Other demyelinating diseases
inflammatory and compressive
Neuromyelitis optica, acute disseminated encephalomyelitis, and acute hemorrhagic leukoencephalopathy are covered in this chapter, together with a group of diseases in which demyelination is probably caused by compression. Demyelinating diseases that occur in other clinical contexts are included in the relevant chapters: progressive multifocal leukoencephalopathy in Chapter 14, central pontine myelinolysis and multifocal necrotizing leukoencephalopathy in Chapter 22, and Marchiafava Bignami disease in Chapter 25. The leukoencephalopathies associated with lysosomal and peroxisomal disorders are covered in Chapter 23.
NEUROMYELITIS OPTICA (DéVIC’S DISEASE)
Neuromyelitis optica (NMO) is characterized by the development of optic neuritis and acute transverse myelitis within weeks of each other (Table 20.1). Approximately two-thirds of patients present with visual loss and subsequently develop paraplegia, sensory loss, and loss of bladder and bowel control, but in the remaining third the order may be reversed. Unlike remissions in MS, recovery in NMO is usually incomplete, even from initial attacks. Some patients die during or soon after the acute syndrome, but others, although severely incapacitated, survive for many years.
Table 20.1
Criteria for diagnosis of neuromyelitis optica
Optic neuritis
+
Acute myelitis
+
At least two of the following three supportive criteria:
Adapted from Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006; 66:1485–1489.
MACROSCOPIC APPEARANCES
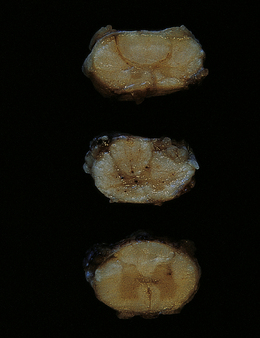
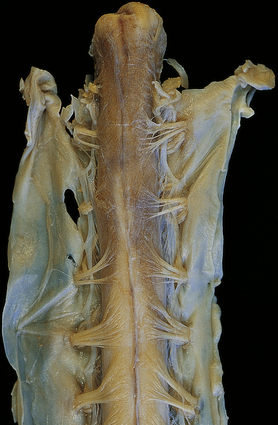
The optic nerves and affected region of the spinal cord are swollen and congested in patients who die during or soon after the acute presentation. The cord may appear necrotic on sectioning (Fig. 20.1). In patients who survive longer, the optic nerves become thin and gray-brown in color, while the cord shows similar discoloration (Fig. 20.2) and may be atrophic.
MICROSCOPIC APPEARANCES
The optic nerves and spinal cord show extensive demyelination (Fig. 20.3). In the acute phase, involved segments of the spinal cord and optic nerve are inflamed, and the cord in particular may be partly necrotic (Fig. 20.4); the inflammatory infiltrates include perivascular neutrophils and eosinophils, and relatively few T cells. There tends to be pronounced perivascular deposition of immunoglobulins (particularly IgM) and C9neo (activated complement), and hyaline fibrosis of small blood vessels. Immunohistochemistry can also be used to demonstrate loss of AQP4 and EEAT2 in the demyelinated regions. The lesions become cavitated and gliotic in those patients who survive the acute stage, and there is usually associated degeneration of ascending and descending tracts.
