Chapter 58B Pancreatic cancer
Clinical aspects, assessment, and management
Clinical Presentation
Ongoing research has produced a multitude of potential biomarkers for pancreatic cancer (Harsha et al, 2009). The only biomarker that has recognized clinical utility is carbohydrate antigen 19-9 (CA19-9); however, its usefulness has two significant limitations. First, it is not specific for pancreatic cancer because it can be elevated in benign conditions, particularly those that cause obstructive jaundice. Second, its sensitivity is reduced by the fact that patients who test negative for Lewis blood group antigens A and B are unable to synthesize CA19-9 and therefore do not express it in their serum. The percentage of pancreatic cancer patients who fall into this group has been reported to range from 10% to 34% (Berger et al, 2008; Tempero et al, 1987). Thus CA19-9 best serves as a marker of treatment response and recurrence in patients who have a pathologic diagnosis of pancreatic cancer.
Diagnosis
Once suspicion is sufficient for pancreatic cancer, high-quality imaging is critical for diagnosis and treatment planning. Many patients initially undergo an abdominal ultrasound (US) or a limited computed tomography (CT) scan of the abdomen. These modalities will suggest processes in the pancreas that require appropriate evaluation. Currently, the best diagnostic modality for imaging the pancreas is a pancreas protocol multidetector CT with dedicated arterial and venous phases and three-dimensional (3D) reconstruction (see Chapter 16; Buchs et al, 2010; Horton & Fishman, 2002). Water is given orally, and contrast is given intravenously. Such scans typically demonstrate the tumor as a low-density (hypodense) lesion within the pancreas, best seen during the venous phase of contrast enhancement. Importantly, these high-quality imaging studies show the important relationship between the tumor and the surrounding visceral vessels, including the superior mesenteric vein, portal vein, splenic vein, superior mesenteric artery (SMA), and the branches of the celiac axis. Such high-quality CT imaging can reliably predict visceral vessel involvement, and thereby surgical respectability, approximately 80% to 90% of the time (House et al, 2004; Karmazanovsky et al, 2005). Additionally, the arterial phase with 3D reconstruction can assist in surgical planning by identifying variations in hepatic arterial anatomy and other vascular anomalies preoperatively, most notably a “replaced” right hepatic artery originating from the SMA, which is seen in one of six patients (see Chapter 1B).
Continuing improvement in the technology that produces magnetic resonance imaging (MRI) has resulted in image quality and resultant diagnostic sensitivities that approach those of CT (see Chapter 17). A recent meta-analysis of 68 articles calculated an overall diagnostic sensitivity of 91% for CT, compared with 84% for MRI, and reported diagnostic specificities of 85% and 82%, respectively (Bipat et al, 2005). Regarding determination of resectability, CT had a sensitivity and specificity of 81% and 82% compared with MRI at 82% and 78%, respectively. No advantage has been demonstrated by obtaining both CT and MRI for uncomplicated patients who display classic symptoms of pancreatic cancer, and this practice should be discouraged. We typically reserve the use of MRI for patients with renal impairment or sensitivity to the intravenous contrast used for CT. The use of MR cholangiopancreatography (MRCP) is valuable in evaluating and following small cystic lesions of the pancreas, but a detailed discussion of these lesions is outside the scope of this chapter (see Chapters 17 and 57).
Endoscopic evaluation of patients with classic symptoms of pancreatic cancer is appropriate in specific situations. Esophagogastroduodenoscopy (EGD) is appropriate in cases of gastric outlet obstruction to assess the anatomy and presence of other diagnoses. In the presence of metastatic or unresectable disease, or when a patient is not physiologically appropriate for immediate surgical intervention, a therapeutic EGD combined with duodenal stenting can be of some benefit, allowing the patient to return to oral intake (see Chapter 27). Endoscopic retrograde cholangiopancreatography (ERCP) was once commonly used as a diagnostic modality for patients with a suspected periampullary pancreatic cancer. With the improvement of cross-sectional imaging, the use of strictly diagnostic ERCP is currently unsupported. Therapeutic ERCP with stenting of biliary strictures can be of benefit in the same patient population (metastatic, unresectable, or physiologically unfit) as therapeutic EGD (see Chapters 14, 18, and 27). However, the routine placement of a biliary endoprosthesis for all patients seen with jaundice without cholangitis should be discouraged. Multiple studies have shown a doubling of the wound infection risk and a slight increase in overall complication risk with preoperative biliary stenting (Pisters et al, 2001a; Sohn et al, 2000). For most patients seen initially with a pancreatic mass and jaundice, early surgery is preferable to endoscopic stenting and delayed surgical resection (Kennedy et al, 2010).
