assessment.41 The Clinical Pulmonary Infection Score (CPIS) was originally introduced for diagnoses in patients with VAP (described later in the text), but it has also been used to follow the response of VAP patients to therapy.42 The value of APACHE II, SOFA score, and CPIS in the prediction of mortality risk during VAP episodes was assessed in a prospective observational study, which found that an APACHE II score greater than 16 (determined at the time of VAP diagnosis) was the only independent predictor of the mortality risk (OR 5; p = 0.019) in a logistic regression analysis.43 In a prospective study by Lisboa and colleagues from Spain, including 441 patients with VAP in three multidisciplinary ICUs, the mortality risk in patients with VAP was assessed by a simple four-variable VAP PIRO score: comorbid conditions (COPD, immunocompromised state, heart failure, cirrhosis, or chronic renal failure); bacteremia; systolic blood pressure less than 90 mm Hg; and ARDS.44 Patients were stratified into three levels of risk: (1) mild, 0 to 1 points; (2) high, 2 points (hazard ratio, 2.14); and (3) very high, 3 to 4 points (hazard ratio, 4.63). More recently, a newer and easier scoring system, the IBMP-10, was proposed and is based on the presence of immunodeficiency; (2) blood pressure less than 90 mm Hg (systolic) or less than 60 mm Hg (diastolic); multilobar infiltrates noted on a chest radiograph; platelet count less than 100,000/µL; and duration of hospitalization before the onset of VAP of more than 10 days.45 IBMP-10 score was comparable to the APACHE II score in its ability to predict mortality risk in patients with VAP but needs further validation in prospective studies.
Risk Factors Associated with Nosocomial Pneumonia
The 2005 ATS/IDSA guidelines characterized the predisposing factors for developing nosocomial pneumonia as modifiable and nonmodifiable. Mechanical ventilation (for > 2 days) is the most important risk factor for nosocomial pneumonia, but other identified risks include age older than 60, malnutrition (serum albumin < 2.2 g/dL), acute lung injury (ARDS), coma, burns, recent abdominal or thoracic surgery, multiple organ failure, transfusion of more than 4 units of blood, transport from the ICU, prior antibiotic therapy, elevation of gastric pH (by antacids or histamine-type 2 blocking agents), large volume aspiration, use of a nasogastric tube (rather than a tube placed in the jejunum or a tube inserted through the mouth), use of inadequate endotracheal tube cuff pressure, prolonged sedation and paralysis, maintaining patients in the supine position in bed, use of total parenteral nutrition (TPN) feeding rather than enteral feeding, and repeated reintubation.2 When a patient is mechanically ventilated, the risk of pneumonia is greatest in the first 5 days (3% per day), and declines thereafter to a risk of 2% per day for days 6 to 10, and to a rate of 1% per day or lower after this.46 Noninvasive ventilation for respiratory failure is associated with a much lower risk of pneumonia than endotracheal intubation.
The relation between pneumonia and ARDS is particularly interesting, because up to one third of all cases of ARDS may be the result of pneumonia, and in some series, pneumonia is the most common cause of acute lung injury. In addition, secondary nosocomial pneumonia is the most common infection complicating the course of established ARDS.47,48 Seidenfeld and coworkers reported better survival in patients with ARDS in the absence of infection, but a subanalysis for pneumonia was not available.48 In a study by Chastre and colleagues of 243 consecutive patients who required mechanical ventilation 48 or more hours, 55% of the ARDS patients developed VAP compared to 28% without ARDS (p = 0.0005).49 Most patients who developed VAP had been treated with prior broad-spectrum antibiotics, and were infected with MDR pathogens (MRSA, nonfermenting gram-negative bacilli, and Enterobacteriaceae).
Mortality Risk from Ventilator-Associated Pneumonia
Mortality rates from VAP can be as high as 50% to 70%, and case-control studies have documented death directly attributable to the presence of pneumonia.50 The patients with HAP often have other associated comorbid illnesses that predispose them to a high risk of dying, independent of the presence of pneumonia. Attributable mortality rate, defined as death directly related to infection and not due to underlying conditions, is challenging to measure, but older studies reported a higher mortality rate for patients with VAP than for similarly ill ventilated patients without VAP (52.4% with VAP, compared to 22.4% for patients without).51 Recently, however, Bekaert and colleagues assessed the population-attributable risk of ICU VAP by taking into account the confounding that is caused by time-dependent severity-of-illness indicators.52 They estimated that 4.4% (95% confidence interval, 1.6-7.0%) of the deaths in the ICU on day 30 and 5.9% (95% confidence interval, 2.5-9.1%) on day 60 were attributable to VAP. This corresponds to an ICU attributable mortality rate for VAP of about 1% on day 30 and 1.5% on day 60.
Antibiotic-resistant organisms may add to the mortality risk of VAP, not because of increased virulence, but rather because these organisms are often not anticipated, and when present, are often initially treated with ineffective antibiotic regimens.53 The factor associated with the greatest impact on attributable mortality rate is the accuracy and timeliness of initial antibiotic therapy. Use of the wrong therapy, or delays in the initiation of therapy, are the most important predictors of VAP mortality rate.54 Initial appropriate therapy (using an agent to which the etiologic pathogen is sensitive) can reduce mortality rate, but administration of correct therapy at a later date, after initially incorrect therapy, may not effectively reduce mortality rate.54 The benefit of accurate empiric therapy may not apply to all patients, but may be greatest for those infected with P. aeruginosa or S. aureus55 and for those without the most severe degree of multiple organ dysfunction at the time of therapy.56 For some patients, even using the correct therapy does not reduce the risk of death if it is not given in adequate doses and if the therapy does not reach the site of infection.
Although a number of host and bacteriologic factors enhance the mortality risk of nosocomial pneumonia, development of a superinfection, as opposed to primary nosocomial pneumonia, is a particularly ominous finding. Rello and associates observed that pulmonary superinfection had a 67% mortality rate, whereas primary nosocomial pneumonia had a 38% mortality rate.57 In earlier studies, Graybill and coworkers observed a 62% mortality rate with superinfection pneumonia, compared to a 40% mortality rate for primary nosocomial lung infection.58 These data, as well as information from Fagon and colleagues, emphasize the important role of prior antibiotics in enhancing mortality risk, an outcome that is likely the result of secondary infection by more virulent pathogens.59,60 As a result, antibiotic use has two pivotal roles in prognosticating outcome from nosocomial pneumonia: outcome is improved if the correct therapy is chosen, but if this therapy is followed by superinfection, then mortality risk is increased, generally because these infections involve difficult-to-treat drug-resistant organisms. Closely related to antibiotic resistance is the presence of bacteremia in patients with VAP, which is associated with increased mortality risk.61
When therapy is given, it is important to decrease the number and spectrum of antimicrobial therapy once culture data become available, referred to as “de-escalation.” Several recent studies have demonstrated that the use of de-escalation is associated with lower mortality risk compared to escalation or compared to a strategy of making no effort to reduce antibiotic therapy.62–64 The choice of how to administer a specific agent can also affect outcome, and one study of MRSA VAP found that the mortality risk with intermittent infusion of vancomycin was twice as high as when this agent was administered by continuous infusion.65 Other risk factors for mortality (Box 42.5) include prolonged duration of ventilation, coma on admission, creatinine greater than 1.5, transfer from another ward to the ICU, the presence of certain high-risk pathogens (particularly an antibiotic-resistant organism such as P. aeruginosa, Acinetobacter spp., or S. aureus), bilateral radiographic abnormalities, age older than 60 years, ultimately fatal underlying condition, shock, prior antibiotic therapy, multiple system organ failure, nonsurgical primary diagnosis, and a rising APACHE II score during pneumonia therapy.2,66
Health Care–Associated Pneumonia
The ATS/IDSA guidelines included HCAP as a form of nosocomial pneumonia because these patients were at risk for infection with MDR pathogens because of recent contact with the health care environment. HCAP includes patients who were hospitalized in an acute care hospital for 2 or more days within 90 days of the infection; those who reside in a nursing home or long-term care facility; individuals who received recent intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days; and those attending a hospital or hemodialysis clinic. Recent clinical investigations have shown that HCAP includes a diverse group of patients, with some at risk for MDR organisms and others not. In fact, many HCAP patients have been treated successfully with a monotherapy regimen or with regimens used for CAP.13 Patients in the HCAP population who are at risk for MDR pathogens were those with severe illness or those with other risk factors including hospitalization in the past 90 days, antibiotic therapy in the past 6 months, poor functional status as defined by activities of daily living score, and immune suppression.
Pathogenesis
General Overview
The respiratory system has both innate and adaptive immunity, which helps prevent potentially harmful pathogens from adhering to the respiratory mucosa and proliferating. Multiple defense barriers in the conducting and gas exchange surfaces of the respiratory tract filter out invading pathogens. The combined effects of the physical, innate, and acquired host defense systems serve to recognize, localize, kill, and remove pathogens. The pathogens that reach the conducting airways are exposed to the soluble constituents in airway fluids. Multiple antimicrobial peptides, such as lysozyme (lytic to bacterial membranes); lactoferrin (which excludes iron from bacterial metabolism); IgA and IgG; and defensins are present in the airway mucosa and pathogens are expelled by the coordinated mucociliary system and cough reflex. Particles 1 µm and smaller reach the alveolar surface and interact with the alveolar macrophages and other components such as IgG, complement, surfactant, and surfactant-associated proteins. Bacteria are opsonized by IgG, complement, or surfactant proteins SP-A and SP-D and are ingested by alveolar macrophages. Pathogen recognition is mediated by toll-like receptors. They initiate local inflammatory response by releasing interleukins and cytokines and carry microbial antigens into the interstitium and to regional lymph nodes where they are taken up by specialized dendritic cells and presented to responding lymphocytes to initiate adaptive immune responses.67
Pneumonia develops when these host defenses are overwhelmed by the invading microorganisms (Fig. 42.1). This may occur because the patient has an inadequate immune response, often as the result of underlying comorbid illness, which can lead to anatomic abnormalities (endobronchial obstruction, bronchiectasis), disease-associated immune impairment, or therapy-induced dysfunction of the immune system (corticosteroids, endotracheal intubation).2,68,69 Pneumonia can also occur in patients who have an adequate immune system if the host defense system is overwhelmed by a large inoculum of bacteria (massive aspiration) or by a particularly virulent organism to which the patient has no preexisting immunity (such as an endemic virus) or to which the patient has an inability to form an adequate immune response. With this paradigm in mind, it is easy to understand why previously healthy individuals develop infection with virulent pathogens such as viruses (influenza), Legionella pneumophila, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and S. pneumoniae. However, for chronically ill patients, it is possible for them to be infected not only by these virulent organisms but also by organisms that are not highly virulent. Owing to host defense impairments, organisms that commonly colonize these patients can cause infection as a result of an inadequate immune response. These organisms include enteric gram-negative bacteria (E. coli, Klebsiella pneumoniae, P. aeruginosa, Acinetobacter spp.) and fungi (Aspergillus and Candida spp.). There can also be genetic variations in the immune response, making some patients prone to overwhelming infection, due to an inadequate response, and others prone to acute lung injury, due to an excessive immune response.32 In fact, the failure to localize the immune response to the respiratory site of initial infection may explain why some patients develop acute lung injury and sepsis as the inflammatory response extends to the entire lung and systemic circulation.70
Route of Entry
Bacteria can enter the lung via several routes, but aspiration from a previously colonized oropharynx is the most common way that patients develop pneumonia. Although most pneumonias result from microaspiration, patients can also aspirate large volumes of bacteria if they have impaired neurologic protection of the upper airway (stroke, seizure), or if they have gastrointestinal illnesses that predispose to vomiting. Other routes of entry include inhalation, which applies primarily to viruses, L. pneumophila, and Mycobacterium tuberculosis; hematogenous dissemination from extrapulmonary sites of infection (right-sided endocarditis); and direct extension from contiguous sites of infection. In critically ill hospitalized patients, bacteria can also enter the lung from a colonized stomach (spreading retrogradely to the oropharynx, followed by aspiration), from a colonized or infected maxillary sinus, from colonization of dental plaque, or directly via the endotracheal tube (from the hands of staff members). Studies have also shown that the use of nasal tubes (into the stomach or trachea), can predispose to sinusitis and pneumonia, but that a gastric source of pneumonia pathogens in ventilated patients is not common.71,72
Colonization of the Upper Respiratory System and Digestive Tract
