Phagocytosis and Intracellular Killing
Learning Objectives
• Restate the role of precipitin reaction in phagocytosis
• Define the zone of equivalence
• Identify the three different antibody Fc receptors in phagocytic cell membranes
• Compare and contrast pinocytosis and phagocytosis
• Explain the respiratory burst
• Identify the role of superoxide dismutase in the generation of hydrogen peroxide (H2O2)
• Define the reactive oxygen species
• Identify the role of myeloperoxidase in intracellular killing
• Recognize the constituents of primary, secondary, and tertiary granules
• Explain the relationship between chronic inflammation and cancer
• Understand the alternative mechanism for generating mutagenic nitrosoamines or N-nitrosoamides
• Recognize the immunologic defect in Gaucher’s disease
• Design a therapeutic regimen to treat Gaucher’s disease
• Recognize the immunologic defect in chronic granulomatous disease (CGD)
• Analyze the importance of cytochrome b588 in respiratory burst
• Design acceptable treatment modalities for CGD
• Identify the fungal species that commonly infect patients with myeloperoxidase (MPO) deficiency
• Explain the relationship between CR3 and leukocyte adhesion deficiency type I
Key Terms
Catalase
Chronic granulomatous disease
Gaucher’s disease
Myeloperoxidase
Oxygen-dependent intracellular killing
Oxygen-independent intracellular killing
Peroxidase
Phagocytosis
Pinocytosis
Reactive oxygen species
Respiratory burst
Singlet oxygen
Superoxide dismutase
Introduction
Phagocytosis and intracellular killing comprise the final step in the resolution of extracellular microbial infections. Phagocytic cells express receptors for the Fc domain of bound antibody and opsonizing complement fragments. After binding to the receptors, antigens or intact microbes are ingested and destroyed by oxygen-dependent and oxygen-independent killing mechanisms. As a prelude to phagocytosis, microbes are rendered insoluble by the chemical properties of the antigen–antibody complexes and the action of anaphylatoxins.
Precipitation of Antigen–Antibody Complexes
Phagocytosis is more efficient when antigen–antibody complexes are insoluble. The molar concentration of antigens and antibodies in plasma determines immune complex solubility. In the early phase of the immune response to the foreign protein, few antibodies are directed at antigens, and the very small immune complexes can pass through the kidney. As the concentration of antibodies increases, the ratio of antibodies to antigens decreases, and very large immune complexes are formed. When equimolar concentrations of antigens and antibodies are present (the equivalence zone), large complexes are deposited in small capillaries and in the renal glomeruli. Deposition of immune complexes is augmented by anaphylatoxins released during complement activation.
Cellular Receptors for Complement
CR1 Complement Receptor
The CR1 complement receptor (CD35) is expressed on a number of blood cells, including polymorphonuclear leukocytes, monocytes or macrophages, and follicular dendritic cells. These receptors are single-chain membrane proteins with multiple short consensus receptors (SCRs). Binding of complement fragments occurs some distance from the cell. The CR1 complement receptor recognizes C3b, iC3b, and C4b.
CR2 Complement Receptor (CD21)
The CR2 membrane glycoprotein receptor (CD21) binds the complement decay fragments iC3b and C3dg. CR2 is expressed by follicular dendritic cells in the germinal centers and serves to trap antigen–antibody complexes on iccosomes.
CR3 Complement Receptor (Mac-1, CD11b/CD18)
Complement receptor type 3 (CR3, Mac-1, and CD11b/CD18) is found on monocytes, neutrophils, natural killer (NK) cells, and dendritic cells. CR3 is composed of α and β chains. The CD11b receptor interacts with cell-bound iC3b to initiate phagocytosis. CD18 forms the β2-chain of CD11a, CD11b, and CD11c (leukocyte adhesion molecule). CD18 is the major component of lymphocyte function–associated antigen 1 (LFA-1), macrophage antigen 1 (Mac-1), and CD4 integrins, which bind leukocytes to the capillary endothelium.
Fc Receptors for Immunoglobulin G
Three different immunoglobulin G (IgG) receptors (CD64, CD16, and CD32) are expressed on phagocytic cells. Fc-gamma receptor I (FcγRI or CD64) is a high-affinity, isotype-restricted receptor that is present on most phagocytic cells, eosinophils, and dendritic cells. CD16 (Fc receptors FcγRIIIa and FcγRIIIb) is a low-affinity Fc receptor expressed on neutrophils, NK cells, eosinophils, and macrophages. The CD32 receptor is expressed on B lymphocytes and cells of macrophage or monocyte lineage. It is also known as a low-affinity Fc-gamma receptor II (FcγRII), which binds immune complexes.
