Pericardial Tamponade
Clinical Presentation, Diagnosis, and Catheter-Based Therapies
ETIOLOGY OF PERICARDIAL EFFUSION AND TAMPONADE
DIAGNOSTIC ROLE OF ECHOCARDIOGRAPHY
CATHETER-BASED DIAGNOSTIC AND THERAPEUTIC STRATEGIES
OPEN SURGICAL PERICARDIAL WINDOW
Etiology of Pericardial Effusion and Tamponade
Pericarditis or pericardial effusion or both may result from an infectious, metabolic, inflammatory, autoimmune, or neoplastic process (Box 6.1).1–3 The frequency of specific causes depends on the geographic location, time period, and characteristics of the populations studied. In one European series of patients presenting with moderate and severe pericardial effusions, acute idiopathic pericarditis and iatrogenic causes accounted for most cases.1 In a smaller series in the United States comprising patients presenting to a tertiary medical center with large pericardial effusions, malignancy was the most common cause.4 Pericardial effusions occurring after radiation therapy, myocardial infarction, and surgical and interventional cardiac procedures are increasing in incidence. Uremia and hypothyroidism remain important causes but are seen less frequently given the prompt diagnosis and treatment of these disorders.
Pericardial fluid can be either a transudate or an exudate. Although transudative effusions typically occur in patients with congestive heart failure, exudative effusions may occur with most types of pericarditis and are characterized by a high concentration of proteins and fibrin. Pericardial effusions may be serous (or serosanguineous), suppurative, or hemorrhagic. Although the presence of suppurative effusion is pathognomonic for an acute infectious cause, usually bacterial, hemorrhagic pericardial effusion is commonly related to chronic infections, with tuberculosis a classic example, particularly in developing countries. In developed countries, hemorrhagic pericardial effusions are likely to be iatrogenic or malignant in origin. In a retrospective analysis of 150 patients in the United States who underwent pericardiocentesis for relieving cardiac tamponade, 64% of patients had a hemorrhagic pericardial effusion (with iatrogenic causes and malignancy accounting for most cases).5
Clinical Presentation
The normal pericardium is a fibroelastic sac composed of visceral and parietal layers separated by the pericardial cavity and containing a thin layer (20 to 50 mL) of straw-colored fluid surrounding the heart.3 The normal pericardium has a steep pressure-volume curve: It is distensible when the intrapericardial volume is small, but becomes gradually inextensible when the volume increases. In the presence of pericardial effusion, the intrapericardial pressure depends on the relationship between the absolute volume of the effusion, the speed of fluid accumulation, and pericardial elasticity. Although the rapid accumulation of small amounts of fluid (150 to 200 mL) can result in cardiac tamponade, the slow accumulation of larger effusions (>1 L, as in uremic pericardial effusions) is usually well tolerated.6,7 The clinical presentation is not only related to the size of the effusion, but also, and more importantly, to the rapidity of fluid accumulation.
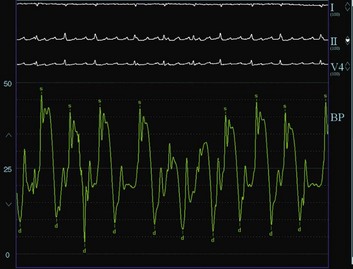
Pericardial tamponade is a clinical syndrome with defined hemodynamic and echocardiographic abnormalities, which result from the accumulation of intrapericardial fluid and impairment of ventricular diastolic filling.7,8 The ultimate mechanism of hemodynamic compromise is the compression of cardiac chambers secondary to increased intrapericardial pressure.8 Pericardial tamponade is usually a clinical diagnosis, with patients showing elevated systemic venous pressure, tachycardia, dyspnea, arterial pulsus paradoxus, muffled heart sounds, and evidence of electrical alternans on electrocardiogram (ECG).3 Pulsus paradoxus, which describes the exaggerated inspiratory decline in arterial blood pressure (>10 mm Hg), is largely attributed to interventricular dependence within the confined pericardial space. Although its diagnostic utility was recognized many decades earlier,9 various conditions may lead to its absence in patients with cardiac tamponade (e.g., in patients with concomitant aortic regurgitation, atrial septal defects, severe left ventricular dysfunction, aortic regurgitation, severe hypotension, pericardial adhesions, pulmonary artery obstruction, or positive-pressure ventilation).8
The ECG shows sinus tachycardia and low voltage. Electrical alternans, which describes the beat-to-beat alterations in the QRS complex reflecting cardiac swinging in the pericardial fluid, is a relatively specific sign for tamponade and is rarely seen with very large pericardial effusions alone.10 Patients with pericardial effusions have an enlarged cardiac silhouette with clear lung fields on chest radiograph. The pericardial effusion has to reach 200 mL in volume to appear on the chest radiograph, and this volume occurs usually in slowly accumulating pericardial effusions (which are less likely to cause tamponade).11 Rapidly accumulating small pericardial effusions may cause tamponade and have a normal chest radiograph.
