Pericardiocentesis
Anatomy and Physiology
Pericardium and Pericardial Space
The pericardium is a two-layered fibroelastic sac surrounding the heart.1 The pericardium is avascular but well innervated, so inflammation induces severe pain. The visceral pericardium is a single-cell layer that adheres to the epicardium. The outer parietal pericardium consists mostly of collagen with some elastin. These two layers create the pericardial space, which normally contains 15 to 50 mL of serous fluid.2 Pericardial fluid provides lubrication for cardiac contractility and acts as a “shock absorber” for deceleration forces.
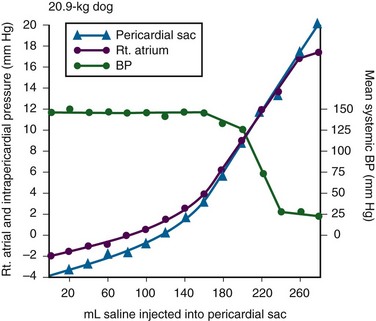
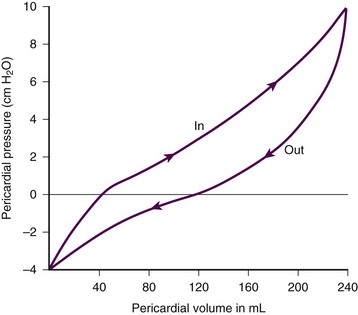
The pericardium is a tense structure, but it also has some elasticity. These properties limit the amount of cardiac dilation that is possible during diastole and enhance mechanical interactions between the atria and ventricles during systole.3 This semi-elastic property can also tolerate an acute (i.e., over a period of hours to days) accumulation of pericardial fluid (80 to 120 mL) without significantly increasing intrapericardial pressure, which is the flat portion of the pressure-volume curve for pericardial pressure (Fig. 16-1).4,5 Once a critical volume is reached, adding as little as 20 to 40 mL can double intrapericardial pressure (the steep portion of the pressure-volume curve [see Fig. 16-1]) and cause clinical decompensation (cardiac tamponade). Cardiac tamponade typically occurs with an intrapericardial pressure of 15 to 20 mm Hg.6
Pathophysiology of Pericardial Tamponade
Elevated intrapericardial pressure (i.e., early tamponade) results in abnormal RV filling and then abnormal LV filling. In this situation, the free wall of the RV cannot expand against the pericardial fluid during inspiration. To accommodate the filling, the interventricular septum bows abnormally into the LV, which reduces its volume. LV filling, stroke volume, and ultimately cardiac output are reduced.7 This phenomenon is responsible for pulsus paradoxus (PP, described later in this chapter), which is sometimes observed with tamponade.8 LV filling is also reduced by the collapse of right-sided structures. After a critical volume is reached on the pressure-volume curve (see Fig. 16-1), the intrapericardial pressure is transmitted to the inferior vena cava (IVC) and right atrium. These thin-walled structures then become compressed and reduce filling of the RV.
The atria and pulmonary circulation are at much lower pressure than systemic arterial pressure and are also vulnerable to rising intrapericardial pressure (Fig. 16-2). Late in tamponade a “pressure plateau” occurs in which right atrial pressure, RV diastolic pressure, pulmonary artery diastolic pressure, and pulmonary capillary wedge pressure are virtually identical. This equalization of chamber pressure leads to a reduction in venous return and the echocardiographic hallmark of tamponade: diastolic collapse of the RV. At this point, hemodynamic collapse is imminent, with severe hypotension, bradycardia, and potentially pulseless electrical activity (PEA) developing. Unless intrapericardial pressure is decreased immediately, cardiac arrest will ensue.9
Compensatory Mechanisms and Pericardiocentesis
Compensatory mechanisms also preserve normal cardiac contractility and myocardial perfusion.7,10,11 However, when pericardial pressure overwhelms the compensatory mechanisms, coronary perfusion pressure is reduced, which leads to myocardial ischemia. Experimental induction of severe tamponade demonstrated microscopic ischemic cardiac injury.12 Lactic acidosis (resulting from reduced cardiac output and systemic hypoperfusion) may directly cause cardiac depression and thereby reduce cardiac contractility and, ultimately, cardiac output.9
Removal of pericardial fluid (i.e., pericardiocentesis) reverses the pathophysiologic processes just described by improving cardiac filling and output. Interestingly, the pressure-volume relationship of the pericardial space demonstrates hysteresis; that is, withdrawing a certain quantity of fluid reduces intrapericardial pressure more than addition of the same amount of fluid increases intrapericardial pressure. This effect, however, is not universal and may vary among patients and in various disease states (Fig. 16-3).2
Special Considerations in Patients with Pericardial Effusion and Tamponade
Under normal circumstances, positive pressure ventilation (e.g., mechanical ventilation) reduces venous return to the right side of the heart by increasing intrathoracic pressure. This could be detrimental for patients with tamponade because right-sided filling is already compromised and further reductions can lead to severe hemodynamic instability.13 Therefore, positive pressure ventilation should be avoided in patients with known or suspected tamponade unless it is absolutely necessary.
Low-pressure pericardial tamponade is defined as a hemodynamically significant effusion with lower than expected intrapericardial pressure.14 This category of tamponade occurs in certain hypovolemic patients with subacute or chronic effusions (e.g., associated with long-term diuretic use, dehydration, excessive dialysis).15 The diagnosis may be challenging because the classic symptoms and findings on physical examination (e.g., distended neck veins) may be absent.16 Fluid boluses may temporize the hemodynamic compromise while pericardial decompression is being arranged.
Epidemiology
The major categories of pericardial effusion include infection, malignancy, trauma, and metabolic abnormalities. Effusion may also be associated with aortic disease, connective tissue disease, or idiopathic causes. It is often difficult to report the exact incidence of each type of pericardial effusion because of variations in patient populations, local epidemiology, and the diagnostic protocols used during evaluation. The prevalence of a chronic effusion is also difficult to ascertain because it is often asymptomatic and underreported. General autopsy studies demonstrate an overall prevalence of 3.4%.17
Causes of Pericardial Effusion (Box 16-1)
Traumatic Hemopericardium
Penetrating Trauma: Penetrating cardiac trauma can cause acute hemopericardium by either external forces (e.g., a stab wound to the heart) or internal forces (e.g., iatrogenic injury during placement of a pacemaker). Cardiac perforation can lead to rapid clinical deterioration and PEA.
External cardiac puncture is associated with stab wounds or projectile injuries (e.g., gunshot wounds). Tamponade develops in 80% to 90% of patients with cardiac stab wounds as opposed to 20% of those with gunshot wounds.18,19 Stab wounds cause tamponade more frequently because if the pericardial injury is small, it can reseal and trap blood within the pericardial space.20 On the other hand, a gunshot typically produces large pericardial wounds that allow continuous drainage into the pleural space.21 Clinical deterioration is usually secondary to hypovolemia.21 Any penetrating injury to the chest, back, or upper part of the abdomen may injure the pericardium and cause tamponade.
Internal penetrating trauma is typically caused by invasive diagnostic or therapeutic procedures. The procedures most often associated with this injury are pacemaker insertion and cardiac catheterization (angioplasty or valvuloplasty).22–24 Hemopericardium results from puncturing the cardiac chamber, a coronary artery, or a great vessel (e.g., the superior vena cava). Ironically, pericardiocentesis itself (treatment of a pericardial effusion) can cause hemopericardium if coronary vessels or the myocardium is injured during the procedure.25,26
Internal jugular and subclavian venous catheters (e.g., central venous or hemodialysis catheters) are commonly inserted in the emergency department (ED). During such procedures, hemopericardium results from perforation of the superior vena cava, right atrium, or RV. Hemopericardium can occur immediately or can be delayed up to 2 days subsequent to erosion of the catheter through myocardial or vascular tissue.27,28 Although this complication seldom occurs, it should always be considered when a patient experiences sudden hemodynamic deterioration following an invasive procedure.
Blunt Trauma: Major blunt chest trauma can cause hemopericardium with or without obvious signs of injury.29 Myocardial rupture can be uncontained or contained.30,31 Patients with uncontained rupture do not typically survive long enough to reach the hospital.32 Contained rupture may be found soon after injury or may be a late finding (in some cases up to 12 days later).33 Tamponade can also be caused by a deceleration mechanism of injury that induces either aortic or vena caval disruption.34 In one case series the incidence of tamponade following deceleration injury was found to be 2.3% (1 in 43 patients).35
Miscellaneous Trauma: Chest compressions during cardiopulmonary resuscitation (CPR) can also cause hemopericardium from broken ribs, bleeding intercostal vessels, or penetrating injury (e.g., intracardiac injection of medication, which is rarely performed today).36 Hemopericardium following CPR has been described in case reports37,38 but is unlikely to be significant, much less to cause tamponade.
Atraumatic Hemopericardium
Bleeding diathesis is an important cause of spontaneous hemopericardium and may be associated with the use of anticoagulants (reported incidence of 2.5% to 11%)22 or thrombolytic therapy (incidence <1%).39 Patients who have undergone cardiac surgery are at increased risk because of the anticoagulative effects of the cardiopulmonary bypass machine and medications started postoperatively (e.g., clopidogrel, warfarin).40 Fortunately, tamponade has a low incidence and is generally detected in the postoperative period before discharge.41,42 This complication is usually prevented by the intraoperative placement of mediastinal or pericardial drains.22,43
Hemopericardium can develop following MI. Early after a transmural MI (1 to 3 days), the necrotic myocardium causes inflammation of the overlying pericardium and then effusions can form. Late-developing effusions (weeks after an MI) are caused by an autoimmune pericarditis called Dressler’s syndrome.1 Improved reperfusion techniques have reduced the incidence of post-MI pericarditis and effusion.44
Ascending aortic dissection causes rapid and usually fatal hemopericardium. The dissection may expand in a retrograde fashion by extending to the base of the aorta and into the pericardial sac. Risk factors for aortic dissection include hypertension, atherosclerosis, vasculitis (e.g., giant cell arteritis, syphilis), collagen vascular disease (e.g., Marfan’s syndrome), and the use of sympathomimetics (e.g., cocaine).45–47
Ventricular free-wall rupture is a rapidly fatal cause of acute hemopericardium that can occur after MI. This complication is less common today than in the past (<1%)39 secondary to improved revascularization techniques, better therapeutic medications, and faster intervention times (shorter door-to-balloon times) for coronary ischemia. Despite a reduction in its overall incidence, 7% of all deaths related to MI are caused by this complication.48,49 Survival is possible with prompt recognition and treatment, but the prognosis is grim once tamponade occurs.50,51
Nonhemorrhagic Effusions
Nonhemorrhagic pericardial effusions usually accumulate slower than acute hemopericardium does (over a period of weeks to months). Chronic fluid accumulation allows the pericardium to stretch circumferentially and accommodate up to 2000 mL of fluid without any hemodynamic compromise.52 Effusions that grow slowly allow the circulatory system to adapt to the intrapericardial pressure, thereby further maintaining hemodynamic stability. Thus, asymptomatic patients with moderate to large effusions may not need emergency pericardiocentesis, in contrast to patients with acute hemopericardium.53,54
Nonhemorrhagic effusions have several causes (see Box 16-1), and the exact one may not be obvious during the initial evaluation without diagnostic pericardiocentesis. Common causes of nonhemorrhagic effusions are discussed in the following sections.
