CHAPTER 1 Normal blood cells
Erythrocytes
Morphology
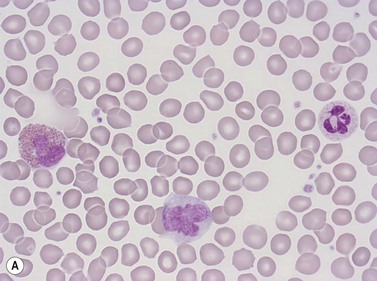
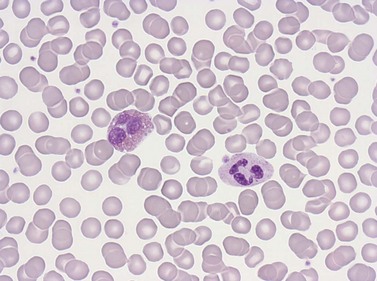
Erythrocytes are highly differentiated cells that have no nuclei or cytoplasmic organelles. Normal erythrocytes are circular biconcave discs with a mean diameter of 7.2 µm (range 6.7–7.7 µm) in dried fixed smears and about 7.5 µm in the living state. They are eosinophilic and consequently appear red with a central area of pallor in Romanowsky-stained smears (Fig. 1.1 A,B).
Red cell parameters
The three basic red blood cell parameters which can be measured are:1
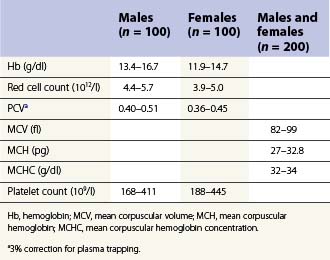
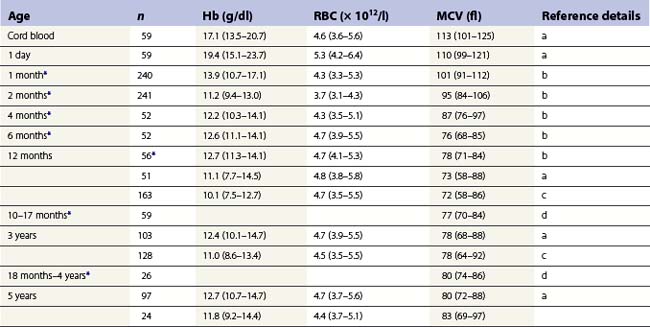
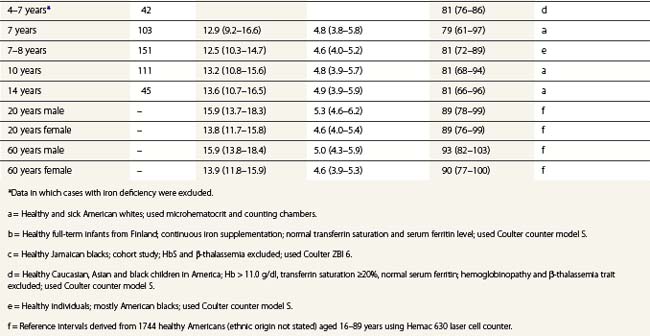
From the values obtained for the hemoglobin concentration, red cell count and hematocrit, it is possible to calculate the mean cell volume (MCV), mean cell hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC) as shown in Table 1.1. Some automated blood-counting machines determine the MCV using electrical impedance or light-scattering techniques and calculate the hematocrit from the measured MCV and red cell count. Others determine the hematocrit directly by summing all the pulses in the red cell channel. The normal values for various red cell parameters at different ages are given in Tables 1.2 and 1.3; however, there are some differences based on the analyzer used and the method of measurement. Between the age of 2 years and the onset of puberty there is a gradual rise in the hemoglobin concentration in both males and females. There is a subsequent further rise in males but not in females with the result that the mean hemoglobin is higher in adult males than in adult females. In healthy infants aged 4 months and over, and in healthy young children, the average MCV is lower than in healthy adults. Whereas the lower limit for the MCV in unselected healthy adults is 82 fl, the corresponding figure for children between 1 and 7 years (who show no biochemical evidence of iron deficiency) is about 70 fl. The MCV increases progressively with age both in children and, to a much lesser extent, in adults.
| MCV (fl) | = Hcta ÷ RBC per liter × 1015 |
| MCH (pg) | = Hbb ÷ RBC per liter × 1013 |
| MCHC (g/dl) | = Hbb ÷ Hcta |
Hb, hemoglobin; Hct, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cells.
Red cell life span
The critical change that causes a red cell to be destroyed at the end of its life span appears to be the formation of denatured/oxidized hemoglobin (hemichromes) which induces clustering of the integral membrane protein, band 3. This clustering generates an epitope on the red cell surface that binds naturally occurring IgG anti-band 3 antibodies and the antibody-coated aged erythrocytes are recognized and phagocytosed by macrophages.2,3 A second mechanism that may be involved in the elimination of aged red cells by macrophages is the exposure of phosphatidylserine on the outer surface of their cell membrane; this is recognized by the macrophage scavenger receptor CD36 or, after combination with lactadherin, by macrophage integrin.3,4 Aging red cells also extrude microvesicles containing denatured hemoglobin that have the same membrane changes and are phagocytosed by the same mechanisms as the residual red cell.5
Functions of red cells
Normal function of the erythrocyte requires a normal red cell membrane and normal enzyme systems to provide energy and protect against oxidant damage. The erythrocyte membrane is composed of a lipid bilayer (containing integral proteins) and is bound to a submembranous cytoskeletal network of protein molecules including spectrin, actin and the proteins constituting bands 4.1a and 4.1b6 (Chapter 7). This cytoskeletal network is responsible for maintaining the biconcave shape of a normal erythrocyte. The membrane also contains adenosine triphosphate (ATP)-dependent cation pumps that continuously pump Na+ out and K+ into the red cell, against concentration gradients, thereby counteracting a continuous passive diffusion of ions across the membrane in the opposite direction. Mature erythrocytes derive their energy from glycolysis by the Embden–Meyerhof pathway (Chapter 8). They can also metabolize glucose through the pentose phosphate pathway, which generates the reduction potential of the cell and protects the membrane, the hemoglobin and erythrocyte enzymes from oxidant damage (Chapter 8). Both a normal cell membrane and normal energy production are required to enable the biconcave red cells to repeatedly and reversibly deform during numerous transits through the microcirculation.
