CHAPTER 295 Nucleoplasty and Posterior Dynamic Stabilization Systems
Nucleoplasty
Lumbar disk disease is a pervasive problem throughout industrialized countries. Both radiculopathy and diskogenic lumbago can be attributed to tears within the annulus fibrosus. Although herniation of the nucleus pulposus has been a known surgical entity since Mixter and Barr’s original work,1 the cause and treatment of diskogenic pain is less well understood. For more than 50 years, open discectomy has been the “gold standard” for disk decompression in the treatment of sciatica.2,3 More recently, radical discectomy with fusion has become an aggressive form of surgical treatment for diskogenic pain. Despite the success of discectomy, complications from the open procedure include: reherniation in up to 10% of patients, epidural fibrosis, loss of height, instability, and residual back pain.4–7 Alternatively, an array of minimally invasive disk-based techniques has arisen in parallel to discectomy, such as chemonucleolysis, automated percutaneous discectomy, laser discectomy, intradiscal electrothermal therapy (IDT), and coblation nucleoplasty. Advantages of these treatments include minimal scarring, avoidance of canal structures, and short hospital times. This section will briefly discuss some of these methods, with an emphasis on nucleoplasty, which perhaps has the greatest promise. The following section will address the concept of dynamic stabilization, a related but separate topic in the treatment of motion preservation lumbar spine surgery.
As the trends within surgery have shifted to minimally invasive, reductionist methods, the concept of definitive percutaneous treatment of disk disease has become increasingly attractive. In general terms, nucleoplasty is a technique in which intervertebral disk material is typically removed, rather than inserted, via a percutaneous route. The lumbar levels below the conus medullaris are the primary targets, in particular, L4-5 and L5-S1, and rarely L3-4.8 Additionally, the cervical spine can be treated.9 More specifically, nucleoplasty refers to the structural modification and treatment of the nucleus pulposus. The coblation form of nucleoplasty was introduced in July 2000 with the PercDLE SpineWand (ArthroCare, Austin, TX). Technically this method differs from other methods such as IDET annuloplasty and percutaneous discectomy. However, an understanding of all of these treatment options is relevant to the field.
Nucleoplasty is ideally used for small, contained disk herniations. Historically, there has been limited data examining disk size and annular characteristics in relation to discectomy outcome; for example, the large SPORT trial’s requirements for disk herniation were “protrusion, extrusion, or sequestered fragment.”10 However, several studies have suggested that treatment for smaller, contained disk herniations is particularly challenging. Patients with contained fragments and less than 6-mm herniation have been shown to respond poorly to diskectomy.11–15 One prospective study of 187 patients examined postlumbar discectomy outcomes based on annular competence and presence of a fragment.13 Notably, 38% of patients with no discrete fragment or annular tear were likely to experience residual sciatica, compared with only 1% of patients with a fragment and small annular tear. The small, contained herniation group had milder but more protracted symptoms and a smaller herniation size on magnetic resonance imaging (MRI). The authors stated that this group of patients with sciatica more closely resembled patients with chronic back pain, with symptoms disproportionate to anatomic findings. Thus, the contained disk herniation with radicular and/or diskogenic symptoms remains a difficult clinical entity to treat with standard discectomy.
Chemothermal Ablation: Chemonucleolysis and intradiscal electrothermal therapy
Chemonucleolysis
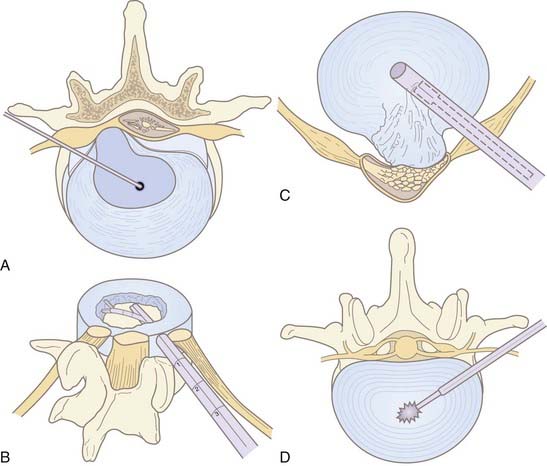
Chemonucleolysis is one of the oldest, least invasive methods available to treat lumbar disk disease. The technique uses chymopapain for enzymatic lysis of the nucleus and was first described by Lyman Smith in 1964.16 It was largely abandoned in North America with the rise of microdiscectomy and reports of anaphylactic complications. Chymopapain is no longer commercially available in the United States. However, the technique is still used in Europe and Asia. Multiple recent studies have reported success rates of 85% or greater in a carefully selected population.17,18 The best prognostic criteria are similar to microdiscectomy: leg pain greater than back pain, positive straight leg raise, and soft, noncalcified disk herniation on imaging.17 Discography determines the symptomatic level in the case of multiple disk herniations. Immediate epidural extravasation of contrast is a contraindication. However, delayed extravasation is acceptable. In a typical protocol, 4000 U of chymopapain dissolved in 1 to 2 mL of saline is injected into the center of the symptomatic disk (Fig. 295-1, A). The enzyme may potentially dissolve annular structures; there is no additional aspiration of lysate. Complications include back stiffness (up to 50%) and pain that can last for weeks. Anaphylactic reactions to the protein occur in less than 0.5% of patients.19 Test dosing of the skin should be done before the procedure.