The diagnosis of pericardial tamponade is best confirmed by a two-dimensional echocardiogram that shows a pericardial effusion, right atrial compression, and abnormal respiratory variations in the right and left ventricular dimensions and in the tricuspid and mitral valve flow velocities (Fig. 6.1).12 The classic hemodynamic findings of pericardial tamponade include arterial pulsus paradoxus, elevation and diastolic equalization of right and left ventricular diastolic pressures with pericardial pressure, and depression of cardiac output.8 Because patients with critical tamponade operate on the steep portion of the pericardial pressure-volume curve, drainage of even a small pericardial volume causes a dramatic reduction in intrapericardial pressure and rapid clinical and hemodynamic improvement (by shifting the stretched pericardium back to the flat portion of the pericardium pressure-volume curve).8
Diagnostic Role of Echocardiography
Echocardiography is recognized as a particularly useful imaging modality for pericardial disease.13,14 Currently, two-dimensional echocardiography has become the gold standard diagnostic modality because it provides a highly sensitive and specific noninvasive imaging technique for pericardial disease.12,15 It is also an important tool for the longitudinal follow-up of pericardial effusions over time (given a class IIa recommendation in the American Heart Association/American College of Cardiology guidelines for the clinical application of echocardiography).12 Classically, a persistent echo-free space throughout the cardiac cycle between the parietal pericardium and the epicardium is pathognomonic for pericardial effusion by M-mode echocardiography.13
Two-dimensional echocardiography allows delineation of the size and distribution of the effusion, including loculated effusions, and helps assess the success of pericardiocentesis. The echocardiogram also can provide a reasonable estimate of the total volume of the effusion.15 Circumferential effusion greater than 1 cm in width is considered large (>500 mL). Moderate effusions (100 to 500 mL) are usually circumferential but less than 1 cm, whereas small effusions (<100 mL) are usually localized posterior to the left ventricle and measure less than 1 cm. Classification criteria differ significantly among various echocardiographers and institutions. The typical echocardiographic signs of pericardial tamponade are listed in Box 6.2.
The nature of the pericardial fluid is difficult to identify by echocardiography. Increased echogenicity is suspicious, however, for the presence of proteins or cells or both in the pericardial fluid. Fibrin deposits localized in the epicardial surface can be identified as echogenic masses. In one study of 42 patients with tuberculous and viral or idiopathic pericardial effusions, intrapericardial echocardiogram abnormalities, such as a greater degree of pericardial thickening, frequency and thickness of exudative coating or deposits, and strands crossing the pericardial space, were useful criteria in the diagnosis of tuberculous pericardial effusion and in differentiating it from chronic idiopathic pericardial effusion.16
The classic echocardiographic signs of cardiac tamponade are right atrial and right ventricular diastolic collapse. The right atrium and right ventricle are compliant structures. As a result, increased intrapericardial pressure leads to their collapse when intracavitary pressures are only slightly exceeded by those in the pericardium. At end diastole (i.e., during atrial relaxation), right atrial volume is minimal, but pericardial pressure is maximal, causing the right atrium to buckle. Right atrial collapse, especially when it persists for more than one third of the cardiac cycle, is a highly sensitive but less specific sign for tamponade. Early diastolic collapse of the right ventricle (usually occurs in early diastole when the ventricular volume is still low) is present when the intrapericardial pressure exceeds the right ventricular pressure and is a highly specific sign for tamponade. Right ventricular collapse may not occur when the right ventricle is hypertrophied, or its diastolic pressure is greatly elevated. Left atrial collapse is seen in nearly 25% of patients and is specific for tamponade. Left ventricular collapse is less