Chapter 32 Pneumonia
The management of pneumonia is based on four findings and premises:
The net result is that the differential diagnosis is wide and treatment should be started before the aetiological agent is known. The differential diagnosis and the likely causative organisms can be narrowed by using epidemiological clues, the most important of which are whether the pneumonia is community-acquired or health care-associated and whether the patient is immunocompromised. Note that the flora and antibiotic resistance patterns vary from country to country, hospital to hospital and even intensive care unit (ICU) to ICU within a hospital6 and this must be taken into account.
COMMUNITY-ACQUIRED PNEUMONIA
Recent evidence-based guidelines have been issued by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS)7 and the European Respiratory Society.8 Links to these and other pneumonia-related guidelines can be found at the following link page: http://www.aic.cuhk.edu.hk/web8/Pneumoniaguidelines.htm.
DEFINITION
Community-acquired pneumonia is an acute infection of the pulmonary parenchyma that is associated with at least some symptoms of acute infection, accompanied by an acute infiltrate on a chest X-ray (CXR) or auscultatory findings consistent with pneumonia (e.g. altered breath sounds, localised crackles) in a patient not hospitalised or residing in a long-term care facility for ≥ 14 days prior to the onset of symptoms.9
AETIOLOGY
Table 32.1 gives possible aetiological agents based on epidemiological clues. Streptococcus pneumoniae is the most commonly isolated organism. The next most common pathogens in patients admitted to ICU are: Legionella species, Haemophilus influenzae, Enterobacteriaceae species, Staphylococcus aureus and Pseudomonas species.10
Table 32.1 Possible aetiological agents based on epidemiological clues2,3,7,9
| Exposure | Organism |
|---|---|
| Exposure to animals | |
| Handling turkeys, chickens, ducks or psittacine birds or their excreta | Chlamydia psittaci |
| Exposure to birds in countries in which avian flu has been identified in birds | Influenza A H5N1 |
| Handling infected parturient cats, cattle, goats or sheep or their hides | Coxiella burnetii |
| Handling infected wool | Bacillus anthracis |
| Handling infected cattle, pigs, goats or sheep or their milk | Brucella spp. |
| Insect bite. Transmission from rodents and wild animals (e.g. rabbits) to laboratory workers, farmers and hunters | Francisella tularensis |
| Insect bites or scratches. Transmission from infected rodents or cats to laboratory workers and hunters | Yersinia pestis |
| Contact with infected horses (very rare) | Pseudomonas mallei |
| Exposure to mice or mice droppings | Hantavirus |
| Geographical factors | |
| Immigration from or residence in countries with high prevalence of tuberculosis | Mycobacterium tuberculosis |
| North America. Contact with infected bats or birds or their excreta. Excavation in endemic areas | Histoplasma capsulatum |
| South-west USA | Coccidiodes species, Hantavirus |
| USA. Inhalation of spores from soil | Blastomyces dermatitidis |
| Asia, Pacific, Caribbean, north Australia. Contact with local animals or contaminated skin abrasions | Burkholderia pseudomallei |
| Host factors | |
| Diabetic ketoacidosis | Streptococcus pneumoniae, Staphylococcus aureus |
| Alcoholism | Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, oral anaerobes, Mycobacterium tuberculosis, Acinetobacter spp. |
| Chronic obstructive pulmonary disease or smoking | Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella spp., Pseudomonas aeruginosa |
| Sickle-cell disease | Streptococcus pneumoniae |
| Pneumonia complicating whooping cough | Bordatella pertussis |
| Pneumonia complicating influenza | Streptococcus pneumoniae, Staphylococcus aureus |
| Pneumonia severe enough to necessitate artificial ventilation | Streptococcus pneumoniae, Legionella spp., Staphylococcus aureus, Haemophilus influenzae, Mycoplasma pneumoniae, enteric Gram-negative bacilli, Chlamydia pneumoniae, Mycobacterium tuberculosis, viral infection, endemic fungi |
