CHAPTER 166 Peripheral Nerve Stimulation for Neuropathic Pain
Electrical stimulation of peripheral nerves is used in a variety of medical applications. The most common ones include testing neuromuscular conduction in anesthesia and intensive care units1,2; motor stimulation of phrenic nerves in cases of diaphragmal palsy3 and somatic nerves of the extremities in patients with hemiplegia4,5 and paraplegia4,6; vagal nerve stimulation for treatment of intractable epilepsy7 and refractory depression8; autonomic stimulation for urinary9 and gastrointestinal disorders10; and, finally, the stimulation of peripheral nerve for control of neuropathic pain.11
Peripheral nerve stimulation (PNS) has been a part of the neurosurgical armamentarium for a long time. It presents an excellent modality for the treatment of various neuropathic pain conditions and is used to complement the other electrical neuromodulation procedures—spinal cord stimulation (SCS) and deep brain stimulation (DBS). Similar to SCS, PNS is thought to be based on the gate-control theory of pain, suggested by Melzack and Wall more than 40 years ago.12 In terms of approved indications, SCS is by far more accepted than PNS, and frequently PNS is considered a novel technique in the continuum of treatment modalities for medically refractory neuropathic pain. This, however, is a misconception. Although the interest in PNS has been increasing over the past few years, the use of electrical stimulation of the peripheral nerves for pain control is anything but new. When about 40 years ago Wall and Sweet13,14 tried to find a new approach to suppression of neuropathic pain, they inserted an electrode into their own infraorbital foramina and obtained decrease in pain perception during the entire episode of electrical stimulation. Moreover, the first articles dedicated to the idea of PNS with implantable devices (even before the dorsal column stimulation, later known as SCS, was introduced) were published back in 1966 and 1967.13,15 In eight patients with neuropathic pain, the stimulation resulted in lasting pain suppression as long as the stimulator was “on.”13 At about same time, Shelden15 implanted PNS electrodes and stimulated them through an implanted receiver at 14,000 Hz, achieving temporary pain relief.
During the following 20 years, multiple reports appeared in the literature dealing with various applications of PNS, summarizing experience with various types of equipment, and describing different surgical techniques for PNS electrode implantation. The articles that appeared in the 1970s,16–21 1980s,22–28 and 1990s29–32 mostly represented single-institution series with use of different electrodes that were implanted in the vicinity of, or in direct contact with, the peripheral nerve, the same nerve that was thought to be responsible for generation of pain either as a result of direct traumatic or iatrogenic injury or as a part of a complex regional pain syndrome. In most cases, the electrode implantation involved surgical exploration of the peripheral nerve and placement of a flat plate (paddle-type) multicontact electrode immediately next to it. Unfortunately, the reported results of the PNS approach were not extremely encouraging in terms of pain relief. For example, Sweet in 197620 reported an overall long-term success rate of 25%, and in 1982, Nashold and colleagues24 reported successful outcomes in 53% of patients with upper extremity nerve implants and in only 31% of patients with sciatic nerve implantation—a total success rate of less than 43%. In addition, the reports of nerve injury from electrode insertion or stimulation-related fibrosis made PNS less attractive,17,33 particularly because the SCS approach became universally accepted as means of long-term treatment of medically intractable neuropathic pain of various etiologies. Few enthusiastic centers continued using PNS for certain neuropathic pain syndromes, but the lack of wide interest among implanters resulted in little effort from the device manufacturers to get appropriate U.S. Food and Drug Administration (FDA) approval for use of their implantable generators in PNS. Even now, according to the manufacturers’ manuals, the only device specifically approved for peripheral nerve stimulation is a radiofrequency system made by Medtronic (Minneapolis, MN), whereas all other systems, including implantable pulse generators made by Medtronic, as well as devices made by Advanced Neuromodulation Systems (ANS; Plano, TX) and Advanced Bionics (Sylmar, CA), are used for PNS on an off-label basis.
