Chapter 61 Pediatric Retinal Vascular Diseases
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Retinopathy of prematurity
Historical perspective
Early history
ROP was first described in 1942,1,2 and quickly became the primary cause of childhood blindness throughout the developed world.3 Terry’s original reports designated the condition retrolental fibroplasia (RLF), based on his impression that it involved a proliferation of the embryonic hyaloid system, but Owens and Owens4 found that the hyaloid system was normal at birth and that RLF developed postnatally. As the pathogenesis and clinical manifestations became better understood, the term “retinopathy of prematurity” was adopted.
The discovery of the relationship between supplementary oxygen and ROP in the 1950s5–9 led to rigid curtailment of oxygen supplementation in the nursery, and a dramatic decrease in ROP incidence followed. Unfortunately this had an adverse effect on infant morbidity and mortality (e.g., respiratory distress syndrome, cerebral palsy, neurologic disorders).10–12
Retinopathy of prematurity and contemporary nursery practices
By the early 1970s, arterial blood gas analysis had come into general use, and the oxygen requirements of premature infants with respiratory distress syndrome were better documented.13 This enabled pediatricians to titrate the incubator oxygen concentration to more nearly meet the individual premature infant’s oxygen needs.
Modern transcutaneous oxygen monitoring and continuous pulse oximetry have provided additional noninvasive tools, allowing neonatologists to monitor babies in real time more closely. These have stimulated studies, including large mutlicenter oygen restriction trials, which have found that lower target oxygen saturation levels (e.g., 85–89%) correlate with significantly improved rates of severe ROP but are also associated with increased mortality rates.14–16 At this time, general consensus regarding target oxygen saturation levels that balance ROP risk and mortality has not been reached.
With improvements in neonatology practice, more of the smallest premature infants are now surviving. Eight percent of low-birth-weight infants survived in 1950, but with ventilators, surfactant, intravenous nutrition, and other gains in knowledge, survival has risen to 37–72%.17–19 Clearly, infants are surviving today with more immature retinal vasculature and therefore higher ROP risk. For this reason, and because of common pathophysiology with other proliferative vascular diseases such as diabetic retinopathy, there has been mounting interest in ROP.20–22
The role of oxygen
Clinical findings
Results of controlled nursery studies5,8 that suggested supplementary oxygen to be the principal cause of ROP in the epidemic of the early 1950s were confirmed, and the role of prolonged oxygen was documented in a collaborative randomized controlled trial.9
Since that time, attempts to delineate the critical blood oxygen levels producing ROP have not resulted in definitive conclusions. In a prospective study of 589 infants monitored by intermittent blood gas measurements, and where clinical goals were to avoid elevated arterial oxygen, the occurrence of ROP was not related to arterial oxygen levels.23 Only the duration of oxygen exposure was a risk factor. Somewhat unexpectedly, continuous transcutaneous monitoring of blood oxygen levels was found in one study to be of no more value than intermittent monitoring in preventing visual disability.24 It was suggested by Lucey and Dangman25 that therapeutic oxygen, although important, has been overemphasized as a cause of ROP in contemporary neonatal practice.* They emphasized that other factors related to very low birth weight are also important, especially in view of current nursery oxygen monitoring.
Recent studies, including large mutlicenter oygen restriction trials, have found that lower target oxygen saturation levels (e.g., 85–89%) correlate with significantly improved rates of severe ROP, but are associated with increased mortality rates.14–16 At this time, a consensus about optimal target oxygen saturation levels has not been reached.
Experimental findings
In the early 1950s the laboratory kitten model, which produced lesions resembling the early stages of human ROP, was used extensively because it demonstrated selective response to oxygen by the immature retinal vessels.6,7 In the full-term newborn kitten, the immature retinal vascularization is comparable to that of a human fetus at 6 months’ gestation, thus providing the unique opportunity to study the response to oxygen of the immature retina, albeit in a full-term, healthy animal.3 When hyperoxia studies were extended to other animal models, such as the young mouse and puppy,6 the general concept of oxygen toxicity to immature retinal vessels was reinforced.
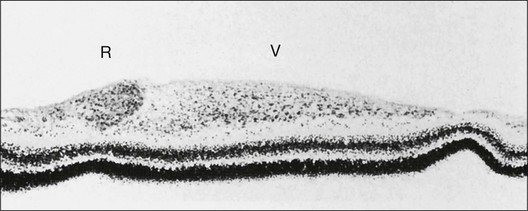
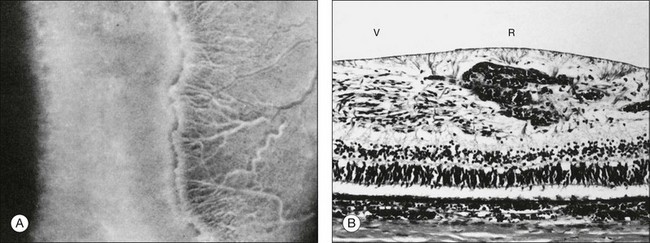
Investigators26,27 have pointed out the histologic differences in the retinas of these animal models from the human but were unable to explain why progression to retinal detachment did not occur. It is noteworthy that McLeod et al. reported the production of intravitreal neovascularization with traction retinal folds in young dogs exposed to hyperoxia.28 These findings add to the potential application of the canine model to investigate these stages of ROP (Figs 61.1 and 61.2 online).
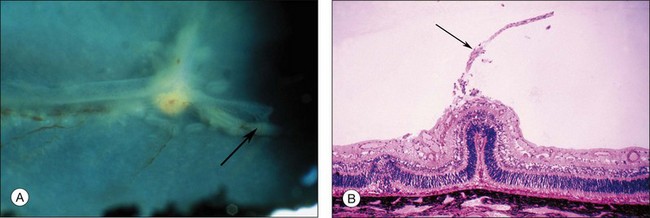
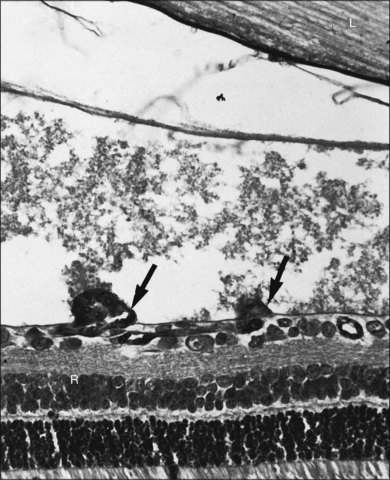
Fig. 61.2 (A) Gross appearance of the areas of neovascularization extending temporally and two smaller areas in the same 45-day-old dog shown in Figure 61.1. A denser 2 × 0.5 mm area is present inferior nasally (arrow). (B) Area from A (marked by arrow) discloses retinal neovascularization (arrow) attached to the apex of a retinal fold (periodic acid–Schiff and hematoxylin; ×125).
The hyperoxic animal models demonstrated that only the incompletely vascularized retina was susceptible to oxygen’s adverse effect, and that the more immature the vascularization, the greater the pathologic response to oxygen.29 These findings supported the clinical observation that the infant with a less mature retina has greater ROP susceptibility, and that the infant with a fully vascularized retina has no risk. Accordingly, the temporal retina, the last part to vascularize, remains susceptible to ROP the longest (Fig. 61.3 online).
Mechanism of oxygen’s effects on the immature retina
Primary stage of retinal vasoconstriction and vascular occlusion
The primary effect of elevated blood oxygen in any retina is vasoconstriction, which, if sustained, is followed by some degree of vascular closure. In young kittens, initial vasoconstriction occurs within several minutes after oxygen exposure. Vascular caliber is reduced by approximately 50% initially, but then rebounds to its original dimensions. Continued oxygen exposure results in gradual vasospasm during the next 4–6 hours, until the vessels are approximately 80% constricted.30 At this stage, constriction is still reversible. However, if significantly elevated arterial oxygen partial pressure levels persist for an additional period (e.g., 10–15 hours), some immature peripheral vessels are permanently occluded.7,23
This occlusion progresses as the duration of hyperoxia increases, and local vascular obliteration is complete after 2–3 days of exposure. In the dog, after 4 days of exposure to hyperoxia, most capillaries are lost and only major blood vessels survive.31
Electron microscopic observations demonstrate selective hyperoxic injury to the endothelial cells of the most immature vessels, without obvious changes in the neuronal elements of the retina.32
Secondary stage of retinal neovascularization
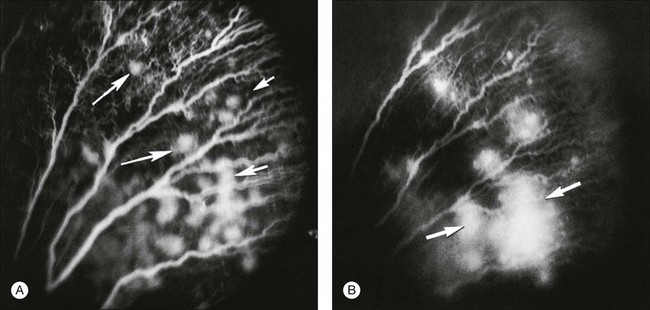
After removal of the laboratory animal to ambient air following sustained hyperoxia, marked endothelial proliferation arises from the residual vascular complexes adjacent to retinal capillaries ablated during hyperoxia (Fig. 61.4 online). This can be demonstrated on fluorescein angiography (Fig. 61.5). Nodules of proliferating endothelial cells canalize to form new vessels that not only grow within the retina, but also erupt through the internal limiting membrane to grow on its surface, similar to the neovascularization in other proliferative retinopathies (Figs 61.6–61.9). In the dog and cat, the initial preretinal neovascular formations are like angioblastic masses with few lumens (formations sometimes called “popcorn”), which mature into neovascular formations that include vessels invested with pericytes.33,34 Although the neovascularization may be extensive, this is generally the maximum response to oxygen in the kitten model and is followed by progressive vascular remodeling and involution of abnormalities. The preretinal neovascularization in the dog persists and can develop into tented membranes which create tractional retinal folds in the retina (Figs 61.1 and 61.2).28 The mouse and rat preretinal neovascular formations, however, will regress after 5 days.30–35–37 Although regression is rapid and spontaneous in mice, the mouse model has been useful in evaluating topical38 and systemic drugs,39,40 experimental gene therapy strategies41,42 and endogenous inhibitors like pigment epithelial growth factor.43
Oxygen exerts an important effect on the remodeling of the original primitive capillary network that develops in the retina.44 Capillaries regress from areas of higher oxygen concentration and grow toward areas of lower oxygen. Penn et al.45 used experimentally alternating periods of high and low oxygen in the rat pup model to produce a more proliferative form of retinopathy. Pierce and colleagues21 used hyperoxia and hypoxia in a mouse pup model to demonstrate the correlation of vascular endothelial growth factor (VEGF) protein production with periods of low oxygen, and its disappearance during oxygenation.
