Chapter 93 Optic Disc Anomalies, Pits, and Associated Serous Macular Detachment
Optic disc anomalies
Megalopapilla
Megalopapilla is a rare anomaly of the optic disc, involving thinning of the nerve fiber across a large optic nerve head, and is often associated with large refractive errors and with midline congenital deformities.1
Aplasia
Aplasia of the optic nerve head is extremely rare, and probably represents an extreme form of optic nerve head hypoplasia, and may be associated with the absence or gross maldevelopment of the globe.1
Hypoplasia
Optic disc hypoplasia is a congenital underdevelopment of the optic nerve head with a reduced number of axons. Hypoplastic optic discs are often underdiagnosed and may vary in level of development, leading to variable levels of visual acuity and visual field defects.2
Cavities in the optic nerve head
Excavated and colobomatous defects of the optic nerve head encompass a spectrum of abnormalities, including tilted discs, peripapillary staphyloma, morning-glory disc anomaly, colobomas, and congenital optic disc pits. Optic disc pits were regarded as atypical colobomas by Grear,3 who reviewed the subject in 1942.
Optic disc pits should probably be considered one manifestation along a spectrum of cavitary optic disc anomalies. Slusher and coworkers4 described a family of 35 members spanning five generations with an autosomal dominant pattern of congenital optic disc abnormalities. Remarkably, a myriad of morphologic variations of phenotype were expressed, including optic disc pits, morning-glory syndrome, and coloboma of the optic nerve. One gene defect can result in a variety of optic disc abnormalities; therefore the traditional classification schemes that describe varieties of cavitary optic disc anomalies should be reconsidered.
Anatomy
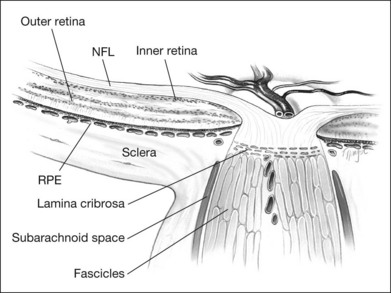
The retinal ganglion cells of each retina contribute approximately 1.2 million unmyelinated axons that converge at a point approximately 4 mm nasal to the foveola, through which they exit the globe, acquire a myelin sheath, and form the optic nerve. These axons project to various primary visual nuclei in the brain,5 constitute a fiber tract rather than a nerve, and, as such, have histologic and functional similarities to brain tissue. The optic nerve is enclosed by three meningeal sheaths that are contiguous with the meningeal coverings of the brain. Before exiting the eye, however, the axons of the retinal ganglion cells must converge centripetally, make a sharp turn, traverse the lamina cribrosa, form nerve bundles enclosed by connective tissue septa, and then, once posterior to the lamina cribrosa, become ensheathed by myelin (Fig. 93.1).6
Thus, the optic nerve head is remarkable in several respects. Axons deriving from the retina become part of the nerve, go from an unmyelinated to a myelinated state, traverse the sieve-like lamina cribrosa, are partitioned into groups by glial columns, and go from an area of high (intraocular) pressure to relatively low interstitial pressure. Not surprisingly, anomalies of structure at this critical juncture often lead to marked physiologic consequences.7
Optic disc pits
In 1882, Wiethe8 described abnormalities in both optic discs of a 62-year-old woman. His description of dark-gray depressions in the optic nerve heads was probably the first report of optic disc pits. Since Wiethe’s initial description, excavations of the optic nerve head have variously been described as craters, holes, cavities, and, most recently, congenital pits of the optic nerve head.
