Influence of Surgical Volume on Operative Failures for Hyperparathyroidism
Parathyroidectomy is the mainstay of treatment for hyperparathyroidism. Operative intervention in a previously unexplored neck can yield cure rates greater than 95% [1,2]. However, once a patient has undergone neck surgery, such as in the case of failed parathyroidectomy, reoperation leads to cure rates of only 80% [3,4]. Similarly, complication rates associated with parathyroidectomy have been found to be much greater during reoperations than during initial surgeries [3]. This significantly lower success rate for reoperation combined with the higher complication rate illustrates the need for a surgeon to achieve eucalcemia at the initial operation.
Hyperparathyroidism
Hyperparathyroidism is as an elevation in parathyroid hormone (PTH) levels (the normal level being 10–65 pg/mL) causing hypercalcemia (the normal level being 8.5–10.5 mg/dL). The symptoms of hyperparathyroidism are varied and at times vague, affecting multiple organ systems (Box 1) [5]. Surgical intervention is warranted for patients with distinct symptoms that can be attributed to the elevation in calcium levels, such as nephrolithiasis, bone disease, and cardiovascular abnormalities. Many other patients with hyperparathyroidism are asymptomatic and are found to have an elevated calcium level on routine blood testing. Although these patients seem asymptomatic, studies have shown that they have vague neurologic symptoms that improve with surgery [6,7]. Furthermore, after parathyroidectomy, the quality of life markedly improves in asymptomatic patients [8]. Similarly, data have shown that the bone mass increases significantly after parathyroidectomy [9]. In 2009, guidelines were established for the timing of surgery in asymptomatic patients (Box 2) [10].
Box 1 The symptoms of hyperparathyroidism are nonspecific and varied, affecting multiple organ systems
Data from Doherty GM. Parathyroid Glands. In: Mulholland MW, editor. Greenfield’s surgery: scientific principles and practice. 4th edition. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. p. 1316.
Box 2 Guidelines for surgical intervention in patients found to be hyperparathyroid, who seem to have no clinical symptoms of the disease
Data from Bilezikian J, Khan A, Potts JJ. Hyperthyroidism TIWotMoAP. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 2009;94(2):335–9.
Most commonly, hyperparathyroidism is caused by a primary disorder of the parathyroid glands. A single parathyroid adenoma accounts for around 80% of the cases of primary hyperparathyroidism, with double adenomas and multigland hyperplasia making up the remaining 20% [11,12]. Rarely, primary hyperparathyroidism is because of parathyroid carcinoma. In the United States, primary hyperparathyroidism is a fairly common disease with an annual incidence of approximately 100,000 individuals [13]. The incidence is increased in the elderly and in women, with 2 of 1000 women older than 60 years being affected yearly [13]. The mainstay of treatment for patients with primary hyperparathyroidism is parathyroidectomy.
Secondary hyperparathyroidism is the excess secretion of PTH that occurs in response to hypocalcemia, most often because of chronic renal failure. It has been estimated that as many as 90% of patients with renal failure who require hemodialysis suffer from secondary hyperparathyroidism [14]. Malabsorption and other disorders causing calcium and vitamin D deficiencies, such as rickets and osteomalacia, are the less-common causes of secondary hyperparathyroidism. Up to 2% of patients with secondary hyperparathyroidism require parathyroidectomy [15].
Tertiary hyperparathyroidism occurs in patients with secondary hyperparathyroidism due to chronic renal failure who undergo renal transplantation and continue to have persistent hyperparathyroidism. Tertiary hyperparathyroidism affects up to 30% of kidney transplant recipients, and 1% to 5% of such patients require surgical management [15–17].
Parathyroidectomy is the standard treatment for patients with primary hyperparathyroidism and for those with secondary and tertiary hyperparathyroidisms requiring surgical intervention. Therefore, regardless of the cause, the ability to perform a safe and successful operation is imperative for surgeons treating this population of patients.