| Nursing-home residency | Treat as health care-associated pneumonia |
| Poor dental hygiene | Anaerobes |
| Suspected large-volume aspiration | Oral anaerobes, Gram-negative enteric bacteria |
| Structural disease of lung (e.g. bronchiectasis, cystic fibrosis) | Pseudomonas aeruginosa, Burkholderia cepacia, Staphylococcus aureus |
| Lung abscess | Community-acquired methicillin-resistant Staphylococcus aureus, oral anaerobes, endemic fungi, Mycobacterium tuberculosis, atypical mycobacteria |
| Endobronchial obstruction | Anaerobes, Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus |
| Intravenous drug addict | Staphylococcus aureus, anaerobes, Mycobacterium tuberculosis, Streptococcus pneumoniae |
| Others | |
| Epidemic | Mycoplasma pneumoniae, influenza virus |
| Air-conditioning cooling towers, hot tubs or hotel or cruise ship stay in previous 2 weeks | Legionella pneumophilia |
| Presentation of a cluster of cases over a very short period of time | Bioterrorist agents: Bacillus anthracis, Franciscella tularensis, Yersinia pestis |
INVESTIGATIONS7,8
Investigations should not delay administration of antibiotics as delays are associated with an increase in mortality.3 Important investigations include:
Table 32.2 Organisms which are virtually always pathogens when recovered from respiratory secretions9
| Legionella |
| Chlamydia |
| Tuberculosis |
| Influenza, para-influenza virus, respiratory syncytial virus, adenovirus, Hantavirus, severe acute respiratory syndrome (SARS), coronavirus |
| Stronglyloides stercoralis |
| Toxoplasma gondi |
| Pneumocystis carinii |
| Histoplasma capsulatum |
| Coccidiodes immitis |
| Blastomycoses dermatitidis |
| Cryptococcus neoformans |
Table 32.3 Risk factors for pulmonary tuberculosis
| Living in or originating from a developing country |
| Age (< 5 years, middle-aged and elderly men) |
| Alcoholism and/or drug addiction |
| Human immunodeficiency virus (HIV) infection |
| Diabetes mellitus |
| Lodging-house dwellers |
| Immunosuppression |
| Close contact with smear-positive patients |
| Silicosis |
| Poverty and/or malnutrition |
| Previous gastrectomy |
| Smoking |
Other investigations should be considered in patients with risk factors for infection with unusual organisms. Bronchoalveolar lavage may be useful in immunocompromised patients, those who fail to respond to antibiotics or those in whom sputum samples cannot be obtained.11
MANAGEMENT
ANTIMICROBIAL REGIMES
Each unit should have its own regimens tailored to the local flora and antibiotic resistance patterns. In the absence of such regimens the regimen outlined in Figure 32.1 may be helpful. This should be modified in the light of risk factors (see Table 32.1). Quinolones may be less appropriate in areas with a high prevalence of TB as their use may mask concurrent TB infection. Appropriate antimicrobial therapy should be administered within 1 hour of diagnosis.8,14 There is controversy regarding the appropriate change to empiric therapy based on microbiological findings.7,8 Changing to narrower-spectrum antimicrobial cover may result in inadequate treatment of the 5–38% of patients with polymicrobial infection. Furthermore, dual therapy may be more effective than monotherapy, even when the identified pathogen is sensitive to the agent chosen, particularly in severely ill patients with bacteraemic pneumococcal pneumonia.15 For the treatment of drug-resistant Streptococcus pneumoniae, the regimes in Figure 32.1 are probably suitable for isolates with a penicillin minimum inhibitory concentration (MIC) < 4 mg/l.7 If the MIC is ≥ 4 mg/l an antipneumococcal fluoroquinolone, vancomycin, teicoplanin or linezolid should be given.8
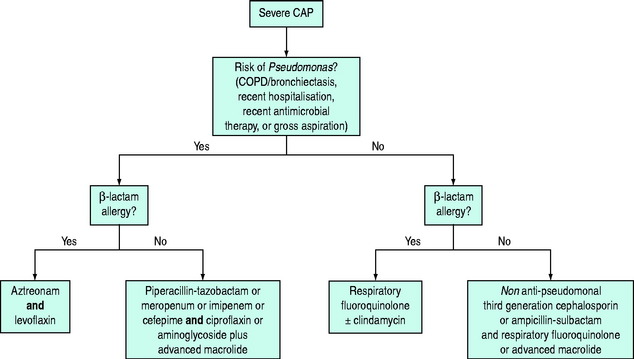
Figure 32.1 Antibiotic regimes for treatment of severe community-acquired pneumonia in critically ill patients.7,8 Respiratory fluoroquinolones include moxifloxacin and levofloxacin. Advanced macrolides include azithromycin and clarithromycin. Cefotaxime is a suitable non-antipseudomonal third-generation cephalosporin.