Studies suggest that optic pits occur in approximately 1 in 10 000 eyes, although there is considerable variance among studies.3,9 Men and women are equally affected. Approximately 10–15% of optic disc pits are bilateral. Most optic disc pits are nonfamilial; however there are a few reports with an autosomal dominant pattern of inheritance.10 One such report describes a family for which several members had small iris colobomas, some in combination with the pit, providing insight as to the etiology.11 About 70% of the pits are on the temporal side of the disc, and about 20% are situated centrally; the remainder are found inferiorly, superiorly, and nasally.12
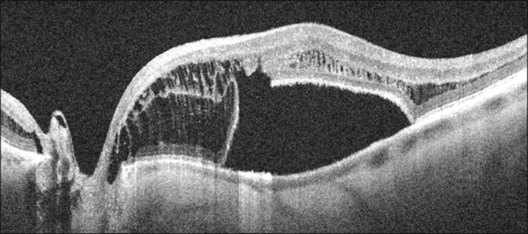
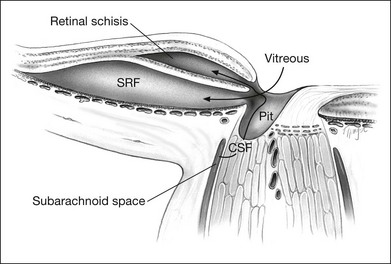
Serous retinal detachments can be associated with optic disc pits. These may occur at any age but are most frequent in early adulthood. However, there have been reports of associated retinal detachment occurring as early as 6 years of age and in patients as old as in the ninth decade of life.12 Some have suggested that the clinical course differs and leads to better visual acuity in children as spontaneous resolution is the rule.13,14 Through the analysis of stereoscopic transparencies, it has been proposed that the fluid that enters through the optic disc pit actually travels between the inner and outer layers of the retina to produce a retinal schisis.15,16 Optical coherence tomography (OCT) has shown such inner retinal schises preceding outer-layer detachment.17 Following this, detachment of the outer retinal layer may occur as a secondary process.17 Although no histopathologic studies have confirmed this, the application of OCT has provided compelling evidence for at least two levels of retinal separation (Fig. 93.2).18 OCT has also been used to demonstrate a marked reduction in thickness of the retinal nerve fiber layer in the quadrant corresponding to the optic nerve pit.19
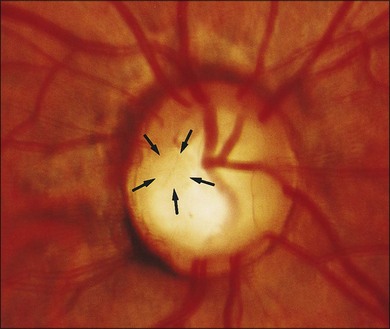
In the series of Brown et al.,12 most optic disc pits were gray in color, although they varied from yellow to black. Their size can range from minute to large, occupying most of the surface of the optic disc.
Visual defects
The optic disc pit is most often associated with two types of visual field defect.20 The first type is typified by arcuate scotomas that probably reflect the absence of the wedge of nerve fibers displaced by the optic disc pit. Larger pits may be associated with large Bjerrum-type scotomas or even altitudinal visual field defects. Nasal or temporal steps are often detectable; less frequently, paracentral scotomas and generalized constriction may be seen.12 However, Walsh and Hoyt21 reviewed several studies that demonstrated only an enlarged blind spot as a forme fruste of the visual field defect in association with optic pits.
The second type of visual field defect is that associated with serous detachment of the macula. In 1960 Kranenburg22 described the association of optic nerve pits and central serous retinopathy. He found that 16 of his 24 patients with optic disc pits had serous detachments of the macula, with corresponding central scotomas or other central visual field changes.
Associated retinal changes
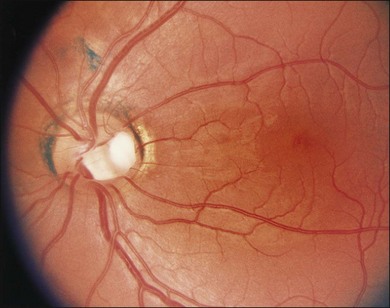
Optic nerve head pits that are centrally located are least likely to be associated with retinal changes. Optic disc pits along the rim of the optic disc are usually seen in association with peripapillary chorioretinal atrophy and retinal pigment epithelium changes (Fig. 93.3). These peripapillary changes may develop over time with or without central serous retinal detachments. In following the development of a serous macular detachment, Walsh and Hoyt21 described the appearance of what they termed an “occult hole” in the optic nerve head.