Intradiscal Electrothermal Therapy
IDET annuloplasty involves the fluoroscopic insertion of a thermal resistance probe into the disk space via a cannula from a contralateral approach to the symptomatic side (Fig. 295-2). The treatment consists of radiofrequency ablation of the annulus fibrosus in a controlled manner. The probe is heated to a temperature required for coagulation of nerve endings and contraction of collagen. IDET is typically used for diskogenic back pain as opposed to radiculopathy from contained disk herniation. It may be used in place of interbody fusion in the treatment of diskogenic pain.8,20 In this regard, IDET does not afford a decompressive benefit to the same degree as percutaneous diskectomy or nucleoplasty (see later discussion). Multiple outcome trials and series have shown modest benefit of IDET in select patients with discography-concordant chronic low back pain.20–23 Two randomized controlled trials reported conflicting findings when IDET was compared with a placebo.24,25 Repeat IDET has been reported as successful but the literature is scant.26
Minimally Invasive Disk Decompression
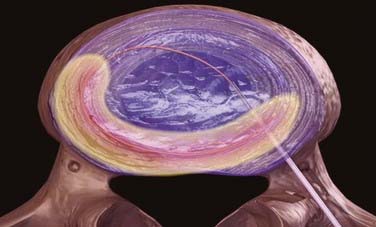
The principle of central disk decompression governs most of the techniques that remove nuclear material from the disk space. If the space is even partially decompressed, radiculopathy can improve from a reduction in volume, which may not be radiographically evident.27 A small reduction in volume can create a disproportionately large reduction in pressure based on hydrostatic forces.28,29 The partial vacuum created by nucleus aspiration causes a retraction of the disk and regeneration of annular fibers to contain nuclear material that may be protruding into the canal. Thus the process is effective only in select patients who still have an intact annulus fibrosus without large or free fragments. Broad, circumscribed disk protrusions on MRI are typical radiographic findings. Discography can be helpful in distinguishing annular fissures from intact external fibers and true extrusions (see Fig. 295-1). Relief of intradiscal pressure may also decrease leaching of inflammatory mediators, thereby alleviating both chemical and mechanical causes of diskogenic pain.30,31
Typical inclusion criteria for percutaneous discectomy (and nucleoplasty) are: relatively young age (<50 years), failure of conservative measures including physical therapy (>6 weeks), radiculopathy and/or lumbago, absence of significant motor deficit, and concordance of a provocative discogram (although not essential in cases of clear radiculopathy). In general, exclusion criteria include greater than 50% loss of disk height, sequestered disk herniation, greater than one third canal occupation, spinal instability or fracture, morbid obesity, infection, or spinal stenosis from osteophytic disease.32 Relative contraindications include motor weakness, previous open surgery, spinal stenosis, or compressive facet arthropathy. Typically patients will have a 6-month or greater history of symptoms, with exacerbations under loading conditions that increase the pressure and diameter of the disk.33 Ideal candidates have a diffuse disk protrusion and radicular pain greater than back pain.8 However, the procedure may be performed for diskogenic pain as well. Decompression and subsequent scar formation may reinforce the annulus with a fibrous scar, facilitate closure of radial tears, and contribute to stabilization.34
Automated Percutaneous Lumbar Discectomy
