Chapter 229 Timing of Decompression Surgery for Traumatic Spinal Cord Injury in a Patient with Complete Myelopathy
Presurgical Management
The majority of spinal cord injuries (SCI) each year occur in young patients. Trends are moving toward a greater incidence in older patients, while the over all incidence remains quite stable1,2 (Fig. 229-1). The devastating morbidity and mortality that results from these injuries affects not only the patients, but also their families and society as a whole.3 The early management of SCI is predicated on treating life-threatening injuries as the top priority.2 Advanced Trauma Life Support guidelines support initially treating the greatest physiologic threats, such as respiratory derangements and hypovolemic shock, while providing provisional spine stabilization until definitive treatment can be safely administered. Even in highly unstable injuries that eventually will require complex surgical reconstruction, nonoperative care is the key to initial management.2 The timing of surgical treatment for cervical SCI is still being debated. There are no standards with respect to timing of decompression or definitive stabilization for acute SCI.2 When considering the timing of surgical treatment, one must factor in its potential effects on a host of other important outcome measures, such as neurologic recovery, mortality, hospital length of stay, ICU stay, and infection, to name a few.
Primary and Secondary Spinal Cord Injury
Injury to the spinal cord is the result of a series of mechanisms, including the initial traumatic event and a secondary biologic response to injury.3,4 In the primary mechanism, the energy of the trauma is transferred to the spinal cord, resulting in local deformation, contusion (due to dislocation or encroachment of bony fragments), distraction, or transection/laceration. The spinal cord then suffers secondary injury by a cascade of microvascular, cellular, and molecular events that unfold in the ensuing minutes, hours, and days after the initial SCI.4 Current SCI research that is focused on pharmacologic treatments that might modify this secondary mechanism of injury is beyond the scope of this chapter.
Of the secondary mechanisms of injury that have been identified, persistent compression of the spinal cord represents a principal area of focus that spine surgeons can modify now that might possibly have an effect on patient outcome. Studies have shown that decompression and stabilization of the spine enhance neuronal function and reverse secondary molecular mechanisms of injury.5 While controversy may exist over operative versus nonoperative management and the timing of surgery, there is no debate about the goals of care in the SCI patient. Treatment goals continue to include spine stabilization, realignment, and neurologic decompression. For optimal treatment of the patient, these goals should be addressed at all times throughout the patient’s care from time of injury to definitive management.
Experimental evidence in animals suggests that persistent compression of the spinal cord is a potentially reversible form of secondary injury and that earlier timing of decompression can lead to improved neurologic outcomes.6–8 To date, the best data in support of timing of spinal cord decompression in the setting of SCI have come from studies on animal models of SCI. There is still a lack of consensus on the role of timing of surgical decompression and fixation in SCI patients. Fehlings and Perrin published an extensive review of the literature regarding role and the effect of timing on surgical treatment of SCI. Most studies suggested that surgical decompression and/or stabilization can influence neurologic recovery. Reversal of compression and stabilization of the spine appeared to enhance neuronal function and reverse secondary molecular mechanisms of injury.4 However, a recent systematic review of the literature failed to establish guidelines for timing of surgical decompression and fixation in the setting of SCI due to lack of quality of evidence.3
Despite basic science studies demonstrating that early decompression reverses the secondary injury to the spinal cord and enhances neurologic recovery, historical series suggest that neurologic recovery is possible with nonoperative management as well.9 In these historical studies, the majority of patients were treated nonoperatively. Spontaneous neurologic improvement was reported even in the setting of nonoperative treatment that might not have fully decompressed or realigned the spine.9 Most of these series were retrospective and nonrandomized and suffer from methodologic concerns. However, Frankel et al. reported on a cohort of over 600 patients who were managed nonoperatively. The report showed that almost 30% of the ASIA A injuries improved at least one grade and that neurologic deterioration was rare.9 This landmark study is the standard to which all further studies must be compared. The findings of Frankel et al. were reiterated by many other studies.10–12 In 1981, Kiwerski and Weiss found that 20% of patients with complete SCI showed some neurologic improvement following nonoperative management.10–12 Tator et al. reported on a prospective, nonrandomized case control group of SCI patients to assess outcomes of operative versus nonoperative treatment; they found no difference in neurologic outcome when comparing these two groups but found that operatively treated patients had a lower overall mortality rate.13 Young and Dexter compared two groups of SCI patients and concluded that there is limited hope for recovery from paralysis in those with severe SCI, whether treated operatively or nonoperatively.14
These studies are not meant to support nonoperative treatment over operative treatment in cervical SCI but rather to illustrate the “control” standard to which current interventions must be compared. Current literature suggests that SCIs should be treated operatively.15–18 The basic science studies demonstrating reversal of secondary SCI by early decompression continues to be the foundation that supports many clinicians’ rationale for performing urgent decompression and fixation in patients with SCI.7,8,19 Trends reported over the last decade reveal that the majority of cervical SCIs are being definitively treated surgically.16 Beyond neurology and stability as reasoning for early decompression and fusion, the literature suggests that surgical management of cervical SCIs leads to decreased mortality, ventilation, and time in the ICU.20,21 It is also clear that early surgical stabilization of SCI patients allows for quicker mobilization and transition to rehabilitative efforts, leading to decreased overall hospital length of stay.21
Initial Management (Nonoperative Treatments)
Traction
The application of traction and the timing of its application in the setting of spinal cord compression make up another highly debated area. The role of traction for early indirect reduction, decompression, and stabilization is well founded.22,23 In the Surgical Timing in Acute Spinal Cord Injury (STASCIS) trial, the largest multicenter study on SCI investigating the effect of surgical timing, which is currently ongoing, traction is defined as an acceptable mode of indirect neurologic decompression if meaningful realignment is achieved. Therefore, cervical traction can be thought of as a means of providing rapid spinal cord decompression and temporary stabilization in the early phases of injury.