Endoscopic ultrasound (EUS) is a relatively new imaging and diagnostic technology compared with those previously discussed (see Chapter 14). Well established at specialty referral centers, it is rapidly becoming more widely available. Interpretation of EUS imaging is operator dependent, a factor that may account for the wide range of diagnostic sensitivities reported in the literature and encountered in real-world clinical settings (Ahmad et al, 2000; Gress et al, 1999; Hunt & Faigel, 2002; Palazzo et al, 1993; Rösch et al, 1991; Varadarajulu & Eloubeidi, 2010). Reported sensitivities for the diagnosis of pancreatic neoplasia have ranged from 69% to 94%. Several studies have found EUS to be superior to CT for the detection of lesions smaller than 2 cm. EUS is of particular utility when a patient has a presentation consistent with pancreatic cancer (biliary or pancreatic duct obstruction) but no evidence of mass on cross-sectional imaging. The ability of EUS to accurately estimate T-stage decreases compared with CT for larger lesions. Additionally, EUS has shown some superiority over CT for the detection of venous invasion, but it is less useful for the determination of arterial involvement (Varadarajulu & Eloubeidi, 2010). An important additional advantage of EUS is the ability to obtain tissue via fine needle aspiration (FNA) for diagnostic purposes at the time of evaluation. This can be of great benefit to unresectable or marginally resectable patients who need a confirmed tissue diagnosis prior to the initiation of chemotherapy. However, for the vast majority of patients with a symptomatic pancreatic mass that appears completely resectable by CT or MR imaging, EUS with FNA is not needed; in most cases the mass will require resection regardless of the EUS and FNA results.
18Fluorodeoxyglucose-positron emission tomography (18FDG-PET) is a functional imaging modality based on the observation that neoplastic cells exhibit accelerated glucose uptake and metabolism (see Chapter 15). Once transported into the cell and phosphorylated by hexokinase, the first step in glycolysis, 18FDG remains trapped within the cell. Although studies suggest 18FDG-PET is sensitive for the detection of pancreatic cancer, the precise role of this technique in the diagnosis and staging of patients with localized pancreatic cancer is limited. Its use is confounded by the difficulty 18FDG-PET has in distinguishing benign from malignant masses as a result of localization, both to sites of inflammation and infection and to neoplasms. 18FDG-PET is of greater utility in evaluating indeterminate masses in the liver that are not amenable to biopsy. A tissue diagnosis of a primary mass made by EUS, when combined with a PET-positive liver lesion, is adequate to initiate palliative chemotherapy at many centers.
Staging
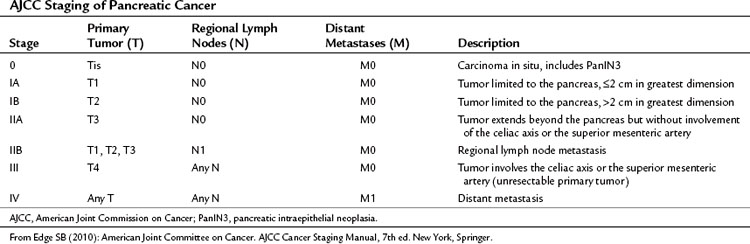
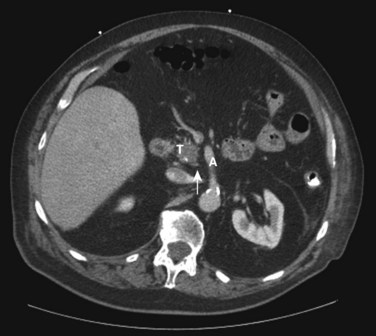
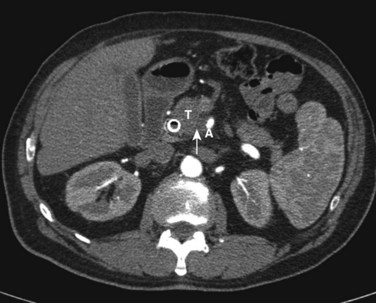
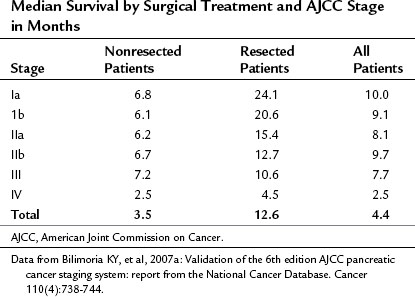
Pancreatic cancer is staged by the seventh edition (2010) of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, according to clinical parameters that are available from high-quality CT imaging (Edge, 2010). Classic T, N, and M parameters are used for tumor size, nodal involvement, and distant metastases, but stage grouping is performed according to resectability. Patients are classified as having localized resectable disease (stages I and II), locally advanced unresectable disease (stage III), or distant metastatic disease (stage IV; Table 58B.1). A resectable tumor is characterized on CT by no evidence of