The role of zanamivir and oseltamivir in severe influenza pneumonia is not clear but early treatment of patients with less severe symptoms results in a reduction of the duration of symptoms if treatment is started early (< 48 hours from onset).16 Oseltamivir is recommended as first-line therapy for patients with suspected avian influenza A/H5N1.17
Recommended treatment for other pathogens can be found at http://www.journals.uchicago.edu/CID/journal/issues/v44nS2/41620/41620.tb9.html.
DURATION OF THERAPY
There are no clinical trials that have specifically addressed this issue. Courses as short as 5 days may be sufficient18 but antibiotics should be continued until the patient has been afebrile for 48–72 hours and organ dysfunction has largely resolved.7 Short courses may be suboptimal for patients with bacteraemic Staphylococcus aureus pneumonia, meningitis or endocarditis complicating pneumonia or infection with less common organisms (e.g. Burkholderia pseudomallei or fungi) or Pseudomonas aeruginosa.
RESPONSE TO THERAPY7,9,19
If the patient fails to respond, consider the following questions:
Useful investigations include computed tomography of the chest, bronchoalveolar lavage (Table 32.4) and transbronchial or open-lung biopsy.
Table 32.4 Procedure for obtaining microbiological samples using bronchoscopy and protected specimen brushing (PSB) and/or bronchoalveolar lavage (BAL)12,13
| Infection control | In patients suspected of having a disease which is transmitted by the airborne route (e.g. tuberculosis): |
ADMISSION TO ICU
This will largely be determined by the need for organ support and the availability of beds. However the presence of three of the following should prompt referral for admission: systolic blood pressure ≤ 90 mmHg, multilobar disease, PaO2/FiO2 ratio ≤ 250 (PaO2 in mmHg) or ≤ 33 (PaO2 in kPa), multilobar infiltrates, confusion, urea > 7 mmol/l (20 mg/dl), leukopenia, thrombocytopenia, hypothermia and need for aggressive fluid resuscitation.7
HOSPITAL-ACQUIRED PNEUMONIA
Hospital-acquired pneumonia occurs in 0.5–5% of hospital patients, with a higher incidence in certain groups, e.g. postoperative patients and patients in ICU. Diagnosis may be difficult: the clinical features of pneumonia are non-specific and many non-infectious conditions (e.g. atelectasis, pulmonary embolus, aspiration, heart failure and cancer) can cause infiltrates on a CXR. Identification of the organism responsible is even more difficult than in patients with community-acquired pneumonia due to the high incidence of oropharyngeal colonisation by Gram-negative bacteria. Blood cultures are only positive in about 6% of cases of nosocomial pneumonia. Ventilator-associated pneumonia is nosocomial pneumonia arising > 48–72 hours after intubation. It is associated with a higher incidence of multidrug-resistant organisms.1
CLINICAL DIAGNOSIS
Health care-associated pneumonia is defined on the basis of time of onset (developing more than 48 hours after admission to a health care facility1), CXR changes (new or progressive infiltrates) and either clinical features and simple laboratory investigations or the results of quantitative microbiology. Using a clinical approach, pneumonia is diagnosed by the finding of a new infiltrate or a change in an infiltrate on CXR and growth of pathogenic organisms from sputum plus one of the following: WBC count greater than 12 × 105/litre, core temperature ≥ 38.3°C, sputum Gram stain with scores of more than two on a scale of four of polymorphonuclear leukocytes and bacteria.
INVESTIGATIONS
These are broadly similar to those required in community-acquired pneumonia:
MANAGEMENT
Management is based on the finding that early treatment with antimicrobials that cover all likely pathogens results in a reduction in morbidity and mortality.5 If quantitative microbiology is not used the initial selection of antimicrobials is made on the basis of epidemiological clues (Figure 32.2; Table 32.5). Antimicrobials should be administered within 1 hour of diagnosis.14 The results of microbiological investigations are used to narrow antimicrobial cover later. Treatment should be reassessed after 2–3 days or sooner if the patient deteriorates (Figure 32.3). An outline of management based on an invasive approach is given in Figure 32.4.