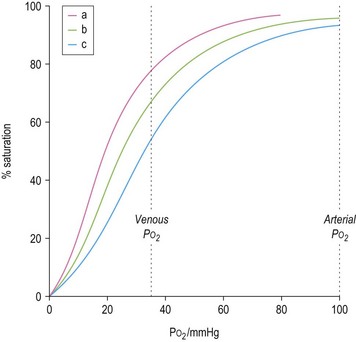
The ability of red cells to combine with and release oxygen is illustrated in the oxygen dissociation curve (Fig. 1.2). The shape of the oxygen dissociation curve of HbA is sigmoid and this is a function of the interaction between the four monomers which make up its tetrameric structure (heme–heme interaction); the combination of one oxygen molecule with one heme group causes a slight shape change in the Hb molecule due to movement at the α1–β2 contact facilitating the binding of oxygen to the next heme group. The shape of the oxygen dissociation curve of the monomer, myoglobin, is hyperbolic. The advantage of the sigmoid curve over the hyperbolic curve is that much more oxygen is released from the hemoprotein at the low PO2 values obtained in tissues (35–40 mmHg) with the former than with the latter. The percentage saturation of hemoglobin at this PO2 is about 70%. The capacity of hemoglobin to combine with O2 is referred to as its oxygen affinity and is expressed as the PO2 required to cause 50% saturation (P50). A decrease in pH leads to a shift of the oxygen dissociation curve to the right and a decrease in oxygen affinity. This effect, which is known as the Bohr effect, facilitates the release of oxygen at the low pH of tissues. A shift of the oxygen dissociation curve to the right also results from the combination of deoxyhemoglobin with 2,3-diphosphoglycerate (2,3-DPG) that is produced as a result of the metabolism of glucose via the Rapoport–Luebering shunt of the Embden–Meyerhof pathway (Chapter 8). In deoxyhemoglobin, the two β chains are separated slightly allowing one molecule of 2,3-DPG to enter and bind to the β chains; when hemoglobin combines with oxygen, the 2,3-DPG is ejected.
The biconcave shape of normal erythrocytes facilitates the diffusion of gases in and out of the cytoplasm and also imparts adequate flexibility and deformability to enable these cells repeatedly to traverse the microcirculation. The hemoglobin molecules within erythrocyes inactivate some of the endothelial cell-derived nitric oxide and consequently regulate the bioavailability of nitric oxide in the circulation. The inactivation results from the reaction of nitric oxide with oxyhemoglobin resulting in the formation of nitrite. Plasma nitrite may also be converted to nitric oxide by deoxyhemoglobin which has a nitrite reductase activity and by a nitric oxide synthase (NOS) located in the plasma membrane and cytoplasm of red cells. These three mechanisms affect nitric oxide-dependent vascular tone and nitric oxide generated by red cell NOS may affect red cell deformability.6–8
Reticulocytes
These are the immediate precursors of mature erythrocytes. They are rounded anucleate cells that are about 20% larger in volume than mature red blood cells and appear faintly polychromatic when stained by a Romanowsky method. When stained with a supravital stain such as new methylene blue or brilliant cresyl blue, the diffuse basophilic material responsible for the polychromasia (i.e. ribosomal RNA) appears as a basophilic reticulum. Electron-microscope studies have shown that reticulocytes are rounded cells with a tortuous surface and that in addition to ribosomes they contain mitochondria and autophagic vacuoles. Circulating reticulocytes mature into red cells over a period of 1–2 days during which there is progressive degradation of ribosomes and mitochondria and the acquisition of a biconcave shape. Reticulocytes actively synthesize hemoglobin and non-hemoglobin proteins. They contain enzymes of the Embden–Meyerhof pathway and the pentose phosphate shunt and, unlike the mature red cells, can also derive energy aerobically via the Krebs cycle that operates in the mitochondria and oxidizes pyruvate to CO2 and water. Supravitally stained preparations were traditionally used and are still frequently used to assess reticulocyte numbers by microscopy with an eyepiece micrometer disc to facilitate counting. In normal adults, the reference range for reticulocytes counted in this way is widely accepted to be 0.5–2.0% of the total circulating erythrocyte plus reticulocyte population. The usefulness of the reticulocyte percentage is increased by applying a correction for the hematocrit and the corrected reticulocyte percentage (usually corrected to a hematocrit of 0.45) is obtained by multiplying the observed percentage by [patient’s hematocrit ÷ 0.45]. Although several laboratories still express reticulocyte counts as a percentage, the absolute reticulocyte count (i.e. the total number per liter of blood) is clinically more useful. The latter is directly proportional both to the rate of effective erythropoiesis and to the average maturation time of blood reticulocytes. In normal adults the absolute reticulocyte count determined by microscopy is 20–110 × 109/l. Reticulocytes can be counted using automated machines employing flow cytometry and laser light after staining their RNA with fluorescent reagents such as acridine orange, thioflavin T, thiazol orange or auramine O. There are also automated methods in which the RNA is stained with supravital basic dyes and the extent of staining quantified using light absorbance or scatter. Results obtained by these automated methods are more reproducible than when counted by the traditional manual method as much larger numbers of reticulocytes are counted. The accepted reference range for reticulocytes in adults when counted by automated fluorescent-based methods is 20–120 × 109/l.9,10 The absolute reticulocyte count has also been shown to be higher in men than women. Reference values do depend on the method of measurement used and each laboratory should determine its own reference range. Semi-automated and fully automated discrete reticulocyte counters and some fully automated multiparameter hematology analyzers also provide various reticulocyte maturation parameters based primarily on the intensity of fluorescence (i.e. the amount of RNA), or, in the case of cells stained supravitally with a basic dye, on the extent of absorbance or scatter. These parameters include the immature reticulocyte fraction (immature reticulocytes have more RNA than mature ones), mean reticulocyte hemoglobin content and concentration and mean reticulocyte volume.11–12 Although these parameters have been shown to be of value in the assessment of certain clinical situations, they are not in regular use in clinical practice.
Granulocytes (polymorphonuclear leukocytes)
Neutrophil granulocytes
Morphology and composition
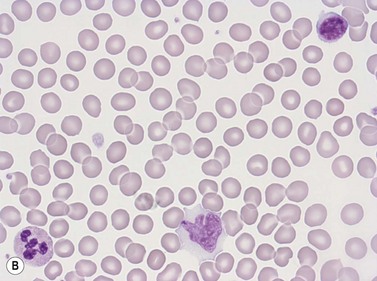
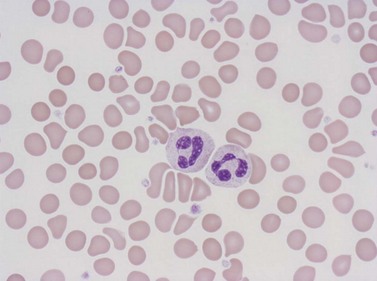
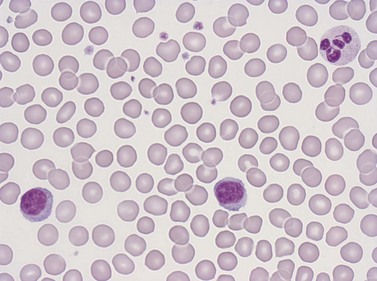
Neutrophil granulocytes have a mean volume of 500 fl and, in dried fixed smears, a diameter of 9–15 µm. Their cytoplasm is slightly acidophilic and contains many very fine granules that stain with neutral dyes; the granules stain a faint purple color with Romanowsky stains (Figs 1.1 and 1.3). The nucleus usually contains two to five nuclear segments; the percentages of neutrophils with two, three, four and five or more segments are 32, 45, 20 and 3%, respectively, with a mean of 2.9 segments. In the female up to 17% of neutrophls contain a drumstick-like appendage attached by a fine chromatin strand to one of the nuclear segments. These appendages correspond to Barr bodies (inactivated X-chromosomes). Neutrophils possess a variety of surface receptors including those for C3 and IgG-Fc and the CXC chemokine receptors.
