3 PET Measurements of OEF for Cerebral Revascularization
PET imaging physics
PET imaging requires three components: a positron-emitting isotope (radiotracer), a tomographic imaging system to detect the location and to measure the quantity of radiation, and a mathematical model relating the physiological process under study to the detected radiation.1,2 For example, the method used in our laboratory for the measurement of cerebral blood flow uses a bolus injection of O-15 labeled water (H215O, the radiotracer).3 The PET camera system records the location and number of counts during the circulation of the water through the brain. Finally, the tomographic PET images of raw counts are converted into maps of regional quantitative CBF using computer algorithms. This processing requires measurement of arterial blood counts and incorporates models and assumptions regarding the transit of water through the cerebral circulation.
The most important limitations of PET imaging of physiologic processes relate to the phenomenon of full-width, half-maximum (FWHM) and a related phenomenon of partial-volume averaging. Detected radiation is observed over a larger area than the actual source. The spread or distribution of activity is approximately Gaussian for a point source of radiation, with the maximum located at the original point. The FWHM describes the degree of smearing of radioactivity in a reconstructed image. The ability of a PET scanner to discriminate between two small adjacent structures or accurately measure the activity in a small region will depend on the FWHM of the system as well as the amount and distribution of activity within the region of interest and the surrounding areas. Because of the smearing or redistribution of detected radioactivity, any given region in the reconstructed image will not contain all the activity actually within the region. Some of the activity will spill over into adjacent areas. This phenomenon is known as the partial volume effect. An important consequence of this principle is that PET will always measure a gradual change in activity where an abrupt change actually exists, such as in an infarct or hemorrhage, or at the border of different structures like brain and CSF or gray and white matter.4
Normal cerebral hemodynamics and metabolism
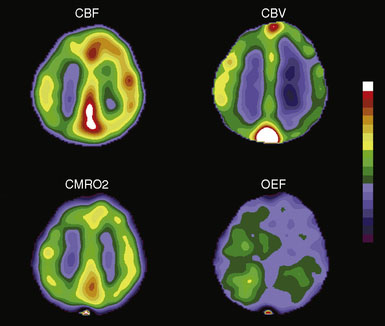
A brief introduction and definition of the common physiologic parameters measured with PET is useful prior to the discussion of normal hemodynamics and metabolism. Cerebral blood flow (CBF) the volume of blood delivered to a defined mass of tissue per unit time, generally in milliliters of blood per 100 g of brain per minute (ml/100g/min) (Figure 3–1). 15O-labeled water is the most commonly used tracer for measurements of CBF and the method used in our laboratory.3 Cerebral blood volume (CBV) is the volume of blood within a given mass of tissue and is expressed as milliliters of blood per 100 g of brain tissue. Regional CBV measurements may serve as an indicator of the degree of cerebrovascular vasodilatation, as discussed further in this chapter. CBV can be measured by PET with either trace amounts of 15O-labeled carbon monoxide or 11CO.5 Both carbon monoxide tracers label the red blood cells. Blood volume is then calculated using a correction factor for the difference between peripheral vessel and cerebral vessel hematocrit. Mean transit time (MTT) is usually calculated as the ratio of CBV/CBF. By the central volume theorem, this ratio yields mean transit time, the hypothetical mean time for a particle to pass through the cerebral circulation. Increased MTT is used as an indicator of autoregulatory vasodilation. Some PET groups have advocated the use of the inverse of this ratio instead.6
Oxygen extraction fraction (OEF) is the proportion of oxygen delivered that is extracted by tissue for metabolism. In the brain, OEF normally varies between 0.25 and 0.5, with values over 0.5 signifying increased extraction. It is measured in our laboratory by an O15O inhalation scan and independent measurements of CBF and CBV7 (Figure 3–1). The CBF accounts for the amount of oxygen delivered to the brain. The CBV corrects for oxygen in the blood that is not extracted. An alternative count based method uses the ratio of the counts after an O15O inhalation scan to the counts from an O15O water scan, without CBV correction.8–11 Other similar methods are also in common use. Cerebral metabolic rate of oxygen (CMRO2) is the amount of oxygen consumed by tissue metabolism, measured in milliliters of oxygen per 100 g of brain tissue per minute7 (Figure 3–1). CMRO2 is equal to the CBF multiplied by OEF and the CaO2 (delivery of oxygen times the fraction extracted times the amount of available oxygen).