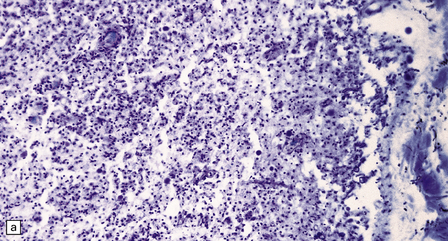
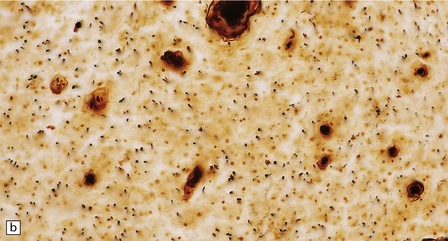
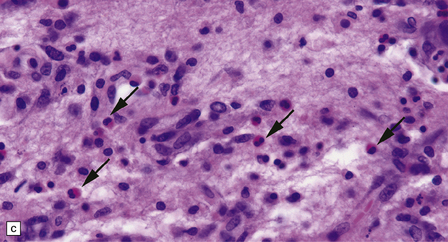
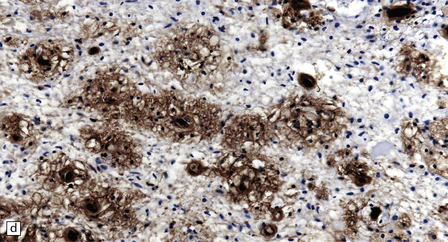
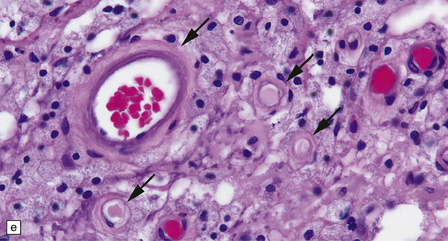
20.4 Spinal white matter in acute neuromyelitis optica.
(a) This shows demyelinated spinal white matter largely replaced by sheets of foamy macrophages. (b) A few surviving axons are demonstrable by silver impregnation. (c) Scattered eosinophils (arrows) are present in some lesions. (d) Immunohistochemistry reveals perivascular deposition of IgM around many small blood vessels in the spinal cord in this example of acute neuromyelitis optica. (e) Hyaline thickening of many of the small blood vessels (arrows) within the demyelinated spinal cord.
ACUTE DISSEMINATED ENCEPHALOMYELITIS (ADEM)
MACROSCOPIC APPEARANCES
Apart from some congestion and swelling, the brain and spinal cord may look macroscopically normal. In some cases, scattered small foci of yellow or gray discoloration, some obviously centered on a small blood vessel, are evident in the white matter (Fig. 20.5). The gray matter may also be involved, although usually to a lesser degree.
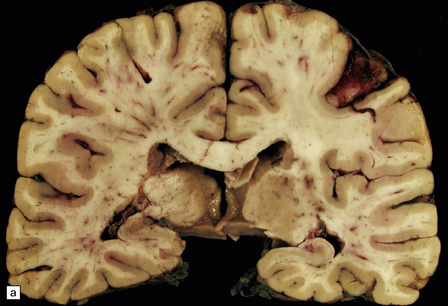
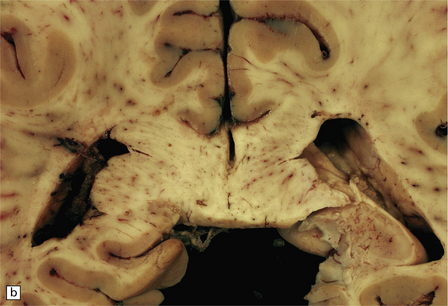
20.5 Macroscopic appearance of brain in ADEM.
(a) Congested blood vessels in the white matter are surrounded by ill-defined zones of gray discoloration. These are most prominent in the left cerebral hemisphere. (b) The deep parietal white matter and corpus callosum have a mottled appearance due to the gray perivascular discoloration.
MICROSCOPIC APPEARANCES
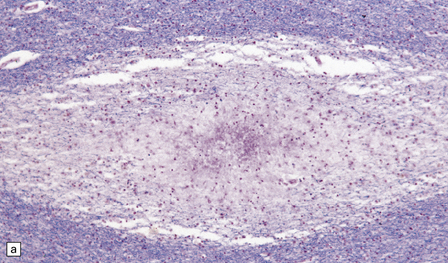
Many small veins and venules within the brain parenchyma are surrounded by an infiltrate of lymphocytes, macrophages, and occasional plasma cells. The inflammatory infiltrate extends a variable distance into the surrounding tissue and is associated with a corresponding zone of demyelination (Fig. 20.6). There may be small perivascular hemorrhages. Although loss of myelin predominates, there may be some axonal destruction. Arteries are relatively free of inflammation (Fig. 20.6), but there are often inflammatory cells in the leptomeninges. Subpial inflammation and demyelination may occur in the brain stem and spinal cord.
