Obstetric Issues, Labor, and Delivery
Amniocentesis is a procedure that involves the aspiration of amniotic fluid from the amniotic sac during pregnancy. It is generally carried out with a spinal needle (20–22 gauge) in a transabdominal approach, using a sterile technique under continuous ultrasound guidance. ∗
In Rh and other blood group isoimmunizations, amniocentesis has traditionally been used for bilirubin assessment using ΔOD 450, but it is being done far less frequently now because middle cerebral artery Doppler has been found to be extremely accurate in predicting the degree of fetal anemia. In this setting amniocentesis is used to determine whether the fetus is Rh positive or positive for the sensitized antigen so that testing can be avoided if the fetus is not at risk. ∗
3. What are the complications of amniocentesis?
 Miscarriage and fetal loss (less than 0.1%) above the background rate of spontaneous loss (0.5–1%) in specialized centers with high procedure volumes (Eddleman, Towner). The rate is higher in low-volume centers (Tabor).
Miscarriage and fetal loss (less than 0.1%) above the background rate of spontaneous loss (0.5–1%) in specialized centers with high procedure volumes (Eddleman, Towner). The rate is higher in low-volume centers (Tabor).
 Transient leakage of amniotic fluid (about 1%)
Transient leakage of amniotic fluid (about 1%)
 Persistent rupture of membranes (rare)
Persistent rupture of membranes (rare)
 Failure to achieve diagnosis (i.e., cell culture failure, which occurs in 1% of cases)
Failure to achieve diagnosis (i.e., cell culture failure, which occurs in 1% of cases)
 Increased Rhesus isoimmunization, especially if the placenta is transversed. In Rh-negative women, Rh immune globulin (e.g., RhoGAM) is given to prevent sensitization. ∗†‡
Increased Rhesus isoimmunization, especially if the placenta is transversed. In Rh-negative women, Rh immune globulin (e.g., RhoGAM) is given to prevent sensitization. ∗†‡
5. What options, aside from amniocentesis, are available for prenatal diagnosis?
 Available tests for fetal chromosome evaluation are classified as “diagnostic” (the result is a definitive karyotype) or “screening” (the result quantifies the risk of aneuploidy).
Available tests for fetal chromosome evaluation are classified as “diagnostic” (the result is a definitive karyotype) or “screening” (the result quantifies the risk of aneuploidy).
 Chorionic villus sampling: This is the only alternative to amniocentesis that is considered “diagnostic”; it is performed in the first trimester (9 to 12 weeks). This procedure involves either transvaginal or transabdominal ultrasound-guided needle aspiration of a small amount of placental tissue and can be used for cytogenetic, biochemical, or DNA testing. The procedure-related loss rate is 0.8%.
Chorionic villus sampling: This is the only alternative to amniocentesis that is considered “diagnostic”; it is performed in the first trimester (9 to 12 weeks). This procedure involves either transvaginal or transabdominal ultrasound-guided needle aspiration of a small amount of placental tissue and can be used for cytogenetic, biochemical, or DNA testing. The procedure-related loss rate is 0.8%.
 Preimplantation genetic diagnosis: This is an adjunct to in vitro fertilization. One or more cells are removed from the developing embryo 2 to 4 days after fertilization and then analyzed. Only normal embryos are selected for implantation. When the parents are carriers of an adverse genetic trait, it may obviate the need for testing during pregnancy. It is not considered “diagnostic” for karyotype, however, because of the high rate of mosaicism.
Preimplantation genetic diagnosis: This is an adjunct to in vitro fertilization. One or more cells are removed from the developing embryo 2 to 4 days after fertilization and then analyzed. Only normal embryos are selected for implantation. When the parents are carriers of an adverse genetic trait, it may obviate the need for testing during pregnancy. It is not considered “diagnostic” for karyotype, however, because of the high rate of mosaicism.
 Fetal free DNA screening from maternal blood: This is considered an “advanced screening test” because of very high sensitivity and specificity (>99%) for trisomy 21 and other common aneuploidies. Introduced in late 2011, this testing is currently very expensive and recommended only for women who have one or more risk factors for aneuploidy (based on maternal serum screening, ultrasound screening, advanced maternal age, family history). In women without risk factors, the positive predictive value is not yet known.
Fetal free DNA screening from maternal blood: This is considered an “advanced screening test” because of very high sensitivity and specificity (>99%) for trisomy 21 and other common aneuploidies. Introduced in late 2011, this testing is currently very expensive and recommended only for women who have one or more risk factors for aneuploidy (based on maternal serum screening, ultrasound screening, advanced maternal age, family history). In women without risk factors, the positive predictive value is not yet known.
