Pleural Diseases
After reading this chapter you will be able to:
 Describe important anatomic features and physiologic function of the visceral and parietal pleural membranes.
Describe important anatomic features and physiologic function of the visceral and parietal pleural membranes.
 Describe how pleural effusions occur and the difference between transudative and exudative effusions.
Describe how pleural effusions occur and the difference between transudative and exudative effusions.
 Identify common causes of transudative and exudative pleural effusions.
Identify common causes of transudative and exudative pleural effusions.
 Write definitions of chylothorax, hemothorax, and pneumothorax.
Write definitions of chylothorax, hemothorax, and pneumothorax.
 Describe the impact of moderate to large pleural effusions on lung function.
Describe the impact of moderate to large pleural effusions on lung function.
 State the role of a chest radiograph in recognizing pleural effusions.
State the role of a chest radiograph in recognizing pleural effusions.
 State the purpose of thoracentesis and the potential complications.
State the purpose of thoracentesis and the potential complications.
 Identify the definitions of spontaneous, secondary, and tension pneumothorax.
Identify the definitions of spontaneous, secondary, and tension pneumothorax.
Pleural Space
Overview and Definitions
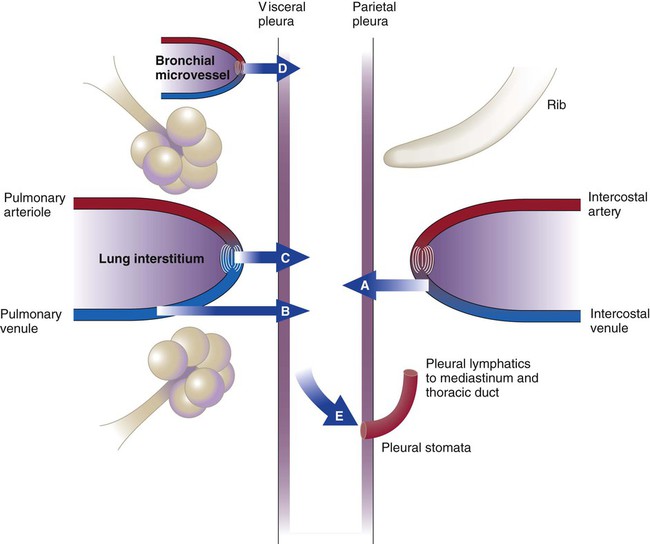
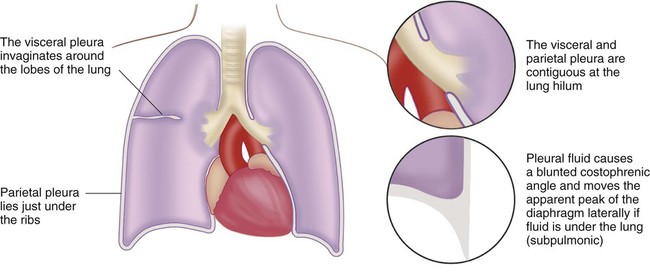
The blood vessels and airways that enter the lung connect to the mediastinum at the lung hilum. At this juncture, the visceral pleura meets the mediastinal parietal pleura to form a single, continuous pleural membrane (Figure 25-1).
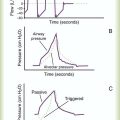
The average person has approximately 8 ml of pleural fluid per hemithorax.1 The pleural fluid is estimated to have a total protein concentration similar to that of interstitial fluid elsewhere in the body: between 1.3 g/dl and 1.4.2
Pleural Effusions
Any abnormal amount of pleural fluid in the pleural space is called pleural effusion. The many causes of pleural effusion are categorized according to etiologic factor and the content of the fluid.3
Transudative Effusions
Any pleural effusion that forms when the integrity of the pleural space is undamaged is called a transudative pleural effusion. A pleural fluid total protein concentration less than 50% of the serum total protein level and lactate dehydrogenase values in the pleural fluid less than 60% of the serum value indicate the presence of a transudative pleural effusion. In the absence of serum values, an absolute pleural fluid lactate dehydrogenase level less than two-thirds normal for serum suggests the presence of a transudate. These numbers were derived from large patient series in which pleural fluid and serum protein concentrations were measured while the cause of the effusion was being determined and corrected.4
The classification system listed in Box 25-1 is not perfect, and refinements continue to be proposed. For practical purposes, these numbers help narrow the possible causes of pleural fluid formation. Transudative pleural effusions form when hydrostatic and oncotic pressures are abnormal (Figure 25-2).5 The list of diseases that cause transudative pleural effusions is short. These diseases remain relatively easy to diagnose.