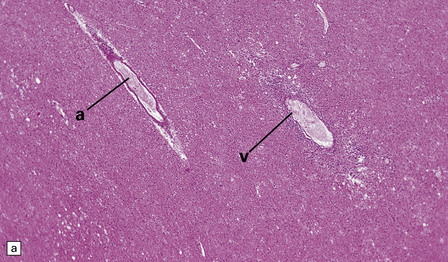
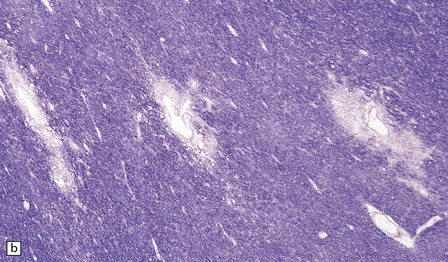
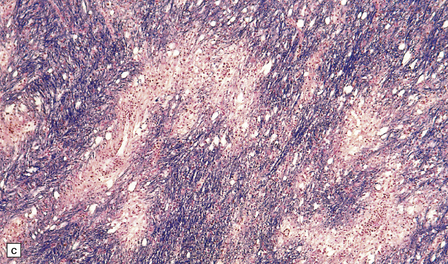


20.6 Histology of ADEM.
(a) Infiltrate of lymphocytes and macrophages around a vein (v) in the cerebral white matter. Note the paucity of inflammation in relation to the adjacent artery. (b) A zone of demyelination surrounds the affected blood vessels. (c)Because the demyelination is so closely related to veins and venules, it tends to form a more irregular pattern than is usually seen in multiple sclerosis. (d) A combined solochrome cyanin stain for myelin and neurofilament immunostain for axons reveals a perivascular sleeve of (brown) axons that lack a (blue) myelin sheath (arrows). (e) Slightly larger zone of perivenular demyelination.
ACUTE HEMORRHAGIC LEUKOENCEPHALOPATHY (AHL)
MACROSCOPIC APPEARANCES
The brain is soft and swollen. Sectioning reveals numerous small and occasional larger foci of hemorrhage, which are most prominent in the cerebral and cerebellar white matter and in the pons (Fig. 20.7).
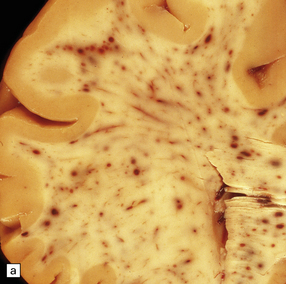
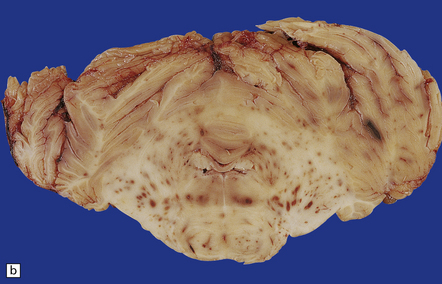
20.7 Macroscopic appearance of brain in AHL.
Section through the cerebrum (a) and the cerebellum and pons (b)of a patient with Crohn’s disease who developed AHL. Perivascular hemorrhages and foci of gray-brown discoloration are scattered throughout the white matter in the cerebrum, and the brain stem and cerebellum. (Courtesy of Dr DA Hilton, Derriford Hospital, Plymouth, UK.)