Colonization of the upper respiratory and digestive tracts with pathogenic microorganisms is a major risk factor for the development of pneumonia.73 Factors enhancing airway colonization include antibiotic therapy, endotracheal intubation, smoking, malnutrition, general surgery, dental plaque, and therapies that elevate the gastric pH.74 The stomach can be the source of 30% of the enteric gram-negative bacteria that colonize the trachea of intubated patients, but it is difficult to decide if such colonization leads to pneumonia. In a recent multicenter randomized controlled study by Lacherade and colleagues, the use of intermittent subglottic secretion drainage resulted in a significant reduction in microbiologically confirmed VAP compared to control subjects (14.8% vs. 25.6%; P = 0.02),75 without a significant difference in the duration of mechanical ventilation and hospital mortality rate. The findings suggest that interruption of gastric to oral to tracheal transmission of bacteria can help to prevent VAP. The importance of oral colonization in VAP pathogenesis was shown in a study by DeRiso and coworkers, which demonstrated that the use of the oral antiseptic chlorhexidine in 353 patients undergoing coronary artery bypass surgery significantly reduced the incidence of respiratory tract infections by 69%.76 It has become difficult to separate colonization from infection in intubated patients, particularly with the recognition of ventilator-associated tracheobronchitis (VAT).77 VAT patients have an infection, with clinical signs (fever, leukocytosis, and purulent sputum) and microbiologic findings (Gram stain with bacteria and leukocytes, with either a positive semiquantitative or a quantitative sputum culture) but the absence of a new infiltrate on chest radiograph. It remains uncertain if VAT can progress to VAP, or if the two events are independent of one another. If VAT is a precursor of VAP, then serial surveillance cultures of endotracheal aspirates could identify MDR pathogens for targeted antibiotic treatment when VAT is present, in an effort to prevent VAP.78
The Role of Respiratory Therapy Equipment and Endotracheal Tubes
The endotracheal tube bypasses the filtration and host defense functions of the upper airway and can act as a conduit for direct inoculation of bacteria into the lung. This route may be particularly important if bacteria form a biofilm and colonize the inside of the endotracheal tube itself.79,80 This can occur if tracheobronchial organisms reach the endotracheal tube, a site where they are able to proliferate free from any impediment by the host defense system. Bacteria commonly do grow at this location in a biofilm, which promotes the growth of MDR organisms.80 The biofilm represents a “sequestered nidus” of infection on the inside of the endotracheal tube, and particles can be dislodged every time the patient is suctioned. This is one of the mechanisms explaining the strong association between endotracheal intubation and pneumonia. Given the presence of biofilm in endotracheal tubes, it may be tempting to regularly reintubate patients and use a fresh tube, but this approach is not recommended because reintubation is itself a risk factor for VAP.81 Afessa and colleagues reported that the use of the silver-coated tube reduced the mortality rate of patients who developed VAP despite having the silver tube in place, compared with the mortality rate of patients who developed VAP with a standard tube in place (14% vs. 36%; P = 0.03).82 However, the overall mortality rate was high in patients with the silver tube, and the rate of death from respiratory failure was higher in the silver tube–treated patients than those with the standard tube (19% vs. 11%, P = 0.02), raising doubts on the impact of using the silver-coated tube on VAP patients. Other interventions, such as use of devices to remove biofilm from the tube interior and the coating of tubes with mimics of antimicrobial peptides (ceragenins), are in development to interrupt the pathogenesis of VAP.83
Just as a patient’s own tracheobronchial flora can spread to the endotracheal tube and amplify to large numbers, a similar phenomenon can occur in respiratory therapy equipment and in ventilator circuits.84,85 Ventilator circuit colonization has been studied and the greatest bacterial numbers are found at sites nearest to the patient, not the ventilator, suggesting that circuit contamination originates from the patient.84 One highly contaminated site is the condensate in the tubing, and this material can inadvertently be inoculated into patients if the tubing is not handled carefully. Because condensate colonization occurs in 80% of tubings within 24 hours, it does not appear that frequent ventilator circuit changes are useful or even able to reduce the risk of pneumonia; in one study, tubing changes every 24 hours (rather than every 48 hours) served as a risk factor for pneumonia.86 Although most patients have ventilator tubing changed every 48 hours, several studies have shown no increased risk of infection if tubing is never changed or changed infrequently.87,88 The use of heat moisture exchangers may be one way to avoid this problem, but they have had an inconsistent effect on preventing VAP. In addition, frequent changes of heat moisture exchangers (i.e., every 24 hours) have not been shown to have an impact on the incidence of VAP, and heat moisture exchangers should be changed no more frequently than every 48 hours.89
Clinical Features
Historical Information
Pneumonia is generally characterized by symptoms of fever, cough, purulent sputum production, and dyspnea in a patient with a new or progressive lung infiltrate, with or without an associated pleural effusion. In nonventilated patients, cough is the most common finding and is present in up to 80% of all CAP patients, but is less common in those who are elderly, those with serious comorbid conditions, and individuals coming from nursing homes. Patients with CAP and an intact immune system generally have classic pneumonia symptoms, but the elderly patient can have a nonrespiratory presentation with symptoms of confusion, falling, failure to thrive, altered functional capacity, or deterioration in a preexisting medical illness, such as congestive heart failure.90 The absence of clear-cut respiratory symptoms and an afebrile status have themselves been identified as predictors of an increased risk of death. Pleuritic chest pain is also commonly seen in patients with CAP, and in one study, its absence was also identified as a poor prognostic finding.91 A recent study pointed out that nonsteroidal anti-inflammatory drugs prior to hospitalization were associated with a higher chance of developing pleuropulmonary complications (OR = 8.1).92
Certain clinical conditions are associated with specific pathogens in patients with CAP, and these associations should be evaluated when obtaining a history (Box 42.6).19 For example, if the presentation is subacute, following contact with birds, rats, or rabbits, then the possibility of psittacosis, leptospirosis, tularemia, or plague, respectively, should be considered. Coxiella burnetti (Q fever) is a concern with exposure to parturient cats, cattle, sheep, or goats; hantavirus with exposure to mice droppings in endemic areas; and Legionella with exposure to contaminated water sources (saunas). Following influenza, superinfection with pneumococcus, S. aureus (including MRSA), and H. influenzae should be considered. With travel to endemic areas in Asia, the onset of respiratory failure after a preceding viral illness should lead to suspicion of a viral pneumonia, which could be severe acute respiratory syndrome (SARS) or avian influenza.93 Endemic fungi (coccidioidomycosis, histoplasmosis, and blastomycosis) occur in well-defined geographic areas and may present acutely with symptoms that overlap with acute bacterial pneumonia. Clinicians should also be cognizant of the risk of bioterrorism and be able to detect clinical features of Bacillus anthracis, Yersinia pestis, and Francisella tularensis.
Nosocomial pneumonia often presents with less definitive clinical findings, particularly in those who are mechanically ventilated, in which the clinical diagnosis is made in patients with a new or progressive radiographic infiltrate, along with some indication that infection is present (fever, purulent sputum, or leukocytosis). In addition, some patients can have purulent sputum and fever, without a new infiltrate, and be diagnosed as having purulent tracheobronchitis, an infectious complication of mechanical ventilation (VAT) that may also require antibiotic therapy, but is not pneumonia.2 In taking a history from a patient with nosocomial pneumonia, it is important to identify if there are risk factors present for drug-resistant organisms. For ventilated patients, these factors include prolonged ICU stay (≥5 days), recent antibiotic therapy, and the presence of HCAP risks.2,60 In CAP patients, risk factors for drug-resistant pneumococcus include recent β-lactam therapy, exposure to a child in day care, alcoholism, immune suppression, and multiple medical comorbid conditions.94,95
Physical Examination
Physical findings of pneumonia include tachypnea, crackles, rhonchi, and signs of consolidation (egophony, bronchial breath sounds, dullness to percussion). Patients should also be evaluated for signs of pleural effusion. In addition, extrapulmonary findings should be sought to rule out metastatic infection (arthritis, endocarditis, meningitis) or to add to the suspicion of an “atypical” pathogen, such as M. pneumoniae or C. pneumoniae, which can lead to such complications as bullous myringitis, skin rash, pericarditis, hepatitis, hemolytic anemia, or meningoencephalitis. One of the most important ways to recognize severe CAP early in the course of illness is to carefully count the respiratory rate.96,97 In the elderly, an elevation of respiratory rate can be the initial presenting sign of pneumonia, preceding other clinical findings by as much as 1 to 2 days and tachypnea is present in over 60% of all patients, being present more often in the elderly than in younger patients with pneumonia.97 In addition, the counting of respiratory rate can identify the patient with severe illness, who commonly has a rate more than 30 breaths/minute. Gurgling sounds heard during quiet breathing or speech have been independently associated with HAP.98 Overall, the clinical diagnosis of pneumonia has moderate sensitivity (79%) and specificity (66%),99 and in general, definitive imaging studies are required in critically ill patients.
Etiologic Pathogens
Community-Acquired Pneumonia
The microorganisms causing CAP may vary according to geographic area and underlying patient risk factors. Even with extensive diagnostic testing, an etiologic agent is defined in only about half of all patients with CAP, pointing out the limited value of diagnostic testing, and the possibility that we do not know all the organisms that can cause CAP. The most common cause of CAP is pneumococcus (S. pneumoniae), an organism that is frequently (at least 40% of the time) resistant to penicillin or other antibiotics, leading to the term drug-resistant Streptococcus pneumoniae (DRSP). Fortunately, most penicillin resistance in the United States is still more commonly of the “intermediate” type (penicillin minimum inhibitory concentration, or MIC, of 0.1 to 1.0 mg/L) and not of the high-level type (penicillin MIC of 2.0 mg/L or more).100 Pneumococcal resistance to other antibiotics is also common, including macrolides and trimethoprim-sulfamethoxazole, but the clinical relevance and impact on outcome of these in vitro findings is uncertain, and most experts believe that only organisms with a penicillin MIC of 4 mg/L or more are associated with an increased risk of death.101 Recently, the U.S. definitions of resistance have changed for nonmeningeal infection, with sensitive being defined by a penicillin MIC of 2 mg/L or less, intermediate as an MIC of 4 mg/L, and resistant as an MIC of 8 mg/L or more. With these new definitions of resistance, very few pathogens will be defined as resistant, but those that are may affect outcome.
All patients with severe CAP should be considered to be at risk for DRSP, and in addition, those admitted to the ICU can have infection with atypical pathogens, which account for up to 20% of infections, either as primary infection or as copathogens. The identity of these organisms varies over time and geography. In some areas, Legionella is a common cause of severe CAP, although in others C. pneumoniae or M. pneumoniae predominate.30 Other important causes of severe CAP include H. influenzae, S. aureus, which includes MRSA (especially after influenza), and enteric gram-negative organisms (including P. aeruginosa) in patients with appropriate risk factors (particularly bronchiectasis and steroid-treated COPD).
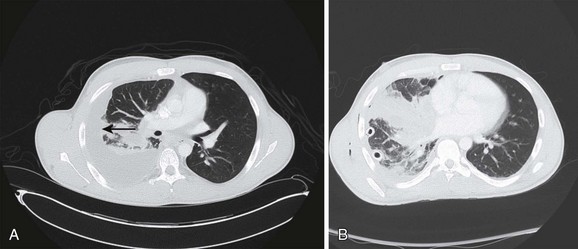
Recently, a toxin-producing strain of MRSA has been described to cause CAP in patients after influenza and other viral infections (Fig. 42.2). This community-acquired MRSA (CA-MRSA) is biologically and genetically distinct from the MRSA that causes nosocomial pneumonia, being more virulent and necrotizing, and associated with the production of the Panton-Valentine leukocidin (PVL).102,103 Viruses can be a cause of severe CAP, including influenza virus, as well as parainfluenza virus and epidemic viruses such as coronavirus (which caused SARS) and avian influenza.93 Viral pneumonia (SARS and influenza) can lead to respiratory failure, and occasionally tuberculosis or endemic fungi can result in severe pneumonia. Recent experience with the 2009 pandemic influenza A (H1N1) infection showed that critical illness was associated with severe hypoxemia and that multisystem organ failure occurred rapidly after hospital admission and mostly in young adults and pregnant patients.104
Unusual etiologic organisms should be considered, especially in patients who have epidemiologic risk factors for specific pathogens, as discussed earlier. In addition, certain “modifying factors” may be present that increase the likelihood of CAP caused by certain pathogens.2 Thus, the risk factors for DRSP include β-lactam therapy in the past 3 months, alcoholism, age older than 65 years, immune suppression, multiple medical comorbid conditions, and contact with a child in day care.94 Risk factors for gram-negative infections include residence in a nursing home, underlying cardiopulmonary disease, multiple medical comorbid conditions, probable aspiration, recent hospitalization, and recent antibiotic therapy. Many of these patients who are at risk for gram-negative infections would now be reclassified as having HCAP.2,105
Some ICU patients are at risk for pseudomonal infection, although others are not, and the risk factors for P. aeruginosa infection are structural lung disease (bronchiectasis), corticosteroid therapy (>10 mg prednisone/day), broad-spectrum antibiotic therapy for more than 7 days in the past month, previous hospitalization, and malnutrition.2 Although aspiration has often been considered a risk factor for anaerobic infection, a study of severe CAP in elderly patients with aspiration risk factors found that this population was very likely to have gram-negative infection, and that, using sensitive microbiologic methods, anaerobes were uncommon.106 Previous studies looking at microbiologic causes in patients admitted with CAP to the ICU have shown wide variation in the results, with a limitation being that not all microbiologic tests were applied systematically for all patients. A recent prospective observational study by Cillóniz and coworkers on 362 consecutive adult patients with CAP admitted to the ICU within 24 hours of presentation reported that 11% of all cases were polymicrobial, with S. pneumoniae, respiratory viruses, and P. aeruginosa being the commonest isolated pathogens.107 Other studies have also shown a high frequency of multiple pathogens, including a mixture of bacterial and “atypical” pathogens, which may explain why most patients with severe CAP need to be treated empirically for both groups of organisms. Chronic respiratory disease and ARDS criteria were independent predictors of polymicrobial causes and inappropriate initial antimicrobial treatment was more frequent in the polymicrobial infection group compared with the monomicrobial infection group (39% vs. 10%, P < 0.001).