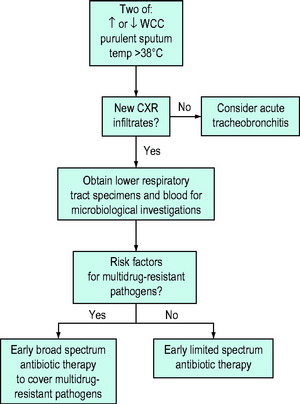
Figure 32.2 An outline of initial management of nosocomial pneumonia based on a non-invasive clinical approach.1
Table 32.5 Recommended initial empiric treatment for nosocomial pneumonia1*
| Situation | Antibiotics |
|---|---|
| No risk factors for multidrug-resistant pathogens | Cefotaxime or |
| Levofloxacin, moxifloxacin or ciprofloxacin or | |
| Ampicillin/sulbactam or | |
| Ertapenem | |
| Antimicrobial therapy in previous 90 days or | |
| Current hospitalisation for ≥ 5 days or | One of: |
| High frequency of antibiotic resistance in the specific hospital unit or | Antipseudomonal cephalosporin (cefepime or ceftazidime) or |
| Antipseudomonal carbapenem (meropenem or imipenem-cilastin) or | |
| Hospitalisation for 2 days or more in previous 90 days or | β-lactam/β-lactamase inhibitor (e.g. piperacillin-tazobactam or cefaperazone-sulbactam) |
| Residence in nursing home or extended-care facility or | plus one of: |
| Home infusion therapy (including antibiotics) or | Aminoglycoside or |
| Chronic dialysis within 30 days or | Antipseudomonal quinolone (levofloxacin or ciprofloxacin) plus one of the following for patients at high risk of methicillin-resistant Staphylococcus aureus (MRSA) infection: |
| Home wound care or | |
| Family member with multidrug-resistant pathogen or | |
| Immunosuppression or | Linezolid or vancomycin or teicoplanin |
| Bronchiectasis |
* The use of dual therapy is not well supported by evidence but it does reduce the probability that the pathogen is resistant to the drugs being given. If an extended-spectrum β-lactamase-producing strain or an Acinetobacter sp. is suspected, a carbapenem should be given. If Legionella pneumophilia is suspected, use a quinolone. Risk factors for MRSA infection in areas with a high incidence of MRSA include diabetes mellitus, head trauma, coma and renal failure.
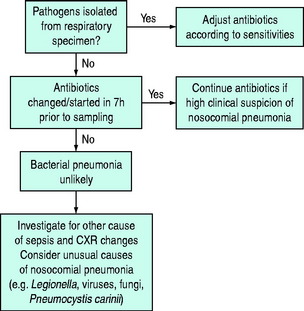
Figure 32.3 Subsequent management of nosocomial pneumonia based on a non-invasive clinical approach.1
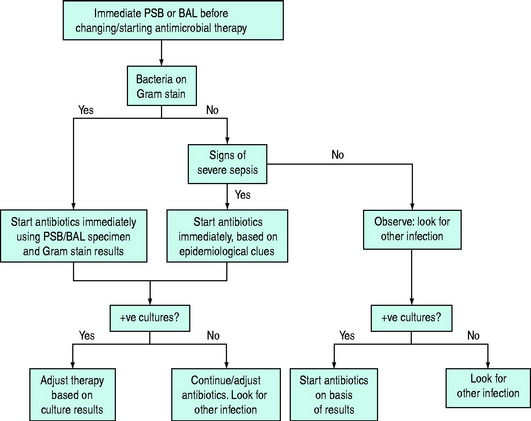
Figure 32.4 Management of suspected nosocomial pneumonia based on invasive sampling of respiratory secretions.
DURATION OF THERAPY
Current ATS guidelines recommend 7 days’ treatment provided the aetiological agent is not P. aeruginosa and the patient has a good clinical response with resolution of clinical features of infection.1 The outcome of patients who receive appropriate initial empiric therapy for ventilator-associated pneumonia for 8 days is similar to those who receive treatment for 14 days.24
RESPONSE TO THERAPY
Clinical improvement is usually not apparent for 48–72 hours and therapy should not be changed in this time. The CXR is of limited value for assessing response: initial deterioration is common and improvement often lags behind clinical response. However a rapidly deteriorating CXR pattern with a > 50% increase in size of infiltrate in 48 hours, new cavitation or a significant new pleural effusion should raise concern. If the patient fails to respond consider the diagnosis, host factors (e.g. immunosuppressed, debilitated), bacterial factors (e.g. virulent organism) and therapeutic factors (e.g. wrong drug, inadequate dose). Review the antibiotics and repeat cultures. It may be useful to broaden the antimicrobial cover while waiting for the results of investigations. Consider invasive sampling of respiratory secretions, computed tomography or ultrasound of the chest (to look for an empyema or abscess), another source of infection, open-lung biopsy to establish diagnosis and aetiology, or administration of steroids.