Surgical failure
Surgical failure for hyperparathyroidism results in persistent disease, whereby hypercalcemia either continues after the initial surgery or recurs within 6 months of the operation. In contrast, recurrent hyperparathyroidism occurs when a patient becomes hypercalcemic after 6 months of normal postoperative calcium levels. Although there are instances in which reoperation is unavoidable, for many patients, their second operations are because of inadequate or inappropriate initial surgeries. Mitchell and colleagues [18] examined this fact in a study on patients undergoing reoperations for thyroid and parathyroid surgery. They established criteria for avoidable and unavoidable parathyroid reoperations (Box 3). According to them, avoidable reoperations occurred because of errors in judgment, illustrated by a surgeon performing a focal exploration or reexploration without appropriate preoperative localization, which then leads to persistent hyperparathyroidism. Technical errors are also responsible for avoidable reoperative parathyroidectomy, such as a missed gland in its normal anatomic location. In a review of 130 patients undergoing reoperative parathyroidectomy, Udelsman and Donovan [19] reported that 91 glands were found in their normal anatomic locations. The remaining glands were found in the retroesophageal space, mediastinal thymus, carotid sheath, submandibular space, or aortopulmonary window or were intrathyroidal. Regardless of the location of the abnormal gland, the investigators were able to achieve a 95% success rate, demonstrating that experienced surgeons with knowledge of the ectopic locations of parathyroid glands can perform successful parathyroidectomies and that had they operated on these patients initially, most reoperations could have been avoided.
Box 3 Classification of parathyroid reoperations as either avoidable or unavoidable. Avoidable operations were either because of technical errors during the case or because of errors in judgment occurring preoperatively or during the operation
 Persistent hyperparathyroidism after exploration without localization or intraoperative drop in PTH levels
Persistent hyperparathyroidism after exploration without localization or intraoperative drop in PTH levelsData from Mitchell J, Milas M, Barbosa G, et al. Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery 2008;144(6):899–906 [discussion: 906–7].
In the early nineties, there began to be an increased interest in the correlation between hospital and/or surgeon volume and clinical outcomes. Several studies of patients undergoing coronary artery bypass surgery, colectomy, gastrectomy, and pancreatic resection demonstrated that high-volume centers and surgeons had better outcomes than those considered to be less experienced [20–22]. Similar studies have been performed by multiple investigators regarding hospital and/or clinician volume as it correlates to outcomes in endocrine surgery. One of the first studies by Sosa and colleagues [3] in 1998 demonstrated that high-volume surgeons performing more than 50 parathyroidectomies yearly had significantly lower complication rates after both initial and reoperative parathyroidectomy. In the same study, the investigators also showed a significantly higher in-hospital mortality rate if the parathyroidecotmy was performed by a low-volume surgeon. Several years later, Stavrakis and colleagues [23] showed a decrease in morbidity, mortality, and length of stay after thyroidectomy and parathyroidecotmy with an increase in hospital and surgeon experience.
More recently, researchers have begun to examine whether surgeon and hospital volume affects surgical cure rates in parathyroidectomy. Mitchell and colleagues [18], after defining avoidable and unavoidable parathyroid reoperations, sought to determine if hospital volume correlated with the incidence of avoidable reoperations. They defined low-volume centers as those performing less than 20 cases per year and high-volume centers as those performing more than 20 cases annually. They examined 146 cases of reoperative parathyroidectomies and found that the low-volume centers had significantly higher rates of avoidable reoperations when compared with the high-volume centers (76% vs 22%, P<.001).When they compared high-volume centers performing 21 to 50 cases per year with those performing more than 50 per year, they found that the high-volume centers performing the fewest cases were responsible for most avoidable parathyroid reoperations. These findings are supported by Chen and colleagues [24] who defined preventable operative failures as those occurring because of a missed abnormal gland in a normal anatomic location. They defined high-volume hospitals as those performing more than 50 cases yearly and low-volume centers as performing less than 50 cases per year. They identified 159 patients who underwent initial unsuccessful parathyroidectomy who were later cured with reoperation. Patients operated on at low-volume centers were more likely to have a missed parathyroid gland in a normal anatomic location compared with those undergoing parathyroidectomy at a high-volume hospital (89% vs 13%, P<.001). During their follow-up, all 159 patients undergoing remedial parathyroid operations were cured of their disease. These studies illustrate the importance of surgical experience not only in being able to perform a curative parathyroidectomy at the initial operation but also in having a high-volume surgeon perform the reoperation.
Reoperation for hyperparathyroidism
Reoperative parathyroid surgery is technically challenging because of the presence of scar tissue, loss of previous tissue planes, and changes in normal anatomy. These factors contribute not only to a lower cure rate but also to an increase in injuries to the recurrent laryngeal nerves and remaining parathyroid glands. To achieve a higher cure rate and lower complication rate, experts in endocrine surgery who perform many reoperative parathyroidectomies annually recommend adjuncts to improve outcomes. Before undertaking reoperation, a detailed preoperative assessment should be performed in an attempt to localize the aberrant tissue. If this assessment is unsuccessful, several intraoperative adjuncts can aide a surgeon in executing a successful parathyroid reoperation.