Serous detachment of the macula is now known as a common complication of the optic disc pit. The natural history of this complication has been well described by Sobol et al.23 They followed 15 patients with optic disc pits and macular detachments for an average of 9 years and found that 80% lost vision to 20/200 or worse. The visual loss was generally complete within 6 months of presentation. Long-term macular changes included full-thickness or laminar (through the outer retina) retinal holes, retinal pigment epithelium mottling, and general cystic changes of the macula.23
Vascular telangiectasis has been reported in connection with intraschistic hemorrhage from a temporal optic disc pit.24
A gray fibroglial membrane appears to overlie the pit in many cases (Fig. 93.4). This membrane may be intact or may incompletely cover the pit. The fact that patients with serous macular detachments almost invariably have defects in their diaphanous membrane has prompted theories on how the optic nerve pit leads to the development of serous macular detachment.
Macular detachment
Several investigators have estimated that between 40 and 50% of patients with optic nerve pits have either an associated nonrhegmatogenous, serous retinal detachment or retinal changes suggestive of previous detachment.12,25 The macular serous detachment (or retinoschisis) seen in association with optic disc pits appears most commonly when the pit is located in the temporal region of the optic disc and in larger pits. Conversely, small pits and those located more centrally are less likely to lead to serous retinal detachments.12,22
Appearance of maculopathy
In 1908, Reis26 described a case of an optic nerve pit with associated maculopathy. However, this association was not taken seriously until Petersen,27 in 1958, described several patients with what he called crater-like holes in the optic disc; these patients also had a central serous chorioretinopathy. This relationship was firmly emphasized by Kranenburg22 in 1960, who described 24 cases of optic disc pits. One-third of these patients had serous retinal detachments, and another third had macular changes that he interpreted as reflecting a previous episode of nonrhegmatogenous serous retinal detachment.
The serous macular detachments are generally low (less than 1.0 mm in height). The elevated retina often contains cystic regions that have been demonstrated on histologic examination to exist within the inner nuclear layer.9 Occasionally the cystic areas rupture outward, producing a lamellar macular hole that, unlike idiopathic lamellar macular holes, retains an intact internal limiting membrane.
The variability of the retinal separation is also consistent with an alternative description of the maculopathy proposed by Lincoff and colleagues.28 In a case report they provide clear OCTs to show a schisis cavity between the inner and outer retina and a larger outer-layer retinal detachment. The two are connected by a hole in the outer layer near the fovea.28
Course of associated serous macular detachment
It is difficult to determine the time interval between the beginning of a serous macular detachment and the earliest visual changes, because the patient usually seeks evaluation after symptoms of blurred vision and metamorphopsia occur secondary to foveal involvement. However, Brown and Tasman29 described one case in which the retinal detachment started at the temporal margin of the optic disc. This serous retinal detachment expanded slowly in a temporal direction until, after several months, it covered the entire macular area. They also described small yellow precipitates seen under the elevated retina late in the course of serous macular detachment.
In analyzing their 15 patients followed over an average of 9 years, Sobol et al.23 found that most eyes with optic disc pits presented with visual acuities of about 20/40–20/60. However, each patient lost three or more lines of vision within the next 6 months. After 6 months, a few of these patients got worse and even fewer got better. Ultimately, only 20% of the patients maintained visual acuities of better than 20/200.23 Generally, however, patients with optic disc pits present later in the course of their macular detachments when their visual acuities are already worse than 20/70.30
Theories of pathophysiology
By 1960 it was clear that serous macular detachments often occurred as complications of optic disc pits. Ferry9 had the opportunity to examine histologically two eyes with optic disc pits associated with macular detachments. He suggested that progressive gliosis and “contraction of the retinal elements” contained in the pit produced a traction detachment of the macula. In 1964 Sugar31 suggested that fluid from the vitreous cavity could enter the subretinal space through a macular hole. However, this is most unlikely because macular holes seen with optic pits are usually lamellar and are only infrequently seen in association with the serous detachment.
It has been reported that fluorescein angiographic examination reveals late hyperfluorescence of the optic disc pit.25 It was therefore considered possible that blood vessels in this area leaked fluid, which then entered the subretinal space.25 However, Brown et al.12 reported that many patients with serous macular detachments had no leakage on their fluorescein angiogram.
