Chapter 19 Osteointegration (Osseointegration)
Recent advances in spine surgery have led to expanded use of synthetic biomaterials. The interface between host bone and a synthetic device has an important influence on the clinical efficacy of that device. These interfaces have been described as abutting (e.g., interbody bone graft, interbody cement), penetrating (e.g., nail, staple, screw), gripping (e.g., hook, wire), conforming (e.g., polymethylmethacrylate), and osteointegrating (e.g., some types of metal and ceramics).1 The word osteointegration is derived from the Latin word integratus and the Greek osteon, meaning renewing or making new bone. However, because the prefix osseo- is also derived from the Latin for bone,2 the term osseointegration is often (or preferably) used instead of osteointegration.
Since Brånemark, a Swedish dentist, introduced the term osseointegration to describe the process by which some oral implants interface with bone,3 this term has been widely used in the dental and orthopaedic arenas. Brånemark originally defined osseointegration as “direct structural and functional connection between ordered, living bone and the surface of a load carrying implant.”3 During the past 30 years, however, the term osseointegration has been used in a number of scientific publications regarding both structural (morphologic) and functional (physiologic) senses. Various factors influence this process at the implant-bone interface, including preparation of the surrounding bone, the surface preparation and sterilization procedures to remove organic residues from the implant, surface topography, overall implant design and composition, and load transmission.
Osteoconductivity
An osteoconductive material promotes bone apposition along its surface. The term osteoconduction is not absolute and is best understood in the context of a comparison in which variables of the substrate material, porosity, surface geometry, and surface chemistry are highly controlled and defined.4 For example, when matched by size, shape, and surface texture, hydroxyapatite is more osteoconductive than titanium, but titanium is more osteoconductive than a similar segment of cobalt-chromium alloy, or stainless steel; and rough stainless steel is more osteoconductive than polished stainless steel. Thus, several different factors influence the extent to which osteoblasts bind to a surface and produce bone matrix.
Biomaterials
Metals
Metals have been used in various forms as implants, including stainless steels, cobalt-based alloys, pure titanium, and titanium-based alloys. Each metal has different characteristics and behaves differently in vivo. For example, titanium alloys differ from stainless steel by having less resistance to abrasive wear, but provide better corrosion resistance, biocompatibility, less MRI distortion, and increased modulus of elasticity. Because of these advantages, titanium alloy is often used for orthopaedic and spine implants. As described by Wolff’s law, bone grows in response to applied stress and often is resorbed if a mechanical stimulus is lacking.5 Note, however, that the stiffness of many metals may shield the underlying bone from stress. Alloys with elastic moduli less than that of stainless steel, such as Ti6Al4V, have been successfully used in fracture fixation, but stress shielding is still observed.6,7 At the implant-bone interface, most of these metals demonstrate variable osteoconductivity. Titanium implants generally have a better biocompatibility and osteoconductivity than many other metals, and their surface chemistry and texture are more influential during bone ingrowth.
Surface Texture
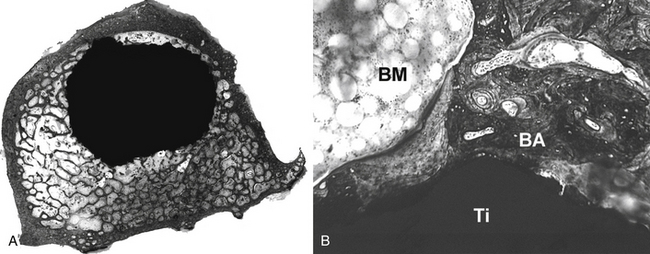
Early investigations were undertaken by Smith in the 1960s, using a porous surface ceramic.8 Currently, a wide variety of surface textures have been utilized to help achieve bone ingrowth into prosthetic devices in both dental and orthopaedic implant applications. Three dimensionally porous surfaces of sintered beads or wire, roughened surfaces created by etching the implant surface, and rough surfaces created by the application of metal by plasma spray or other methods have been tested in a number of animal and clinical studies9–12 (Figs. 19-1 and 19-2). For example, Friedman et al. tested various biomaterials with different surfaces in the rabbit femur and showed that the shear strength and bone apposition of implants with arc-deposited titanium coating and with one and three layers of cobalt-chromium beads were significantly greater than those of implants with plasma-sprayed cobalt-chromium texture and grid-blasted titanium alloy.13 Moreover, previous studies have suggested that the metal surface texture of a biomaterial can influence cell attachment and bone apposition. Martin et al. showed that surface texture affects cell attachment as well as cell morphology, proliferation, and differentiation.14 Thomas and Cook showed that roughened implants yielded more direct bone apposition in vivo than smooth implants of the same materials.15 Similarly, Turner et al. demonstrated greater bone apposition to titanium canine hip implants with an average texture of 45 μm than implants with texture of 8, 4, and 1 μm.16