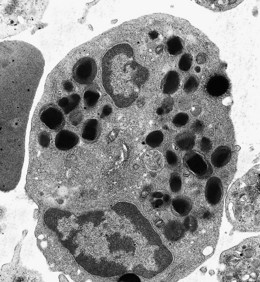
Neutrophils contain primary granules and specific (secondary) granules. Primary granules, first formed at the promyelocyte stage of differentiation, contain myeloperoxidase, lysozyme (muramidase), defensins, bacterial permeability inducer, acid phosphatase, β-glucuronidase, α-mannosidase, elastase, cathepsins B, D and G, and proteinase 3. On electron microscopy they are electron-dense, 0.5–1.0 µm in their long axis, and ellipsoidal in shape (Fig. 1.4). Specific granules are formed at the myelocyte (secondary granules) and metamyelocyte (tertiary granules) stages. They are less electron-dense and are very pleomorphic. Specific granules vary considerably in size, being frequently quite small (0.2–0.5 µm long), and the granule membrane contains NADPH oxidase (cytochrome b558), vitronectin and laminin receptors, formylpeptide receptors and CR3. The granule proteins include lysozyme, transcobalamin I (vitamin B12 binding protein), collagenase, β2 microglobulin, lactoferrin or lactoferrin and gelatinase, SGP28 (specific granule protein of 28 kDa), hCAP-18 (human cationic antimicrobial protein) and NGAL (a matrix protein). A third type of granule contains gelatinase but little or no lactoferrin (gelatinase granules) and neutrophils also contain secretory vesicles with molecules such as β2-integrins, formylpeptide receptors and CD14. The secretory vesicles are involved in adhesion of neutrophils to the endothelium, the gelatinase granules in migration through basement membrane and the primary and specific granules mainly in phagocytosis, killing and digestion of microorganisms.13–14 The alkaline phosphatase activity of neutrophils is present within membrane-bound intracytoplasmic vesicles called phosphosomes. In addition to the various organelles mentioned above, the cytoplasm contains a centrosome, a poorly developed Golgi apparatus, microtubules and microfilaments, a few small mitochondria, a few ribosomes, a little endoplasmic reticulum, occasional multivesicular bodies and numerous glycogen particles.
Number and life span
The number of neutrophil granulocytes in the peripheral venous blood of healthy Caucasians of different ages and genders are given in Tables 1.4 and 1.5. Healthy blacks have lower neutrophil counts than Caucasians (Table 1.4); Chinese and Indians have similar counts to those in Europeans.15 A single nucleotide polymorphism in the Duffy antigen receptor/chemokine gene is strongly associated with the neutropenia in Afro-Caribbeans and Africans but the mechanism by which this mutation causes neutropenia is not yet clear.16 Ethnic neutropenia has also been described in Yemenite Jews, Falashah Jews, black Bedouin and Jordanian Arabs.17 Considerably lower total leukocyte and neutrophil counts have been reported from East Africa than those shown in Table 1.4 for black Americans, and black West Indians and Africans living in England. However, the former studies have not allowed for the skewed distribution of leukocyte numbers in calculating reference ranges, and thus have exaggerated the difference between the black and Caucasian populations. Despite this, total white cell and neutrophil counts are probably genuinely lower in Africans living in African countries, particularly if taking an African diet, than in Africans living in Western countries.
Table 1.4 95% reference limits for the concentration of circulating leukocytes in peripheral venous blood of healthy adults15,48
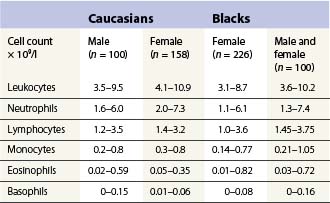
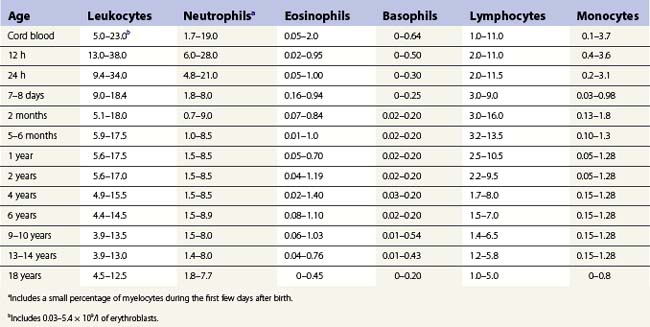
Table 1.5 Age-related ranges for the concentration of circulating white blood cells (× 109/l) in normal individuals.
Once formed, the mature neutrophil is retained in the bone marrow through interaction of stromal cell-derived CXCL12 with its receptor CXCR4 on neutrophils.18 After entering the blood by migration through the sinusoidal endothelium, neutrophil granulocytes leave the circulation in an exponential fashion with a T1/2 of 2.6–11.8 h (mean 7.2 h) and appear in normal secretions (saliva, secretions of the respiratory and gastrointestinal tracts and urine) and in various tissues. They probably survive outside the blood for up to 30 h.
Functions
Neutrophils are highly motile cells. They move towards, phagocytose and degrade various types of particulate material such as bacteria and damaged tissue cells. Neutrophils are attracted to sites of infection or inflammation as a result of chemotactic gradients generated around such sites. The chemotactic factors include activated complement components (C3a, C5a, C567), membrane phospholipids and other factors released from tissue cells, lymphokines released from activated lymphocytes, products of mononuclear phagocytes (e.g. tumor necrosis factor, IL-8), platelet-derived factors (platelet factor 4, the β-thromboglobulin neutrophil-activating peptide 2 (NAP-2), platelet-derived growth factor) and products of certain bacteria. IL-8, platelet factor 4 and NAP-2 bind to CXC chemokine receptors on the surface of neutrophils and activate these cells. Activated neutrophils adhere to endothelial cells via adhesion molecules on their cell membrane (Chapter 17). The arrival of neutrophils at sites of inflammation is probably facilitated by an increased permeability of adjoining blood vessels caused by activated complement components such as C3a and C5a.
The first stage in the phagocytosis of a particle such as a bacterium is the adherence of the neutrophil to the particle. The adherence is mediated through specific receptors on the neutrophil cell membrane: these include Fc (IgG1, IgG3) and C3 receptors. Both the adherence and the subsequent ingestion of such particles are enhanced by their interaction with opsonizing factors such as C3 generated via the classical or alternative complement activation pathway, antibody and mannose-binding lectin (Chapter 17). Following adhesion, pseudopodia form around the particle and progressively encircle it, probably via a zipper-like mechanism dependent on the interaction between receptors on the cell membrane and opsonizing factors present all over the particle. Both the movement of neutrophils towards a particle and the act of phagocytosis may be dependent on the activity of intracytoplasmic microfilaments composed of actin. The act of phagocytosis is associated with a burst of oxygen consumption (respiratory burst) and the production of hydrogen peroxide.