common because the wall of the left ventricle is more muscular. Dilation of the inferior vena cava with lack of inspiratory collapse (usually <50% reduction in its diameter) and swinging of the heart also are seen in patients with pericardial tamponade. Doppler echocardiography provides direct assessment of the ventricular filling patterns in pericardial tamponade.11,12,17,18 Patients with pericardial tamponade have a marked increase in tricuspid and pulmonary valve flow velocities and a marked decrease in mitral and aortic valve flow velocities during inspiration compared with normal subjects and patients with effusions but not tamponade. Changes in left atrial inflow pattern and exaggerated respiratory variations in pulmonary venous flow velocity also are observed. In one study aiming to correlate clinical and echocardiographic findings prospectively, the highest specificity (98%) was seen in patients with right atrial and right ventricular collapse plus abnormal venous flow.19 The sensitivity and specificity of any chamber collapse were 90% and 65%.19
Catheter-Based Diagnostic and Therapeutic Strategies
Cardiac catheterization historically has been the standard diagnostic modality for cardiac tamponade. Right-sided heart catheterization can confirm the significance of a pericardial effusion and allows evaluation of hemodynamic changes occurring after pericardiocentesis. It usually shows two major findings in patients with pericardial tamponade: (1) elevation and equilibration of intracardiac diastolic pressures (usually 10 to 30 mm Hg) and (2) inspiratory increase in right-sided pressures and reduction in left-sided pressures (ventricular disconcordance), which are responsible for the presence of a pulsus paradoxus (Box 6.3).8 With equalization of intrapericardial pressures, the mean right atrial, left atrial, diastolic pulmonary artery, and right and left ventricular end-diastolic pressures all are within 5 mm Hg of each other. In addition to producing elevation in the central venous pressure, cardiac tamponade produces characteristic changes in the waveforms of the hemodynamic tracings. With increasing severity of cardiac tamponade, the “y descent” and the early diastolic dip in the ventricular pressure tracings are gradually obliterated and eventually disappear. The absence of the y descent in the right atrial tracing is an important finding in pericardial tamponade. As pericardial fluid is removed, the intrapericardial pressure usually returns to the intrapleural pressure level, and the right atrial waveform normalizes with the reappearance of the diastolic y descent. If the right atrial pressure remains elevated after the pericardiocentesis, however, and a prominent y descent appears, the diagnosis of effusive-constrictive disease must be considered.20 Although the latter condition is infrequent, it may be missed in some patients presenting with tamponade in whom it usually causes significant morbidity until they undergo surgical epicardiectomy. Pulsus paradoxus is another hallmark of pericardial tamponade; however, it may be absent in many conditions, or alternatively may be present in patients without cardiac tamponade, as previously stated.
Pericardiocentesis
Indications
When the diagnosis of pericardial effusion has been made, it is important to determine whether the effusion is creating significant hemodynamic compromise. Many asymptomatic patients with large effusions do not require pericardiocentesis if they have no hemodynamic compromise, unless there is a need for fluid analysis for diagnostic purposes. In a prospective long-term follow-up of large idiopathic chronic pericardial effusion (up to 20 years), Sagrista-Sauleda and colleagues21 concluded that large idiopathic chronic pericardial effusions were usually well tolerated for long periods in most patients with severe tamponade; however, they may develop unexpectedly at any time. Although pericardiocentesis was effective in resolving these effusions, recurrences were common, prompting the authors to recommend referral of these patients for pericardiectomy when recurrence occurs.21 When cardiac tamponade occurs, the emergency drainage of pericardial fluid by pericardiocentesis is a lifesaving therapy in a patient who would otherwise develop pulseless electrical activity and cardiac arrest.