Others have speculated that there may be a direct source of fluid from the choroid that penetrates through Bruch’s membrane under the macular detachment; it was hypothesized that peripapillary chorioretinal atrophic changes permitted this leakage.32 However, fluorescein angiographic findings do not support this theory. Moreover, many other diseases produce extensive chorioretinal atrophy that does not lead to serous macular detachment.12
A few investigators,33 including Gass,34 have suggested that cerebrospinal fluid may leak from the optic nerve subarachnoid space into the optic pit and from there into the subretinal space. However, intrathecal fluorescein injections in humans and in animals and histologic studies have failed to demonstrate any such connection.9,10,35,36
Currently, the most widely accepted explanation is that originally proposed by Sugar37 in 1962 and later endorsed by Brockhurst38 in 1975. Sugar proposed that fluid from the vitreous leaked through the optic disc pit to fill the subretinal space (Fig. 93.5). In corroboration of this theory, Brown et al.12 demonstrated that more than three-fourths of patients with optic disc pits and associated serous macular detachments have posterior vitreous detachments. This would allow liquefied vitreous to be contiguous with the optic disc cavity. Moreover, most of the patients in their series who had pits without macular serous elevations did not have posterior vitreous detachments.12 Additionally, Brown and his colleagues36 demonstrated experimentally in dogs a direct connection between the posterior vitreous space and the subretinal space via a congenital optic disc pit. Irvine et al.33 demonstrated in vivo that there is a continuity between the posterior vitreous cavity and the optic nerve subarachnoid space by observing bubbles percolating out of an optic nerve sheath window after pars plana vitrectomy and gas injection.
The most recent variation on these theories of pathophysiology is that proposed by Lincoff et al.,28 who suggest that the primary communication from the optic disc pit is to the retina temporal to the optic disc.15 Fluid slips under the inner retina, lifting it and the nerve fiber layer up and away from the outer retina. This has been corroborated by OCT18 and extended to show both this retinal schisis and an outer retinal detachment connected by a hole in the outer retinal layer (Fig. 93.2).
Although the theory of direct vitreous fluid entry via the optic pit into the subretinal space is appealing, it does not explain why serous macular detachments tend to occur first in young adulthood. Brown and Tasman29 suggest that posterior vitreous detachments may be a precipitating factor. Another possibility is that the impelling factor is macular traction, which occurs with age.
Prognosis
Although the optic nerve head pits, being congenital, are stationary, their associated retinal abnormalities may be progressive. The prognosis for return of vision after serous macular detachment is variable. Walsh and Hoyt21 described a patient followed between the ages of 14 and 23 years who developed multiple serous macular detachments that remitted to near-normal vision after each episode. A more rigorous study was conducted by Brown et al.12 in a group of 20 eyes with optic disc pits and serous macular detachments. These eyes were followed for 5 years, untreated. The mean visual acuity at the end of this time was about 20/80. The authors found very little correlation between the visual acuity at the time of detachment and the long-term visual outcome. They also noted that some detachments resolved spontaneously, whereas others persisted for years. Most patients still had some subretinal macular fluid present after 5 years. Other macular changes, as described above, also persisted. Long-term studies confirm the earlier impressions that untreated macular detachments caused by optic disc pits have an overall poor prognosis.23
Treatment
Given the rarity of optic disc pits, most studies examining purported treatments are small and nonrandomized, leading to difficulty in coming to a consensus on the best treatment for the associated serous macular detachments. Observation, systemic steroids, optic nerve sheath decompression, and scleral buckling procedures have not been demonstrated to be very effective.38,39 However, several series have favorably compared the outcome of photocoagulated eyes with untreated eyes in the resolution of the serous macular detachment and in final visual outcome.33,38–40 The argon laser procedure was used in most of these series to produce photocoagulation burns in one or several rows between the area of serous retinal detachment and the optic disc. Usually, the burns are only applied to areas of elevated retina. Brockhurst,38 Gass,40 and Theodossiadis41 used similar photocoagulation protocols, and all reported that their patients were likely to have good resolution of the serous detachment to a flat macula.