PREVENTION
A number of measures have been shown to reduce the incidence of ventilator-associated pneumonia.25–31 Measures recommended by the Centers for Disease Control (CDC) include hand-washing, nursing patients in a 30° head-up position, subglottic aspiration of secretions, orotracheal rather than nasotracheal intubation, changing the breathing circuit only when visibly soiled or mechanically malfunctioning and preferential use of non-invasive ventilation. CDC guidelines can be accessed via the link page: http://www.aic.cuhk.edu.hk/web8/Pneumoniaguidelines.htm.
TUBERCULOSIS
The main risk factors are listed in Table 32.3. Typical clinical features include fever, sweating, weight loss, lassitude, anorexia, cough productive of mucoid or purulent sputum, haemoptysis, chest wall pain, dyspnoea, localised wheeze and apical crackles. Patients may also present with unresolved pneumonia, pleural effusions, spontaneous pneumothorax and hoarseness or with enlarged cervical nodes or other manifestations of extrapulmonary disease. Clinical disease is seldom found in asymptomatic individuals, even those with strongly positive tuberculin test (Heaf grade III or IV). Older patients, who may have coexistent chronic bronchitis, can be missed unless a CXR is taken. The outlook for patients with TB who require ICU admission is poor. In one retrospective study the in-hospital mortality for all patients with TB requiring ICU admission was 67% but in those with acute respiratory failure it rose to 81%.32 The presentation and management of TB in HIV-positive patients is different (see below).
INVESTIGATION OF PULMONARY TUBERCULOSIS
IDENTIFICATION OF MYCOBACTERIA33
Multiple (3–6) sputum samples should be collected, preferably on different days, for microscopy for AFB and culture. If sputum is not available bronchial washings taken at bronchoscopy and gastric lavage or aspirate samples should be obtained. Gastric aspirates need to be neutralised immediately on collection. Bronchoscopy and transbronchial biopsy may be useful in patients with suspected TB but negative sputum smear. Pleural biopsy is often helpful and mediastinoscopy is occasionally needed in patients with mediastinal lymphadenopathy. Part of any biopsy specimen should always be sent for culture. Nucleic acid amplification tests on sputum have a sensitivity similar to culture in smear-negative patients with pulmonary TB but have the advantage of a much more rapid result. There is, however, a significant false-negative rate.34
TREATMENT OF PULMONARY TUBERCULOSIS34,35
The most commonly used regimen consists of a 6-month course of rifampicin 600 mg/day (450 mg for patients < 50 kg) and isoniazid 300 mg/day plus a 2-month course of pyrazinamide 2 g/day (1.5 g for patients < 50 kg) and ethambutol 15 mg/kg daily (streptomycin can be substituted for ethambutol). Ethambutol should only be used in patients who have reasonable visual acuity and who are able to appreciate and report visual disturbances. Visual acuity and colour perception must be assessed (if ethambutol is to be used) and liver and renal function checked before treatment is started. Steroids are recommended for children with endobronchial disease and, possibly, for patients with tuberculous pleural effusions. Pyrazinamide 10 mg/day should be given to prevent isoniazid-induced neuropathy to those at increased risk (e.g. patients with diabetes mellitus, chronic renal failure or malnutrition or alcoholic or HIV-positive patients).
INFECTION CONTROL
Patients admitted to an ICU with infectious TB or suspected of having active pulmonary TB should be managed in an isolation room with special ventilation characteristics, including negative pressure. Patients should be considered infectious if they are coughing or undergoing cough-inducing procedures or if they have positive AFB smears and they are not on or have just started chemotherapy or have a poor clinical or bacteriologic response to chemotherapy.13,34 Patients with non-drug-resistant TB should be non-infectious after 2 weeks of treatment, which includes rifampicin and isoniazid.34 As TB is spread through aerosols it is probably appropriate to isolate patients who are intubated even if only their bronchial washings are smear-positive. Staff caring for patients who are smear-positive should wear personal protective equipment including a fit-tested negative-pressure respirator (N95, FFP2 or higher). Use of a powered air-purifying respirator should be considered when bronchoscopy is being performed.13 Detailed infection control advice can be obtained via the link page http://www.aic.cuhk.edu.hk/web8/Pneumoniaguidelines.htm-.