Excellent clinical results for joint arthroplasty have been reported with several surface treatments.10,11 However, the optimal surface texture for each implant remains controversial. A number of manufacturers continue to investigate new surfaces in an attempt to improve fixation and lower cost. Thus, a surface topography that incorporates such surface modifications can alter the tissue and/or cell interactions with bone and appears to affect biomechanical interactions as well.17
Other Materials
Various other biomaterials have been used at the bone-implant interface for spine surgery, including polymethylmethacrylate (PMMA), calcium phosphate cement, ceramics (hydroxyapatite, bioactive glasses), and polymers (polylactic acid [PLA], polyglycolic acid [PGA], hydrogels, carbon fiber-reinforced polymer, and polyetheretherketone [PEEK]). All foreign materials induce some response when implanted in a host; so strictly speaking, all materials are bioactive. This response is often inflammatory, but some materials induce relatively little inflammation and instead promote bone formation by osteogenic, osteoconductive, or osteoinductive processes. Although PMMA and carbon have excellent biocompatibility, both are less osteoconductive than calcium phosphates or some metals. PMMA has been used for years to help stabilize pathologic fractures, but its exothermic curing and poor osteoconductivity are disadvantages for some clinical applications. Carbon fiber-reinforced polymer and PEEK can yield wear debris,18–21 but in the spine, carbon fiber-reinforced polymer has been used as an interbody stabilization device and has been associated with clinically successful outcomes without significant particle-induced osteolysis.22,23 Nevertheless, there is no evidence that these cages have direct bone apposition around them.
Hydroxyapatite (HA) is an osteoconductive calcium phosphate that can be prepared as granules, blocks, or a coating on implants.24 When placed in a suitable host site, HA is osteoconductive and has some compressive strength, but in general blocks of sintered HA are difficult to machine. In addition, they are brittle and very slow to resorb. Injectable cements are composed of either calcium phosphates or bioglass derivatives. The calcium phosphate cements are highly osteoconductive, develop about 55 MPa compressive strength, cure isothermically, are very slow to resorb, and very weak in tension and shear. The bioglass cements are not as osteoconductive, but offer greater shear strength.25 Bioabsorbable materials like PLA and PGA, are less osteoconductive in general, but since Kulkarni et al. introduced resorbable polymers for use in surgical implants, these materials have been used successfully in selected applications.26–30 The use of resorbable polymers in spine surgery has only been advocated recently. The main theoretical advantage of a resorbable material is that it confers initial and intermediate stability without having any of such long-term complications as stress shielding or migration of the implant, but this requires degradation of the implant at a rate that coincides with new bone formation. The gradual degradation of bioabsorbable spinal implants can theoretically allow axial loads that were initially borne by the implant to be progressively transferred to the bone.31 Another advantage of such materials is that they do not interfere with radiographic studies. Resorbable polymers have been used as plates and interbody fusion devices,32–34 but again there is little histologic evidence of direct bone apposition to the implant, and the rate of degradation has not always been coupled with new bone formation.
Surgical Applications
Interbody Fusion
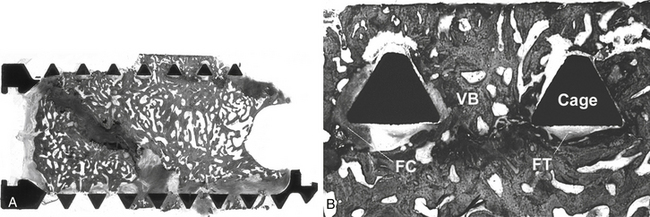
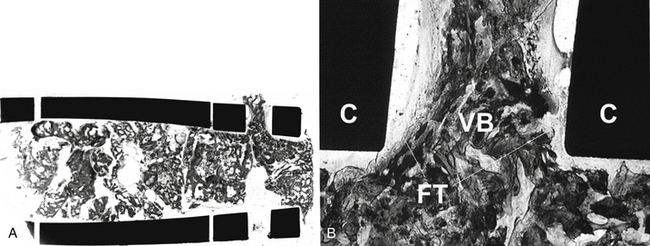
Interbody fusion devices are widely used for spinal arthrodesis and have demonstrated their clinical effectiveness for various degenerative disorders of the spine. Numerous types of spinal fusion cages have been developed from titanium and carbon fiber-reinforced or bioabsorbable polymer composites.35–39 They also have been created in many shapes: horizontal cylinders; vertical rings; or mesh, rectangular, and open boxes. All can be packed with bone graft or graft materials to promote interbody fusion. Variations in cage design in the extent of the end plate, material stiffness, and other characteristics may be factors affecting success. Successful spinal fusion with interbody cage devices has been radiographically confirmed in a number of clinical studies.22,38,40,41 The results of some animal studies have shown histologic evidence of bone graft incorporation and good connectivity between the bone inside the cages and adjacent vertebral bodies.36,42–44 The extent of direct bone apposition to clinically satisfactory cages is unknown, but clinically failed cages show no direct bone apposition, even when viable bone is present in the center of the cages21,45 (Figs. 19-3 and 19-4).