A number of studies have found no neurologic benefit to early closed reduction of cervical SCI.11,24,25 Fehlings and Perrin concluded in their systematic review that the evidence does not support standards or guidelines for the initial management of SCI with the use of traction and the timing of its application.4 Traction is not without pitfalls.26,27 Tator et al. demonstrated up to an 8% neurologic deterioration rate in patients undergoing traction.26 Others advocate for traction as a necessary means of applying the earliest possible mode of neurologic decompression and advocate immediate application for enhanced chance of neurologic recovery.17,28 Cotler et al. found that patients who underwent cervical spine reduction by traction within the first 8 hours after injury had greater neurologic recovery compared to similar patients who underwent reduction later than 8 hours after the initial injury.28 This timeline was further defined when Aebi et al. reported that patients with cervical dislocations who were reduced less than 6 hours after injury improve to a greater extent than those who were reduced later.17 Brunette and Rockswold and Hadley et al. also provide evidence in studies with small numbers of patients that rapid reduction of traumatic cervical deformity due to fracture can lead to profound neurologic improvement.29,30 These were all retrospective studies, and some provided no comparison groups and are thus limited by their methodology. They still provide useful reference points for assessing treatment guidelines and designing studies.
Kinetic Therapy
In addition to the preservation of life, the main priority of initial SCI treatment is to prevent neurologic deterioration, provide provisional spinal stability, and create the potential for return of neurologic function. Once a patient has been brought under the care of medical personnel, there is still a 6% to 10% risk of neurologic deterioration.27 One of the potential causes of further neurologic deterioration is motion of the spine. The initial form of cervical spine immobilization in the trauma bay or ICU is typically a rigid orthosis or traction and bedrest. However, previous studies have shown that the spine-injured patient who is subjected to bedrest is at risk for pulmonary complications, skin breakdown, deep vein thrombosis, and muscle atrophy.31–35 Frequent patient repositioning has been shown to decrease the morbidity associated with bedrest.32,34,36 However, it also creates the potential for motion of the spine. Kinetic therapy is one way of providing controlled motion to the patient while also maintaining spine immobilization. The various forms of kinetic beds available include Stryker frame (Stryker Corporation, Kalamazoo, MI), RotoRest bed (KCI, San Antonio, TX), CircOlectric Bed (Ferndale Surgical, Inc., Ferndale, MI), and Foster frames.37 Conrad et al. and Rechtine et al. performed biomechanics studies on cadavers with cervical and thoracolumbar instability and compared logroll to kinetic bed treatment. They found that kinetic beds generated significantly less cervical and thoracolumbar spine motion when compared to logroll.38–40 Therefore, kinetic therapy is an excellent mechanism to prevent the complications of immobility while maintaining spine immobility and pursuing potential return of neurologic function.
Patient Positioning in the Operating Room
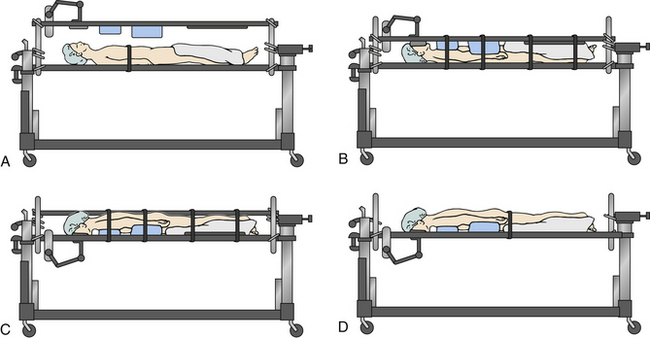
Prone positioning prior to operative intervention is another potential mechanism of generating further neurologic deterioration due to the generation of motion in the unstable spine.27 The cervical collar does not adequately immobilize the cervical spine when the patient is transitioned from supine to prone positioning, and the manual logroll produces a significant amount of cervical motion that could put neurologic structures at risk. This has been demonstrated in cadavers. In these scenarios, the Jackson table turning technique immobilizes the cervical spine to a significantly greater degree than does manual prone positioning when one focuses on a series of motion parameters for angular displacement (axial rotation, lateral bending and flexion-extension) and linear displacement (anteroposterior, axial, and medial-lateral) whether a rigid collar is used or not.41 The Jackson table prone positioning technique involves positioning a patient supine on the flat Jackson table and then applying the frame, with pads over the ventral surface of the patient. The frame is compressed, and the patient is held in place by the locking pins and compression of the system. Safety straps are applied around the entire setup. The patient is flipped prone by unlocking the manual lock at the head and foot of the bed and then rotating the whole system42 (Fig. 229-2).
Definitive Treatment (Surgery)
Timing of Surgery and Decompression
Although animal studies demonstrate that early cord decompression results in reversal of secondary injury and enhancement of neurologic recovery, the clinical data evaluating the timing of surgical decompression are less clear. Trauma patients often present to SCI centers late and with multiple injuries that confound those findings. According to Tator et al., only 50% of patients with SCI in North America are admitted to SCI centers within 24 hours of their injury.26 This severely limits the patient pool that would even be eligible for what is considered early intervention. In addition, there is not a clear consensus as to the role that timing of spinal cord decompression plays in neurologic recovery. Another significant concern in favor of early operative treatment in patients who present with presumed ASIA A injuries is the difficulty in determining whether the SCI is complete or incomplete in the early phase of injury. It is often challenging to distinguish which patients definitively have complete injuries and which patients are still in spinal shock and therefore might be incomplete (though it is very rare to see a patient who appears to be ASIA A and then, once out of spinal shock, converts to ASIA C or D). Evidence suggests that some patients who were previously identified as having complete SCIs eventually converted to incomplete injuries, occasionally in a delayed fashion.43 Thus, nonoperative management of presumed complete SCIs, as suggested by historical studies, may result in neurologic deterioration if the patient actually had an incomplete SCI. Therefore, some surgeons may choose to treat all cervical SCIs as if they are potentially incomplete (and therefore may decide to operate more quickly) if they weigh the preceding factors more heavily in favor of rapid decompression.