Nosocomial Pneumonia
All patients with this illness are at risk for infection with a group of bacteria referred to as “core pathogens,” which include pneumococcus, H. influenzae, MSSA, and nonresistant gram-negative organisms (E. coli, Klebsiella spp., Enterobacter spp., Proteus spp., and Serratia marcescens). In addition, some patients are also at risk for infection with other organisms, depending on the presence of risk factors such as prolonged hospitalization (≥5 days), prior antibiotic therapy, recent hospitalization (within 90 days), recent antibiotic therapy, residence in a nursing home, or need for chronic care outside the hospital.2,60 Patients with these risk factors can possibly be infected with MDR gram-positive and gram-negative organisms including MRSA, P. aeruginosa and Acinetobacter spp. Recognition of the multiple risk factors associated with these resistant pathogens has made it clear that patients with either early-onset or late-onset nosocomial pneumonia can be infected with MDR organisms. In addition, up to 40% of patients with VAP have polymicrobial infection, involving multiple pathogens.108 In immunosuppressed patients uncommon pathogens such as Aspergillus species, Candida species, L. pneumophila, Pneumocystis jiroveci, Nocardia species, and viruses such as cytomegalovirus should be suspected.74
Most data on nosocomial pneumonia bacteriology come from patients with VAP, and the cause in nonventilated patients is presumed to be similar, based on the presence of risk factors for drug-resistant pathogens. In patients with VAP, infection with enteric gram-negative organisms is more common than infection with gram-positive organisms, although the frequency of MRSA infection is increasing in this population, as is infection with Acinetobacter spp.109 In a recent prospective study comparing the clinical and microbiologic characteristics of 315 episodes of ICU-acquired pneumonia (VAP 52% vs. non-VAP 48%) the types of etiologic pathogens and outcome were similar regardless of whether pneumonia is or is not acquired during mechanical ventilation.110
HCAP patients have been included in the nosocomial pneumonia guidelines as being a group at risk for infection with MDR gram-positive and gram-negative organisms.2 Although many ICU-admitted patients with this illness are infected with these organisms, one study of nursing home patients requiring mechanical ventilation for severe pneumonia showed that these organisms were not present if the patient with severe pneumonia had not received antibiotics in the preceding 6 months and was also of a good functional status (as defined by activities of daily living).111 In approaching the bacteriology of nosocomial pneumonia, it is important to recognize that each hospital, and each ICU within a given hospital, can have its own unique flora and antibiotic susceptibility patterns, and thus therapy needs to be adapted to the organisms in a given institution, which can change over time.112 In addition, it is especially important to know this information because antibiotic resistance is a common factor contributing to initially inappropriate empiric antibiotic therapy. Choosing the wrong empiric therapy has been a particular problem for organisms such as P. aeruginosa, Acinetobacter spp., and MRSA.53 These highly resistant organisms can be present in as many as 60% of patients who develop VAP after at least 7 days of ventilation and who have also received prior antibiotic therapy.2,60
Diagnostic Issues
General Considerations
Therapy of severe pneumonia should be started empirically, and laboratory and imaging studies should be done to corroborate the diagnosis and identify the etiologic agent when possible. A specific causal diagnosis is obtained in less than 50% of patients with CAP and the major focus of diagnostic testing is to assess the severity of illness and allow early identification of pneumonia-related complications.14 However, in most cases of VAP an etiologic agent can be demonstrated, but the presence of a positive culture cannot reliably distinguish infection from colonization.
Radiographic Evaluation
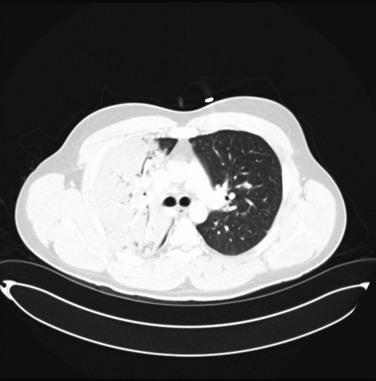
In all forms of pneumonia, a chest radiograph is used to identify the presence of a lung infiltrate, but in some clinical settings, especially in suspected VAP, there can be noninfectious causes for the radiographic abnormality. A chest radiograph is used to identify complicated and severe illness, such as multilobar infiltrates, cavitation, or a loculated pleural effusion (suggesting an empyema). Chest radiographic patterns are generally not useful for identifying the cause of CAP, although hematogenous dissemination will have bilateral peripheral infiltrates, and aspiration most commonly involves the superior segment of the right lower lobe or posterior segment of the right upper lobe. Findings such as pleural effusion (pneumococcus, H. influenzae, M. pneumoniae, pyogenic streptococci) and cavitation (P. aeruginosa, S. aureus, anaerobes, MRSA, tuberculosis) can suggest certain groups of organisms. The chest radiograph may be suboptimal in patients with early infection, severe granulocytopenia, and bullous emphysema and in obese patients. In those instances, it is reasonable to repeat a chest radiograph in 24 to 48 hours.19 A computed tomography (CT) scan of the chest has better sensitivity in diagnosing an infiltrate (Fig. 42.3), but routine CT scan has not been shown to be associated with improved outcomes. Thin-section CT cannot reliably distinguish bacterial pneumonias from other causes, especially in patients with underlying lung diseases or immunocompromised conditions except in cases of Pneumocystis pneumonia, which has a characteristic pattern.113,114 Chest ultrasound is progressively being used to identify the safe site for sampling of the pleural fluid. In the ICU, radiographs are often done at the bedside, and are of such poor quality that pneumonia may be missed or confused with other diagnoses.
Routine Laboratory Tests
A complete blood count, chemistry panel, and arterial blood gas analysis are necessary for all patients admitted to the ICU. Leukopenia is seen in patients with severe pneumonia and sepsis caused by pneumococcus or by gram-negative organisms. Both leukocytosis and leukopenia are associated with poor prognosis in patients with CAP19 and with thrombocytosis and thrombocytopenia.115 Hyponatremia (serum sodium <130 mEq/L) on admission is associated with poor outcome in CAP patients.116 Elevated liver function tests can be seen in a variety of viral and bacterial pneumonias secondary to atypical agents such as Legionella spp. and Mycoplasma spp., Q fever, tularemia, and psittacosis, as well as in pneumococcal infection.
Blood Culture
The ATS/IDSA 2007 guidelines recommend that patients with severe CAP and patients with other risk factors, listed later, should have two sets of blood cultures. These results are more likely to be positive if the patient has not received antibiotics at the time of sampling or has any of the following risk factors: underlying liver disease, systolic blood pressure less than 90 mm Hg, fever less than 35° C or greater than 40° C, pulse greater than 125 beats/minute, BUN greater than 10.71 mmol/L (30 mg/dL), serum sodium less than 130 mmol, and leukocyte count less than 5000 or greater than 20,000 cells/mL.117 Blood cultures should also be considered in patients with asplenia, cavitary infiltrates, or a history of ongoing alcohol abuse. The presence of bacteremia may not worsen prognosis but does allow identification of drug-resistant organisms, and most positive blood cultures in CAP reveal pneumococcus. However, in nosocomial pneumonia, the presence of bacteremia does not necessarily mean pneumonia, and some other extrapulmonary source of infection should be ruled out, especially if the organism present in the blood culture is different from the one in the respiratory tract. In a recent prospective, multicenter trial involving 689 patients with nosocomial pneumonia, a positive blood culture was associated with higher mortality rate (57.1% vs. 33%, P < 0.001) and a prolonged length of ICU stay compared to nonbacteremic patients.61
Sputum Examination
Sputum culture should be accompanied by a Gram stain to guide interpretation of the culture results but not to focus initial antibiotic therapy. In some situations, Gram stain can be used to broaden initial empiric therapy by enhancing the suspicion for organisms that are not covered in routine empiric therapy (such as S. aureus being suggested by the presence of clusters of gram-positive cocci, especially during a time of epidemic influenza). A good specimen contains fewer than 10 squamous epithelial cells and more than 25 polymorphonuclear cells per low-power field. The routine testing of expectorated sputum is not useful in the absence of a helpful Gram stain in patients with CAP. In ventilated patients the presence of pathogenic organisms in sputum culture is not diagnostic, because this finding cannot separate oropharyngeal and tracheobronchial colonization from parenchymal lung infection. In addition, some ventilated patients can have VAT, an illness with all the clinical features of pneumonia, but with no new lung infiltrate, and this illness may require antibiotic therapy and involve the same pathogens, present in high concentrations, as are present in VAP.2
Invasive Cultures
Bronchoscopy is not indicated as a routine diagnostic test, but may be needed in some patients with severe forms of CAP to establish an etiologic diagnosis. In these patients, the results of diagnostic testing can often be used to focus the initially broad-spectrum empiric therapy to a simpler regimen.118 In patients with HAP the sputum culture is often not reliable, and in an effort to make the diagnosis more secure, and to avoid the treatment of colonization and not infection, some investigators have used quantitative sampling of lower respiratory secretions collected either bronchoscopically (bronchoalveolar lavage, protected specimen brush) or nonbronchoscopically (endotracheal aspirate, nonbronchoscopic catheter lavage) in patients with suspected VAP. When quantitative cultures are collected, the presence of pneumonia is defined by the growth of bacteria above a predefined threshold concentration.119,120 Although the results can guide therapy decisions, most clinicians use antibiotic therapy, regardless of quantitative culture data, in patients who have clinical signs of sepsis and suspected pneumonia. Regardless of whether quantitative cultures are used, all patients with suspected nosocomial pneumonia should have a lower respiratory tract culture collected prior to the start of antibiotic therapy. If this is not a quantitative culture, then a sputum or tracheal aspirate should be obtained and the findings reported “semiquantitatively” as light, moderate, or heavy growth of bacteria.2,120 Unfortunately, a negative culture is difficult to interpret if the patient has had initiation or change in antibiotic therapy in the preceding 72 hours. If, however, either a quantitative or semiquantitative culture is negative, or does not show a highly resistant pathogen, and antibiotics have not been changed in the past 72 hours, then the therapy can often be stopped or focused to a narrower spectrum.2,121 A number of studies have examined the ability of quantitative cultures to impact outcome in VAP, and in general, although results are conflicting, there is no consistent evidence that they improve patient outcome or lead to more de-escalation of antibiotics.122
Urinary Antigen, Serologic, and Polymerase Chain Reaction Testing
Routine serologic testing is not recommended, and a serologic diagnosis of a specific pathogen is based on acute and convalescent blood serologic examinations showing a fourfold increase in titers. This type of testing is used for epidemiologic diagnosis of atypical agents such as C. pneumoniae, C. psittaci, Coxiella burnetii (Q fever), and M. pneumoniae. However, in patients with severe illness, the diagnosis of Legionella infection can be made by urinary antigen testing, which is the test that is most likely to be positive at the time of admission, but a test that is specific only for serogroup I infection.19,123 Examination of concentrated urine for pneumococcal antigen may also be valuable. Urinary pneumococcal antigen has a sensitivity of 50% to 80% and specificity of over 90%. False-positive tests are seen in patients who had pneumonia within the previous 3 months. Polymerase chain reaction (PCR) assays are used for the detection of viruses (as was the case in the H1N1 epidemic) and other agents such as M. tuberculosis. Direct immunofluorescence or enzyme immunoassay is used for detection of viral antigens such as influenza, parainfluenza, RSV, and adenovirus. The impact of a positive test on management is still unclear, but a negative test is valuable in directing a focused antibiotic regimen.