metastatic disease, a clear tissue (fat) plane between the tumor and the visceral arteries (celiac axis and SMA), and 180-degree or less circumferential involvement of the superior mesenteric vein–portal vein confluence (Fig. 58B.1). In contrast, patients with unresectable disease exhibit distant metastases, ascites, or involvement of the celiac axis or SMA (Fig. 58B.2). Venous vascular involvement is considered potentially resectable in the AJCC system and is therefore categorized as stage II disease, and arterial involvement is considered unresectable and is categorized as stage III disease. A small subset of patients with complete occlusion of the superior mesenteric vein–portal vein confluence and significant collateralization that precludes safe venous resection and reconstruction are typically deemed unresectable but are still classified as stage II in the current AJCC system. This system has been validated by correlation to overall survival (OS) using the National Cancer Database (Table 58B.2; Bilimoria et al, 2007a).
Laparoscopy
Diagnostic laparoscopy for suspected or confirmed pancreatic cancer has the potential to detect small surface liver or peritoneal metastases (see Chapter 21). It is limited, however, in its ability to evaluate locally advanced disease. Prior to the development of current imaging techniques, namely three-dimensional MDCT, unresectability rates at laparotomy exceeded 30%. Modern cross-sectional imaging has reduced that rate considerably, as discussed above.
Disagreement exists in the literature about the role of diagnostic laparoscopy in the evaluation of pancreatic cancer. Multiple authors have reported series evaluating the utility of diagnostic laparoscopy. The rate at which diagnostic laparoscopy alters subsequent management is reported at between 4% and 40% (Pisters et al, 2001b; Shah et al, 2008; Stefanidis et al, 2006). Taking the data as a whole, diagnostic laparoscopy seems best reserved for selected patients, in whom an increased likelihood of intraabdominal dissemination exists. Such cases include tumors greater than 4 cm, particularly those on the left side of the pancreas; ascites; markedly elevated CA19-9 (>1000 U/mL); and small, indeterminate liver or peritoneal lesions seen on CT, which are too small to investigate with percutaneous biopsy or PET imaging. We rarely use diagnostic laparoscopy for right-sided lesions, and our use of diagnostic laparoscopy for left-sided tumors has declined in recent years, as CT staging has become more accurate.
Surgical Treatment
Resectable Disease (See Chapters 62A and 62B)
The goal of the initial evaluation process for patients with pancreatic cancer is classification into one of three broad groups: resectable, locally advanced/borderline resectable, or unresectable. This determination should be made in consultation with an expert in pancreatic surgery. Standards as to what constitutes resectable disease have broadened, as more experience is gained with pancreatic resection. A 2007 study by Bilimoria and colleagues revealed that of the more than 9500 U.S. patients identified in the National Cancer Database as having clinical stage 1 pancreatic cancer, more than 70% did not undergo surgery. In this group, the reason for not undergoing surgery could not be identified in more than 50% of patients, and a minority were deemed too old or unfit for pancreatectomy. This represents a gross underutilization of surgical intervention for potentially curable pancreatic cancer in the United States, which the authors postulate may be due to a nihilistic attitude toward pancreatic cancer care.