 Second-trimester ultrasound: Many structural fetal defects (e.g., anencephaly, omphalocele) can routinely be seen in patients who undergo ultrasound scanning during the second trimester. Other defects, such as major cardiac defects, can be seen most of the time depending on the sophistication of the center, type of equipment, patient body habitus, and other factors. In addition, many fetuses with chromosome abnormalities including trisomy 13, 18, and 21 syndromes will have findings that will lead to subsequent amniocentesis to confirm the diagnosis.
Second-trimester ultrasound: Many structural fetal defects (e.g., anencephaly, omphalocele) can routinely be seen in patients who undergo ultrasound scanning during the second trimester. Other defects, such as major cardiac defects, can be seen most of the time depending on the sophistication of the center, type of equipment, patient body habitus, and other factors. In addition, many fetuses with chromosome abnormalities including trisomy 13, 18, and 21 syndromes will have findings that will lead to subsequent amniocentesis to confirm the diagnosis.
 Combinations of first-trimester ultrasound and first-trimester or second-trimester maternal serum screening: These screening tests involve ultrasound evaluation of nuchal translucency at 11 to 14 weeks’ gestation, maternal serum levels of human chorionic gonadotropin (hCG) and pregnancy-associated plasma protein A (PAPP-A) at 10 to 14 weeks’ gestation, and “triple
Combinations of first-trimester ultrasound and first-trimester or second-trimester maternal serum screening: These screening tests involve ultrasound evaluation of nuchal translucency at 11 to 14 weeks’ gestation, maternal serum levels of human chorionic gonadotropin (hCG) and pregnancy-associated plasma protein A (PAPP-A) at 10 to 14 weeks’ gestation, and “triple
marker” (alpha-fetoprotein (AFP), hCG, estriol) or “quadruple marker” (triple marker plus inhibin-A) at 15 to 20 weeks’ gestation. Depending on which combination of tests is performed, detection of Down syndrome is 60% to 95% with a 5% screen positive rate. Reasonable detection rates are also achieved for trisomy 18 and open neural tube defects. ∗†‡§¶ ∗∗††
∗∗††
Third-trimester hemorrhage refers to any bleeding from the genital tract during the third trimester of pregnancy. In practice, it refers to any bleeding that occurs from the time of viability, (i.e., 23 to 24 weeks’ gestation). The common causes are classified as placenta previa (7%), placental abruption (13%), and other bleeding (80%), including local lesions of the lower genital tract, vasa previa, early labor, trauma, neoplasia, and marginal placental separation. Such bleeding complicates about 6% of pregnancies. ∗
Ultrasound visualization is the method of choice for diagnosis of placenta previa. Multiple reports show a transvaginal approach to be safe and superior in its accuracy compared with transabdominal ultrasound. ∗
9. How is placenta previa classified?
 Complete: The placenta symmetrically covers the entire internal os of the cervix.
Complete: The placenta symmetrically covers the entire internal os of the cervix.
 Partial: The placenta lies asymmetrically toward one wall of the uterus and crosses part of the internal os.
Partial: The placenta lies asymmetrically toward one wall of the uterus and crosses part of the internal os.
 Marginal: The placental edge just reaches the edge of the internal cervical os.
Marginal: The placental edge just reaches the edge of the internal cervical os.
 Low-lying placenta: Placental edge is within 2 centimeters of the internal os.
Low-lying placenta: Placental edge is within 2 centimeters of the internal os.
12. What are the complications of placenta previa?
 Hemorrhage in third trimester, possibly catastrophic: Uterine contractions, cervical shortening/dilation, and sexual intercourse are common triggers for hemorrhage.
Hemorrhage in third trimester, possibly catastrophic: Uterine contractions, cervical shortening/dilation, and sexual intercourse are common triggers for hemorrhage.
 Preterm delivery: 50%—the vast majority of increased perinatal mortality with placenta previa is caused by prematurity.
Preterm delivery: 50%—the vast majority of increased perinatal mortality with placenta previa is caused by prematurity.
 Placenta accreta: when the placenta infiltrates the uterine wall (1% to 5%; 25% with one previous uterine surgery; 45% if more than one previous surgery)
Placenta accreta: when the placenta infiltrates the uterine wall (1% to 5%; 25% with one previous uterine surgery; 45% if more than one previous surgery)
 Increased risk of postpartum hemorrhage
Increased risk of postpartum hemorrhage
 Fetal growth restriction: Studies are inconsistent, and the majority show no increased rates of IUGR with placenta previa
Fetal growth restriction: Studies are inconsistent, and the majority show no increased rates of IUGR with placenta previa
Placental abruption is the separation of the normally implanted placenta before the birth of the fetus. It results from bleeding from a small arterial vessel into the decidua basalis. It is termed a revealed abruption when vaginal bleeding is present (90%) and a concealed abruption if no bleeding is visible (10%). It is uniquely dangerous to the fetus and the mother because of its serious pathophysiologic sequelae. The incidence varies but averages about 0.83% or 1 in 120 deliveries. Abruption severe enough to cause fetal death is less common (approximately 1 in 420 deliveries). ∗†