Whole-brain, mean CBF of the adult human brain is approximately 50 ml per 100 g per minute. Functional activation increases local or regional CBF, but global CBF generally remains unchanged. Under normal conditions any change in regional CBF must be caused by a change in regional vascular resistance. Vascular resistance is mediated by alterations in the diameter of small arteries or arterioles. In the resting brain with normal perfusion pressure, CBF is closely matched to the metabolic rate of the tissue. Regions with higher metabolic rates have higher levels of CBF. For example, gray matter has a higher CBF than white matter. While there is wide variation in levels of flow and metabolism, the ratio between regional CBF(rCBF) and metabolism is nearly constant in all areas of the brain. Consequently, the maps of OEF from the blood show little regional variation.12 One exception to this is seen with physiological activation, where blood flow increases well beyond the metabolic needs of the tissue. This leads to a relative decrease of OEF and a reduction in local venous deoxyhemoglobin.13 This phenomenon is the basis for the use of magnetic resonance imaging (MRI) as a means to map brain function.
Responses to Reductions in Cerebral Perfusion Pressure: Oligemia and Ischemia
Cerebral perfusion pressure (CPP) is the difference between mean arterial pressure and venous back pressure (or intracranial pressure). An arterial stenosis or occlusion may cause a reduction in perfusion pressure if collateral sources of flow are not adequate.14 The presence of arterial stenosis or occlusion does not equate with hemodynamic impairment: up to 50% of patients with complete carotid artery occlusion and prior ischemic symptoms have no evidence of reduced CPP.15 The adequacy of collateral sources of flow determines whether an occlusive lesion will cause a reduction in perfusion pressure. When perfusion pressure falls owing to an occlusive lesion and an inadequate collateral system, the brain and its vasculature will maintain the normal delivery of oxygen and glucose through two mechanisms—autoregulatory vasodilation and increased OEF.16 The presence of these mechanisms has been extensively studied, primarily in animal models employing acute reductions in perfusion pressure. The extent to which these models are applicable to humans with chronic regional reductions in perfusion pressure is not completely known. Autoregulatory vasodilation and increased OEF may also occur in response to reduced cerebral perfusion pressure owing to increases in venous back pressure.17–20
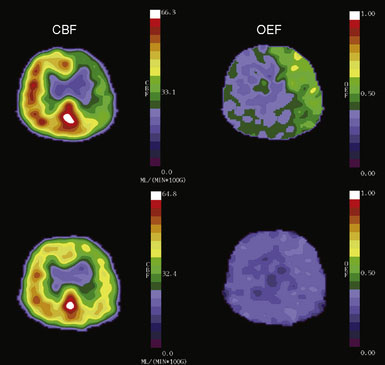
Changes in perfusion pressure have little effect on CBF over a wide range of pressure owing to vascular autoregulation. Increases in mean arterial pressure produce vasoconstriction of the pial arterioles, serving to increase vascular resistance and maintain CBF at a constant level.21 Conversely, when the pressure falls, reflex vasodilation will maintain CBF at near normal levels.22,23 Two measurable parameters that indicate autoregulatory vasodilation are increases in mean transit time and CBV (Figure 3–2). Despite vasodilation, there is some slight reduction in CBF through the autoregulatory range as perfusion falls, leading to a slight increase in oxygen extraction to compensate for the reduced delivery of oxygen.16,24
At some point the capacity for autoregulatory vasodilation can be exceeded. The threshold value for autoregulatory failure is variable between patients and can be shifted higher or lower by prior ischemic injury or longstanding hypertension. Beyond this point, CBF falls linearly as a function of pressure. Direct measurements of arteriovenous oxygen differences (CaO2 × OEF) using jugular venous oximetry have demonstrated the brain’s capacity to increase OEF and maintain normal CMRO2 in circumstances where oxygen delivery diminishes due to decreasing CBF25 (Figure 3–1). The precise mechanism by which OEF increases is not completely understood. Oxygen passively diffuses from the blood to the tissue. The best current hypothesis is that more of the oxygen that diffuses into the tissue is used for oxidative metabolism, thus reducing the amount of oxygen available to diffuse back to the capillaries.26
