Chapter 22 Ophthalmology
Ophthalmic Prostaglandins
MOA (Mechanism of Action)
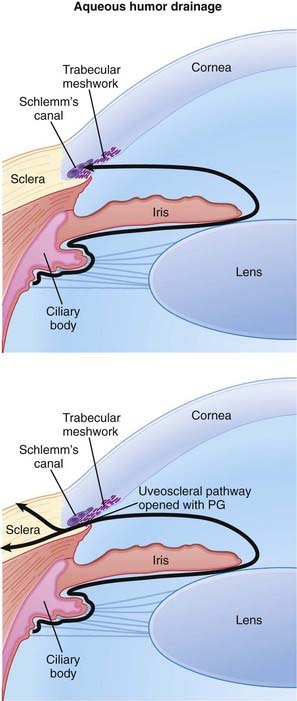
 Glaucoma is characterized by increased intraocular pressure (IOP) and can lead to blindness if not treated. Strategies to reduce IOP include reducing the production and secretion of aqueous humor and facilitating its drainage.
Glaucoma is characterized by increased intraocular pressure (IOP) and can lead to blindness if not treated. Strategies to reduce IOP include reducing the production and secretion of aqueous humor and facilitating its drainage. PGs bind prostaglandin F (FP) receptors that are located on the ciliary muscle and reduce IOP by increasing outflow of aqueous humor through the uveoscleral pathway. This is thought to be accomplished through the following mechanisms:
PGs bind prostaglandin F (FP) receptors that are located on the ciliary muscle and reduce IOP by increasing outflow of aqueous humor through the uveoscleral pathway. This is thought to be accomplished through the following mechanisms:
Side Effects
 Local irritation: Conjunctival hyperemia (redness), itching, foreign body sensation, tearing, and eye pain are side effects.
Local irritation: Conjunctival hyperemia (redness), itching, foreign body sensation, tearing, and eye pain are side effects. Pigmentation of the iris: This occurs in patients with mixed-color irises (green-brown or blue-brown) and is a result of increased deposition of melanin. Pigmentation of the palpebral skin (eyelids) also occurs.
Pigmentation of the iris: This occurs in patients with mixed-color irises (green-brown or blue-brown) and is a result of increased deposition of melanin. Pigmentation of the palpebral skin (eyelids) also occurs.Important Notes
 Glaucoma is often asymptomatic in early stages of the disease, and when symptoms develop they are irreversible. Therefore patients with no special risk factors should be assessed every 2 years by an optometrist or ophthalmologist after the age of 40 for IOP changes. If present, glaucoma must be treated by an ophthalmologist.
Glaucoma is often asymptomatic in early stages of the disease, and when symptoms develop they are irreversible. Therefore patients with no special risk factors should be assessed every 2 years by an optometrist or ophthalmologist after the age of 40 for IOP changes. If present, glaucoma must be treated by an ophthalmologist.Evidence
Prostaglandin versus Other Therapy for Glaucoma
 A systematic review in 2008 (eight randomized controlled trials [RCTs], 1722 patients) did not find a significant reduction in the mean IOP in patients receiving latanoprost compared with those receiving brimonidine (α2 agonist) but did find a significant reduction in mean IOP compared with the dorzolamide (carbonic anhydrase) group (WMD = 22.64). The number of ocular adverse events (excluding hyperemia) was significantly higher in the brimonidine group compared with the latanoprost group (relative risk [RR] = 0.66). The authors concluded that latanoprost was superior to dorzolamide but not brimonidine and those ocular adverse events were significantly fewer in latanoprost users than in brimonidine users. Neither travoprost nor bimatoprost was compared with dorzolamide or brimonidine in the present literature.
A systematic review in 2008 (eight randomized controlled trials [RCTs], 1722 patients) did not find a significant reduction in the mean IOP in patients receiving latanoprost compared with those receiving brimonidine (α2 agonist) but did find a significant reduction in mean IOP compared with the dorzolamide (carbonic anhydrase) group (WMD = 22.64). The number of ocular adverse events (excluding hyperemia) was significantly higher in the brimonidine group compared with the latanoprost group (relative risk [RR] = 0.66). The authors concluded that latanoprost was superior to dorzolamide but not brimonidine and those ocular adverse events were significantly fewer in latanoprost users than in brimonidine users. Neither travoprost nor bimatoprost was compared with dorzolamide or brimonidine in the present literature.Aptamers
Description
Aptamers are a new approach to drug design that uses nucleotide sequences to bind to targets.