14. What are the main risk factors for a placental abruption? Is placental abruption a recurrent disease?
 Maternal mortality: less than 1%
Maternal mortality: less than 1%
 Disseminated intravascular coagulation
Disseminated intravascular coagulation
 Severe Rhesus isoimmunization: occurs in Rh-negative mothers unless there is adequate treatment with anti-D immunoglobulin
Severe Rhesus isoimmunization: occurs in Rh-negative mothers unless there is adequate treatment with anti-D immunoglobulin
Fetal and neonatal complications
 IUGR: especially in preterm deliveries
IUGR: especially in preterm deliveries
 Increased risk of congenital malformations: 4.4%
Increased risk of congenital malformations: 4.4%
 Greater incidence of abnormal neurodevelopment at 2 years
Greater incidence of abnormal neurodevelopment at 2 years
 Perinatal mortality: 14.4 to 67% higher rates occur at earlier gestations; high rate of stillbirth, fetal distress, and asphyxia. Most mortality is caused by prematurity
Perinatal mortality: 14.4 to 67% higher rates occur at earlier gestations; high rate of stillbirth, fetal distress, and asphyxia. Most mortality is caused by prematurity
 Fetomaternal hemorrhage with resultant fetal anemia: more common in abruption associated with maternal trauma
Fetomaternal hemorrhage with resultant fetal anemia: more common in abruption associated with maternal trauma
Fetomaternal hemorrhage is caused by a disruption of the normal barrier at the placental-decidual interface. It may occur with abruptio placentae; however, it occurs more commonly with abruptio placentae associated with maternal trauma, with maternal trauma without abruptio placentae, or spontaneously without an apparent precipitating event. Approximately 5% of stillbirths without apparent cause are the result of fetomaternal hemorrhage. The diagnosis is made by performing a Kleihauer–Betke test on maternal blood, which allows quantification of fetal cells in maternal serum. In patients with spontaneous fetomaternal hemorrhage, the presenting symptom is decreased fetal movement. If the fetus is still alive and the hemorrhage is severe enough, the diagnosis is often made because of a sinusoidal fetal heart rate (FHR) tracing. Treatment can consist of immediate delivery if the fetus is near term or intrauterine transfusion if the fetus is premature and no abruption is apparent. ∗
17. A 32-year-old, G2P1001 woman at term gestation presents in labor. Her membranes are intact, she is afebrile, and the fetal heart tracing is reassuring. Reviewing her prenatal records, you notice that she had a positive Group B streptococcal culture obtained at 34 weeks’ gestation. She is allergic to penicillin and “had difficulty breathing and swelled up” when she received it many years ago. What therapy is appropriate?
For pencillin-allergic women with high risk of anaphylaxis (as in the present case), the CDC’s 2010 guidelines recommend susceptibility testing against both erythromycin and clindamycin. Prophylaxis with erythromycin is no longer recommended, even if sensitivity is documented. Prophylaxis with clindamycin is recommended if GBS is proved sensitive to both clindamycin and erythromycin and if there is no inducible resistance to clindamycin using D-zone testing. If sensitivity is unknown, or if all these requirements are not met, vancomycin is recommended. ∗†‡
Fetal fibronectin is an extracellular matrix protein, the presence of which in cervicovaginal secretions is a predictor of preterm birth. This predictor has a high negative predictive accuracy (>99% negative predictive value; i.e., the absence of fetal fibronectin indicates <1% chance of delivery within 2 weeks) but only a mediocre positive predictive accuracy. ∗†
Most commonly, this test is used in patients with preterm contractions in which the diagnosis of preterm labor is uncertain. A negative test result allows greater than 99% reassurance that the patient will not deliver in the next 2 weeks and often prevents unnecessary treatment. ∗