Rebirth of the PNS approach may be credited to pioneering work of Weiner and Reed34 that described the percutaneous technique of electrode insertion in the vicinity of the occipital nerves to treat occipital neuralgia. Soon thereafter, Slavin and Burchiel11,35 described use of this technique in both occipital and trigeminal areas, and afterward, the approach was modified by many implanters in terms of the electrode type, insertion procedure, indications, and other features.36–61
The procedure was not limited to the upper neck and face area; other reports detailed the use of PNS in other parts of the body. For example, percutaneously inserted PNS electrodes were used to control inguinal pain after herniorrhaphy,62 and paraspinal electrodes have been used for treatment of low back pain and sacroiliac pain,63 thoracic postherpetic pain,64 and coccydynia.65
Indications
The usual indications for PNS in neuropathic pain are very similar to those used for SCS procedures. The pain is chronic and severe, negatively affects the patient’s functionality, and is refractory to the usual medical treatment, including medications, physical therapy, and less invasive interventions, such as local anesthetic and sympathetic blocks, application of transcutaneous electrical nerve stimulation (TENS), and injections of botulinum toxin preparations, if these interventions are even considered by the treating physicians. For the purposes of this chapter, the neuropathic pain is broadly defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system,”66 and this wide definition includes, among other syndromes, such debatable nosologic conditions as fibromyalgia, migraines, and coccydynia.
Two other criteria are considered before implantation of a PNS system: the patient’s psychological evaluation and results of a short-term trial with externalized electrodes. Both these steps are taken as the standard approach to SCS; the psychological evaluation details and their predictive values in SCS were recently reviewed.67 Outpatient stimulation trial is also a routine step in SCS surgical procedure, and the trial length usually varies between 2 and 14 days. Usually, a 50% improvement in pain intensity serves as a cutoff limit for considering the trial successful. In some places, the patient satisfaction with pain relief, even if the pain intensity decrease is less than 50%, justifies proceeding with permanent implantation.
In terms of specific indications, there are at least 11 distinct pain conditions for which PNS has been recently reported: (1) postherpetic neuralgia40,45,64; (2) posttraumatic or postsurgical neuropathic pain that is related to underlying dysfunction of a certain nerve, including the infraorbital, supraorbital, or occipital nerves45,48,52; (3) classic migraine, “transformed migraine” presenting with occipital pain and discomfort, and hemicrania continua39,43,46,47,53,54,56,61; (4) occipital neuralgia or cervicogenic occipital pain34,38,47,49,51; (5) complex regional pain syndrome28,30,68; (6) cluster headaches54,58,59,69; (7) chronic daily headaches39,53,60; (8) inguinal pain after herniorrhaphy62; (9) coccydynia65; and (10) fibromyalgia.57,70
Surgical Technique
The hardware for PNS is usually implanted with a straightforward set of guidelines. The electrode (lead) is implanted in the vicinity of the stimulated nerve. The approach may include either direct exposure of the nerve,32 so that the electrode may be placed next to it (or even wrapped around it—similar to vagal nerve stimulation system),7,8,71 or percutaneous electrode implantation through a needle inserted perpendicular to the nerve course.34,48,52,59 Between these two approaches is the technique of surgical lead insertion toward the nerve without actual nerve exposure.44,47,49,58,61
The direction of electrode insertion may be chosen based on implanter’s preference; for example, we routinely insert electrodes from lateral to medial not only in the supraorbital and infraorbital regions (where it is probably the only way to place them) but also in the occipital area,51,52,55 whereas others prefer to insert electrodes from medial to lateral.41,43 Standard 4-, 8- or even 16-contact electrodes61 are used (Figs. 166-1 to 166-3); the electrodes are passed in the epifascial plane under the skin but above the muscles. Our general approach is to have the electrode cross the path of the nerve chosen as a stimulation target. As long as this nerve happens to be either under one of the electrode’s contacts or between the two contacts, the stimulation can be steered toward it to achieve adequate coverage. For the trial insertion, we do not implant any deep anchors or extensions. The electrodes are sutured to the skin with plastic anchors and fine nylon, and a strain-relief loop is created around the insertion site to avoid inadvertent electrode pullout.