Congestive Heart Failure
Elevation of pressure in the left atrium and pulmonary veins is the hallmark of congestive heart failure (CHF). Elevation of pulmonary venous pressure increases the amount of interstitial fluid in the lung. In severe cases, flooding of the alveoli causes pulmonary edema, but in less severe cases, interstitial lung water increases and decompresses into the pleural space. Because systemic venous pressure also is elevated, there is limited capability to remove pleural fluid through the intercostal veins. Pleural fluid must be predominantly removed by the lymphatic vessels. Pleural effusions result when the capacity of pleural lymphatic drainage is overcome.6
CHF is the most common cause of clinical pleural effusions. The effusions can be massive, filling the entire hemithorax and compressing the lung. More commonly, they are small and bilateral. The effusions are rarely drained because outcome is heavily influenced by successful management of the underlying CHF, which also clears the effusions.7
Liver Disease
All ascitic fluid can end up in the chest because of the pressure gradient, and true ascites can be absent. This condition often is quite difficult to manage except with methods that limit ascites formation, such as sodium restriction and diuretics. Excessive pleural fluid is present in approximately 6% of patients with ascites, and 70% of these fluid collections are on the right side.8
Exudative Effusions
An exudative pleural effusion is caused by inflammation in the lung or pleura. This type of pleural effusion has more protein and inflammatory cells present than a transudative effusion. Because therapy for pleural effusion depends on the cause, thoracentesis often is performed to determine the specific biochemical and cellular characteristics of the pleural effusion. Box 25-1 lists the common causes of exudative pleural effusion. These account for approximately 70% of all pleural effusions.
Parapneumonic Effusion
Pleural effusions form in pneumonia because inflammation in the lung increases interstitial lung water and pleural fluid production. Most effusions are small and resolve with resolution of bacterial pneumonia.9 Complicated parapneumonic pleural effusion develops when the pleural fluid has a high enough protein content to clot. The clotting causes fibrin strands to span the visceral and parietal pleurae. The net result is collection of pleural fluid into different loculi within the pleural cavity. These often cannot be drained by a single chest tube.
Progression to empyema is marked by the presence of bacteria within the pleural space, seen as pus or bacteria on Gram stain. Empyema necessitates drainage. Whether complicated parapneumonic effusions necessitate drainage is controversial, although most physicians perform drainage because some of these effusions can progress to empyema.10
Tuberculous Pleurisy
Although these patients require respiratory isolation, only 25% of them have sputum that subsequently grows Mycobacterium tuberculosis. The PPD skin test result is negative in 30% of patients when they come to medical attention but turns positive in 6 to 8 weeks in almost everyone.11
Postoperative Causes
Various operations involving the chest or upper abdomen produce pleural fluid.12 Effusions following cardiac surgery usually are predominant on the left side and tend to be bloody. These effusions are particularly prevalent after a cutdown of the internal mammary artery for coronary artery bypass.
Chylothorax
The thoracic duct is a lymphatic channel that runs from the abdomen through the mediastinum to enter the left subclavian vein. Disruption of the thoracic duct anywhere along its course can cause leakage of chyle into the mediastinum, which may rupture into the pleural space and cause a chylothorax. The most common causes of rupture are malignancy (50%), surgery (20%), and trauma (5%).13 The thoracic duct courses through the right side of the mediastinum in the lower thoracic cavity before crossing to the left side of the mediastinum at T4 to T6. Rupture below this level causes right-sided pleural effusion, whereas rupture above this level causes left-sided pleural effusion.