23. What are the common pharmacologic agents used for the inhibition of preterm labor and their mechanisms of action?
TABLE 3-1
COMMON PHARMACOLOGIC AGENTS USED FOR THE INHIBITION OF PRETERM LABOR
| PHARMACOLOGIC AGENT | MECHANISM OF ACTION |
| Beta-adrenergic agonists | Adenylate cyclase inhibitor—sequesters intracellular calcium (e.g., terbutaline, ritodrine) |
| Magnesium sulfate | Uncertain—magnesium suppresses muscle contraction of myometrial strips in vitro, decreases intracellular calcium, and affects acetylcholine release |
| Prostaglandin synthase inhibitors (indomethacin) | Inhibition of the cyclooxygenase enzyme responsible for prostaglandins that promote uterine contractions |
| Calcium antagonists (nifedipine) | Inhibition of influx of calcium through the cell membrane |
TABLE 3-2
ADVERSE EFFECTS OF TOCOLYTIC AGENTS ON THE FETUS AND NEONATE
| PHARMACOLOGIC AGENT | ADVERSE EFFECTS |
| Beta-adrenergic agonists | Fetal tachycardia, neonatal hypoglycemia, hypocalcemia, and hypotension |
| Magnesium | Fetal demineralization with prolonged use, neonatal respiratory and motor depression at higher serum levels, ileus |
| Prostaglandin synthase inhibitors | Constriction of fetal ductus arteriosus leading to pulmonary hypertension, oligohydramnios, decreased fetal urine production, and spontaneous intestinal perforation |
| Calcium antagonists | No known human effects—decreases fetal arterial PO2 and pH in animal studies |
There is no question that tocolysis is effective over short-term intervals; however, clinical trials have not consistently demonstrated that gestation can be prolonged significantly or that respiratory distress syndrome can be consistently prevented with tocolysis. ∗
 Preterm labor: PROM accounts for 25% to 50% of preterm deliveries.
Preterm labor: PROM accounts for 25% to 50% of preterm deliveries.
 Maternal and neonatal infections
Maternal and neonatal infections
 Increased rates of cord accidents and stillbirth
Increased rates of cord accidents and stillbirth
 Fetal deformation sequence: In the very preterm gestation this includes pulmonary hypoplasia, IUGR, and limb deformities.
Fetal deformation sequence: In the very preterm gestation this includes pulmonary hypoplasia, IUGR, and limb deformities.
27. A patient makes inquiries regarding multiple courses of steroids to enhance fetal lung maturity. What should you tell her about this approach?
Multiple courses of antenatal steroids (more than three) are associated with suppression of the fetal adrenal gland and decreased response to stress in a critically ill neonate. In addition, animal and human data suggest less brain growth and developmental delay in childhood after multiple doses of steroids. The benefit of more than one course of antenatal steroids is controversial. A National Institutes of Health consensus conference on antenatal steroids recommended that only a single course of steroids be used and that the use of subsequent courses be limited to patients in research studies that address this question. Several clinical trials tested weekly repeated courses of steroids versus a single course. A Cochrane review concluded that repeated courses may result in a modest reduction in neonatal respiratory distress syndrome. Still, more than three courses can result in other problems, as noted previously. A reasonable compromise is the use of a “rescue course” of steroids—that is, a single repeat course targeted at those most likely to deliver within a week. ∗†
28. During a review of the perinatal outcomes for premature infants at your hospital, the nurse manager for the intensive care nursery inquires whether there is an effective method to detect women at risk for premature delivery before they present in active preterm labor. What do current data indicate?
Many strategies have been used to identify patients who are destined to deliver prematurely. Risk assessment scoring using the modified Creasy score ( Table 3-3) or other similar systems works well in some populations but not in others. The Creasy score looks at a series of variables in an attempt to define clinical indicators that are likely to result in preterm labor. A major limitation of most clinical risk scoring systems is that they rely heavily on a history of preterm birth in a prior pregnancy, yet the majority of preterm births occur in women without such a history.
TABLE 3-3
RISK FACTORS IN THE PREDICTION OF SPONTANEOUS PRETERM LABOR (MODIFIED CREASY SCORE)
| MAJOR RISK FACTORS | MINOR RISK FACTORS |
| Multiple gestation | Febrile illness |
| DES exposure | Bleeding after 12 weeks’ gestation |
| Hydramnios | History of pyelonephritis |
| Uterine anomaly | Cigarette smoking >10 cigarettes/day |
| Cervix dilated >1 cm at 32 weeks’ gestation | One second-trimester abortion |
| ≥ Two second-trimester abortions | More than two first-trimester abortions |
| Previous preterm delivery | |
| Previous preterm labor, term delivery | |
| Abdominal surgery during pregnancy | |
| History of cone biopsy | |
| Cervical shortening <1 cm at 32 weeks’ gestation | |
| Uterine irritability | |
| Cocaine abuse |
Fetal fibronectin screening can identify a subgroup of women at high risk for preterm birth, but there is no known therapy that will consistently prevent preterm delivery in women with positive fibronectin screening. ∗
Since 2003, there have been over a dozen trials evaluating prophylactic use of progesterone agents, either vaginal or oral micronized progesterone or intramuscular 17-hydroxyprogesterone caproate (17Pc). In women with prior preterm birth, weekly 17Pc reduced the recurrence of preterm birth by 33% to 45% and vaginal micronized progesterone showed similar benefit in one large trial but not another. In women with short cervix detected by endovaginal ultrasound screening, vaginal micronized progesterone reduced early preterm delivery by 40% to 50% in two large trials. The ACOG has endorsed progesterone therapy in such patients. Several trials showed that these agents are not effective in twin or triplet pregnancies. ∗†‡§¶