Pathogenesis
Normal retinal vasculogenesis
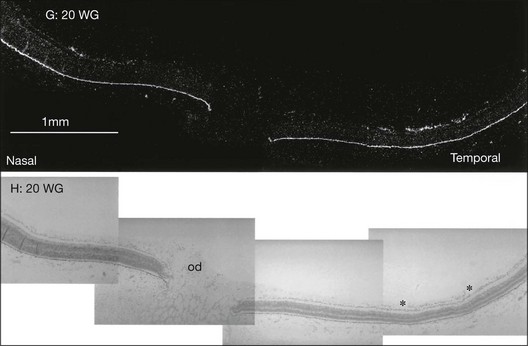
It is appropriate to review normal retinal vascular development as background for understanding ROP pathogenesis. Michaelson33 originally suggested that retinal capillaries arise by budding from pre-existent arteries and veins that originate from the hyaloid vessels at the optic nerve head. Cogan34 proposed a similar mechanism, except for the hypothesis of budding of solid endothelial cords from hyaloid vessels. Ashton37 suggested that mesenchyme, the blood vessel precursor, grows from the optic disc through the nerve fiber layer to the periphery of the retina. Mesenchymal precursors have recently been observed far in advance of formed blood vessels in human fetal retinas.46 On the posterior edge of the advancing mesenchyme, a “chicken-wire” meshwork of capillaries develops, and undergoes absorption and remodeling to produce mature retinal arteries and veins that are surrounded by the capillary meshwork.37,44 Variations in capillary development may be species-specific. However, across all species studied to date, VEGF appears to be a key factor guiding vascular growth, which most closely fits the identity of Michaelson’s proposed “factor X.”33 In the kitten, Chan-Ling and Stone demonstrated the role of astrocytes leading to growth of the capillary network.47–49 Provis et al.50 demonstrated the expression of VEGF message in the predicted location in the developing normal human retina, just anterior to the developing vessels (Fig. 61.10).
Figure 61.11 shows the normal rate of progression of the retinal vessels into the far retinal periphery in human premature infants without ROP according to their postconceptional age (gestational age at birth plus chronologic age). More than 80% of prematurely born infants have been observed to develop this relatively mature retinal vasculature by the time they reach full term.
Pathogenesis of ROP
Initial changes in the developing vessels are described above, and historically this was believed to be an injury initiated by “excess” oxygen. Alon et al. demonstrated that hyperoxia caused downregulation of VEGF and death of endothelial cells, suggesting that VEGF is an endothelial survival factor.51 In the time that follows closure of these growing vessels, the differentiating retina becomes increasingly ischemic and hypoxic and VEGF is upregulated52,53 driving the neovascularization.48
Theoretically, the provision of increased oxygen should downregulate the release of such growth factor(s) and permit neovascularization to remodel and regress in an orderly fashion. Szewczyk54 proposed this, and treated infants with significant ROP by returning them to oxygen. With no controls, it is difficult to know from his report if this success was simply due to spontaneous involution of ROP. This hypothesis was tested in the kitten model of oxygen-induced retinopathy. Systemic mild hypoxia was found to worsen the retinopathy,55 whereas mild hyperoxia improved it.56 With National Institutes of Health sponsorship, the multicenter Supplemental Therapeutic Oxygen for Prethreshold ROP (STOP-ROP) trial, chaired by Dale L. Phelps, found that, once the ROP was established, raising the oxygen saturation mildly did not harm the ROP, but neither was it of clear benefit.57
The clinical and histopathologic observations of Flynn and coworkers58–62 led them to postulate the following sequence of events in human ROP pathogenesis:
1. Endothelial injury occurs where it has just differentiated from mesenchyme to form the primitive capillary meshwork. This is reminiscent of animal studies in which a short duration of hyperoxia resulted in capillary damage limited to the most recently differentiated vascular complexes (Fig. 61.4A). It is currently believed that environmental factors other than oxygen also are involved. For example, Brooks and associates found that nitric oxide may contribute to the vaso-obliterative stage of ROP,63 while Alon et al. found that reduced VEGF may result in death of endothelial cells51 because of its role as a survival factor.
2. After this injury to the vascular endothelium, the mesenchyme and mature arteries and veins survive and merge via the few remaining vascular channels to form a mesenchymal arteriovenous shunt which replaces the destroyed or damaged capillary bed.
3. The mesenchymal arteriovenous shunt is located at the demarcation between the avascular and vascularized retina. It consists of a nest of primitive mesenchymal and maturing endothelial cells that are fed by mature arteries and veins. No capillaries are found in the region of the shunt. Flynn60 suggested that this structure represents the pathognomonic lesion of acute ROP.
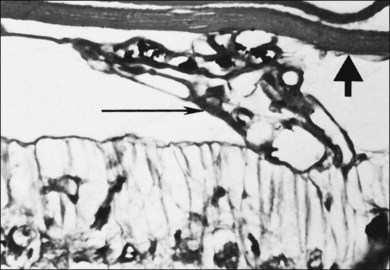
Flynn described a dormant period after the injury (days to months), during which retinal findings are relatively stable. The tissues comprising the shunt may thicken, and the gray-white initial color of the structure turns from pink to salmon to red. He stated: “during this period when vasculogenic activity resumes in the retina, the fate of the eye is decided.”60 Flynn pointed out that when the cells inside the shunt divide and differentiate into normal capillary endothelium, they form primitive endothelial tubes that send forth a brush border of capillaries that grows anteriorly into the avascular retina. This represents ROP involution, which he observed to occur in more than 90% of cases at this early stage. In progressive disease, however, the primitive cells inside the shunt proliferate and erupt through the internal limiting membrane, growing on the surface of the retina and into the vitreous body. Flynn stated: “it is this lack of differentiation and destructive proliferation of cells and their invasion into spaces and tissues where they do not belong that is the chief event in the process of membrane proliferation leading to traction detachment.”60
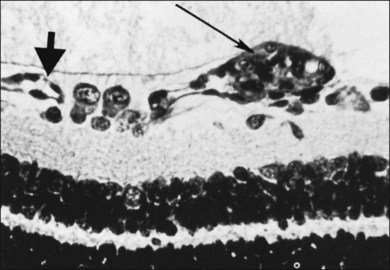
Foos64–66 suggested a pathogenesis of ROP based on examination of histopathologic material. He used the terms “vanguard” and “rearguard” to describe cellular components of the developing retina. The vanguard (anterior) component contains spindle-shaped cells thought to be glia, which play a role in nourishing the immature retina during development.67 The rearguard contains primitive endothelial cells. As the retina matures, the endothelial cells aggregate into cords that, according to Foos,66 subsequently lumenize and become the primordial capillaries of the retina. It is from the rearguard and primitive endothelial cells that neovascularization of ROP develops (Figs 61.12 and 61.13). Foos noted that, as the developing vasculature reaches its most anterior extent and matures, the spindle cells of the vanguard disappear. The work of Chan-Ling et al.,46 McLeod et al.,68 and Provis et al.50 showed that spindle cells are endothelial precursors and, in fetal human and neonatal dog retina,68 the precursors organize and differentiate to form the initial retinal vasculature.46
International classification
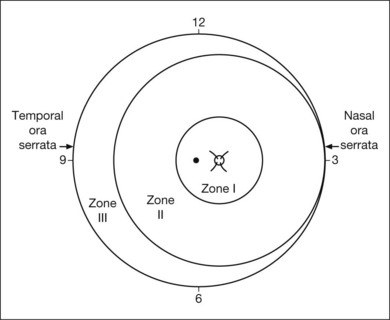
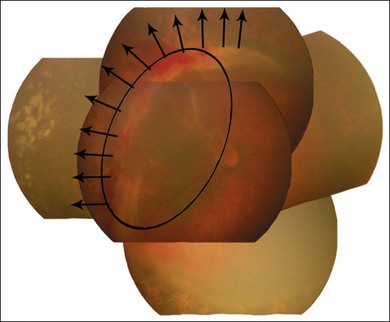
The international classification of ROP divided the retina into three anteroposterior zones and describes the extent of disease by the 30° meridians (clock-hours) involved (Fig. 61.14). Retinal changes are divided into stages of severity, based on descriptive and photographic standards.69
Zones of involved retina
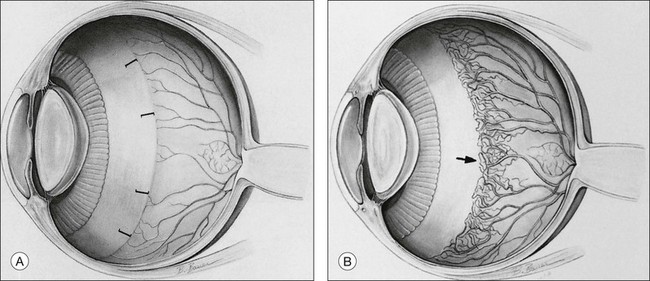
Each of the three zones of the retina is centered on the optic disc (Fig. 61.14). Zone I includes the posterior pole, and is defined as a circle, centered on the disc, whose radius is twice the distance from the disc to the macula. It subtends an arc of about 60° (Fig. 61.15). Zone II extends from the peripheral border of zone I to a concentric circle tangential to the nasal ora serrata. Temporally, this boundary corresponds approximately to the anatomic equator. Once the nasal vessels have reached the ora serrata, zone III is the remaining temporal crescent of retina anterior to zone II. Zone III, which is the farthest from the disc, is the last zone to become vascularized. It is clinically important to continue classifying ROP as zone II if there remains any active ROP or immature vessels in the nasal retina.
Extent of retinopathy of prematurity
The extent of the ROP changes is described according to the 12 30° sectors involved, labeled as hours of the clock (Fig. 61.14): the nasal side of the right eye is at 3:00, and the nasal side of the left eye is at 9:00.
Staging
Abnormal peripheral changes are divided into three stages, which may progress to retinal detachment (stages 4–5) (Figs 61.16 and 61.17).
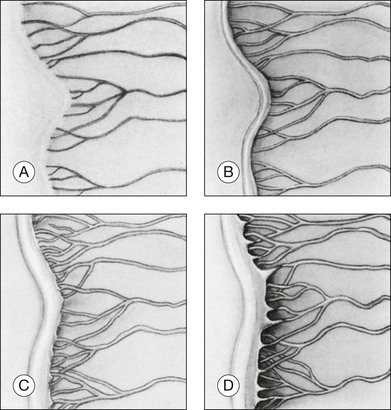
Stage 1: demarcation line
Stage 1 is characterized by the presence of a demarcation line, the first ophthalmoscopic sign of ROP (Fig. 61.16A). This represents a structure separating the anterior, avascular retina from the posterior, vascularized retina. It appears flat and white, and lies within the plane of the retina. Abnormal branching or arcading of vessels leads up to the line. Stage 1 is relatively evanescent, generally either progressing to stage 2 or involuting to normal vascularization within several weeks. According to Garner,70 the stage 1 demarcation line morphologically comprises two relatively distinct zones. The more anterior vanguard zone is formed by a mass of spindle-shaped cells, which are the progenitors of the differentiated vascular endothelium. As such it corresponds to the primitive mesenchyme (spindle cells) seen in normal fetal development but with a considerable increase in the number of cells. It is this hyperplasia, involving both thickening and widening, that makes the demarcation line visible.70
Stage 2: ridge
In stage 2, the demarcation line has grown into a ridge with height and width, which extends centripetally within the globe (Fig. 61.16B). The ridge may be white or pink and, rarely, vessels may even leave the surface of the retina to enter it. Small tufts of new vessels (“popcorn” lesions) may be seen located posterior to the ridge structure but not attached to it. The absence of fibrovascular growth from the surface of the ridge separates this stage from stage 3. According to Garner, the stage 2 retinal ridge results from the proliferation of endothelial cells “with some evidence of organization into recognizable vascular channels.”70 Flynn et al.61 demonstrated that in this stage these channels leak fluorescein on angiographic examination.