NADPH serves as the electron donor in the biochemical processes leading to the reduction of O2 to O2− and oxygen-dependent killing; the bactericidal agents derived from O2− include hydrogen peroxide, hydroxyl radicals, hypochlorite ions (generated from halides by hydrogen peroxide in the presence of the enzyme myeloperoxidase) and chloramines. The generation of O2− requires the membrane-associated enzyme known as the respiratory burst oxidase, the components of which only assemble when the neutrophil is activated by various stimuli, including the phagocytosis of opsonized bacteria. These components are cytochrome b558 (the electron transferring oxidase), three phosphoproteins (p40-phox, p47-phox and p67-phox) and two GTP-binding proteins (Rac2 and Rap1a) (see also Chapter 17).
Eosinophil granulocytes
Morphology and composition
Eosinophil granulocytes (eosinophils) have a diameter of 12–17 µm in fixed smears. Their cytoplasm is packed with large rounded granules which stain red-orange with Romanowsky stains (see Figs 1.1A and 1.5). The percentage of cells with one, two, three and four nuclear segments are 6, 68, 22 and 4%, respectively (mean 2.2). Eosinophils possess surface receptors for IgG-Fc (FcγRII, FcγRIII), IgE, IgA, IgM, C4, C3b, C3d, cytokines (GM-CSF, IL-3 and IL-5) and the CC chemokine receptor 3.19–20
There are two types of eosinophil granules: a few rounded homogeneously electron-dense granules and many rounded, elongated or oval crystalloid-containing granules (Fig. 1.6) (see also Chapter 2).21 Both homogeneous and crystalloid-containing granules contain an arginine- and zinc-rich basic protein, a peroxidase (distinct from neutrophil peroxidase) and acid phosphatase. Eosinophil granules also contain phospholipase B and D, histaminase, ribonuclease, β-glucuronidase, cathepsin and collagenase but not lysozyme. The eosinophil ribonucleases include eosinophil-derived neurotoxin (Rnase2) and eosinophil cationic protein (Rnase3). The Charcot–Leyden crystal protein, which has lysophospholipase activity and carbohydrate-binding properties, is found both in the cytosol and in some of the eosinophil granules.22
Number and life span
Table 1.4 gives the venous blood eosinophil counts for normal healthy adults. The eosinophil counts of healthy blacks and people from the Indian subcontinent do not differ from those of Caucasians.15 Eosinophils leave the circulation in a random manner with a T1/2 of about 4.5–8 h; they probably survive in the tissues for 8–12 days.
Functions
Eosinophils share several functions with neutrophils: both cell types are motile, respond to specific chemotactic agents and phagocytose and kill similar types of microorganisms.23–24 Eosinophils tend to be slower at ingesting and killing bacteria than neutrophils but appear to be metabolically more active than these cells. Eosinophil granule contents are transported to and discharged at the surface via large vesicular-tubular structures (piecemeal degranulation).24 Eosinophils also function as the effector cell (killer cell) in antibody-dependent damage to metazoal parasites. Eosinophils bind to IgG- and C3-coated helminths via their corresponding surface receptors and discharge their granule contents around the parasite. The killing of the parasite is caused by the eosinophilic cationic proteins which generate defects and pores in the cuticle and cell membrane and the major basic protein which is a potent toxin for helminths, as well as eosinophil peroxidase, which exerts its effect through the production of H2O2 and hypochlorous acid and superoxide. All three molecules are also toxic towards human tissues. The two eosinophil ribonucleases, Rnase2 and Rnase3, may be involved in defense against viruses.25 Not only the stimulation of FcγRII and complement receptors but also binding of IgA to IgA receptors triggers degranulation and the respiratory burst. Eosinophils also have a role in regulating immediate-type hypersensitivity reactions. In these reactions chemical mediators of anaphylaxis such as histamine and leukotriene C4 (LTC4) and LTB4 (a component of a mixture of small peptides known as eosinophil chemotactic factor of anaphylaxis or ECF-A) are released from mast cells and basophils as a result of the interaction between specific antigen and IgE on the surface of these cells. Eosinophils are attracted to the site of the activated mast cells or basophils by mast cell- and basophil-derived ECF-A, platelet-activating factor (PAF) and leukotriene B4 (LTB4) and by several other chemoattractants (chemokines) produced at sites of allergic inflammation; some of the chemokines are also activators of eosinophils. The most potent eosinophil chemoattractants are eotaxin (produced by macrophages and some other tissue cells), RANTES (released from thrombin-activated platelets), the 5-lipoxygenase product 5-oxo-6,8,11,14-eicosatetraenoic acid and monocyte chemoattractant protein 3 (MCP3) (released from endothelial and other cells).26 Eotaxin is a ligand of the CC chemokine receptor 3 (CCR3) that is expressed on eosinophils, basophils and TH2 lymphocytes. C5a, monocyte-derived LTB4 and PAF, and some lymphokines are also involved in attracting eosinophils. The attracted eosinophils then release prostaglandin E2 (PGE2) which inhibits further release of basophil- and mast-cell-derived mediators. Eosinophils also release specific enzymes that inactivate these mediators, including histaminase and phospholipase B and D which break down histamine and PAF, respectively. Eosinophil-derived arylsulphatase inactivates various chemotactic peptides and LTC4. Eosinophils may enhance hypersensitivity reactions by their phospholipase-A2-dependent synthesis and release of LTC4 and PAF, and also via the release of eosinophil-derived major basic protein, peroxidase and cationic proteins that activate basophils and mast cells and cause histamine release. Recent studies have shown that eosinophils also function as antigen-presenting cells, constitutively express TH1- and TH2-associated cytokines including IL-4, IL-13, IL-6, IL-10, IL-12, IFN-γ and TNF-α, differentially release these cytokines, and modulate the function of T cells (promoting either a TH1 or TH2 response) as well as of dendritic cells, B cells, mast cells, neutrophils and basophils.27,28