30. What are some of the increased risks of twin pregnancies?
31. Why are monozygotic twins considered to be at higher risk for complications than dizygotic twins?
 Thoracopagus: joined at the thorax
Thoracopagus: joined at the thorax
 Xiphopagus: joined at the anterior abdominal wall (from the xiphoid to the umbilicus)
Xiphopagus: joined at the anterior abdominal wall (from the xiphoid to the umbilicus)
 Pygopagus: joined at the buttocks or rump
Pygopagus: joined at the buttocks or rump
 Arterial cord pH sample below 7.0
Arterial cord pH sample below 7.0
 Apgar scores of 4 or less for at least 5 minutes
Apgar scores of 4 or less for at least 5 minutes
 Evidence of altered neurologic status (e.g., obtundation, seizures, altered level of consciousness)
Evidence of altered neurologic status (e.g., obtundation, seizures, altered level of consciousness)
34. Why has the term nonreassuring fetal status been used to replace the term fetal distress in practice?
In labor electronic FHR monitoring is the primary modality used to determine fetal oxygenation. Although this method is quite reliable when results are normal, when marked abnormalities occur on the FHR tracing the infant is more often vigorous and not acidotic at birth. Thus the term fetal distress is more often than not inaccurate. A more accurate expression is that the FHR is no longer reassuring and that either other information must be used to establish fetal well-being or, failing that option, the fetus must be delivered (Fig. 3-1).
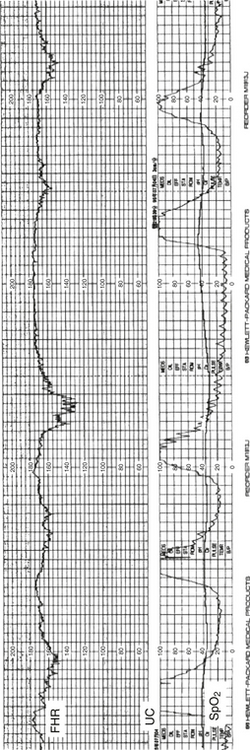
Figure 3-1 A fetal heart rate strip showing late decelerations of the heart rate following intrauterine contractions.
A CST was one of the earliest techniques to assess fetal well-being. In this test uterine contractions are induced, either by maternal nipple stimulation or by an intravenous infusion of oxytocin (oxytocin challenge test [OCT]). The former method may be quicker and removes the need to establish an intravenous infusion; the latter is the traditional, time-honored technique. Results are interpreted the same, regardless of the method of inducing contractions. The mother is monitored with a tocodynamometer and a FHR transducer while uterine contractions are stimulated until adequate, which is defined as three contractions within 10 minutes. In a negative test result, there are no late decelerations of the FHR. In a positive test result, in which there are late decelerations, the risk of mortality and morbidity for the fetus increases, with some reports of mortality as high as 15%. There are, however, many false-positive instances of CST results. In such situations the obstetrician often faces a difficult decision of how aggressively to proceed with delivery of the fetus because the cervix may not be in a favorable condition at that time, and a cesarean section may be required. If the test results are equivocal, it may be reasonable to wait an additional 24 hours to repeat the test.
Neonatal depression is not always caused by hypoxia and acidosis. Furthermore, given the litigious environment surrounding the issue of perinatal brain damage, the issue of documenting the fetal blood gas status at birth is critical to an objective assessment of the baby’s condition. The following are indications for assessing blood gas status at birth:
 Operative intervention for nonreassuring fetal status
Operative intervention for nonreassuring fetal status
 Fetuses that had nonreassuring fetal status but did not have operative intervention
Fetuses that had nonreassuring fetal status but did not have operative intervention
 Anticonvulsants: Infants of mothers using anticonvulsants have twice the risk of malformations compared with the general population, especially cleft lip and palate and congenital cardiac defects. Valproic acid may cause neural tube defects, and diphenylhydantoin is associated with fetal hydantoin syndrome (i.e., microcephaly, developmental delay, growth failure, mental retardation, dysmorphic facies, and nail hypoplasia). Carbamazepine may also produce dysmorphism.