Stage 3: ridge with extraretinal fibrovascular proliferation
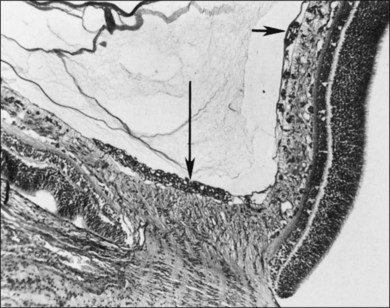
Stage 3 is characterized by the addition of extraretinal, fibrovascular tissue proliferating from the former ridge (Fig. 61.16C and D). This proliferating tissue is localized continuous with the posterior and interior aspect of the ridge, causing a ragged appearance of the ridge as proliferation increases into the vitreous. As in stage 2, vessels may leave the surface of the retina to enter the ridge and could be mistaken for retinoschisis or even detachment. The presence of elevated retinal vessels coursing from the retinal surface to the height of the ridge does not alone constitute a retinal detachment;69 however, this may signify presence of vitreous traction. According to Foos,66 the stage 3 “extraretinal vascularization” may appear placoid, polypoid, or pedunculated on histological examination. The placoid pattern is the most common and also the most important because it correlates with subsequent development of retinal detachment. Foos demonstrated that these extraretinal vessels are apparently derived from proliferating endothelial cells and not from the vasoformative mesenchymal “spindle” cells based on his factor VIII preparations. He also observed significant synchysis and condensation of the vitreous body in stage 3. Foos suggested that a condensation of the vitreous body over the ridge is related to depolymerization of hyaluronic acid and collapse of the collagenous framework into optically visible structures.66
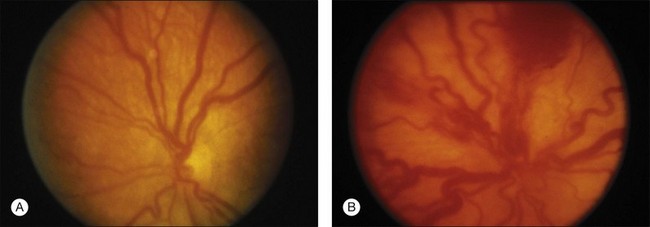
“Plus” and “pre-plus” disease
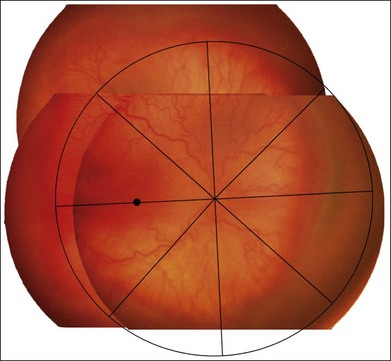
Plus disease signifies a more florid form of ROP. Increasing dilation and tortuosity of the retinal vessels, iris vascular engorgement, pupillary rigidity, and vitreous haze indicate progressive vascular incompetence. When vascular changes are so marked that the posterior veins are enlarged and the arterioles tortuous, this represents plus disease, and a plus sign is added to the ROP stage number. This finding is a key sign of worse prognosis.71 A published standard photograph selected by expert consensus, which has been used in four multicenter clinical trials and represents the minimum arteriolar tortuosity and venous dilation required for plus disease, is shown in Figure 61.18A. There has been increasing recognition of a spectrum of retinal vascular abnormalities in ROP. In 2005, the revised international classification defined an intermediate “pre-plus” categorization as abnormal arteriolar tortuosity and venous dilation of the posterior pole which is insufficient for diagnosis of plus disease.72 Studies have shown that the diagnosis of plus disease may be subjective and qualitative, even among experts, and future definitions may be based on more quantitative measures.73–75
Zone I ROP
ROP located in zone I can be dangerously deceptive, in that the proliferation signifying stage 3 can appear spread out “flat” on the retina posterior to the ridge, rather than elevated.36 In severe plus disease cases inside zone I, centripetal proliferation from the ridge may occur virtually simultaneously with detachment of the retina. Flynn and Chan-Ling examined the distinction between vasculogenesis (de novo formation of new vessels by transformation of vascular precursor cells) and angiogenesis (budding from existing vessels) with regard to the distinction of zone I and zone II ROP. They proposed that zone I ROP correlates with vasculogenesis, and is therefore less sensitive to treatment by laser or cryotherapy because the disease mechanism is not VEGF-mediated. They proposed that zone II ROP corelates with angiogenesis, is mediated by hypoxia-induced VEGF-165, and is therefore more sensitive to treatment by laser or cryotherapy.76
Aggressive posterior ROP
The 2005 revised international classification of ROP designated an uncommon, severe form of disease as “aggressive posterior ROP.” This rapidly progressive disease variant had been previously termed “rush disease,” and is characterized by its location in zone I or posterior zone II, ill-defined nature of the peripheral retinopathy, and prominent plus disease out of proportion to the peripheral findings.72 This diagnosis can be made by a single examination without serial evaluation, and may not progress through the class stages 1–3. In fact, the peripheral disease may appear as a flat area of neovascularization at the junction of vascular and avascular retina.
Classification of retinal detachment
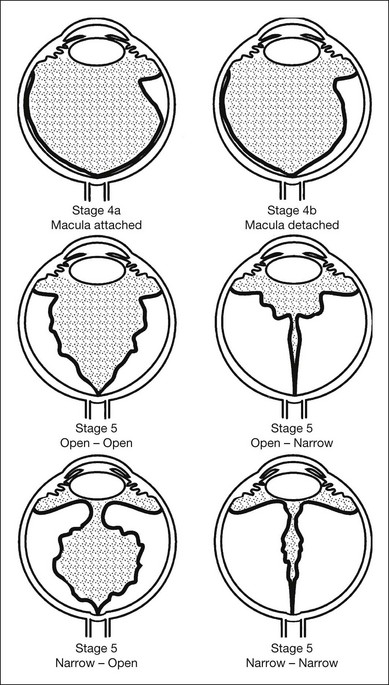
In 1987, ophthalmologists and pathologists established a second international committee, which expanded the original 1984 international classification to describe the morphology, location, and extent of retinal detachment (Fig. 61.17).77 This classification was based on an understanding of the development of severe ROP gained from surgical experience78 and pathology.66 Stage 4 (subtotal) retinal detachment is usually tractional elevation added to findings in stage 3, although there may also be exudative effusion from adjacent active stage 3 neovascularization.
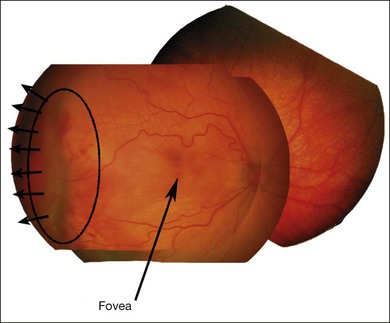
Stage 4A: extrafoveal retinal detachment
Typically this is a concave, traction detachment in the peripheral retina without involvement of the central macula (Fig. 61.19). Generally, these detachments are located at the sites of extraretinal fibrovascular proliferation with associated vitreous traction. Elevation may start in any zone where there was stage 3 disease that incompletely involuted following ablative treatment with laser photocoagulation or cryotherapy, and they may become circumferential. They may extend for 360° in the periphery without elevation of the macula, or they may be segmental, occupying only a portion of the periphery. The prognosis anatomically and visually is relatively good in the absence of posterior extension. Frequently these areas will reattach spontaneously, without affecting macular function.
Stage 4B: partial retinal detachment including the fovea
This can follow extension of stage 4A, or may appear as a fold from the disc through zone I to zones II and III (Fig. 61.20). Once a stage 4 detachment involves the fovea, the prognosis for recovery of good visual acuity is poor.
Stage 5: total retinal detachment
This is virtually always funnel-shaped. The classification of stage 5 detachments divides the funnel into an anterior and a posterior part (Fig. 61.17). When open both anteriorly and posteriorly, the detachment has a concave configuration and extends to the optic disc. An alternative configuration is one in which the funnel is narrow both anteriorly and posteriorly, and the detached retina is located just behind the lens. A third, less common type is one in which the funnel is open anteriorly but narrowed posteriorly. Least common is a funnel that is narrow anteriorly and open posteriorly. The configuration of the funnel-shaped detachment may be appreciated by ultrasonography.
Other factors related to retinal detachment
1. Appearance of the retrolenticular space. This space may be occupied by heavily vascularized translucent tissue, which represents disease activity. As the disease subsides, the tissue occupying this space becomes white, with a scarcity of blood vessels. This is the appearance that gave rise to the original term “retrolental fibroplasia.”
2. Peripheral trough. The presence of a peripheral red reflex in combination with apparent narrow funnel stage 5 retinal detachment indicates the presence of attached or shallowly detached avascular, stretched, and nonfunctioning peripheral retina. Occasionally, we have seen a peripheral red reflex coming from choroid beneath detached retina that has become adherent to the posterior lens capsule.
3. Anterior segment. In more severe cases of ROP, the anterior segment may become involved as follows:
4. Other tissues. Subretinal blood and exudate may be identifiable by ultrasonography or optical coherence tomography, but it may be difficult to distinguish one from another. Subretinal fibrotic membranes may be present but usually are recognized only during surgery.
Involution of retinopathy of prematurity
Involution of ROP typically begins after 38 weeks’ postconceptional/postmenstrual age, and may be characterized by a downgrading of staging and/or growth of retinal vessels into a more peripheral zone.79
Regressed rop: retinal detachment, strabismus, and amblyopia
Although active ROP usually involutes without progressing to retinal detachment, cicatricial sequelae can remain even in those cases.80,81 The relatively stable state of the eye after retinopathy has run its course is referred to as regressed ROP. In Box 61.1 the residual changes have been classified into those affecting the retinal periphery and those affecting the posterior fundus. Retinal pigmentary changes may be mistaken for side-effects of treatment.82
Box 61.1
Regressed retinopathy of prematurity
Peripheral changes
The most serious complications of regressed ROP are late development of retinal detachment and angle closure glaucoma. At almost any age after the neonatal period, but especially several years after birth, retinal detachment remains a risk in eyes with sequelae from ROP. Eyes with high myopia, peripheral retinal pigmentary changes or lattice-like degeneration, vitreoretinal interface changes, vitreous condensation, and stretching and folding of the retina are at special risk of developing retinal breaks and detachment. Eyes with partial retinal detachment present at about 3 months after threshold retinopathy remain at risk for progression of the detachment.83 Overall visual outcomes for 61 eyes studied were poor: only six eyes had better than 20/200 visual acuity.83 These patients and their parents should be alert to the symptoms of retinal detachment as soon as the child is old enough to appreciate and report them.
Patients with regressed ROP are at risk for developing strabismus and amblyopia.84–88 In the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study, 200 (6.6%) of 3030 infants who had weighed <1251 g at birth were strabismic at the 3-month examination. Presence of ROP was found to be a significant predictor of strabismus at 3 months. Subgroup analysis determined that the risk for strabismus increased as the zone of ROP became more posterior and the stage more severe.89 Regular examinations and attention to refractive, visual, and extraocular muscle status are indicated for all infants who have had ROP until about age 18 months, and thereafter as clinically indicated.
In the multicenter Early Treatment for ROP (ETROP) study, the prevalence of strabismus at 6 months corrected age was 20% among infants with high-risk prethreshold ROP, and 10% among infants with low-risk prethreshold ROP. At 9 months corrected age, 30% of infants with high-risk prethreshold ROP had strabismus, and risk factors associated with development of strabismus included abnormal fixation behavior, presence of amblyopia, and outborn birth status (i.e., birth outside a study-affiliated hospital).90 Overall, ophthalmologists should be aware of significant variability in ocular alignment early in life among infants with a history of severe ROP.