Role of Biomarkers
Serum Biomarkers
Inflammatory biomarkers like CRP, PCT, midregional proadrenomedullin, midregional proatrial natriuretic peptide (MR-proANP), proarginine-vasopressin, proendothelin-1, and the interleukins have been used to distinguish bacterial infection from viral infection. Ramirez and associates showed that inflammatory biomarkers like PCT, CRP, tumor necrosis factor-α, and interleukin 6 levels were higher in patients admitted to the ICU, including those with delayed ICU admission, than those not needing ICU care.28
PCT is a “hormokine” (hormone with cytokine-like behavior), a peptide precursor of the hormone calcitonin, with a half-life of 25 to 30 hours. It is produced in response to bacterial microbial toxins and inhibited by virus-related cytokines. In patients with CAP, PCT levels on admission correlate with disease severity and identify high-risk patients with similar accuracy as the CURB-65 score and with a higher prognostic accuracy compared with CRP and leukocyte count.27,124 In fact, if the PCT level on admission is low, prognosis is good, regardless of PSI class or CURB-65 score.124 Serum levels tend to be high in patients with severe bacteremic CAP, and serial measurements help guide the duration of antibiotic therapy.125 Luyt and colleagues reported that PCT levels decreased during the clinical course of VAP but were significantly higher from day 1 to day 7 in patients with unfavorable outcomes.126 A recent prospective study by Bloos and colleagues on 175 patients with severe pneumonia admitted to the ICU (CAP, HAP, and VAP, equally distributed) showed that PCT levels were higher and remained persistently elevated in nonsurvivors compared to survivors.127 The initial and maximum PCT levels correlated with maximum SOFA score and the initial PCT levels were higher in CAP than in VAP patients (median; 2.4 vs. 0.7 ng/mL, P < 0.001) but not significantly different from HAP patients (2.2 ng/mL), but patients with CAP were more severely ill, with a higher APACHE II score. In another multicenter, prospective, parallel-group study in the ICU setting, patients were randomly assigned in a 1:1 ratio to PCT-guided (n = 311 patients) or control (n = 319) groups and antibiotic regimens were instituted based on a protocol with predefined cutoff levels of PCT. There was no difference in mortality rate between PCT group and control group at day 28 (21.2% vs. 20.4%),10 but patients in the PCT group had significantly more days without antibiotics than those in the control group (14.3 days vs. 11.6 days, P < 0.0001). This supports earlier data that a drop in PCT levels could be used to define when antibiotic treatment can be safely withdrawn.9
Recommended Testing for Community-Acquired Pneumonia
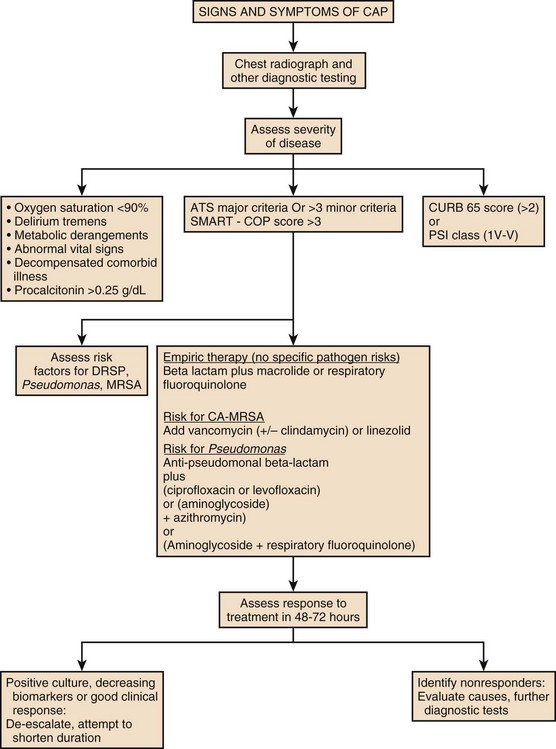
Severity assessment scores such as ATS/IDSA 2007 guidelines or SMART-COP can be used to determine the severity of the disease and serum biomarkers like PCT should be used to guide site of care decisions. In patients with no response to treatment or unrelenting high levels of biomarkers, further imaging or invasive diagnostic methods such as bronchoscopy and thoracentesis should be performed and an alternate diagnosis other than CAP should be considered. A suggested diagnostic/treatment approach is provided in Figure 42.4.
Recommended Testing for Nosocomial Pneumonia
Nosocomial pneumonia is diagnosed when a patient has been in the hospital for at least 48 to 72 hours and then develops a new or progressive infiltrate on chest radiograph, accompanied by at least two of the following three: fever, leukocytosis, and purulent sputum. As mentioned, these clinical findings may be sensitive but not specific for infection, and efforts to improve the clinical diagnosis of pneumonia have involved the previously mentioned CPIS.128 The CPIS uses six criteria (fever, purulence of sputum, white blood cell count, oxygenation, degree of radiographic abnormality, and presence of pathogens in the sputum), with each scored on a scale from 0 to 2, and pneumonia is diagnosed with a total score of at least 6 (out of a maximum of 12). The CPIS was modified by Singh and coworkers to include radiographic progression in order to improve diagnostic accuracy (Table 42.1).129 The CPIS at 72 hours was calculated on the basis of all seven variables and took into consideration the progression of the infiltrate and culture results of the tracheal aspirate specimen. A CPIS greater than 6 at baseline and at 72 hours was considered suggestive of pneumonia and indicated the need for a full course of antibiotic therapy. However, subsequent studies showed that CPIS has a low sensitivity and specificity for diagnosing VAP compared to quantitative cultures of bronchoalveolar lavage fluid, with considerable interobserver variability.130 Thus, the use of CPIS remains controversial and has been most successfully used in guiding treatment decisions for patients with a low likelihood of VAP and in guiding the duration of therapy and defining the response to treatment.42,131
Table 42.1
Modified Clinical Pulmonary Infection Score
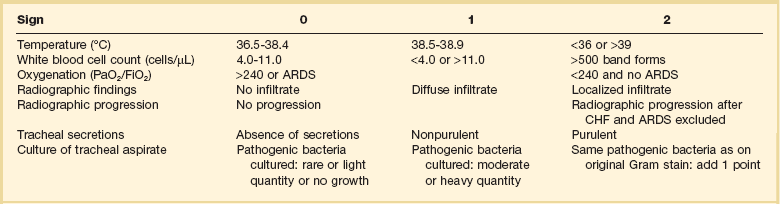
ARDS, acute respiratory syndrome; CHF, congestive heart failure.
Many studies have documented that VAP is diagnosed more often clinically than can be confirmed microbiologically, and the diagnosis is further obscured by the fact that most mechanically ventilated patients are colonized by enteric gram-negative bacteria, and thus the finding of potential pathogens in the sputum has limited diagnostic value. Many patients with suspected nosocomial pneumonia can have other diagnoses, which can be suggested by the rapidity of the clinical response and by the nature of the clinical findings. These diagnoses include atelectasis and congestive heart failure (very rapid clinical resolution), or in the case of a lack of response to therapy, inflammatory lung diseases, extrapulmonary infection (sinusitis, central-line infection, intra-abdominal infection), or the presence of an unusual or drug-resistant pathogen. The ATS/IDSA guidelines for nosocomial pneumonia have recommended that all patients have a lower respiratory tract sample collected prior to starting therapy and that the technique and culture method be one that the clinician is expert at performing and interpreting. Lower respiratory tract cultures can be obtained bronchoscopically or nonbronchoscopically and can be cultured quantitatively or semiquantitatively. A suggested algorithm for diagnosis and treatment of nosocomial pneumonia is provided in Figure 42.5.
Therapy
General Considerations
Timely initiation of appropriate antibiotics has significant mortality benefit for both severe CAP and VAP patients. The ATS and IDSA have developed algorithms for initial empiric therapy, based on the most likely etiologic pathogens in a given clinical setting.2,19 If diagnostic testing reveals a specific etiologic pathogen, then therapy can be focused on those results. De-escalation of antibiotics is valuable in the management of VAP because most patients are at risk for infection with MDR pathogens, requiring empiric broad-spectrum antibiotics, and this can lead to antibiotic overuse and further development of resistance if therapy is not adjusted once culture and clinical response data become available.64
Microbial Resistance
Patients in the ICU are at a high risk of developing infection, which is often due to antibiotic-resistant pathogens. The incidence of resistance is related to several factors: (1) induction of resistant strains (e.g., emergence of resistance during treatment because of the selection of new mutations), (2) selection of resistant strains (e.g., antimicrobial treatment may select and favor overgrowth of preexisting resistant flora), (3) introduction of resistant strains (e.g., cross-transmission from other patients or health care workers), and (4) dissemination of resistant strains (e.g., suboptimal infection control).132 There is growing concern about MDR pathogens in the United States, belonging to the group of “ESKAPE” organisms (Enterococcus faecium, S. aureus, K. pneumoniae, Acinetobacter baumanii, P. aeruginosa, and Enterobacter species).133,134 A recent prospective interventional study evaluated the effect of antimicrobial diversity on resistance caused by the ESKAPE pathogens in patients with VAP. The study was conducted over 44 months and examined three different antimicrobial strategies implemented consecutively: (1) a patient-specific period, when therapy was based on preexisting risk factors; (2) a 24-month scheduling period, in which antipseudomonal β-lactams were selected and prioritized quarterly during the first 12 months (prioritization periods) and restricted during the next 12 months (restriction periods); and (3) a mixing period over the next 10 months. VAP due to the ESKAPE pathogens increased significantly during scheduling compared with patient-specific and mixing periods (RR, 2.67 and 3.84, respectively).135 During the periods in which a diverse prescription pattern was implemented, there was a lower incidence of VAP due to ESKAPE organisms and the authors concluded that antibiotic strategies promoting diversity may prevent the emergence of MDR organisms.