Pedicle Screws
Pedicle screw, rod, or plate systems utilized in conjunction with a dorsal intertransverse bone graft maintain spinal alignment and provide immediate structural stability, thereby allowing early mobilization of the patient while promoting arthrodesis. However, pedicle integrity can be poor in osteoporotic vertebrae, in part due to low screw-bone interface strength. Different methods of improving the purchase of these screws have been investigated, including modifications of the design of the thread, its shape, and surface properties.46–51 Sandén et al. investigated the effects on purchase of both partial and total HA coating of pedicle screws in a series of the patients with lumbar and lumbosacral degenerative disorders.52 Approximately 1 year after the surgery, the instruments were removed in some patients, and the authors measured both insertion and extraction torques. The results demonstrated that both insertion and extraction torques for fully coated screws were significantly higher than for uncoated or partly coated screws. Of particular note, the extraction torques exceeded the upper limit of the torque wrench (600 Ncm) for many HA-coated screws, suggesting that HA-coated pedicle screws improved fixation with reduced risk of loosening of the screw.
Augmenting cancellous bone with cement to increase its stiffness and strength represents another modification to improve the chances of successful fixation with pedicle screws.1,53,54 For example, Turner et al. tested an HA composite resin cement (Kuraray Co., Krashiki, Japan) to determine whether it could stiffen the screw-bone interface in a human cadaveric study.53 Cement augmentation significantly improved the initial load-carrying capacity (116%), the load-carrying capacity after mechanical testing (165%), and the initial rate of decrease of the implant-bone interface (159%). These results suggested that cement augmentation of pedicle screws increased the stiffness and stability of the screw-bone interface. Furthermore, the pressurized injection of cement into the screw hole causes the cement to penetrate into the trabecular bone, effectively increasing the diameter of the screw.1
Spacers, Scaffold, Carrier
Sintered blocks and granules of HA have been occasionally used as a spacer, especially for ventral cervical spine fusions and laminoplasty procedures.55–61 Radiographic evaluation in these studies have suggested excellent osteoconductivity of these HA spacers. Reported clinical results appear to be good, and there seem to be few complications. Histologic evaluation of three spinous processes of the cervical spine removed from a patient with a recurrent intramedullary tumor 1 year after laminoplasty confirmed direct bone apposition of bone to an HA spacer at three of the six bone-hydroxyapatite interfaces.61
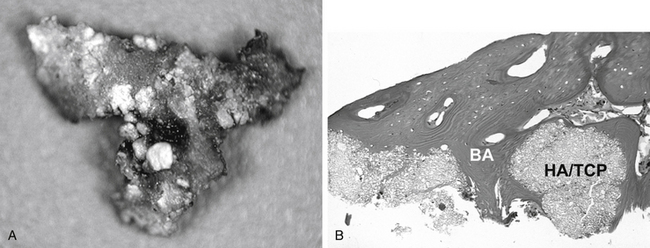
Clinically, HA and tricalcium phosphate (TCP) have been shown to be effective as bone graft expanders in dorsal spinal fusion surgery,62,63 but it may be difficult to distinguish bone from residual synthetic calcium phosphates when radiographs are used as outcome measures in fusion studies. Human biopsies obtained from fusion mass 1 year after dorsolateral fusion using HA/TCP granules show extensive bone apposition64 (Fig. 19-5).
HA, TCP, and collagen can also be used as carriers of bone morphogenetic proteins (BMPs).65–68 The combination matrices mixed with HA could have some compression resistance and act as a carrier for BMPs.69 Akamaru et al. used granules (15% HA, 85% TCP) combined with human recombinant BMP-2 in an adult monkey dorsolateral spine fusion model.69 Histologic results showed that most of the ceramic had resorbed by 24 weeks after surgery, but some was still present and encased in normal bone, suggesting that the ceramic served as a scaffold and became incorporated with new bone before it could be resorbed.