Idiopathic Effusions
Most idiopathic effusions are believed to be viral in origin and most commonly caused by infection with coxsackievirus, echovirus, or enterovirus. Idiopathic pericardial effusions may be asymptomatic or have an associated component of pericarditis (e.g., positional pain or diffuse ST-segment changes on the electrocardiogram [ECG]).55 These effusions are often labeled “idiopathic” because the diagnosis cannot be made noninvasively (i.e., based on the history, physical examination, or serum testing) and the risk associated with diagnostic pericardiocentesis outweighs the risk of observation in asymptomatic adults who appear to be well.56 Diagnostic pericardiocentesis may be recommended for idiopathic effusions that are persistent or symptomatic without a known cause.57
Neoplastic Effusion
Tumors of the pericardium or myocardium may cause nonhemorrhagic effusions.54,58 Primary cardiac tumors are less common (0.001% to 0.003%) than metastases from another site (2% to 18%), but either may cause a malignant effusion.59 Although no malignancy preferentially metastasizes to the heart, certain tumors commonly involve the heart when they metastasize; frequently implicated are lung cancer, breast cancer, mediastinal tumors, malignant melanoma, leukemia, and lymphoma.46
Cardiac metastasis is usually a late finding in cancer; other foci are generally evident first.47 The classic signs and symptoms of tamponade (e.g., chest pain and dyspnea) may not be obvious with malignant tamponade. When present, they may be mistakenly attributed to the underlying malignancy.46 Thus, in the relevant clinical scenario, consider screening patients with malignancy for pericardial effusion (e.g., ultrasound) before the clinical findings of tamponade appear.
Congestive Heart Failure
Congestive heart failure (CHF) is a cause of pericardial effusion. Diagnosis may be difficult because of overlapping signs and symptoms with exacerbations of CHF (e.g., chest pain or dyspnea). Adding to the diagnostic complexity is that 12% to 20% of patients with CHF have a coexisting pericardial effusion.60 Fortunately, treatment of CHF-associated pericardial effusion does not differ from that for an effusion from other causes: treat the underlying cause unless the patient has evidence of hemodynamic compromise.
Radiation
Pericardial effusions (secondary to radiation-induced pericarditis) can develop acutely during radiation therapy or nay be delayed for years. Risk factors include the radiation dose, duration of exposure, and age of the patient. Patients treated with radiation for Hodgkin’s disease have the highest association of radiation-induced pericarditis and subsequent effusions.22 These effusions can be serous, hemorrhagic, or fibrinous.57
HIV-Associated Effusions
Human immunodeficiency virus (HIV) can cause nonhemorrhagic pericardial effusion and tamponade (see Review Box 16-1).61,62 The incidence has been reported to be approximately 11% in patients with HIV infection or acquired immunodeficiency syndrome (AIDS), and 13% of cases are classified as moderate to severe. It is unclear whether antiretroviral therapy has affected these data.17
HIV-related effusions have been attributed to bacterial (e.g., Staphylococcus aureus), viral (e.g., cytomegalovirus), fungal (Cryptococcus neoformans), and mycobacterial causes (e.g., tuberculosis, which is the most common cause of HIV-related effusions worldwide).63 Kaposi’s sarcoma and lymphoma can cause noninfectious pericardial effusions in HIV patients.64,65
Renal Failure and Uremia
Pericardial effusion develops in approximately 15% to 20% of dialysis patients, and tamponade may eventually occur in as many as 35% of that group.66,67 Up to 7% of chronic dialysis patients have effusions with volumes of 1000 mL or greater.68 In many cases, effusions secondary to renal failure can be managed solely with aggressive dialysis without pericardiocentesis. Any sign of hemodynamic compromise, however, warrants strong consideration of pericardiocentesis.
Hypothyroidism
Hypothyroid patients are at risk for pericardial effusions (up to 30%), but the fluid accumulates gradually, so tamponade develops in only a few patients.54 If pericardial effusions are present, other areas of the body usually demonstrate serositis (e.g., pleural effusions). Treating the underlying hypothyroidism often reverses the effusion without the need for pericardiocentesis.
Special Considerations in Pericardial Disease
Pericardial tamponade is classically described as being secondary to circumferential effusion, which causes a generalized increase in pericardial pressure and compression of multiple cardiac chambers. Loculated effusions (caused by a local hematoma or an infectious process) or pericardial adhesions (from previous inflammation) can compress one or two cardiac chambers and thus reduce both cardiac filling and cardiac output.69,70
Constrictive pericarditis occurs following chronic pericardial inflammation, infection, or mediastinal irradiation. These processes cause scarring, fibrosis, or calcification, and the pericardium eventually becomes a nonelastic and “constrictive” sac around the heart. Myocardial relaxation and cardiac filling are impaired, and diastolic dysfunction ensues. Without echocardiography, constrictive pericarditis can be difficult to distinguish from pericardial tamponade.1
Effusive-constrictive pericarditis is defined by the presence of both pericardial effusion and pericardial constriction. It may be quite difficult to differentiate between effusive-constrictive pericarditis and pericardial tamponade in stable patients because both are associated with effusions.71 Fortunately, distinguishing between these diagnoses is less important in hemodynamically unstable patients because they are treated identically (i.e., with pericardiocentesis).72
Pneumopericardium is an interesting, though rare cause of cardiac tamponade. It is most commonly associated with pneumothorax caused by barotrauma (e.g., mechanical ventilation).73 It also occurs spontaneously during acute asthma exacerbations,74 and it can follow blunt chest injury.75,76 Although typically benign, tension pneumopericardium has been reported as a cause of life-threatening tamponade after blunt77,78 and penetrating chest trauma.79,80
Diagnosing Cardiac Tamponade
Diagnosis of pericardial effusions requires integration of the patient’s history, findings on physical examination, and diagnostic testing. Unfortunately, even experienced clinicians may not initially consider pericardial effusion because the clinical findings are often vague and nonspecific. Nonspecific symptoms, such as chest pain and dyspnea, can be ascribed to more common conditions (e.g., CHF or pulmonary pathology), so the diagnosis might be delayed until diagnostic testing is performed (e.g., computed tomography [CT] of the chest for pulmonary embolism)81 or until hypotension develops and bedside ultrasound is performed.82
Acute pericardial tamponade (e.g., secondary to blunt chest wall trauma) is usually challenging to diagnose because the findings on physical examination may resemble those of other life-threatening conditions (e.g., tension pneumothorax, hemothorax, hypovolemia, pulmonary edema, severe contusion of the RV, aortic dissection, or pulmonary embolism).67 In hemodynamically unstable patients, diagnostic (e.g., bedside ultrasound) and therapeutic (e.g., pericardiocentesis) interventions must be performed even with a paucity of findings on physical examination because rapid clinical deterioration and cardiac arrest can occur before a definitive diagnosis can be made.
Once a pericardial effusion is suspected (or diagnosed), the next step is to determine its size and hemodynamic significance and presence of underlying or associated diseases.83 Specific therapy will hinge on this information and is discussed in the following sections.
History: Patient Profile and Symptoms
The historical features of pericardial effusions are nonspecific and the diagnosis may be overlooked initially. However, an astute clinician might be suspicious based on comorbid conditions (e.g., warfarin therapy or a history of myxedema) and the time course of the symptoms (e.g., free-wall rupture several days after MI, dyspnea in a patient with uremic pericarditis). Box 16-2 lists important details to be ascertained from the history when pericardial effusion is suspected.84
Physical Examination
In 1935, Beck characterized the physical manifestations of tamponade with two triads—one for chronic and one for acute tamponade.85 Beck’s “chronic” triad consists of increased central venous pressure (CVP) (i.e., distended neck veins), ascites, and a small, quiet heart. “Beck’s triad” is a classic description of acute cardiac compression, which includes increased CVP, decreased arterial pressure, and muffled heart sounds. Almost 90% of patients have one or more of these “acute” signs,86 but only about 33% demonstrate the complete triad.9,87 The utility of this triad is further limited because all three signs are usually observed shortly before cardiac arrest.
It would be clinically desirable to identify patients in early tamponade, before hemodynamic collapse. Unfortunately, findings on physical examination in early tamponade are nonspecific and may be indistinguishable from those of other critical diseases (e.g., septic shock, right heart failure).57 Patients initially seen in late tamponade also have nonspecific findings. They may be agitated, panic-stricken, confused, uncooperative, restless, cyanotic, diaphoretic, acutely dyspneic, or hemodynamically unstable. Such patients should undergo a brief and focused physical examination because the time between initial evaluation and full arrest may be brief.
Some of the findings on physical examination associated with tamponade are described below. A more comprehensive list is presented in Box 16-3.
Vital Sign Abnormalities
The three stages occur sequentially and reflect the natural history of acute tamponade (Table 16-1).88 The time course within each stage varies from patient to patient. Some patients are stable within a given stage for hours, whereas others proceed through all three stages and cardiac arrest within minutes.9,88 Grade I tamponade is characterized by normal blood pressure and cardiac output with an increase in the heart rate and CVP (measured invasively with a central venous catheter). Grade II tamponade is defined by normal or slightly reduced blood pressure; CVP and the heart rate remain increased. Grade III tamponade is identified on the basis of Beck’s triad: hypotension, tachycardia, and elevated CVP.
TABLE 16-1
Shoemaker System of Grading Cardiac Tamponade
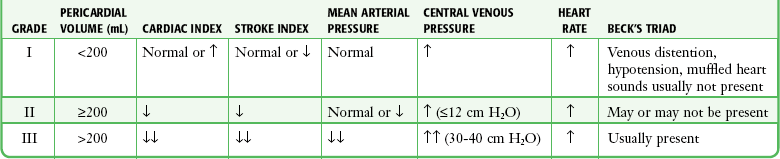
From Shoemaker WC, Carey SJ, Yao ST, et al. Hemodynamic monitoring for physiologic evaluation, diagnosis, and therapy of acute hemopericardial tamponade from penetrating wounds. J Trauma. 1973;13:36.
Nearly all patients with tamponade have sinus tachycardia, although its specificity is low.89 The physiologic purpose of tachycardia is to maintain normal cardiac output despite reductions in stroke volume from worsening tamponade. Exceptions to the pairing of tachycardia with tamponade usually relate to the underlying cause of the effusion (e.g., myxedema) or the concomitant use of certain medications (e.g., β-blockers).
Adding to the diagnostic complexity, not all patients in tamponade have a reduction in blood pressure. In fact, Brown and coworkers90 described several tamponade patients with elevated blood pressure. These patients were previously hypertensive and paradoxically had reduced systolic blood pressure following pericardiocentesis.