PNEUMONIA IN THE IMMUNOCOMPROMISED
The speed of progression of pneumonia, the CXR changes (Table 32.6) and the type of immune defect provide clues to the aetiology. Bacterial pneumonias progress rapidly (1–2 days) whereas fungal and protozoal pneumonias are less fulminant (several days to a week or more). Viral pneumonias are not usually fulminant but on occasions may develop quite rapidly. Bronchoscopy is a major component of the investigation of these patients. Empiric management based on CXR appearances is outlined in Table 32.6. There is some evidence that early non-invasive ventilation may improve outcome amongst immunocompromised patients with fever and bilateral infiltrates.36
Table 32.6 Causes of chest X-ray changes and empiric treatment of pneumonia in the immunocompromised
| Chest X-ray appearance | Causes | Empiric treatment for suspected pneumonia |
|---|---|---|
| Diffuse infiltrate | Cytomegalovirus and other herpesviruses | Broad-spectrum antibiotics for at least 48 hours (e.g. third-generation cephalosporin and aminoglycoside) |
| Pneumocystis carinii | ||
| Bacteria | ||
| Aspergillus (advanced) | Co-trimoxazole | |
| Cryptococcus (uncommon) | Lung biopsy or lavage within 48 hours or full 2-week course of co-trimoxazole (depends on patient tolerance of invasive procedure) | |
| Non-infectious causes, e.g. drug reaction, non-specific interstitial pneumonitis, radiation pneumonitis (uncommon), malignancy, leukoagglutinin reaction | ||
| Focal infiltrate | Gram-negative rods | Broad-spectrum antibiotics |
| Staphylococcus aureus | If response seen, continue treatment for 2 weeks | |
| Aspergillus | ||
| Cryptococcus | If disease progresses, lung biopsy/aspirate within 48–72 hours or empiric trial of antifungal ± macrolide | |
| Nocardia | ||
| Mucor | ||
| Pneumocystis carinii (uncommon) | ||
| Tuberculosis | ||
| Legionella | ||
| Non-infectious casues (e.g. malignancy, non-specific interstitial pneumonitis, radiation pneumonitis) |
PNEUMOCYSTIS JIROVECI PNEUMONIA (PCP)37
The incidence of this common opportunistic infection has fallen substantially in patients with AIDS who are receiving prophylaxis and effective antiretroviral therapy, with most cases occurring in patients who are not receiving HIV care or among patients with advanced immunosuppression. The onset is usually insidious with dry cough, dyspnoea and fever on a background of fatigue and weight loss. Crackles in the chest are rare. Approximately 15% of patients have a concurrent cause for respiratory failure (e.g. Kaposi sarcoma, TB, bacterial pneumonia). Useful investigations are:
Response to treatment is usually excellent, with a response time of 4–7 days. If the patient deteriorates or fails to improve: consider (re-) bronchoscopy (is the diagnosis correct?), treat co-pathogens and consider a short course of high-dose intravenous methylprednisolone and/or diuretics (patients are often fluid-overloaded). Approximately 40% of patients with HIV-related PCP who require mechanical ventilation survive to hospital discharge.38
TUBERCULOSIS
Response to treatment is usually rapid. Complex interactions occur between rifamycins (e.g. rifampicin and rifabutin) and protease inhibitors and non-nucleosidase reverse transcriptase inhibitors used to treat patients infected with HIV. The choice of rifampicin or rifabutin depends on a number of factors, including the unique and synergistic adverse effects for each individual combination of rifampicin and anti-HIV drugs and consultation with a physician with experience in treating both TB and HIV is advised.39 Infectious Diseases Society of America-recommended dosage adjustment for patients receiving antiretrovirals and rifabutin37 can be obtained via the link page: http://www.aic.cuhk.edu.hk/web8/Pneumoniaguidelines.htm. The optimal time for initiating antiretroviral therapy in patients with TB is controversial. Early therapy may decrease HIV disease progression but may be associated with a high incidence of adverse effects and an immune reconstitution reaction.37
CYTOMEGALOVIRUS (CMV) PNEUMONITIS40,41
Risk of infection is highest following allogeneic stem cell transplantation, followed by lung transplantation, pancreas transplantation and then liver, heart and renal transplantation and advanced AIDS. If both the recipient and the donor are seronegative then the risk of both infection and disease is negligible. If the recipient is seropositive the risk of infection is approximately 70% but the risk of disease is only 20%, regardless of the serostatus of the donor. However, if the recipient is seronegative and the donor is seropositive, the risk of disease is 70%. If steroid pulses and antilymphocyte globulin are given for treatment of acute rejection the risk of developing disease is markedly increased. Infection may be the result of primary infection or reactivation of latent infection. It is clinically important, but often difficult, to distinguish between CMV infection and CMV disease and a definitive diagnosis can only be made histologically. Detection of CMV-pp65 antigen in peripheral WBCs and detection of CMV DNA or RNA in the blood by quantitative polymerase chain reaction are the most useful tests for demonstrating CMV disease. Using thresholds of 10/300 000–50/200 000 positive circulating peripheral WBC, the positive predictive value for CMV-pp65 ranges from 64% to 82% and the negative predictive value from 70% to 95%.42–44 Treatment consists of intravenous ganciclovir for at least 14 days. Foscarnet can be used if ganciclovir fails.