Artificial Discs
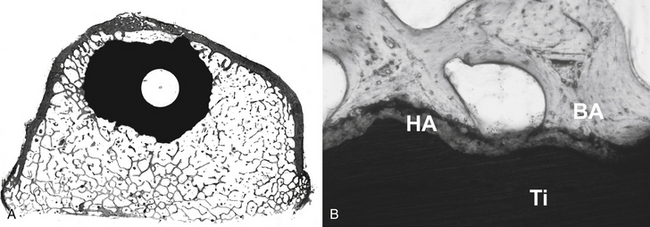
A functional disc interspace prosthesis has been sought at least since the 1950s, and several devices are currently available in Europe and in the United States.70,71 The Charité artificial disc prosthesis (DePuy Spine, Inc., Raynham, MA) was developed in Germany in the early 1980s,72 and the third generation of the Charité lumbar total disc replacement prosthesis has been used in more than 5000 patients worldwide since 1987. Originally available as just a grit-blasted titanium ongrowth surface, recent models include calcium phosphate coating. Theoretically, if adequate initial stability is achieved, then the unconstrained design of the prosthesis should reduce the stress at the bone-metal interface and lead to more favorable porous ingrowth characteristics than a constrained prosthesis. In their baboon study, McAfee et al. reported that 14 of 14 hydroxyapatite-coated SB Charité prosthetic vertebral end plates were well-fixed with no evidence of loosening.73 And a coronal histologic section illustrated in that study shows excellent bone apposition to the HA-coated end plates without evidence of fibrous tissue or synovium 6 months after surgery. Early clinical results, however, have demonstrated migration of some SB Charité implants, suggesting that achieving consistent fixation in humans may still be a problem in some cases.
The few published studies with long duration of follow-up have provided conflicting information, with some reporting success74 and others describing multiple complications and unfavorable outcome.75,76 For example, Devin et al. reported a patient who developed osteolysis and failure of a lumbar total disc replacement (AcroFlex, DePuy Spine, Inc.) after 19-year follow-up.77 Long-term implant fixation depends on bone ingrowth (osseointegration) into the surface of the prosthesis. This case report suggested that although the short-term results were rated as good due to good osteointegration, in long-term follow-up the clinical course can be poor due to associated osteolysis (wear and particles).
Vertebral Augmentation
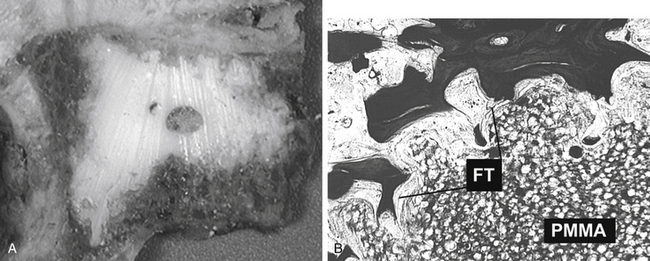
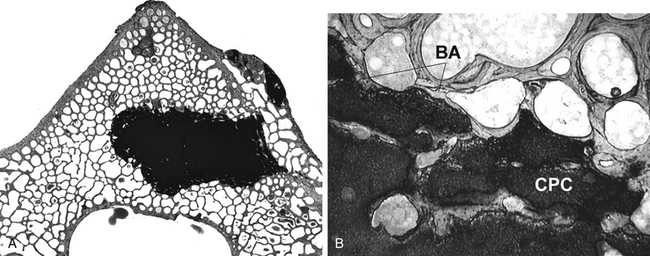
Vertebral augmentation has been extensively used to treat vertebral bodies involved with osteolytic metastases, myeloma, and osteoporotic compression fractures. PMMA is the most commonly used material for such procedures. Since PMMA is not as osteoconductive as HA, it cannot be expected to promote bone apposition. Technically, under fluoroscopic guidance, PMMA is injected into the weakened vertebrae until the cement is interdigitated into trabecular bone within the vertebrae. A recent report describing the histology of excised human specimens shows absent or only very limited direct bone apposition to the cement. Instead, a thin membrane of fibrous tissue separated bone from the PMMA78 (Fig. 19-6). To bypass perceived limitations of PMMA, alternative cements that have variable osteoconductive properties have been tested. For example, several animal studies with injectable calcium phosphate cements confirm their feasibility, mechanical effectiveness, biocompatibility, and osteoconductivity79,80 (Fig. 19-7). Grafe et al. reported a prospective trial comparing 3-year clinical and morphologic outcomes after kyphoplasty to treat painful osteoporotic vertebral fractures with a calcium phosphate cement (CaP, Calcibon, Biomet Merck, Darmstadt, Germany) or with PMMA cement.81 Their results showed no significant differences between the CaP and the PMMA cement regarding visual analogue scale scores, mobility scores, or height restoration at any time point. Furthermore, there was no significant difference in the occurrence of subsequent compression fractures during the 3-year follow-up period. On the other hand, Blattert et al. reported unfavorable results in kyphoplasty using CaP cement (Norian SRS, Norian Corp., Cupertino, CA) in a prospective randomized controlled clinical study.82 They reported subtotal cement washout and radiographic loss of correction due to cement failure in the CaP cement group, while there was no case of cement failure in PMMA group.