In a patient who has eaten recently, the effusions are milky white as a result of the presence of chylomicrons (microscopic fat particles) absorbed by abdominal lymphatic vessels. In a fasting patient, these effusions usually are yellow. The effusions may be bloody. A pleural fluid triglyceride concentration greater than 110 mg/dl confirms the diagnosis.14 Computed tomography (CT) should be performed to evaluate the cause of the chylothorax.
Hemothorax
Although hemothorax is seen most commonly after blunt or penetrating chest trauma, numerous medical conditions can give rise to blood in the pleural space. These conditions should be considered in the absence of trauma. Any vein or artery in the thorax can bleed into the pleural space. A chest tube usually is inserted to monitor the rate of bleeding and determine whether the source is arterial or venous.15
Physiologic Importance
Mechanics of Ventilation
Hypoxemia
Most patients with a pleural effusion have an increased alveolar-arterial gradient resulting from the pathologic changes in the lung that are causing the effusion. Oxygenation can worsen after thoracentesis because changes in ventilation/perfusion matching are not instantaneous. Recovery to baseline PO2 and subsequent small improvement usually occur within 90 minutes.16
Diagnostic Tests
Chest Radiography
Thoracentesis
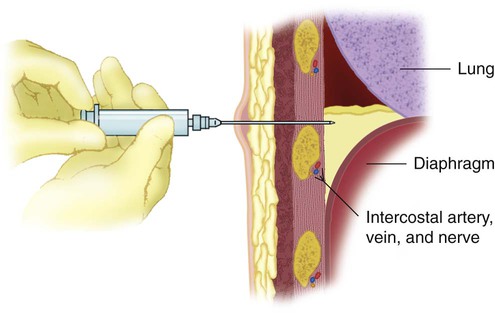
In thoracentesis, pleural fluid is sampled percutaneously by means of insertion of a needle into the pleural space (Figure 25-3). Administration of adequate local anesthetic ensures a painless procedure if care is taken to place lidocaine at the skin insertion site, along the periosteum of the involved rib, and at the parietal pleura, which is richly innervated with sensory nerve fibers. Diagnostic sampling of pleural fluid for cell counts, cultures, chemistries, and cytologic examination usually can be performed with a single syringe and a small needle. Samples for pleural pH should be kept from contact with room air. Pleural fluid drainage for lung reexpansion generously involves placing a larger catheter into the pleural space for a longer time.
Thoracentesis involves the following three major risks: (1) intercostal artery laceration, (2) infection, and (3) pneumothorax.17 Both an artery and a vein course under every rib, and the vessels become increasingly serpiginous with aging. Ensuring needle passage just superior to the rib margin makes bleeding during thoracentesis rare. Ideally, anticoagulants should be stopped before the procedure to lessen bleeding risk.
Because infection can be introduced into the pleural space, a totally sterile procedure is necessary. In some situations, the risk of infection is so high that thoracentesis rarely should be performed. When a lung is surgically removed, the space fills with sterile fluid. An infection introduced into this space usually necessitates open surgical drainage. Any trapped lung also carries a high risk of empyema because of the inability of the visceral and parietal pleurae to meet and contain any infectious process. Needle puncture is one of the most common causes of pneumothorax (see discussion of pneumothorax later in this chapter).