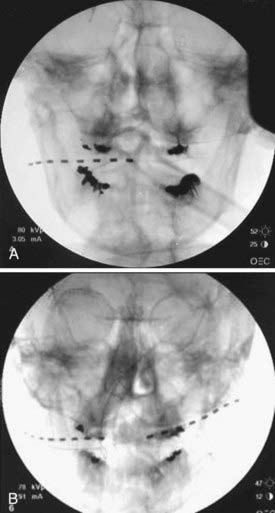
FIGURE 166-1 Anteroposterior radiographs of unilateral (A) and bilateral (B) 8-contact occipital nerve stimulation electrodes.
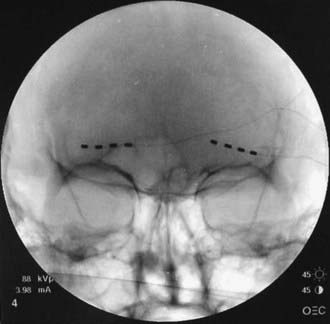
FIGURE 166-2 Anteroposterior radiograph of bilateral 4-contact supraorbital nerve stimulation electrodes.
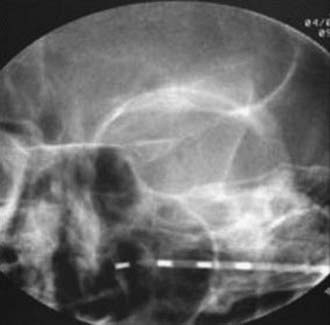
FIGURE 166-3 Anteroposterior radiograph of unilateral 4-contact infraorbital nerve stimulation electrode.
When the trial is completed, the temporary system is replaced with the permanent one. We prefer to remove the temporary electrode in the operating room after confirming its position with fluoroscopy, and then we insert a new permanent electrode that is connected either directly to the generator or to an extension cable that connects to the generator. The electrodes that we use are cylindrical wire type (e.g., Quad, Octad, Quad Plus, or Quad Compact, Medtronic; Qattrode, Octrode, or Axxess, ANS; and Linear, Advanced Bionics). Other groups reported using plate-type (paddle-type) electrodes (e.g., Resume, Resume II, or Resume TL, Medtronic; or Artesan, Advanced Bionics) for stimulation of the occipital nerves. Implantation of such electrodes may be preceded by a trial with wire-type electrodes,49 or the trial may be done with plate-type electrodes connected to a temporary extension.47 In all cases, the electrodes are placed over the course of the peripheral nerve that supplies the painful area and may be involved in the generation of pain.
The electrodes or extension cables are tunneled toward the generator pocket. The tunneling step is painful and, in our practice, necessitates the use of general anesthesia. Location of this pocket is chosen based on the patient’s and surgeon’s preference. Placement of the generator into the gluteal area,49,50 abdominal wall,38,44 and infraclavicular areas34,45,47,48,51,55,58 has been described. In our opinion, the infraclavicular area, which is routinely used for placement of deep brain stimulation generators, is the preferred location for both trigeminal and occipital nerve stimulation systems (as documented by a recent analysis of clinical experience of patients with infraclavicular generators that found extremely high levels of patient satisfaction with this particular location).72 Independently of location, the pocket should satisfy certain requirements: it has to be deep enough to avoid hardware erosion; it should not be too deep to interfere with reprogramming or, in the case of rechargeable devices, their regular charging; and it should be located in a relatively immobile region because the hardware may fail if subjected to repetitive mechanical stress.