First described by Onik in 1985, automated percutaneous lumbar discectomy (APLD) is the most widely performed percutaneous decompressive technique, preceding nucleoplasty by 15 years. It has been performed with modest success over the past 2 decades. The technique involves insertion of a mechanical (automated) probe to perform a nucleotomy via a “suction and cutting” mechanism (see Fig. 295-1, C). A 2.8-mm outer cannula is inserted fluoroscopically via the posterolateral approach on the predominantly painful ipsilateral side and positioned against the annulus. Then, a rounded-tip aspiration probe with a side port is passed into the disk space. Disk material is sheared off by the pneumatically driven guillotine effect of the sharp inner cannula (see Fig. 295-1, C). Suction aspiration of nuclear material is stopped when there is blood return or decreased flow. Approximately 1 to 3 g are removed.35 Aspiration times average less than 20 minutes. Afterward, a steroid or topical anesthetic may be injected along the same tract. Iatrogenic fragment herniation is a rare complication (<1%), which may occur from annular weakening or direct pressure of the probe.34,36
Unfortunately, randomized trials of APLD have shown limited to no benefit.35,37,38 Proponents of APLD have criticized these studies for small sample sizes, poor patient selection, and the authors’ learning curve of the technique.34 A large prospective trial with a 1-year follow-up found a 75% successful treatment rate.39 Proper patient selection is critical; sequestered or free fragments and canal compromise greater than 50% are predictors of poor outcome. Overall, several hundred thousand patients have undergone APLD. In general, however, the success rates of APLD for radiculopathy seem lower than standard microdiscectomy.40
Laser Discectomy
Intradiscal laser discectomy is another method that works via reduction of intradiscal pressures. Between 1100 to 1200 J of energy from the laser vaporizes a portion of the nucleus pulposus (see Fig. 295-1, D). Various lasers have been used, including Nd:YAG and KTP.532 Despite the technical limitations of thermal spread, several large series have reported improvement in approximately 70% of patients, including those with diskogenic pain.41–44 However, no randomized controlled trials (RCTs) have been performed. Laser-induced steam can be seen in the disk space afterward if it is not removed via the cannula, but this has not resulted in complications.
Nucleoplasty
Radiofrequency (RF) nucleoplasty (coblation nucleoplasty or plasma disk decompression) is a method of decompressive nucleotomy that uses current to dissolve portions of the nucleus pulposus (see Fig. 295-4).
Unlike IDET and laser discectomy, in which thermal complications or limitations are potential drawbacks,45 coblation does not in itself generate heat; rather, the RF current isolates and dissociates components of surrounding tissues, resulting in their dissolution. The catheter creates a low thermal plasma field in which there is minimal thermal injury to adjacent tissue.46 Histologically, the coblation channels create clean coagulation borders of the nucleus pulposus. Chen and coworkers found that the annulus, end plates, and neural elements were histologically normal at the level of the procedure in a cadaver study.46 Surgically, coblation technology has gained prominence in the otorhinolaryngology field for tonsillectomy and turbinoplasty procedures for its sparing of vital neighboring structures.47,48
Method
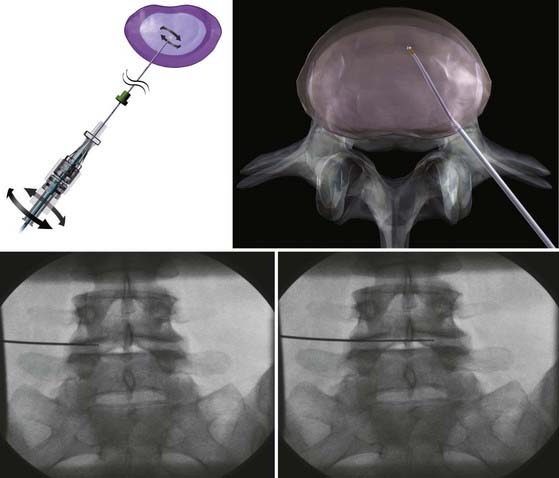
Under general anesthesia or conscious sedation, the patient is placed in a prone or lateral decubitus position. Conscious sedation must be used in discography. Under fluoroscopic guidance, a 17-gauge, 6-inch spinal access cannula is inserted into the disk space via a posterolateral extrapedicular approach (Fig. 295-3).49 The cannula is placed at the annular-nuclear junction. The Perc-DLE SpineWand (ArthroCare, Sunnyvale, CA.) is advanced down the access cannula until the probe tip is 5 mm beyond the cannula edge, ensuring that the active probe is within the nucleus pulposus and past the inner layer of annulus fibrosus.