Preoperative assessment
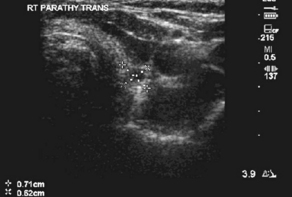
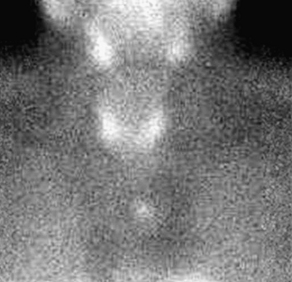
Many studies on reoperative parathyroidectomy have demonstrated improved cure rates with the use of various preoperative imaging modalities (Box 4). One of the most commonly used studies is ultrasonography because of its ease of use, low cost, and noninvasive nature. Ultrasonography can visualize intrathyroidal parathyroid glands and those in the area of the carotid sheath and jugular vein, but glands that are retroesophageal or retrotracheal are difficult to localize (Fig. 1). The sensitivity and positive predictive value of ultrasonography varies depending on operator experience, size of the parathyroid gland, and image resolution but have been found to be as high as 86% and 100%, respectively [25,26]. Sestamibi scintigraphy is another commonly used noninvasive technique for localizing parathyroid tissue and can visualize glands in ectopic locations (Fig. 2). Similar to neck ultrasonographies, the sensitivity and positive predictive value of sestamibi ultrasonography is variable but can reach 90% and 100%, respectively [25,26]. For glands that cannot be localized by ultrasonography or sestamibi scanning, computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) can be used. These modalities are more expensive, but they may be the only method for identifying disease in the mediastinum and other ectopic locations (Fig. 3).
Box 4 Multiple noninvasive imaging techniques are available for the preoperative assessments of patients undergoing reoperations for hyperparathyroidism. If the noninvasive techniques are unsuccessful, invasive modalities such as fine-needle aspiration biopsy, arteriography, and venous sampling may be undertaken
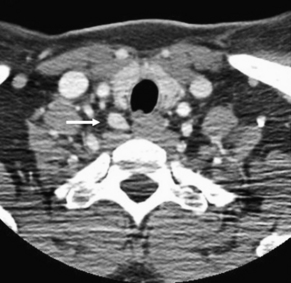
Fig. 3 CT scan demonstrating a right-sided parathyroid adenoma (arrow) in the tracheoesophageal groove.
(From Powell A, Alexander H, Chang R, et al. Reoperation for parathyroid adenoma: a contemporary experience. Surgery 2009;146(6):1149; with permission.)
When noninvasive imaging studies are unable to localize an aberrant gland, more invasive tests need to be undertaken. If a suspicious gland is visualized on ultrasonography, the diagnosis can be confirmed with fine-needle aspiration biopsy. A PTH level greater than 1000 pg/mL indicates an abnormal gland [27]. Arteriography and venous sampling can be done preoperatively as well as in the operating room (Fig. 4). These 2 invasive techniques are expensive and associated with complications such as arterial injuries and venous thrombosis and are therefore used less frequently than other studies.
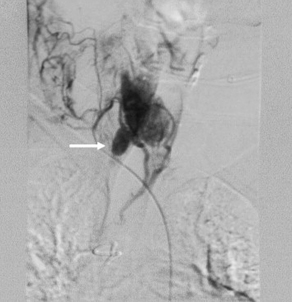
Fig. 4 Arteriogram demonstrating a parathyroid gland (arrow) in the right tracheoesophageal groove.
(From Powell A, Alexander H, Chang R, et al. Reoperation for parathyroid adenoma: a contemporary experience. Surgery 2009;146(6):1149; with permission.)