FUNGAL PNEUMONIA
CANDIDIASIS
This is effectively a combination of the two types of fungal infection in that impaired cell-mediated immunity predisposes to mucosal overgrowth with Candida but impaired phagocytic function or numbers is usually required before deep invasion of tissues occurs. Primary Candida pneumonia (i.e. isolated lung infection) is uncommon40,45,46 and more commonly pulmonary lesions are only one manifestation of disseminated candidiasis. Even more common is benign colonisation of the airway with Candida. In most reported cases of primary Candida pneumonia amphotericin B has been used. In disseminated candidiasis treatment should be directed to treatment of disseminated disease rather than Candida pneumonia per se.46
INVASIVE ASPERGILLOSIS47
In acutely ill immunocompromised patients intravenous therapy should be initiated if there is suggestive evidence of invasive aspergillosis while further investigations to confirm or refute the diagnosis are carried out. First-line therapy is voriaconazole.48 Caspofungin and amphotericin are alternatives.
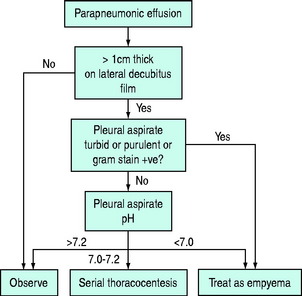
PARAPNEUMONIC EFFUSION
This may be an uncomplicated effusion which resolves with appropriate treatment of the underlying pneumonia or a complicated effusion which develops into an empyema unless drained. Complicated effusions tend to develop 7–14 days after initial fluid formation. They are characterised by increasing pleural fluid volume, continued fever and pleural fluid of low pH (< 7.3) which contains a large number of neutrophils and may reveal organisms on Gram staining or culture. An outline of management is given in Figure 32.5.
EMPYEMA49
TREATMENT
The mainstay of treatment is drainage either by intercostal drain or by surgical intervention. Patients who present before the pus is loculated and a fibrinous peel has formed on the lung can usually be treated by simple drainage. The combination with intrapleural fibrinolysis may be beneficial.50 Optimal surgical management, which consists of decortication (open or thoracoscopic), is indicated if the empyema is more advanced or if simple drainage fails. This is a major procedure and many patients with cardiac or chronic respiratory disease will not tolerate it. Alternatives for these patients are instillation of thrombolytics into the pleural space or thoracostomy. Antibiotics have only an adjunctive role. Broad-spectrum antibiotic regimes with anaerobic cover should be used until the results of microbiological analysis of the aspirated pus are available.
ACKNOWLEDGEMENTS
All tables and figures are reproduced from http://www.aic.cuhk.edu.hk/web8 with permission of the author.
1 American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
2 Mandell LA, Marrie TJ, Grossman RF, et al. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis. 2001;31:383-421.
3 American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 2001;163:1730-1754.
4 Fang GD, Fine M, Orloff J, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore). 1990;69:307-316.
5 Dupont H, Mentec H, Sollet JP, et al. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intens Care Med. 2001;27:355-362.
6 Namias N, Samiian L, Nino D, et al. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J Trauma. 2001;49:638-646.
7 Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27-72.
8 Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138-1180.
9 Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis. 2000;31:347-382.
10 File TMJr. Community-acquired pneumonia. Lancet. 2003;362:1991-2001.
11 van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur J Clin Micro Infect Dis. 2005;24:241-249.
12 Meduri GU, Chastre J. The standardization of bronchoscopic techniques for ventilator-associated pneumonia. Chest. 1992;102:557S-564S.
13 Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morbid Mortal Week Rep. 2005;54:1-141.