Surgical resection of pancreatic cancer remains the only potentially curative therapy. Unfortunately, only a minority of patients (approximately 20% to 30%) diagnosed with pancreatic cancer are candidates for curative resection at the time of diagnosis. Hopefully, as early detection schemes improve and gain widespread use, and our ability to identify high-risk groups improves, the percentage of patients who are candidates for resection will increase. Resectional approaches are based on tumor location and extent. Resection of right-sided tumors typically requires pancreatoduodenectomy, most often performed with pylorus preservation. Distal pancreatectomy is used to resect left-sided tumors. In a small group of patients with extensive parenchymal involvement of the pancreas, total pancreatectomy may be required. The specific techniques of such resectional procedures can be found in Chapter 62A, Chapter 62B .
Results
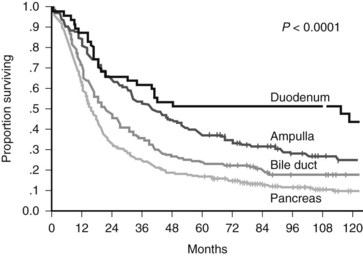
Recurrent controversies have persisted over the effectiveness of surgical resection for pancreatic cancer (Crile, 1970; Gudjonsson, 1995). The preponderance of recent data that have emerged from large specialized centers refute claims of futility and lack of long-term survival. A recent publication by Riall and colleagues (2006) examines the actual 5-year survival rates after pancreatoduodenectomy, all stages combined, for pancreatic and periampullary cancer and reports actuarial 10-year survival. The actual 5-year survival for resected pancreatic cancer (all stages combined) was 17%, but the actuarial 10-year survival was 9% (Fig. 58B.3). This analysis included a positive lymph node rate of 48% and a positive margin rate of 8% in the overall periampullary cohort. A similar study by Ferrone and colleagues (2008) revealed an actual 5-year survival rate of 23% for resected stage Ia disease, and all-stage actual 5-year and 10-year survival rates of 12% and 5%, respectively, were reported (Ferrone et al, 2008).
Adjuvant Therapy
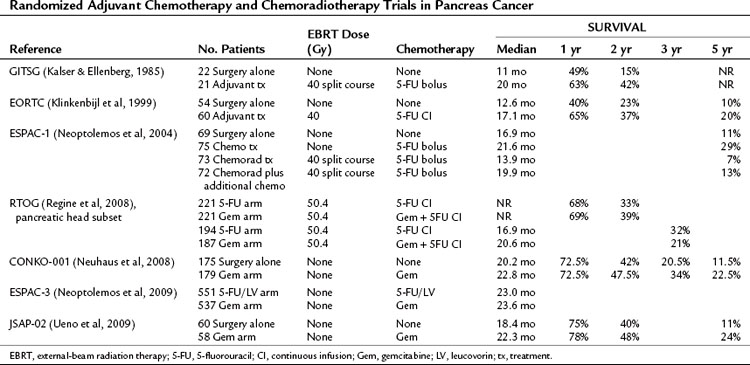
Because of the great propensity of pancreatic cancer to recur or persist even after optimal surgical resection, clinicians have used adjuvant chemotherapy with or without radiotherapy in an attempt to prolong disease-free survival and increase OS (see Chapter 63A). The most noteworthy of the early trials was a prospective, randomized trial from the Gastrointestinal Tumor Study Group (GITSG) using split-course irradiation (40 Gy) with concurrent bolus 5-fluorouracil (5-FU) followed by maintenance 5-FU for a duration of up to 2 years (Kalser & Ellenberg, 1985). Patients were randomized to treatment versus a no-treatment control arm. The trial showed a survival advantage for adjuvant chemoradiotherapy (median survival, 20 months) compared with surgery alone (median survival, 11 months; P = .01). Although criticized for its small numbers (43 total patients), slow accrual, and failure to meet accrual targets, the GITSG trial was the first trial to document a survival advantage for adjuvant therapy in pancreatic adenocarcinoma.
Subsequent to this initial study, six additional randomized adjuvant chemotherapy or chemoradiotherapy trials have been published (Table 58B.3; Klinkenbijl et al, 1999; Neoptolemos et al, 2004, 2009; Neuhaus et al, 2008; Regine et al, 2008; Ueno et al, 2009). A randomized controlled trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) showed a trend toward improved survival with adjuvant 5-FU–based chemoradiation compared with surgery alone in patients with periampullary and pancreatic cancer (Klinkenbijl et al, 1999). However, this study was statistically underpowered and initially reported as a negative trial.