Anticonvulsants: Infants of mothers using anticonvulsants have twice the risk of malformations compared with the general population, especially cleft lip and palate and congenital cardiac defects. Valproic acid may cause neural tube defects, and diphenylhydantoin is associated with fetal hydantoin syndrome (i.e., microcephaly, developmental delay, growth failure, mental retardation, dysmorphic facies, and nail hypoplasia). Carbamazepine may also produce dysmorphism.
 Psychoactive medications: Lithium has been associated with a slightly increased risk of cardiac defects. In addition, lithium can produce polyhydramnios and fetal diabetes insipidus. Hypotonia, lethargy, and feeding problems are also seen in some infants. The effects of other psychotropic agents on the fetus appear minimal, but some cases of teratogenesis have been reported, especially with some benzodiazepines. The critical issue that remains unresolved, however, is whether these drugs alter the development of the maturing fetal central nervous system.
Psychoactive medications: Lithium has been associated with a slightly increased risk of cardiac defects. In addition, lithium can produce polyhydramnios and fetal diabetes insipidus. Hypotonia, lethargy, and feeding problems are also seen in some infants. The effects of other psychotropic agents on the fetus appear minimal, but some cases of teratogenesis have been reported, especially with some benzodiazepines. The critical issue that remains unresolved, however, is whether these drugs alter the development of the maturing fetal central nervous system.
 Anticoagulants: Warfarin is known to produce teratogenic effects in the fetus. Approximately 5% of pregnancies result in fetal warfarin syndrome (i.e., mental retardation, bone stippling, dysmorphic characteristics, ophthalmologic abnormalities). If necessary, warfarin should be replaced by heparin during pregnancy.
Anticoagulants: Warfarin is known to produce teratogenic effects in the fetus. Approximately 5% of pregnancies result in fetal warfarin syndrome (i.e., mental retardation, bone stippling, dysmorphic characteristics, ophthalmologic abnormalities). If necessary, warfarin should be replaced by heparin during pregnancy.
 Antihypertensive medications: Angiotensin-converting enzyme inhibitors may cause fetal renal failure in later stages of gestation, leading to oligohydramnios, pulmonary hypoplasia, and fetal deformities.
Antihypertensive medications: Angiotensin-converting enzyme inhibitors may cause fetal renal failure in later stages of gestation, leading to oligohydramnios, pulmonary hypoplasia, and fetal deformities.
 Thyroid drugs: Propylthiouracil and methimazole (Tapazole) cross the placenta and can cause a fetal goiter and fetal hypothyroidism. Use of thyroid hormone appears, generally, to be safe. Maternal Graves disease can result in neonatal thyroid storm and hyperthyroidism in rare cases.
Thyroid drugs: Propylthiouracil and methimazole (Tapazole) cross the placenta and can cause a fetal goiter and fetal hypothyroidism. Use of thyroid hormone appears, generally, to be safe. Maternal Graves disease can result in neonatal thyroid storm and hyperthyroidism in rare cases.
 Acne medications: Isotretinoin (Accutane) is a significant human teratogen that should be avoided in women planning to become pregnant. It is associated with a high risk of both structural abnormalities and mental retardation in the newborn. The use of topical tretinoin (Retin-A) appears to be safe.
Acne medications: Isotretinoin (Accutane) is a significant human teratogen that should be avoided in women planning to become pregnant. It is associated with a high risk of both structural abnormalities and mental retardation in the newborn. The use of topical tretinoin (Retin-A) appears to be safe.
 Antineoplastic drugs: The anticancer drugs that appear to have the greatest significance for teratogenesis are methotrexate and cyclophosphamide. Both can cause malformations of the skull and bones as well as mental retardation.
Antineoplastic drugs: The anticancer drugs that appear to have the greatest significance for teratogenesis are methotrexate and cyclophosphamide. Both can cause malformations of the skull and bones as well as mental retardation.
 Steroids: The value of steroids for lung maturation is well established. Chronic exposure to steroids has been reported to inhibit neuronal development. Prednisone and prednisolone cross the placenta to a small degree and therefore are the drugs of choice during gestation.
Steroids: The value of steroids for lung maturation is well established. Chronic exposure to steroids has been reported to inhibit neuronal development. Prednisone and prednisolone cross the placenta to a small degree and therefore are the drugs of choice during gestation.
 Antibiotics: Tetracycline is the most notorious drug for producing both skeletal and dental abnormalities in pregnant women. Sulfa drugs may accentuate hyperbilirubinemia during the neonatal period by displacing bilirubin from binding sites. Sulfamethoxazole/trimethoprim has been associated with congenital cardiac defects. Kanamycin and streptomycin (rarely used today) have produced congenital deafness. It is unclear whether gentamicin has the same potential. Careful drug monitoring appears to reduce the likelihood of hearing loss. Some cephalosporins (e.g., cefaclor, cephalexin, cephradine) have been associated with congenital defects, but the association is weak. Most other antibiotics (including acyclovir) appear to be safe for use during pregnancy.