If the perfusion pressure of the brain continues to fall beyond the capacity for increases in OEF to compensate for the reduced delivery of oxygen, oxygen extraction will become insufficient to meet the energy requirements of the brain and true ischemia ensues.27 CMRO2 begins to fall and neurological dysfunction occurs. This may be reversible if oxygen delivery is rapidly restored. Persistent or further declines in flow can lead to permanent tissue damage, depending on the duration and degree of ischemia.28
Once tissue damage has occurred, the normal mechanisms of cerebrovascular control may no longer operate.29 Therefore, in some patients who have had transient ischemic attacks (TIA) or mild ischemic strokes with subsequent recanalization, autoregulation or the normal cerebrovascular response to partial pressure of carbon dioxide (PaCO2) may be abnormal for up to several weeks.30 Over time, flow will fall to match the metabolic needs of the tissue and autoregulatory capacity will be regained. Following reperfusion, the biochemical and ionic abnormalities resolve to a degree dependent on the severity of the initial ischemic insult. The acidosis of anaerobic glycolysis may be replaced by alkalosis.
Chronic oligemia may lead to other compensatory mechanisms, in addition to autoregulatory vasodilation and increased OEF. These include possible reversible metabolic downregulation, accompanied by a reversible cognitive impairment.31,32 This phenomenon remains an unproven hypothesis and is being evaluated in ongoing trials.
PET studies of OEF in chronic arterial occlusive disease (oligemia)
Atherosclerotic Carotid Occlusion
The patient population that has been the focus of the most investigation has been atherosclerotic carotid artery occlusion. The presence of increased OEF as measured by PET has been established as a powerful and independent risk factor for future stroke in these patients.15 Based on this information, a clinical trial of surgical revascularization is underway—the Carotid Occlusion Surgery Study (COSS).33 The details of these natural history studies and the design and rationale for the current trial will be described in this section.
Patients with complete atherosclerotic occlusion of the carotid artery are at high risk for future stroke.34 A randomized trial of extracranial to intracranial arterial bypass (the EC-IC Bypass Trial) failed to show a benefit of surgical revascularization in over 800 patients randomized to surgery or aspirin.35 One possible reason for the failure of this study to show a benefit was the lack of an effective tool to establish whether flow was normal or impaired. A procedure intended to improve flow is unlikely to provide any benefit if flow at baseline is normal. It is possible that a benefit of bypass was missed for a subgroup at particularly high risk due to hemodynamic impairment.
The St. Louis Carotid Occlusion Study was designed to determine if such a subgroup existed.15 This was a blinded, prospective study of stroke risk designed to test the hypothesis that increased OEF in patients with symptomatic atherosclerotic carotid occlusion predicted future stroke risk. Eighty-one patients with complete carotid occlusion and ipsilateral ischemic symptoms were enrolled. At baseline, 17 clinical, epidemiologic, and laboratory stroke risk factors were recorded. PET measurements of oxygen extraction were obtained.36 Thirty-nine of the 81 patients had increased OEF. All 81 patients were followed for a mean duration of 3.1 years. Fifteen total and 13 ipsilateral ischemic strokes occurred during this period. Eleven of the 13 ipsilateral strokes occurred in the 39 patients with increased OEF. Multivariate analysis found only age and OEF as predictors of stroke risk. Log rank analysis demonstrated increased OEF to be a powerful predictor of subsequent stroke (p = 0.004). Similar results were found by Yamauchi and coworkers.37
Prior studies with PET have shown that the superficial temporal artery to middle cerebral artery bypass procedure is capable of reversing the OEF abnormality29,38 (Figure 3–2). Based on these facts, the COSS was funded by the National Institutes of Health and is underway.33 Patients with complete atherosclerotic carotid artery occlusion and recent (120 days) ipsilateral cerebral ischemic symptoms are eligible for enrollment. PET studies are obtained on enrollment to identify patients with increased OEF for randomization to surgery or best medical therapy. The primary hypothesis is that bypass surgery will prevent stroke in this high-risk group.