Pulsus Paradoxus
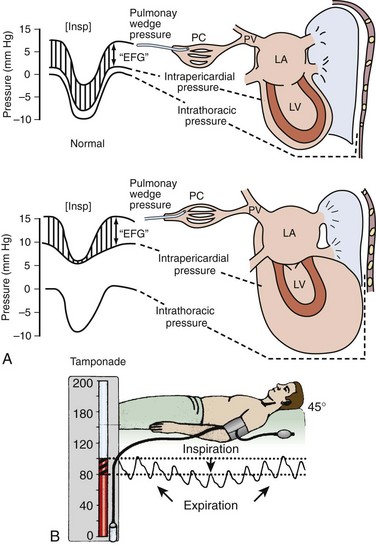
PP is an exaggerated decrease in systolic blood pressure (>12 mm Hg) during inspiration secondary to reduced stroke volume (Fig. 16-4A).30,91,92 Patients with moderate to severe tamponade typically demonstrate PP greater than 20 mm Hg.9,86,93 Unfortunately, PP is not pathognomonic for tamponade. It is observed in other conditions, such as hypotension associated with labored breathing (secondary to extreme reductions in intrathoracic pressure [Fig. 16-4B]), severe emphysema, severe asthma, obesity, cardiac failure, constrictive pericarditis, pulmonary embolism, and cardiogenic shock.9,86,93
The absence of PP does not rule out tamponade because it can occur with several conditions: atrial septal defects, aortic insufficiency, positive pressure ventilation, loculated pericardial effusions, and elevated left ventricular diastolic pressure (e.g., poor left ventricular compliance secondary to chronic hypertension).14 Finally, PP should be interpreted with caution in patients with traumatic tamponade because it can be unreliable.94–96 In a study of 197 patients with traumatic tamponade, only 8.6% had PP.97
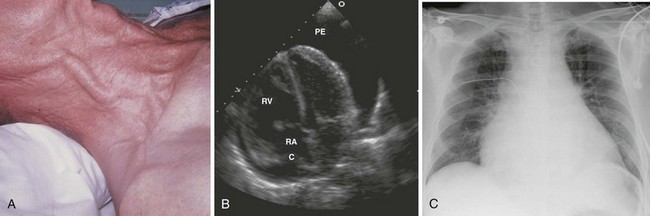
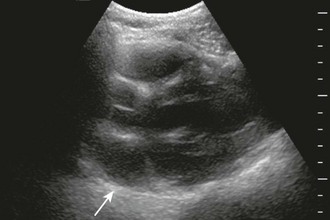
Neck Vein Distention and Elevated CVP
Neck vein distention (a surrogate for measuring CVP) occurs late in tamponade, when right-sided chambers (e.g., the RV) collapse. Neck vein distention may be obvious on examination (Fig. 16-5A), but visualization of such distention is less accurate than measuring CVP by central venous catheter or evaluation with ultrasound (Table 16-2). Patients with significant tamponade typically have a CVP of 12 cm H2O or higher.95 Finally, although initial CVP readings are useful and diagnostic when grossly elevated (e.g., 20 to 30 cm H2O),95,98 upward CVP trends can be a more sensitive diagnostic tool.95
TABLE 16-2
Noninvasive Estimation of Right Atrial Pressure with Ultrasound
| DIAMETER (cm) OF INFERIOR VENA CAVA | CHANGE IN DIAMETER WITH RESPIRATION | ESTIMATED RIGHT ATRIAL PRESSURE (mm Hg) |
| Normal (<2.1) | Decrease >50% | ~3 (normal, 0-5) |
| Dilated (<2.1) | Decrease <50% | ~8 (normal, 5-10) |
| Dilated (>2.1) | Decrease >50% | ~8 (normal, 10-15) |
| Dilated (>2.1) | Decrease <50% | >15 |
Based on Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685.
Overreliance on increased CVP and venous distention should be avoided because they do not always indicate tamponade. For example, increased intrathoracic pressure (as induced by positive pressure ventilation or Valsalva maneuvers) increases CVP and causes neck vein distention even without pericardial effusion. Conversely, hypovolemic patients may have reduced CVP and no neck vein distention despite having clinical tamponade. The absence of distended neck veins may also result from severe venoconstriction secondary to intrinsic sympathetic discharge, vasopressor use, or severe hypovolemia.9,88,93,95
Diagnostic Testing
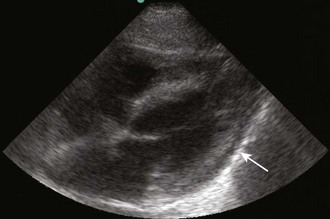
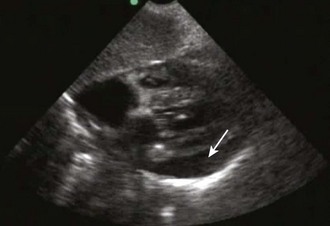
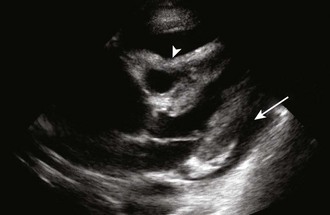
Diagnostic testing should be initiated when pericardial effusion or tamponade is suspected. Definitive diagnosis requires imaging such as CT or, preferably, cardiac ultrasound (Fig. 16-5B). Bedside ultrasound is the fastest and most reliable diagnostic tool because it is noninvasive, does not emit radiation, and can be performed at the bedside without transporting unstable patients outside the ED.
Chest Radiography
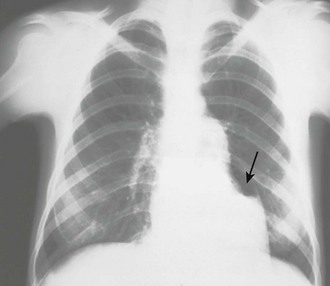
Chest radiographs are not diagnostically useful in patients with acute traumatic tamponade because the pericardium does not have sufficient time to change size or shape (see “Pathophysiology of Pericardial Tamponade”). Radiographs, however, may reveal other associated findings such as hemothorax, bullets in the thorax, or even pneumopericardium. Chest radiography may also be helpful when other diagnoses (e.g., CHF) have clinical findings similar to those of pericardial effusion. For example, a dyspneic patient with a clear chest film is less likely to have decompensated CHF than tamponade.
In patients with chronic pericardial effusions, chest films often demonstrate an enlarged, saclike, “water-bottle” cardiac shadow or pleural effusion (Fig. 16-5C). Unfortunately, it is difficult to differentiate a large effusion from myocardial enlargement (e.g., dilated cardiomyopathy) because radiographs demonstrate only the cardiac silhouette and do not reveal the physiologic differences between these two diagnoses.
Electrocardiography
Pericardial effusion secondary to acute pericarditis is suspected on the basis of typical changes on the ECG. Pericarditis has four stages: (1) diffuse ST-segment elevation with PR depression, (2) ST- and PR-segment normalization, (3) diffuse T-wave inversion, and (4) normalization of T waves.99
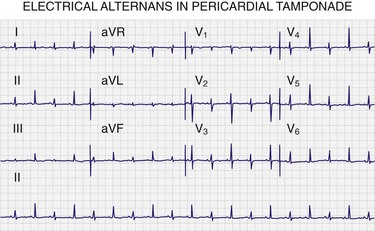
Electrocardiography has acceptable specificity but poor sensitivity93,100,101 in diagnosing pericardial effusion and tamponade. In a study of patients with pericardial effusion, electrocardiography had an overall sensitivity of 1% to 17% and a specificity of 89% to 100%.93 Therefore, an ECG may suggest but should never be the only means of diagnosing a pericardial effusion (Fig. 16-6). Furthermore, electrocardiography cannot reliably differentiate tamponade from effusion.102
The three most commonly described electrocardiographic findings in pericardial effusion are PR depression, low-voltage QRS complexes, and electrical alternans. PR-segment depression is the most common finding and is defined as depression of 1 mV or greater in at least one lead other than aVR. Low-voltage QRS complexes (most frequently associated with moderate to large effusions) are defined by a QRS complex with an amplitude of 5 mV or less across all limb leads. Alternatively, low-voltage QRS complexes can be identified by a sum of 30 mV or less for all limb lead QRS amplitudes. Electrical alternans is a beat-to-beat alternation in QRS amplitude caused by the pendulum motion of the heart within the fluid-filled pericardial sac.103 Alternans has been observed in 22% of medical patients with tamponade104 and in 5% of patients with tamponade secondary to cancer.105 Electrical alternans of P waves and QRS complexes (i.e., total electrical alternans) is a rare finding but, when seen, is pathognomonic of tamponade (see Fig. 16-6).93,106
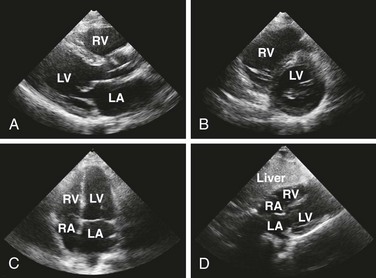
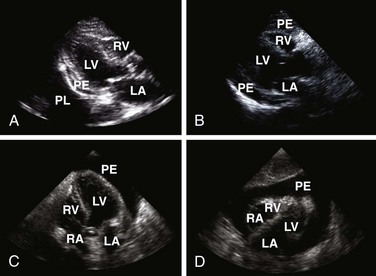
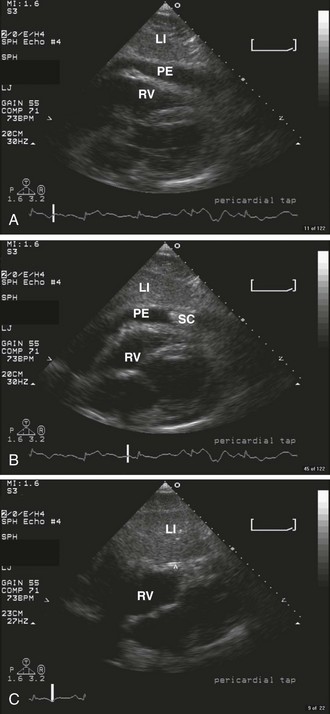
Echocardiography
Echocardiography is the best tool for diagnosing pericardial effusion or tamponade (see Figs. 16-7 to 16-9 and the Ultrasound Box). It not only demonstrates the presence of a pericardial effusion but also detects hemodynamic abnormalities. Ultrasonography (used interchangeably with echocardiography from here on) has the advantage that it is noninvasive and portable for use at the bedside and involves no ionizing radiation.9 Echocardiography is a very sensitive and specific tool for the diagnosis of pericardial effusion and tamponade,57,87,89 and its use in diagnosing pericardial effusions has been endorsed by several academic societies.107–110
Diagnosing Pericardial Effusions and Tamponade: Fluid within the pericardial space is not always pathologic—the pericardial space normally holds 15 to 50 mL of fluid. Small effusions may be clinically insignificant. The sonographer/clinician should exercise caution to not overread an effusion, particularly when the patient is hemodynamically stable.111
Once a pericardial effusion is discovered, evaluate the patient for evidence of hemodynamic compromise. Echocardiographic signs include (1) diastolic collapse of the right atrium (highly specific and sensitive for tamponade, especially when the collapse occurs for more than a third of the cardiac cycle)112; (2) early diastolic collapse of the right ventricular free wall (less sensitive than right atrial collapse but specific for tamponade)113; (3) left atrial collapse (a very specific sign of tamponade)114; (4) a small, slitlike, hyperkinetic left ventricle; (5) dilation of the hepatic veins and IVC; and (6) swinging of the heart to and fro within the pericardial sac.115 The presence of any of these signs should alert the clinician to the possibility of hemodynamic instability. Obtain expert consultation if there is any uncertainty about the ultrasound findings.
Limitations of Ultrasound: Ultrasound is the best diagnostic tool for pericardial effusion and tamponade, but great care must be taken when it is used as the only diagnostic modality. In a postoperative series of cardiac surgery patients, 60% of loculated effusions causing tamponade were missed on transthoracic echocardiography but were visualized with transesophageal echocardiography.116
Many ultrasound findings that suggest pericardial effusion are actually false positives. Examples include pericardial thickening, large pleural effusions, atelectasis, and mediastinal lesions.17 Epicardial (or anterior) fat pads can also be misinterpreted as pericardial effusions, although several details help distinguish between the two entities. First, epicardial fat pads tend to occur anteriorly, unlike circumferential effusions, which occur posteriorly. If a fat pad is suspected, multiple ultrasound views should be obtained to rule out a posterior effusion. Second, the echocardiographic appearance of an epicardial fat pad is isoechoic and homogeneous. This differs from blood in the pericardial space, which may look like fronds of clot waving within an anechoic (black) pericardial space. Third, an epicardial fat pad does not alter hemodynamics like tamponade does—it should not cause diastolic collapse of the right ventricular free wall, dilation of the IVC, or any signs indicating hemodynamic compromise. After careful examination, if doubt still exists regarding the presence of an effusion, hemodynamically stable patients should have a formal echocardiogram or CT scan.