Combining their results, 15 of the 18 patients in these three series had reattachments of their maculas, as opposed to only 5 of 20 untreated patients in the series of Brown et al.12 However, the difference in final visual outcome between the treated and untreated groups was less pronounced. The photocoagulated eyes in the three studies had ultimate visual acuities that averaged a little worse than 20/80. This does not compare favorably with the 20/80 final visual outcome in the series of Brown et al. In making a similar comparison, Brown and Tasman29 concluded that photocoagulation therapy is effective for flattening the retinal detachment, but not for improving final visual outcome.
A macular buckling procedure successfully treated cases of serous detachment in optic disc pit. In such cases, OCT showed a resultant closure of the connection between the pit and a retinal schisis with resolution of the schisis.42 Multifocal electroretinography was performed in 10 patients with optic disc pit with serous macular detachment before and after treatment with macular buckling procedures.43 Improvement was measured in all eyes at 12 months, though often this was not accompanied by an increase in visual acuity.43 Silicon oil has been used successfully in cases of macular hole in association with the optic disc pit.44
More recent attempts to combine photocoagulation therapy with posterior vitrectomy and gas–fluid exchange have led to more encouraging long-term visual outcomes.45 Bonnet46 looked at 25 eyes with optic disc pits in 24 patients who presented with visual loss due to serous macular detachments. High-magnification biomicroscopy and fluorescein angiography of these eyes revealed evidence of vitreous traction on the retina and on the optic nerve head. Most particularly, Bonnet noted that none of the patients had posterior vitreous detachments at presentation and that in the 2 cases that subsequently developed a posterior vitreous detachment, reattachment of the macula occurred spontaneously. Fluorescein dye was seen to stain the optic nerve head, especially at the pit and temporal margin of the disc. This dye leakage was not seen in cases that underwent surgical peeling of the posterior vitreous face. Moreover, Bonnet observed a small hole in the roof of the optic pit in several cases, and small bubbles of gas passing from the vitreous cavity into the subretinal space via the optic disc pit in a case that underwent vitrectomy and gas injection but not photocoagulation. Hence, Bonnet concluded that macular detachments in optic disc pits have a rhegmatogenous component (at the optic disc), that they are associated with vitreous traction, and that the subretinal fluid comes from the vitreous space via the optic disc pit.46
Cox and colleagues47 looked at three treatment methods. They concluded that the combination of vitrectomy plus gas tamponade plus photocoagulation of the retina temporal to the disc was more effective than vitrectomy and gas or photocoagulation alone. They obtained short-term surgical success in all of their 8 eyes and long-term attachment in 4 out of 8 eyes using the three-part combination therapy.47 Others have also obtained good anatomic results in optic disc pit with macular detachment using the combination of vitrectomy, air–fluid exchange, and photocoagulation in patients whose final visual acuities averaged 20/60.48 Various precipitating events may combine with the mechanism described above, leading to macular detachment by vitreous fluid entry into the subretinal space via the optic disc pit.
Novel techniques for treatment of optic disc pit-associated serous macular detachments have been described. Spaide et al.49 published a case report describing the use of a bent 25-gauge needle to create half-thickness cuts to the retina. This maneuver was combined with a vitrectromy in an attempt to allow intra- and subretinal fluid to escape. This procedure did not use intraocular gas to avoid premature closure of the surgical fenestrations and the posterior hyaloid was left intact. One month after surgery, the patient’s visual acuity improved from 8/400 preoperatively to 20/20. Schaal et al.50 described a similar procedure with the use of a 27-gauge cannula to make three-quarter depth cuts, in combination with a limited preretinal vitrectomy.
Jalil et al.51 actively drained subretinal fluid with a 42-gauge cannula. This procedure included vitrectomy, induction of posterior vitreous detachment, and internal limiting membrane peel, but without the use of laser photocoagulation around the retinotomy site. Fluid–air exchange followed by internal gas tamponade with 14% C3F8 concluded the procedure. The patient’s preoperative visual acuity of 1.00 logMAR improved at 8-months follow-up to 0.4 logMAR. Ziahosseini et al.52 utilized a similar approach with the addition of argon laser to the cannula insertion site.
1 Sadun AA, Yanoff M. Pathology of the optic nerve. Biomedical foundations of ophthalmology. Duane TD, Jaeger EA, eds. Biomedical foundations of ophthalmology. Philadelphia: Harper & Row; 1986;vol. 3.