Neurologic Recovery
Duh et al. analyzed data from NASCIS II and reported that patients who underwent surgery less than 25 hours after the initial SCI had neurologic outcomes similar to those of patients who underwent surgery at more than 200 hours after injury.20 Wagner and Chehrazi also showed that timing did not affect neurologic recovery, as they found no difference in neurologic outcome in patients who had surgery within 48 hours of injury compared with those who had surgery after 48 hours from injury.44 Finally, Vaccaro et al. prospectively studied a randomized group of patients with SCI who underwent either early (<72 hours, mean of 1.8 days) or late (>5 days, mean of 16.8 days) surgery.45 There was no significant difference in ASIA motor scores between the two groups at all follow-up time points. Thus, these studies suggest that timing of operative management of complete SCI does not affect neurologic recovery.
However, the study by Mirza et al. provides evidence to the contrary.43 Mirza et al. performed a comparative cohort analysis in which two different centers employed their standard protocols. Members of both institutions performed traction reductions for immediate indirect decompression and stabilization. One of the institutions performed surgery as soon as feasible for the patient, and the other had a protocol of performing delayed surgical fixation 10 to 14 days after injury. Mirza et al. found that the patients in the early-surgery protocol (<72 hours) tended to have higher rates of neurologic recovery during their hospitalization compared to the delayed-surgery group. They hypothesized that surgery provided better stabilization than traction and bedrest. This study was limited by the retrospective nature and nonstandardized treatment algorithms or earlier defined time points for surgery. Also, the study failed to provide data regarding neurologic exams for either patient population after discharge from the acute setting; therefore, it is not known how the long-term neurologic outcomes of these patients compare. Papadopoulos et al. reviewed the effect of treatment timing on 91 nonrandomized patients with acute cervical SCI.15 They found that half of the patients who underwent immediate traction and subsequent surgical decompression had neurologic improvement compared to about one quarter of those who were treated in a nonstandardized manner, typically with delayed surgery. The protocol group had eight patients that recovered from Frankel grade A or B to grade D or E and became ambulatory. These apparent findings of a lower frequency of neurologic improvement in the delayed-treatment group may be confounded by the severity of injury in these patients. Many of the “control” patients had a contraindication to immediate surgery, had other life-threatening injuries that required more immediate care, or were considered futile cases with respect to neurologic recovery.
Non-Neurologic Outcomes
Timing of surgery has been debated not only for its role in neurologic recovery, but also for its role in perioperative and medical complications. Multiple studies have concluded that time of hospitalization and complication rates tend to be related to the severity of injury and multisystem problems.46,47 Many authors have historically argued against surgical treatment (especially early surgery) owing to high risk in critically ill patients.3 Because of improved surgical and anesthetic techniques, more patients are undergoing surgery with little difference in complication rates.2,3
Some studies have found no difference in non-neurologic outcome measures when comparing early and late surgery. Mirza et al. found no difference in major or minor complication rates, ventilator time, and ICU stay between early (mean, 1.8 days) and late (mean, 14 days) surgery groups.43 Vaccaro et al. also found no difference in length of postoperative ICU stay or inpatient rehabilitation time between early and late surgery groups.45 Still other researchers claim that earlier operative treatment helps to improve non-neurologic outcome measures. McKinley et al. reported that early surgery leads to decreased hospital length of stay and reduced pulmonary complications.21 Duh et al. showed that patients who underwent operative treatment less than 24 hours after injury had fewer complications than those who underwent surgery more than 24 hours after injury.20 More recent literature and trends suggest that earlier spine fracture fixation (within 3 days) decreases hospital stay, ventilator days, pneumonia, and hospital costs.16,48–50 Kerwin et al. caution against applying a rigid protocol for early spine stabilization to all patients.48 The timing of surgery should always be individualized to achieve optimization of the patient’s medical and physiologic condition.
Conclusion
There appears to be substantial biologic evidence in the animal literature to support rapid spine stabilization and decompression,3,4 while the relevant timing and patient selection in humans remain unclear.51 It is clear that the goals of treatment for SCI injuries should be to stabilize the spine and decompress the neural elements, but the mechanism and protocols for obtaining these goals are far from rigid. On the basis of current research, there are no clear protocols defining the use of traction and the timing of its application. The same current research appears to indicate that early operative management of spinal cord injuries is safe, but the role for early surgery has yet to be fully defined. It is quite difficult to differentiate a complete and an incomplete SCI in the early phases of injury.43,52 Therefore, some authors recommended treating all SCIs as potentially incomplete SCIs.43,52 Other clinical studies indicate that operative management of SCI leads to improved non-neurologic benefits such as decreased pulmonary complications, time in the ICU, and decreased hospital length of stay.20,21 Therefore, surgical treatment of cervical SCI is generally well accepted to achieve rigid stabilization and direct spinal cord decompression. The importance of the timing of surgery remains unclear. The meta-analysis by La Rosa et al. analyzing SCI decompression at less than 24 hours and more than 24 hours after injury found enough evidence to support loose guidelines but not enough evidence to provide a concrete protocol for early surgery in patients with incomplete SCI and found no firm evidence to support early surgery in complete SCI.53 Fehlings and Perrin concluded that surgery within 24 hours after injury remains a valid practice option whenever possible but did not define firm treatment protocols.3 The evidence suggests that early surgical intervention is safe and effective and may confer neurologic benefit. However, other studies suggest that delayed decompression may also confer neurologic benefit. These discrepancies define the need for further study. They have generated the impetus for multicenter clinical trials such as STASCIS, which at the time of the writing of this chapter is ongoing.
Fehlings M.G., Perrin R.G. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-B26.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
Fisher C.M., Harris M., Hurlbert J., et al. Summary statement: early management of spinal cord and column injury. Spine (Phila Pa 1976). 2006;31:S36.
La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323-332.
1. National Spinal Cord Injury Statistics Center. Facts and figures at a glance. 2009. Available from https://www.nscisc.uab.edu/public_content/facts_figures_2009.aspx
2. Fisher C.M., Harris M., Hurlbert J., et al. Summary statement: early management of spinal cord and column injury. Spine (Phila Pa 1976). 2006;31:S36.
3. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
4. Fehlings M.G., Perrin R.G. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-B26.
5. Carlson G.D., Gorden C. Current developments in spinal cord injury research. Spine J. 2002;2:116-128.
6. Carlson G.D., Minato Y., Okada A., et al. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J Neurotrauma. 1997;14:951-962.
7. Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77:1042-1049.
8. Dimar J.R.2nd, Glassman S.D., Raque G.H., et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24:1623-1633.
9. Frankel H.L., Hancock D.O., Hyslop G., et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179-192.
10. Shrosbree R.D. Neurological sequelae of reduction of fracture dislocations of the cervical spine. Paraplegia. 1979;17:212-221.
11. Waters R.L., Adkins R.H., Yakura J.S., Sie I. Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord. 1996;34:188-192.
12. Kiwerski J., Weiss M. Neurological improvement in traumatic injuries of cervical spinal cord. Paraplegia. 1981;19:31-37.
13. Tator C.H., Duncan E.G., Edmonds V.E., et al. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurol Sci.. 1987;14:60-69.
14. Young J.S., Dexter W.R. Neurological recovery distal to the zone of injury in 172 cases of closed, traumatic spinal cord injury. Paraplegia. 1978;16:39-49.
15. Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323-332.
16. Fisher C.G., Noonan V.K., Dvorak M.F. Changing face of spine trauma care in North America. Spine (Phila Pa 1976). 2006;31:S2-S8. discussion S36
17. Aebi M., Mohler J., Zach G.A., Morscher E. Indication, surgical technique, and results of 100 surgically-treated fractures and fracture-dislocations of the cervical spine. Clin Orthop Relat Res. 1986:244-257.
18. Pateder D., Carbone J. Cervical spine trauma. J Surg Orthop Adv. 2005;14:8-16.
19. Ducker T.B., Salcman M., Perot P.L.Jr., Ballantine D. Experimental spinal cord trauma, I: Correlation of blood flow, tissue oxygen and neurologic status in the dog. Surg Neurol. 1978;10:60-63.
20. Duh M.S., Shepard M.J., Wilberger J.E., Bracken M.B. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240-248. discussion 248–249
21. McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818-1825.
22. Darsaut T.E., Ashforth R., Bhargava R., et al. A pilot study of magnetic resonance imaging-guided closed reduction of cervical spine fractures. Spine (Phila Pa 1976). 2006;31:2085-2090.
23. Darsaut T.E., Sartawi M.M., Dhaliwal P., Fox R.J. Rapid magnetic resonance imaging-guided reduction of craniovertebral junction deformities. J Neurosurg Spine. 2009;10:27-32.
24. Dall D.M. Injuries of the cervical spine. II. Does anatomical reduction of the bony injuries improve the prognosis for spinal cord recovery? S Afr Med J. 1972;46:1083-1090.
25. Harvey C., Rothschild B.B., Asmann A.J., Stripling T. New estimates of traumatic SCI prevalence: a survey-based approach. Paraplegia. 1990;28:537-544.
26. Tator C.H., Fehlings M.G., Thorpe K., Taylor W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J Neurosurg. 1999;91:12-18.
27. Harrop J.S., Sharan A.D., Vaccaro A.R., Przybylski G.J. The cause of neurologic deterioration after acute cervical spinal cord injury. Spine. 2001;26:340-346.
28. Cotler J.M., Herbison G.J., Nasuti J.F., et al. Closed reduction of traumatic cervical spine dislocation using traction weights up to 140 pounds. Spine (Phila Pa 1976). 1993;18:386-390.
29. Brunette D.D., Rockswold G.L. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445-447.
30. Hadley M.N., Fitzpatrick B.C., Sonntag V.K., Browner C.M. Facet fracture-dislocation injuries of the cervical spine. Neurosurgery. 1992;30:661-666.
31. Chulay M., Brown J., Summer W. Effect of postoperative immobilization after coronary artery bypass surgery. Crit Care Med. 1982;10:176-179.
32. Convertino V.A., Bloomfield S.A., Greenleaf J.E. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187-190.
33. Curry K., Casady L. The relationship between extended periods of immobility and decubitus ulcer formation in the acutely spinal cord-injured individual. J Neurosci Nurs. 1992;24:185-189.
34. Dittmer D.K., Teasell R. Complications of immobilization and bed rest. 1: Musculoskeletal and cardiovascular complications. Can Fam Physician. 1993;39:1428-1432. 1435–1437
35. Krishnagopalan S., Johnson E.W., Low L.L., Kaufman L.J. Body positioning of intensive care patients: clinical practice versus standards. Crit Care Med. 2002;30:2588-2592.
36. Teasell R., Dittmer D.K. Complications of immobilization and bed rest. 2: Other complications. Can Fam Physician. 1993;39:1440-1442. 1445–1446
37. McGuire R.A., Green B.A., Eismont F.J., Watts C. Comparison of stability provided to the unstable spine by the kinetic therapy table and the Stryker frame. Neurosurgery. 1988;22:842-845.
38. Conrad B.P., Horodyski M., Wright J., et al. Log-rolling technique producing unacceptable motion during body position changes in patients with traumatic spinal cord injury. J Neurosurg Spine. 2007;6:540-543.
39. Rechtine G.R., Del Rossi G., Conrad B.P., Horodyski M. Motion generated in the unstable spine during hospital bed transfers. J Trauma. 2004;57:609-611. discussion 611–612
40. Rechtine G.R., Conrad B.P., Bearden B.G., Horodyski M. Biomechanical analysis of cervical and thoracolumbar spine motion in intact and partially and completely unstable cadaver spine models with kinetic bed therapy or traditional log roll. J Trauma. 2007;62:383-388. discussion 388
41. Bearden B.G., Conrad B.P., Horodyski M., Rechtine G.R. Motion in the unstable cervical spine: comparison of manual turning and use of the Jackson table in prone positioning. J Neurosurg Spine. 2007;7:161-164.
42. DiPaola M.J., DiPaola C.P., Conrad B.P., et al. Cervical spine motion in manual versus Jackson table turning methods in a cadaveric global instability model. J Spinal Disord Tech. 2008;21:273-280.