Ocular findings of regressed retinopathy of prematurity
Myopia
In the CRYO-ROP study, 20% of infants with birth weight <1251 g were found to develop myopia in the first 2 years of life. The lower the birth weight, the higher the chance of myopia. Among infants with ROP, the incidence of myopia increased in direct relationship to the severity of ROP. For example, in patients who developed zone II, stage 3 ROP (without plus disease), 44–45% were myopic at 12 and 24 months postterm. In contrast, infants of this same birth weight group who never developed ROP had a 13% incidence of myopia.91
In the ETROP study, infants treated for high-risk prethreshold ROP were found to have 58% prevalence of myopia (defined as spherical equivalent ≥0.25 D) at age 6 months postterm, 68% prevalence of myopia at 9 months postterm, and little change thereafter until 3 years postnatal age. The prevalence of high myopia increased steadily between ages 6 months and 3 years. There was little difference in prevalence of myopia or high myopia between eyes with zone I versus zone II ROP, or between eyes with plus disease versus without plus disease. However, prevalence of myopia and high myopia was higher in eyes with retinal residual of ROP such as straightened temporal vessels or macular heterotopia.92
The exact mechanism of the myopia remains unclear. Fletcher and Brandon93 suggested that it might be due to an elongation of the globe, alteration of the lens or the corneal curvature, or a combination of these factors. We have adopted the practice of questioning every new patient with moderate to high degrees of myopia regarding a past history of prematurity and have detected several previously unsuspected cases of regressed ROP.
Other refractive and binocular defects
Astigmatism and anisometropia are relatively common in patients with regressed ROP. In the CRYO-ROP study, 2518 infants born weighing <1251 g were refracted at 12 months postterm, and 3.3% had anisometropia. Of the 1548 who had ROP of some degree, 4.8% had anisometropia.91 In the ETROP study, 401 infants with prethreshold ROP in one or both eyes were randomized to early treatment (laser photocoagulation at high-risk prethreshold ROP) versus conventional treatment only if threshold ROP developed. All infants were refracted at 6 and 9 months correct age, and at 2 and 3 years postnatal age. The prevalence of astigmatism was similar at each test age in the early treatment and conventional management groups. For both groups, there was an increase in prevalence of astigmatism (defined as >1.00 D) from 32% at 6 months to 42% at 3 years.94
Approximately 20% of ROP cases are asymmetric at the time they reach threshold for treatment, and this asymmetry may well contribute to anisometropia. Amblyopia, nystagmus, and strabismus are also common after ROP has regressed.84,85,89,95 Taken together, these findings highlight the importance of follow-up ophthalmological examinations in infants with a history of severe ROP.
Lens and corneal changes
At the 12-month examination of the CRYO-ROP study, there was an overall incidence of cataract of 0.3% in the natural history population. The incidence of cataract among eyes with a history of zone I ROP or zone II stage 3+ ROP was approximately 2.5%.96 At the final 6-year examination of the ETROP study, cataract or aphakia was found in 4.9% of early-treated eyes and in 7.2% of conventionally managed eyes in 271 children with symmetric ROP.97 Kushner95 pointed out that early development of cataract may seriously compromise vision in the presence of retinal abnormalities. Results can be satisfactory from cataract surgery in adults with a history of ROP.98 Patients with ROP also have an increased risk of developing irregularities of corneal curvature, band keratopathy, and acute hydrops.71
Glaucoma in retinopathy of prematurity
Glaucoma in patients with advanced retinopathy
Patients with advanced retinopathy who develop a shallow anterior chamber occasionally develop acute or subacute glaucoma later. In the CRYO-ROP study, 20.3% of 195 patients with threshold ROP developed shallow anterior chambers in the control eye that did not receive cryotherapy, compared with 12% in the eye that received cryotherapy, when examined at about 12 months postterm.99 By that time, 1.5% of those control eyes had been noted to have glaucoma.100 This complication, which does not always look typical of iris bombé, may occur at any time: in the nursery, shortly after discharge, and throughout childhood. Where feasible, parents should be instructed to recognize the appearance of corneal haze and episcleral injection, and to seek ophthalmic consultation for these concerns. A trial of topical steroids and cycloplegic agents is recommended in suitable cases of glaucoma in the setting of ocular damage from ROP,101 and further glaucoma management may be required.
Angle closure glaucoma in regressed retinopathy of prematurity
Eyes with regressed ROP are at increased risk of developing acute angle closure glaucoma, even in adulthood.102,103 Kushner101 pointed out that certain patients with mild degrees of regressed ROP have a predilection for developing ciliary block glaucoma. Because these forms of glaucoma may be treatable by surgery in selected cases, ophthalmologists and patients should be aware of these potential complications, their associated signs and symptoms, and their management.
Risk factors
In general, prematurity, low birth weight, a complex hospital course, and prolonged supplemental oxygen are today’s established risk factors for the development of ROP.10,72,104,105 Supplemental oxygen given for a period of weeks, without specific indication, was abundantly documented to be a major cause of ROP during the epidemic of the 1950s but is no longer the predominant factor in cases of ROP seen since the mid-1970s. Now, neonatal advances have resulted in improved survival rates of extremely low-birth-weight children. The risk of develpoing treatment-requiring ROP is higher in this group, with as many as 25% of children born at 750 g or less developing plus disease.99
The role of blood carbon dioxide levels in the development of ROP is controversial. Bauer and Widmayer,106 following Flower’s observation107 that carbon dioxide enhanced the oxygen-induced retinal changes in beagles, conducted a retrospective analysis of infants with low birth weights. They reported that higher arterial carbon dioxide values were the most important variable in separating those infants of equal gestation who developed ROP from those without disease. Biglan et al.108 and Brown et al.104,109 failed to confirm this association and, indeed, found that infants with “scarring retinopathy of prematurity” had lower carbon dioxide blood levels. It is likely that this parameter, like many others, is associated with an unstable clinical course – as is ROP – but not necessarily linked with it causally.
Numerous other neonatal health factors have been reported to be associated with ROP, including cyanosis, apnea, mechanical ventilation, intraventricular hemorrhages, seizures, transfusions, septicemia, in utero hypoxia, anemia, patent ductus arteriosus, and vitamin E deficiency.13,25,87,105–108–115 These associations require further investigation to identify causal relationships. In the CRYO-ROP natural history cohort of 4099 infants born weighing less than 1251 g, significant additional factors were identified, including white race, multiple birth, and being transported elsewhere for intensive care. Once ROP develops, greater risk is associated with ROP located in zone I, the presence of plus disease, the severity of stage, and the extent of circumferential involvement.116,117 The risk factors studied during the CRYO-ROP study were consolidated into a mathematical model that can predict the risk of an unfavorable outcome for a particular eye that reaches prethreshold severity.118
Examination procedures in the nursery
General aspects and timing of the examination
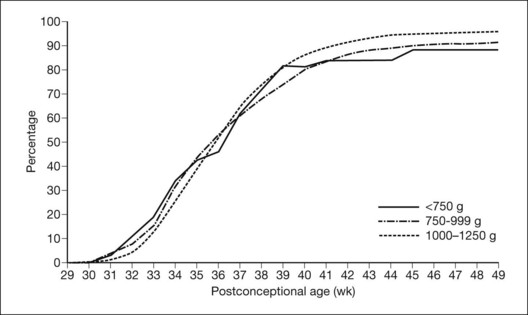
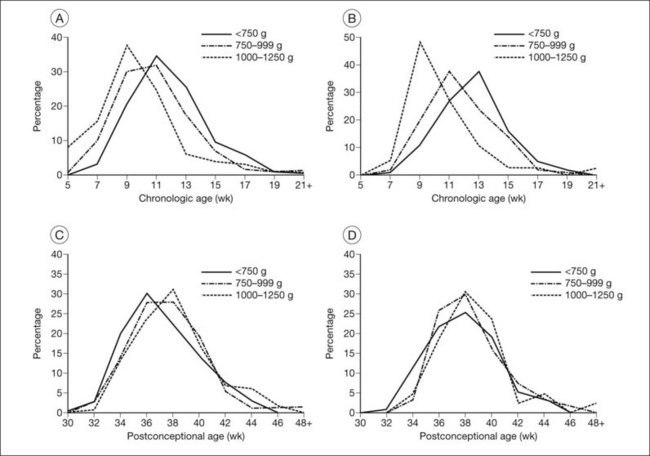
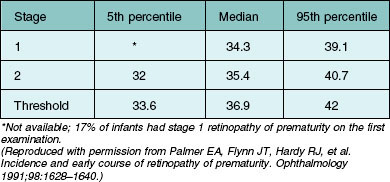
The nursery surveillance carried out in the CRYO-ROP study produced definitive information concerning the early course of ROP. The “natural history” portion of this study recorded data from 4099 infants born weighing less than 1251 g. The study showed that ROP occurs on a schedule according to the infant’s corrected age (postmenstrual age since mother’s last menstrual period, or postconceptional age), rather than the time since birth, the so-called chronologic age (Fig. 61.21).116 For infants in the birth weight category studied, it was found that those who develop stage 1 ROP (and no worse) do so at a median of 34.3 postmenstrual weeks. The median time for onset of stage 2 ROP that progresses no further is 35.4 weeks, and 95% of these cases have onset at 32 weeks or later. For patients with eyes that reached the treatment randomization “threshold” severity of stage 3+ ROP (at least 5 contiguous or 8 interrupted clock-hours in zones I or II), the threshold was reached at a median of 36.9 weeks (90% of cases were in the range of 33.6–42.0 weeks) (Table 61.1).
Screening guidelines
Because ROP can progress to blindness during the first 3 months of life119 and treatment is available to arrest it in many cases, a protocol has been recommended for examining the eyes of premature infants during that time span. In November 2002, Reynolds et al.74 reported an analysis of combined data from the CRYO-ROP study (n = 4099) and the LIGHT-ROP study (n = 361) to develop screening criteria based on the evidence from those two clinical trials. The authors concluded that the initial eye examination should be performed by 31 weeks’ postmenstrual age or 4 weeks from birth, whichever is later, in order to detect prethreshold retinopathy in a timely fashion. Prethreshold ROP is defined as ROP of less severity than the threshold severity in the CRYO-ROP trial, specifically as any ROP in zone I, or zone II ROP of stage 2+ or stage 3 with or without plus disease. It appeared that most risk had passed not only whenever full vascularization had been achieved, but also whenever vessels reached the nasal ora serrata without any ROP development prior to that. If the infant reaches 45 weeks’ gestational age without developing prethreshold ROP or worse, the risk of visual loss from ROP is minimal.74 The authors caution that recommendations for infants born prior to 24 weeks are by extrapolation. They also point out that the database excluded infants born weighing more than 1250 g, and some of those larger infants are at risk for ROP. Guidelines for those larger infants would need to be derived from different studies. It should also be noted that these are data from the USA, and the natural history of ROP may be different in other parts of the world. The current recommendations from the American Academies of Ophthalmology and Pediatrics are that children born at 30 weeks or less, or at less than 1500 g, should be screened for ROP. Specifically those born at a gestational age of 27 weeks or less should have their first exam at 31 weeks and children born from 28 to 32 weeks should have their first exam 4 weeks after birth. The subsequent examination schedule is determined by findings on the initial examination, as shown at the end of this chapter.120
Side-effects of the examination
Very-low-birth-weight infants, while they are still in a precarious general condition, must be managed with care. The stress of an indirect ophthalmoscopic examination is necessary whenever the risk of treatable disease capable of progressing to blindness exists or when information is needed to assist in the general medical evaluation.121 Screening programs must be designed around the consideration that the procedure may be stressful for the infant.
Techniques of eye examination
Eye examinations should be performed at the request of, or with the approval of, an attending neonatologist. Pupils may be effectively dilated in most infants with Cyclomydril eye drops (cyclopentolate 0.2% and phenylephrine 1%), with the excess drops immediately blotted from the lids to minimize systemic side-effects such as hypertension and intestinal ileus.121 The examination is performed about 25–30 minutes later using a binocular indirect ophthalmoscope and condensing lens. More heavily pigmented infants sometimes fail to respond adequately to the mydriatic drops, in which case 0.5% cyclopentolate or 1% tropicamide, or both, and 2.5% phenylephrine may be substituted and instilled twice. Most examiners generally use a lid speculum, and there are now a variety of designs suitable for premature infants (e.g., Barraquer, Sauer, Alfonso specula). The infant’s hands should be physically restrained, and a nurse ordinarily assists with the exam. As a precaution against viral or chlamydial transfer, the lid speculum must be sterile for each infant and the examination lens should be wiped with an alcohol sponge between cases whenever it has touched the infant’s face. The universal precaution of wearing gloves during examination is recommended.