Composite cements (acrylic cements in conjunction with ceramics) are bioactive, highly radiopaque, and feature excellent mechanical properties. One such cement, Cortoss (Orthovita, Malvern, PA), could be a potentially valuable alternative to PMMA. Palussiere et al. reported that vertebral augmentation with Cortoss rapidly reduced pain, decreased disability, and improved physical functioning in patients with painful vertebral compression fractures.83 The degree, timing, and maintenance of pain relief seen following Cortoss augmentation in this investigation is similar to that previously reported with PMMA treatment of vertebral fractures.
Summary
In this chapter, the terminology of osseointegration and osteoconductive materials and their clinical applications were discussed. The term osteointegration (osseointegration) has been used in both structural and functional applications. In order to satisfy both conditions, the material must achieve direct bone apposition and be clinically effective. Many types of spine implants have achieved osseointegration in the broadest functional sense, but it is often unknown whether direct bone apposition to the implant has been achieved; or for that matter, if it is even necessary. Thus, osseointegration is desirable, but it may not always be necessary. It is necessary for surgeons to understand the importance of material properties, implant characteristics, implant design, and the properties of the local environment (blood flow, load transmission) to achieve clinically successful osseointegration in spine surgery.
Brånemark P.I., Zarb G., Albrektsson T. Tissue integrated prosthesis: osseointegration in clinical dentistry. Chicago: Quintessence; 1985.
McAfee P.C., Cunningham B.W., Orbegoso C.M., et al. Analysis of porous ingrowth in intervertebral disc prostheses: a nonhuman primate model. Spine (Phila Pa 1976). 2003;28:332-340.
Phillips F.M., Garfin S.R. Cervical disc replacement. Spine (Phila Pa 1976). 2005;30:S27-S33.
Sanden B., Olerud C., Petren-Mallmin M., et al. Hydroxyapatite coating improves fixation of pedicle screws. A clinical study. J Bone Joint Surg [Br]. 2002;84:387-391.
Thomas K.A., Cook S.D. An evaluation of variables influencing implant fixation by direct bone apposition. J Biomed Mater Res. 1985;19:875-901.
Togawa D., Bauer T.W., Lieberman I.H., et al. Histologic evaluation of human vertebral bodies after vertebral augmentation with polymethylmethacrylate. Spine (Phila Pa 1976). 2003;28:1521-1527.
Watts T.L. Osseointegration is Latin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:532.
1. Benzel E.C. Implant-bone interface. In Benzel EC, editor: Biomechanics of spine stabilization. New York: Thieme; 2001. pp 155–170
2. Watts T.L. Osseointegration is latin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:532.
3. Brånemark P.I., Zarb G., Albrektsson T. Tissue integrated prosthesis; osseointegration in clinical dentistry. Chicago: Quintessence; 1985.
4. Bauer T.W., Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10-27.
5. Uhthoff H.K., Dubuc F.L. Bone structure changes in the dog under rigid internal fixation. Clin Orthop Relat Res. 1971;81:165-170.
6. Uhthoff H.K., Finnegan M. The effects of metal plates on post-traumatic remodelling and bone mass. J Bone Joint Surg [Br]. 1983;65:66-71.
7. Woo S.L., Lothringer K.S., Akeson W.H., et al. Less rigid internal fixation plates: historical perspectives and new concepts. J Orthop Res. 1984;1:431-449.
8. Smith L. Ceramic-plastic material as a bone substitute. Arch Surg. 1963;87:653-661.
9. Collier J.P., Mayor M.B., Chae J.C., et al. Macroscopic and microscopic evidence of prosthetic fixation with porous-coated materials. Clin Orthop Relat Res. 1988;235:173-180.