CT Scan
CT may be the only diagnostic option at institutions where ultrasound or formal echocardiography is not available in the ED. CT can demonstrate dilated hepatic veins, a plethoric IVC, and interventricular septal bowing.117 This modality is less desirable than bedside ultrasound because it requires patients to be transported to the radiology suite. Thus, patient safety and cardiopulmonary stability must be considered before the decision is made to transport the patient. Unstable patients should never be transported until they are fully stabilized.
For stable patients, CT is effective in defining the presence, severity, and extent of pericardial effusions (i.e., circumferential versus loculated). In certain circumstances it even provides a more definitive diagnosis than echocardiography does because it may reveal the type of pericardial fluid (by differences in tissue density) and pericardial disease (e.g., constrictive pericarditis).118 In one series, eight equivocal echocardiograms were evaluated by follow-up CT.119 Two patients thought to have pericardial effusion by ultrasonography were found to have pleural effusions. Another patient in whom a pericardial effusion was diagnosed on ultrasonography was found by CT to have an epicardial lipoma. CT defined three loculated pericardial effusions not identified by ultrasonography. Finally, two patients had hemopericardium visualized by CT but not by ultrasonography.
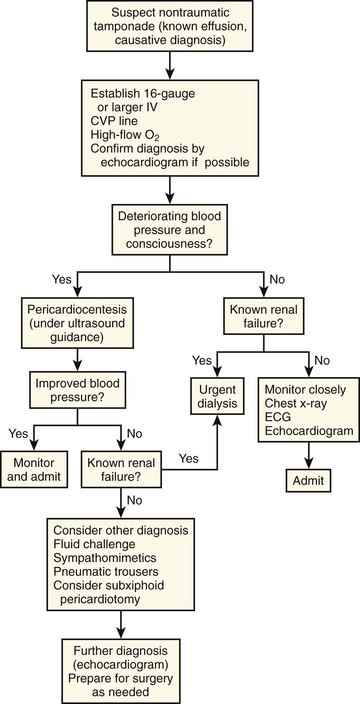
Treating Pericardial Effusions and Tamponade
Treatment of pericardial effusions in the ED depends on the degree of hemodynamic compromise. Patients with stable effusions should be treated supportively while the underlying cause (known or suspected) is addressed. For example, stable patients with pericardial effusions secondary to uremia may best be treated with dialysis, observation, and serial echocardiograms.120 Even when the diagnosis is unknown there may be no need to perform emergency pericardiocentesis if the effusion is small to moderate.121 Deferring diagnostic pericardiocentesis to the inpatient setting may be preferred because the cardiac catheterization laboratory or operating room is a more sterile and controlled environment than the ED.57
Patients with evidence of tamponade need urgent pericardiocentesis (discussed in the next section). Even those with early tamponade who are stable can decompensate quickly with little warning. Fluid boluses may improve hemodynamics temporarily, especially in patients with concomitant hypovolemia.89 Administration of vasopressors and an inotrope is a temporizing measure and should be initiated while preparing for emergency pericardiocentesis. Finally, positive pressure ventilation (e.g., mechanical ventilation) should be avoided if possible because as discussed in the section on physiology, it can lead to hemodynamic collapse secondary to changes in intrathoracic pressure.
Indications for Pericardiocentesis
Diagnostic Pericardiocentesis
Use of pericardiocentesis to determine the cause of nonhemorrhagic effusions is common practice, even though opinions on its utility vary.52,122,123 Recovery of neoplastic cells, blood, bacteria, and viruses from pericardial fluid can be valuable in making the diagnosis. Measurement of pericardial fluid pH can also be helpful because inflammatory fluid is significantly more acidotic than noninflammatory fluid.124 When a specific cause is suspected, additional diagnostic testing may be useful (e.g., adenosine deaminase in patients with tuberculosis and carcinoembryonic antigen in those with suspected malignancy).125
The diagnostic accuracy of pericardiocentesis is variable, and certain diagnoses are unlikely to be made from pericardial fluid. In one large series, fluid samples were obtained in 90% of aspirations, but the specific cause was determined from only 24% of those specimens.126 Another series demonstrated false-negative cytologic results in certain cases of lymphoma and mesothelioma.126 In some HIV patients, effusions secondary to Kaposi’s sarcoma and cytomegalovirus infection have been diagnosed by pericardial biopsy following nondiagnostic fluid analysis.127,128
The most helpful clinical predictors of diagnosis before pericardiocentesis are the size of the effusion (larger effusions and tamponade are more likely to yield a diagnosis) and signs of inflammation (e.g., fever, pericardial friction rub, ST-segment elevation).58 Unfortunately, the only definitive methods of diagnosis are pericardial fluid aspiration and analysis and pericardial biopsy.
Subxiphoid pericardiotomy can also be used in stable patients with pericardial effusion. This technique collects both pericardial fluid and a pericardial biopsy specimen, and because a tissue sample is obtained, a definitive diagnosis is more likely.129 This procedure can be performed safely in the operating suite without general anesthesia.130 In a prospective series of 57 patients who underwent subxiphoid pericardiotomy, a definitive diagnosis was obtained in 36%, a probable diagnosis in 40%, a possible diagnosis in 16%, and no diagnosis in 7%. It is uncertain whether this technique is safer than ultrasound-guided pericardiocentesis, but published reports show low rates of complications in experienced hands.58
Diagnostic pericardiocentesis has limited utility for hemopericardium secondary to traumatic causes. When used diagnostically after trauma to assess for the presence of pericardial bleeding, the procedure has a false-negative rate (i.e., no blood aspirated) of between 20% and 40%.95,131–133 The high false-negative rate is due to the fact that posttraumatic blood tends to clot within the pericardial space and therefore cannot be aspirated.19 Furthermore, pericardiocentesis should not delay emergency thoracotomy if cardiac tamponade is suspected.134 If there is uncertainty about the presence of tamponade, the focused abdominal sonography in trauma (FAST) examination rapidly and noninvasively identifies pericardial fluid.135–137
Therapeutic Pericardiocentesis
Tamponade of Uncertain Cause: Pulseless Electrical Activity
A major indication for emergency pericardiocentesis is a patient in cardiac arrest with PEA. Always consider cardiac tamponade in the differential diagnosis for PEA, especially if jugular venous pressure is elevated or a pericardial effusion is demonstrated on ultrasound. In a series of 20 patients with PEA, 3 had tamponade and another 5 had some degree of pericardial effusion.138 In this setting, blind (i.e., landmark method), ECG-guided, or ultrasound-guided pericardiocentesis can be lifesaving.
Tamponade Caused by Nonhemorrhagic Effusions
Most nonhemorrhagic effusions are liquid. They can be drained by pericardiocentesis with a small needle or by a catheter left in the pericardial space. Removal of even a small amount of fluid can cause immediate and dramatic improvement in blood pressure and cardiac output. Pericardiocentesis relieves tamponade caused by nonhemorrhagic effusions in 60% to 90% of cases.54,126,139
If small-needle pericardiocentesis fails, the patient might have a purulent, malignant, or well-organized effusion. Placement of a pericardial catheter may be more useful in the long-term management of these patients. In Krikorian and Hancock’s series,126 24% of patients were managed successfully with a single pericardiocentesis procedure, but 37% needed multiple aspirations or an indwelling catheter (39% required surgical drainage and 55% of these patients had traumatic hemopericardium). Therefore, if time and patient stability permit, consider pericardiocentesis with a catheter to reduce the necessity for repeated aspirations.
Patients with renal failure and tamponade require urgent pericardiocentesis. Without signs of tamponade, however, these patients may be better managed by dialysis. In one series, 63% of renal failure patients were managed successfully with only dialysis.126 There is some evidence that needle pericardiocentesis is a poor choice in patients who need pericardial drainage; in one series, 9 of 10 patients had serious complications following this procedure.66 Consultation with specialists is advised when there is no evidence of hemodynamic collapse but pericardial drainage is still being considered.
An algorithm for the urgent management of nonhemorrhagic cardiac tamponade is presented in Figure 16-10.
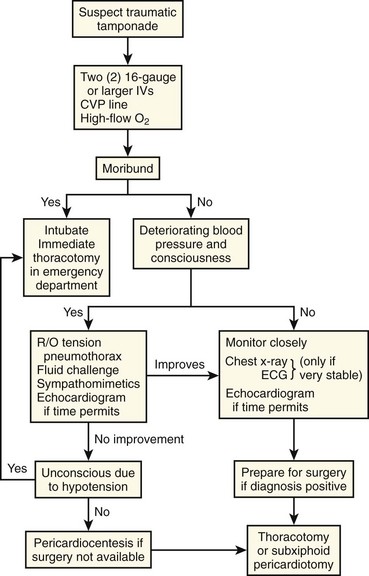
Pericardiocentesis in Patients with Hemorrhagic Tamponade
For hemorrhagic tamponade, pericardiocentesis is never the definitive treatment because this strategy has several drawbacks.140,141 Aspiration of a small quantity of fluid may cause a dramatic improvement in hemodynamics, but pericardial clots can prevent adequate drainage, so blood usually reaccumulates.93,106 Patients with traumatic pericardial hemorrhage ultimately require thoracotomy to explore and repair the cardiac injury. Pericardiocentesis simply delays this definitive procedure. A study investigating traumatic cardiac injury found that all patients who underwent surgery within 2 hours of the injury survived but the mortality rate was higher in patients who experienced longer operating room delays.140
Sugg and associates documented a 43% mortality rate when pericardiocentesis was the sole treatment of traumatic tamponade as compared with 16% in those who also underwent surgical intervention.132 All patients managed by pericardiocentesis had repairable wounds at autopsy, thus suggesting that in this population, operative repair is preferable. The number of deaths from stab wounds has been decreasing over time in response to a shift in trauma philosophy in which early thoracotomy is supported rather than repeated pericardiocentesis.95,133,141,142
Pericardiocentesis may improve a trauma patient’s hemodynamics temporarily, and benefit may be derived from this approach, but only when definitive surgical treatment is being arranged (Fig. 16-11). A study of penetrating trauma patients with tamponade found that preoperative pericardiocentesis decreased the mortality rate from 25% to 11%.143
Contraindications
Ideally, pericardiocentesis is performed in the cardiac catheterization laboratory under fluoroscopic or echocardiographic guidance. With the advent of bedside ultrasound and immediate visualization of large pericardial effusions, pericardiocentesis is being performed in the ED more frequently. Ultrasound can accurately identify the area of the heart with the greatest fluid accumulation and clarify its relationship to the body wall.40,144,145 This allows the physician to choose an entry site and angle of penetration with the greatest likelihood of obtaining fluid while avoiding vital structures.