MICROSCOPIC APPEARANCES
Many small blood vessels undergo fibrinoid necrosis and are surrounded by a narrow zone of necrotic tissue containing nuclear debris and, in some cases, a larger zone of hemorrhage (Fig. 20.8). The classic description is of ring- and ball-shaped perivascular hemorrhages. Other blood vessels are still recognizable as veins or venules, but are surrounded by fibrin and a mixed inflammatory infiltrate, including neutrophils and mononuclear cells. Some fibers within the infiltrates are demyelinated, but others show axonal fragmentation (Fig. 20.9).
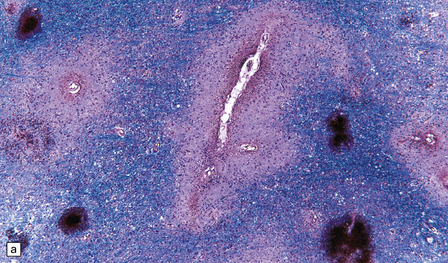
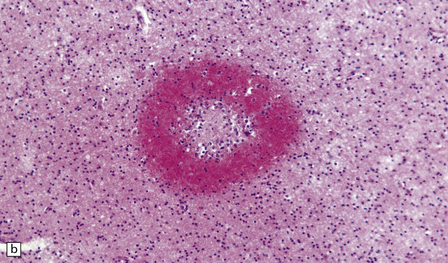
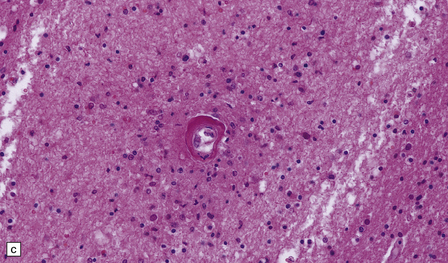
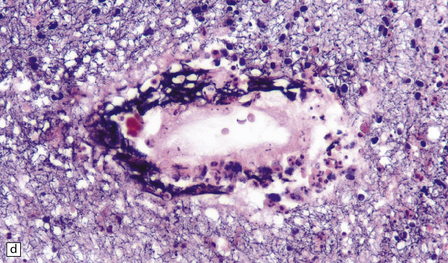
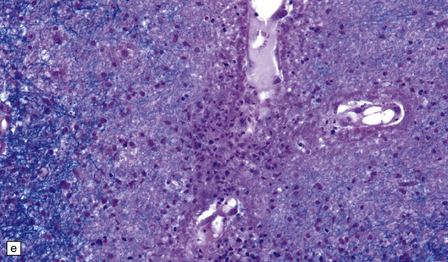
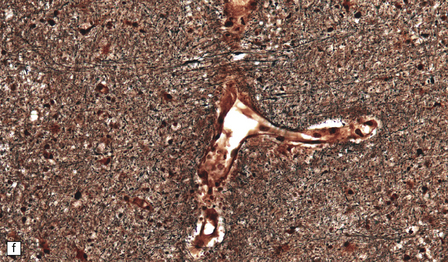
20.8 Histology of AHL.
(a) Scattered ball-shaped perivascular hemorrhages and areas of demyelination in the white matter. (b)Ring-shaped hemorrhage around a zone of fibrinoid necrosis. (c) Blood vessel showing fibrinoid necrosis and surrounded by a zone of demyelinated tissue with a predominantly mononuclear cell inflammatory infiltrate. (d) Acute fibrinoid vascular necrosis. The vessel wall is partly infiltrated by neutrophils. (e) Section through a region of perivenous demyelination. Note the vascular necrosis and inflammatory infiltrate. (f) An adjacent section shows relative axonal preservation.
TRIGEMINAL NEURALGIA (TN)
MACROSCOPIC AND MICROSCOPIC APPEARANCES
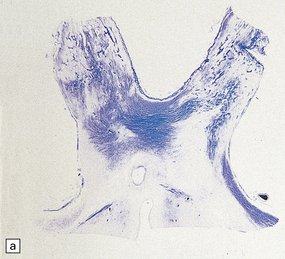
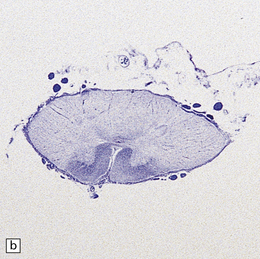
The compression typically involves the proximal, CNS part of the trigeminal nerve root, close to its entry into the brain stem. The root generally appears macroscopically normal but when examined in vivo through an operating microscope there may be slight gray discoloration of the nerve root where it is indented (Fig. 20.10).