Border-zone hemodynamics
Acute reductions in perfusion pressure can cause ischemic infarction of the cortex and adjacent subcortical white matter located at the border zones between major cerebral arterial territories, such as the middle and anterior cerebral arteries.39,40 Severe systemic hypotension is a well-recognized cause of multiple bilateral discrete cortical border-zone infarctions.39 However, the mechanism of cortical border-zone infarction in most patients with carotid atherosclerotic disease is likely embolic and not purely hemodynamic.41–44
In addition to this cortical arterial border zone, there is good evidence for an arterial border zone within the white matter of the centrum semiovale and corona radiata.45,46 This has been called the internal arterial border zone (between lenticulostriate perforators and deep penetrating branches of the distal middle cerebral artery).45 There is a strong association between hemodynamic impairment of the hemisphere and prior stroke in the white matter, but not cortical border zone.42 Interestingly, the degree of oligemia as indicated by increased OEF is not higher in noninfarcted white matter regions than the overlying cortex in patients with chronic carotid disease.47 This suggests that these white matter infarctions may occur at the time of occlusion or soon after (when some selective increase in OEF is present) and not in the chronic situation.
Improvement in hemodynamics over time
In some patients with atherosclerotic carotid occlusion, hemodynamic impairment can improve over time, as collateral flow increases.48 We repeated PET measurements in 10 patients with complete atherosclerotic carotid artery occlusion who exhibited increased OEF by PET and had no interval stroke 12 to 59 months after the initial examination. Quantitative regional measurements of CBF, CBV, CMRO2, and OEF were obtained. Regional measurements of the cerebral rate of glucose metabolism (CMRGlc) were also made on follow-up in five patients. As a group, the ratio of ipsilateral to contralateral OEF declined from a mean of 1.16 to 1.08 (p = 0.022). Greater reductions were seen with longer duration of follow-up (p = 0.023, r = 0.707). The CBF ratio improved from 0.81 to 0.85 (p = 0.021). No change in CBV or CMRO2 was observed. CMRGlc was reduced in the ipsilateral hemisphere (p = 0.001 compared with normal), but the CMRO2/CMRGlc ratio was normal. These findings allowed us to conclude that increased glucose transport was not a compensatory response to chronic hemodynamic impairment.
This improvement in collateral sources of flow over time may be a factor that accounts for the reduction in stroke risk over time in all the major cerebral revascularization trials. The greatest risk for stroke in medically treated patients in the North American Symptomatic Carotid Stenosis Trial, the EC-IC Bypass Trial, the European Carotid Stenosis Trial, as well as the St. Louis Carotid Occlusion Study was in the first 2 years after stroke.35,49,50
Moyamoya disease
Moyamoya disease is an obliterative vasculopathy of unknown etiology affecting the anterior circulation at the circle of Willis. In North America, it most frequently affects women in their third and fourth decades. Ischemic symptoms of stroke or TIAs are the most common presentation.51 It is highly likely that hemodynamic mechanisms play a role in the pathogenesis of stroke in these patients. Hemodynamic assessment may be able to provide prognostic information regarding stroke risk in this patient population, analogous to the atherosclerotic carotid occlusion.
We have used PET to study 42 patients with Moyamoya disease and found the frequency of hemodynamic impairment to be quite variable despite uniformly severe vasculopathy: 29 had normal OEF, eight had elevated unilateral OEF, and five had elevated bilateral OEF. Interval improvement in CBF and OEF was observed in one patient with increased OEF at baseline who underwent surgical revascularization. Whether increased OEF predicts stroke risk in this patient population (as it does in atherosclerotic carotid occlusion) is an area of ongoing study.52
Acknowledgments
This work is supported by the National Institutes of Health grants NINDS P50 55977 and R01 NS051631.