Basophil granulocytes
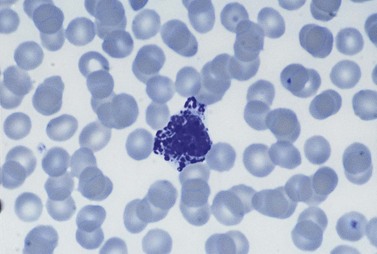
Basophil granulocytes (basophils) represent the most infrequent type of leukocyte in the blood (see Tables 1.4 and 1.5). In Romanowsky-stained blood smears, basophil granulocytes have an average diameter of about 12 µm and display large round purple-black cytoplasmic granules (Fig. 1.7), some of which overlie the nucleus. The granules stain metachromatically (i.e. reddish-violet) with toluidine blue or methylene blue. The nucleus usually has two segments, although these can be difficult to see by light microscopy. Basophils stain strongly by the periodic acid-Schiff (PAS) reaction (due to the presence of glycogen aggregates) and do not stain for acid or alkaline phosphatase. Basophil granules undergo varying degrees of extraction during processing for electron microscopy and characteristically show a particulate substructure with each particle measuring about 20 nm in diameter (Fig. 1.8).29 Basophils possess at their cell surface high-affinity receptors for IgE (FcεRI), low-affinity receptors for IgG (FcγRIIIB and FcγRII), receptors for C5a and histamine, and CC chemokine receptors (CCR3 and CCR2).30
Basophil granules contain histamine (which is synthesized by the cell), sulphated mucopolysaccharides (predominantly chondroitin sulphate), peroxidase, low levels of chymase (a serine protease) and negligible amounts of tryptase. The mucopolysaccharides account for the metachromatic staining of the granules. Basophils also contain Charcot–Leyden crystal protein and possibly PAF (which causes platelets to aggregate and release their contents) and eosinophil chemotactic factor of anaphylaxis (ECF-A).22
Functions
Basophils play a key role in immediate-type hypersensitivity reactions and in the immune response to helminthic infections.29–31 When IgE binds to FcεRI and the bound IgE reacts with specific antigen, basophils degranulate releasing histamine and chemotactic factors such as eosinophil chemotactic factor of anaphylaxis (ECF-A) and generate and release metabolites of arachidonic acid such as LTC4 (that stimulate secretion of mucus and contraction of smooth muscle) as well as cytokines, especially TNF-α, IL-4, IL-5 and IL-6. Basophils and mast cells may also be activated to release histamine by the binding of monocyte chemoattractant protein-1 (MCP-1) (produced by endothelial and other cells) to CCR2 on their surface and to a lesser degree the binding of ligands to FcγRIIIB and C5a receptors. Basophils also have FcγRIIB on their surface and stimulation via this receptor generates inhibitory signals. The release of histamine and other substances from basophils (and mast cells) is mediated via the transport of vesicles between the secretory granules and plasma membrane (piecemeal degranulation).32 The released histamine causes contraction of bronchial and gastrointestinal smooth muscle, inhibition of cytotoxic T-cell activity and lymphokine release, chemotactic attraction of other granulocytes, upregulation of C3b receptors on eosinophils and release of lysosomal enzymes from neutrophils. The accumulation of basophils at sites of hypersensitivity reactions is mediated by chemokines such as MCP-1 and eotaxins (produced by macrophages and some other cells such as fibroblasts, endothelial cells and epithelial cells). Eotaxin receptors (e.g. CCR3) are present not only on basophils but also on mast cells, eosinophils and T-helper type 2 cells (TH2 cells) which have IL-4-induced CCR3, leading to the attraction of eosinophils and TH2 cells to sites of allergic inflammation and parasitic infection. Basophils appear to be a major source of the immunomodulatory cytokine IL-4 in the body; mast cells do not produce this cytokine. Basophil-derived IL-4 may be important for the development of type 2 immunity via promotion of the development of TH2 cells and consequently antibody synthesis, particularly IgE synthesis, by B-cells.
Monocytes
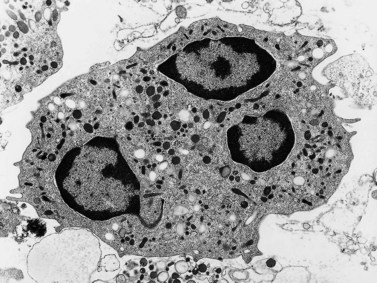
These are the largest leukocytes in peripheral blood. In stained smears, they vary considerably in diameter (15–30 µm) and in morphology (Figs 1.1 and 1.9). The nucleus is large and eccentric and may be rounded, kidney-shaped, horseshoe-shaped or lobulated. The nuclear chromatin has a skein-like or lacy appearance. The cytoplasm is plentiful, stains grayish-blue and contains few to many fine azurophilic granules. One or more intracytoplasmic vacuoles may be present. Cytochemical studies with the light microscope have shown the presence of many hydrolytic enzymes, including acid phosphatase, NaF-resistant esterase, galactosidases and lysozyme. Monocytes also contain defensins, myeloperoxidase, collagenase, elastase and coagulation system proteins (tissue factor, factors V, VII, IX, X and XIII, plasminogen activator) and have membrane receptors for IgG-Fc and C3. In addition, they have two CC chemokine receptors, CCR2 and CCR5, that bind various CC chemokines such as monocyte chemoattractant protein-1 (MCP-1), MCP-2, MCP-3, RANTES, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β.
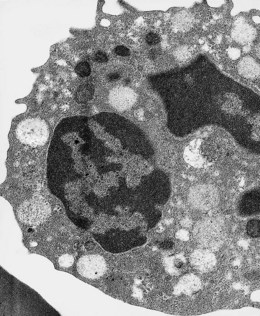
Under the electron microscope, monocyte granules vary considerably in size and shape and are relatively homogeneously electron-dense (Fig. 1.10). Some of the granules contain acid phosphatase and peroxidase. The peroxidase-positive granules are characteristically smaller than those of neutrophils. In thin sections, monocytes display finger-like projections of their cell membrane. Their cytoplasm contains appreciable amounts of rough endoplasmic reticulum, moderate numbers of dispersed ribosomes, a well-developed Golgi apparatus, several mitochondria and bundles of microfibrils. The nucleus has moderate quantities of heterochromatin and nucleoli are commonly seen by electron microscopy.
Blood monocytes are, like neutrophils, distributed between a circulating and a marginated pool; there are, on average, 3.6 times more marginated than circulating cells. The number of circulating monocytes in the peripheral venous blood of healthy adults is given in Table 1.4. Monocytes leave the circulation in an exponential manner, with an average T1/2 of 71 h. They then transform into macrophages in various tissues and may survive in this form for several months.