2 Nelson M, Lessell S, Sadun AA. Optic nerve hypoplasia and maternal diabetes mellitus. Arch Neurol. 1986;43:20–25.
3 Grear JN, Jr. Pits, or crater-like holes in the optic disk. Arch Ophthalmol. 1942;28:467–483.
4 Slusher MM, Weaver RG, Greven CM, et al. The spectrum of cavitary optic disc anomalies in a family. Ophthalmology. 1989;96:342–347.
5 Sadun AA, Schaecter JD. Tracing axons in the human brain: a method utilizing light and TEM techniques. J Electron Microsc Tech. 1985;2:175–186.
6 Hogan MJ, Alvarado JA, Weddell JE. Histology of the human eye: an atlas and textbook. Philadelphia, PA: WB Saunders; 1971.
7 Sadun AA, Currie JN, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol. 1984;16:489–494.
8 Wiethe T. Ein Fall von angeborener Deformitat der Sehnervenpapille. Arch Augenheilkd. 1882;11:4–19.
9 Ferry AP. Macular detachment associated with congenital pit of the optic nerve head: pathologic findings in two cases simulating malignant melanoma of the choroid. Arch Ophthalmol. 1963;70:346–357.
10 Kalina RE, Conrad WC. Intrathecal fluorescein for serous macular detachment. Arch Ophthalmol. 1976;94:1421.
11 Singerman LJ, Mittra RA. Hereditary optic pit and iris coloboma in three generations of a single family. Retina. 2001;21:273–275.
12 Brown GC, Shields JA, Goldberg RE. Congenital pits of the optic nerve head. II. Clinical studies in humans. Ophthalmology. 1980;87:51–65.
13 Brodsky MC. Congenital optic pit with serous maculopathy in childhood. J AAPOS. 2003;2:150.
14 Yuen CHW, Kaye SB. Spontaneous resolution of serous maculopathy associated with optic disc pit in a child: a case report. J AAPOS. 2002;6:330–331.
15 Lincoff H, Lopez R, Kreissig I, et al. Retinoschisis associated with optic nerve pits. Arch Ophthalmol. 1988;106:61–67.
16 Lincoff H, Yannuzzi L, Singerman L, et al. Improvement in visual function after displacement of the retinal elevations emanating from optic pits. Arch Ophthalmol. 1993;111:1071–1079.
17 Lincoff H, Kreissig I. Optical coherence tomography of pneumatic displacement of optic disc pit maculopathy. Br J Ophthalmol. 1998;82:367–372.
18 Krivoy D, Gentile R, Liebmann JM, et al. Imaging congenital optic disc pits and associated maculopathy using optic coherence tomography. Arch Ophthalmol. 1996;114:165–170.
19 Myer CH, Rodrigues EB, Schmidt JC. Congenital optic nerve head pit associated with reduced retinal nerve fibre thickness at the papillomacular bundle. Br J Ophthalmol. 2003;87:1300–1301.
20 Simpson DE. Optic nerve pit. J Am Optom Assoc. 1987;58:118–120.
21 Walsh FB, Hoyt WF. Clinical neuro-ophthalmology, 3rd ed. Baltimore, MD: Williams & Wilkins; 1969.
22 Kranenburg EW. Crater-like holes in the optic disc and central serous retinopathy. Arch Ophthalmol. 1960;64:912–924.
23 Sobol WM, Boldi CF, Folk JC, et al. Long-term visual outcome in patients with optic nerve pit and serous retinal detachment of the macula. Ophthalmology. 1990;97:1539–1542.
24 Quinn SM, Charles SJ. Telangiectasis as a cause of intra-schitic haemorrhage in optic disc pit maculopathy. Acta Ophthalmol Scand. 2004;82:93–95.
25 Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. Br J Ophthalmol. 1969;53:481–489.
26 Reis W. Eine wenig bekannte typische Missbildung am Sehnerveneintritt: umschriebene Grubenbildung auf der Papilla n. optici. Z Augenheilkd. 1908;19:505–528.