43. Mirza S.K., Krengel W.F.3rd, Chapman J.R., et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999:104-114.
44. Wagner F.C.Jr., Chehrazi B. Early decompression and neurological outcome in acute cervical spinal cord injuries. J Neurosurg. 1982;56:699-705.
45. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
46. Kiwerski J. The results of early conservative and surgical treatment of cervical spinal cord injured patients. Int J Rehabil Res.. 1986;9:149-154.
47. Donovan W.H., Kopaniky D., Stolzmann E., Carter R.E. The neurological and skeletal outcome in patients with closed cervical spinal cord injury. J Neurosurg. 1987;66:690-694.
48. Kerwin A.J., Frykberg E.R., Schinco M.A., et al. The effect of early spine fixation on non-neurologic outcome. J Trauma. 2005;58:15-21.
49. Croce M.A., Bee T.K., Pritchard E., et al. Does optimal timing for spine fracture fixation exist? Ann Surg. 2001;233:851-858.
50. Kerwin A.J., Griffen M.M., Tepas J.J.3rd, et al. Best practice determination of timing of spinal fracture fixation as defined by analysis of the National Trauma Data Bank. J Trauma. 2008;65:824-830. discussion 830–831
51. Bono C.M., Vaccaro A.R., Fehlings M., et al. Measurement techniques for upper cervical spine injuries: consensus statement of the Spine Trauma Study Group. Spine (Phila Pa 1976). 2007;32:593-600.
52. Waters R.L., Yakura J.S., Adkins R.H., Sie I. Recovery following complete paraplegia. Arch Phys Med Rehabil. 1992;73:784-789.
53. La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
Emergent Surgery
Pathophysiology of Spinal Cord Injury
The neurologic dysfunction that occurs in acute SCI is the end result of primary and secondary injury. The primary injury occurs at the time of trauma and refers to irreversible damage caused by disruption of neural elements and vascular supply. The initial injury fosters a cascade of events, including ischemia, electrolyte disturbances, and lipid peroxidation, which produce additional neural injury.1–3 Spinal cord compression and instability also provoke secondary mechanical injury and ischemia.1–3 It is important to understand that, unlike primary injury, secondary injury may be preventable or reversible. In fact, ongoing compression of the neural elements, compromised vascular supply, and mechanical instability at the site of injury exacerbate secondary injury. Many medical interventions, such as the administration of methylprednisolone and the maintenance of a mean arterial blood pressure of 85 mmHg or greater, aim to prevent neurologic damage due to secondary injury. Evidence suggests that the effectiveness of these measures is partially dependent on the timing of administration. For example, the National Acute Spinal Cord Injury Studies (NASCIS II and NASCIS III) showed that management aimed at reducing secondary injury is time sensitive.4 Similarly, the neurologic impact of surgical decompression of the acutely injured spinal cord may be sensitive to the timing of intervention.
Early Surgical Decompression in Spinal Cord Injury
Studies investigating the role of early surgery for acute SCI report variable clinical results. In a systematic review of the relevant experimental and clinical literature, Fehlings and Baptiste concluded that there is a biologic basis for early decompression in SCI and that early surgical decompression is safe.1 Animal models of SCI demonstrate smaller lesion volumes and improve locomotor and neurologic recovery following early decompression.3–5 In a recent preclinical study, Rabinowitz et al.5 compared early surgical decompression (6 hours) with or without methylprednisolone compared to methylprednisolone alone in a dog SCI model. The authors report that early surgical decompression, with or without methylprednisolone administration, offers greater neurologic improvement than does the use of methylprednisolone alone. Overall, experimental results are promising, though outcomes in the human SCI population are less clear, largely owing to the lack of high-quality prospective trials. The senior author, however, is currently completing a large prospective trial (STASCIS), which will be available in the near future.
Before discussing the evidence for early surgical decompression in humans, it is necessary to recognize the logistical challenges to delivering acute SCI patient to the surgical theater. Transfer to an appropriate center may be delayed by rescue, resuscitation, and transportation resources. Medical stabilization, along with obtaining the appropriate tests, may further delay time to surgical decompression. As a result, most studies define early surgery as less than 72 hours after injury. 6–11 However, there is concern that surgery within 72 hours is not early enough to mitigate the effects of secondary injury. This is highlighted by the results of an international survey of spine surgeons conducted by Fehlings et al.,12 in which the majority of respondents preferred to decompress the injured spinal cord within 24 hours of injury, regardless of injury severity or anatomic level.
Vaccaro et al.7 examined the role of early surgery in acute cervical SCI by following two groups of patients (early surgery, <72 hours; late surgery, >5 days) for change in ASIA grade or motor score as well as length of ICU and rehabilitation stay. They concluded that there was no difference between groups in terms of neurologic recovery. However, as the authors themselves acknowledge, it is questionable whether 72 hours truly represents an “early” time point. Vale et al.13 studied the outcome of acute SCI patients with both aggressive medical management, focusing on volume resuscitation and maintenance of blood pressure, and early surgical decompression. They demonstrated that aggressive medical management affords an optimal chance for recovery and that surgical treatment affords little additional advantage. Pointillart et al.11 studied the effects of two medical agents, methylprednisolone and nimodipine, administered in the first 24 hours, as an adjunct to early surgery in a prospective randomized manner. They found that the only predictor of outcome was the presence of an incomplete SCI, as compared to complete injury. Neither the medical therapy nor the surgical decompression offered benefit in terms of ASIA score at 1 year.
In contrast to the aforementioned studies, Papadopoulos et al.6 evaluated 91 patients with cervical SCI and demonstrated that early surgery led to improvement in 39 of 66 patients in the early surgery group compared to 6 of 25 in the control group. In addition, patients in the early surgery group had both shorter ICU stay and shorter overall hospital stay. Moreover, some patients improved from complete SCI following surgery. Waters et al.9and Ng et al.10 examined the rate of complications and feasibility of early surgery, respectively. It was shown that the rate of medical complications following early surgery is not different from that after late surgery.