Antibiotic Considerations
All patients with severe pneumonia treated in the ICU should receive initial empiric combination therapy. The rationale for this approach is to provide broad antimicrobial coverage to assure appropriate therapy. In addition, there has been the hope that, in the therapy of nosocomial pneumonia, combination therapy could prevent the emergence of resistance during therapy and potentially provide synergistic activity if a β-lactam antibiotic is combined with an aminoglycoside (for P. aeruginosa pneumonia). However, only with bacteremic P. aeruginosa pneumonia has combination therapy (generally with an aminoglycoside and a β-lactam) been shown to be superior to monotherapy.136,137 In the absence of bacteremia, an older meta analysis found no therapeutic benefit to adding an aminoglycoside to a β-lactam in critically ill patients, but the impact on appropriateness of therapy was not evaluated.137 Currently, with the high prevalence of MDR pathogens in nosocomial pneumonia, combination therapy increases the likelihood of appropriate therapy, compared to monotherapy. In fact, in the Canadian Clinical Trials group study of VAP, even though combination therapy did not reduce mortality rates, compared to monotherapy, it led to appropriate therapy 84% of the time when MDR pathogens were present, compared to 11% of the time when these organisms were treated with monotherapy.138
One practical problem to using aminoglycosides is that they have a narrow therapeutic-to-toxic ratio and a high incidence of nephrotoxicity, particularly in elderly patients. When these drugs are used, it is important to achieve high peak serum levels to optimize efficacy, but to also avoid elevated trough levels, which correlate with toxicity. When peak serum levels have been monitored, levels of more than 7 µg/mL for gentamicin and tobramycin and more than 28 µg/mL for amikacin have been associated with more favorable outcomes.139 One other limitation of aminoglycosides is their relatively poor penetration into bronchial secretions, achieving only 40% of the serum concentrations in the lung. In addition, antimicrobial activity is reduced at the low pH levels that are common in the bronchial secretions of patients with pneumonia.140 To address some of these concerns, it has now become standard to administer aminoglycosides by combining the total 24-hour dose into a single dose, rather than in divided doses. This approach is theoretically possible because bactericidal activity of aminoglycosides is optimized by high peak concentrations, and once-daily dosing relies on the prolonged postantibiotic effect of aminoglycosides. This approach might not only improve efficacy but also reduce (or at least not increase) toxicity because of low trough levels, and reduce the need for monitoring of serum levels. In one meta-analysis, this approach proved to have little advantage with regard to efficacy or safety.141 However, once-daily dosing is now standard, and optimizing drug pharmacokinetics and pharmacodynamics is important in ICU patients, and this goal can be achieved by using maximal doses of antibiotics while choosing proper drug delivery schemes. This means using once-daily dosing of aminoglycosides, and prolonged infusion of high-dose β-lactams, the latter taking advantage of the time-dependent killing of β-lactams, in contrast to the concentration-dependent killing by aminoglycosides. In one study that used an optimized approach to antibiotic dosing and delivery versus standard therapy, infection-related mortality rate was reduced (8.5% vs. 21.6%).142
Although initial therapy of severe pneumonia usually requires multiple agents, once culture data are available, as part of a de-escalation strategy, monotherapy can often be used. Even patients with severe nosocomial pneumonia can be converted to monotherapy, provided that certain high-risk organisms are absent (P. aeruginosa, Acinetobacter spp., and MRSA), but the antibiotics that have been effective as monotherapy for severe VAP include imipenem, meropenem, doripenem, cefepime, ciprofloxacin, high-dose levofloxacin (750 mg daily), and piperacillin/tazobactam.2,121,143–147 Circumstances in which monotherapy should not be used include the following: (1) in any patient with severe CAP, in which the efficacy of this approach has not been demonstrated; (2) in suspected or documented bacteremic infection with P. aeruginosa; (3) in the empiric therapy of VAP if the patient has risk factors for infection with MDR pathogens; and (4) if the patient has nosocomial pneumonia and both S. aureus and P. aeruginosa are identified in culture as the etiologic pathogens. Monotherapy of nosocomial infection should never be attempted with a third-generation cephalosporin because of the possibility of emergence of resistance during therapy as a result of production of chromosomal β-lactamases by the Enterobacteriaceae group of organisms.2
In patients with suspected MRSA pneumonia, therapy should be with either vancomycin or linezolid (a bacteriostatic, oxazolidinone antibiotic). Early subgroup analysis of two prospective randomized, controlled trials suggested that linezolid led to higher cure and survival rates, compared to vancomycin in patients with documented MRSA VAP.148 A subsequent prospective study of patients with suspected MRSA VAP, randomized to receive either linezolid, 600 mg, or vancomycin, 1 g every 12 hours, showed trends in favor of linezolid for bacteriologic cure and survival that were not statistically significant.149 The comparison between linezolid and vancomycin has been evaluated by several meta analyses, which showed no definitive difference, except for a trend in favor of linezolid for clinical success.150 Recently, a large multicenter trial comparing linezolid to optimally dosed vancomycin was completed, including nearly 400 patients with documented MRSA VAP and HCAP. In that trial, linezolid led to a significantly higher rate of clinical response than vancomycin, but no difference in mortality rate.151 In the study, vancomycin caused more nephrotoxicity, a problem that has been increasingly common when trough levels are pushed above 15 mg/L. Telavancin is another agent that has been studied in MRSA VAP, but when compared to vancomycin, it had no clear advantage and a similar rate of nephrotoxicity but did lead to better clinical responses when MRSA was intermediately sensitive to vancomycin, with MIC values higher than 1 mg/L.152 In fact, recent studies have shown that the frequency of MRSA with these higher MIC values is increasing, and this is further challenging the efficacy of vancomycin.153 Tigecycline has efficacy against MRSA, but has not shown efficacy in VAP, although daptomycin is active against MRSA when it causes bacteremia but is inactivated by pulmonary surfactant, and thus cannot be used to treat pneumonia.
Acinetobacter spp. are inherently resistant to cephalosporins, penicillins, and aminoglycosides.154 In the past, the most reliable therapy for these agents was a carbapenem, but now resistance to carbapenems is more common, necessitating the use of polymixin B and E (colistin). Acinetobacter is sensitive in vitro to tigecycline, but in a clinical trial, tigecycline monotherapy was inferior to imipenem in the therapy of VAP, including when Acinetobacter was present, and thus if this agent is used, it is probably best as part of a combination regimen, along with a carbapenem, sulbactam, colistin, or an aminoglycoside.155
Role of Corticosteroids
The fact that inflammatory markers such as IL-6, IL-8, and IL-10 are elevated in patients with severe CAP and decrease in the first few days of appropriate antibiotic therapy favored the possible use of immunomodulators and anti-inflammatory medications such as macrolides, or steroids as an adjunct to reduce the proinflammatory cytokines. One randomized controlled trial of 48 patients compared hydrocortisone infusion (240 mg/day) to placebo, and found that steroid therapy reduced mortality rates, length of stay, and duration of mechanical ventilation.156 Subsequent studies have not consistently shown benefit for the use of corticosteroids as routine therapy in severe CAP. A meta analysis of available data concluded that there was no definite benefit but also no adverse consequences,157 although a prospective randomized controlled trial showed a higher frequency of late clinical failure when steroids were used as adjunctive therapy in CAP.158 Corticosteroids were also used as adjunctive therapy in patients with severe H1N1 pneumonia with ARDS, and were associated with an increased mortality rate, suggesting harm from their use.159 Given the currently available data, corticosteroids should not be part of routine therapy of severe CAP. In special situations, however, steroids may have value. Patients with pneumonia and pneumococcal meningitis may benefit from adjunctive corticosteroid therapy (dexamethasone) if it is started prior to the initiation of antibiotic therapy, because it may lead to improved long-term neurologic outcomes.160,161 Patients with P. jiroveci pneumonia also benefit from corticosteroid therapy, if the initial arterial oxygen tension is less than 70 mm Hg, by attenuating some of the inflammation that is induced by antibiotic killing of the organisms. In patients with pneumonia and refractory shock some investigators have considered using corticosteroids to treat relative adrenal insufficiency, but a large prospective randomized trial could not demonstrate benefit.162
Community-Acquired Pneumonia Therapy Algorithm (see Fig. 42.4)
Monotherapy Versus Combination Therapy
In all treatment algorithms, no ICU-admitted CAP patient should receive empiric monotherapy, even with a quinolone.95 In a study comparing levofloxacin to a β-lactam/quinolone combination, the single-agent regimen was not tested in patients with septic shock and was not optimally effective for those treated with mechanical ventilation.163 Rodríguez and colleagues reported in a subset of patients with CAP and shock that combination therapy with either a β-lactam and a macrolide or a β-lactam and a quinolone had a 28-day survival advantage compared to patients receiving monotherapy with a quinolone or a β-lactam alone.164 In contrast, another prospective observational study of 218 mechanically ventilated patients with CAP found that the use of a macrolide, but not a quinolone, was associated with reduced mortality rate.165 Several other studies have specifically shown benefit for combination therapy in patients with documented pneumococcal bacteremia. In one study that examined data from 2209 Medicare patients with bacteremic pneumonia, the initial use of antibiotic with atypical pathogen coverage, particularly a macrolide, was independently associated with a decreased 30-day mortality risk (odds ratio [OR] = 0.76; P = 0.03).166 This confirmed earlier data from a prospective study of bacteremic pneumococcal pneumonia patients, showing that combination antibiotic therapy (usually by adding a macrolide antibiotic) was associated with lower 14-day mortality rate compared to monotherapy (23.4 vs. 55.3%, P = 0.0015) for patients with severe illness.167
ATS/IDSA 2007 Guidelines
Recommended therapy for severe CAP, in the absence of pseudomonal risk factors, should be with a selected intravenous β-lactam (cefotaxime, ceftriaxone, ertapenem, a β-lactam/β-lactamase inhibitor combination), combined with either an intravenous macrolide or an intravenous antipneumococcal quinolone (levofloxacin or moxifloxacin). For patients with pseudomonal risk factors, therapy can be with a two-drug regimen, using an antipseudomonal β-lactam (imipenem, meropenem, piperacillin/tazobactam, cefepime) plus ciprofloxacin (the most active antipseudomonal quinolone) or levofloxacin (750 mg daily); or alternatively with a three-drug regimen, using an antipseudomonal β-lactam plus an aminoglycoside plus either an intravenous antipneumococcal quinolone (levofloxacin, or moxifloxacin) or a macrolide.95,168 In patients with bacteremic pneumococcal pneumonia, particularly in those with severe illness, as discussed earlier, dual therapy including a macrolide has been associated with improved outcomes.167
In patients with risk factors for MRSA, vancomycin (and possibly clindamycin) or linezolid alone should be added to the regimen. Most experts recommend that CA-MRSA be targeted in patients with severe, necrotizing CAP including those with empyema, following a viral illness, particularly influenza. Optimal therapy has not been defined, and vancomycin alone may not be sufficient, and has led to clinical failure, presumably because it is not active against the PVL toxin production that accompanies CA-MRSA. For that reason, it may be necessary to add clindamycin to vancomycin or to use linezolid, because both of these latter agents can inhibit toxin production.103
Timing of Antibiotics
In addition to the antibiotic approach to therapy outlined previously, there are several other considerations in the management of severe CAP. These plans include providing the first dose of therapy as soon as possible and providing coverage in all patients for atypical pathogens using either a macrolide or a quinolone in the regimen, based on data that such an approach reduces mortality rate.169–171
Duration of Treatment
There is little information on the proper duration of therapy in patients with CAP, especially those with severe illness. Even in the presence of pneumococcal bacteremia, short durations of therapy may be possible, with a rapid switch from intravenous to oral therapy in responding patients. Generally, S. pneumoniae can be treated for 5 to 7 days if the patient is responding rapidly and has received accurate empiric therapy at the correct dose. The presence of extrapulmonary infection (such as meningitis), and the identification of certain pathogens (such as bacteremic S. aureus, and P. aeruginosa) may require longer durations of therapy. Identification of L. pneumophila pneumonia may require at least 14 days of therapy, depending on severity of illness and host defense impairments, although recent data have shown that quinolone therapy may be the best approach to management and that durations as short as 5 days with levofloxacin 750 mg may be effective.172 Biomarkers, especially PCT, can be followed serially to guide duration of therapy for severe CAP. Although early studies of PCT did not involve patients with severe pneumonia, one large randomized controlled trial, including 326 ICU patients with CAP, showed that serial measurement of PCT, compared to standard care, led to a reduction in duration of therapy of 3.3 days.10 The switch to oral therapy, even in severely ill patients, may be facilitated by the use of quinolones, which are highly bioavailable and achieve the same serum levels with oral therapy as with intravenous therapy.
Nosocomial Pneumonia Therapy Algorithm (see Fig. 42.5)
In defining therapy for patients with nosocomial pneumonia, it is important to evaluate (a) time of onset of infection, (b) underlying comorbid conditions, (c) severity of disease—based on scoring systems, and (d) risk factors for MDR pathogens. Antibiotic therapy should be given at the first clinical suspicion of infection, and empiric therapy should be dictated by dividing patients into a group not at risk for MDR pathogens, and a group that is at risk. Those who are not at risk for MDR pathogens include patients with both early onset of infection, and no risks for HCAP such as recent hospitalization, treatment in a health care–associated facility (nursing home, dialysis center, etc.), and no history of recent antibiotic therapy in the past month. Patients with either late-onset infection or the presence of any of the other MDR risk factors are treated empirically for infection with MDR gram-negative and gram-positive pathogens. Not all HCAP patients are at risk for MDR pathogens, and those who are not can receive a narrow-spectrum regimen, similar to that used for CAP. The HCAP patient who is at risk for MDR pathogens is the patient with severe pneumonia and any additional MDR risk such as antibiotic therapy or hospitalization in the past 3 months, poor functional status, and immune suppression. MDR pathogens are not likely in HCAP patients with severe pneumonia who do not have any of these other risks present.106
Choosing the Appropriate Regimen
Patients who have no MDR risks can be treated for the “core pathogens” listed earlier, generally with a monotherapy regimen of a second- or nonpseudomonal third-generation cephalosporin, a β-lactam/β-lactamase inhibitor combination, ertapenem, or a quinolone (levofloxacin or moxifloxacin).2 If the patient is penicillin-allergic, therapy can be with a quinolone or the combination of clindamycin and aztreonam. As mentioned earlier HCAP patients without MDR risks should also receive monotherapy, an approach that has been successful in this well-defined subgroup of individuals.
Patients at risk for MDR pathogens generally require combination therapy, rather than monotherapy, which is effective against the core pathogens, as well as MDR gram-negative organisms and MRSA. As discussed, combination therapy is necessary for this population because it provides broad-spectrum coverage, thereby minimizing the chance of initially inappropriate therapy.173 The empiric therapy for patients at risk for MDR pathogens should include an aminoglycoside or quinolone (ciprofloxacin or high-dose levofloxacin) plus an antipseudomonal β-lactam (imipenem, meropenem, doripenem, piperacillin/tazobactam, aztreonam, or cefepime). Because most patients are at risk for a second ICU-acquired infection, it may be prudent to use an aminoglycoside for the first episode of infection, reserving the quinolone for any subsequent infection, because of concern about quinolone induction of multidrug resistance, which could limit subsequent therapy options.174 In addition, the efficacy of quinolones against MDR gram-negative organisms in the ICU is not as good as in years past, making an aminoglycoside a more reliable empiric choice in most hospitals. In one study, when a quinolone was added to the best β-lactams, the combination was only minimally better than monotherapy, covering less than 85% of the gram-negative organisms, but when amikacin was added to the same β-lactams, coverage was over 95%.175 Empiric therapy of MRSA is needed when the patient is at high risk for this pathogen because of a tracheal aspirate Gram stain showing gram-positive organisms or because of other risk factors. In this setting, a third drug should be added, either linezolid or vancomycin, but as discussed previously, recent data have suggested an advantage for linezolid in patients with documented MRSA VAP, because of higher clinical efficacy and a lower risk of nephrotoxicity.151 When Acinetobacter spp. or ESBL-producing Enterobacteriaceae are present, therapy should be with a carbapenem if the pathogen is sensitive; if not, then therapy could be with colistin, or a combination of agents, including tigecycline with sulbactam, colistin, or an aminoglycoside.