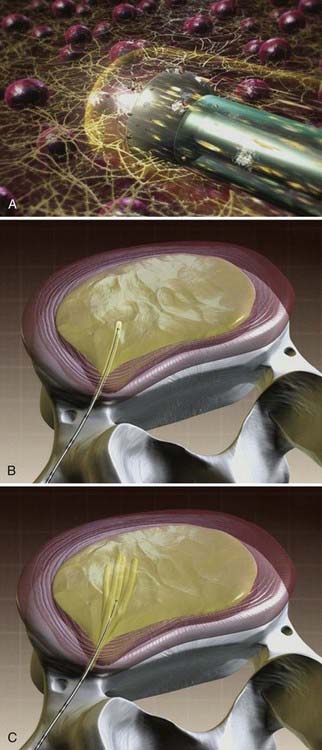
The wand is a 1-mm diameter, bipolar instrument. In ablation mode, the wand generates approximately 120 V of energy, with resultant temperatures of 50° to 70° C at the wand tip.50 An ionized plasma field is created. The decompression is performed by advancing the wand in ablation mode to create a channel from the posterolateral annulus to the anteromedial annulus (Fig. 295-4, A). When the wand is withdrawn, it is switched to coagulation mode, which allows for widening of the channel to approximately 1 mm by denaturing type II collagen and proteoglycans, with resultant shrinkage of the surrounding collagen (Fig. 295-4, B). Six channels are created at the 2, 4, 6, 8, 10, and 12 o’clock positions, forming a cone-shaped area of nucleotomy (Fig. 295-4, C). The entire radiofrequency ablation process requires 2 to 3 minutes and results in disk volume reduction of 10% to 20%.51 Approximately 1 mL of tissue volume is removed with the six channels. A bilateral approach may be used for maximal nucleus pulposus removal or for centrally herniated disks.
Postoperatively, patients may be discharged on the day of the procedure or the following day. Patients are typically instructed to follow postdiscectomy guidelines (i.e., limiting heavy lifting, bending, and twisting movements for 2 to 4 weeks. Side effects and complications include local needle site pain, transient paresthesias, and increased back pain and muscle spasms in a minority of patients. There has also been one report of epidural fibrosis.52
There have been no RCTs published regarding nucleoplasty. Large series reported symptomatic improvements for both low back and sciatic pain of between 50% and 80% as much as 1 year posttreatment.53–57 However, Gerstzen and coworkers reported negative outcome results.49 Therefore, patient selection is critical for therapeutic benefit. The principles of percutaneous discectomy apply to nucleoplasty patient selection. It is important to consider not only whether the herniation is contained, but also the degree of disk degeneration. Chen and coworkers measured intradiscal pressures postnucleoplasty in a cadaver model,28 demonstrating that nucleoplasty was largely ineffective at reducing pressures in highly degenerative disks. Nondegenerative disks had a marked reduction in pressure. The clinical implication is that older patients with more calcified disks or patients with smaller disk spaces may not respond well to nucleoplasty. Older patients are less likely to have radial fissures in the annulus because of decomposition of mucoid matrix,58 making nucleoplasty potentially less successful for diskogenic pain in this group.
More recent European studies have continued to report the safety and efficacy of nucleoplasty in nonplacebo controlled cohorts.59–61 Cervical nucleoplasty has also been reported with complete or partial resolution of symptoms in up to 90% of patients.9 The largest series of nucleoplasty procedures to date describes 82% significant improvement at 1-year follow-up in nearly 1400 patients.51 There was a corresponding radiographic reduction in disk bulge in 82% of cases. However, Gerszten and coworkers49 found mixed results in an outcomes study on a smaller longitudinal cohort of 67 patients. Although patients had a significant improvement in quality of life at 6 months by the Short Form-36 physical component scale, they were no better in terms of pain as measured by the VAS (visual analogue scale).
Dynamic Stabilization
The concept of dynamic stabilization rests on intervertebral motion preservation or restoration to the normal biomechanical limits of the healthy spine. This review will focus on posterior dynamic stabilization (PDS) in the lumbar spine, using primarily pedicle screw-based systems. Table 295-1 outlines a proposed classification scheme for PDS devices that includes interspinous process-based systems (ISP) and total facet replacement systems, which generally have pedicle bone anchors, but pedicle screw–based systems have a longer track record and are further along in the FDA approval process than the ISP and total facet replacement systems.
TABLE 295-1 Proposed Classification Scheme for Posterior Dynamic Stabilization (PDS)
| CLASSIFICATION | INDICATIONS | DEVICE |
|---|---|---|
| Interspinous spacer devices | Neurogenic claudication | Wallis, X STOP, DIAM, Coflex, ExtendSure |
| Pedicle screw/rod-based devices | Unloading disk/facets; promote fusion; prevent adjacent-segment disease; control motion in destabilized spine | Graf ligament, Dynesys, Accuflex rod, Medtronic PEEK rod, Scient’X Isobar |
| Total facet replacement systems | Replacement for facet disease; functional spine unit reconstruction; prevent adjacent segment disease; control motion in destabilized spine | TFAS, TOPS, Stabilimax NZ |
From Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22(1):E3.