The Surgical Treatment of Acute Spinal Cord Injury Study (STASCIS) is a multicentered prospective cohort study started in 2003.14 It was designed to collect detailed information about neurologic outcomes in patients with traumatic SCI without imposing a specific treatment regimen. Preliminary 1-year results for 170 patients with subaxial SCI were presented at the 76th annual meeting of the American Association of Neurological Surgeons in April 2008. Approximately 44% of these patients had complete SCI (ASIA A) at the time of admission. Patient outcomes were assessed at 6 months and 1 year after the initial injury. Results showed an average of two grade improvement on the ASIA score in 24% of the patients who underwent early surgery (<24 hours after injury) compared to only 4% in patients who underwent decompression 24 hours after injury. There was a statistically significant decrease in cardiopulmonary and urinary complications in the early-surgery group.
Overall, these results indicate that there is a population of SCI patients that may greatly benefit from early surgical decompression. The severity of initial neurologic injury is one of the most important determinants of neurologic recovery, with less favorable outcomes in patients with a complete SCI.15 Despite this, results from a recent international survey of spine surgeons indicate that 46.2% of respondents would ideally decompress the spine within 6 hours and 85.2% would decompress within 24 hours for a patient with a complete SCI.12 The same patient with an incomplete SCI would be taken to the operating room within 6 hours by 72.9% of respondents. Early decompressive surgery, within 24 hours of injury, is an important component of our clinical arsenal and may produce significant improvement in neurologic outcome in patients with complete SCI. The authors recommend surgical decompression within 24 hours of injury in any patient with a complete SCI, particularly patients with a complete cervical spine injury.
Clinical Vignette
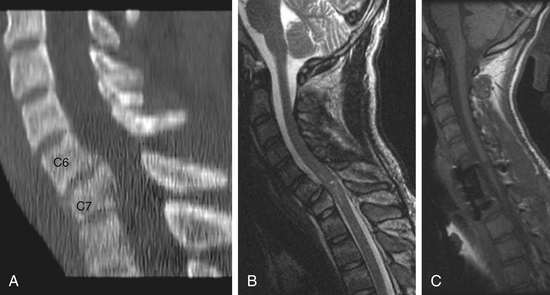
CT scan revealed bony injury involving the C7 vertebral body with ventral wedging of this vertebral body and fractures involving the lamina on the right and left sides. MRI revealed mild compression of the spinal cord at C7 with apparent swelling of the cord and increased cord signal. Representative images are shown in Figure 229-3.
Postoperatively, the patient remained stable and was discharged from the hospital to a rehabilitation facility with an unchanged ASIA B clinical exam. At 1-year follow-up, the patient had improved to an ASIA C: C7 was graded as 5/5 bilaterally and C8/T1 was 2 to 3 in most muscle groups bilaterally. The lower extremity muscle groups also improved to 3 to 4/5 in most muscle groups on the right and 2 to 3/5 in most muscle groups on the left. This examination remained stable beyond 2 years. The postoperative MRI at 1 year is shown in Figure 229-3C.
Conclusions
Patients suffering from SCI should be maintained with adequate oxygenation and a mean arterial blood pressure of 85 mmHg. Arrangements should be made for transfer to a specialized center where decompressive surgery can be undertaken if deemed appropriate by the treating surgeon. The authors recommend, when medically feasible, to undertake decompressive and reconstructive surgery in patients with cervical SCI (both complete and incomplete) and selected patients with thoracic SCI (those with incomplete lesions) within 24 hours. The benefits of early decompressive surgery in patients with clinically complete (ASIA A) thoracic SCI are less clear.
Fehlings M.G., Perrin R.G. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(suppl 2):B13-B26.
Fehlings M.G., Rabin D., Sears W., et al. Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976). 2010;35(21 Suppl):S166-S173.
Fehlings M.G., Sekhon L.H., Tator C. The role and timing of decompression in acute spinal cord injury: What do we know? What should we do? Spine (Phila Pa 1976). 2001;26:S101-S110.
Fehlings M.G., Tator C.H. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91:1-11.
1. Fehlings M.G., Baptiste D.C. Current status of clinical trials for acute spinal cord injury. Injury. 2005;36(Suppl 2):B113-B122.
2. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
3. Fehlings M.G., Tator C.H. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91:1-11.
4. Bracken M.B. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine (Phila Pa 1976). 2001;26:S47-S54.
5. Rabinowitz R.S., Eck J.C., Harper C.M.Jr., et al. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine (Phila Pa 1976). 2008;33:2260-2268.
6. Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323-332.
7. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
8. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
9. Waters R.L., Meyer P.R.Jr., Adkins R.H., Felton D. Emergency, acute, and surgical management of spine trauma. Arch Phys Med Rehabil. 1999;80:1383-1390.
10. Ng W.P., Fehlings M.G., Cuddy B., et al. Surgical treatment for acute spinal cord injury study pilot study #2: evaluation of protocol for decompressive surgery within 8 hours of injury. Neurosurg Focus. 1999;6:E3.
11. Pointillart V., Petitjean M.E., Wiart L., et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71-76.
12. Fehlings M.G., Rabin D., Sears W., et al. Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976). 2010;35(Suppl 21):S166-S173.
13. Vale F.L., Burns J., Jackson A.B., Hadley M.N. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239-246.
14. Fehlings MG. STASCIS: Early Surgery in Spinal Cord Injury Improves Outcomes, Lowers Complications. Presented at the 76th Annual Meeting of the American Association of Neurological Surgeons, 2008.