Overview of Techniques and Equipment
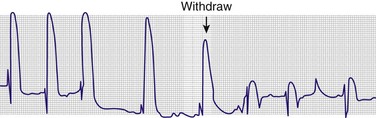
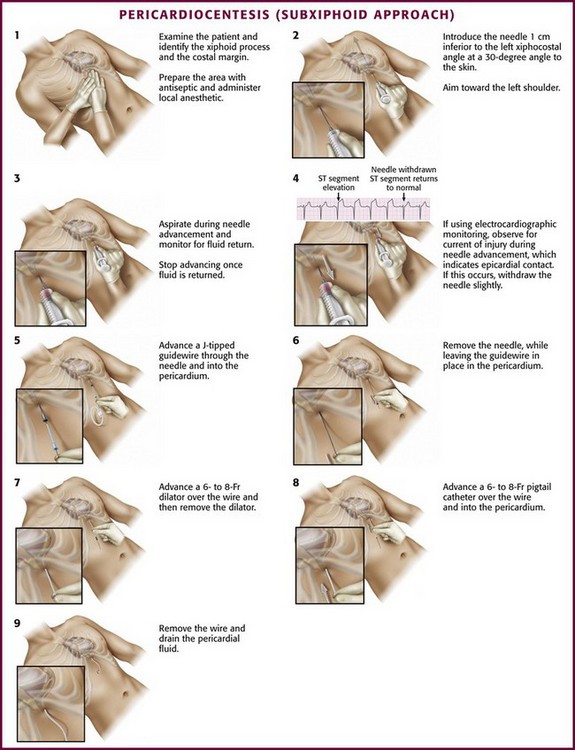
Before the introduction of real-time sonography to guide needle placement, electrocardiographic monitoring was used to indicate appropriate needle placement. This was done by connecting the electrocardiographic machine to one of the precordial leads (e.g., V1). That precordial lead was then attached to the distal end of a spinal needle with an alligator clamp (Fig. 16-12). The precordial lead was used as a rhythm strip to monitor the needle tip continuously.
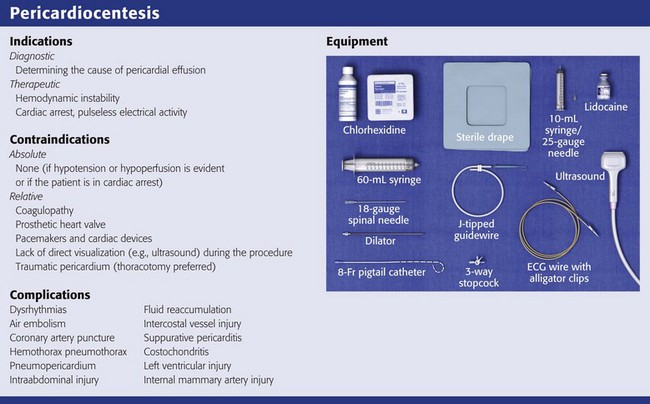
Other tools that are desirable for urgent pericardiocentesis can be found easily in the ED or in a pericardiocentesis kit (see Review Box 16-1): a finder needle; Seldinger wire; dilator; flexible catheter guide; 6- to 8-Fr pigtail catheter; plastic drainage tube; extra syringes; sterile hat, gown, gloves, and drape; and local anesthetic.
Procedure
Cardiac tamponade is an emergency that requires urgent therapy. Therapy typically consists of either pericardial drainage by needle aspiration or placement of a pericardial window. These procedures cannot be readily performed in an ED, so temporizing methods are the mainstay of therapy unless the patient is unstable (e.g., in PEA arrest). The most common therapeutic procedures used as temporizing measures in the setting of tamponade are intravascular volume expansion and administration of vasopressors.5,14,146 Although most textbooks and protocols encourage the use of these temporizing methods, they are backed by only sparse scientific evidence.147,148 Studies in animals have shown an increase in cardiac output and improvement in blood pressure with expansion of central blood volume; the validity of this in humans with cardiac tamponade is uncertain. Fluid resuscitation in a trauma patient with penetrating cardiac injury might cause deterioration. Animal experiments indicate that the response depends on whether fluid boluses produce recurrent bleeding from the cardiac wound.149 Despite the lack of evidence, judicious volume expansion with or without an adjunctive vasopressor before definitive therapy may be the only option for patients with tamponade.
Norepinephrine, isoproterenol, dopamine, and dobutamine have all been evaluated as the vasopressor of choice in patients with cardiac tamponade. Norepinephrine and isoproterenol increased cardiac output in animal models of tamponade but failed to increase it in humans.150,151 Dopamine and dobutamine increased cardiac output and improved hemodynamics in the setting of tamponade.33,148 Either of these agents may be helpful as a temporizing agent, but theoretically, dobutamine might be preferable because of its greater β-adrenergic activity.151
Preparation
Every effort should be made to ensure aseptic technique. Prepare the chest and upper part of the abdomen with a chlorhexidine-based solution. Drape the patient and ensure that all care providers involved in the procedure are wearing a sterile hat, mask, gown, and gloves. If the patient is awake, anesthetize the skin and the proposed route with 1% lidocaine (see Review Box 16-1). Because the pericardium is extremely sensitive, it should be anesthetized.152
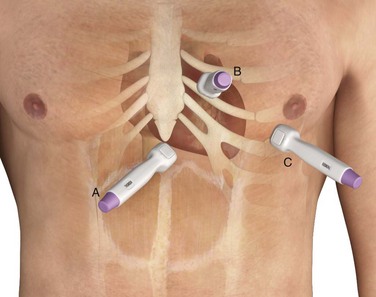
The approach to pericardiocentesis depends on the clinical status of the patient, the availability of ultrasound, and the distribution of pericardial fluid. Pericardial fluid is not always distributed circumferentially in the pericardial sac, so ultrasound can quickly identify the maximal effusion pocket and demarcate the appropriate site for needle placement. Pericardiocentesis with ultrasound guidance is currently the safest and most reliable method for the diagnosis and treatment of pericardial effusion and tamponade.153 Studies of echocardiography-directed pericardiocentesis have found that the apical approach is the best site for puncture.145,154,155 Cadaver studies have corroborated this finding and demonstrated greater safety with an apical approach. However, these studies also revealed that an apical approach is associated with a greater incidence of pneumothorax than a traditional subcostal approach is. Before the advent of ultrasound guidance, the subxiphoid approach (discussed later in this chapter) was the preferred method of pericardiocentesis. It is still used frequently during cardiac arrest and when ultrasound is not readily available.
ECG Monitoring
After all equipment is sterile, attach an alligator clip from one of the precordial leads (e.g., V1) to the distal end of the spinal needle. Record a rhythm strip of this lead (the “exploring electrode”) continuously. Advance the needle through the skin while remembering that any contact with the epicardium will cause a current-of-injury pattern that can be seen on the ECG. Typically, this is represented as a wide-complex premature ventricular contraction with an elevated ST segment (Fig. 16-13). When a current-of-injury pattern is seen, the needle is probably touching the epicardium. Withdraw it several millimeters to prevent laceration of the myocardium or coronary vessels. After slight withdrawal, the needle should be within the pericardial space. Aspirate any fluid, but watch for any changes on the ECG. Electrocardiographic monitoring is not infallible: if the patient has an abnormal myocardium from conditions such as a previous MI or the formation of scar tissue, no current-of-injury pattern will be generated on the rhythm strip.
Subxiphoid/Subcostal Approach
As mentioned earlier, the traditional blind subxiphoid approach can still be used for pericardiocentesis in the ED (e.g., for patients in cardiac arrest and when ultrasound is not available). The technique is performed as follows: introduce the needle 1 cm inferior to the left xiphocostal angle at a 30-degree angle to the skin (Fig. 16-14). Because the heart is an anterior structure, angles greater than 45 degrees may lacerate the liver or stomach. Aim toward the left shoulder and advance the needle slowly while continuously maintaining negative pressure on the syringe to aspirate any fluid. Aspirate with an “in-and-out” vector only, not “side to side,” which may lacerate tissue. If no fluid is aspirated, withdraw the needle completely and redirect it in a deeper posterior trajectory. If no fluid is aspirated after redirecting the needle, withdraw the needle and redirect it, working from the patient’s left to right, until it is aimed at the right shoulder. Recommendations regarding needle trajectory vary widely, including toward the right shoulder, sternal notch, and left shoulder.146,152
Apical Approach
The apical approach is sometimes used as an alternative to the subcostal approach to drain a pericardial effusion when ultrasound is available. Use ultrasound to identify the largest area of the apical effusion (Fig. 16-15A) or simply feel for the apex. If the apex cannot be palpated, it typically lies within the area of cardiac dullness, often between the fifth, sixth, or seventh intercostal space, between the midclavicular and midaxillary lines. Introduce the needle 1 cm lateral to and into the intercostal space below the apical heartbeat. Advance the needle over the cephalad border of the rib and aim it toward the right shoulder to avoid the neurovascular bundle located caudal to the rib space. This area is close to the lingula and the left pleural space, thus making pneumothorax a frequent complication. Theoretically, this technique is used because the coronary vessels are small at the apex; therefore, if a ventricle is entered, it is the thick-walled LV, which is more likely to seal off after ventricular injury. With echocardiographic guidance, the apical approach is used more commonly.156
Parasternal Approach
The parasternal approach is an alternative approach to the previously described techniques. First, identity the largest area of the parasternal effusion on ultrasound if possible. If ultrasound is not available or if the effusion is not clearly identified on the ultrasound image, proceed by introducing the needle 1 cm lateral to the sternal border at the left fifth or sixth intercostal interspace. Advance the needle over the cephalad border of the rib to avoid the neurovascular bundle on the caudal aspect of the rib. Avoid going too far laterally from the sternal border because of potential injury to the internal mammary artery.157 Occasionally, a right parasternal approach may be used when ultrasound predicts superior access to an effusion from this direction.
Tsang and coworkers158 described this technique for ultrasound-guided pericardiocentesis in 1998. The ideal site for skin puncture is where the largest area of fluid accumulation is closest to the skin surface. On ultrasound, this is indicated by a large anechoic (black) area at the top of the screen, usually corresponding to the left anterior chest wall (rather than the subcostal region). This approach also avoids injury to the liver (common with the subcostal approach). Inadvertent puncture of the lung is also prevented with this approach because air in the lung will not conduct sound waves and will prevent visualization of the heart when located immediately beneath the probe. Avoid choosing a site that could puncture the internal mammary artery, which lies 3 to 5 cm from either parasternal border, or the neurovascular bundle, which is located at the inferior border of the rib. Mark the best site with a sterile pen.
Procedure and Technique
Once the pericardial space is entered, inject agitated saline to confirm needle placement, particularly if the pericardial fluid is grossly bloody or if there is any question about needle position (see Fig. 16-15B). Prepare a saline echocardiographic contrast medium by using two 5-mL syringes, one with saline and the other with air. Connect them via a three-way stopcock to the needle and catheter. Rapidly inject saline between the syringes and then inject it into the sheath. Monitor the entrance of the agitated saline into the pericardial space sonographically—it appears as a brightly echogenic stream. If the use of agitated saline proves to be inconclusive or suboptimal, use an echocardiographic contrast agent (e.g., Definity) as a safe and successful alternative.159,160 Contrast agents contain gas microbubbles, which markedly enhance the fluid echo by introducing multiple liquid-gas interfaces. Inject this solution as a bolus. If the contrast material clears immediately after administration (as occurs with agitated saline) or persists temporarily within the cardiac chambers, an intracardiac location is suggested.
Fluid Aspiration and Evaluation
Removal of even a small amount of pericardial fluid (e.g., 30 to 50 mL) usually results in either return of spontaneous circulation or hemodynamic improvement. After any approach used for pericardiocentesis, place a temporary drain not only to ensure rapid access into the pericardial sac but also to allow more fluid to be removed quickly if hemodynamic collapse recurs. After needle placement is confirmed, a temporary drain can be placed by the Seldinger technique, described in Chapter 22.