Lymphocytes
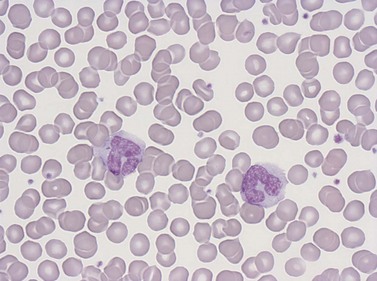
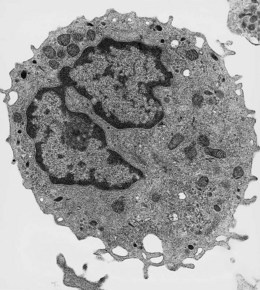
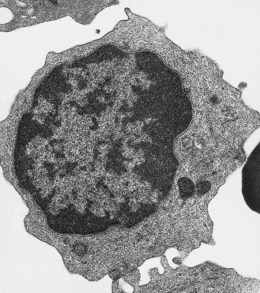
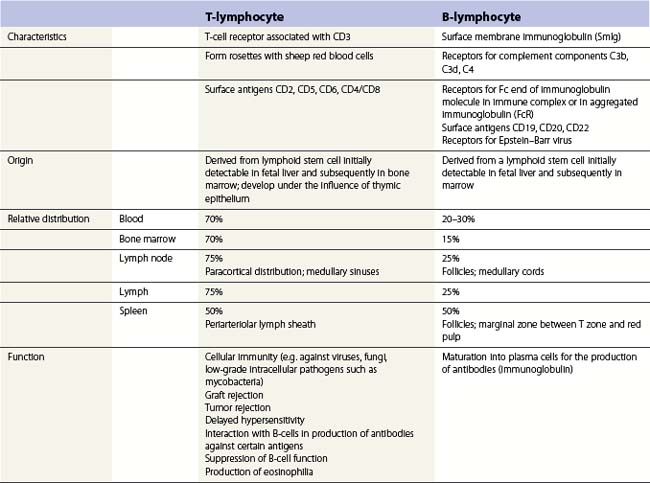
Lymphocytes have an average volume of approximately 180 fl and in stained smears have a diameter of 7–12 µm. Most of the lymphocytes in normal blood are small (Figs 1.1B and 1.11). In Romanowsky-stained smears, they have scanty bluish cytoplasm; the nucleus is round or slightly indented and there is considerable condensation of nuclear chromatin. The cytoplasm, which sometimes merely consists of a narrow rim around the nucleus, may contain a few azurophilic granules. Ultrastructural studies reveal that small lymphocytes contain a few scattered monoribosomes, an inactive Golgi apparatus, a few mitochondria, a few lysosomal granules and a small nucleolus (Fig. 1.12). About 10% of lymphocytes are large lymphocytes. These are about 12–16 µm in diameter and contain more cytoplasm and less condensed chromatin than small lymphocytes. In normal blood an occasional large lymphocyte has voluminous cytoplasm and several coarse azurophilic granules (large granular lymphocytes).
The concentration of lymphocytes in the blood is age-dependent: normal values are given in Tables 1.4 and 1.5. Lymphocytes leave the blood through endothelial cells of the postcapillary venules of lymphoid organs and eventually find their way back into lymphatic channels and re-enter the blood via the thoracic duct. The life span of lymphocytes varies considerably. The average life span in humans appears to be about 4 years but some cells survive for over 10 years.
Although most mature lymphocytes are morphologically similar to one another they can be divided into two major functionally distinct groups, B-lymphocytes (B-cells) and T-lymphocytes (T-cells).33 Some characteristics of these two types of cell, including their various functions, are summarized in Table 1.6. On the basis of the nature of the two disulfide-linked chains of the T-cell receptor (TcR), T-cells are divided into αβ-T-cells (with αβ-TcR) and γδ-T-cells (with γδ-TcR); most T-cells are αβ-T-cells. Four functionally different groups of αβ-T-cells exist, termed helper cells or TH cells, cytotoxic/suppressor T-cells or TC cells, T-regulatory cells or Treg cells and TH17 cells.34 TH cells are CD4-positive, recognize antigen and release lymphokines involved in promoting the functions of B-cells and the maturation of other kinds of T-cells including TC cells. TH cells are subdivided into TH1 cells and TH2 cells. When the TcR of TH1 cells reacts with antigen fragments on class II MHC molecules on dendritic cells, the antigen-presenting cells produce IL-12, IL-18 and IFN-γ which in turn stimulates TH1 cells to secrete the inflammatory cytokines TNF-β and IFN-γ. These cytokines activate macrophages, thus promoting the killing of intracellular pathogens such as Mycobacterium tuberculosis, and also attract leukocytes. When activated by antigen fragments on dendritic cells, TH2 cells produce cytokines such as IL-4 that affect growth and differentiation of B-cells (promoting the synthesis of antibody, including IgE), IL-13 that promotes IgE synthesis and recruits and activates basophils, and IL-5 that recruits and activates eosinophils. In this way they are involved in killing extracellular pathogens. After reaction with specific peptide antigens on class II MHC molecules, some CD4-positive T-cells may function directly as cytotoxic cells.35 TC cells are CD8-positive, inhibit the functions of other lymphocytes and also have cytotoxic capability against malignant or virus-infected cells. They react with and are activated by peptides presented with class I MHC molecules on the abnormal cell. Activated TC cells acquire azurophilic cytoplasmic granules (lysosomes) containing perforin that forms pores in the target cell membrane and granzymes (serine proteases) that enter the cell via the pores and mediate target cell apoptosis. They also express more Fas ligand on their surface; this reacts with Fas on the target cell surface resulting in apoptosis. When activated by peptides presented on class II MHC molecules, another subset of T-cells, the T-regulatory cells (Treg cells), produce IL-10 that inhibits TH1-mediated stimulatory effects on inflammation and TH2-mediated stimulatory effects on antibody synthesis; these effects operate towards the end of an immune response.36 A further subset of T-cells, TH17 cells, is located near the skin and mucosal surfaces and when activated these produce TGF-β and IL-21 and eventually IL-17.37 The antibacterial effects of TH17 cells are mediated via the secretion of defensins and the attraction of neutrophils to the site of inflammation. Lymphocytes that are neither B-cells nor T-cells also exist. These were originally called null cells but are now called NK (natural killer) cells; they have the appearance of large granular lymphocytes.38 NK cells lack antigen-specific receptors but have NK receptors that have an innate capacity to recognize virus-infected and tumor cells with low expression of class I MHC molecules, and kill such cells by exocytosis of perforin and granzymes. They also secrete the antiviral cytokine IFN-γ and the proinflammatory cytokine TNF-α.
TH lymphocytes regulate normal hemopoiesis including eosinophil granulocytopoiesis and erythropoiesis.39 Furthermore, abnormalities in T-cell subpopulations seem to play a role in the pathogenesis of the cytopenias in some cases of aplastic anemia, pure red cell aplasia associated with chronic lymphocytic leukemia and chronic idiopathic neutropenia (Chapters 17 and 28).
Platelets
Morphology and composition
Platelets are small fragments of megakaryocyte cytoplasm with an average volume of 7–8 fl.40,41 When seen in Romanowsky-stained blood smears, most platelets have a diameter of 2–3 µm. They have an irregular outline, stain light blue and contain a number of small azurophilic granules that are usually concentrated at the center (Fig. 1.1). Newly formed platelets are larger than more mature ones and have a higher mean platelet volume (MPV).
Electron microscope studies have revealed that non-activated (resting) platelets are shaped like biconvex discs, have a convoluted surface and contain mitochondria, granules, two systems of cytoplasmic membranes (a surface-connected canalicular system and a dense tubular system), microfilaments, microtubules and many glycogen molecules (Fig. 1.13). The discoid shape is actively maintained by a cytoskeleton consisting of many short contractile microfilaments composed of actomyosin and an equatorial bundle of microtubules composed of tubulin. The microfilaments are situated between various organelles and may be attached to specific proteins at the inner surface of the cell membrane. In addition to maintaining cell shape, the microfilaments are probably involved in clot retraction. The equatorial bundle of microtubules is situated in an organelle-free sol-gel zone just beneath the cell membrane and appears to be connected to this membrane by filaments. When platelets change shape during activation, the microtubules break their connections with the cell membrane and contract inwards; the platelet granules also become concentrated at the center of the cell. The cell membrane of the resting platelet is extensively invaginated to form a surface-connected open canalicular system. This canalicular system provides a large surface area through which various substances, including the contents of platelet granules, can be released to the exterior via multiple openings in the cell membrane. It is thought that the contraction of the microfilaments during platelet activation brings the platelet granules close to special areas of this canalicular system which are capable of fusing with granules. The contraction of microtubules may also play a role in this process. The platelet also contains a specialized form of endoplasmic reticulum known as the dense tubular system, elements of which are found adjacent to the bundle of microtubules and in between the invaginations of the open canalicular system. This system is the main site of synthesis of thromboxane A2 which plays an important role in the reactions leading to the release of the contents of platelet granules. In addition, the dense tubular system contains a high concentration of calcium ions when compared with that elsewhere in the cytoplasm and may regulate the activity of several calcium-dependent reversible cytoplasmic processes such as the activation of actomyosin, depolymerization of microtubules and glycogenolysis.