15. Tator C.H. Spine-spinal cord relationships in spinal cord trauma. Clin Neurosurg. 1983;30:479-494.
Nonemergent Surgery
The timing of decompressive surgery for traumatic spinal cord injury (SCI) patients remains a controversial topic. A significant amount of research has produced several studies that have assessed the timing of surgery and whether or not decompression should be performed for patients presenting with incomplete SCI. There has been little evidence, though, to support the role of decompressive surgery for a patient with complete SCI.1
SCIs cause myelopathy or damage to white matter through either direct or indirect mechanisms. SCI, depending on mechanism or severity of injury, could also damage the gray matter, damaging cell bodies, interneurons and motor neurons. SCI has further been divided into complete or partial. The most common causes of SCI since 2005 are motor vehicle crashes, accounting for 42.1% of cases, the next most common cause being falls, followed by acts of violence, which consist primarily of gunshot wounds.2
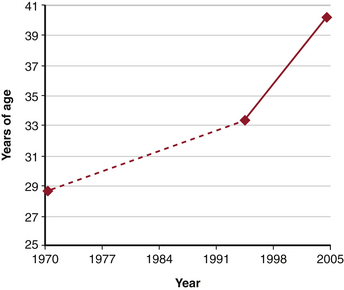
In 2004, the National Spinal Cord Injury Statistical Center (NSCISC) at the University of Alabama at Birmingham reported an annual incidence of 11,000 new cases of traumatic SCI each year, with a rate of 40 cases per million population.3–4 The prevalence of SCI was 259,000 in 2008 and is increasing because of increasing survival rates. While the average age at injury has steadily increased over time to 40.2 years in 2005,2,5 the average patient with SCI remains young, making the personal and socioeconomic impacts enormous. Total direct costs associated with all causes of SCI in the United States are estimated to be $7.736 billion.6 Overall rates of employment for people after SCI range from 13% to 58%, with 35.4% being employed at postinjury year 20.2,7 The rates of employment also depend on functional status; patients with paraplegia are employed more frequently (32.8%) than those with tetraplegia (24.7%).3,8 Owing to the enormous societal and personal costs associated with SCI, much attention has been given to the role of early versus late surgical intervention.
Studies of Decompression in Spinal Cord Injury in Animal Models
Research in animal models has shown that early decompressive surgery can improve neurologic recovery after SCI. Dimar et al.9 used the New York University weight-drop model to produce a thoracic SCI followed by an epidural spacer placement to simulate a compression in rats. Their results demonstrated that the tolerance for spinal canal narrowing with a contused cord appears diminished, indicating that an injured spinal cord may benefit from early decompression.9 Delamarter et al.10 also evaluated the timing of decompression after 50% compression of the diameter of the spinal canal at L4 level in 30 dogs. All of them were initially paraplegic, though all the dogs that underwent decompression immediately or within 1 hour recovered the ability to walk and bowel and bladder control. When decompression was performed at 6 hours or later, there was no neurologic recovery, and there was evidence of spinal cord necrosis.10
Recently, Rahimi-Movaghar et al.11 evaluated whether spinal cord decompression improves functional recovery and decreases size of the lesion and what decompression timeline is most efficacious in rats. Compression in 63 rats was accomplished at the T9 level by an aneurysm clip. The results of the study showed that immediate (<3 seconds) spinal cord decompression improves functional recovery and decreases lesion size. The studies by Rahimi-Movaghar et al. and Delamarter et al. both demonstrated that urgent decompression of the spinal cord can improve functional outcomes. Extrapolating their conclusions regarding immediate decompression, which in the case of Rahimi-Movaghar et al. was less than 3 seconds, and for Delamarter et al. was less than 1 hour, to SCI cases in humans would not necessarily be feasible.
Surgery for Spinal Cord Injury
The studies that have looked at merely the role of immediate surgical management versus delayed or nonsurgical management of patients with SCI have been limited to noncontrolled, retrospective studies. McKinley et al.,12 in their retrospective series of SCI patients who underwent either early (<72 hours from injury), late (>72 hours), or no surgery, found that ASIA motor index improvements were observed in patients who underwent no surgery but that this group was more likely to have patients with incomplete SCI. They also found no significant difference between early and late surgical groups for either neurologic level of injury or ASIA motor changes.12 Other authors have also shown that delayed or nonoperative management can lead to spontaneous neurologic improvement with the exception of patients with incomplete SCI, who can deteriorate neurologically with nonoperative treatment.13 Thus, while it would be reasonable to conclude that early surgical intervention be recommended for incomplete SCI with deteriorating neurologic status,14 a distinction must be drawn for patients with complete SCI, who can improve neurologically with delayed surgery or without surgery. Since no neurologic improvement is likely from early surgery, nonemergent surgery is a viable option.
Spinal Cord Injury Decompression Surgery
While the role of surgery versus conservative therapy (e.g., no surgery) for SCI has been mostly analyzed with retrospective studies, the role of decompressive surgery for acute SCI has been examined with both retrospective and prospective studies. La Rosa et al.14 conducted a meta-analysis of all studies from 1966 to 2000 that focused on the role of early surgical decompression for acute SCI. They found that in patients undergoing early surgical decompression (<24 hours), the percentage of patients with a complete SCI presenting with an improvement of cord function was 42%, and for that incomplete SCI was 89.7%. The rate of improvement was 8.3% in the delayed surgery group and 58.5% for complete and incomplete SCI. La Rosa et al. concluded that timing may play a role in surgical decompression or offer some neurologic advantage as seen in animal studies. They were unable to draw a definite conclusion, owing to the number of variables that can influence overall neurologic outcome for which they could not account.14
Several prospective studies have been conducted for acute decompression of acute SCI. Vaccaro et al.15 in a prospective, randomized trial did not show a significant neurologic or functional benefit for patients who underwent early (<72 hours) versus late (>5 days) decompression after cervical spine trauma. While other prospective studies have shown a clear benefit of acute decompression, they did not distinguish between complete and incomplete SCI patients. Papadopoulos et al.,16 for example, examined 91 consecutive patients with acute, traumatic SCI. The study reported that 39 of 66 patients in the protocol group, including some with complete SCI, had improvement with early surgery, in which the mean time to decompression was 12.6 hours. Fehlings and Perrin,17 in their review of studies that have examined decompression in SCI patients (which were limited to class II and III evidence), concluded that surgery remains a valid practice option but that there exist no conclusive data that it offers a benefit over conservative treatment.