Antibiotics: Tetracycline is the most notorious drug for producing both skeletal and dental abnormalities in pregnant women. Sulfa drugs may accentuate hyperbilirubinemia during the neonatal period by displacing bilirubin from binding sites. Sulfamethoxazole/trimethoprim has been associated with congenital cardiac defects. Kanamycin and streptomycin (rarely used today) have produced congenital deafness. It is unclear whether gentamicin has the same potential. Careful drug monitoring appears to reduce the likelihood of hearing loss. Some cephalosporins (e.g., cefaclor, cephalexin, cephradine) have been associated with congenital defects, but the association is weak. Most other antibiotics (including acyclovir) appear to be safe for use during pregnancy.
 Prostaglandin synthase inhibitors: Aspirin, ibuprofen, and naproxen may cause in utero constriction of the ductus arteriosus in rare cases and probably should be avoided if possible. Indomethacin has been used frequently as a tocolytic agent and is also reported to produce ductal closure, but it appears to be reasonably safe with careful fetal monitoring. These drugs do not appear to be teratogens; however, platelet aggregation is also reduced by many of these agents and may increase the potential for bleeding.
Prostaglandin synthase inhibitors: Aspirin, ibuprofen, and naproxen may cause in utero constriction of the ductus arteriosus in rare cases and probably should be avoided if possible. Indomethacin has been used frequently as a tocolytic agent and is also reported to produce ductal closure, but it appears to be reasonably safe with careful fetal monitoring. These drugs do not appear to be teratogens; however, platelet aggregation is also reduced by many of these agents and may increase the potential for bleeding.
 Alcohol: Fetal alcohol syndrome may occur with even minimal ingestion of alcohol. Symptoms include mental retardation, craniofacial abnormalities, and growth failure.
Alcohol: Fetal alcohol syndrome may occur with even minimal ingestion of alcohol. Symptoms include mental retardation, craniofacial abnormalities, and growth failure.
 Narcotics: The use of narcotics results in significant problems for the neonate, of which the most classic is neonatal drug withdrawal. Withdrawal typically begins in the immediate newborn period and lasts for days to weeks. With some narcotics, such as methadone, withdrawal may not be seen for several days. Babies of mothers who use narcotics appear to have an increased risk of abortion, prematurity, and growth failure.
Narcotics: The use of narcotics results in significant problems for the neonate, of which the most classic is neonatal drug withdrawal. Withdrawal typically begins in the immediate newborn period and lasts for days to weeks. With some narcotics, such as methadone, withdrawal may not be seen for several days. Babies of mothers who use narcotics appear to have an increased risk of abortion, prematurity, and growth failure.
 Cocaine: Cocaine use appears to result in a higher risk of abortion and stillbirth. Birth weight is generally slightly lower than normal, and there is an increased risk of prematurity. Microcephaly does occur in rare instances with cocaine use during pregnancy. Organ infarction may lead to bowel atresia, porencephaly, and limb maldevelopment.
Cocaine: Cocaine use appears to result in a higher risk of abortion and stillbirth. Birth weight is generally slightly lower than normal, and there is an increased risk of prematurity. Microcephaly does occur in rare instances with cocaine use during pregnancy. Organ infarction may lead to bowel atresia, porencephaly, and limb maldevelopment.
 Nicotine: Exposure to cigarette smoke in utero reduces birth weight by an average of 300 grams if the mother consistently smokes throughout gestation. The risk of apnea and sudden infant death syndrome is increased. The incidence of abruptio placentae also increases.
Nicotine: Exposure to cigarette smoke in utero reduces birth weight by an average of 300 grams if the mother consistently smokes throughout gestation. The risk of apnea and sudden infant death syndrome is increased. The incidence of abruptio placentae also increases.
 Selective serotonin uptake inhibitors: Commonly used as antidepressants, these agents carry a small risk of neonatal pulmonary hypertension. Obstetricians must balance this risk against the serious maternal and fetal risks of untreated depression.
Selective serotonin uptake inhibitors: Commonly used as antidepressants, these agents carry a small risk of neonatal pulmonary hypertension. Obstetricians must balance this risk against the serious maternal and fetal risks of untreated depression.
∗National Institutes of Child Health and Human Development National Registry for Amniocentesis Study Group. Amniocentesis for prenatal diagnosis: safety and accuracy. JAMA 1976;236:1471–1476.