There are four types of platelet granules (Table 1.7):
Table 1.7 Characteristics of the four types of platelet granules
| Granule | Contents | Appearance |
|---|---|---|
| Dense bodies (δ granules) |
Serotonin, calcium, storage pool of ATP and ADP, pyrophosphate | Very dense, may have ‘bull’s eye’ appearance |
| α granulesa | β-thromboglobulin, platelet factor 4, platelet-derived growth factor, fibrinogen, fibronectin, factor-VIII-related antigen (vWF), thrombospondin | Less electron-dense than dense bodies |
| Lysosomal granulesa (λ granules) | Acid phosphatase, cathepsin, β-glucuronidase, β-galactosidase, arylsulphatase | Less electron-dense than dense bodies |
| Peroxisomes | Catalase | Smaller than α and λ granules |
a Distinguished from each other by ultrastructural cytochemistry.
Disorders of platelet granules are discussed in more detail in Chapter 32.
Number and life span
The normal range for the platelet count in peripheral blood is about 150–450 × 109/l (see Table 1.2); slightly lower values are seen during the first 3 months of life. Small cyclical variations in the platelet count may be seen in some individuals of both sexes, with a periodicity of 21–35 days; in premenopausal women the fall usually occurs during the 2 weeks preceding menstruation. The platelet counts of women are slightly higher than those of men.42,43 There are also slight racial variations in the normal platelet count. For example, values lower than those quoted above have been reported in Australians of Mediterranean descent. In addition, Nigerians have lower platelet counts than Caucasians, as have Africans and West Indians living in the UK.44 The life span of normal platelets is 8–10 days.
Functions
Platelets play an essential role in the hemostatic mechanism. When endothelial cells of vessel walls are damaged and shed, platelets adhere to subendothelial connective tissue (basement membrane and non-collagen microfibrils) via von Willebrand factor (vWF) attached to a specific receptor on the platelet membrane, glycoprotein Ib-IX. This adhesion requires calcium ions. Platelets may also adhere to collagen via other specific membrane receptors. Adhesion is followed within seconds by the transformation of the platelet from its original discoid shape to a spiny sphere (a potentially reversible process) and within a few minutes by the release of the contents of some platelet granules (the release reaction). The release reaction may be mediated through thromboxane A2 synthesized in the platelet from arachidonic acid released from membrane phospholipids (the conversion of arachidonic acid to thromboxane A2 requires the enzymes cyclo-oxygenase and thromboxane synthase). Initially, the contents of the dense bodies are released; with stronger stimulation, some α granules are also discharged. The ADP released from the dense bodies, and possibly also traces of thrombin generated by the activation of the clotting cascade, cause an interaction of other platelets with the adherent platelets and with each other (secondary platelet aggregation) with further release of ADP from the aggregating platelets. Aggregation induced by ADP (and by adrenaline and collagen) is preceded by an alteration of the cell membrane leading to calcium-dependent binding of fibrinogen to specific platelet receptors on membrane glycoprotein IIb–IIIa; the fibrinogen molecules link adjacent platelets. To a lesser extent, aggregation may also be mediated by binding of vWF and vitronectin to glycoprotein IIb–IIIa. In addition, platelet- and endothelial-cell-derived thrombospondin stabilize aggregation after binding to receptors on glycoprotein IV. The process of secondary aggregation continues until a platelet plug occludes the damaged vessel. The formation of a fibrin clot around the platelet plug is initiated by tissue factor (TF)-VIIa complex; the TF is expressed and factor VII is activated at the site of injury (Chapter 31). The exposure of certain membrane phospholipids (platelet factor 3) in aggregated platelets plays a role in the formation of this fibrin clot. These platelet phospholipids participate in:
Platelet function may be tested in vivo or in vitro. Platelet functions that may be investigated in vitro include adhesion, aggregation, clot retraction and contribution to the intrinsic coagulation pathway (also see Chapter 31). Both adhesion and aggregation may be tested by passing blood through a glass bead column and determining the percentage of retained platelets; this test is difficult to standardize. Automated equipment has been developed to test these linked functions using whole blood. Aggregation is most readily tested by the use of an aggregometer which measures optical density of platelet-rich plasma; as aggregation is induced (e.g. by ADP, adrenaline [epinephrine], collagen or the antibiotic ristocetin) the optical density falls; if platelets disaggregate the optical density rises again. It is also possible to measure ATP release during platelet aggregation. Clot retraction is assessed by measuring the volume of serum expressed by whole blood that is allowed to clot in a glass tube at 37°C for 1 h; a high hematocrit may interfere with clot retraction. The contribution of the platelet to the intrinsic pathway of blood coagulation may be tested by the prothrombin consumption test (which shows defective conversion of prothrombin to thrombin when there is a deficiency of platelet number or function) or the platelet factor 3 availability test or the thromboplastin generation test (which test for the ability of the platelet to accelerate the intrinsic pathway of coagulation). A number of machines are available to test platelet function and coagulation at the bedside (see Chapter 31). For example, the platelet function analyzer (PFA100) measures the time taken for whole blood to occlude ADP- or epinephrine-impregnated membranes. Thromboelastography is a global test for hemostasis that measures viscoelastic changes induced by fibrin polymerization and evaluates platelet function as well as the rate of formation of a clot, its strength, stability, retraction and lysis.