Technique
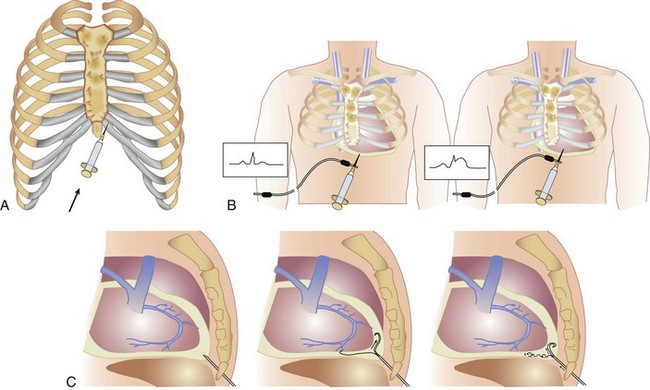
Pericardiocentesis is most commonly performed via a subxiphoid approach under ECG and fluoroscopy guidance (Fig. 6.2A). Traditionally, pericardiocentesis has been performed in the cardiac catheterization laboratory with arterial and right-sided heart pressure monitoring. Today the procedure also is performed in the noninvasive laboratory, intensive care units, or even at the bedside under echocardiographic guidance.22,23 Whichever modality is used, it is a safe procedure when performed by appropriately trained personnel.
Pericardiocentesis is a procedure based on the Seldinger technique of percutaneous catheter insertion. After the administration of local anesthesia (1% to 2% lidocaine) to the skin and deeper tissues of the left xiphocostal area, the pericardial needle is connected to an ECG lead. The needle is advanced from the left of the subxiphoid area while aiming toward the left shoulder (usually under fluoroscopic or echocardiographic guidance; however, blinded procedures are undertaken in cases of extreme emergencies). Often, a discrete pop is felt as the needle enters the pericardial space. ST-segment elevation is seen on the ECG lead tracing when the needle touches the epicardium and helps confirm the needle position (see Fig. 6.2B). The needle should be withdrawn slightly until the ST-segment elevation disappears. When the pericardial space is entered, a stiff guidewire is introduced into the pericardial space through the needle, which is thereafter removed, and a catheter is inserted into the pericardial sac over the guidewire (see Fig. 6.2C). The drainage catheter used (often a pigtail catheter, denoting its shape) has an end hole and multiple side holes. Intrapericardial pressure is measured by connecting a pressure transducer system to the intrapericardial catheter. Pericardial fluid is then removed. Samples of pericardial fluid should be sent for appropriate biochemical, cytologic, bacteriologic, and immunologic analyses to assist in the diagnosis of the cause of the effusion (the first sample is usually reserved for microbiologic studies).
Complications
Echocardiographically guided pericardiocentesis is a safe and effective technique.23,24 In a series of 1127 therapeutic echocardiographically guided pericardiocenteses performed in 977 patients at the Mayo Clinic between 1979 and 1998, the procedural success rate was 97% overall, with a total complication rate of 4.7%.24 Echocardiography allows identification of the ideal site of needle entry and trajectory and is especially useful in patients with loculated effusions. In contrast to pericardiocenteses performed in the cardiac catheterization laboratory, the left chest wall, rather than the subcostal, approach is often used with echocardiographically guided pericardiocenteses.
Prevention of Recurrent Tamponade
For many patients with pericardial effusion and tamponade, standard percutaneous pericardial drainage with an indwelling pericardial catheter is sufficient to avoid recurrence of pericardial effusion and tamponade. Patients who continue to drain more than 100 mL/24 hours 3 days after standard catheter drainage should be considered for more aggressive therapy. Reaccumulation of the pericardial fluid is particularly common in patients with malignant pericardial effusions. Additional therapeutic approaches are available to prevent pericardial fluid reaccumulation, including intrapericardial instillation of sclerosing agents, use of chemotherapy, radiotherapy, percutaneous balloon pericardial window, and surgical intervention.25–28 Reaccumulation of fluid with recurrence of cardiac tamponade has been considered a definitive indication for an open surgical pericardial window or for percutaneous balloon pericardiotomy.29
Percutaneous Balloon Pericardiotomy
Patients with a malignant pericardial effusion and tamponade are likely to be suboptimal surgical candidates because of their overall poor health conditions and limited life expectancies. Palacios and colleagues29,30 pioneered at Massachusetts General Hospital in Boston the technique of percutaneous balloon pericardial window (also called percutaneous balloon pericardiotomy) as an alternative and less invasive technique to surgical pericardial window. With this modality, adequate drainage of pericardial effusion is performed, and a pericardial window is created percutaneously under fluoroscopic guidance using a balloon-dilation catheter. The technique of percutaneous pericardial window is relatively simple and safe and is performed in the catheterization laboratory under local anesthesia with minimal discomfort. Conscious sedation with intravenous narcotics and a short-acting benzodiazepine is generally used.