In the selection of an empiric therapy regimen, it is also necessary to know which antibiotic the patient has recently received (within the past 14 days) and to choose an agent that is in a different class, because repeated use of the same class of antibiotic may drive resistance to that class, especially if the pathogen is P. aeruginosa.176 Similar findings have been made for patients with bacteremic pneumococcal pneumonia and CAP, and repeat use of an agent within 3 months may mean that the patient is being treated with an agent to which pneumococcus is more likely to be resistant.177 In addition, the recent use of quinolones may present a particular problem, because in the ICU, recent quinolone therapy may predispose to not only quinolone-resistant organisms but also to infection with MDR pathogens, extended-spectrum β-lactamase-producing gram-negative organisms, and MRSA.178 For all patients with VAP, it is important to use the correct dose of antibiotic, and the recommended doses for patients with normal renal function appear in Box 42.7.
Although it is possible to use risk factors to identify the patient who is likely to be infected with MDR pathogens, it is important to realize that each hospital, and each ICU has its own unique organisms, and patterns of antimicrobial resistance, and that these patterns change over time. Therefore, it is necessary to monitor local patterns of resistance and to choose empiric therapy that is likely to be effective in a given clinical setting.112 One other therapeutic concept that has been considered is that of “antibiotic rotation,” an idea that means that the standard empiric regimens are intentionally varied over time to expose bacteria to different antibiotics and potentially minimize the selection pressure for resistance. In some studies, this approach has been effective in reducing the incidence of infection with resistant organisms.179 One of the limitations of antibiotic rotation is that it may mean the use of the same regimen repeatedly in the same patient, and this may itself be a risk factor for selecting for resistance.180 Currently, this approach is not widely used or recommended.
Duration of Treatment
There are limited data regarding the optimal duration of treatment for nosocomial pneumonia. Most patients in the past have received 10 to 14 days of treatment, although those infected with non-lactose-fermenting organisms such as P. aeruginosa have received 14 to 21 days of treatment.74 If the lower respiratory tract cultures are negative, it may be possible to stop therapy (especially if an alternative diagnosis is suspected) or to shorten the duration of therapy. In addition, if cultures show that the initial empiric regimen was appropriate, and if the patient has a good clinical response (reflected by a drop in the CPIS or decreased levels of biomarkers), then it may be possible to reduce the duration of therapy to as little as 7 to 8 days, although this may not be possible if the etiologic pathogen is P. aeruginosa or MRSA.181 One large study in ICU patients has shown that it is possible to reduce the duration of therapy for pneumonia, including nosocomial pneumonia, by following serial measurements of PCT. In this prospective, randomized, controlled trial, 295 patients with HAP were randomized to have therapy duration defined by clinical evaluation or a PCT algorithm, and with PCT guidance the duration of therapy was reduced by 2.3 days.10
De-escalation of Treatment
Many patients with nosocomial pneumonia will get an initial empiric therapy that is broad spectrum, and thus it is important to consider de-escalation of the initial regimen as serial clinical and microbiologic data become available.121 If the patient has received a broad-spectrum regimen and the cultures do not show MDR organisms, then the patient can finish therapy with any of 7 monotherapy regimens that have been documented to be effective for severe VAP, in the absence of MDR organisms: ciprofloxacin, doripenem, imipenem, meropenem, piperacillin/tazobactam, cefepime, and high-dose levofloxacin. If P. aeruginosa is present, combination therapy with a β-lactam and aminoglycoside should continue for no more than 5 days, after which the patient can be switched to monotherapy with an agent to which the organism is sensitive.2 When de-escalation has been used, meaning either the switch to a more narrow-spectrum regimen, the use of fewer drugs, or both, mortality rate in VAP has been reduced, compared to when patients do not have de-escalation.62,63,121 There are many unrealized opportunities for using this approach, including patients with P. aeruginosa infection and sensitive pathogens, and in those with a good clinical response and negative respiratory tract cultures.121 Studies of de-escalation have found that the frequency of this practice varies from 22% to 74% of nosocomial pneumonia patients.64 Factors associated with a higher rate of de-escalation are use of a protocol for when to narrow antibiotic therapy, use of initially appropriate therapy, the finding of a positive (rather than negative) respiratory tract culture, the use of an empiric broad-spectrum and multidrug regimen, and a low incidence of MDR pathogens in the ICU. The diagnostic method for pneumonia is not consistently related to the rate of de-escalation.
Localized Treatment
For selected patients who are infected with highly resistant organisms, and not responding to systemic antibiotics, it may be valuable to add aerosolized antibiotics (such as gentamicin, tobramycin, colistin, and ceftazidime). Aerosolized administration of antibiotics offers the advantage of achieving high concentrations of antibiotics at the site of infection, and as a result, it may be possible to overcome the problems of poor lung penetration of certain agents (aminoglycosides) and in addition, to provide the high levels of antibiotics that are needed to kill certain resistant organisms. Locally administered antibiotics are rarely absorbed, and systemic toxicity is minimized. In spite of these theoretical advantages, many efficacy questions remain to be answered by clinical trials. Aerosolized antibiotics are not usually recommended for routine treatment of pneumonia but may have a role as adjunctive therapy in patients with MDR organisms not responding to systemic therapy.182 However, new data suggest that the use of adjunctive aerosolized amikacin, delivered with a special small-particle nebulizer, in patients with a high frequency of MDR gram-negative organisms causing VAP, may lead to an earlier clinical response, resulting in a shorter duration of systemic antibiotic therapy and a lower need to escalate antibiotic therapy.183
Evaluation of Nonresponding Patients
With effective therapy, most patients with CAP become afebrile by day 3 to 5, and most have a clinical response by day 3. Similarly, even with VAP, most patients have some improvement, particularly in oxygenation, by day 3.2,42 Nonresponding patients with either CAP or VAP should be evaluated for alternative diagnoses (inflammatory lung disease, atelectasis, heart failure, malignancy, pulmonary hemorrhage, pulmonary embolus, a nonpneumonic infection); a resistant or unusual pathogen (including tuberculosis and fungal infection); a pneumonia complication (empyema, lung abscess, drug fever, antibiotic-induced colitis, bronchopleural fistula) (Fig. 42.6); or a secondary site of infection (central-line infection, intra-abdominal infection). The evaluation of a nonresponding patient should be individualized but may include CT scanning of the chest, pulmonary angiography, bronchoscopy, and occasionally open lung biopsy.
Prevention
Community-Acquired Pneumonia
Pneumococcal Vaccination
All high-risk patients and patients older than 65 years should be vaccinated with the 23-valent polysaccharide vaccine. It should be considered in all patients with chronic illnesses such as congestive heart failure, COPD, diabetes, asthma, chronic liver disease, functional or anatomic asplenia, and alcoholism. It is also provided to cigarette smokers, and immunocompromised adults, although the efficacy is somewhat reduced in the latter group. Immunocompromised vaccine candidates include those with HIV infection, malignancy, immune-suppressing therapy (including corticosteroids), and chronic renal failure. In those who were initially vaccinated before the age of 65 years, revaccination is provided once after 5 years. In immunocompromised patients, revaccination is given 5 years after initial vaccination. If there is uncertainty about whether the patient has recently been vaccinated, it is probably best to give a pneumococcal vaccination, because repeat administration, even more often than recommended, is not generally associated with an adverse reaction.184 In a study of 62,918 adults with CAP hospitalized across 109 centers in the United States, prior vaccination against pneumococcus showed improved survival, decreased chance of respiratory failure or other complications, and decreased length of stay compared with patients who did not receive vaccination.185
Hospital-based immunization is recommended, and one study found that among 1633 patients with pneumonia treated in the hospital, 62% had been hospitalized in the preceding 4 years.186 In addition, 80% of these patients had a high-risk condition that would have qualified them to receive pneumococcal vaccine. Based on these observations, it seems likely that many cases of CAP could be prevented if pneumococcal vaccine was given to all hospitalized patients who qualify for the vaccine, regardless of why they are hospitalized.
Nosocomial Pneumonia
Although no single method is able to prevent nosocomial pneumonia reliably, multiple small interventions may have benefit, especially those focused on modifiable risk factors for infection. Recently, these interventions have been combined into “ventilator bundles,” which have been demonstrated to reduce the incidence of VAP, if applied carefully.187,188 Most of these bundles include multiple interventions, so it is difficult to know which individual manipulations are most valuable. Successful bundles have included interventions such as elevation of the head of the bed to 30 degrees (to avoid the risk of aspiration present with the supine position), daily interruption of sedation to attempt weaning, peptic ulcer disease prophylaxis, endotracheal tube suctioning (possibly with a closed suction system), hand washing, careful oral care (including oral chlorhexidine), and tight control of blood glucose.40 In spite of the success of this approach, one recent randomized study has demonstrated a lack of benefit and feasibility of routine elevation of the head of the bed.189 The efficacy of this approach has led to a movement to aim for “zero VAP” in all ICUs, but it remains uncertain if this goal is really achievable, and if hospitals that consistently report this result have simply changed the diagnostic criteria for VAP, because in many instances, the incidence of VAP has been reduced, without a reduction in associated outcomes such as mortality rate and ICU length of stay.
Other widely used measures in mechanically ventilated patients are avoidance of large inocula of bacteria into the lung (careful handling of ventilator circuit tubing), mobilization of respiratory secretions (frequent suctioning, use of rotational bed therapy in selected individuals), nutritional support (enteral preferred over parenteral), placing of feeding tubes into the small bowel (to avoid aspiration, which is more likely with stomach tubes), and avoidance of large gastric residuals when giving enteral feeding. In addition, any tube inserted into the stomach or trachea should be inserted through the mouth and not the nose, whenever possible, to avoid obstructing the nasal sinuses, and to prevent nosocomial sinusitis, which can lead to nosocomial pneumonia.40 A specially adapted endotracheal tube that allows for continuous aspiration of subglottic secretions may interrupt the oropharyngeal to tracheal transfer of bacteria and reduce the incidence of pneumonia.190 In addition, modifications of the endotracheal tube have been developed, including silver coating of the tube and a focus on maintaining endotracheal tube cuff pressure to avoid leakage of tracheal secretions to the lung, and both approaches have had some success. In addition, new devices to remove biofilm from the surface of the endotracheal tube are in development, but their clinical benefit is not yet defined. Because endotracheal intubation is a risk for pneumonia, noninvasive positive-pressure ventilation should be used whenever possible, and this approach is associated with a lower pneumonia risk than traditional mechanical ventilation. Early tracheostomy has been considered a potentially effective means of VAP prevention, but a large randomized trial did not confirm such a benefit.191 There is no specific role for prophylactic systemic or topical antibiotics, but some data suggest that patients with coma due to stroke or head trauma and those who may have aspirated during an emergent intubation may benefit from a 24-hour course of systemic antibiotics.192 The use of selective digestive decontamination (SDD), which involves the use of prophylactic systemic and topical (mouth and intestinal tract) antimicrobials, has been a controversial strategy for VAP prevention for many years. Although one large trial has recently reported benefit, there are many concerns with this approach, including the development of antibiotic resistance, and the possibility that the use of oral chlorhexidine and ventilator bundles may be just as effective as the entire SDD regimen.193,194
References
1. Restrepo, MI, Mortensen, EM, Velez, JA, et al. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008; 133:610–617.
2. Niederman, MS, Craven, DE, Bonten, MJ, et al. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388–416.
3. Kaplan, V, Clermont, G, Griffin, MF, et al. Pneumonia: Still the old man’s friend? Arch Intern Med. 2003; 163:317–323.
4. File, TM, Jr., Marrie, TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010; 122:130–141.
5. Fine, MJ, Smith, MA, Carson, CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996; 275:134–141.
6. Safdar, N, Dezfulian, C, Collard, HR, Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit Care Med. 2005; 33:2184–2193.