In their series of 130 patients undergoing reoperative exploration for primary hyperparathyroidism, Udelsman and Donovan [19] described the use of multiple preoperative imaging techniques in planning their surgeries. They routinely used sestamibi scanning, ultrasonography, MRI, CT, venous localization, and fine-needle aspiration biopsy with sensitivities of 79%, 74%, 47%, 50%, 93%, and 78%, respectively. Similarly, Yen and colleagues [28] reported in their series of 39 patients undergoing reoperation for recurrent or persistent hyperparathyroidism a success rate of 92%, higher than that of most published reports for reoperative parathyroidectomy. They advocated that all patients undergoing reoperative surgery for hyperparathyroidism should undergo both sestamibi scanning and ultrasonography of the neck. Powell and colleagues [29] have also shown improved cure rates of 92% and a decrease in the use of invasive imaging with the use of both sestamibi scanning and ultrasonographic imaging. In their review of 46 patients, Hessman and colleagues [26] used sestamibi scanning, PET, ultrasonography, fine-needle aspiration biopsy, and selective venous sampling to achieve a 98% cure rate, emphasizing that with appropriate preoperative imaging and planning, surgical cure for reoperative hyperparathyroidism is attainable.
Operative technique
Once the decision to take a patient back to the operating room has been made, a surgeon has several ways to approach the operation. Many surgeons favor a medial approach through the patient’s previous incision, which allows for bilateral neck exploration [30]. This approach can be technically difficult because of adhesions and distortion of the normal anatomy. The lateral approach is another option, which avoids the previous operative planes by dissecting lateral to the strap muscles, but if the abnormal parathyroid gland is not located, further dissection will be necessary. Preoperative imaging allows for a surgeon to better plan the operative approach, and at times, if the abnormal gland cannot be located, both a medial and lateral approach may be necessary. There are patients in whom the abnormal parathyroid gland cannot be resected via a neck incision and either a median sternotomy or a transthoracic approach may be necessary to retrieve an intrathymic gland or one near the aortopulmonary window [31,32].
There are 2 intraoperative adjuncts available to surgeons that can improve cure rates in reoperative parathyroidectomy. Use of the intraoperative PTH (IoPTH) assayallows for the rapid identification of a decrease in PTH levels after the abnormal gland is resected [2]. Before undergoing surgical exploration, a baseline PTH value is obtained, and once the abnormal gland is excised, PTH values are drawn at 5, 10, and 15 minutes after excision. By definition, the PTH level must decrease by at least 50% from the baseline or highest value to be considered adequate [33]. If a decrease of 50% is not achieved, further exploration should be performed looking for another abnormal gland or for hyperplasia. The use of IoPTH assay in improving outcomes in initial parathyroidectomy has been reported in multiple studies [2,34] Similarly, IoPTH assay in remedial parathyroid surgery has been shown to be equally effective. Powell and colleagues [29] reported the sensitivity and specificity of IoPTH assay to be 99% and 80%, respectively and the overall cure rate to be 92%. Irvin and colleagues [35] demonstrated an improvement in their cure rates for reoperation from 76% to 94% with the introduction of IoPTH assay.
The radioguided gamma probe is another useful intraoperative adjunct that has been shown to increase cure rates in reoperative parathyroidectomy. With this technique, patients are injected with radiolabeled sestamibi 1 to 2 hours before parathyroidectomy. Background counts are obtained by placing the probe on the skin overlying the thyroid isthmus before incision. Once the operation is begun, the probe is used to scan the field for counts greater than the initial background count. When the abnormal gland is identified and excised, the ex vivo count should be greater than 20% of the background count [36,37]. Cayo and Chen [38] reported the case of a patient in whom preoperative sestamibi scanning had identified a right inferior gland, but the patient had previously undergone 2 failed parathyroid explorations. With the use of the gamma probe, they were able to locate an enlarged parathyroid gland on the right, directly on the spine behind the esophagus, and the patient was subsequently cured. In a series of 110 patients undergoing reoperation, Pitt and colleagues [39] reported a cure rate of 96% with the use of the radioguided gamma probe. Concern has been raised on whether the gamma probe is useful in the setting of a negative sestamibi scan result, but Chen and colleagues [40] were able to demonstrate the utility of the radioguided gamma probe regardless of the preoperative sestamibi scan findings. In this study, all 769 patients had their abnormal glands localized by the gamma probe, regardless of the preoperative sestamibi scan findings with a final cure rate of 98%.