10. Engh C.A., Hooten J.P., Zettl-Schaffer K.F., et al. Porous coated total hip replacement. Clin Orthop Relat Res. 1994;298:89-96.
11. Mallory T.H., Head W.C., Lombardi A.V., et al. Clinical and radiographic outcome of a cementless, titanium plasma spray-coated total hip arthroplasty femoral component. J Arthroplasty. 1996;11:653-660.
12. Togawa D., Bauer T.W., Mochida Y., et al. Bone apposition to three femoral stem surfaces in canine total hip arthroplasty. Transactions of the Society for Biomaterials. 2001:251.
13. Friedman R.J., An Y.H., Ming J., et al. Influence of biomaterial surface texture on bone ingrowth in the rabbit femur. J Orthop Res. 1996;14:455-464.
14. Martin J.Y., Schwartz Z., Hummert T.W., et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29:389-401.
15. Thomas K.A., Cook S.D. An evaluation of variables influencing implant fixation by direct bone apposition. J Biomed Mater Res. 1985;19:875-901.
16. Turner T.M., Urban R.M., Hall D.J., et al. The effect of femoral stem surface roughness on the extent of fibrous membrane formation and bone in a canine total hip replacement model. Transactions of the Society for Biomaterials. 2001:298.
17. Hayashi K., Matsuguchi N., Uenoyama K., et al. Evaluation of metal implants coated with several types of ceramics as biomaterials. J Biomed Mater Res. 1989;23:1247-1259.
18. Jockisch K.A., Brown S.A., Bauer T.W., et al. Biological response to chopped-carbon-fiber-reinforced peek. J Biomed Mater Res. 1992;26:133-146.
19. Togawa D., Bauer T.W., Brantigan J.W., et al. Bone graft incorporation in radiographically successful human intervertebral body fusion cages. Spine (Phila Pa 1976). 2001;26:2744-2750.
20. Togawa D., Bauer T.W., Lieberman I.H., et al. Histology of tissues within retrieved human titanium mesh cages. Spine (Phila Pa 1976). 2003;28:246-253. discussion 254
21. Togawa D., Bauer T.W., Lieberman I.H., et al. Lumbar intervertebral body fusion cages—histological evaluation of human retrievals. J Bone Joint Surg [Am]. 2003;86:70-79.
22. Brantigan J.W., Steffee A.D., Lewis M.L., et al. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976). 2000;25:1437-1446.
23. Hashimoto T., Shigenobu K., Kanayama M., et al. Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine (Phila Pa 1976). 2002;27:258-262.
24. Bauer T.W., Smith S.T. Bioactive materials in orthopaedic surgery: overview and regulatory considerations. Clin Orthop Relat Res. 2002;395:11-22.
25. Bauer T.W., Togawa D. Bone graft substitute: towards a more perfect union. Orthopedics. 2003;26:925-926.
26. Kulkarni R.K., Pani K.C., Neuman C., et al. Polylactic acid for surgical implants. Arch Surg. 1966;93:839-843.
27. Bostman O.M. Absorbable implants for the fixation of fractures. J Bone Joint Surg [Am]. 1991;73:148-153.
28. Bucholz R.W., Henry S., Henley M.B. Fixation with bioabsorbable screws for the treatment of fractures of the ankle. J Bone Joint Surg [Am]. 1994;76:319-324.
29. Caborn D.N., Coen M., Neef R., et al. Quadrupled semitendinosus-gracilis autograft fixation in the femoral tunnel: a comparison between a metal and a bioabsorbable interference screw. Arthroscopy. 1998;14:241-245.
30. Cizek G.R., Boyd L.M. Imaging pitfalls of interbody spinal implants. Spine (Phila Pa 1976). 2000;25:2633-2636.
31. Ciccone W.J.2nd, Motz C., Bentley C., et al. Bioabsorbable implants in orthopaedics: new developments and clinical applications. J Am Acad Orthop Surg. 2001;9:280-288.
32. van Dijk M., Smit T.H., Arnoe M.F., et al. The use of poly-L-lactic acid in lumbar interbody cages: design and biomechanical evaluation in vitro. Eur Spine J. 2003;12:34-40.
33. Vaccaro A.R., Carrino J.A., Venger B.H., et al. Use of a bioabsorbable anterior cervical plate in the treatment of cervical degenerative and traumatic disc disruption. J Neurosurg. 2002;97:473-480.