Remove the syringe from the needle, advance a guidewire through the needle, and then remove the needle. Position a dilator (6- to 8-Fr Cordis) over the wire. If a dilator is not used, particularly with the subxiphoid approach, the pigtail catheter tip may get caught in the subcutaneous tissue and make placement of the catheter difficult. Remove the dilator and slide an introducer sheath dilator (6- to 8-Fr Cordis) over the wire. Remove the wire and the dilator while leaving the introducer sheath in place. Insert the pigtail angiocatheter through the introducer sheath, and aspirate fluid to confirm placement.154,158 After the catheter is advanced into place, it should be secured with a suture to ensure that it does not migrate after the procedure; an appropriate catheter dressing should be applied. The catheter should be attached to a three-way stopcock and connected to a water seal to drain by gravity. The pigtail catheter allows prolonged drainage and safe access into the pericardial sac without requiring the introduction of another needle.161,162 If drainage of pericardial fluid becomes sluggish, flush the catheter with a heparinized saline solution to ensure patency of the lumen.152
Immediately following the procedure, obtain a chest film to ensure the absence of pneumothorax and free air under the diaphragm. Place the patient on continuous cardiac monitoring for 24 hours and watch for signs of reaccumulating fluid or iatrogenic complications. Repeating the ultrasound examination in 24 hours is recommended. Diagnostic evaluation of nonhemorrhagic fluid is similar to that for pleural fluid (see Chapter 9).
Complications
Emergency physicians often perform pericardiocentesis under duress on a patient in PEA arrest. Many also perform the technique blindly because they have little or no time to gather adjunctive assistance or tools. It is critical for the emergency physician to be aware of both the traditional and contemporary methods of performing the procedure and the complications that can be associated with these methods (see Review Box 16-1).
With the advent of ultrasound- and CT-guided pericardiocentesis, the complication rate has been greatly reduced. Complication rates as low as 4% have been reported in large observational studies. Earlier studies of blind pericardiocentesis documented morbidity rates of 20% to 40% and mortality rates as high as 6%.163 Because pericardiocentesis is performed in moribund patients, the likelihood of cardiac arrest and death is high. However, they are not usually a direct complication of pericardiocentesis but of poor cardiopulmonary reserve. Cardiac arrest and death are rarely associated with echocardiographically guided pericardiocentesis. When blind or electrocardiographically guided pericardiocentesis is performed, the patient is usually already in full arrest and attributing the cause of death to the procedure is nearly impossible. In a series of 52 patients the only death occurred in a patient in cardiogenic shock in whom pericardiocentesis was nonproductive and who was found to have severe arteriosclerotic heart disease, not tamponade, on postmortem examination.164 In a series of 352 fluoroscopically guided pericardiocenteses, two deaths were documented.165 Ultrasonographic or CT confirmation of effusion was used in all but 15 cases. The two deaths occurred during or after the procedure, but whether they could be attributed to the procedure is unclear. One patient with aortic rupture that penetrated into the pericardial space died of cardiac arrest immediately after the puncture. The other death, in a post-MI patient with a left ventricular aneurysm, was caused by ventricular fibrillation that occurred about 15 minutes after the procedure.
Preventricular contractions are frequently noted after the needle enters the pericardial sac; however, no serious dysrhythmias resulting in hemodynamic compromise have been mentioned in the literature. Several case series report no dysrhythmias.54,67,154 Krikorian and Hancock126 reported one episode of ventricular tachycardia and several “hypotensive vasovagal reactions” that were associated with bradycardia and responded to atropine and fluid loading. Duvernoy and associates165 reported 1 case of ventricular tachycardia and 1 case of atrial fibrillation in 352 procedures. Maggiolini and coworkers reported transient third-degree heart block in a single patient.166 The traditional subxiphoid approach carries a risk for liver laceration. Fortunately, inadvertent needle passage into the liver has not been reported to cause significant hemorrhage or death.167
The parasternal and apical approaches have been documented as causing pneumothorax and pneumopericardium in several case series, but without any clinical consequence (Fig. 16-16). The pneumothoraces were treated with 100% oxygen or thoracostomy. There have also been infrequent reports of pneumopericardium after removal of a pericardiocentesis catheter. The cause of the pneumopericardium is thought to be the formation of a bronchopericardial fistula, but the exact mechanism is unclear. The mortality rate associated with tension pneumopericardium is about 50%, so consider pneumopericardium when patients complain of dyspnea and hypotension after removal of their catheter.168–170
Very few studies have reported ventricular or coronary vessel laceration during pericardiocentesis. These complications occur more frequently during blind or electrocardiographically guided procedures. Most cardiac perforations occur in the RV, but punctures in the LV and atria have also been reported.25 When these perforations occur, they tend to be silent and result in hemopericardium and death. In patients taking anticoagulants, it is important to check coagulation factors and monitor them closely after a seemingly insignificant pericardiocentesis because hemopericardium could develop just from the procedure itself.
In the series compiled by Krikorian and Hancock,126 hemopericardium developed in 13 of 123 patients as a result of pericardiocentesis, 1 as a result of a lacerated coronary artery. One patient died of a punctured ventricle. Surgical control was necessary in four patients in whom tamponade developed, whereas it did not develop in eight patients with hemopericardium, and they were managed conservatively. Guberman and colleagues54 reported three lacerations of the RV in 46 patients; one was fatal. Wong and colleagues164 found five punctures of the RV, four in patients with nonproductive pericardiocentesis, but none caused any adverse sequelae. In their series of 352 procedures, Duvernoy and associates165 reported 23 penetrations. In two cases both the RV and LV had been perforated, and in all other cases the RV had been entered.
Researchers differ in their opinions regarding the adverse effects of ventricular puncture. Most ventricular punctures involve the lower aspect of the RV. The wall of the RV is thin and therefore vulnerable to laceration. However, pressure in the RV is low,2 so a puncture should cause little bleeding. In a series of patients who underwent ultrasound-directed pericardiocentesis, ventricular puncture occurred in 1.5% but was without consequence.154 In another study, laceration of the RV occurred in 1 patient despite the use of echocardiography; it resulted in tamponade and necessitated emergency surgery.123 Of the 23 perforations in the series by Duvernoy and associates, 3 were considered major complications (two patients required thoracotomy). Left ventricular pseudoaneurysm typically occurs as a complication of MI. It is rarely seen after surgery, trauma, or infection. Rare cases of severe left ventricular pseudoaneurysm after pericardiocentesis have been reported recently.171,172
Even when pericardiocentesis has induced no physical injury, adverse events have been documented. Most have to do with the fact that during pericardiocentesis the stroke volume of the previously collapsed RV increases 75% after the first 200 mL of fluid is removed.7 In general, this increase in stroke volume is greater initially than that demonstrated by the LV. This imbalance can cause significant consequences for both right and left ventricular function. Three of six patients in whom large effusions were removed by pericardiocentesis experienced right ventricular dilation and overload, abnormal septal motion, and either no increase or a decrease in the right ventricular ejection fraction.173 These patients subsequently and slowly returned to normal hemodynamic status.
Pulmonary edema following pericardiocentesis has also been reported, presumably caused by a sudden increase in venous return to the LV when peripheral vascular resistance is still high from compensatory catecholamine secretion.174–178 Supporting evidence for this explanation is that right ventricular stroke volume increases more than left ventricular stroke volume after relief of tamponade.10 Circulatory collapse with persistently low arterial blood pressure has been reported in a patient from whom 700 mL of clear fluid was drained at a rate of 100 mL/min.179 Thus, many authors recommend that the pericardial drainage rate not exceed 50 mL/min.
References
1. Little, WC, Freeman, GL. Pericardial disease. Circulation. 2006;113:1622.
2. Baue, A, Blakemore, W. The pericardium. Ann Thorac Surg. 1972;14:81.
3. Applegate, R, Johnston, WE, Vinten-Johansen, J, et al. Restraining effect of intact pericardium during acute volume leaning. Am J Physiol. 1992;262:H1725.
4. Lee, M, Fung, Y, Shabetai, R. Biaxial mechanical properties of human pericardium and canine comparisons. Am J Physiol. 1987;253:H75.
5. Shabetai, R, Fowler, N, Guntheroth, W. The hemodynamics of cardiac tamponade and constrictive pericarditis. Am J Cardiol. 1970;26:480.
6. Spodick, D. The normal and diseased pericardium: current concepts of pericardial physiology, diagnosis, and treatment. J Am Coll Cardiol. 1983;1:240.
7. Grose, R, Greenberg, M, Steingart, R, et al. Left ventricular volume and function during relief of cardiac tamponade in man. Circulation. 1982;66:149.
8. Bodson, L, Boufferache, K, Vieillard-Baron, A. Cardiac tamponade. Curr Opin Crit Care. 2011;17:416–424.
9. Shoemaker, W, Carey, S, Yao, S. Hemodynamic monitoring for physiologic evaluation, diagnosis, and therapy of acute hemopericardial tamponade from penetrating wounds. J Trauma. 1973;13:36.
10. Manyari, D, Kostuk, W, Purves, P. Effect of pericardiocentesis on right and left ventricular function and volumes in pericardial effusion. Am J Cardiol. 1983;52:159.
11. Crystal, G, Bashour, F, Downey, H, et al. Myocardial blood flow and oxygen consumption during moderate cardiac tamponade: role of reflex vasoconstriction. Proc Soc Exp Biol Med. 1979;160:65.
12. Wertheimer, W, Bloom, S, Hughes, R. Myocardial effects of pericardial tamponade. Ann Thorac Surg. 1972;14:494.
13. Moller, C, Schoonbee, C, Rosendorff, C. Haemodynamics of cardiac tamponade during various modes of ventilation. Br J Anaesth. 1979;51:409.
14. Antman, E, Cargill, V, Grossman, W. Low-pressure cardiac tamponade. Ann Intern Med. 1979;91:403.
15. Hayes, S, Freeman, WK, Gersh, BJ. Low pressure cardiac tamponade: diagnosis facilitated by Doppler echocardiography. Br Heart J. 1990;63:1136.
16. Sagristà-Sauleda, J, Angel, J, Sambola, A, et al. Low-pressure cardiac tamponade: clinical and hemodynamic profile. Circulation. 2006;114:945.
17. Strimel, WJ. Pericardial effusion. Available at http://emedicine.medscape.com/article/157325-overview#a0199, 2010. [Updated June 30].
18. Symbas, P, Harlafhs, N, Waldo, W. Penetrating cardiac wounds: a comparison of different therapeutic methods. Ann Surg. 1976;183:377.
19. Borja, A, Lansing, A, Randell, H. Immediate operative treatment for stab wounds of the heart. Ann Thorac Cardiovasc Surg. 1970;59:662.
20. Clarke, D. The heart and great vessels. In: Dudley H, ed. Hamilton Bailey’s Emergency Surgery. 11th ed. Bristol, England: Wright; 1986:235.
21. Blair, E, Tapuzla, C, Dean, R. Chest trauma. In: Hardy J, ed. Critical Surgical Illness. Philadelphia: Saunders; 1971:175.
22. Thomas, T. Emergency evacuation of acute pericardial tamponade. Ann Thorac Surg. 1970;10:566.
23. Isner, JM. Acute catastrophic complications of balloon aortic valvuloplasty. J Am Coll Cardiol. 1991;17:1436.
24. Friedrich, SP, Berman, AD, Baim, DS, et al. Myocardial perforation in the cardiac catheterization laboratory: incidence, presentation, diagnosis and management. Catheter Cardiovasc Diagn. 1994;32:99.
25. Kerber, R, Ridges, J, Harrison, D. Electrocardiographic indications of atrial puncture during pericardiocentesis. N Engl J Med. 1979;282:1142.
26. Sobol, S, Thomas, H, Evans, R. Myocardial laceration not demonstrated by continuous electrocardiographic monitoring occurring during peri cardiocentesis. N Engl J Med. 1979;292:1222.
27. Edwards, H, King, T. Cardiac tamponade from central venous catheters. Arch Surg. 1982;117:965.
28. Barton, B, Hermann, G, Weil, R. Cardiothoracic emergencies associated with subclavian hemodialysis catheters. JAMA. 1983;250:2660.