7. Woodhead, M, Welch, CA, Harrison, DA, et al. Community-acquired pneumonia on the intensive care unit: Secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 10(Suppl 2), 2006.
8. Christ-Crain, M, Jaccard-Stolz, D, Bingisser, R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet. 2004; 363:600–607.
9. Schuetz, P, Christ-Crain, M, Thomann, R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009; 302:1059–1066.
10. Bouadma, L, Luyt, CE, Tubach, F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet. 2010; 375:463–474.
11. Niederman, MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: Definitions and challenges in trial design. Clin Infect Dis. 2010; 51:S12–S17.
12. Mehr, DR, Binder, EF, Kruse, RL, et al. Predicting mortality in nursing home residents with lower respiratory tract infection: The Missouri LRI Study. JAMA. 2001; 286:2427–2436.
13. Brito, V, Niederman, MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009; 22:316–325.
14. Nair, GB, Niederman, MS. Community-acquired pneumonia: An unfinished battle. Med Clin North Am. 2011; 95:1143–1161.
15. Fine, MJ, Auble, TE, Yealy, DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997; 336:243–250.
16. Brito, V, Niederman, MS. Predicting mortality in the elderly with community-acquired pneumonia: Should we design a new car or set a new “speed limit”? Thorax. 2010; 65:944–945.
17. Lim, WS, van der Eerden, MM, Laing, R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003; 58:377–382.
18. Rello, J, Rodriguez, A, Lisboa, T, et al. PIRO score for community-acquired pneumonia: A new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med. 2009; 37:456–462.
19. Mandell, LA, Wunderink, RG, Anzueto, A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44:S27–S72.
20. Liapikou, A, Ferrer, M, Polverino, E, et al. Severe community-acquired pneumonia: Validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis. 2009; 48:377–385.
21. Brown, SM, Jones, BE, Jephson, AR, Dean, NC. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009; 37:3010–3016.
22. Chalmers, JD, Taylor, JK, Mandal, P, et al. Validation of the Infectious Diseases Society of America/American Thoratic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis. 2011; 53:503–511.
23. Charles, PG, Wolfe, R, Whitby, M, et al. SMART-COP: A tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008; 47:375–384.
24. Niederman, MS. Making sense of scoring systems in community acquired pneumonia. Respirology. 2009; 14:327–335.
25. Renaud, B, Labarere, J, Coma, E, et al. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community-acquired pneumonia: Development of an international prediction rule. Crit Care. 2009; 13:R54.
26. Kruger, S, Ewig, S, Giersdorf, S, et al. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010; 182:1426–1434.
27. Huang, DT, Weissfeld, LA, Kellum, JA, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008; 52:48–58.
28. Ramirez, P, Ferrer, M, Marti, V, et al. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med. 2011; 39:2211–2217.
29. Narvaez-Rivera, RM, Rendon, A, Salinas-Carmona, MC, Rosas-Taraco, AG. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis. 2012; 12:15.
30. Ruiz, M, Ewig, S, Torres, A, et al. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999; 160:923–929.
31. Nuorti, JP, Butler, JC, Farley, MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000; 342:681–689.
32. Waterer, GW, Quasney, MW, Cantor, RM, Wunderink, RG. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. Am J Respir Crit Care Med. 2001; 163:1599–1604.
33. Garcia-Laorden, MI, Rodriguez de Castro, F, Sole-Violan, J, et al. Influence of genetic variability at the surfactant proteins A and D in community-acquired pneumonia: A prospective, observational, genetic study. Crit Care. 2011; 15:R57.
34. Singh, S, Amin, AV, Loke, YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: A meta-analysis. Arch Intern Med. 2009; 169:219–229.
35. Houck, PM, Bratzler, DW, Nsa, W, et al. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004; 164:637–644.
36. Waterer, GW, Kessler, LA, Wunderink, RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006; 130:11–15.
37. Metersky, ML, Sweeney, TA, Getzow, MB, et al. Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: Is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006; 130:16–21.
38. Renaud, B, Santin, A, Coma, E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009; 37:2867–2874.
39. Restrepo, MI, Mortensen, EM, Rello, J, et al. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010; 137:552–557.
40. Tablan, OC, Anderson, LJ, Besser, R, et al. Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004; 53:1–36.
41. Napolitano, LM. Use of severity scoring and stratification factors in clinical trials of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010; 51:S67–S80.
42. Luna, CM, Blanzaco, D, Niederman, MS, et al. Resolution of ventilator-associated pneumonia: Prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003; 31:676–682.
43. Gursel, G, Demirtas, S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration. 2006; 73:503–508.
44. Lisboa, T, Diaz, E, Sa-Borges, M, et al. The ventilator-associated pneumonia PIRO score: A tool for predicting ICU mortality and health-care resources use in ventilator-associated pneumonia. Chest. 2008; 134:1208–1216.
45. Mirsaeidi, M, Peyrani, P, Ramirez, JA. Predicting mortality in patients with ventilator-associated pneumonia: The APACHE II score versus the new IBMP-10 score. Clin Infect Dis. 2009; 49:72–77.
46. Cook, DJ, Walter, SD, Cook, RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998; 129:433–440.
47. Sutherland, KR, Steinberg, KP, Maunder, RJ, et al. Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995; 152:550–556.
48. Seidenfeld, JJ, Pohl, DF, Bell, RC, et al. Incidence, site, and outcome of infections in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1986; 134:12–16.
49. Chastre, J, Trouillet, JL, Vuagnat, A, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998; 157:1165–1172.
50. Heyland, DK, Cook, DJ, Griffith, L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999; 159:1249–1256.
51. Fagon, JY, Chastre, J, Vuagnat, A, et al. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996; 275:866–869.
52. Bekaert, M, Timsit, JF, Vansteelandt, S, et al. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am J Respir Crit Care Med. 2011; 184:1133–1139.
53. Kollef, MH. Inadequate antimicrobial treatment: An important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000; 31:S131–S138.
54. Luna, CM, Vujacich, P, Niederman, MS, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997; 111:676–685.
55. Dupont, H, Mentec, H, Sollet, JP, Bleichner, G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001; 27:355–362.
56. Clec’h, C, Timsit, JF, De Lassence, A, et al. Efficacy of adequate early antibiotic therapy in ventilator-associated pneumonia: Influence of disease severity. Intensive Care Med. 2004; 30:1327–1333.
57. Rello, J, Quintana, E, Ausina, V, et al. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest. 1991; 100:439–444.
58. Graybill, JR, Marshall, LW, Charache, P, et al. Nosocomial pneumonia. A continuing major problem. Am Rev Respir Dis. 1973; 108:1130–1140.
59. Fagon, JY, Chastre, J, Hance, AJ, et al. Nosocomial pneumonia in ventilated patients: A cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993; 94:281–288.
60. Trouillet, JL, Chastre, J, Vuagnat, A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998; 157:531–539.
61. Magret, M, Lisboa, T, Martin-Loeches, I, et al. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: A prospective and observational multicenter study. Crit Care. 15, 2011.
62. Kollef, MH, Morrow, LE, Niederman, MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006; 129:1210–1218.
63. Soo Hoo, GW, Wen, YE, Nguyen, TV, Goetz, MB. Impact of clinical guidelines in the management of severe hospital-acquired pneumonia. Chest. 2005; 128:2778–2787.
64. Niederman, MS, Soulountsi, V. De-escalation therapy: Is it valuable for the management of ventilator-associated pneumonia? Clin Chest Med. 2011; 32:517–534.
65. Rello, J, Sole-Violan, J, Sa-Borges, M, et al. Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit Care Med. 2005; 33:1983–1987.
66. Chastre, J, Fagon, JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002; 165:867–903.
67. Martin, TR, Frevert, CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005; 2:403–411.
68. Skerrett, SJ, Niederman, MS, Fein, AM. Respiratory infections and acute lung injury in systemic illness. Clin Chest Med. 1989; 10:469–502.
69. Hospital-acquired pneumonia in adults: Diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996; 153:1711–1725.
70. Niederman, MS, Ahmed, QA. Inflammation in severe pneumonia: Act locally, not globally. Crit Care Med. 1999; 27:2030–2032.
71. Niederman, MS, Craven, DE. Devising strategies for preventing nosocomial pneumonia—Should we ignore the stomach? Clin Infect Dis. 1997; 24:320–323.
72. Holzapfel, L, Chastang, C, Demingeon, G, et al. A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999; 159:695–701.
73. Bonten, MJ, Bergmans, DC, Ambergen, AW, et al. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med. 1996; 154:1339–1346.
74. Rotstein, C, Evans, G, Born, A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008; 19:19–53.
75. Lacherade, JC, De Jonghe, B, Guezennec, P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: A multicenter trial. Am J Respir Crit Care Med. 2010; 182:910–917.
76. DeRiso, AJ, 2nd., Ladowski, JS, Dillon, TA, et al. Chlorhexidine gluconate 0. 12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996; 109:1556–1561.
77. Craven, DE, Chroneou, A, Zias, N, Hjalmarson, KI. Ventilator-associated tracheobronchitis: The impact of targeted antibiotic therapy on patient outcomes. Chest. 2009; 135:521–528.
78. Craven, DE, Hjalmarson, KI. Ventilator-associated tracheobronchitis and pneumonia: Thinking outside the box. Clin Infect Dis. 2010; 51:S59–S66.
79. Sottile, FD, Marrie, TJ, Prough, DS, et al. Nosocomial pulmonary infection: Possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med. 1986; 14:265–270.
80. Prince, AS. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med. 2002; 347:1110–1111.
81. Torres, A, Gatell, JM, Aznar, E, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995; 152:137–141.
82. Afessa, B, Shorr, AF, Anzueto, AR, et al. Association between a silver-coated endotracheal tube and reduced mortality in patients with ventilator-associated pneumonia. Chest. 2010; 137:1015–1021.
83. Niederman, MS. Fighting vampires and ventilator-associated pneumonia: Is silver the magic bullet? Chest. 2010; 137:1007–1009.
84. Craven, DE, Goularte, TA, Make, BJ. Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia? Am Rev Respir Dis. 1984; 129:625–628.
85. Craven, DE, Lichtenberg, DA, Goularte, TA, et al. Contaminated medication nebulizers in mechanical ventilator circuits. Source of bacterial aerosols. Am J Med. 1984; 77:834–838.
86. Craven, DE, Connolly, MG, Jr., Lichtenberg, DA, et al. Contamination of mechanical ventilators with tubing changes every 24 or 48 hours. N Engl J Med. 1982; 306:1505–1509.
87. Dreyfuss, D, Djedaini, K, Weber, P, et al. Prospective study of nosocomial pneumonia and of patient and circuit colonization during mechanical ventilation with circuit changes every 48 hours versus no change. Am Rev Respir Dis. 1991; 143:738–743.
88. Hess, D, Burns, E, Romagnoli, D, Kacmarek, RM. Weekly ventilator circuit changes. A strategy to reduce costs without affecting pneumonia rates. Anesthesiology. 1995; 82:903–911.
89. Djedaini, K, Billiard, M, Mier, L, et al. Changing heat and moisture exchangers every 48 hours rather than 24 hours does not affect their efficacy and the incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995; 152:1562–1569.
90. Metlay, JP, Schulz, R, Li, YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997; 157:1453–1459.
91. Fine, MJ, Orloff, JJ, Arisumi, D, et al. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med. 1990; 88:1N–8N.
92. Voiriot, G, Dury, S, Parrot, A, et al. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest. 2011; 139:387–394.
93. Lapinsky, SE, Hawryluck, L. ICU management of severe acute respiratory syndrome. Intensive Care Med. 2003; 29:870–875.
94. Clavo-Sanchez, AJ, Giron-Gonzalez, JA, Lopez-Prieto, D, et al. Multivariate analysis of risk factors for infection due to penicillin-resistant and multidrug-resistant Streptococcus pneumoniae: A multicenter study. Clin Infect Dis. 1997; 24:1052–1059.
95. Niederman, MS, Mandell, LA, Anzueto, A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001; 163:1730–1754.
96. van Eeden, SF, Coetzee, AR, Joubert, JR. Community-acquired pneumonia—Factors influencing intensive care admission. S Afr Med J. 1988; 73:77–81.
97. McFadden, JP, Price, RC, Eastwood, HD, Briggs, RS. Raised respiratory rate in elderly patients: A valuable physical sign. Br Med J (Clin Res Ed). 1982; 284:626–627.
98. Vazquez, R, Gheorghe, C, Ramos, F, et al. Gurgling breath sounds may predict hospital-acquired pneumonia. Chest. 2010; 138:284–288.
99. Saldias, PF, Cabrera, TD, de Solminihac, LI, et al. [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Rev Med Chil. 2007; 135:143–152.