The major advantages of posterior dynamic stabilization are as follows: (1) the posterior approach is commonly used by spine surgeons and the techniques are translational in their application; (2) the systems can be revised to fusion if necessary; (3) the instrumentation is pedicle screw–based; (4) the systems control segmental motion and may act as augmentation to lost physiologic constraints; (5) combinations of fusion and nonfusion (“hybrid constructs”) instrumentation can be used in the patient to customize and adapt the system to the patient’s needs (Fig. 295-5); and (6) minimally invasive techniques of device insertion are possible.
The indications for posterior dynamic stabilization are still not completely defined.62 These systems have been used outside of the United States for more than 15 years, and have been used to treat a wide range of pathologies, including lumbar spinal stenosis, recurrent disk rupture, lumbar pain, facet arthropathy, and others. In the United States, the recently completed IDE trial examining the Dynesys system (Zimmer Spine, Minneapolis, MN) required the presence of a stenosing lumbar lesion (either spinal canal stenosis or neuroforaminal stenosis) in patients with greater leg symptoms than lower back symptoms.63 These patients were considered for study entry if they had instability (up to grade I degenerative spondylolisthesis without pars interarticularis defect) and were thought to be appropriate candidates for pediclescrew augmented lumbar fusion. An example would be a patient with grade I spondylolisthesis and lumbar spinal stenosis at the same level. Such a patient might be considered for decompression with fusion. This patient might be considered for fusion if extensive facet joint resection for neural decompression was performed. In lieu of fusion, the patient might be appropriate for a nonfusion PDS construct.
The conceptual goals for all pedicle screw–based PDS systems is to off-load some of the stresses placed on the disks and facet joints, provide some form of controlled motion, decompress neural elements, and relieve radicular and lumbar pain.64 Although the surgeon and patient may theoretically hope for restoration or preservation of motion, the most thoroughly clinically tested instrumentation, Dynesys, reduces lumbar motion.65
The inherent risk with PDS techniques is that the spinal stresses are at least partially assumed by the posterior instrumentation. Furthermore, because fusion is not expected to occur, the stresses will be borne by the instrumentation for many years, with associated stress cycles. This may lead to device failure as was seen with the Agile system (Medtronic, Memphis, TN), prompting its withdrawal from further development. A secondary site of failure of the system is at the bone-screw interface. Screw loosening has been identified in approximately 8% of Dynesys patients.66 As a potential treatment for this problem, hydroxyapatite-coated screws are now available in the Dynesys system. Another potential failure point is the bone screw itself. Fortunately, improved screw design has made this occurrence a rarity (0.14% of all Dynesys screws placed during the U.S. IDE [Investigational Device Exemption] trial).
The 1-year clinical outcomes of the Dynesys IDE trial have been reported.63 The 2-year outcomes study is being prepared for submission. Regarding the 1-year outcomes, the primary indication was leg symptoms greater than lower back pain, spondylolisthesis, and canal stenosis. Patients were treated at a single level and were randomized to either instrumented fusion with semirigid pedicle screw fixation and iliac crest autograft or Dynesys. In the Dynesys group, both the VAS scores for lower back pain and leg pain improved from 55 to 30 and 80 to 25, respectively. Oswestry disability scores also improved considerably from 55.6% to 26.3%. SF-12 scores improved significantly statistically as well. The most common complication was dural tear.
Methods
Decompression and discectomy are performed as necessary. Interbody fusion is performed in fusion cases at the surgeon’s discretion. In both fusion and nonfusion cases, the screws are placed in a lateral-to-medial direction starting lateral to the facet joint in the trough formed by the lateral facet joint and the medial transverse process (Fig. 295-6). All efforts are made to maximize the bone-screw interface. We try to use a special pedicle probe, which creates a large enough hole in the pedicle to obviate the need for tapping. We prefer to do this with fluoroscopic guidance. The screws are placed and driven into the bone as far as possible to reduce the profile of the system and to improve the relationship of the instrumentation to the preoperative instantaneous axis of rotation.