MOA (Mechanism of Action)
 Aptamers are nucleotide sequences that bind to biologic targets, typically proteins. Pegaptanib, the first aptamer approved for human use, targets a protein called vascular endothelial growth factor (VEGF).
Aptamers are nucleotide sequences that bind to biologic targets, typically proteins. Pegaptanib, the first aptamer approved for human use, targets a protein called vascular endothelial growth factor (VEGF). Age-related macular degeneration is characterized by an overgrowth of blood vessels in the macula of the eye. These vessels function poorly and also leak, which obscures vision and eventually leads to degeneration of the macula and blindness.
Age-related macular degeneration is characterized by an overgrowth of blood vessels in the macula of the eye. These vessels function poorly and also leak, which obscures vision and eventually leads to degeneration of the macula and blindness. VEGF is an angiogenic growth factor whose function is to promote new blood vessel growth; it is believed to be largely responsible for this overgrowth of leaky, dysfunctional vessels in the macula. In addition to stimulating the growth of new blood vessels, VEGF also increases vascular permeability.
VEGF is an angiogenic growth factor whose function is to promote new blood vessel growth; it is believed to be largely responsible for this overgrowth of leaky, dysfunctional vessels in the macula. In addition to stimulating the growth of new blood vessels, VEGF also increases vascular permeability.Side Effects
 Increased IOP is most likely a result simply of the administration of liquid into an enclosed space.
Increased IOP is most likely a result simply of the administration of liquid into an enclosed space.Evidence
Aptamers versus Other Vascular Endothelial Growth Factor Inhibitors or Sham for Treatment of Macular Degeneration
 A 2008 Cochrane review (five trials, N = 2500 participants) examined the effects of anti-VEGF therapies for neovascular macular degeneration. Two of the included studies compared pegaptanib versus sham treatment. A key outcome in assessing interventions in macular degeneration is deterioration of vision, with the cutoff for deterioration being a loss of ≥15 letters of visual acuity on an eye chart. Compared with sham-treated patients, fewer pegaptanib-treated patients experienced this loss of visual acuity (numbers needed to treat [NNTs] ranged from 7 to 14, depending on dose). Endophthalmitis occurred in 1.3% of pegaptanib-treated patients.
A 2008 Cochrane review (five trials, N = 2500 participants) examined the effects of anti-VEGF therapies for neovascular macular degeneration. Two of the included studies compared pegaptanib versus sham treatment. A key outcome in assessing interventions in macular degeneration is deterioration of vision, with the cutoff for deterioration being a loss of ≥15 letters of visual acuity on an eye chart. Compared with sham-treated patients, fewer pegaptanib-treated patients experienced this loss of visual acuity (numbers needed to treat [NNTs] ranged from 7 to 14, depending on dose). Endophthalmitis occurred in 1.3% of pegaptanib-treated patients.Important Notes
 An important potential advantage of aptamers over monoclonal antibodies is that aptamers are unlikely to elicit an immune response, as they are not antibodies. Hypersensitivity reactions are an important limitation to the use of monoclonal antibodies in some patients.
An important potential advantage of aptamers over monoclonal antibodies is that aptamers are unlikely to elicit an immune response, as they are not antibodies. Hypersensitivity reactions are an important limitation to the use of monoclonal antibodies in some patients.FYI
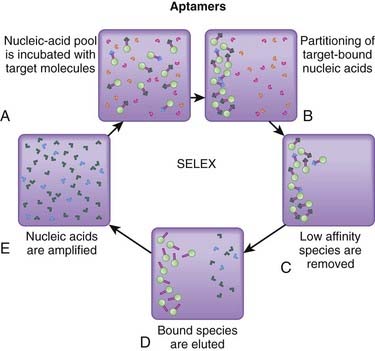
 The SELEX process is an automated technique for manufacturing aptamers. It begins with a library of nucleotide sequences of varying lengths, which are then exposed to a ligand target. The sequences that do not bind to the ligand are then washed away. The remaining sequences are then amplified and subjected to another round of binding and removal. With each successive round, the techniques that are used to remove the less tightly bound sequences are intensified, such that with the end of each round, the remaining sequences have increased affinity for the ligand over those of the previous round (Figure 22-2).
The SELEX process is an automated technique for manufacturing aptamers. It begins with a library of nucleotide sequences of varying lengths, which are then exposed to a ligand target. The sequences that do not bind to the ligand are then washed away. The remaining sequences are then amplified and subjected to another round of binding and removal. With each successive round, the techniques that are used to remove the less tightly bound sequences are intensified, such that with the end of each round, the remaining sequences have increased affinity for the ligand over those of the previous round (Figure 22-2).