In general, ROP severe enough to cause serious concern will be visible far enough posteriorly in the fundus to bring it into view without scleral indentation. However, to determine the final maturity of retinal vascularization requires either serial examinations past full term or, preferably, examination of the nasal retina to the ends of the growing vessels to determine whether vascularization has advanced into zone III.77,116 For this far-nasal peripheral retinal examination, scleral indentation or eye positioning is generally needed. An aluminum-wired Calgiswab nasopharyngeal culture swab can be used as an inexpensive, sterile, and relatively gentle tool for this. The tip can be bent to any desired angle, even to resemble a fine muscle hook. Scleral depressors designed for infant examinations (e.g., Flynn depressor) are also commercially available. For scleral depression, topical anesthetic, such as proparacaine, is typically used. It is recommended that a member of the nursery staff be present during the entire examination to monitor the infant’s airway, vital signs, and behavior and to deal with apnea or other adverse reactions that may occur.
Prophylaxis and therapy
The role of vitamin E
Vitamin E was considered as a potential agent to prevent ROP due to its antioxidant properties. It was evaluated by Johnson et al.122,123 with subsequent controlled clinical trials having tested the role of large doses of vitamin E.124–131 The results were equivocal and a report from the Institute of Medicine published in 1986 concluded: “Vitamin E as prophylaxis for retinopathy of prematurity was subject to a detailed analysis. This committee found no conclusive evidence either of benefit or harm from vitamin E administration. Risks from vitamin E appear to be minimal for premature infants provided that doses are kept moderate to achieve a blood level no higher than 3 mg/dl.”132 Currently, there is no formal recommendation on the use of vitamin E in the management of ROP.
The role of light
Historically there has been interest in a possible relationship between light and ROP. In his original descriptions of RLF, Terry133 considered premature exposure of the eye to light as an important etiologic possibility.
Before the importance of the role of inspired oxygen levels was recognized in ROP, two studies addressed the question of the effect of light. In the late 1940s, Hepner et al.134 patched the eyes of five premature infants from birth until they weighed 2000 g. They found that four of the five infants developed ROP and they concluded that light was not a factor in its development. In 1952, Locke and Reese135 reported on a series of 22 premature infants (birth weight less than 2000 g) in which they had patched one eye of each baby. Both found that there was no difference in the incidence of ROP between the patched eyes and the unpatched eyes.134,135
To examine the relationship between light exposure and ROP more definitively, a feasibility trial of light-reducing goggles (LIGHT-ROP study), chaired by James D. Reynolds, was sponsored by the National Eye Institute in 1995 at three nurseries in the USA. Half of 409 infants with birth weights of less than 1250 g were randomly selected either to wear goggles containing 97% near-neutral density filters until 31 weeks’ postconceptional age or to undergo no extraordinary light reduction. The study concluded that there is no clinically important effect of light on the occurrence or severity of ROP.136 Neither the American Academy of Ophthalmology nor the American Academy of Pediatrics has made any recommendations about restricting ambient light from the eyes of premature infants.
Cryotherapy
From 1968, reports suggested that ablative treatment of the peripheral retina of premature infants with ROP may ameliorate the course of the disease. Those early reports suggested that photocoagulation137,138 or cryotherapy139,140 may accomplish this goal. Throughout the early 1980s, studies produced conflicting results and opinions regarding the efficacy and role of cryotherapy for severe ROP.141–146 The need for a large-scale clinical trial was apparent.
The multicenter trial of cryotherapy
The CRYO-ROP study was organized in 1985 under the chairmanship of Earl A. Palmer. Supported by the National Eye Institute, the study began enrolling premature infants weighing 1250 g or less at birth in 1986. Although enrollment was scheduled to continue until mid-1988, it was stopped in January 1988 because preliminary results showed a compelling favorable effect of cryotherapy in improving the anatomic outcome for the macula of treated eyes.99 The study has been continued for longer-term follow-up, and a final examination was carried out when the children were about 15 years old.147
Treatment
Infants eligible for the cryotherapy trial had stage 3 ROP, involving five or more clock-hours of retina posterior to zone III in the presence of a standardized plus disease.69,99 Outlines of the protocol have been published,100,116,148 as well as the manual of procedures.149 In brief, contiguous, nonoverlapping spots of transscleral cryotherapy were directed at the entire anterior cuff of avascular retina. No infant received cryotherapy to both eyes during the study, and the eye to receive cryotherapy was randomly determined. Laser photocoagulation was not studied, since no practical laser delivery instrument had been developed at that time.
Results
The results of the CRYO-ROP study were evaluated through a masked comparison by fundus photographs of the incidence of objectively visible macular fold, retinal detachment, or retrolental mass in the eyes that received cryotherapy, with those eyes not receiving it.150 Age-appropriate visual acuity outcomes were performed as the children grew and developed.81,100,150 Cryotherapy was found to reduce the listed unfavorable fundus outcomes over the serial examination visits. At the 10-year outcome assessment, 247 of the original randomized cohort were examined and total retinal detachments had continued to occur in control eyes that had received cryotherapy, increasing from 38.6% at 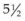 years to 41.4% at 10 years, while treated eyes remained stable at 22%. Unfavorable fundus outcomes were present in 27% of treated eyes versus 48% of control eyes, and visual acuity was 20/200 or worse in 44% of treated eyes versus 62% of control eyes.150 By the close of the 20th century, level 3 and level 2 NICUs had become organized to provide screening ophthalmic examinations for all high-risk infants and to provide peripheral retinal ablative therapy for cases of severe ROP.
years to 41.4% at 10 years, while treated eyes remained stable at 22%. Unfavorable fundus outcomes were present in 27% of treated eyes versus 48% of control eyes, and visual acuity was 20/200 or worse in 44% of treated eyes versus 62% of control eyes.150 By the close of the 20th century, level 3 and level 2 NICUs had become organized to provide screening ophthalmic examinations for all high-risk infants and to provide peripheral retinal ablative therapy for cases of severe ROP.
Current concepts in management of retinopathy of prematurity
Treatment techniques
Cryotherapy – special considerations
Cryotherapy was performed during the CRYO-ROP study with the use of general anesthesia in 27.5% of the patients; for the rest, cryotherapy was performed in a room within or adjacent to the NICU using local or even topical anesthesia.99 The average number of individual freezes used in the CRYO-ROP study was 50. As with other forms of eye surgery, a number of factors are considered in determining the method of analgesia or anesthesia, including the physical arrangement of the nursery, proximity to operating or procedure rooms, experience of the anesthesiologist, current medical stability of the infant, “track record” of the infant in tolerating previous stressful procedures, experience of the cryosurgeon, and posterior extent of retinopathy.
Laser – special considerations
In an effort to reduce the time and stress accompanying cryotherapy, refinements of ablative therapeutic technique were studied – in particular, laser therapy, using the binocular laser indirect ophthalmoscope (LIO) delivery system.151–153 During the early 1990s laser ablation gained acceptance as an alternative to cryotherapy. In general, ophthalmologists have found that the LIO delivery system is technically easier than cryotherapy and creates fewer postoperative sequelae, such as inflammation and swelling. Furthermore, it seemed apparent that the outcomes of treatment of threshold disease in zone I and posterior zone II were superior to cryotherapy, and at least equivalent to cryotherapy results for zone II disease.152–154–161
The Early Treatment for Retinopathy of Prematurity trial
In 1999, the National Eye Institute funded a clinical trial, under the chairmanship of William V. Good, to study optimal ROP treatment indications. In this trial, called the ETROP study, eyes were randomized to early peripheral retinal ablation or conventional management (observation until threshold criteria developed) once they achieved a high-risk level of prethreshold ROP. The ETROP study showed a significant benefit of earlier treatment intervention as measured by visual acuity outcome at a corrected age of 9 months and in the structural outcome of the retina at corrected ages of 6 and 9 months.162 In the selected high-risk eyes that were studied, unfavorable acuity results were reduced by earlier treatment intervention to 14.5%, from 19.5% in the conventionally treated control group (P = 0.01). Unfavorable structural outcomes were reduced from 15.6% in the control group to 9.1% in the early treatment eyes (P < 0.001).
The ETROP study results, published in December 2003, produced a new clinical algorithm as a guide for treatment intervention in eyes with severe ROP.162 Prompt treatment is indicated for eyes with type 1 ROP and continued serial observations without treatment are recommended for eyes with type 2 ROP, as shown in Table 61.2. The ETROP group cautions that plus disease should involve at least two quadrants of the fundus (usually six or more clock-hour segments) with dilation and tortuosity of the posterior retinal blood vessels meeting the published standard (Fig. 61.18A).
| Type 1 ROP (“new threshold”) | Type 2 ROP |
|---|---|
| Administer peripheral ablation treatment | Wait and watch for progression |
| Zone II: | Zone II: |
| plus disease with stage 2 or 3 | stage 3 without plus disease |
| Zone I: | Zone I: |
| plus disease with stage 1, 2, or 3 | stage 1 or 2 without plus disease |
| stage 3 without plus disease |
ETROP, Early Treatment for ROP; ROP, retinopathy of prematurity.
At the final study outcome examinations performed at age 6 years, unfavorable visual acuity outcomes in eyes with type 1 ROP were reduced by early treatment to 25.1% from 32.8% in the conventionally treated control group (P = 0.02). Interestingly, unfavorable visual acuity outcomes in eyes with type 2 ROP increased in the early treatment group to 23.6% from 19.4% in the conventionally treated group, although this difference was not statistically significant (P = 0.37).97
Retinal detachment
There is a need for randomized trials of treatment approaches for retinal detachment from ROP. Current clinical thinking about the treatment of retinal detachment from ROP is discussed in Chapter 114 Retinopathy of prematurity.
The ETROP study: better outcomes, changing clinical strategy
In the ETROP trial, only 66% of the high-risk eyes selected at random to be treated conventionally went on to receive laser therapy (cryotherapy was rarely used). Secondary analysis of the large database produced a simplified revision of the indications for treatment, which was a great practical improvement over the computer-generated algorithm used to select the research subjects for the study118 (Table 61.2).
Some of the advantages of an earlier treatment policy may be lost if newborn eye examinations do not occur, as in the ETROP study. Careful reading of the methods used in the trial163 reveals a real impact on an intensive care unit’s policy for serial ROP examinations. Therefore, consider the following schedule for infants who do not meet criteria for treatment:120
Favorable signs, with respect to progression or involution of ROP, include attainment of postmenstrual age of 45 weeks without developing at least type 2 (as defined above) ROP, and either the completion of full retinal vascularization or progression of retinal vascularization into zone III without previous zone II ROP.74
Anti-VEGF therapy for posterior ROP
There have been numerous trials demonstrating the benefits of bevacizumab in adult patients suffering from choroidal neovascular membranes in the setting of wet age-related macular degeneration. Based on this experience, many investigators have been interested in using a similar approach for the treatment of aggressive ROP. A number of recent case series have reported that intravitreal injections of anti-VEGF antibodies (e.g., bevacizumab) are a very promising approach for the treatment of aggressive ROP, with the possibility of easier administration and improved preservation of peripheral retina compared to laser.164–169 A prospective multicenter trial has been performed in which 150 infants with bilateral stage 3+ disease in zone I or posterior zone II were randomized to intravitreal bevacizumab (0.625 mg) versus conventional laser treatment. This showed that infants treated with bevacizumab for stage 3+ disease in zone I had significantly fewer disease recurrences and better structural outcomes at 54 weeks’ postmenstrual age, although there was no difference for infants with ROP in posterior zone II.170
Although there is the possibility of improved treatment efficacy with bevacizumab, ROP recurrences have been reported several months postinjection.170 Unlike laser treatment, where the regression is often durable and permanent, the potential for recurrence after bevacizumab injection emphasizes the need for prolonged follow-up examinations.