∗Canadian Early and Mid-trimester Amniocentesis Trial (CEMAT) Group. Randomised trial to assess safety and fetal outcome of early and mid-trimester amniocentesis. Lancet 1998;351:242–247.
∗Eddleman KA, Malone FD, Sullivan L, et al. Pregnancy loss rates after midtrimester amniocentesis. Obstet Gynecol 2006;108:1067–72.
†Towner D, Currier RJ, Lorey FW, et al. Miscarriage risk from amniocentesis performed for abnormal maternal serum screening. Am J Obstet Gynecol 2007 Jun;196(6):608.e1–5
†Tabor A, Vestergaard CH, Lidegaard ø. Fetal loss rate after chorionic villus sampling and amniocentesis: an 11-year national registry study. Ultrasound Obstet Gynecol 2009 Jul;34(1):19–24.
∗Sundberg K, Bang J, Smidt-Jensen S, et al. Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling. Lancet 1997;350:697–703.
†Goldenberg RL, Andrews WW, Guerrant RL, et al. The preterm prediction study: cervical lactoferrin concentration, other markers of lower genital tract infection, and preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:631–635.
†Goldenberg RL, Andrews WW, Mercer BM, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Units Network. Am J Obstet Gynecol 2000;182:625–630.
§Goldenberg RL, Iams JD, Das A, et al. The preterm prediction study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:636–643.
¶Iams JD, Goldenberg RL, Mercer BM, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 2001;184:652–5.
 Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol 2011;204:205.e1–11.
Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol 2011;204:205.e1–11.
∗∗Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med 2011;13:913–20.
††Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012;207:137.e1–8.
∗Konje JC, Walley RJ. Bleeding in late pregnancy. In: James DK, Steer PJ, Weiner CP, Gonik B, editors. High risk pregnancy: management options. Philadelphia: Saunders; 1994. p. 119–136.
∗Rao KP, Belogolovkin V, Yankowitz J, et al. Abnormal placentation: evidence-based diagnosis and management of placenta previa, placenta accreta, and vasa previa. Obstet Gynecol Surv 2012 Aug;67(8):503–19.
∗Ananth CV, Berkowitz GS, Savitz DA, et al. Placental abruption and adverse perinatal outcome. JAMA 1999;17:1646–1651.
†Han CS, Schatz F, Lockwood CJ. Abruption-associated prematurity. Clin Perinatol 2011 Sep;38(3):407–21.
∗Kim YA, Makar RS. Detection of fetomaternal hemorrhage. Am J Hematol 2012;87:417–23.
∗Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010 Nov 19;59(RR-10):1–36.
†Byington CL, Polin RA. Policy statement—recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Committee on Infectious Diseases; Committee on Fetus and Newborn. Pediatrics 2011;128:611–6.
†Randis TM, Polin RA. Early-onset group B Streptococcal sepsis: new recommendations from the Centres for Disease Control and Prevention. Arch Dis Child Fetal Neonatal Ed 2012;97:F291–4.
∗McParland PC. Obstetric management of moderate and late preterm labour. Semin Fetal Neonatal Med 2012;17:138–42.
∗Joffe GM, Jacques D, Bernis-Heys R, et al. Impact of the fetal fibronectin assay on admissions for preterm labor. Am J Obstet Gynecol 1999;180:581–586.
†Goldenberg RL, Iams JD, Das A, et al. The preterm prediction study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:636–643.
∗Sanchez-Ramos L, Delke I, Zamora J, et al. Fetal fibronectin as a short-term predictor of preterm birth in symptomatic patients: a meta-analysis. Obstet Gynecol 2009;114:631–40.
∗Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol 2009;200:595–609.
∗Abramovici A, Cantu J, Jenkins SM. Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am 2012;39:77–87.
∗NIH Consensus Statement: Effect of Cortiocosteroids for Fetal Maturation on Perinatal Outcomes. NIH Consensus Statement, volume 12. February 28–March 2, 1994.
†Garite TJ, Kurtzman J, Maurel K, et al. Obstetrix Collaborative Research Network. Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol 2009;200:248.e1–9. Erratum in: Am J Obstet Gynecol 2009;201:428.
¶Iams JD, Goldenberg RL, Mercer BM, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 2001 Mar;184:652–5.
∗Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;12;348:2379–85. Erratum in: N Engl J Med 2003;25;349:1299.
†da Fonseca EB, Bittar RE, Carvalho MH, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003 Feb;188(2):419–24.
†da Fonseca EB, Celik E, Parra M, et al. Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;2;357:462–9.
§Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 2011;204:221.e1–8.
¶Combs CA, Garite T, Maurel K, et al. Obstetrix Collaborative Research Network. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol 2011;204:221.e1–8.