A completely different setup is used with a new revolutionary device, BION (Advanced Bionics), that integrates the generator and electrode into a single (and very unique) apparatus.73 Initially developed for functional electrical stimulation, the BION has been tried for PNS in the treatment of overactive bladder74 and headaches54,56 in multiple clinical centers. Several published reports have been made of its clinical use,54,56,74 although the device has not yet received formal FDA approval. In essence, BION is a miniature leadless implantable neurostimulator that is designed to be implanted through a small incision as a part of a minimally invasive procedure. Because the stimulating electrodes are mounted directly on the neurostimulator, the possible complications associated with the use of leads (e.g., migration, disconnection) are avoided. The currently tested device is the shape of a cylinder measuring 3.3 mm in diameter and 27 mm in length. It has a cathode on the tip of the stimulator and an anode in its distal surface; it contains a programming and telemetry module and a rechargeable battery. BION allows delivery of a wide range of stimulation parameters with pulse width up to 1000 µsec, stimulation rate up to 1000 Hz, and electrical current amplitude up to 12 mA. Similar to other devices on the market, BION is programmed by trained clinicians; a remote control is given to the patient, allowing adjustment of the stimulation parameters within a preset range. The charging process takes less than 2 hours, and the interval between charging depends on the device use, ranging from daily to every other week, with total battery life expectation of up to 20 years.75
Results and Future Directions
So far, the use of PNS for neuropathic pain has been associated with consistently positive results.53–60,62–64 With the only exception of postherpetic trigeminal neuralgia, in which PNS has shown only minor improvement in less than half of patients,45 the other indications have either been shown to respond to PNS or are currently investigated in prospective fashion.
The complication rate of PNS is generally low, but both minor and major complications, such as local infections, hardware erosions, component disconnections, electrode fractures and displacements,32,48,51,55 and even sepsis,18,22 have been reported in the literature. The complications of perineural fibrosis, described in the past with the use of plate-type or wrap-around electrodes,18,24,33 are essentially unheard of with the use of modern wire-type electrodes. Most complications require system revision or replacement; lasting side effects are overall unlikely.
The mechanism of PNS is still largely unknown. Several animal studies proposed certain explanations that are related to direct excitation of the central pain-processing system and increase in the excitability of the system,76,77 although limited human research indicated activation of the dorsal rostral pons, anterior cingulate cortex, and cuneus in response to PNS in the suboccipital area.46 Better understanding of the PNS mechanism may result in refinement of surgical indications, individual tailoring of appropriate treatment approach, and perhaps optimization of the hardware choice.
Development of special devices for PNS is yet another potential direction of progress in this rapidly growing area. New electrodes with different spacing options and lower profile may be particularly useful for PNS. Significant research in the area of nerve-electrode interface is taking place,78 but the devices for PNS have not been developed or approved for widespread clinical use. It is possible that newly developed electrodes will be used not only for PNS in the strict sense of its definition but also in the neighboring area of stimulation of spinal nerves79 and the gasserian ganglion.80 Undoubtedly, BION73,75 and similar devices, dedicated to PNS, may widen the indications and further decrease the rate of complications and side effects. Getting approval for these devices to use with PNS procedures may facilitate the acceptance of the approach and better reimbursement of procedures that are done now only as part of research protocols or on an off-label basis.
Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099-1106.
Carbunaru R, Whitehurst T, Jaax K, et al. Rechargeable battery-powered Bion microstimulators for neuromodulation. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4193-4196.
Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21:E1.
Haque R, Winfree CJ. Spinal nerve root stimulation. Neurosurg Focus. 2006;21:E4.
Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55:135-142.
Kothari S. Neuromodulatory approaches to chronic pelvic pain and coccygodynia. Acta Neurochir Suppl. 2007;97:365-371.
Loeb GE, Peck RA, Moore WH, Hood K. BION system for distribute neural prosthetic interfaces. Med Eng Physics. 2001;23:9-18.
Machado A, Ogrin M, Rosenow JM, Henderson JM. A 12-month prospective study of gasserian ganglion stimulation for trigeminal neuropathic pain. Stereotact Funct Neurosurg. 2007;85:216-224.
Magis D, Allena M, Bolla M, et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314-321.
Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
Melzack RA, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
Oh MY, Ortega J, Bellotte JB, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a C1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103-112.
Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: preliminary results of long-term follow-up: a case series. Neuromodulation. 2007;10:279-290.
Popeney CA, Aló KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369-375.
Rogers LL, Swidan S. Stimulation of the occipital nerve for the treatment of migraine: current state and future prospects. Acta Neurochir Suppl. 2007;97:121-128.
Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache: long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
Slavin KV, Colpan ME, Munawar N, et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21:E5.
Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:128-132.
Slavin KV, Wess C. Trigeminal branch stimulation for intractable neuropathic pain: technical note. Neuromodulation. 2005;8:7-13.
Stinson LWJr, Roderer GT, Cross NE, Davis BE. Peripheral subcutaneous electrostimulation for control of intractable post-operative inguinal pain: a case report series. Neuromodulation. 2001;4:99-104.
Thimineur M, De Ridder D. C2 area neurostimulation: a surgical treatment for fibromyalgia. Pain Med. 2007;8:639-646.
Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109.
Weiner RL. Occipital neurostimulation (ONS) for treatment of intractable headache disorders. Pain Med. 2006;7:S137-S139.
Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
White JC, Sweet WH. Pain and the Neurosurgeon. A 40-Year Experience. Springfield, IL: Thomas; 1969. 894-899
1 Beemer GH, Reeves JH, Bjorksten AR. Accurate monitoring of neuromuscular blockade using a peripheral nerve stimulator: a review. Anaesth Intensive Care. 1990;18:490-496.
2 Rowlee SC. Monitoring neuromuscular blockade in the intensive care unit: the peripheral nerve stimulator. Heart Lung. 1999;28:352-364.
3 Chervin RD, Guilleminault C. Diaphragm pacing for respiratory insufficiency. J Clin Neurophysiol. 1997;14:369-377.
4 Donaldson Nde N, Rushton DN, Perkins TA, et al. Recruitment by motor nerve root stimulators: significance for implant design. Med Eng Phys. 2003;25:527-537.
5 Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24:443-452.
6 Graupe D. An overview of the state of the art of noninvasive FES for independent ambulation by thoracic level paraplegics. Neurol Res. 2002;24:431-442.
7 Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493-500.
8 Walsh SP, Kling MA. VNS and depression: current status and future directions. Expert Rev Med Devices. 2004;1:155-160.
9 Oerlemans DJ, van Kerrebroeck PE. Sacral nerve stimulation for neuromodulation of the lower urinary tract. Neurourol Urodyn. 2008;27:28-33.
10 Holzer B, Rosen HR, Novi G, et al. Sacral nerve stimulation for neurogenic faecal incontinence. Br J Surg. 2007;94:749-753.
11 Slavin KV, Burchiel KJ. Peripheral nerve stimulation for painful nerve injuries. Contemp Neurosurg. 1999;21:1-6.
12 Melzack RA, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
13 Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109.
14 White JC, Sweet WH. Pain and the Neurosurgeon: A 40-Year Experience. Springfield, IL: Thomas; 1969. 894-899
15 Shelden CH. Depolarization in the treatment of trigeminal neuralgia. Evaluation of compression and electrical methods; clinical concept of neurophysiological mechanism. In: Knighton RS, Dumke PR, editors. Pain. Boston: Little, Brown; 1966:373-386.
16 Cauthen JC, Renner EJ. Transcutaneous and peripheral nerve stimulation for chronic pain states. Surg Neurol. 1975;4:102-104.
17 Kirsch WM, Lewis JA, Simon RH. Experiences with electrical stimulation devices for the control of chronic pain. Med Instrum. 1975;4:167-170.
18 Picaza JA, Cannon BW, Hunter SE, et al. Pain suppression by peripheral nerve stimulation. II. Observations with implanted devices. Surg Neurol. 1975;4:115-126.
19 Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976;45:692-699.
20 Sweet WH. Control of pain by direct stimulation of peripheral nerves. Clin Neurosurg. 1976;23:103-111.
21 Nashold BSJr, Mellen JB, Avery R. Peripheral nerve stimulation for pain relief using a multicontact electrode system. J Neurosurg. 1979;51:872-873.