Percutaneous Balloon Pericardiotomy Technique
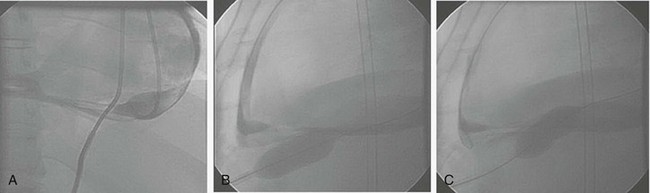
Percutaneous balloon pericardial window is offered as an alternative technique to the surgical pericardial window procedure for patients with persistent drainage from their indwelling intrapericardial catheter (≥3 days of >100 mL/24 hours drainage) or as primary therapy at the time of initial pericardiocentesis. The subxiphoid area around the indwelling pigtail pericardial catheter is infiltrated with local anesthesia (1% to 2% lidocaine). A small amount (5 to 10 mL) of iodinated contrast agent is injected in the pericardial space to help outline the parietal pericardium (Fig. 6.3A). A 0.038-inch stiff guidewire with a preshaped curve at the tip is advanced through the pigtail catheter into the pericardial space. The catheter is removed, leaving the guidewire in the pericardial space. After predilation of the skin and subcutaneous tissue along the track of the wire using a 10F dilator, a 20-mm-diameter × 3-cm-long balloon-dilation catheter (Boston Scientific, Watertown, MA) is advanced over the guidewire and positioned to straddle the parietal pericardium. Care should be taken to advance the proximal end of the balloon beyond the skin and the subcutaneous tissue to avoid dilation of the skin and subcutaneous tissue (and the resultant formation of a pericardial-cutaneous fistula) (see Fig. 6.3B). The balloon is inflated manually until the waist produced by the parietal pericardium disappears (see Fig. 6.3C). Biplane fluoroscopy is helpful to ascertain the correct position of the balloon straddling the parietal pericardium with the left lateral projection being particularly useful (see Fig. 6.3B and C). Two to three inflations are usually performed to have adequate opening of the parietal pericardium. The balloon-dilation catheter is removed, leaving the stiff guidewire in the pericardial space, where a new pigtail catheter is advanced over it and left indwelling in the pericardial space.
Outcome Data After Percutaneous Balloon Pericardiotomy
Palacios and colleagues29 reported the first human experience with the technique of percutaneous balloon pericardiotomy in eight patients with malignant pericardial effusion and tamponade. The technique was successful in all patients with no immediate or late procedure-related complications. The mean time to radiologic development of a new or a significantly increased pleural effusion was 2.9 ± 0.4 days (range 2 to 5 days). No patient developed recurrence of the pericardial effusion or tamponade at a mean follow-up of 6 ± 2 months (range 1 to 11 months). Five patients died from their primary malignancy at 1, 4, 9, 10, and 11 months. A success rate of 87% was reported in the multicenter percutaneous balloon pericardial window registry, which enrolled 130 patients between 1987 and 1994 in 16 centers.31,32 In this registry, three patients sustained pericardial bleeding and were considered to have a failed procedure and ended up undergoing surgical window procedures. Eight patients had recurrence of pericardial effusion (mean time to recurrence 54 ± 65 days), of whom seven ended up having surgical window procedures (with recurrence occurring in four of those patients).