27 Petersen HP. Pits or crater-like holes in the optic disc. Acta Ophthalmol. 1958;36:345–443.
28 Lincoff H, Schiff W, Krivoy D, et al. Optic coherence tomography of optic disk pit maculopathy. Am J Ophthalmol. 1996;122:264–266.
29 Brown GC, Tasman WS. Congenital anomalies of the optic disk. New York: Grune & Stratton; 1983.
30 Theodossiadis GP. Visual acuity in patients with optic nerve pit. Ophthalmology. 1991;98:563.
31 Sugar HS. An explanation for the acquired macular pathology associated with congenital pits of the optic disc. Am J Ophthalmol. 1964;57:833–835.
32 Wise G, Dollery C, Henkind P. The retinal circulation. New York: Harper & Row; 1971.
33 Irvine AR, Crawford JB, Sullivan JH. The pathogenesis of retinal detachment with morning glory disk and optic pit. Retina. 1986;6:146–150.
34 Gass JDM. Serous detachment of the macular secondary to congenital pit of the optic nerve head. Am J Ophthalmol. 1969;67:821–841.
35 Brown GC, Shields JA, Patty BE, et al. Congenital pits of the optic nerve head. I. Experimental studies in collie dogs. Arch Ophthalmol. 1979;97:1341–1344.
36 Brown GC, Shields JA, Patty BE, et al. Congenital optic pits and serous retinal detachment. Trans Pa Acad Ophthalmol Otolaryngol. 1979;32:151–154.
37 Sugar HS. Congenital pits in the optic disc with acquired macular pathology. Am J Ophthalmol. 1962;53:307–311.
38 Brockhurst RJ. Optic pits and posterior retinal detachment. Trans Am Ophthalmol Soc. 1975;73:264–291.
39 Mustonen E, Varonen T. Congenital pit of the optic nerve head associated with serous detachment of the macular. Acta Ophthalmol. 1972;50:689–698.
40 Gass JDM. Stereoscopic atlas of macular diseases. St Louis: Mosby; 1977.
41 Theodossiadis G. Evolution of congenital pit of the optic disk with macular detachment in photocoagulated and nonphotocoagulated eyes. Am J Ophthalmol. 1977;84:620–631.
42 Theodossiadis GP, Theodossiadis PG. Optical coherence tomography in optic disk pit maculopathy treated by the macular buckling procedure. Am J Ophthalmol. 2001;132:184–190.
43 Theodossiadis G, Theodossiadis P, Lalias J, et al. Preoperative and postoperative assessment by multifocal electroretinography in the management of optic disc pits with serous macular detachment. Ophthalmology. 2002;109:2295–2302.
44 Bechmann M, Mueller AJ, Gandorfer A, et al. Macular hole surgery in an eye with an optic pit. Am J Ophthalmol. 2001;132:263–264.
45 Pahwa V. Optic pit and central serous detachment. Ind J Ophthalmol. 1985;33:175–176.
46 Bonnet M. Serous macular detachment associated with optic nerve pits. Graefes Arch Clin Exp Ophthalmol. 1991;229:526–532.
47 Cox MS, Witherspoon CD, Morris RE, et al. Evolving techniques in the treatment of macular detachment caused by optic nerve pits. Ophthalmology. 1988;95:889–896.
48 Schatz H, McDonald HR. Treatment of sensory retinal detachment associated with optic nerve pit or coloboma. Ophthalmology. 1988;95:178–186.
49 Spaide RF, Fisher Y, Ober M, et al. Surgical hypothesis: inner retinal fenestration as a treatment for optic disc pit maculopathy. Retina. 2006;26:89–91.
50 Schaal KB, Wrede J, Dithmar S. Internal drainage in optic pit maculopathy. Br J Ophthalmol. 2007;91:1093.
51 Jalil A, Stavrakas P, Dhawahir-Scala FE, et al. Drainage of subretinal fluid in optic disc pit maculopathy using subretinal 42-gauge cannula: a new surgical approach. Graefes Arch Clin Exp Ophthalmol. 2010;248:751–753.
52 Ziahosseini K, Sanghvi C, Muzaffar W, et al. Successful surgical treatment of optic disc pit maculopathy. Eye (Lond). 2009;23:1477–1479.