22 Law JD, Sweet J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg. 1980;52:482-485.
23 Long DM, Erickson D, Campbell J, North R. Electrical stimulation of the spinal cord and peripheral nerves for pain control: a ten year experience. Appl Neurophysiol. 1981;44:207-217.
24 Nashold BSJr, Goldner L, Mullen JB, Bright DS. Long-term pain control by direct peripheral nerve stimulation. J Bone Joint Surg Am. 1982;64:1-10.
25 Waisbrod H, Panhans C, Hansen D, Gerbeshagen HU. Direct nerve stimulation for painful peripheral neuropathies. J Bone Joint Surg Br. 1985;67:470-472.
26 Long DM. Stimulation of the peripheral nervous system for pain control. Clin Neurosurg. 1986;33:323-343.
27 Iacono RP, Linford J, Sandyk R. Pain management after lower extremity amputation. Neurosurgery. 1987;20:496-500.
28 Racz GB, Browne T, Lewis RJr. Peripheral stimulator implant for treatment of causalgia caused by electrical burns. Tex Med. 1988;84:45-50.
29 Heavner JE, Racz G, Diede JM. Peripheral nerve stimulation: current concepts. In: Waldman SD, Winnie AP, editors. Interventional Pain Management. Philadelphia: Saunders; 1996:423-425.
30 Hassenbusch SJ, Stanton-Hicks M, Schoppa D, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415-423.
31 Stanton-Hicks M, Salamon J. Stimulation of the central and peripheral nervous system for the control of pain. J Clin Neurophysiol. 1997;14:46-62.
32 Shetter AG, Racz GB, Lewis R, Heavner JE. Peripheral nerve stimulation. In: North RB, Levy RM, editors. Neurosurgical Management of Pain. New York: Springer; 1997:261-270.
33 Nielson KD, Watts C, Clark WK. Peripheral nerve injury from implantation of chronic stimulating electrodes for pain control. Surg Neurol. 1976;5:51-53.
34 Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
35 Slavin KV, Burchiel KJ. Use of long-term nerve stimulation with implanted electrodes in the treatment of intractable craniofacial pain. J Neurosurg. 2000;92:576.
36 Weiner RL. The future of peripheral nerve stimulation. Neurol Res. 2000;22:299-304.
37 Lou L. Uncommon areas of electrical stimulation. Curr Rev Pain. 2000;4:407-412.
38 Hammer M, Doleys DM. Perineuromal stimulation in the treatment of occipital neuralgia: a case study. Neuromodulation. 2001;4:47-51.
39 Weiner RL, Aló KM, Reed KL, Fuller ML. Subcutaneous neurostimulation for intractable C-2-mediated headaches. J Neurosurg. 2001;94:398A.
40 Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002;5:32-37.
41 Aló KM, Holsheimer J. New trends in neuromodulation for the management of neuropathic pain. Neurosurgery. 2002;50:690-704.
42 Weiner RL. Peripheral nerve neurostimulation. Neurosurg Clin N Am. 2003;14:401-408.
43 Popeney CA, Aló KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369-375.
44 Jones RL. Occipital nerve stimulation using a Medtronic Resume II ® electrode array. Pain Physician. 2003;6:507-508.
45 Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55:135-142.
46 Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
47 Oh MY, Ortega J, Bellotte JB, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a C1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103-112.
48 Slavin KV, Wess C. Trigeminal branch stimulation for intractable neuropathic pain: technical note. Neuromodulation. 2005;8:7-13.
49 Kapural L, Mekhail N, Hayek SM, et al. Occipital nerve electrical stimulation via the midline approach and subcutaneous surgical leads for treatment of severe occipital neuralgia: a pilot study. Anesth Analg. 2005;101:171-174.
50 Rodrigo-Royo MD, Azcona JM, Quero J, et al. Peripheral neurostimulation in the management of cervicogenic headaches: four case reports. Neuromodulation. 2005;4:241-248.