The results of the European Study Group for Pancreatic Cancer (ESPAC-1) trial were met with controversy (Neoptolemos et al, 2004). In this randomized controlled trial of adjuvant therapy for pancreatic cancer, patients were assigned to one of four groups: treatment with 5-FU–based chemoradiation alone, chemotherapy alone (5-FU plus leucovorin), chemoradiation followed by chemotherapy, or observation. Using a two-by-two factorial design, the authors concluded that adjuvant chemotherapy conferred a survival benefit, whereas adjuvant chemoradiation had a deleterious effect on survival. This study is often criticized for methodologic problems, such as a high rate of nonadherence for the patients assigned to receive chemotherapy, and for radiation therapy quality control issues (Choti, 2004). Because of the study design, statistical power was insufficient to compare each of the four treatment groups individually. However, when compared to the observation group, patients who received chemoradiation alone appeared to have a worse median survival, suggesting a possible role for treatment-related toxic radiation effects.
The Radiation Therapy Oncology Group (RTOG) protocol R97-04 was designed to determine whether the addition of gemcitabine to postoperative adjuvant 5-FU chemoradiotherapy improved survival for patients with resected pancreatic adenocarcinoma (Regine et al, 2008). This phase III multi-institution trial randomized resected pancreatic adenocarcinoma patients to either one cycle of continuous infusion 5-FU, followed by continuous infusion 5-FU during radiotherapy (50.4 Gy), followed by two additional cycles of continuous 5-FU or one cycle of gemcitabine, followed by continuous infusion 5-FU during radiotherapy (50.4 Gy), followed by three additional cycles of gemcitabine. Patients with pancreatic head tumors had a median survival of 20.5 months and a 3-year survival of 31% in the gemcitabine group versus 16.9 months and 22% in the 5-FU group (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.65 to 1.03; P = .09). After adjusting for the protocol-specified stratification variables of nodal status, which strongly affected survival (P = .001), tumor diameter, and surgical margin status, the multivariate analysis for patients with pancreatic head tumors treated with gemcitabine yielded an HR of 0.80 (95% CI, 0.63 to 1.00; P = .05), reflecting improved survival for the gemcitabine group.
The Charité Onkologie study (CONKO-001) compared adjuvant gemcitabine therapy to no postoperative anticancer therapy in patients undergoing surgical resection of pancreatic cancer (Neuhaus et al, 2008). Patients in the treatment arm received six cycles of gemcitabine therapy over 6 months with acceptable toxicity. Overall, 368 patients were randomized and 354 were included in the intention-to-treat analysis. Patients in the treatment arm had a statistically significant increase in OS on final analysis, with a median OS of 22.8 months and a 5-year survival of 21% compared with a 20.2-month median survival and a 5-year survival of 9.0% for the control arm (P = .005).
ESPAC-3 is the largest randomized trial of adjuvant postresection treatment of pancreas cancer to date (Neoptolemos et al, 2009). The trial randomized patients who had undergone either an R0 or R1 resection to adjuvant 5-FU plus leucovorin versus adjuvant gemcitabine. Radiation therapy was not used. Over a 6.5-year period, 1088 patients from 16 countries were randomized. Median survival for the 5-FU/leucovorin arm was 23.0 months, and median survival for the gemcitabine arm was 23.6 months. The authors concluded that no significant difference in survival was evident between the two treatment arms.
A study recently published by the Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer (JSAP) ran concurrent with the CONKO-001 trial and was of similar design (Ueno et al, 2009). It also randomized resected pancreas cancer patients to either observation or adjuvant gemcitabine therapy. OS was extended in the gemcitabine group (22.3 months vs. 18.4 months in the control arm), but the difference did not reach statistical significance. The authors reported their belief that their study was underpowered to detect a difference, given its smaller enrollment than the CONKO-001 trial.