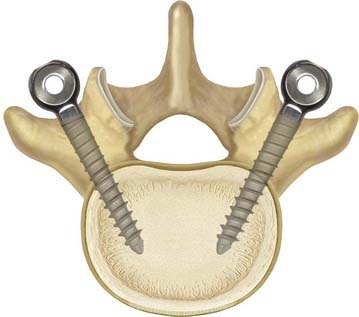
FIGURE 295-6 Demonstration of the preferred pedicle screw angle for Dynesys screw placement.
(Courtesy Zimmer Spine.)
Once the screws are placed, they can be linked with solid rods, polyethylene terephthalate (PET) cables, and polyurethane bumpers (Fig. 295-7) or combinations of the two for hybrid constructs. The use of solid rods is straightforward and similar to many other commercially available lumbar instrumentation systems. The nonfusion construct consists of PET cables tensioned against polyurethane bumpers. This is done by advancing the PET cable through the pedicle screw and clamping the cable in place. The distance between the pedicle screw heads is measured and a polyurethane bumper is cut to length. The bumper is placed over the cable, and the cable is advanced into the next pedicle screw. Tension is placed across the pedicle screws with a tensioning device and the cable is clamped in place. The process is repeated on the other side of the spine and at the other levels as determined by the surgeon. The hybrid construct provides a connection pedicle screw that essentially clamps the rod against the PET cable. The remainder of the procedure is unchanged from the above description. When used as a fusion adjunct, the surgeon may place bone on the transverse processes for arthrodesis. Excess cable is trimmed and the wound is closed in the usual fashion. We generally put patients in lumbar braces for a period of time after surgery and monitor the system radiographically at 6, 12, 16, 52, and 104 weeks.
Alexandre A, Coro L, Azuelos A, et al. Percutaneous nucleoplasty for discoradicular conflict. Acta Neurochir Suppl. 2005;92:83-86.
Carragee EJ, Kim DH. A prospective analysis of magnetic resonance imaging findings in patients with sciatica and lumbar disc herniation. Correlation of outcomes with disc fragment and canal morphology. Spine. 1997;22:1650-1660.
Chen YC, Lee SH, Chen D. Intradiscal pressure study of percutaneous disc decompression with nucleoplasty in human cadavers. Spine. 2003;28:661-665.
Chen YC, Lee SH, Saenz Y, et al. Histologic findings of disc, end plate and neural elements after coblation of nucleus pulposus: an experimental nucleoplasty study. Spine J. 2003;3:466-470.
Cheng BC, Gordon J, Cheng J, et al. Immediate biomechanical effects of a lumbar posterior dynamic stabilization above a circumferential fusion. Spine. 2007;32:2551-2557.
Freeman Freeman BJ, Fraser RD, Cain CM, et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2777.
Gerszten PC, Welch WC, King JTJr. Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine. 2006;4:36-42.
Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22(1):E3.
Kieffer SA, Stadlan EM, Mohandas A, et al. Discographic-anatomical correlation of developmental changes with age in the intervertebral disc. Acta Radiol Diagn (Stockh). 1969;9:733-739.
Kim YS, Chin DK, Yoon DH, et al. Predictors of successful outcome for lumbar chemonucleolysis: analysis of 3000 cases during the past 14 years. Neurosurgery. 2002;51:S123-S128.
Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002:S198-S205.
Onik G, Mooney V, Maroon JC, et al. Automated percutaneous discectomy: a prospective multi-institutional study. Neurosurgery. 1990;26:228-232.
Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
Schmoelz W, Huber JF, Nydegger T, et al. Influence of a dynamic stabilization system on load bearing of a bridged disc: an in vitro study of intradiscal pressure. Eur Spine J. 2006;15:1276-1285.
Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11:S170-S178.
Welch WC, Cheng BC, Awad TE, et al. Clinical outcomes of the Dynesys dynamic neutralization system: 1-year preliminary results. Neurosurg Focus. 2007;22(E8):1-8.
Welch WC, Gerszten PC. Alternative strategies for lumbar discectomy: intradiscal electrothermy and nucleoplasty. Neurosurg Focus. 2002;13:E7.
Welch WC, Gerszten PC, McGrath P. Intradiscal electrothermy: indications, techniques, and clinical results. Clin Neurosurg. 2001;48:219-225.
1 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
2 Williams RW. Microlumbar discectomy: a conservative surgical approach to the virgin herniated lumbar disc. Spine. 1978;3:175-182.
3 Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane review. Spine. 2007;32:1735-1747.