Ingestion of Antigens or Microbes
Endocytosis is a general term describing a process by which cells absorb external material by engulfing it with the cell membrane. Endocytosis is usually subdivided into pinocytosis and phagocytosis. If the antigen is a small-molecular-weight protein or a polysaccharide, the cell membrane invaginates in a process called pinocytosis, and the protein or carbohydrate is placed in a fluid-filled sack called a vesicle. Large-molecular-weight antigens or intact microbes are internalized by a different form of endocytosis, which is called phagocytosis. During phagocytosis, the membrane envelopes the particle to form an internal vacuole termed phagosome.
Intracellular Killing of Bacteria
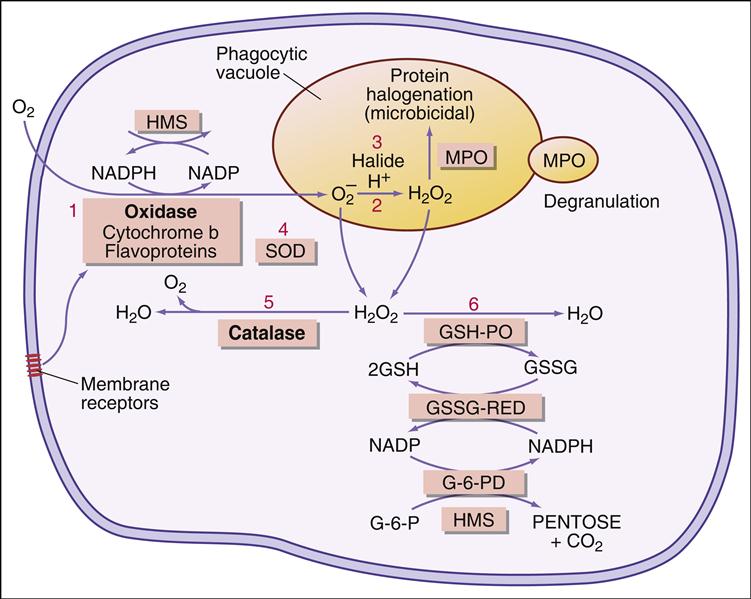
In the phagosome, bacteria are killed by two different mechanisms. One mechanism is dependent on the presence of oxygen, a respiratory burst, and the generation of reactive oxygen species. The second mechanism (which is independent of oxygen) uses preformed granules containing proteolytic enzymes to kill microbes.
Oxygen-Dependent Respiratory Burst
Following the ingestion of microbes, an oxygen-dependent respiratory burst occurs, with rapid production of singlet oxygen ( ) and hydrogen peroxide (H2O2) and energy in the form of adenosine triphosphate (ATP). In the oxidative phosphorylation pathway, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generates singlet oxygen as it transfers electrons to the cytochrome system.
) and hydrogen peroxide (H2O2) and energy in the form of adenosine triphosphate (ATP). In the oxidative phosphorylation pathway, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generates singlet oxygen as it transfers electrons to the cytochrome system.
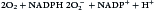
Electrons are transported through the cytochrome system by a series of oxidation reduction reactions to generate both singlet oxygen ( ) and ATP. A cytochrome known as cytochrome b588 is the major producer of singlet oxygen.
) and ATP. A cytochrome known as cytochrome b588 is the major producer of singlet oxygen.
Some singlet oxygen is released into the phagosome as free radical oxygen. Most singlet oxygen, however, interacts with an enzyme called superoxide dismutase, which converts singlet oxygen to oxygen (O2) and H2O2
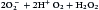
Additional dismutations occur between  and H2O2 to form hydroxyl (OH–) radicals. Singlet oxygen can also participate in other reactions.
and H2O2 to form hydroxyl (OH–) radicals. Singlet oxygen can also participate in other reactions.  can react with nitric oxide (NO–) to form peroxynitrite anion (ONOO–) or the conjugate acid (ONOOH). The reactive peroxynitrite molecule also can react with phenolic compounds, proteins, lipids, and deoxyribonucleic acid (DNA). A slow decomposition of protonated peroxynitrite (ONOOH+) produces hydroxyl radicals, protons, and peroxynitrous acid.
can react with nitric oxide (NO–) to form peroxynitrite anion (ONOO–) or the conjugate acid (ONOOH). The reactive peroxynitrite molecule also can react with phenolic compounds, proteins, lipids, and deoxyribonucleic acid (DNA). A slow decomposition of protonated peroxynitrite (ONOOH+) produces hydroxyl radicals, protons, and peroxynitrous acid.