Chest Thoracotomy Tubes
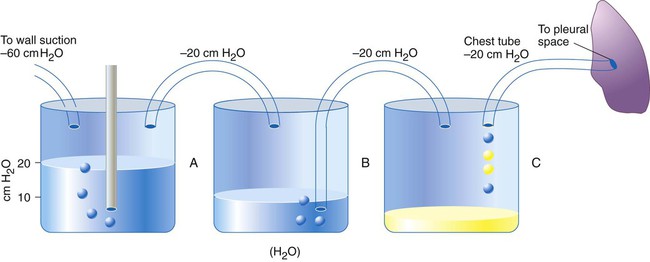
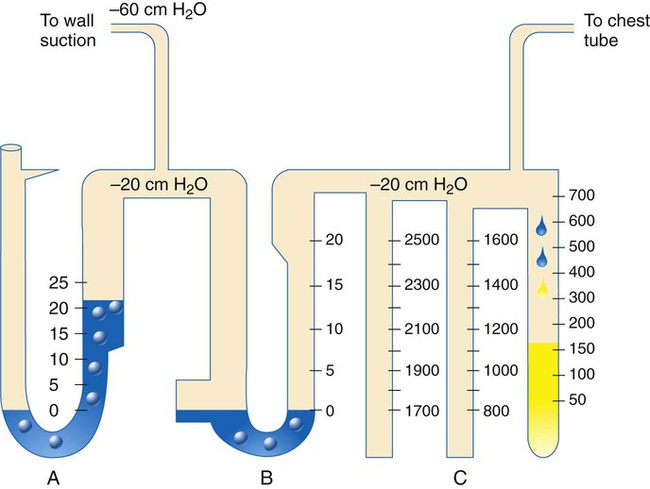
Intercostal placement is designed for the skin and soft tissue to approximate the tube and prevent air from entering the pleural space from the outside. The chest tube is connected to a water-sealed chamber, which usually is contained within a commercially marketed three-bottle system that also regulates pleural pressure and is used to measure pleural fluid volume (Figure 25-4).
Pleurodesis
Pleurodesis is the process of fusing the parietal and visceral pleurae with a fibrotic reaction that prevents further pleural fluid formation. Methods to produce pleural symphysis include surgical abrasion and the application of intrapleural chemicals such as doxycycline, minocycline, and talc. Talc has been applied as a powder suspended in sterile saline solution and injected through the chest tube (talc slurry) or dusted through a thoracoscope (talc insufflation). The success of talc pleurodesis, approximately 90%, is higher than that of all alternatives except surgical abrasion.18,19 Pleurodesis is used most commonly in the management of symptomatic pleural effusions caused by cancer. Although pleurodesis of benign effusions, such as effusions occurring with CHF, nephrotic syndrome, and idiopathic chylothorax, have been performed successfully, the procedure is discouraged for pleural effusions that are not malignant. Most pleural effusions are best managed by control of the underlying condition.20
Pneumothorax
1. Primary spontaneous pneumothorax, in which there is no underlying lung disease
2. Secondary spontaneous pneumothorax, in which lung disease is present
Traumatic Pneumothorax
Blunt and Penetrating Chest Trauma
Neonatal Pneumothorax
In radiographic series, spontaneous pneumothorax occurs in 1% to 2% of all infants soon after birth.21 The cause of pneumothorax is likely high transpulmonary pressure during birth coupled with transient bronchial blockade caused by meconium, mucus, or aspiration of blood; transpulmonary pressure gradients of 100 cm H2O can be produced.
Spontaneous Pneumothorax
Primary Spontaneous Pneumothorax
Primary spontaneous pneumothorax occurs without underlying lung disease. In a way, this term is a misnomer because CT scans have shown the presence of small subpleural blebs in more than 80% of patients.22
Primary spontaneous pneumothorax usually occurs in patients in their late teenage years or early 20s. Patients often are tall and slender, and the lungs and pleural membrane may not have grown at the same pace; the result is airspace enlargement and a thin pleural membrane. Results of some studies suggest that cigarette smoking is a risk factor in more than 90% of cases of primary spontaneous pneumothorax.23 The smoking history is typically short, and smoking cessation is recommended.