More recently, with the increased use of IoPTH assay and the radioguided gamma probe, surgeons have been performing minimally invasive parathyroidectomy. With this approach, a small incision is made to allow for a limited dissection to resect the parathyroid gland. This approach is only recommended if preoperative imaging has definitively identified the abnormal parathyroid tissue, but with the use of these intraoperative adjuncts, success rates comparable to those of open parathyroidectomy have been reported. In their series of 656 consecutive patients, Udelsman [1] performed 61% of surgeries using the standard technique and 39% via the minimally invasive approach. There was an overall 98% success rate for the series with no difference between the 2 approaches. Similarly, Chen and colleagues [36] also demonstrated a 98% success rate with the minimally invasive approach with the combination of radioguided gamma probe and IoPTH assay.
Despite a surgeon’s best efforts, there are patients in whom the abnormal parathyroid gland remains unidentified. In this instance, many surgeons advise ligation of the blood supply to the missing gland [19]. Because the parathyroid glands have an end-organ blood supply, they can be eliminated by tying off the ipsilateral inferior thyroidal artery. Udelsman and Donovan [19] reported their success with this technique, which led to surgical cure in 3 patients undergoing reoperative parathyroidectomy.
Summary
Hyperparathyroidism is a disease that is often seen in the United States. Patients may present with a wide variety of symptoms affecting multiple organs, but frequently, they are found to be hyperparathyroid on a routine blood examination. Although these patients may be asymptomatic, new consensus guidelines exist for when they should undergo surgery, and several studies have shown multiple benefits from operative intervention. Surgical cure rates can be greater than 95%, but if the initial surgery is unsuccessful, the cure rate becomes 80%. In the hands of experienced surgeons, both initial cure rates and those for reoperations are much higher, illustrating that the surgical volume does affect failure in parathyroid surgery.
References
[1] R. Udelsman. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235(5):665-670. [discussion: 670–2]
[2] H. Chen, Z. Pruhs, J. Starling, et al. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery. 2005;138(4):583-587. [discussion: 587–90]
[3] J. Sosa, N. Powe, M. Levine, et al. Profile of a clinical practice: thresholds for surgery and surgical outcomes for patients with primary hyperparathyroidism: a national survey of endocrine surgeons. J Clin Endocrinol Metab. 1998;83(8):2658-2665.
[4] G. Thompson, C. Grant, N. Perrier, et al. Reoperative parathyroid surgery in the era of sestamibi scanning and intraoperative parathyroid hormone monitoring. Arch Surg. 1999;134(7):699-704. [discussion: 704–5]
[5] G.M. Doherty. Parathyroid glands. In M.W. Mulholland, editor: Greenfield’s surgery: scientific principles and practice, 4th edition, Philadelphia (PA): Lippincott Williams & Wilkins, 2006.
[6] O. Clark, W. Wilkes, A. Siperstein, et al. Diagnosis and management of asymptomatic hyperparathyroidism: safety, efficacy, and deficiencies in our knowledge. J Bone Miner Res. 1991;6(Suppl 2):S135-S142. [discussion: 151–2]
[7] B. Solomon, M. Schaaf, R. Smallridge. Psychologic symptoms before and after parathyroid surgery. Am J Med. 1994;96(2):101-106.
[8] J. Adler, R. Sippel, S. Schaefer, et al. Surgery improves quality of life in patients with “mild” hyperparathyroidism. Am J Surg. 2009;197(3):284-290.
[9] S. Silverberg, F. Gartenberg, T. Jacobs, et al. Increased bone mineral density after parathyroidectomy in primary hyperparathyroidism. J Clin Endocrinol Metab. 1995;80(3):729-734.
[10] J. Bilezikian, A. Khan, J.J. Potts, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335-339.
[11] H. Chen, M. Zeiger, T. Gordon, et al. Parathyroidectomy in Maryland: effects of an endocrine center. Surgery. 1996;120(6):948-952. [discussion: 952–3]
[12] J. Lowney, B. Weber, S. Johnson, et al. Minimal incision parathyroidectomy: cure, cosmesis, and cost. World J Surg. 2000;24(11):1442-1445.
[13] Health NIo. Hyperparathyroidism. Available at: http://endocrine.niddk.nih.gov/pubs/hyper/hyper.htm Accessed October 1, 2010
[14] D. Memmos, G. Williams, J. Eastwood, et al. The role of parathyroidectomy in the management of hyperparathyroidism in patients on maintenance haemodialysis and after renal transplantation. Nephron. 1982;30(2):143-148.
[15] F. Triponez, O. Clark, Y. Vanrenthergem, et al. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248(1):18-30.
[16] J. Kerby, L. Rue, H. Blair, et al. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg. 1998;227(6):878-886.