100. Doern, GV, Richter, SS, Miller, A, et al. Antimicrobial resistance among Streptococcus pneumoniae in the United States: Have we begun to turn the corner on resistance to certain antimicrobial classes? Clin Infect Dis. 2005; 41:139–148.
101. Feikin, DR, Schuchat, A, Kolczak, M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am J Public Health. 2000; 90:223–229.
102. Francis, JS, Doherty, MC, Lopatin, U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005; 40:100–107.
103. Micek, ST, Dunne, M, Kollef, MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: Importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005; 128:2732–2738.
104. Kumar, A, Zarychanski, R, Pinto, R, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009; 302:1872–1879.
105. Kollef, MH, Shorr, A, Tabak, YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest. 2005; 128:3854–3862.
106. El-Solh, AA, Pietrantoni, C, Bhat, A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003; 167:1650–1654.
107. Cilloniz, C, Ewig, S, Ferrer, M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: Etiology and prognosis. Crit Care. 2011; 15:R209.
108. Fagon, JY, Chastre, J, Domart, Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989; 139:877–884.
109. Gaynes, R, Edwards, JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005; 41:848–854.
110. Esperatti, M, Ferrer, M, Theessen, A, et al. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010; 182:1533–1539.
111. El Solh, AA, Pietrantoni, C, Bhat, A, et al. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004; 39:474–480.
112. Rello, J, Sa-Borges, M, Correa, H, et al. Variations in etiology of ventilator-associated pneumonia across four treatment sites: Implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999; 160:608–613.
113. Ito, I, Ishida, T, Togashi, K, et al. Differentiation of bacterial and non-bacterial community-acquired pneumonia by thin-section computed tomography. Eur J Radiol. 2009; 72:388–395.
114. Reittner, P, Ward, S, Heyneman, L, et al. Pneumonia: High-resolution CT findings in 114 patients. Eur Radiol. 2003; 13:515–521.
115. Mirsaeidi, M, Peyrani, P, Aliberti, S, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010; 137:416–420.
116. Nair, V, Niederman, MS, Masani, N, Fishbane, S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007; 27:184–190.
117. Metersky, ML, Ma, A, Bratzler, DW, Houck, PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004; 169:342–347.
118. Rello, J, Bodi, M, Mariscal, D, et al. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest. 2003; 123:174–180.
119. Fagon, JY, Chastre, J, Wolff, M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000; 132:621–630.
120. Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006; 355:2619–2630.
121. Niederman, MS. The importance of de-escalating antimicrobial therapy in patients with ventilator-associated pneumonia. Semin Respir Crit Care Med. 2006; 27:45–50.
122. Niederman, MS. The argument against using quantitative cultures in clinical trials and for the management of ventilator-associated pneumonia. Clin Infect Dis. 2010; 51:S93–S99.
123. Plouffe, JF, File, TM, Jr., Breiman, RF, et al. Reevaluation of the definition of legionnaires’ disease: Use of the urinary antigen assay. Community Based Pneumonia Incidence Study Group. Clin Infect Dis. 1995; 20:1286–1291.
124. Kruger, S, Ewig, S, Marre, R, et al. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008; 31:349–355.
125. Niederman, MS. Biological markers to determine eligibility in trials for community-acquired pneumonia: A focus on procalcitonin. Clin Infect Dis. 2008; 47(Suppl 3):S127–S132.
126. Luyt, CE, Guerin, V, Combes, A, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:48–53.
127. Bloos, F, Marshall, JC, Dellinger, RP, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: A multicenter observational study. Crit Care. 2011; 15:R88.
128. Pugin, J, Auckenthaler, R, Mili, N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991; 143:1121–1129.
129. Singh, N, Rogers, P, Atwood, CW, et al. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000; 162:505–511.
130. Schurink, CA, Van Nieuwenhoven, CA, Jacobs, JA, et al. Clinical pulmonary infection score for ventilator-associated pneumonia: Accuracy and inter-observer variability. Intensive Care Med. 2004; 30:217–224.
131. Zilberberg, MD, Shorr, AF. Ventilator-associated pneumonia: The clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010; 51:S131–S135.
132. Bonten, MJ, Mascini, EM. The hidden faces of the epidemiology of antibiotic resistance. Intensive Care Med. 2003; 29:1–2.
133. Boucher, HW, Talbot, GH, Bradley, JS, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:1–12.
134. Rice, LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis. 2008; 197:1079–1081.
135. Sandiumenge, A, Lisboa, T, Gomez, F, et al. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organisms. Chest. 2011; 140:643–651.
136. Hilf, M, Yu, VL, Sharp, J, et al. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: Outcome correlations in a prospective study of 200 patients. Am J Med. 1989; 87:540–546.
137. Paul, M, Benuri-Silbiger, I, Soares-Weiser, K, Leibovici, L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: Systematic review and meta-analysis of randomised trials. BMJ. 2004; 328:668.
138. Heyland, DK, Dodek, P, Muscedere, J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008; 36:737–744.
139. Moore, RD, Smith, CR, Lietman, PS. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984; 77:657–662.
140. Cometta, A, Baumgartner, JD, Lew, D, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother. 1994; 38:1309–1313.
141. Hatala, R, Dinh, T, Cook, DJ. Once-daily aminoglycoside dosing in immunocompetent adults: A meta-analysis. Ann Intern Med. 1996; 124:717–725.
142. Nicasio, AM, Eagye, KJ, Nicolau, DP, et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care. 2010; 25:69–77.
143. Fink, MP, Snydman, DR, Niederman, MS, et al. Treatment of severe pneumonia in hospitalized patients: Results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother. 1994; 38:547–557.
144. Jaccard, C, Troillet, N, Harbarth, S, et al. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob Agents Chemother. 1998; 42:2966–2972.
145. West, M, Boulanger, BR, Fogarty, C, et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: A multicenter, prospective, randomized, open-label study. Clin Ther. 2003; 25:485–506.
146. Chapman, TM, Perry, CM. Cefepime: A review of its use in the management of hospitalized patients with pneumonia. Am J Respir Med. 2003; 2:75–107.
147. Sieger, B, Berman, SJ, Geckler, RW, Farkas, SA. Empiric treatment of hospital-acquired lower respiratory tract infections with meropenem or ceftazidime with tobramycin: A randomized study. Meropenem Lower Respiratory Infection Group. Crit Care Med. 1997; 25:1663–1670.
148. Wunderink, RG, Rello, J, Cammarata, SK, et al. Linezolid vs vancomycin: Analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003; 124:1789–1797.
149. Wunderink, RG, Mendelson, MH, Somero, MS, et al. Early microbiological response to linezolid vs. vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest. 2008; 134:1200–1207.
150. Walkey, AJ, O’Donnell, MR, Wiener, RS. Linezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A meta-analysis of randomized controlled trials. Chest. 2011; 139:1148–1155.
151. Wunderink, RG, Niederman, MS, Kollef, MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin Infect Dis. 2012; 54:621–629.
152. Rubinstein, E, Lalani, T, Corey, GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011; 52:31–40.
153. Haque, NZ, Zuniga, LC, Peyrani, P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010; 138:1356–1362.
154. Paterson, DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006; 43:S43–S48.
155. Freire, AT, Melnyk, V, Kim, MJ, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010; 68:140–151.
156. Confalonieri, M, Urbino, R, Potena, A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: A preliminary randomized study. Am J Respir Crit Care Med. 2005; 171:242–248.
157. Salluh, JI, Povoa, P, Soares, M, et al. The role of corticosteroids in severe community-acquired pneumonia: A systematic review. Crit Care. 12, 2008.
158. Snijders, D, Daniels, JM, de Graaff, CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: A randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010; 181:975–982.
159. Brun-Buisson, C, Richard, JC, Mercat, A, et al. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011; 183:1200–1206.
160. de Gans, J, van de Beek, D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002; 347:1549–1556.
161. van de Beek, D, de Gans, J, McIntyre, P, Prasad, K. Steroids in adults with acute bacterial meningitis: A systematic review. Lancet Infect Dis. 2004; 4:139–143.
162. Sprung, CL, Annane, D, Keh, D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008; 358:111–124.
163. Leroy, O, Saux, P, Bedos, JP, Caulin, E. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005; 128:172–183.
164. Rodriguez, A, Mendia, A, Sirvent, JM, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007; 35:1493–1498.
165. Martin-Loeches, I, Lisboa, T, Rodriguez, A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010; 36:612–620.
166. Metersky, ML, Ma, A, Houck, PM, Bratzler, DW. Antibiotics for bacteremic pneumonia: Improved outcomes with macrolides but not fluoroquinolones. Chest. 2007; 131:466–473.
167. Baddour, LM, Yu, VL, Klugman, KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004; 170:440–444.
168. File, TM, Jr., Niederman, MS. Antimicrobial therapy of community-acquired pneumonia. Infect Dis Clin North Am. 2004; 18:993–1016.
169. Gleason, PP, Meehan, TP, Fine, JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med. 1999; 159:2562–2572.
170. Houck, PM, MacLehose, RF, Niederman, MS, Lowery, JK. Empiric antibiotic therapy and mortality among medicare pneumonia inpatients in 10 western states: 1993, 1995, and 1997. Chest. 2001; 119:1420–1426.
171. Waterer, GW, Somes, GW, Wunderink, RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001; 161:1837–1842.
172. Yu, VL, Greenberg, RN, Zadeikis, N, et al. Levofloxacin efficacy in the treatment of community-acquired legionellosis. Chest. 2004; 125:2135–2139.
173. Niederman, MS. Can one plus one ever equal three in the antibiotic therapy of sepsis? Crit Care Med. 2010; 38:1906–1908.
174. Niederman, MS. Reexamining quinolone use in the intensive care unit: Use them right or lose the fight against resistant bacteria. Crit Care Med. 2005; 33:443–444.
175. Beardsley, JR, Williamson, JC, Johnson, JW, et al. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006; 130:787–793.
176. Trouillet, JL, Vuagnat, A, Combes, A, et al. Pseudomonas aeruginosa ventilator-associated pneumonia: Comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis. 2002; 34:1047–1054.
177. Vanderkooi, OG, Low, DE, Green, K, et al. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005; 40:1288–1297.
178. Nseir, S, Di Pompeo, C, Soubrier, S, et al. First-generation fluoroquinolone use and subsequent emergence of multiple drug-resistant bacteria in the intensive care unit. Crit Care Med. 2005; 33:283–289.
179. Kollef, MH, Vlasnik, J, Sharpless, L, et al. Scheduled change of antibiotic classes: A strategy to decrease the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997; 156:1040–1048.
180. Niederman, MS. Is “crop rotation” of antibiotics the solution to a “resistant” problem in the ICU? Am J Respir Crit Care Med. 1997; 156:1029–1031.
181. Chastre, J, Wolff, M, Fagon, JY, et al. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA. 2003; 290:2588–2598.
182. Michalopoulos, A, Kasiakou, SK, Mastora, Z, et al. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005; 9:R53–59.
183. Niederman, MS, Chastre, J, Corkery, K, et al. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with gram-negative pneumonia. Intensive Care Med. 2012; 38:263–271.
184. Walker, FJ, Singleton, RJ, Bulkow, LR, et al. Reactions after 3 or more doses of pneumococcal polysaccharide vaccine in adults in Alaska. Clin Infect Dis. 2005; 40:1730–1735.
185. Fisman, DN, Abrutyn, E, Spaude, KA, et al. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006; 42:1093–1101.
186. Fedson, DS, Harward, MP, Reid, RA, Kaiser, DL. Hospital-based pneumococcal immunization. Epidemiologic rationale from the Shenandoah study. JAMA. 1990; 264:1117–1122.
187. Zack, JE, Garrison, T, Trovillion, E, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002; 30:2407–2412.
188. Cocanour, CS, Peninger, M, Domonoske, BD, et al. Decreasing ventilator-associated pneumonia in a trauma ICU. J Trauma. 2006; 61:122–129.
189. van Nieuwenhoven, CA, Vandenbroucke-Grauls, C, van Tiel, FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: A randomized study. Crit Care Med. 2006; 34:396–402.
190. Valles, J, Artigas, A, Rello, J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995; 122:179–186.
191. Terragni, PP, Antonelli, M, Fumagalli, R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: A randomized controlled trial. JAMA. 2010; 303:1483–1489.
192. Sirvent, JM, Torres, A, El-Ebiary, M, et al. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med. 1997; 155:1729–1734.
193. de Smet, AM, Kluytmans, JA, Cooper, BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009; 360:20–31.
194. Brar, N, Niederman, MS. Should all ICU patients receive systemic digestive decontamination? Curr Respir Med Rev. 2010; 6:45–51.