Other pediatric retinal vascular diseases
Coats disease
Coats disease arises from abnormal telangiectatic retinal vessels that result in profuse leakage, leading to retinal edema and exudative detachments. It was originally described in 1908 by George Coats, who noted that patients with this condition had unilateral telangiectatic vessels with associated lipid deposits.171 Interestingly, none of the patients described in the original paper were children, and it was not until subsequent observations that its incidence in the pediatric population was appreciated.
In 2001, Shields et al. proposed a classification system based on response to treatment. Stage 1 eyes have telangiectasias only. Stage 2 eyes have in addition exudation in either extrafoveal (2a) or subfoveal (2b) locations. Stage 3 eyes have an exudative detachment that is subtotal (3a) or total (3b). Of the stage 3a eyes, if it involves the fovea then it is stage 3a1 and if it remains outside the fovea it is stage 3a2. Stage 4 is a detachment with glaucoma, and stage 5 is end-stage disease.172
The primary treatment of Coats disease involves laser photocoagulation directly to telangiectatic vessels. It is helpful to have wide-angle fluorescein angiography to identify the full extent of the disease. By using a green 532-nm frequency-doubled yttrium aluminum garnet (YAG) laser indirect, it is possible to coagulate the vessels even in areas where the retina is fully detached.173 This often requires multiple treatment sessions under anesthesia over successive months. In more recalcitrant cases, subretinal fluid drainage can help expose more retina that may be hidden anteriorly due to the extent of the detachment. In severe cases, vitrectomy with external drainage may be necessary. Prior to considering any invasive treatment, retinoblastoma must be clearly ruled out.
Persistent fetal vasculature
Originally labeled as persistent hyperplastic primary vitreous (PHPV), PFV is a result of incomplete regression of the vascular primary vitreous leaving a stalk of fibrovascuar tissue extending from the optic nerve to the posterior lens capsule.174 This often leads to a white opaque membrane behind the lens, resulting in dense amblyopia. The ciliary processes are often drawn towards the center and are associated with some degree of microphthalmia. The retina around the optic nerve can be drawn up into the stalk and in some cases can involve the entire length of the stalk, making surgical amputation of the stalk a risk.
Treatment for PFV can vary from observation to surgical amputation of the stalk. In some cases, the stalk is mild and the macula can be visualized. If the retina is intact and there are macular structures, a surgical approach to clear the visual access can be helpful. In some cases, the posterior plaque is quite dense and upon removal the stalk ends up being less severe with relatively normal retinal architecture. Some have advocated surgical treatment in the setting of significantly deformed retina, even when the vision is at light perception. Amputation of the stalk is thought in these cases to prevent phthisis and allow for continued eye growth.175
Little is known about the pathogenesis of PFV. The primary vitreous undergoes a well-described programmed involution, and in patients with PFV, it is believed that this process is altered. Recent work suggests that astrocytes may play a role in altering how this process happens and may prevent the hayloid artery from underdoing a macrophage-mediated involution.176
Incontinentia pigmenti
Previously called Bloch–Sulzberger syndrome, after the two dermatologists who first recognized the condition, IP is an X-linked dominant disease with ocular, central nervous system, dermatologic, and dental abnormalities. It is associated with a mutation in the NEMO gene located on Xq28. The resulting protein regulates the activity of NF-κB, increasing a cell’s sensitivity to apoptotic signals and resulting in increase endothelial cell death.177
Ocular findings include retinal ischemia with neovascularization that can lead to vitreous hemorrhage and tractional detachment. The differential diagnosis based solely on the ocular findings includes ROP, FEVR, and Norrie disease. However, unlike these diseases, the ischemia in IP does not mimic a developmental vascular pattern with distinct areas of vascularized retina posteriorly and avascular retina anteriorly. Instead, the peripheral ischemia is often accompanied by ischemia in posterior vascularized retina and in some cases the macula as well. Usually, the diagnosis of IP is made soon after birth based on the presence of the skin blisters. Approximately one in three children with IP have obvious retinal abnormalities on exam, and one in four have tractional retinal detachments.178
Familial exudative vitreoretinopathy and Norrie disease
Management of any patient where FEVR or Norrie disease is being considered should include careful examination under anesthesia and, when possible, fluorescein angiography. Both conditions can have an asymmetric presentation and can mimic PFV. In these cases the presumed unaffected eye may have subtle fundoscopic abnormalities that can be overlooked in an outpatient clinic setting and even under anesthesia. Wide-angle fluorescein angiography can provide a very definitive assessment of the vasculature in both eyes and may demonstrate premature termination of the peripheral vessels and subclinical neovascularization. In eyes with ischemia and neovascularization but no detachment, laser photocoagulation to the avascular retina can induce regression and prevent a detachment. When tractional retinal detachment is present, some have advocated vitrectomy to release stress on the retina and ciliary body to reduce the likelihood of hypotony and maintain light perception vision when present.179
In the case of FEVR, the mutation occurs in the FZD4 gene that encodes the frizzled-4 receptor. This receptor binds Wnt ligands 3, 5a, and 8a as well as Norrin and triggers the translocation of β-catenin to the nucleus where it can activate transcription of genes involved in cell proliferation. In Norrie disease, the mutation occurs in the NDP gene that produces norrin, a secreted protein that can bind frizzled-4.180,181 Recently, another gene, Tspan12, was found to facilitate norrin binding to frizzled-4 and loss of Tspan12 was found in some patients with FEVR.182 There is currently ongoing investigation as to whether mutations in these genes may also increase the risk of developing aggressive ROP.
![]() Bonus images for this chapter can be found online at http://www.expertconsult.com
Bonus images for this chapter can be found online at http://www.expertconsult.com
Fig. 61.1(A) Ophthalmoscopic examination of a 45-day-old dog exposed to 100% oxygen for the first 4 days of life disclosed a 2-mm-wide area of retinal neovascularization (arrow) extending from the disc to the temporal midperiphery. Two similar structures are present at the 2- and 5-o’clock positions. (B) Area of temporal retinal neovascularization (arrow) indicated by arrow in A shows mild folds in the retina (arrowheads) (periodic acid–Schiff and hematoxylin; ×50).
Fig. 61.2(A) Gross appearance of the areas of neovascularization extending temporally and two smaller areas in the same 45-day-old dog shown in Figure 61.1. A denser 2 × 0.5 mm area is present inferior nasally (arrow). (B) Area from A (marked by arrow) discloses retinal neovascularization (arrow) attached to the apex of a retinal fold (periodic acid–Schiff and hematoxylin; ×125).
Fig. 61.3 Schematic diagram of retinal vessel development in humans. At 4 months’ gestation, vessels grow from the disc to reach the ora serrata nasally at 8 months and the ora temporally shortly after term. The vascularization of the newborn kitten corresponds to the 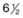 -month-gestation human fetus. N, nasal retina; T, temporal retina; numbers refer to months’ gestational age.
-month-gestation human fetus. N, nasal retina; T, temporal retina; numbers refer to months’ gestational age.
Fig. 61.4(A) Schematic diagram of vascular closure of the most anterior and immature retinal vascular bed (indicated by brackets) of a young kitten exposed to hyperoxia for a relatively short period. The posterior, more mature vessels are unaffected. (B) Three weeks after removal of the subject in A to ambient air, neovascularization has developed immediately posterior to the area of capillary closure (arrow). (Panel B, reproduced with permission from Patz A. Oxygen studies in retrolental fibroplasia. IV. Clinical and experimental observations. Am J Ophthalmol 1954;38:291.)
1 Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. I. Preliminary report. Am J Ophthalmol. 1942;25:203–204.
2 Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. II. Report of cases – clinical aspects. Arch Ophthalmol. 1943;29:36–53.
3 Patz A. The role of oxygen in retrolental fibroplasias. Trans Am Ophthalmol Soc. 1968;66:940–985.
4 Owens WC, Owens EU. Retrolental fibroplasia in premature infants. Am J Ophthalmol. 1949;32:1–29.
5 Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253.
6 Patz A, Eastham A, Higginbotham DH, et al. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522.
7 Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia: a preliminary report. Br J Ophthalmol. 1953;37:513–520.
8 Lanman JT, Guy LP, Dancis J. Retrolental fibroplasia and oxygen therapy. JAMA. 1954;55:223–226.
9 Kinsey VE. Retrolental fibroplasia: cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol. 1956;56:481–543.
10 Bolton DPG, Cross KW. Further observations on cost of preventing retrolental fibroplasias. Lancet. 1974;1:445–448.
11 Avery ME, Oppenheimer EH. Recent increase in mortality from hyaline membrane disease. J Pediatr. 1960;57:553–559.
12 McDonald AD. Cerebral palsy in children of very low birth weight. Arch Dis Child. 1963;38:579–588.
13 Strang LB, MacLeish MH. Ventilatory failure and right-to-left shunt in newborn infants with respiratory distress. Pediatrics. 1961;28:17–27.
14 Chow LC, Wright KW, Sola A, et al. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345.
15 SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med.. 2010;362:1959–1969.
16 Stenson B, Brocklehurst P, Tarnow-Mordi W, et al. Increased 36-week survival with high oxygen saturation target in extremely premature infants. N Engl J Med. 2001;364:1680–1682.
17 Finnstrom O, Olausson PO, Sedin G, et al. The Swedish national prospective study on extremely low birth weight (ELBW) infants – incidence, mortality, morbidity and survival in relation to level of care. Acta Paediatr. 1998;86:503–511.
18 National NeoKnowledge Network. Multi-institutional comparative analysis for births in 1996. Based on 1810 liveborn infants <1000 g birth weight. Wayne, Penn: MDS; 1997.
19 Strebel R, Bucher HU. [Improved chance of survival for very small premature infants in Switzerland.]. Schweiz Med Wochenschr. 1994;124:1653–1659.
20 Smith LE. Through the eyes of a child: understanding retinopathy through ROP. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–5182.
21 Pierce EA, Foley ED, Smith LEH. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. (note: see correction of errata in Arch Ophthalmol 115:427, 1997)
22 Stone J, Maslim J. Mechanisms of retinal angiogenesis. Prog Ret Eye Res. 1996;16:157–181.
23 Patz A. Current concepts of the effect of oxygen on the developing retina. Curr Eye Res. 1984;3:159–163.
24 Flynn JT, Bancalari E, Bawol R, et al. Retinopathy of prematurity: a randomized, prospective trial of transcutaneous oxygen monitoring. Ophthalmology. 1987;94:630–638.
25 Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasias. Pediatrics. 1984;73:82–96.
26 Gole GA. Animal models of retinopathy of prematurity. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:53–96.
27 Kretzer FL, Hittner HM. Initiating events in the development of retinopathy of prematurity. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:121–152.
28 McLeod DS, D’Anna SA, Lutty GA. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Invest Ophthalmol Vis Sci. 1998;39:1918–1932.
29 Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasias. Br J Ophthalmol. 1954;38:397–432.
30 Ashton N, Cook C. Direct observation of the effect of oxygen on developing vessels: a preliminary report. Br J Ophthalmol. 1954;38:433–440.
31 McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311.
32 Ashton N, Pedler C. Studies on developing retinal vessels. IX. Reaction of endothelial cells to oxygen. Br J Ophthalmol. 1962;16:257–276.
33 Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68:137–180.