[17] M. Kilgo, J. Pirsch, T. Warner, et al. Tertiary hyperparathyroidism after renal transplantation: surgical strategy. Surgery. 1998;124(4):677-683. [discussion: 683–4]
[18] J. Mitchell, M. Milas, G. Barbosa, et al. Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery. 2008;144(6):899-906. [discussion: 906–7]
[19] R. Udelsman, P. Donovan. Remedial parathyroid surgery: changing trends in 130 consecutive cases. Ann Surg. 2006;244(3):471-479.
[20] E. Hannan, J. O’Donnell, H.J. Kilburn, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262(4):503-510.
[21] M. Lieberman, H. Kilburn, M. Lindsey, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222(5):638-645.
[22] J. Jollis, E. Peterson, E. DeLong, et al. The relation between the volume of coronary angioplasty procedures at hospitals treating Medicare beneficiaries and short-term mortality. N Engl J Med. 1994;331(24):1625-1629.
[23] A. Stavrakis, P. Ituarte, C. Ko, et al. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142(6):887-899. [discussion: 887–99]
[24] H. Chen, T. Wang, T. Yen, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg. 2010;252(4):691-695.
[25] D. Seehofer, T. Steinmüller, N. Rayes, et al. Parathyroid hormone venous sampling before reoperative surgery in renal hyperparathyroidism: comparison with noninvasive localization procedures and review of the literature. Arch Surg. 2004;139(12):1331-1338.
[26] O. Hessman, P. Stålberg, A. Sundin, et al. High success rate of parathyroid reoperation may be achieved with improved localization diagnosis. World J Surg. 2008;32(5):774-781. [discussion: 782–3]
[27] D. Conrad, J. Olson, H. Hartwig, et al. A prospective evaluation of novel methods to intraoperatively distinguish parathyroid tissue utilizing a parathyroid hormone assay. J Surg Res. 2006;133(1):38-41.
[28] T. Yen, T. Wang, K. Doffek, et al. Reoperative parathyroidectomy: an algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery. 2008;144(4):611-619. [discussion: 619–21]
[29] A. Powell, H. Alexander, R. Chang, et al. Reoperation for parathyroid adenoma: a contemporary experience. Surgery. 2009;146(6):1144-1155.
[30] J. Prescott, R. Udelsman. Remedial operation for primary hyperparathyroidism. World J Surg. 2009;33(11):2324-2334.
[31] J. Gold, P. Donovan, R. Udelsman. Partial median sternotomy: an attractive approach to mediastinal parathyroid disease. World J Surg. 2006;30(7):1234-1239.
[32] A. Chae, A. Perricone, K. Brumund, et al. Outpatient video-assisted thoracoscopic surgery (VATS) for ectopic mediastinal parathyroid adenoma: a case report and review of the literature. J Laparoendosc Adv Surg Tech A. 2008;18(3):383-390.
[33] M. Cook, S. Pitt, S. Schaefer, et al. A rising ioPTH level immediately after parathyroid resection: are additional hyperfunctioning glands always present? An application of the Wisconsin Criteria. Ann Surg. 2010;251(6):1127-1130.
[34] A. Cayo, R. Sippel, S. Schaefer, et al. Utility of intraoperative PTH for primary hyperparathyroidism due to multigland disease. Ann Surg Oncol. 2009;16(12):3450-3454.
[35] G.R. Irvin, A. Molinari, C. Figueroa, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999;229(6):874-878. [discussion: 878–9]
[36] H. Chen, E. Mack, J. Starling. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg. 2005;242(3):375-380. [discussion: 380–3]
[37] C. Murphy, J. Norman. The 20% rule: a simple, instantaneous radioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery. 1999;126(6):1023-1028. [discussion: 1028–9]
[38] A. Cayo, H. Chen. Radioguided reoperative parathyroidectomy for persistent primary hyperparathyroidism. Clin Nucl Med. 2008;33(10):668-670.
[39] S. Pitt, R. Panneerselvan, R. Sippel, et al. Radioguided parathyroidectomy for hyperparathyroidism in the reoperative neck. Surgery. 2009;146(4):592-598. [discussion: 598–9]
[40] H. Chen, R. Sippel, S. Schaefer. The effectiveness of radioguided parathyroidectomy in patients with negative technetium tc 99m-sestamibi scans. Arch Surg. 2009;144(7):643-648.