Role of Surgical Decompression in Complete Spinal Cord Injury
While there have been studies documenting the benefits of spinal cord decompression for patients with partial SCI, there has been no definite evidence to support the role of decompression in patients with complete SCI. Rahimi-Movaghar et al.18 retrospectively evaluated 24 patients who sustained a blunt traumatic SCI to the conus medullari. Sixteen of the 24 patients presented with a Frankel grade A, or functionally complete, SCI. The median time interval from injury to surgery was 6 days. Complete SCI patients improved an average of 1.6 Frankel grades and 15.1 motor points. Rahimi-Movaghar et al. concluded that nerve root recovery was more predictable than was spinal cord recovery. They also identified spinal cord and bowel and bladder recovery in their patient cohort. Unfortunately, they were not able to demonstrate a correlation between decompression and motor improvement according to the Frankel grading system.18
Another study conducted by Rahimi-Movaghar1 assessed the shortcomings of the previous study by evaluating the efficacy of surgical decompression in complete thoracic-level SCI. A retrospective evaluation of 12 patients who presented with a Frankel grade A deficit was undertaken. The median time from injury to decompressive surgery was 11 days. Motor improvement was seen in one patient, who improved from a Frankel grade A to grade C, and sensory without motor improvement was seen in one other patient. The authors concluded that there was no correlation between spinal cord decompression and motor improvement. Unfortunately, the study did not employ a control or have an early surgical intervention group. Most of the surgeries were delayed, which clouded the conclusion that decompression did not lead to motor improvement, as the prohibiting factor for motor improvement may have been the timing of surgery.1
Conclusion
The overall conclusion regarding decompression for SCI patients is based mostly on class II and III studies. Some of the studies have concluded that surgery (with early surgery being more beneficial than late) versus conservative treatment (no surgery) for SCI patients reduces pulmonary complications and decreases overall hospital length of stay, although the data have not been conclusive. Furthermore, class II evidence has shown that early surgery with decompression is effective and safe with improvements in Frankel grades, though most of those studies did not differentiate complete and incomplete SCI patients. Other studies have shown that neurologic recovery can be seen in patients with complete SCI who did not undergo surgical treatment at all. Marshall et al.19 showed in their retrospective review that a small percentage of patients with SCI worsened after early surgery, which they defined as less than 5 days. Knowing that a small percentage of people improve without surgery and possibly others deteriorate with early surgery helps to mitigate the conclusion of level II studies that recommend early surgery.
To better compare the role of emergent versus nonemergent decompressive surgery for complete and incomplete SCI patients, a prospective, controlled, randomized study is needed that will produce level I data. Without such data, early intervention can be considered merely an option available only for sufficiently resuscitated SCI patients. One of the primary reasons such a trial has not been undertaken as yet is that it presents an ethical dilemma for some surgeons, since delaying surgery for a certain cohort of patients may affect their ultimate neurologic recovery. Currently, a prospective, randomized controlled trial is underway that will compare early (<24 hours after injury) versus late (between 24 and 72 hours) surgical decompression in terms of neurologic improvement.20 The results of this study when combined with those of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS), which will allocate but not randomize patients with cervical SCI to either early (<24 hours) versus late (>24 hours), will greatly help to answer the question of timing of surgery for patients with SCI. With the studies that have been completed to date, no standard of care has been established for patients with complete SCI with regard to time to decompressive surgery. Late surgery for complete SCI patients remains a viable option.
Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury: recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77:1042-1049.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35.
La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42(9):503-512.
McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical interventional after acute spinal cord injury. Arch Phys Med Rehab. 2004;85:1818-1825.
Rahimi-Movaghar V., Vaccaro A.R., Mohammadi M. Efficacy of surgical decompression in regard to motor recovery in the setting of conus medullaris injury. J Spinal Cord Med. 2006;29(1):32-38.
Vacarro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
1. Rahimi-Movaghar V. Efficacy of surgical decompression in the setting of complete thoracic spinal cord injury. J Spinal Cord Med. 2005;28(5):415-420.
2. National Spinal Cord Injury Statistical Center. Facts and figures at a glance—April 2009. Available at www.spinalcord.uab.edu Accessed 31 October, 2009
3. Kraus J.F., Franti C.E., Riggins R.S., et al. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471-492.
4. Jackson A.B., Dijkers M., DeVivo M.J., Poczatek R.B. A demographic profile of new traumatic spinal cord injuries: change and stability over 20 years. Arch Phys Med Rehabil. 2004;85:1740-1748.
5. Sekhon L.H., Fehlings M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S2-S12.
6. Devivo M.J. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809-813.
7. Jang Y., Wang Y.H., Wang J.D. Return to work after spinal cord injury: the contribution of functional independence. Arch Phys Med Rehabil. 2005;86:681-686.
8. Priebe M.M., Chiodi A.E., Scelza W.M., et al. Spinal cord injury medicine. 6. Economic and societal issues in spinal cord injury. Arch Phys Med Rehabil. 2007;88:S84-S88.
9. Dimar J.R.2nd, Glassman S.D., Raque G.H., et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24:1623-1633.
10. Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury: recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77:1042-1049.
11. Rahimi-Movaghar V., Yazdi A., Karimi M., et al. Effect of decompression on complete spinal cord injury in rats. Int J Neurosci.. 2008;118(10):1359-1373.
12. McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical interventional after acute spinal cord injury. Arch Phys Med Rehab. 2004;85:1818-1825.
13. Katoh S., el Masry W.S., Jaffray D., et al. Neurologic outcome in conservatively treated patients with incomplete closed traumatic cervical spinal cord injuries. Spine (Phila Pa 1976). 1996;21:2345-2351.
14. La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42(9):503-512.
15. Vacarro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
16. Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52(2):323-332.
17. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35.
18. Rahimi-Movaghar V., Vaccaro A.R., Mohammadi M. Efficacy of surgical decompression in regard to motor recovery in the setting of conus medullaris injury. J Spinal Cord Med. 2006;29(1):32-38.
19. Marshall L.F., Knowlton S., Garfin S.R., et al. Deterioration following spinal cord injury. J. Neurosurg. 1981;66:400-404.
20. Rahimi-Movaghar V., Saadat S., Vaccaro A.R., et al. The efficacy of surgical decompression before 24 hours versus 24 to 72 hours in patients with spinal cord injury from T1 to L1—with specific consideration on ethics: a randomized controlled trial. Trials. 2009;24:10-77.