34 Cogan DG. Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc UK. 1963;83:465–489.
35 Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatric Res. 1994;36:724–731.
36 Smith LEH, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111.
37 Ashton N. Oxygen and the growth and development of retinal vessels: in vivo and in vitro studies. Am J Ophthalmol. 1966;62:412–435.
38 Riecke B, Chavakis E, Bretzel R, et al. Topical application of integrin antagonists inhibits proliferative retinopathy. Horm Metab Res. 2001;33:307–311.
39 Wilkinson-Berka J, Alousis N, Kelly D, et al. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–979.
40 Higgins RD, Hendricks-Munoz KD, Caines VV, et al. Hyperoxia stimulates endothelin-1 secretion from endothelial cells; modulation by captopril and nifedipine. Curr Eye Res. 1998;17:487–493.
41 Raisler BJ, Berns KI, Grant MB, et al. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1–3 of angiostatin reduce retinal neovascularization. Proc Natl Acad Sci USA. 2001;99:8909–8914.
42 Aurricchio A, Behling KC, Maguire A, et al. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002;6:490–494.
43 Stellmach V, Crawford SE, Zhou W, et al. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci. 2001;98:2593–2597.
44 Phelps DL. Oxygen and developmental retinal capillary remodeling in the kitten. Invest Ophthalmol Vis Sci. 1990;31:2194–2200.
45 Penn JS, Tolman BAL, Henry MM. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994;35:3429–3435.
46 Chan-Ling T, McLeod DS, Hughes S, et al. Astrocyte–endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–2032.
47 Chan-Ling T, Tout S, Hollander H, et al. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992;33:2128–2147.
48 Stone J, Itin A, Chan-Ling T, et al. The roles of endothelial growth factor (VEGF) and neuroglia in retinal vascularization during normal development and in retinopathy of prematurity. J Neurochem. 1995;65:121.
49 Stone J, Chan-Ling T, Pe’er J, et al. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37:290–299.
50 Provis JM, Leech J, Diaz CM, et al. Development of the human retinal vasculature – cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–568.
51 Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med. 1995;1:1024–1028.
52 Donahue ML, Phelps DL, Watkins RH, et al. Retinal vascular endothelial growth factor (VEGF) mRNA expression is altered in relation to neovascularization in oxygen-induced retinopathy. Curr Eye Res. 1996;15:175–184.
53 Dorey CK, Aouididi S, Reynaud X, et al. Correlation of vascular permeability factor/vascular endothelial growth factor with extraretinal neovascularization in rat. Arch Ophthalmol. 1996;114:1210–1217.
54 Szewczyk TS. Retrolental fibroplasia and related ocular diseases: classification, etiology, and prophylaxis. Am J Ophthalmol. 1953;36:1333–1361.
55 Phelps DL, Rosenbaum A. Effects of marginal hypoxemia on recovery from oxygen-induced retinopathy in the kitten model. Pediatrics. 1984;73:1–10.
56 Phelps DL. Reduced severity of oxygen-induced retinopathy in kittens recovered in 28% oxygen. Pediatr Res. 1988;24:106–109.
57 STOP-ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I. Primary outcomes. Pediatrics. 2000;105:295–310.
58 Cantolino SJ, O’Grady GE, Herrera JA, et al. Ophthalmoscopic monitoring of oxygen therapy in premature infants: fluorescein angiography in acute retrolental fibroplasias. Am J Ophthalmol. 1971;72:322–331.
59 Flynn JT. Acute proliferative retrolental fibroplasia: evolution of the lesion. Graefes Arch Clin Exp Ophthalmol. 1975;195:101–111.
60 Flynn JT. Retinopathy of prematurity. Pediatr Clin North Am. 1987;34:1487–1515.
61 Flynn JT, Cassady J, Essner D, et al. Fluorescein angiography in retrolental fibroplasia: experience from 1969–1977. Ophthalmology. 1979;86:1700–1723.
62 Flynn JT, O’Grady GE, Herrera J. Retrolental fibroplasia: I. Clinical observations. Arch Ophthalmol. 1977;95:217–223.
63 Brooks SE, Gu X, Samuel S, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228.
64 Foos RY. Acute retrolental fibroplasias. Graefes Arch Clin Exp Ophthalmol. 1975;95:87–100.
65 Foos RY. Chronic retinopathy of prematurity. Ophthalmology. 1985;92:563–574.
66 Foos RY. Retinopathy of prematurity – pathologic correlation of clinical stages. Retina. 1987;7:260–276.
67 Cogan DG, Kuwabara T. Accessory cells in vessels of the perinatal human retina. Arch Ophthalmol. 1986;104:747–752.
68 McLeod DS, Lutty GA, Wajer SD, et al. Visualization of a developing vasculature. Microvasc Res. 1987;33:257–269.
69 Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134.
70 Garner A. The pathology of retinopathy of prematurity. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:19–52.
71 Hittner HM, Rhodes LM, McPherson AR. Anterior segment abnormalities in cicatricial retinopathy of prematurity. Ophthalmology. 1979;86:803–816.
72 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999.
73 Chiang MF, Jiang L, Gelman R, et al. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125:875–880.
74 Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–1476.
75 Wallace DK, Quinn GE, Freedman SF, et al. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. JAAPOS. 2008;12:352–356.
76 Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol. 2006;142:46–59.
77 International Committee for Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity: II. The classification of retinal detachment. Arch Ophthalmol. 1987;105:906–912.
78 Machemer R. Description and pathogenesis of late stages of retinopathy of prematurity. Ophthalmology. 1985;92:1000–1004.
79 Repka MX, Palmer EA. Involution of retinopathy of prematurity. Arch Ophthalmol. 2000;118:645–649.
80 Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural ocular outcome of premature birth and retinopathy: status at one year. Arch Ophthalmol. 1994;112:903–912.
81 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen acuity and structural outcome at 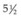 years. Arch Ophthalmol. 1996;114:417–424.
years. Arch Ophthalmol. 1996;114:417–424.
82 Fishburne BC, Winthrop KL, Robertson JE. Atrophic fundus lesions associated with untreated retinopathy of prematurity. Am J Ophthalmol. 1997;124:247–249.
83 Gilbert WS, Quinn GE, Dobson V, et al. Partial retinal detachment at 3 months after threshhold retinopathy of prematurity. Arch Ophthalmol. 1996;114:1085–1091.
84 Kushner BJ. Strabismus and amblyopia associated with regressed retinopathy of prematurity. Arch Ophthalmol. 1982;100:256–261.
85 Schaffer DB, Quinn GE, Johnson L. Sequelae of arrested mild retinopathy of prematurity. Arch Ophthalmol. 1984;102:373–376.
86 Cats BP, Tan KEWP. Prematures with and without regressed retinopathy of prematurity: comparison of long-term (6–10 years) ophthalmological morbidity. J Pediatr Ophthalmol Strabismus. 1989;26:271–275.
87 Robinson R, O’Keefe M. Follow-up study on premature infants with and without retinopathy of prematurity. Br J Ophthalmol. 1993;77:91–94.
88 Snir M, Nissenkorn I, Sherf I, et al. Visual acuity, strabismus, and amblyopia in premature babies with and without retinopathy of prematurity. Ann Ophthalmol. 1988;20:256–258.
89 Bremer D, Fellows RR, Palmer EA, et al. Strabismus in premature infants in the first year of life. Arch Ophthalmol. 1998;116:329–333.
90 Vanderveen DK, Coats DK, Dobson V, et al. Prevalence and course of strabismus in the first year of life for infants with prethreshold retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity Study. Arch Ophthalmol. 2006;124:766–773.
91 Quinn GE, Dobson V, Repka MX, et al. Development of myopia in infants with birth weights less than 1251 grams. Ophthalmology. 1992;99:329–340.
92 Quinn GE, Dobson V, Davitt BV, et al. Progression of myopia and high myopia in the Early Treatment for Retinopathy of Prematurity Study: findings to 3 years of age. Ophthalmology. 2008;115:1058–1064.
93 Fletcher MC, Brandon S. Myopia of prematurity. Am J Ophthalmol. 1955;40:474–481.
94 Davitt BV, Dobson V, Quinn GE, et al. Astigmatism in the Early Treatment for Retinopathy of Prematurity Study. Ophthalmology. 2009;116:332–339.
95 Kushner BJ. The sequelae of regressed retinopathy of prematurity. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:239–248.
96 Summers GC, Phelps DL, Tung B, et al. Ocular cosmesis in retinopathy of prematurity. Arch Ophthalmol. 1992;110:1092–1097.
97 Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the Early Treatment for Retinopathy Study. Arch Ophthalmol. 2010;128:663–671.
98 Krolicki TJ, Tasman W. Cataract extraction in adults with retinopathy of prematurity. Arch Ophthalmol. 1995;113:173–177.
99 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–479.
100 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: one-year outcome-structure and function. Arch Ophthalmol. 1990;108:950–955.
101 Kushner BJ. Ciliary block glaucoma in retinopathy of prematurity. Arch Ophthalmol. 1982;100:1078–1079.
102 Pollard ZF. Secondary angle-closure glaucoma in cicatricial retrolental fibroplasia. Am J Ophthalmol. 1980;89:651–653.
103 Smith J, Shivitz I. Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity. Arch Ophthalmol. 1984;102:371–372.
104 Brown DR, Biglan AW, Stretavsky MAM. Screening criteria for the detection of retinopathy of prematurity in patients in a neonatal intensive care unit. J Pediatr Ophthalmol Strabismus. 1987;24:212–214.
105 Clark C, Gibbs JAH, Maniello R, et al. Blood transfusions: a possible risk factor in retrolental fibroplasias. Acta Paediatr Scand. 1981;70:535–539.
106 Bauer CR, Widmayer SM. A relationship between PaCO2 and retrolental fibroplasia (RLF). Pediatr Res. 1981;15:649.
107 Flower RW. A new perspective on the pathogenesis of retrolental fibroplasia: the influence of elevated arterial CO2. Retinopathy of Prematurity Conference. 1981:4–6. Dec
108 Biglan AW, Brown DR, Reynolds JD, et al. Risk factors associated with retrolental fibroplasias. Ophthalmology. 1981;91:1504–1511.
109 Brown DR, Milley JR, Ripepi U, et al. Retinopathy of prematurity – risk factors in a five-year cohort of critically ill premature neonates. Am J Dis Child. 1987;141:154–160.
110 Kretzer FL, Hittner HM, Johnson AT, et al. Vitamin E and retrolental fibroplasia: ultrastructural support of clinical efficacy. Ann NY Acad Sci. 1982;393:145–166.
111 Aranda JV, Clark TE, Maniello R, et al. Blood transfusions (BT): possible potentiating risk factor in retrolental fibroplasia (RLF). Pediatr Res. 1975;9:362.
112 Bossi E, Koerner F, Zulauf M. Retinopathy of prematurity (ROP): risk factors – a statistical analysis with matched pairs. Retinopathy of Prematurity Conference. 1981:4–6. Dec
113 Procianoy RS, Garcia-Prats JA, Hittner HM, et al. An association between retinopathy of prematurity and interventricular hemorrhage in very low birth weight infants. Acta Paediatr Scand. 1981;70:473–477.
114 Sacks M, Schaffer DB, Anday EK, et al. Retrolental fibroplasia and blood transfusion in very low birth-weight infants. Pediatrics. 1981;68:770–774.
115 Hammer ME, Mullen PW, Ferguson JG, et al. Logistic analysis of risk factors in acute retinopathy of prematurity. Am J Ophthalmol. 1986;102:1–6.
116 Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640.
117 Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–236.