Neoadjuvant Therapy and Borderline Resectable Disease
When preoperative imaging reveals either tumor encroachment with significant compromise of the mesenteric venous structures that is not amenable to reconstruction or encroachment on the superior mesenteric artery or celiac axis, patients are classified as borderline resectable. A skilled pancreatic surgeon can often remove such tumors safely, however, it is statistically unlikely that an R0 resection can be accomplished in such settings. Therefore, neoadjuvant chemotherapy or chemoradiotherapy is usually initiated with the goal of downstaging the tumor such that an R0 resection is possible. In theory, therapy administered in the preoperative neoadjuvant versus the postoperative adjuvant setting offers several potential advantages: the therapy would be given up front, eliminating the risk of delayed or incomplete treatment postoperatively; patients who manifest metastatic disease following neoadjuvant therapy would avoid an operation unlikely to be beneficial; and, because radiation efficacy is dependent on oxygenation, it may be more effective given before tissues are devascularized by surgery. The M.D. Anderson group has recently published reports of two phase II trials of either gemcitabine-based neoadjuvant chemoradiotherapy or preoperative gemcitabine and cisplatin chemotherapy in addition to chemoradiation (Evans et al, 2008; Varadhachary et al, 2008). They report resection rates of 74% and 58%, respectively, for patients entered into each protocol. Median survival for the subset of patients who actually underwent resection is promising, at 34 months and 31 months, respectively.
Another report from the same group described three types of borderline patients: type A is borderline resectable by objective anatomic criteria; type B is borderline resectable because of findings suggestive but not diagnostic of metastatic disease; and type C is borderline operable because of marginal performance status or extensive comorbidity (Katz et al, 2008). The 160-patient group was composed of 84 type A, 44 type B, and 32 type C patients classified as borderline resectable. After neoadjuvant treatment and restaging, 66 patients (41%) underwent pancreatectomy. The median survival was 40 months for the 66 borderline patients who completed all therapy but only 13 months for the 94 patients who did not undergo pancreatectomy (P < .001).
Palliative Therapy
Unresectable Disease (see Chapters 63A and 63B)
The first goal in treating patients with unresectable and/or metastatic disease is to provide adequate palliation for symptoms related to their diagnosis, particularly obstructive jaundice, gastroduodenal obstruction, and abdominal pain. A more detailed discussion of these topics is provided in Chapters 63A and 63B. Modern endoscopic therapy and interventional radiology procedures can often provide substantial palliation of biliary obstruction and gastric outlet obstruction. When patients cannot be palliated in this fashion, surgical palliation through gastrojejunostomy and Roux-en-Y hepaticojejunostomy is indicated to relieve symptoms of duodenal and biliary obstruction and allow physiologic improvement such that patients can subsequently receive palliative chemotherapy or chemoradiotherapy. Additionally, when patients are determined to be unresectable at the time of exploration with curative intent, the opportunity exists to provide surgical palliation of duodenal obstruction or tumor-associated pain, two symptom complexes that can cause suffering and interrupt other cancer-directed therapies. Prophylactic gastrojejunostomy at the time of initial exploration has been shown to predispose to symptomatic duodenal obstruction in this population of patients (Lillemoe et al, 1999).
Another beneficial intervention that can be provided at the time of exploration is celiac plexus nerve block. This simple intervention has been shown to decrease pain and postoperative narcotic use, and, in the subset of unresectable patients who present with pain, to prolong survival (Lillemoe et al, 1993). The procedure is simple and carries minimal risk and expense when done during surgical exploration. It can also be done endoscopically or percutaneously, with pain relief that is often superior to that suggested by the stepwise analgesic ladder provided by the World Health Organization (WHO) (Wong et al, 2004).
Palliative Chemotherapy
Chemotherapy is provided in the unresectable and metastatic setting with the intention of prolonging life while preserving or improving quality of life. The current standard of care for advanced pancreatic cancer was set in 1997 by Burris and colleagues in their landmark study, which showed a significant prolongation in OS, as well as a concurrent clinical benefit response, in patients with advanced pancreatic cancer treated with gemcitabine compared with the previous standard chemotherapy (5-FU). The efficacy measure was clinical benefit response, which was a composite of measurements of pain, specifically, analgesic consumption and pain intensity; Karnofsky performance status; and weight. Clinical benefit required a sustained (≥4 weeks) improvement in at least one parameter without worsening in any others. Since that time, only one other chemotherapeutic, erlotinib, has been granted a Food and Drug Administration (FDA) therapeutic indication for use against pancreatic cancer (Moore et al, 2007). Of note, the inclusion of erlotinib as standard of care has been controversial, given the minimal improvement in OS (only 0.33 months) when evaluated in the context of the drug’s expense and side effects.