4 North RB, Campbell JN, James CS, et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28:685-690.
5 Ramirez LF, Thisted R. Complications and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25:226-230.
6 Stolke D, Sollmann WP, Seifert V. Intra- and postoperative complications in lumbar disc surgery. Spine. 1989;14:56-59.
7 Yorimitsu E, Chiba K, Toyama Y, et al. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine. 2001;26:652-657.
8 Welch WC, Gerszten PC. Alternative strategies for lumbar discectomy: intradiscal electrothermy and nucleoplasty. Neurosurg Focus. 13(E7), 2002.
9 Nardi PV, Cabezas D, Cesaroni A. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochir Suppl. 2005;92:73-78.
10 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT): a randomized trial. JAMA. 2006;296:2441-2450.
11 Knop-Jergas BM, Zucherman JF, Hsu KY, et al. Anatomic position of a herniated nucleus pulposus predicts the outcome of lumbar discectomy. J Spinal Disord. 1996;9:246-250.
12 Carragee EJ, Kim DH. A prospective analysis of magnetic resonance imaging findings in patients with sciatica and lumbar disc herniation. Correlation of outcomes with disc fragment and canal morphology. Spine. 1997;22:1650-1660.
13 Carragee EJ, Han MY, Suen PW, et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85:102-108.
14 Vucetic N, Astrand P, Guntner P, et al. Diagnosis and prognosis in lumbar disc herniation. Clin Orthop Relat Res. 1999;361:116-122.
15 Vucetic N, de Bri E, Svensson O. Clinical history in lumbar disc herniation. A prospective study in 160 patients. Acta Orthop Scand. 1997;68:116-120.
16 Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA. 1964;187:137-140.
17 Kim YS, Chin DK, Yoon DH, et al. Predictors of successful outcome for lumbar chemonucleolysis: analysis of 3000 cases during the past 14 years. Neurosurgery. 2002;51:S123-S128.
18 Guha AR, Debnath UK, D’Souza S. Chemonucleolysis revisited: a prospective outcome study in symptomatic lumbar disc prolapse. J Spinal Disord Tech. 2006;19:167-170.
19 McCulloch JA. Chemonucleolysis: experience with 2000 cases. Clin Orthop Relat Res. 1980;146:128-135.
20 Welch WC, Gerszten PC, McGrath P. Intradiscal electrothermy: indications, techniques, and clinical results. Clin Neurosurg. 2001;48:219-225.
21 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
22 Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty (IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
23 Maurer P, Block JE, Squillante D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. J Spinal Disord Tech. 2008;21:55-62.
24 Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
25 Freeman BJ, Fraser RD, Cain CM, et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2377.
26 Cohen SP, Shockey SM, Carragee EJ. The efficacy of repeat intradiscal electrothermal therapy. Anesth Analg. 2007;105:495-498.
27 Shea M, Takeuchi TY, Wittenberg RH, et al. A comparison of the effects of automated percutaneous diskectomy and conventional diskectomy on intradiscal pressure, disk geometry, and stiffness. J Spinal Disord. 1994;7:317-325.
28 Chen YC, Lee SH, Chen D. Intradiscal pressure study of percutaneous disc decompression with nucleoplasty in human cadavers. Spine. 2003;28:661-665.
29 Case RB, Choy DS, Altman P. Change of intradisc pressure versus volume change. J Clin Laser Med Surg. 1995;13:143-147.
30 Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065-1073.
31 Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218-224.
32 Bhagia SM, Slipman CW, Nirschl M, et al. Side effects and complications after percutaneous disc decompression using coblation technology. Am J Phys Med Rehabil. 2006;85:6-13.
33 Onik G, Helms CA, Ginsburg L, et al. Percutaneous lumbar diskectomy using a new aspiration probe. AJR Am J Roentgenol. 1985;144:1137-1140.
34 Bonaldi G. Automated percutaneous lumbar discectomy: technique, indications and clinical follow-up in over 1000 patients. Neuroradiology. 2003;45:735-743.
35 Revel M, Payan C, Vallee C, et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica. A randomized multicenter trial. Spine. 1993;18:1-7.
36 Dullerud R, Nakstad PH. Side effects and complications of automated percutaneous lumbar nucleotomy. Neuroradiology. 1997;39:282-285.
37 Haines SJ, Jordan N, Boen JR, et al. Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci. 2002;9:411-417.
38 Chatterjee S, Foy PM, Findlay GF. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20:734-738.
39 Onik G, Mooney V, Maroon JC, et al. Automated percutaneous discectomy: a prospective multi-institutional study. Neurosurgery. 1990;26:228-232.