118 Hardy RJ, Palmer EA, Dobson V, et al. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697–1701.
119 Palmer EA. Optimal timing of examination for acute retrolental fibroplasia. Ophthalmology. 1981;88:662–668.
120 Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of prematurity infants for retinopathy of prematurity. Pediatrics. 2006;117:572–576. Erratum in: Pediatrics 2006;118:1324
121 Palmer EA. Risks of dilating a child’s pupils. Trans Pac Coast Oto Ophthalmol Soc. 1982;63:141–145.
122 Johnson L, Schaffer D, Boggs TR. The premature infant, vitamin E deficiency, and retrolental fibroplasia. Am J Clin Nutr. 1974;27:1158–1171.
123 Johnson LH, Schaffer DB, Goldstein DE, et al. Influence of vitamin E treatment (Rx) and adult blood transfusions on mean severity of retrolental fibroplasia (MS-RLF) in premature infants. Pediatr Res. 1977;11:535.
124 Hittner HM, Godio LB, Rudolph AJ, et al. Retrolental fibroplasia: efficacy of vitamin E in a double-blind clinical study of preterm infants. N Engl J Med. 1981;305:1365–1371.
125 Phelps DL. Vitamin E and retinopathy of prematurity. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:181–206.
126 Phelps DL, Rosenbaum AL, Isenberg SJ, et al. Tocopherol efficacy and safety for preventing retinopathy of prematurity: a randomized, controlled, double-masked trial. Pediatrics. 1987;79:489–500.
127 Finer NN, Schindler RF, Peters KL, et al. Vitamin E and retrolental fibroplasia: improved visual outcome with early vitamin E. Ophthalmology. 1983;90:428–435.
128 Puklin JE, Simon RM, Ehrenkranz RA. Influence on retrolental fibroplasia of intramuscular vitamin E administration during respiratory distress syndrome. Ophthalmology. 1982;89:96–103.
129 Milner RA, Watts JL, Paes B, et al. Retrolental fibroplasia in 1500 gram neonates: part of a randomized clinical trial of the effectiveness of vitamin E. Retinopathy of Prematurity Conference. 1981:4–6. Dec
130 Johnson L, Bowen F, Herman N, et al. The relationship of prolonged elevation of serum vitamin E levels to neonatal bacterial sepsis (SEP) and necrotizing enterocolitis (NEC). Pediatr Res. 1983;17:319.
131 Schaffer DB, Johnson L, Quinn GE, et al. Vitamin E and retinopathy of prematurity: follow-up at one year. Ophthalmology. 1985;92:1005–1011.
132 Institute of Medicine. Report of a study. vitamin E and retinopathy of prematurity. Washington, DC: National Academy; 1986.
133 Terry TL. Fibroplastic overgrowth of the persistent tunica vasculosa lentis in premature infants. IV. Etiologic factors. Arch Ophthalmol. 1943;29:54–65.
134 Hepner WR, Krause AC, Davis ME. Retrolental fibroplasia and light. Pediatrics. 1949;3:824–828.
135 Locke JC, Reese AB. Retrolental fibroplasia: the negative role of light, mydriatics, and the ophthalmoscopic examinations in its etiology. Arch Ophthalmol. 1952;48:44–47.
136 Reynolds JD, Hardy RJ, Kennedy KA, et al. Lack of efficacy of light reduction in preventing retinopathy of prematurity. N Engl J Med. 1998;338:1572–1576.
137 Nagata M, Kobayashi Y, Fukuda H. Photocoagulation for the treatment of the retinopathy of prematurity (first report). J Clin Ophthalmol. 1968;22:419.
138 Oshima K, Ikui H, Kano M, et al. Clinical study and photocoagulation of retinopathy of prematurity. Folia Ophthalmol Jpn. 1971;22:700–707.
139 Payne JW, Patz A. Treatment of acute proliferative retrolental fibroplasia. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:1234–1246.
140 Yamashita Y. Studies on retinopathy of prematurity. III. Cryocautery for retinopathy of prematurity. Rinsho Ganka. 1972;26:385–393.
141 Palmer EA, Biglan AW, Hardy RJ. Retinal ablative therapy for active proliferative retinopathy of prematurity: history, current status, and prospects. In: Silverman WA, Flynn JT. Retinopathy of prematurity. Boston: Blackwell; 1985:207–228.
142 Kingham JD. Acute retrolental fibroplasia. II. Treatment by cryosurgery. Arch Ophthalmol. 1978;96:2049–2053.
143 Kalina RE. Treatment of retrolental fibroplasia. Surv Ophthalmol. 1980;24:229–236.
144 Hindle NW. Cryotherapy for retinopathy of prematurity to prevent retrolental fibroplasias. Can J Ophthalmol. 1982;17:207–212.
145 Mousel DK. Cryotherapy for retinopathy of prematurity: a personal retrospective. Ophthalmology. 1985;92:375–378.
146 Tasman W, Brown GC, Schaffer DB, et al. Cryotherapy for active retinopathy of prematurity. Ophthalmology. 1986;93:580–585.
147 Palmer EA, Hardy RJ, Dobson V, et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123:311–318.
148 Palmer EA, Phelps DL. Multicenter trial of cryotherapy for retinopathy of prematurity. Pediatrics. 1986;77:428–429.
149 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Manual of Procedures. Archived at the National Technical Information Service, Springfield, Va: US Department of Commerce, NTIS accession no. PB88–16350; 1988.
150 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110–1118.
151 Landers MB, Semple HC, Ruben JB, et al. Argon laser photocoagulation for advanced retinopathy of prematurity. Am J Ophthalmol. 1990;110:429–431.
152 Landers MB, III., Toth CA, Semple CS, et al. Treatment of retinopathy of prematurity with argon laser photocoagulation. Arch Ophthalmol. 1992;110:44–47.
153 McNamara JA, Tasman WS, Brown GC, et al. Laser photocoagulation for retinopathy of prematurity. Ophthalmology. 1991;98:576–580.
154 O’Keefe M, Burke J, Algawi K, et al. Diode laser photocoagulation to the vascular retina for progressively advancing retinopathy of prematurity. Br J Ophthalmol. 1995;79:1012–1014.
155 Hammer ME, Pusateri TJ, Hess JB, et al. Threshold retinopathy of prematurity. Transition from cryopexy to laser treatment. Retina. 1995;15:486–489.
156 Capone A, Jr., Diaz-Rohena R, Sternberg P, Jr., et al. Diode-laser photocoagulation for zone 1 threshold retinopathy of prematurity. Am J Ophthalmol. 1993;116:444–450.
157 Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993;100:238–244.
158 McNamara JA, Tasman W, Vander JF, et al. Diode laser photocoagulation for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1992;110:1714–1716.
159 Fleming TN, Runge PE, Charles ST. Diode laser photocoagulation for prethreshold, posterior retinopathy of prematurity. Am J Ophthalmol. 1992;114:589–592.
160 DeJoyce MH, Ferrone PJ, Trese MT. Diode laser ablation for threshold retinopathy of prematurity. Arch Ophthalmol. 2000;118:365–367.
161 White JE, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy for threshold retinopathy of prematurity. Three-year outcome. J Pediatr Ophthalmol Strabismus. 1997;34:83–87.
162 Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696.
163 Early Treatment for Retinopathy of Prematurity Cooperative Group. Multicenter trial of early treatment for retinopathy of prematurity: study design. Controlled Clin Trials. 2004;25:311–325.
164 Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008;28(3 Suppl):S19–S25. Erratum in: Retina 2009; 29:127
165 Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38:233–237.
166 Lalwani GA, Berroca AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28(Suppl):S13–S18.
167 Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008;92:1450–1455.
168 Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. JAAPOS. 2010;14:6–10.
169 Wu WC, Yeh PT, Chen SN, et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology. 2011;118:176–183.
170 Mintz-Hittner HA, Kennedy KA, Chuang AZ, et al. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615.
171 Coats G. Forms of retinal disease with massive exudation. R. Lond Ophthalmic Hosp Rep. 1908;17:440–525.
172 Shields JA, Shields CL, Honavar SG, et al. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford memorial lecture. Am J Ophthalmol. 2001;131:561–571.
173 Shapiro MJ, Chow CC, Karth PA, et al. Effects of green diode laser in the treatment of pediatric Coats disease. Am J Ophthalmol. 2011;151:725–731. e2
174 Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson memorial lecture. Am J Ophthalmol. 1997;124:587–626.
175 Shaikh S, Trese MT. Lens-sparing vitrectomy in predominantly posterior persistent fetal vasculature syndrome in eyes with nonaxial lens opacification. Retina. 2003;23:330–334.
176 Zhang C, Asnaghi L, Gongora C, et al. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol. 2011;90:440–448.
177 Jin DY, Jeang KT. Isolation of full-length cDNA and chromosomal localization of human NF-κB modulator NEMO to Xq28. J Biomed Sci. 1999;6:115–120.
178 O’Doherty M, Mc Creery K, Green AJ, et al. Incontinentia pigmenti – ophthalmological observation of a series of cases and review of the literature. Br J Ophthalmol. 2011;95:11–16.
179 Walsh MK, Drenser KA, Capone A, Jr., et al. Early vitrectomy effective for Norrie disease. Arch Ophthalmol.. 2010;128:456–460.
180 Chen ZY, Battinelli EM, Fielder A, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5:180–183.
181 Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by norrin and frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895.
182 Junge HJ, Yang S, Burton JB, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311.
* We agree with the conclusions in that report but do not agree with all the reasons cited by the authors. They point out that, by combining data from the early clinical studies in oxygen, approximately one-third of the “high-oxygen” group infants did not develop ROP. The failure to detect ROP in many of the high-oxygen group could be readily explained by the inability to view the peripheral fundus with the direct ophthalmoscope, the instrument used in studies in the early 1950s. As a result, patients with stages 1 and 2 ROP, particularly in zone III or peripheral zone II, could have been recorded as normal. Furthermore, because the majority of cases of stages 1 and 2 disease undergo spontaneous regression, ROP would not have been detected on follow-up examination.

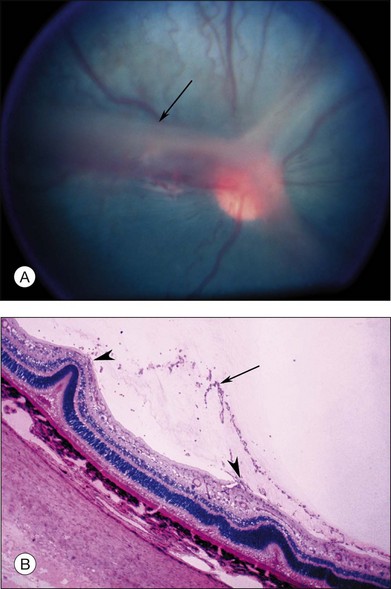
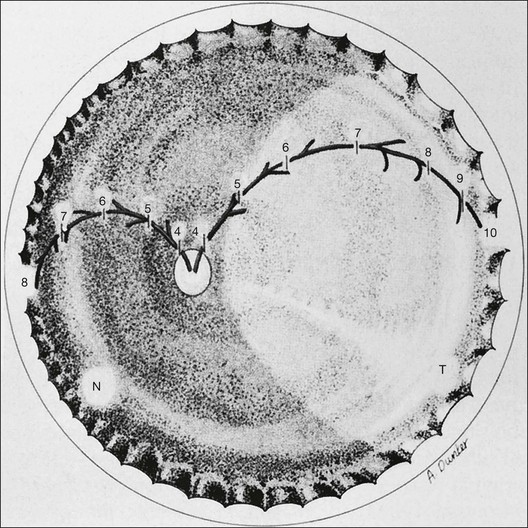
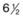 -month-gestation human fetus. N, nasal retina; T, temporal retina; numbers refer to months’ gestational age.
-month-gestation human fetus. N, nasal retina; T, temporal retina; numbers refer to months’ gestational age.