29. Ramp, J, Harkins, J, Mason, G. Cardiac tamponade secondary to blunt trauma. J Trauma. 1974;14:767.
30. Mandavia, DP, Hoffner, RJ, Mahaney, K, et al. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38:377.
31. Namai, A, Sakurai, M, Fujiwara, H. Five cases of blunt traumatic cardiac rupture: success and failure in surgical management. Gen Thorac Cardiovasc Surg. 2007;55:200.
32. Leavitt, BJ, Meyer, JA, Morton, JR, et al. Survival following nonpenetrating traumatic rupture of cardiac chambers. Ann Thorac Surg. 1987;44:532.
33. Gabram, SG, Devanney, J, Jones, D, et al. Delayed hemorrhagic pericardial effusion: case reports of a complication from severe blunt chest trauma. J Trauma. 1992;32:794.
34. Fey, G, Deren, M, Wesolek, J. Intrapericardial caval injury due to blunt trauma. Conn Med. 1999;63:259.
35. Kirsh, M, Behrendt, D, Orringer, M, et al. The treatment of acute traumatic rupture of the aorta: a 10-year experience. Ann Surg. 1976;184:308.
36. Glasser, S, Harrison, E, Amey, B. Echocardiographic incidence of pericardial effusion in patients resuscitated by emergency medical technicians. JACEP. 1979;8:6.
37. Noffsinger, AE, Bilsard, KS, Balko, MG. Cardiac laceration and pericardial tamponade due to cardiopulmonary resuscitation after myocardial infarction. J Forensic Sci. 1991;36:60.
38. Reardon, MJ, Gross, DM, Vallone, AM, et al. Atrial rupture in a child from cardiac massage by his parent. Ann Thorac Surg. 1987;43:557.
39. Figueras, J, Barrabés, JA, Serra, V, et al. Hospital outcome of moderate to severe pericardial effusion complicating ST-elevation acute myocardial infarction. Circulation. 2010;122:1902.
40. Tsang, TSM, Enriquez-Sarano, M, Freeman, WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429.
41. Von Sohsten, R, Kopistansky, C, Cohen, M, et al. Cardiac tamponade in the new device era: evaluation of 6999 consecutive percutaneous interventions. Am Heart J. 2000;140:279.
42. Pepi, M, Muratori, M, Barbier, P, et al. Pericardial effusion after cardiac surgery: Incidence, site, size, and haemodynamic consequences. Br Heart J. 1994;72:327.
43. Frater, R. Intrapericardial pressure and pericardial tamponade in cardiac surgery. Ann Thorac Surg. 1970;10:563.
44. Correale, E, Maggioni, AP, Romano, S, et al. Comparison of frequency, diagnostic, and prognostic significance of pericardial involvement in acute myocardial infarction treated with and without thrombolytics. Am J Cardiol. 1993;71:1377.
45. Hagan, PG, Nienaber, CA, Isselbacher, EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897.
46. Larson, E, Edwards, W. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849.
47. Spittell, PC, Spittell, JA, Jr., Joyce, JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc. 1993;68:642.
48. Figueras, J, Alcalde, O, Barrabés, JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 2008;118:2783.
49. Patel, MR, Meine, TJ, Lindblad, L, et al. Cardiac tamponade in the fibrinolytic era: analysis of >100,000 patients with ST-segment elevation myocardial infarction. Am Heart J. 2006;151:316.
50. Coma-Canella, I, Lopez-Sendon, J, Gonzalez Garcia, A, et al. Hemodynamic effects of dextran, dobutamine and pericardiocentesis in cardiac tamponade secondary to subacute heart rupture. Am Heart J. 1987;114:78.
51. Balakumaran, K, Verbaan, CJ, Essed, CE, et al. Ventricular free wall rupture: sudden, subacute, slow, sealed and stabilized varieties. Eur Heart J. 1984;5:282.
52. Hancock, E. Management of pericardial disease. Mod Concepts Cardiovasc Dis. 1979;48:1.
53. LeWinter, M, Pavelec, R. Influence of the pericardium on left ventricular end-diastolic pressure-segment length relations during early and later phases of experimental chronic volume overload in dogs. Circ Res. 1982;50:401.
54. Guberman, B, Fowler, N, Engel, P. Cardiac tamponade in medical patients. Circulation. 1981;64:633.
55. Sagrista-Sauleda, J, Marcé, J, Permanyer-Miralda, G, et al. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109:95–101.
56. Hoit, BD. Pericardial disease and pericardial tamponade. Crit Care Med. 2007;35(8 suppl):S355.
57. Maisch, B, Seferovi , PM, Risti
, PM, Risti , AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587.
, AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587.
58. Corey, GR, Campbell, PT, Van Trigt, P, et al. Etiology of large pericardial effusions. Am J Med. 1993;95:209.
59. Bussani, R, De-Giorgio, F, Abbate, A, et al. Cardiac metastases. J Clin Pathol. 2007;60:27.
60. Natanzon, A, Kronzon, I. Pericardial and pleural effusions in congestive heart failure: anatomical, pathophysiologic, and clinical considerations. Am J Med Sci. 2009;338:211.
61. Reynolds, MM, Hecht, SR, Berger, M, et al. Large pericardial effusions in the acquired immunodeficiency syndrome. Chest. 1992;102:1746.
62. Kwan, T, Karve, MM, Emerole, O. Cardiac tamponade in patients infected with HIV. Chest. 1993;104:1059.
63. Lewis, W. AIDS: cardiac findings from 115 autopsies. Cardiovasc Dis. 1989;32:207.
64. Stotka, JL, Good, CB, Downer, WR, et al. Pericardial effusion and tamponade due to Kaposi’s sarcoma in acquired immunodeficiency syndrome. Chest. 1989;95:1359.
65. Zakowski, MF, Ianuale-Shanerman, A. Cytology of pericardial effusions in AIDS patients. Diagn Cytopathol. 1993;9:266.
66. Rutsky, E, Rostand, S. Treatment of uremic pericarditis and pericardial effusion. Am J Kidney Dis. 1987;10:2.
67. Kwasnik, E, Kostes, J, Lazarus, J. Conservative management of uremic pericardial effusions. J Thorac Cardiovasc Surg. 1978;76:629.
68. Hanfling, S. Metastatic cancer in the heart. Circulation. 1960;22:474.
69. Spodick, DH. Acute cardiac tamponade. N Engl J Med. 2003;349:684.
70. Shabetai, R. Pericardial effusion: haemodynamic spectrum. Heart. 2004;90:255.
71. Sarrista-Sauleda, J, Angel, J, Sánchez, A, et al. Effusive-constrictive pericarditis. N Engl J Med. 2004;350:469.
72. Hancock, EW. A clearer view of effusive-constrictive pericarditis. N Engl J Med. 2004;350:435.
73. Hurd, T, Novak, R, Gallagher, T. Tension pneumopericardium: a complication of mechanical ventilation. Crit Care Med. 1984;12:200.
74. Toledo, T, Moore, W, Nash, D. Spontaneous pneumopericardium in acute asthma: case report and review of the literature. Chest. 1972;16:118.
75. Hacker, P, Dorsey, D. Pneumopericardium and pneumomediastinum following closed chest injury. JACEP. 1979;8:409.
76. Capizzi, PJ, Martin, M, Bannon, MP. Tension pneumopericardium following blunt injury. J Trauma. 1995;39:775.
77. Frascone, R, Cicero, J, Sturm, J. Pneumopericardium occurring during a high-speed motorcycle ride. J Trauma. 1983;23:163.
78. McDougal, C, Mulder, G, Hoffman, J. Tension pneumopericardium following blunt chest trauma. Ann Emerg Med. 1985;14:167.
79. Robinson, M, Markovchick, V. Traumatic tension pneumopericardium: a case report and literature review. J Emerg Med. 1985;2:409.
80. Lynn, R. Delayed post-traumatic pneumopericardium producing acute cardiac tamponade. Can J Surg. 1983;26:62.
81. Markiewicz, W, Borovik, R, Ecker, S. Cardiac tamponade in medical patients: treatment and prognosis in the echocardiographic era. Am Heart J. 1986;111:1138.
82. Perera, P, Mailhot, T, Riley, D, et al. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28:29.
83. Imazio, M, Mayosi, BM, Brucato, A, et al. Triage and management of pericardial effusion. J Cardiovasc Med. 2010;11:928.
84. Shabetai, R. Diseases of the pericardium. In Walsh R, Fuster V, Harrington R, eds.: Hurst’s the Heart, 13th ed, New York: McGraw-Hill, 2010.
85. Beck, C. Two cardiac compression triads. JAMA. 1935;104:715.
86. Symbas, P, Harlafhs, N, Waldo, W. Penetrating cardiac wounds: a comparison of different therapeutic methods. Ann Surg. 1976;183:377.
87. DiPasquale, J, Pluth, J. Penetrating wounds of the heart and cardiac tamponade. Postgrad Med. 1971;49:114.
88. Shoemaker, W, Carey, J, Jao, S. Hemodynamic alterations in acute cardiac tamponade after penetrating injuries of the heart. Surgery. 1970;67:754.
89. Hoit, BD. Pericardial disease and pericardial tamponade. Crit Care Med. 2007;35(8 suppl):S355–S364.
90. Brown, J, MacKinnon, D, King, A, et al. Elevated arterial blood pressure in cardiac tamponade. N Engl J Med. 1992;327:463.
91. Kilpatrick, Z, Chapman, C. On pericardiocentesis. Am J Cardiol. 1965;16:622.
92. Spodick, D. Acute cardiac tamponade. Pathologic physiology, diagnosis, and management. Prog Cardiovasc Dis. 1967;10:64.
93. Eisenberg, MJ, de Romeral, LM, Heidenreich, PA, et al. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. Chest. 1996;110:318.
94. Shabetai, R. Changing concepts of cardiac tamponade. Mod Concepts Cardiovasc Dis. 1983;52:19.
95. Shoemaker, W. Algorithm for early recognition and management of cardiac tamponade. Crit Care Med. 1975;3:59.
96. Trinkle, J, Marcas, J, Grover, F. Management of the wounded heart. Ann Thorac Surg. 1974;17:230.
97. Breaux, E, Dupont, J, Albert, H. Cardiac tamponade following penetrating mediastinal injuries: improved survival with early pericardiocentesis. J Trauma. 1979;19:461.
98. Khan, R. Air tamponade and tension pneumopericardium. J Thorac Cardiovasc Surg. 1974;68:328.
99. Spodick, D. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150–1153.
100. Meyers, DG, Bagin, RG, Levene, JF. Electrocardiographic changes in pericardial effusion. Chest. 1993;104:1422.
101. Smedema, J, Katjitae, I, Reuter, H, et al. Twelve-lead electrocardiography in tuberculous pericarditis. Cardiovasc J S Afr. 2001;12:31.
102. Bruch, C, Schmermund, A, Dagres, N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38:219.
103. Sotolongo, R, Horton, J. Total electrical alternans in pericardial tamponade. Am Heart J. 1981;101:853.
104. Markiewicz, W, Borovik, R, Ecker, S. Cardiac tamponade in medical patients: treatment and prognosis in the echocardiographic era. Am Heart J. 1986;111:1138.
105. Press, O, Livingston, R. Management of malignant pericardial effusion and tamponade. JAMA. 1987;257:1088.
106. Spodick, D. Electrical alternans of the heart: its relation to the kinetics and physiology of the heart during cardiac tamponade. Am J Cardiol. 1962;10:155.
107. Cheitlin, MD, Armstrong, WF, Aurigemma, GP, et al. ACC/AHA/ASE 2003 guideline for the clinical application of echocardiography. Available at www.acc.org/qualityandscience/clinical/statements.htm. [Accessed August 24, 2006].