1 Derdeyn C.P. Positron emission tomography imaging of cerebral ischemia. Neuroimaging Clin N Am. 2005;15:341-350. x–xi
2 Derdeyn C.P., Powers W.J. Positron emission tomography: experimental and clinical applications. Philadelphia: Lippincott-Raven, 1997;239-253.
3 Raichle M.E., Martin W.R., Herscovitch P., et al. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24:790-798.
4 Videen T.O., Dunford-Shore J.E., Diringer M.N., et al. Correction for partial volume effects in regional blood flow measurements adjacent to hematomas in humans with intracerebral hemorrhage: implementation and validation. J Comput Assist Tomogr. 1999;23:248-256.
5 Martin W.R.W., Powers W.J., Raichle M.E. Cerebral blood volume measured with inhaled C15O and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:421-426.
6 Sette G., Baron J.C., Mazoyer B., et al. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Brain. 1989;113:931-951.
7 Mintun M.A., Raichle M.E., Martin W.R.W., et al. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177-187.
8 Jones T., Chesler D.A., Ter-Pogossian M.M. The continuous inhalation of oxygen-15 for assessing regional oxygen extraction in the brain of man. Br J Radiol. 1976;49:339-343.
9 Derdeyn C.P., Videen T.O., Simmons N.R., et al. Count-based PET method for predicting ischemic stroke in patients with symptomatic carotid arterial occlusion. Radiology. 1999;212:499-506.
10 Derdeyn C.P., Videen T.O., Grubb R.L.Jr, et al. Comparison of PET oxygen extraction fraction methods for the prediction of stroke risk. J Nucl Med. 2001;42:1195-1197.
11 Kobayashi M., Okazawa H., Tsuchida T., et al. Diagnosis of misery perfusion using noninvasive 15O-gas PET. J Nucl Med. 2006;47:1581-1586.
12 Baron J.C., Rougemont D., Soussaline F., et al. Local interrelationships of cerebral oxygen consumption and glucose utilization in normal subjects and in ischemic stroke patients: a positron tomography study. J Cereb Blood Flow Metab. 1984;4:140-149.
13 Fox P.T., Raichle M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140-1144.
14 Powers W.J., Tempel L.W., Grubb R.L.Jr, et al. Clinical correlates of cerebral hemodynamics. Stroke. 1987;18:284.
15 Grubb R.L.Jr, Derdeyn C.P., Fritsch S.M., et al. The importance of hemodynamic factors in the prognosis of carotid artery occlusion. J Am Med Association. 1998;280:1055-1060.
16 Derdeyn C.P., Videen T.O., Yundt K.D., et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125:595-607.
17 Wei E.P., Kontos H.A. Increased venous pressure causes myogenic constriction of cerebral arterioles during local hyperoxia. Circ Res. 1984;55:249-252.
18 Ekstrom-Jodal B. On the relation between blood pressure and blood flow in the canine brain with particular regard to the mechanism responsible for cerebral blood flow autoregulation. Acta Physiol Scand Suppl. 1970;350:1-61.
19 Iwama T., Hashimoto N., Takagi Y., et al. Hemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: positron emission tomography evaluation before and after treatment. J Neurosurg. 1997;86:806-811.
20 McPherson R.W., Koehler R.C., Traystman R.J. Effect of jugular venous pressure on cerebral autoregulation in dogs. Am J Physiol. 1988;255:H1516-H1524.
21 Forbes H.S. The cerebral circulation, I: observation and measurement of pial vessels. Arch Neurol Psychiatry. 1928;19:751-761.
22 Fog M. Cerebral circulation. The reaction of the pial arteries to a fall in blood pressure. Arch Neurol Psychiatry. 1937;24:351-364.
23 Rapela C.E., Green H.D. Autoregulation of canine cerebral blood flow. Circ Res. 1964;15:I205-211.
24 Schumann P., Touzani O., Young A.R., et al. Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain. 1998;121:1369-1379.