The major reactive oxygen species (singlet oxygen, hydrogen peroxide, and hydroxyl radicals) are toxic to microbes. Singlet oxygen disrupts bacterial cell walls. Hydrogen peroxide and hydroxyl radicals attack cell membranes and also cause damage to bacterial DNA.
Myeloperoxidase (MPO) catalyzes an additional microbial killing mechanism. MPO is stored in the azurophilic granules of neutrophils and the lysosomes of monocytes. Fusion of the granules and phagosome membranes deposits MPO into the phagosome. In the presence of a halide, which is usually Cl–, MPO interacts with hydrogen peroxide to form hypochlorous acid. The acid pH kills the microbe and dissolves the cell walls.
The reaction is shown below:
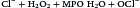
A summary of the oxygen-dependent killing process is shown in Figure 12-1.
Oxygen-Independent Intracellular Killing
Oxygen-independent intracellular killing uses preformed cytoplasmic granules containing antimicrobial agents. Primary, secondary, and tertiary granules fuse with the phagosome membrane and empty their contents into the phagosome. Each type of granule has a different array of cytotoxic molecules that have different microbial targets.
Primary granules fuse with the phagosome membrane and secrete a number of antimicrobial agents, including proteinases, and lysozyme. Lysozyme attacks the bacterial cell wall at the beta 1, 4 glucosidic linkages between N-acetyl muramic acid and N-acetylglucosamine residues and destroys the integrity of the cell wall.
Secondary granules are usually smaller than primary granules and deposit lactoferrin and a unique family of proteins called defensins into the phagosome. Lactoferrin chelates iron and prevents bacterial synthesis of heme-containing cytochromes. Defensins are a family of small cationic proteins that induce osmotic lysis by forming pores in bacterial cell walls.
Tertiary granules also are abundant in phagocytic cells. These granules contain gelatinase, acetyltransferase, and lysozyme.
Inflammation, Tissue Damage, and Cancer
As a consequence of continual or chronic inflammation, reactive oxygen species and hypochlorous acid leak from the phagocytic cell and injure host tissue. Chemical reactions in the injured tissue transform reactive oxygen species into mutagens, which cause changes in host cell DNA. In one chemical reaction, highly mutagenic chloramines also are created when primary amines react with hypochlorous acid. In another reaction, activation of nitric oxide synthase converts L-arginine to citrulline with the liberation of NO–. Reactions between NO– and O2 generate dinitrogen trioxide (N2O3). In turn, this compound reacts with water to form mutagenic nitrates. Other reaction products interact with secondary amines or amides in protein to form mutagenic N-nitrosamines or N-nitrosoamides.
Cancers may arise as a result of rapid cell division, which is usually part of the tissue repair process. In the repair process, normal cells and cells with mutated DNA undergo cell division at the same rate. Expansion of the mutated cell population and subsequent changes in the genome of mutated cells cause the uncontrolled division and development of cancerous cells.
Microbial Evasion of Intracellular Killing
Bacteria have developed mechanisms to prevent intracellular killing. Some aerobic bacteria produce catalase and peroxidase, which break down H2O2 into water and oxygen. These bacteria can live and replicate within the phagosome.
The catalase reaction is as follows:
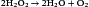
The peroxidase reaction is as follows:
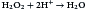
Immunodeficiencies Associated with Phagocytosis
Gaucher’s Disease
In Gaucher’s disease, patients have a deficiency in β-glucosidase or glucosylceramidase, which is necessary for the intracellular degradation of senescent red blood cells—a normal function of macrophages and monocytes. As a consequence, glucocerebroside (a membrane lipid component) accumulates in phagocytic cells in the liver, spleen, bone marrow, lymph nodes, and alveolar capillaries. The high lipid concentration inhibits protein kinase C that is necessary for macrophage activation and phagocytosis.