Secondary Spontaneous Pneumothorax
Depending on the extent of parenchymal lung disease, pneumothorax can be devastating. A Veterans Affairs cooperative study included 185 patients with secondary spontaneous pneumothorax and monitored them for 5 years.24 Although only three patients died of pneumothorax, the mortality rate was 43%.1 Severe underlying lung disease caused most deaths. This finding suggested that most pneumothoraces occur in patients with severe lung dysfunction. The degree of dyspnea is disproportionate to the size of pneumothorax in this group of patients because pulmonary reserve is already diminished. Pneumothorax usually should be evacuated and not observed in this patient cohort.
Complications
Tension Pneumothorax
In one case series of 74 patients with tension pneumothorax, a clinical diagnosis was made for 45 patients; the associated mortality rate was 7%. In the other cases, the diagnosis was delayed from the onset of clinical signs by 30 minutes to 8 hours, resulting in a 31% mortality rate.25 RTS are in the perfect position to make a timely diagnosis because ventilator alarms give early warnings (e.g., high pressure, lower compliance).
Therapy
Oxygen
Observation
Consensus conferences have recommended observation of patients in stable condition with primary spontaneous pneumothorax and of some patients with small secondary spontaneous pneumothorax before recurrence prevention is administered.26 Small iatrogenic pneumothorax also should be managed with observation. Primary spontaneous pneumothorax often is observed for 4 hours in the emergency department before discharge to home follow-up care as long as no pneumothorax enlargement is found on chest radiographs. Discharged patients should have ready access to emergency care facilities.
Simple Aspiration
The goal of aspiration is to reexpand the lung. Many patients have a pneumothorax from air leak that subsequently heals between the time of onset and the time treatment is sought in the emergency department. Patients with primary spontaneous pneumothorax who undergo simple aspiration for lung reexpansion and who have a stable chest radiograph 4 hours after aspiration can go home without hospital admission.26,27 Aspiration can decrease the number of patients admitted to the hospital with no increase in complications.27
Chest Tubes
Large-Bore Chest Tube
Large-bore chest tubes usually are connected to a commercial equivalent of a three-bottle system to collect any pleural fluid present, to determine whether an air leak is ongoing, and to measure intrapleural pressure (Figure 25-5). Insertion of large-bore catheters is accomplished with local anesthetic and blunt dissection of soft tissue down to the parietal pleura. Dissection should be wide enough to allow insertion of a finger into the pleural space to ensure that no adhesions are holding the lung close to the insertion site and to allow unobstructed entrance of the tube into the pleural space, where it can be directed to the position of choice.
Chest tube removal remains a highly variable practice. Removal of a chest tube as soon as an air leak visually ceases is associated with a 25% rate of recurrence of pneumothorax. The recurrence rate is near zero when chest tubes are removed 48 hours after the air leak no longer is seen in the water-seal chamber.28,29 A common practice of clamping the chest tube, with chest radiographs before and after a 4-hour observation period, can be accompanied by the return of pneumothorax. If symptoms develop during chest tube clamping, the clamp should be removed immediately, and the presence of air leak should be assessed.
Bronchopleural Fistula
Because a BPF can leak large quantities of air, more than one chest tube may be used to approximate the lung to the chest wall. This maneuver results in tamponade of the site of the air leak and allows pleural healing to occur. Therapy for BPF involves meticulous monitoring of tidal volume, airway pressures, and positive end-expiratory pressure (PEEP); avoidance of auto-PEEP; and consideration of bronchoscopic closure or thoracoscopic surgery.30 Also, endobronchial valves placed by flexible bronchoscopy have shown high rates of success for patients who are not operative candidates.31
Pleurodesis
More invasive methods have included thoracoscopy with pleural poudrage (blowing talc onto the pleural surface under direct visualization), pleural abrasion through a thoracoscope, and thoracotomy with pleurectomy (removing the pleural surface to ensure lung adhesion). More recent recommendations are for pleurodesis to occur after the first secondary spontaneous pneumothorax with thoracoscopic bullae stapling and talc poudrage.28