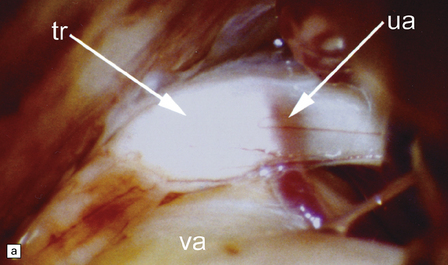
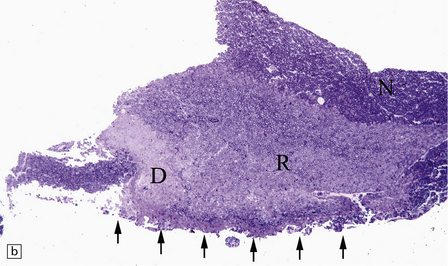
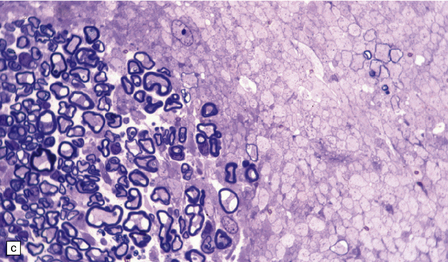
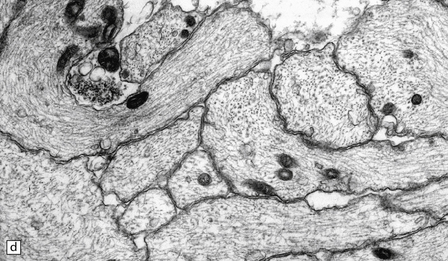
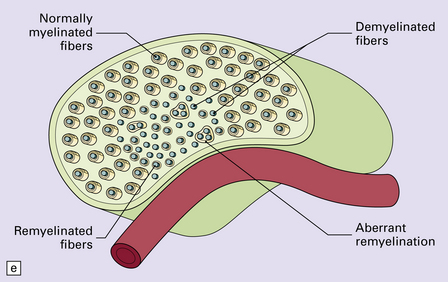
20.10 Trigeminal neuralgia due to vascular compression.
(a) Intraoperative photograph from a patient undergoing partial rhizotomy for TN. The trigeminal nerve root (tr) is focally indented by a small unnamed artery (ua) that passes over the surface of an atherosclerotic, ectatic loop of vertebral artery (va). (b) Trigeminal rhizotomy specimen from another patient with TN due to vascular compression. Arrows indicate the region where the nerve root had been compressed. Areas of demyelination (D), partial remyelination (R) and of normally myelinated (N) nerve root are visible. (c)Higher magnification view through the region of CNS demyelination in the proximal part of the nerve root, with adjacent distal (PNS) nerve fibers that appear normally myelinated. (d) Electron microscopy reveals demyelinated nerve fibers, some of which are juxtaposed without intervening glial processes. (Reproduced with permission from Love et al. Brain Pathol 1998; 8:1–11.) (e)Schematic illustration of the relationship between the region of proximal nerve root demyelination and vascular compression.
In most cases, the abnormalities are too subtle to detect using paraffin histology. Examination of semithin resin sections shows the proximal part of the nerve root to include a zone of demyelination no more than 1–2 mm in diameter, usually surrounded by an ill-defined zone of partial remyelination (Fig. 20.10). Electron microscopy confirms the presence of demyelinated axons, and in most cases some of these are closely juxtaposed, without intervening glial processes. In occasional biopsies there is also evidence of aberrant myelination/remyelination, in which more than one axon is enclosed within a single myelin sheath.