Non-neurologic and neurologic forms of the disease exist. Non-neurologic disease is common in adult Ashkenazi Jews and is characterized by hepatosplenomegaly, pancytopenia, and skeletal disease. Neurologic forms of the disease occur in infants and are characterized by seizures, epilepsy, learning disabilities, and dementia.
Treatment of Gaucher’s Disease
Imiglucerase, a recombinant glucocerebrosidase, can be used in enzyme replacement therapy (ERT) in patients with the non-neurologic form of the disease. ERT reduces the hepatosplenomegaly and increases red cell and platelet counts. In some cases, splenectomy is indicated for refractory splenomegaly. ERT efficacy in patients with the neurologic form of the disease is variable.
Chronic Granulomatous Disease
Patients with chronic granulomatous disease (CGD) cannot generate normal concentrations of singlet oxygen and hydrogen peroxide. Failure to produce reactive oxygen species increases the risk of infection with catalase-producing or peroxidase-producing microbes, which are resistant to oxygen-independent killing. Infection with fungal and bacterial pathogens such as the Aspergillus species, Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, and Nocardia species are common in patients with CGD. Failure to resolve the intracellular infections evokes an inflammatory response that attempts to wall off the infected phagocytic cells. These spherical masses of immunocompetent cells are known as granulomas. In patients with CGD, granulomas usually form in the lymph nodes, liver, lungs, and gastrointestinal tract. Despite aggressive therapy, the disease is often fatal.
The defective respiratory burst is attributed to a defective cytochrome b588. This cytochrome is composed of membrane- bound (gp91phox and gp22phox) and cytosolic (p47phox, p67phox, and p40phox) components. In patients with CGD, gp91phox or p47phox proteins are defective, and the cytochrome is inactive.
Treatment of Chronic Granulomatous Disease
Administration of antibiotics is necessary to control infections. Acceptable medical treatment includes first-line antibiotics such as trimethoprim-sulfamethoxazole (antibacterial), and itraconazole (antimycotic). Patients with active disease and granuloma often benefit from parenteral antibiotics and intravenous corticosteroids. Interferon-gamma (IFN-γ) treatment is recommended for patients who have some residual  production. Human stem cell transplantation is the only available curative therapy. At this juncture, 24 patients have undergone transplantations with moderate success.
production. Human stem cell transplantation is the only available curative therapy. At this juncture, 24 patients have undergone transplantations with moderate success.
Myeloperoxidase Deficiency
MPO deficiency is relatively common (1 per 2000 to 1 per 4000 live births). In most individuals with MPO deficiency, Staphylococcus and Escherichia coli can be killed by using oxygen-independent mechanisms. Fungi, however, pose a more serious problem. Candida species such as C. albicans, C. krusei, C. stellatoidea, and C. tropicalis survive in MPO-deficient phagocytic cells.
Treatment of Myeloperoxidase Deficiency
Because bacterial or fungal infections are rare in patients with MPO deficiency, prophylactic antibiotic treatment is not recommended. However, individuals with both diabetes mellitus and MPO deficiency have a high risk of invasive Candida infections. After identification of the infectious fungi, prompt and aggressive antibiotic therapy should be initiated.
Complement Receptor Deficiency
Expression of CR1 and CR2 receptor is associated with autoimmune diseases such as systemic lupus erythematosus (SLE), autoimmune hemolytic anemia, juvenile rheumatoid arthritis (JRA), and Sjögren syndrome. The role of complement receptors in the pathophysiology of these diseases is unclear. Failure to express CR3 (CD11bCD18) results in recurrent cutaneous infections and gingivitis in leukocyte adhesion deficiency syndrome type I (see Chapter 2).
Treatment of Complement Receptor Deficiency
The treatment of SLE, JRA, and autoimmune hemolytic anemia is discussed in Chapter 16. Leukocyte adhesion deficiency type I is characterized by severe or moderate gingivitis and skin ulcerations caused by Staphylococcus, Pseudomonas, Klebsiella, and Enterococcus species and E. coli. Surgical treatment is indicated for severe necrotic or infected tissue. After identification of the etiologic infective agent, microbe-specific antibiotic therapy should be initiated. Often, patients with severe defects are treated with first-line intravenous antibiotics. Moderate forms of the disease can be treated with appropriate oral antibiotics. Despite surgical intervention and aggressive antibiotic therapy, most patients succumb to virulent infections during the first 2 years of life.
Summary