14 Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intens Care Med. 2004;30:536-555.
15 Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837-1842.
16 Jefferson T, Demicheli V, Deeks J, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2000:CD001265.
17 Gruber PC, Gomersall CD, Joynt GM. Avian influenza (H5N1): implications for intensive care. Intens Care Med. 2006;32:823-829.
18 Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
19 O’Grady NP, Barie PS, Bartlett JG, et al. Practice guidelines for evaluating new fever in critically ill adult patients. Clin Infect Dis. 1998;26:1042-1059.
20 Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621-630.
21 Ruiz M, Torres A, Ewig S, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med. 2000;162:119-125.
22 Kirtland SH, Corley DE, Winterbauer RH, et al. The diagnosis of ventilator-associated pneumonia. A comparison of histologic, microbiologic, and clinical criteria. Chest. 1997;112:445-457.
23 The Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619-2630.
24 Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-2598.
25 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858.
26 Bonten MJ, Kullberg BJ, Van Dalen R, et al. Selective digestive decontamination in patients in intensive care. J Antimicrob Chemother. 2000;46:351-362.
27 Liberati A, D’Amico R, Pifferi S, et al. Antibiotics for preventing respiratory tract infections in adults receiving intensive care (Cochrane Review). In The Cochrane Library. Oxford: Update Software; 2001. Issue 2 2001
28 Nourdine K, Combes P, Carton MJ, et al. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intens Care Med. 1999;25:567-573.
29 Guerin C, Girard R, Chemorin C, et al. Facial mask noninvasive mechanical ventilation reduces the incidence of nosocomial pneumonia. A prospective epidemiological survey from a single ICU. Intens Care Med. 1997;23:1024-1032. [erratum appears in Intens Care Med 1998; 24: 27]
30 Combes P, Fauvage B, Oleyer C. Nosocomial pneumonia in mechanically ventilated patients, a prospective randomised evaluation of the Stericath closed suctioning system. Intens Care Med. 2000;26:878-882.
31 Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995;122:179-186.
32 Frame RN, Johnson MC, Eichenhorn MS, et al. Active tuberculosis in the medical intensive care unit: a 15-year retrospective analysis. Crit Care Med. 1987;15:1012-1014.
33 American Thoracic Society, Centers for Disease Control. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376-1395.
34 National Collaborating Centre for Chronic Conditions. Tuberculosis. Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention. London: Royal College of Physicians, 2006.
35 Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001;345:189-200.
36 Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481-487.
37 Benson CA, Kaplan JE, Masur H, et al. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. Clin Infect Dis. 2005;40:S131-235.
38 Randall CJ, Yarnold PR, Schwartz DN, et al. Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus-related Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 2000;162:393-398.
39 Centers for Disease Control. Updated guidelines for the use of rifabutin or rifampicin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. MMWR Morb Mortal Wkly Rep. 2000;49:185-189.
40 Tamm M. The lung in the immunocompromised patient. Infectious complications part 2. Respiration. 1999;66:199-207.
41 van der Bij W, Speich R. Management of cytomegalovirus infection and disease after solid-organ transplantation. Clin Infect Dis. 2001;33(Suppl. 1):S32-S37.
42 Camargo LF, Uip D, Simpson A, et al. Comparison between antigenemia and a quantitative-competitive polymerase chain reaction for the diagnosis of cytomegalovirus infection after heart transplantation. Transplantation. 2001;71:412-417.
43 Schäfer P, Tenschert W, Cremaschi L, et al. Area under the viraemia curve versus absolute viral load: utility for predicting symptomatic cytomegalovirus infections in kidney transplant patients. J Med Virol. 2001;65:85-89.
44 Meyer-Koenig U, Weidmann M, Kirste G, et al. Cytomegalovirus infection in organ-transplant recipients: diagnostic value of pp65 antigen test, qualitative polymerase chain reaction (PCR) and quantitative Taqman PCR. Transplantation. 2004;77:1692-1698.
45 Baughman RP. The lung in the immunocompromised patient. Infectious complications Part 1. Respiration. 1999;66:95-109.
46 Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-189.
47 Stevens DA, Kan VL, Judson MA, et al. Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis. 2000;30:696-709.
48 Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
49 Peek GJ, Morcos S, Cooper G. The pleural cavity. Br Med J. 2000;320:1318-1321.
50 Misthos P, Sepsas E, Konstantinou M, et al. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. Eur J Cardiothoracic Surg. 2005;28:599-603.