Complications
Minor complications occurred in 13% of the patients.31,32 The development of a large pleural effusion remains the major concern after percutaneous balloon pericardial window. Most patients develop a left pleural effusion within 24 to 48 hours of the procedure, which in most cases resolves spontaneously (presumably owing to the greater resorption capacity of the pleural surface). Thoracocentesis or chest tube placement was required in 15% of patients with preexisting pleural effusions compared with 9% of patients without preexisting pleural effusions.31,32 It is desirable to aspirate most of the pericardial fluid before creating the window to limit the potential volume of fluid that can immediately leak to the pleural space. When the preprocedure chest radiograph reveals a large pleural effusion, the chance of requiring thoracentesis subsequent to the percutaneous pericardial window is higher, and the procedure should be performed only when its benefits outweigh the risks of thoracentesis or chest tube placement or both. It is not advised to perform the procedure in patients with marginal pulmonary reserve, as in postpneumonectomy patients, because the development of a pleural effusion may significantly compromise their respiratory function. Finally, an increased risk of bleeding from the pericardiotomy site occurs in patients with platelet or coagulation abnormalities. In these patients, a surgical procedure under direct visualization may be safer. Thoracoscopic techniques were developed to create a larger pericardial window and carry lower morbidity rates compared with open surgical techniques.33 This technique allows adequate long-term drainage and the ability to obtain specimens for pathologic analysis.33
Other Pericardial Interventions
Percutaneous pericardial biopsy (PPB) is a relatively safe and feasible technique in the cardiac catheterization laboratory, and it can increase the diagnostic yield of pericardiocentesis and pericardial fluid analysis. One clinical scenario in which PPB may prove to be of particular importance is in the setting of tuberculous pericardial effusion because Mycobacterium tuberculosis is rarely cultured and a positive acid-fast stain is infrequently obtained from the pericardial fluid (which makes tuberculosis, unlike malignant pericarditis, a commonly missed diagnosis without the technique of PPB). PPB is also less invasive than surgical biopsy, and can be easily modified to obtain tissue samples from pericardial masses. PPB was initially described in 1988 by Endrys and colleagues,34 who reported a series of 18 patients undergoing pericardial biopsy using an endomyocardial bioptome, and showed that PPB can be safely performed using conventional invasive cardiology techniques. Margey and colleagues35 reported a case series of seven patients undergoing PPB for pericardial effusion, in whom they obtained a total of five biopsy specimens per patient with no complications. They demonstrated that pericardial biopsy adds incremental diagnostic value to the analysis of pericardial fluid alone, as it confirmed the absence of malignant invasion in four patients with neoplastic disease and the presence of lymphocytic and organizing effusive pericarditis in one and two patients, respectively.35 Catheter-based intervention techniques in the pericardial space gained further momentum after a number of patients undergoing arrhythmia catheter ablation were found to have epicardial foci for the arrhythmia that could only be approached from the pericardial surface. Cardiologists also recognized that the pericardial space could be safely approach percutaneously even without a large collection of pericardial fluid present. Epicardial ablation has therefore become a therapeutic strategy for epicardial scar-related reentry, which is recognized as an important cause of ventricular tachycardia (especially in patients with nonischemic cardiomyopathy). Sosa and colleagues36 were the first to show that the pericardial space can be safely entered with a blunt-tipped needle via a subxiphoid approach under fluoroscopic guidance. One should be aware that pericardial effusion and tamponade can occur as a result of these novel pericardial interventions.
References
1. Sagrista-Sauleda, J, Merce, J, Permanyer-Miralda, G, et al. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000; 109:95–101.
2. Troughton, RW, Asher, CR, Klein, AL. Pericarditis. Lancet. 2004; 363:717–727.
3. Lange, RA, Hillis, LD. Clinical practice: Acute pericarditis. N Engl J Med. 2004; 351:2195–2202.
4. Corey, GR, Campbell, PT, Van Trigt, P, et al. Etiology of large pericardial effusions. Am J Med. 1993; 95:209–213.
5. Atar, S, Chiu, J, Forrester, JS, et al. Bloody pericardial effusion in patients with cardiac tamponade: Is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999; 116:1564–1569.
6. Spodick, DH. Pathophysiology of cardiac tamponade. Chest. 1998; 113:1372–1378.
7. Fowler, NO. Cardiac tamponade: A clinical or an echocardiographic diagnosis? Circulation. 1993; 87:1738–1741.
8. Spodick, DH. Acute cardiac tamponade. N Engl J Med. 2003; 349:684–690.
9. Shabetai, R, Fowler, NO, Fenton, JC, et al. Pulsus paradoxus. J Clin Invest. 1965; 44:1882–1898.
10. Bruch, C, Schmermund, A, Dagres, N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: Reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001; 38:219–226.
11. Maisch, B, Seferovic, PM, Ristic, AD, et al. Guidelines on the diagnosis and management of pericardial diseases: Executive summary. The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004; 25:587–610.
12. Cheitlin, MD, Armstrong, WF, Aurigemma, GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003; 108:1146–1162.
13. Feigenbaum, H, Waldhausen, JA, Hyde, LP. Ultrasound diagnosis of pericardial effusion. JAMA. 1965; 191:711–714.
14. Moss, AJ, Bruhn, F. The echocardiogram: An ultrasound technic for the detection of pericardial effusion. N Engl J Med. 1966; 274:380–384.