Multiple studies continue to be done in this area that examine combination therapies involving currently available chemotherapeutics and investigational agents (Stathis & Moore, 2010). Most have failed to show any evidence of benefit to patients with advanced pancreatic cancer, although recently, some enthusiasm attended the preplanned interim analysis of the PRODIGE 4/ACCORD 11 trial, presented at the Spring 2010 American Society of Clinical Oncology meeting (Conroy et al, 2010). This randomized Phase III trial compared a combination of 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) versus gemcitabine as first-line therapy for advanced pancreas cancer. At the preplanned interim analysis, the median OS for the FOLFIRINOX group was 10.5 months compared with 6.9 months for the gemcitabine group (P < .001). FOLFIRINOX is the first therapy not containing gemcitabine that has shown a significantly longer OS, progression-free survival, and higher response rate than gemcitabine alone. Determination of the role of this regimen as standard of care will await the full results of the trial and detailed reporting of the toxicity associated with FOLFIRINOX therapy.
Ahmad NA, et al. EUS in preoperative staging of pancreatic cancer. Gastrointest Endosc. 2000;52(4):463-468.
Berger AC, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26(36):5918-5922.
Bilimoria KY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246(2):173-180.
Bipat S, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29(4):438-445.
Buchs NC, et al. Vascular invasion in pancreatic cancer: imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol. 2010;16(7):818-831.
Burris HIII, et al. Improvements in survival and clinical benefit with gemcitabine as first- line therapy for patients with advanced pancreas cancer: randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
Choti MA. Adjuvant therapy for pancreatic cancer—the debate continues. N Engl J Med. 2004;350(12):1249-1251.
Conroy T, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28(7S):4010.
Crile GJr. The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130(6):1049-1053.
Edge SB. American Joint Committee on Cancer. AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010.
Evans DB, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496-3502.
Ferrone CR, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12(4):701-706.
Gress FG, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50(6):786-791.
Gudjonsson B. Carcinoma of the pancreas: critical analysis of costs, results of resections, and the need for standardized reporting. J Am Coll Surg. 1995;181(6):483-503.
Harsha HC, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6(4):e1000046.
Horton KM, Fishman EK. Multidetector row CT with dual-phase CT angiography in the preoperative evaluation of pancreatic cancer. Crit Rev Comput Tomogr. 2002;43(5):323-360.
House MG, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8(3):280-288.
Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc. 2002;55(2):232-237.
Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899-903.
Karmazanovsky G, et al. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30(4):488-500.
Katz MH, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833-846. discussion 846-848
Kennedy EP, Rosato EL, Yeo CJ. Preoperative drainage in pancreatic cancer. N Engl J Med. 2010;362(14):1344. author reply 1345
Klinkenbijl JH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776-782. discussion 782-784
Lillemoe KD, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer: a prospective randomized trial. Ann Surg. 1993;217(5):447-455. discussion 456-457
Lillemoe KD, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230(3):322-328. discussion 328-330
Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2007;25(15):1960-1966.
Neoptolemos J, et al. ESPAC-3(v2): a multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(18S):LBA4505.
Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200-1210.
Neuhaus P, et al. CONKO-001: final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). J Clin Oncol. 2008;26(May 20 Suppl):LBA4504.
Palazzo L, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma: results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993;25(2):143-150.
Pisters PW, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234(1):47-55.
Pisters PW, et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88(3):325-337.
Regine WF, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019-1026.
Riall TS, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140(5):764-772.
Rösch T, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37(3):347-352.
Shah D, et al. Preoperative prediction of complete resection in pancreatic cancer. J Surg Res. 2008;147(2):216-220.
Sohn TA, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4(3):258-267. discussion 267-268
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163-172.
Stefanidis D, et al. The current role of staging laparoscopy for adenocarcinoma of the pancreas: a review. Ann Oncol. 2006;17(2):189-199.
Tempero MA, et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47(20):5501-5503.
Ueno H, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese study group of adjuvant therapy for pancreatic cancer. Br J Cancer. 2009;101(6):908-915.
Varadarajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Surg Clin North Am. 2010;90(2):251-263.
Varadhachary GR, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487-3495.
Wong GY, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291(9):1092-1099.