34. Warren S.M., Hedrick M.H., Sylvester K., et al. New directions in bioabsorbable technology. J Neurosurg. 2002;97:481-489.
35. Brantigan J.W., Steffee A.D. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976). 1993;18:2106-2107.
36. Toth J.M., Estes B.T., Wang M., et al. Evaluation of 70/30 poly (L-lactide-co-D, L-lactide) for use as a resorbable interbody fusion cage. J Neurosurg. 2002;97:423-432.
37. Kuslich S.D., Danielson G., Dowdle J.D., et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976). 2000;25:2656-2662.
38. Ray C.D. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976). 1997;22:667-679. discussion 679–680
39. Steffen T., Tsantrizos A., Fruth I., et al. Cages: designs and concepts. Eur Spine J. 2000;9(Suppl 1):S89-S94.
40. Kuslich S.D., Ulstrom C.L., Griffith S.L., et al. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976). 1998;23:1267-1278. discussion 1279
41. McAfee P.C., Boden S.D., Brantigan J.W., et al. Symposium: a critical discrepancy—a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976). 2001;26:320-334.
42. Cunningham B.W., Kanayama M., Parker L.M., et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. A comparative endoscopic study using the Bagby and Kuslich interbody fusion device. Spine (Phila Pa 1976). 1999;24:509-518.
43. Toth J.M., Seim H.B.3rd, Schwardt J.D., et al. Direct current electrical stimulation increases the fusion rate of spinal fusion cages. Spine (Phila Pa 1976). 2000;25:2580-2587.
44. Boden S.D., Martin G.J.Jr., Horton W.C., et al. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998;11:95-101.
45. Togawa D., Bauer T.W. The histology of human retrieved body fusion cages: good bone graft incorporation and few particles. Transactions of the 47th Annual Meeting of the Orthopaedic Research Society, San Francisco. 2001:950.
46. DeCoster T.A., Heetderks D.B., Downey D.J., et al. Optimizing bone screw pullout force. J Orthop Trauma. 1990;4:169-174.
47. Krag M.H., Beynnon B.D., Pope M.H., et al. An internal fixator for posterior application to short segments of the thoracic, lumbar, or lumbosacral spine. Design and testing. Clin Orthop Relat Res. 1986;203:75-98.
48. Kwok A.W., Finkelstein J.A., Woodside T., et al. Insertional torque and pull-out strengths of conical and cylindrical pedicle screws in cadaveric bone. Spine (Phila Pa 1976). 1996;21:2429-2434.
49. Sell P., Collins M., Dove J. Pedicle screws: axial pull-out strength in the lumbar spine. Spine (Phila Pa 1976). 1988;13:1075-1076.
50. Cook S.D., Salkeld S.L., Whitecloud T.S.3rd, et al. Biomechanical evaluation and preliminary clinical experience with an expansive pedicle screw design. J Spinal Disord. 2000;13:230-236.
51. Lapresle P., Missenard G. Hydroxylapatite-coated Diapason screws: first clinical report. J Spinal Disord. 1995;8(Suppl 1):S31-S39.
52. Sandén B., Olerud C., Petrén-Mallmin M., et al. Hydroxyapatite coating improves fixation of pedicle screws. A clinical study. J Bone Joint Surg [Br]. 2002;84:387-391.
53. Turner A.W., Gillies R.M., Svehla M.J., et al. Hydroxyapatite composite resin cement augmentation of pedicle screw fixation. Clin Orthop Relat Res. 2003;406:253-261.
54. Moore D.C., Maitra R.S., Farjo L.A., et al. Restoration of pedicle screw fixation with an in situ setting calcium phosphate cement. Spine (Phila Pa 1976). 1997;22:1696-1705.
55. Iguchi T., Kanemura A., Kurihara A., et al. Cervical laminoplasty: evaluation of bone bonding of a high porosity hydroxyapatite spacer. J Neurosurg. 2003;98:137-142.
56. Kubo S., Goel V.K., Yang S.J., et al. Biomechanical evaluation of cervical double-door laminoplasty using hydroxyapatite spacer. Spine (Phila Pa 1976). 2003;28:227-234.
57. Goro T., Ohata K., Takami T., et al. Hydroxyapatite laminar spacers and titanium miniplates in cervical laminoplasty. J Neurosurg. 2002;97:323-329.
58. Takayasu M., Takagi T., Nishizawa T., et al. Bilateral open-door cervical expansive laminoplasty with hydroxyapatite spacers and titanium screws. J Neurosurg. 2002;96:22-28.