15. Prakash, AM, Sun, Y, Chiaramida, SA, et al. Quantitative assessment of pericardial effusion volume by two-dimensional echocardiography. J Am Soc Echocardiogr. 2003; 16:147–153.
16. George, S, Salama, AL, Uthaman, B, et al. Echocardiography in differentiating tuberculous from chronic idiopathic pericardial effusion. Heart. 2004; 90:1338–1339.
17. Appleton, CP, Hatle, LK, Popp, RL. Cardiac tamponade and pericardial effusion: Respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol. 1988; 11:1020–1030.
18. Schutzman, JJ, Obarski, TP, Pearce, GL, et al. Comparison of Doppler and two-dimensional echocardiography for assessment of pericardial effusion. Am J Cardiol. 1992; 70:1353–1357.
19. Merce, J, Sagrista-Sauleda, J, Permanyer-Miralda, G, et al. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: Implications for the diagnosis of cardiac tamponade. Am Heart J. 1999; 138:759–764.
20. Sagrista-Sauleda, J, Angel, J, Sanchez, A, et al. Effusive-constrictive pericarditis. N Engl J Med. 2004; 350:469–475.
21. Sagrista-Sauleda, J, Angel, J, Permanyer-Miralda, G, et al. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999; 341:2054–2059.
22. Ristic, AD, Seferovic, PM, Maisch, B. Management of pericardial effusion the role of echocardiography in establishing the indications and the selection of the approach for drainage. Herz. 2005; 30:144–150.
23. Lindenberger, M, Kjellberg, M, Karlsson, E, et al. Pericardiocentesis guided by 2-D echocardiography: The method of choice for treatment of pericardial effusion. J Intern Med. 2003; 253:411–417.
24. Tsang, TS, Enriquez-Sarano, M, Freeman, WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002; 77:429–436.
25. Shepherd, FA, Morgan, C, Evans, WK, et al. Medical management of malignant pericardial effusion by tetracycline sclerosis. Am J Cardiol. 1987; 60:1161–1166.
26. Davis, S, Sharma, SM, Blumberg, ED, et al. Intrapericardial tetracycline for the management of cardiac tamponade secondary to malignant pericardial effusion. N Engl J Med. 1978; 299:1113–1114.
27. Vaitkus, PT, Herrmann, HC, LeWinter, MM. Treatment of malignant pericardial effusion. JAMA. 1994; 272:59–64.
28. Palatianos, GM, Thurer, RJ, Kaiser, GA. Comparison of effectiveness and safety of operations on the pericardium. Chest. 1985; 88:30–33.
29. Palacios, IF, Tuzcu, EM, Ziskind, AA, et al. Percutaneous balloon pericardial window for patients with malignant pericardial effusion and tamponade. Cathet Cardiovasc Diagn. 1991; 22:244–249.
30. Ziskind, AA, Pearce, AC, Lemmon, CC, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: Description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993; 21:1–5.
31. Ziskind, AA. Final report of the percutaneous balloon pericardiotomy registry for the treatment of effusive pericardial disease. Circulation. 1994; 90:1–121.
32. Ziskind, AA, Rodriguez, S, Lemmon, C, et al. Percutaneous pericardial biopsy as an adjunctive technique for the diagnosis of pericardial disease. Am J Cardiol. 1994; 74:288–291.
33. Ozuner, G, Davidson, PG, Isenberg, JS, et al. Creation of a pericardial window using thoracoscopic techniques. Surg Gynecol Obstet. 1992; 175:69–71.
34. Endrys, J, Simo, M, Shafie, MZ, et al. New nonsurgical technique for multiple pericardial biopsies. Catheter Cardiovasc Diagn. 1988; 15(2):92–94.
35. Margey R, Suh W, Witzke C, et al: Percutaneous pericardial biopsy: A novel interventional technique to aid diagnosis and management of pericardial disease. Paper presented at Transcatheter Cardiovascular Therapeutics (TCT) Meeting, Sept. 2010, Washington, DC.
36. Sosa, E, Scanavacca, M, d’Avila, A, et al. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996; 7(6):531–536.