51 Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:128-132.
52 Slavin KV, Nersesyan H, Wess C. Treatment of neuropathic craniofacial pain using peripheral nerve stimulation approach. In: Meglio M, Krames ES, editors. Proceedings of 7th Meeting of International Neuromodulation Society. Bologna: Medimond; 2005:77-80.
53 Weiner RL. Occipital neurostimulation (ONS) for treatment of intractable headache disorders. Pain Med. 2006;7:S137-S139.
54 Schwedt TJ, Dodick DW, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic cluster headache and hemicrania continua: pain relief and persistence of autonomic features. Cephalalgia. 2006;26:1025-1027.
55 Slavin KV, Colpan ME, Munawar N, et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21:E5.
56 Rogers LL, Swidan S. Stimulation of the occipital nerve for the treatment of migraine: current state and future prospects. Acta Neurochir Suppl. 2007;97:121-128.
57 Thimineur M, De Ridder D. C2 area neurostimulation: A surgical treatment for fibromyalgia. Pain Med. 2007;8:639-646.
58 Magis D, Allena M, Bolla M, et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314-321.
59 Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099-1106.
60 Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache: long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
61 Hagen JE, Bennett DS. Occipital nerve stimulation for treatment of migraine. Pract Pain Manage. 2007;7:43-45. 56
62 Stinson LWJr, Roderer GT, Cross NE, Davis BE. Peripheral subcutaneous electrostimulation for control of intractable post-operative inguinal pain: a case report series. Neuromodulation. 2001;4:99-104.
63 Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: preliminary results of long-term follow-up: a case series. Neuromodulation. 2007;10:279-290.
64 Yakovlev AE, Peterson AT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation. 2007;10:373-375.
65 Kothari S. Neuromodulatory approaches to chronic pelvic pain and coccygodynia. Acta Neurochir Suppl. 2007;97:365-371.
66 Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle, WA: IASP Press; 1994.
67 Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21:E1.
68 Monti E. Peripheral nerve stimulation: a percutaneous minimally invasive approach. Neuromodulation. 2004;7:193-196.
69 Leone M, Franzini A, Cecchini AP, et al. Stimulation of occipital nerve for drug-resistant chronic cluster headache. Lancet Neurol. 2007;6:289-291.
70 Slavin KV. Peripheral neurostimulation in fibromyalgia: a new frontier? Pain Med. 2007;8:621-622.
71 Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82-86.
72 Syed M, Slavin KV. Infraclavicular generator placement for neurostimulation: are the patients satisfied? In: Meglio M, Krames ES, editors. Proceedings of 7th Meeting of International Neuromodulation Society. Bologna: Medimond; 2005:81-84.
73 Loeb GE, Peck RA, Moore WH, Hood K. BION™ system for distribute neural prosthetic interfaces. Med Eng Physics. 2001;23:9-18.
74 Groen J, Amiel C, Bosch JL. Chronic pudendal nerve neuromodulation in women with idiopathic refractory detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodyn. 2005;24:226-230.
75 Carbunaru R, Whitehurst T, Jaax K, et al. Rechargeable battery-powered Bion microstimulators for neuromodulation. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4193-4196.
76 Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002;125:1496-1509.
77 Le Doaré K, Akerman S, Holland PR, et al. Occipital afferent activation of second order neurons in the trigeminocervical complex in rat. Neurosci Lett. 2006;403:73-77.
78 Navarro X, Krueger TB, Lago N, et al. A critical review of interfaces with the peripheral nervous system for the control of neuroprosthesis and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229-258.
79 Haque R, Winfree CJ. Spinal nerve root stimulation. Neurosurg Focus. 2006;21:E4.
80 Machado A, Ogrin M, Rosenow JM, Henderson JM. A 12-month prospective study of gasserian ganglion stimulation for trigeminal neuropathic pain. Stereotact Funct Neurosurg. 2007;85:216-224.