REFERENCES
Graber, D.J., Levy, M., Kerr, D., et al. Neuromyelitis optica pathogenesis and aquaporin 4. J Neuroinflammation.. 2008;5:22.
Hinson, S.R., McKeon, A., Lennon, V.A. Neurological autoimmunity targeting aquaporin-4. Neuroscience.. 2010;168:1009–1018.
Hinson, S.R., Roemer, S.F., Lucchinetti, C.F., et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med.. 2008;205:2473–2481.
Jarius, S., Paul, F., Franciotta, D., et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol.. 2008;4:202–214.
Jarius, S., Wildemann, B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol.. 2010;6:383–392.
Lennon, V.A., Wingerchuk, D.M., Kryzer, T.J., et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet.. 2004;364:2106–2112.
Pittock, S.J., Lennon, V.A., de Seze, J., et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol.. 2008;65:78–83.
Pittock, S.J., Lennon, V.A., Krecke, K., et al. Brain abnormalities in neuromyelitis optica. Arch Neurol.. 2006;63:390–396.
Roemer, S.F., Parisi, J.E., Lennon, V.A., et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain.. 2007;130:1194–1205.
Wingerchuk, D.M., Lennon, V.A., Lucchinetti, C.F., et al. The spectrum of neuromyelitis optica. Lancet Neurol.. 2007;6:805–815.
Wingerchuk, D.M., Lennon, V.A., Pittock, S.J., et al. Revised diagnostic criteria for neuromyelitis optica. Neurology.. 2006;66:1485–1489.
Acute disseminated encephalomyelitis
Dale, R.C., de Sousa, C., Chong, W.K., et al. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain.. 2000;123:2407–2422.
Leake, J.A., Albani, S., Kao, A.S., et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J.. 2004;23:756–764.
Noorbakhsh, F., Johnson, R.T., Emery, D., et al. Acute disseminated encephalomyelitis: clinical and pathogenesis features. Neurol Clin.. 2008;26:759–780.
Sonneville, R., Klein, I., de Broucker, T., et al. Post-infectious encephalitis in adults: diagnosis and management. J Infect.. 2009;58:321–328.
Tenembaum, S., Chamoles, N., Fejerman, N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology.. 2002;59:1224–1231.
VanLandingham, M., Hanigan, W., Vedanarayanan, V., et al. An uncommon illness with a rare presentation: neurosurgical management of ADEM with tumefactive demyelination in children. Childs Nerv Syst.. 2010;26:655–661.
Acute hemorrhagic leukoencephalopathy
Byers, R.K. Acute hemorrhagic leukoencephalitis: report of three cases and review of the literature. Pediatrics.. 1975;56:727–735.
Graham, D.I., Behan, P.O., More, I.A. Brain damage complicating septic shock: acute haemorrhagic leucoencephalitis as a complication of the generalised Shwartzman reaction. J Neurol Neurosurg Psychiatry.. 1979;42:19–28.
Abhinav, K., Love, S., Kalantzis, G., et al. Clinicopathological review of patients with and without multiple sclerosis treated by partial sensory rhizotomy for medically refractory trigeminal neuralgia: a 12-year retrospective study. Clin Neurol Neurosurg.. 2011. [Epub:22130049].
Devor, M., Govrin-Lippmann, R., Rappaport, Z.H. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg.. 2002;96:532–543.
Love, S., Coakham, H.B. Trigeminal neuralgia: pathology and pathogenesis. Brain.. 2001;124:2347–2360.
Love, S., Gradidge, T., Coakham, H.B. Trigeminal neuralgia due to multiple sclerosis: ultrastructural findings in trigeminal rhizotomy specimens. Neuropathol Appl Neurobiol.. 2001;27:238–244.
Love, S., Hilton, D.A., Coakham, H.B. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol.. 1998;8:1–11.