40 Maroon JC. Current concepts in minimally invasive discectomy. Neurosurgery. 2002;51:S137-S145.
41 Hellinger J. Technical aspects of the percutaneous cervical and lumbar laser-disc-decompression and -nucleotomy. Neurol Res. 1999;21:99-102.
42 Knight M, Goswami A. Lumbar percutaneous KTP532 wavelength laser disc decompression and disc ablation in the management of discogenic pain. J Clin Laser Med Surg. 2002;20:9-13.
43 Choy DS. Percutaneous laser disc decompression (PLDD): twelve years’ experience with 752 procedures in 518 patients. J Clin Laser Med Surg. 1998;16:325-331.
44 Gronemeyer DH, Buschkamp H, Braun M, et al. Image-guided percutaneous laser disk decompression for herniated lumbar disks: a 4-year follow-up in 200 patients. J Clin Laser Med Surg. 2003;21:131-138.
45 Scholl BM, Theiss SM, Lopez-Ben R, et al. Vertebral osteonecrosis related to intradiscal electrothermal therapy: a case report. Spine. 2003;28:E161-E164.
46 Chen YC, Lee SH, Saenz Y, et al. Histologic findings of disc, end plate and neural elements after coblation of nucleus pulposus: an experimental nucleoplasty study. Spine J. 2003;3:466-470.
47 Shah UK, Dunham B. Coblation for tonsillectomy: an evidence-based review. ORL J Otorhinolaryngol Relat Spec. 2007;69:349-357.
48 Carney AS, Timms MS, Marnane CN, et al. Radiofrequency coblation for the resection of head and neck malignancies. Otolaryngol Head Neck Surg. 2008;138:81-85.
49 Gerszten PC, Welch WC, King JTJr. Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine. 2006;4:36-42.
50 Nau WH, Diederich CJ. Evaluation of temperature distributions in cadaveric lumbar spine during nucleoplasty. Phys Med Biol. 2004;49:1583-1594.
51 Alexandre A, Coro L, Azuelos A, et al. Percutaneous nucleoplasty for discoradicular conflict. Acta Neurochir Suppl. 2005;92:83-86.
52 Smuck M, Benny B, Han A, et al. Epidural fibrosis following percutaneous disc decompression with coblation technology. Pain Physician. 2007;10:691-696.
53 Sharps LS, Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5:121-126.
54 Singh V, Piryani C, Liao K, et al. Percutaneous disc decompression using coblation (nucleoplasty) in the treatment of chronic discogenic pain. Pain Physician. 2002;5:250-259.
55 Singh V, Piryani C, Liao K. Evaluation of percutaneous disc decompression using coblation in chronic back pain with or without leg pain. Pain Physician. 2003;6:273-280.
56 Singh V, Piryani C, Liao K. Role of percutaneous disc decompression using coblation in managing chronic discogenic low back pain: a prospective, observational study. Pain Physician. 2004;7:419-425.
57 Marin FZ. CAM versus nucleoplasty. Acta Neurochir Suppl. 2005;92:111-114.
58 Kieffer SA, Stadlan EM, Mohandas A, et al. Discographic-anatomical correlation of developmental changes with age in the intervertebral disc. Acta Radiol Diagn (Stockh). 1969;9:733-739.
59 Calisaneller T, Ozdemir O, Karadeli E, et al. Six months post-operative clinical and 24 hour post-operative MRI examinations after nucleoplasty with radiofrequency energy. Acta Neurochir (Wien). 2007;149:495-500.
60 Masala S, Massari F, Fabiano S, et al. Nucleoplasty in the treatment of lumbar diskogenic back pain: one year follow-up. Cardiovasc Intervent Radiol. 2007;30:426-432.
61 Azulay N, Forgerit M, Alava EG, et al. A novel radiofrequency thermocoagulation method for treatment of lower back pain: thermal conduction after instillation of saline solution into the nucleus pulposus-preliminary results. Acta Radiol. 2008:1-6.
62 Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002:S198-S205.
63 Welch WC, Cheng BC, Awad TE, et al. Clinical outcomes of the Dynesys dynamic neutralization system: 1-year preliminary results. Neurosurg Focus. 2007;22(E8):1-8.
64 Schmoelz W, Huber JF, Nydegger T, et al. Influence of a dynamic stabilization system on load bearing of a bridged disc: an in vitro study of intradiscal pressure. Eur Spine J. 2006;15:1276-1285.
65 Cheng BC, Gordon J, Cheng J, et al. Immediate biomechanical effects of a lumbar posterior dynamic stabilization above a circumferential fusion. Spine. 2007;32:2551-2557.
66 Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11:S170-S178.