25 McHenry L.C.Jr, Fazekas J.F., Sullivan J.F. Cerebral hemodynamics of syncope. Am J Med Sci. 1961;80:173-178.
26 Mintun M.A., Lundstrom B.N., Snyder A.Z., et al. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci U S A. 2001;98:6859-6864.
27 Marshall R.S., Lazar R.M., Mohr J.P., et al. Higher cerebral function and hemispheric blood flow during awake carotid artery balloon test occlusions. J Neurol Neurosurg Psychiatry. 1999;66:734-738.
28 Heiss W.D., Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294-301.
29 Powers W.J., Martin W.R., Herscovitch P., et al. Extracranial-intracranial bypass surgery: hemodynamic and metabolic effects. Neurology. 1984;34:1168-1174.
30 Powers W.J. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231-240.
31 Sasoh M., Ogasawara K., Kuroda K., et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003;59:455-460. discussion 460–463
32 Chmayssani M., Festa J.R., Marshall R.S. Chronic Ischemia and Neurocognition. Neuroimaging Clin N Am. 2007;17:313-324.
33 Grubb R.L.Jr, Powers W.J., Derdeyn C.P., et al. The carotid occlusion surgery study. Neurosurg Focus. 2003;14:e9.
34 Klijn C.J.M., Kappelle L.J., Tulleken C.A.F., et al. Symptomatic carotid artery occlusion: a reappraisal of hemodynamic factors. Stroke. 1997;28:2084-2093.
35 The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med. 1985;313:1191-2000.
36 Derdeyn C.P., Yundt K.D., Videen T.O., et al. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke. 1998;29:754-758.
37 Yamauchi H., Fukuyama H., Nagahama Y., et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive disease. J Nucl Med. 1999;40:1992-1998.
38 Baron J.C., Bousser M.G., Rey A., et al. Reversal of focal “misery perfusion syndrome” by extra-intracranial artery bypass in hemodynamic cerebral ischemia. A case study with 0-15 positron emission tomography. Stroke. 1981;12:454-459.
39 Adams J., Brierley J., Connor R., et al. The effects of systemic hypotension upon the human brain. Clinical and neuropathological observations in 11 cases. Brain. 1966;89:235-268.
40 Brierley J.B., Excell B.J. The effects of profound systemic hypotension upon the brain of M. Rhesus: physiological and pathological observations. Brain. 1966;89:269-298.
41 De Reuck J.L. Pathophysiology of carotid artery disease and related clinical syndromes. Acta Chir Belg. 2004;104:30-34.
42 Derdeyn C.P., Khosla A., Videen T.O., et al. Severe hemodynamic impairment and border zone—region infarction. Radiology. 2001;220:195-201.
43 Torvik A., Skellerud K. Wastershed infarcts in the brain caused by microemboli. Clin Neuropath. 1982;1:99-105.
44 Torvik A. The pathogenesis of watershed infarctions in the brain. Stroke. 1984;15:221-223.
45 Zulch K.J. Uber die entstehung und lokalization der hirn-infarkte. Zentralbl Neurochir. 1961;21:158-178.
46 Wodarz R. Watershed infarctions and computed tomography. A topographical study in cases with stenosis or occlusion of the carotid artery. Neuroradiol. 1980;19:245-248.
47 Derdeyn C.P., Simmons N.R., Videen T.O., et al. Absence of selective deep white matter ischemia in chronic carotid disease: a positron emission tomographic study of regional oxygen extraction. Am J Neuroradiol. 2000;21:631-638.
48 Derdeyn C.P., Videen T.O., Fritsch S.M., et al. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke. 1999;30:1019-1024.
49 North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
50 European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70%–99%) or with mild (0%–29%) carotid stenosis. Lancet. 1991;337:1235-1243.
51 Chiu D., Shedden P., Bratina P., et al. Clinical features of Moyamoya disease in the United States. Stroke. 1998;29:1347-1351.
52 Zipfel G.J., Sagar J., Miller J.P., et al. Cerebral hemodynamics as a predictor of stroke in adult patients with moyamoya disease: a prospective observational study. Neurosurg Focus. 2009;26:E6.