59. Hirabayashi S., Kumano K. Contact of hydroxyapatite spacers with split spinous processes in double-door laminoplasty for cervical myelopathy. J Orthop Sci. 1999;4:264-268.
60. Nakano K., Harata S., Suetsuna F., et al. Spinous process-splitting laminoplasty using hydroxyapatite spinous process spacer. Spine (Phila Pa 1976). 1992;17:S41-S43.
61. Kokubun S., Kashimoto O., Tanaka Y. Histological verification of bone bonding and ingrowth into porous hydroxyapatite spinous process spacer for cervical laminoplasty. Tohoku J Exp Med. 1994;173:337-344.
62. Spivak J.M., Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001;10(Suppl 2):S197-S204.
63. Fujibayashi S., Shikata J., Tanaka C., et al. Lumbar posterolateral fusion with biphasic calcium phosphate ceramic. J Spinal Disord. 2001;14:214-221.
64. Togawa D., Bauer T.W., Kanayama M., et al. Histological evaluation of human posterolateral lumbar fusion mass induced by osteogenic protein-1. Transactions of the 71st Annual Meeting of the American Academy of Orthopaedic Surgeons, San Francisco, Poster. 2004. p 396
65. Hassan A.H., Evans C.A., Zaki A.M., et al. Use of bone morphogenetic protein-2 and dentin matrix protein-1 to enhance the osteointegration of the Onplant system. Connect Tissue Res. 2003;44:30-41.
66. Boden S.D., Kang J., Sandhu H., et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662-2673.
67. Minamide A., Kawakami M., Hashizume H., et al. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine (Phila Pa 1976). 2001;26:933-939.
68. Schimandle J.H., Boden S.D., Hutton W.C. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976). 1995;20:1326-1337.
69. Akamaru T., Suh D., Boden S.D., et al. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine (Phila Pa 1976). 2003;28:429-434.
70. Guyer R.D., Ohnmeiss D.D. Intervertebral disc prostheses. Spine (Phila Pa 1976). 2003;28:S15-S23.
71. Phillips F.M., Garfin S.R. Cervical disc replacement. Spine (Phila Pa 1976). 2005;30:S27-S33.
72. Buttner-Janz K., Schellnack K., Zippel H. [An alternative treatment strategy in lumbar intervertebral disk damage using an SB Charité modular type intervertebral disk endoprosthesis]. Z Orthop Ihre Grenzgeb. 1987;125:1-6.
73. McAfee P.C., Cunningham B.W., Orbegoso C.M., et al. Analysis of porous ingrowth in intervertebral disc prostheses: a nonhuman primate model. Spine. 2003;28:332-340.
74. Tropiano P., Huang R.C., Girardi F.P., et al. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg [Am]. 2005;87:490-496.
75. Putzier M., Funk J.F., Schneider S.V., et al. Charité total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183-195.
76. Ross R., Mirza A.H., Norris H.E., et al. Survival and clinical outcome of SB Charité III disc replacement for back pain. J Bone Joint Surg [Br]. 2007;89:785-789.
77. Devin C.J., Myers T.G., Kang J.D. Chronic failure of a lumbar total disc replacement with osteolysis. Report of a case with nineteen-year follow-up. J Bone Joint Surg [Am]. 2008;90:2230-2234.
78. Togawa D., Bauer T.W., Lieberman I.H., et al. Histologic evaluation of human vertebral bodies after vertebral augmentation with polymethyl methacrylate. Spine. 2003;28:1521-1527.
79. Takikawa S., Bauer T.W., Turner A.S., et al. Comparison of injectable calcium phosphate cement and polymethylmethacrylate for use in vertebroplasty: in-vivo evaluation using an osteopenic sheep model. Transactions of the Society for Biomaterials. 2002:231.
80. Turner T.M., Urban R.M., Lim T.H., et al. Vertebroplasty using injectable calcium phosphate cement compared to polymethylmethacrylate in a unique canine vertebral body large defect model. Transactions of the 49th Annual Meeting of the Orthopaedic Research Society, Paper # 0267, New Orleans. 2003.
81. Grafe I.A., Baier M., Noldge G., et al. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine (Phila Pa 1976). 2008;33:1284-1290.
82. Blattert T.R., Jestaedt L., Weckbach A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine (Phila Pa 1976). 2009;34:108-114.
83. Palussiere J., Berge J., Gangi A., et al. Clinical results of an open prospective study of a bis-GMA composite in percutaneous vertebral augmentation. Eur Spine J. 2005;14:982-991.