108. Silvestry, F, Kerber, RE, Brook, MM, et al. Echocardiography-guided interventions. J Am Soc Echocardiogr. 2009;22:213–231.
109. Labovitz, AJ, Noble, VE, Bierig, M, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225–1330.
110. American College of Emergency Physician Policy Statement, Emergency ultrasound guidelines. Available at, 2008, www.acep.org.
111. Schairer, J, Biswas, S, Ketevian, SJ, et al. A systematic approach to the evaluation of pericardial effusion and cardiac tamponade. Cardiol Rev. 2011;19:233–238.
112. Gillam, LD, Guyer, DE, Gibson, TC, et al. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation. 1983;68:294.
113. Kerber, RE, Gascho, JA, Litchfield, R, et al. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982;307:929.
114. Fusman, B, Schwinger, ME, Charney, R, et al. Isolated collapse of left-sided heart chambers in cardiac tamponade: demonstration by two-dimensional echocardiography. Am Heart J. 1991;121:613.
115. Himelman, RB, Kircher, B, Rockey, DC, et al. Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade. J Am Coll Cardiol. 1988;12:1470.
116. Mazurek, B, Jehle, D, Martin, M. Emergency department echocardiography in the diagnosis and therapy of cardiac tamponade. J Emerg Med. 1991;9:27.
117. Restrepo, CS, Lemos, DF, Lemos, JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27:1595.
118. Duvernoy, O, Larsson, SG, Persson, K, et al. Pericardial effusion and pericardial compartments after open heart surgery. An analysis by computed tomography and echocardiography. Acta Radiol. 1990;31:41.
119. Yousem, D, Traill, TT, Wheeler, PS, et al. Illustrative cases in pericardial effusion misdetection: correlation of echocardiography and CT. Cardiovasc Intervent Radiol. 1987;10:162.
120. Mercé, J, Sagristà-Sauleda, J, Permanver-Miralda, G, et al. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138:759–764.
121. Permanyer-Miralda, G. Acute pericardial disease: approach to the aetiologic diagnosis. Heart. 2004;90:252–254.
122. Memon, A, Zawadski, Z. Malignant effusions: diagnostic evaluation and therapeutic strategy. Curr Probl Cancer. 1981;5:1.
123. Permanyer-Miralda, G, Sagrista-Sauleda, J, Soler-Soler, J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985;56:623.
124. Kindig, J, Goodman, M. Clinical utility of pericardial fluid pH determination. Am J Med. 1983;75:1077.
125. Koh, KK, Kim, EJ, Cho, CH, et al. Adenosine deaminase and carcinoembryonic antigen in pericardial effusion diagnosis, especially in suspected tuberculous pericarditis. Circulation. 1994;89:2728.
126. Krikorian, J, Hancock, E. Pericardiocentesis. Am J Med. 1978;65:808.
127. Just, M, Raventos, A, Romeu, J, et al. Cardiac tamponade and Kaposi’s sarcoma. Med Clin. 1994;102:495.
128. Nathan, PE, Arsura, EL, Zappi, M. Pericarditis with tamponade due to cytomegalovirus in the acquired immunodeficiency syndrome. Chest. 1991;99:765.
129. Prager, R, Wilson, C, Bender, H. The subxiphoid approach to pericardial disease. Ann Thorac Surg. 1981;34:6.
130. Alcan, K, Zabetakis, P, Marino, N. Management of acute cardiac tamponade by subxiphoid pericardiotomy. JAMA. 1982;247:1143.
131. Bolanowksi, P, Swaminathan, A, Neville, W. Aggressive surgical management of penetrating cardiac injuries. J Thorac Cardiovasc Surg. 1973;66:52.
132. Sugg, W, Rea, W, Ecker, R. Penetrating wounds of the heart: an analysis of 459 cases. J Thorac Cardiovasc Surg. 1968;56:531.
133. Arom, K, Richardson, J, Webb, G. Subxiphoid pericardial window in patients with suspected traumatic pericardial tamponade. Ann Thorac Surg. 1977;23:545.
134. Hung, KK. Best Evidence Topic Report. BET 3. Use of pericardiocentesis for patients with cardiac tamponade in penetrating chest trauma. Emerg Med J. 2009;26:119–120.
135. Rozycki, GS, Newman, PG. Surgeon-performed ultrasound for the assessment of abdominal injuries. Adv Surg. 1999;33:243.
136. Sisley, AC, Rozycki, GS, Ballard, RB, et al. Rapid detection of traumatic effusion using surgeon-performed ultrasonography. J Trauma. 1998;44:291.
137. Rozycki, GS, Feliciano, DV, Ochsner, MG, et al. The role of ultrasound in patients with possible penetrating cardiac wounds: a prospective multicenter study. J Trauma. 1999;46:543.
138. Tayal, TS, Kline, JA. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation. 2003;59:315.
139. Press, O, Livingston, R. Management of malignant pericardial effusion and tamponade. JAMA. 1987;257:1088.
140. Boyd, T, Strieder, J. Immediate surgery for traumatic heart disease. J Thorac Cardiovasc Surg. 1965;50:305.
141. Siemens, R, Polk, H, Gray, L. Indications for thoracotomy following penetrating thoracic injury. J Trauma. 1977;17:493.
142. Beall, A, Gasior, R, Bricker, D. Gunshot wounds of the heart: changing patterns of surgical management. Ann Thorac Surg. 1972;11:523.
143. Breaux, E, Dupont, J, Albert, H. Cardiac tamponade following penetrating mediastinal injuries: improved survival with early pericardiocentesis. J Trauma. 1979;19:461.
144. Callahan, J, Seward, J, Nishimura, R, et al. Two-dimensional echocardiographically guided pericardiocentesis: experience in 117 consecutive patients. Am J Cardiol. 1985;55:476.
145. Clarke, D, Cosgrove, D. Real-time ultrasound scanning in the planning and guidance of pericardiocentesis. Clin Radiol. 1987;38:119.
146. Fowler, N. Recognition and management of pericardial disease and its complications. In Hurst J, ed.: The Heart, 4th ed, New York: McGraw-Hill, 1978.
147. Gascho, JA, Martins, JB, Marcus, ML, et al. Effects of volume expansion and vasodilators in acute pericardial tamponade. Am J Physiol. 1981;240:H49.
148. Kerber, RE, Gascho, JA, Litchfield, R, et al. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982;307:929.
149. Pierart, J, Gyhra, A, Torres, P, et al. Causes of increasing pericardial pressure in experimental cardiac tamponade induced by ventricular perforation. J Trauma. 1993;35:834.
150. Martins, JB, Manuel, WJ, Marcus, ML, et al. Comparative effects of catecholamines in cardiac tamponade; experimental and clinical studies. Am J Cardiol. 1980;46:459.
151. Zhang, H, Spapen, H, Vincent, JL. Effects of dobutamine and norepinephrine on oxygen availability and tamponade-induced stagnant hypoxia: a prospective, randomized, controlled study. Crit Care Med. 1994;22:299.
152. Treasure, T, Cottler, L. Practical procedures: how to aspirate the pericardium. Br J Hosp Med. 1980;24:488.
153. Tsang, T, Barnes, M, Hayes, S, et al. Clinical and echocardiographic characteristics of significant pericardial effusions following cardiothoracic surgery and outcomes of echo-guided pericardiocentesis for management. Chest. 1999;116:322.
154. Callahan, J, Seward, J, Tajik, A. Cardiac tamponade: pericardiocentesis directed by two-dimensional echocardiography. Mayo Clin Proc. 1985;60:344.
155. Salem, K, Mulji, A, Lonn, E. Echocardiographically guided pericardiocentesis—the gold standard for the management of pericardial effusion and cardiac tamponade. Can J Cardiol. 1999;15:1251–1255.
156. Caspari, G, Bartel, T, Mohlenkamp, S, et al. Contrast medium echocardiography-assisted pericardial drainage. Herz. 2000;25:755.
157. Brown, C, Gurley, H, Hutchins, G, et al. Injuries associated with percutaneous placement of transthoracic pacemakers. Ann Emerg Med. 1985;14:223.
158. Tsang, TSM, Freeman, WK, Sinak, LG, et al. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc. 1998;73:647.
159. Cheng, T. Contrast echocardiography during pericardiocentesis. Heart. 1999;82:534–535.
160. Watzinger, N, Brussee, H, Fruhwald, FM, et al. Pericardiocentesis guided by contrast echocardiography. Echocardiography. 1998;15:635–640.
161. Patel, A, Kosolcharoen, P, Nallasivan, M, et al. Catheter drainage of the pericardium. Practical method to maintain long-term patency. Chest. 1987;92:1018.
162. Stewart, J, Gott, V. The use of a Seldinger wire technique for pericardiocentesis following cardiac surgery. Ann Thorac Surg. 1983;35:467.
163. Tsang, T, Emroquez-Sarano, M, Freeman, WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–436.
164. Wong, B, Murphy, J, Chang, CJ, et al. The risk of pericardiocentesis. Am J Cardiol. 1979;44:1110.
165. Duvernoy, O, Borowiec, J, Helmius, G, et al. Complications of percutaneous pericardiocentesis under fluoroscopic guidance. Acta Radiol. 1992;33:309.
166. Maggiolini, S, Bozzano, A, Russo, P, et al. Echocardiography-guided pericardiocentesis with probe-mounted needle: report of 53 cases. J Am Soc Echocardiogr. 2001;14:82.
167. Inglis, R, King, AJ, Gleave, W, et al. Pericardiocentesis in contemporary practice. J Invasive Cardiol. 2011;23:234–239.
168. Ewer, M, Ali, M, Frazier, O. Open chest resuscitation for cardiopulmonary arrest related to mechanical impairment of the circulation. Crit Care Med. 1982;10:198.
169. Braiteh, F, Malik, I. Pneumopericardium. CMAJ. 2008;179:1087.
170. Haan, J, Scalea, TM. Tension pneumopericardium: a case report and a review of the literature. Am Surg. 2006;72:330–331.
171. Patanà, F, Sansone, F, Centofanti, P, et al. Left ventricular pseudoaneurysm after pericardiocentesis. Interact Cardiovasc Thorac Surg. 2008;7:1112–1113.
172. Moharana, M, Aqarwal, S, Minhas, HS, et al. Delayed presentation of iatrogenic left ventricular pseudoaneurysm. J Cardiac Surg. 2010;25:284–287.
173. Armstrong, W, Feigenbaum, H, Dillon, J. Acute right ventricular dilatation and echocardiographic volume overload following pericardiocentesis for relief of cardiac tamponade. Am Heart J. 1984;107:1266.
174. Vandyke, WJ, Cure, J, Chakko, C, et al. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med. 1983;309:595.
175. Glasser, F, Fein, AM, Feinsilver, SH, et al. Non-cardiogenic pulmonary edema after pericardial drainage for cardiac tamponade. Chest. 1988;94:869.
176. Downey, RJ, Bessler, M, Weissman, C. Acute pulmonary edema following pericardiocentesis for chronic cardiac tamponade secondary to trauma. Crit Care Med. 1991;19:1323.
177. Chamoun, A, Cenz, R, Mager, A, et al. Acute left ventricular failure after large volume pericardiocentesis. Clin Cardiol. 2003;26:588.
178. Angouras, DC, Dosios, T. Pericardial decompression syndrome: a term for a well-defined but rather underreported complication of pericardial drainage. Ann Thorac Surg. 2010;89:1702–1703.
179. Hamaya, Y, Dohi, S, Ueda, N, et al. Severe circulatory collapse immediately after pericardiocentesis in a patient with chronic cardiac tamponade. Anesth Analg. 1993;77:1278.