Alterations in the blood in pregnancy
In most women, the hemoglobin level begins to fall at about the 6th to 8th week of a normal pregnancy, reaches its lowest level at about the 32nd week and increases slightly thereafter. The extent of fall varies markedly from woman to woman but hemoglobin levels less than 10 g/dl are probably abnormal.45 The average fall is about 1.5–2 g/dl. This physiologic ‘anemia’ occurs despite an average increase in the red cell mass of about 300 ml and results from an increase in the plasma volume of about one liter. The reticulocyte count is initially unchanged but increases between week 25 and week 35. The mean corpuscular volume and MCH rise during pregnancy in the absence of any deficiency of vitamin B12 or folic acid. Serum iron falls. Transferrin synthesis increases due to a direct hormonal effect (similar changes are seen in subjects taking oral contraceptives); the transferrin concentration and total iron-binding capacity increase. The serum vitamin B12 level falls steadily throughout pregnancy reaching its lowest level at term; this is a physiological change and is not indicative of deficiency. About 10% of normal women have serum vitamin B12 levels below 100 ng/l during the last trimester. There is a return to non-pregnant levels by 6 weeks postpartum. Red cell and serum folate levels also fall and 20–30% of women have subnormal red cell folate levels at term. Physiologic needs for iron and folic acid are increased, and in subjects with reduced stores and/or poor intake (Chapters 11 and 12) deficiency may occur. The hemoglobin F level increases slightly. The percentage of F-cells is increased at mid-term but returns to non-pregnant levels by term. The erythrocyte sedimentation rate (ESR) rises early in pregnancy and is highest in the third trimester. The white cell count increases, due to an increase of neutrophils and monocytes. Total white blood cell counts (WBCs) of 10–15 × 109/l are common during pregnancy, and postpartum levels may reach 20–40 × 109/l. Metamyelocytes and myelocytes are seen in the blood in about a quarter of subjects and promyelocytes may also be present. ‘Toxic’ granulation and Döhle bodies (see Chapter 16) are common and are a physiologic change. The neutrophil alkaline phosphatase rises early in pregnancy and remains elevated; a further rise occurs during labor, with a return to non-pregnant levels by 6 weeks postpartum. The bactericidal capacity of neutrophils is increased and in 40–60% of subjects in the second and third trimester, an increased proportion of neutrophils are positive in the nitro-blue tetrazolium reduction test. Lymphocyte and eosinophil counts are decreased. The basophil count may rise. Some fall in the platelet count may occur in the third trimester and values in the range 80–150 × 109/l may be observed.46
A prothrombotic state develops during pregnancy. Throughout pregnancy, factors VII, VIIIC, VIIIR:Ag, X and fibrinogen increase progressively and markedly. Factors II and V are not significantly altered apart from a transient increase early in pregnancy and there is some increase in factor IX.46,47 Pregnancy is also associated with a marked increase in α1 antitrypsin, reduced protein S activity and with acquired activated protein C resistance. From 11–15 weeks onwards there is a marked decrease in fibrinolytic activity mainly due to large increases in endothelial cell-derived plasminogen activator inhibitor-1 (PAI-1) and placenta-derived plasminogen activator inhibitor-2 (PAI-2) in the plasma. Fibrin degradation products and D-dimer increase after 21–25 weeks in a proportion of subjects.
Fetal cells, for example fetal red cells and fetal lymphocytes, enter the maternal circulation during pregnancy as well as at delivery. This phenomenon is common enough to be regarded as physiologic, although it may have adverse effects when the mother becomes sensitized to fetal antigens (see Chapters 10 and 37).
1 Lewis S, Bain B, Bates I, editors. Dacie & Lewis Practical Haematology, 10th ed, Philadelphia: Elsevier, 2006.
2 Arese P, Turrini F, Schwarzer E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell Physiol Biochem. 2005;16(4–6):133-146.
3 Pantaleo A, Giribaldi G, Mannu F, et al. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimmun Rev. 2008 Jun;7(6):457-462.
4 Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998 Mar 17;95(6):3077-3081.
5 Bosman GJ, Willekens FL, Werre JM. Erythrocyte aging: a more than superficial resemblance to apoptosis? Cell Physiol Biochem. 2005;16(1–3):1-8.
6 Marchesi VT. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1-11.
7 Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006 Apr 1;107(7):2943-2951.
8 Ozuyaman B, Grau M, Kelm M, et al. RBC NOS: regulatory mechanisms and therapeutic aspects. Trends Mol Med. 2008 Jul;14(7):314-322.
9 Bowen D, Bentley N, Hoy T, Cavill I. Comparison of a modified thiazole orange technique with a fully automated analyser for reticulocyte counting. J Clin Pathol. 1991 Feb;44(2):130-133.
10 Tarallo P, Humbert JC, Mahassen P, et al. Reticulocytes: biological variations and reference limits. Eur J Haematol. 1994 Jul;53(1):11-15.
11 D’Onofrio G, Zini G, Rowan RM. Reticulocyte counting: methods and clinical applications. In: Rowan RM, van Assendelft OW, Preston FE, editors. Advanced Laboratory Methods in Haematology. London: Arnold, 2002.
12 Kim JM, Ihm CH, Kim HJ. Evaluation of reticulocyte haemoglobin content as marker of iron deficiency and predictor of response to intravenous iron in haemodialysis patients. Int J Lab Hematol. 2008 Feb;30(1):46-52.
13 Borregaard N. Development of neutrophil granule diversity. Ann N Y Acad Sci. 1997 Dec 15;832:62-68.
14 Borregaard N, Lollike K, Kjeldsen L, et al. Human neutrophil granules and secretory vesicles. European Journal of Haematology. 1993;51:187-198.
15 Bain B, Seed M, Godsland I. Normal values for peripheral blood white cell counts in women of four different ethnic origins. J Clin Pathol. 1984 Feb;37(2):188-193.
16 Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 5(1), 2009 Jan. e1000360
17 Shoenfeld Y, Alkan ML, Asaly A, et al. Benign familial leukopenia and neutropenia in different ethnic groups. Eur J Haematol. 1988 Sep;41(3):273-277.
18 von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008 Oct 15;181(8):5183-5188.
19 Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol. 2000;76:45-62.
20 Anwar AR, Kay AB. Membrane receptors for IgG and complement (C4, C3b and C3d) on human eosinophils and neutrophils and their relation to eosinophilia. J Immunol. 1977 Sep;119(3):976-982.
21 Dvorak AM, Weller PF. Ultrastructural analysis of human eosinophils. Chem Immunol. 2000;76:1-28.
22 Calafat J, Janssen H, Knol EF, et al. Ultrastructural localization of Charcot–Leyden crystal protein in human eosinophils and basophils. Eur J Haematol. 1997 Jan;58(1):56-66.
23 Butterworth AE, David JR. Eosinophil function. N Engl J Med. 1981 Jan 15;304(3):154-156.
24 Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147-174.
25 Rosenberg HF. Eosinophil-derived neurotoxin/RNase 2: connecting the past, the present and the future. Curr Pharm Biotechnol. 2008 Jun;9(3):135-140.
26 Powell WS, Ahmed S, Gravel S, Rokach J. Eotaxin and RANTES enhance 5-oxo-6,8,11,14-eicosatetraenoic acid-induced eosinophil chemotaxis. J Allergy Clin Immunol. 2001 Feb;107(2):272-278.
27 Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008 Aug;38(8):1254-1263.
28 Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009 Jan;85(1):117-123.
29 Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol Allergy. 2006;90:141-156.
30 Sullivan BM, Locksley RM. Basophils: a nonredundant contributor to host immunity. Immunity. 2009 Jan 16;30(1):12-20.
31 Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009 Mar 19;113(12):2816-2825.
32 Min B, Paul WE. Basophils and type 2 immunity. Curr Opin Hematol. 2008 Jan;15(1):59-63.
33 Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287-320.
34 Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006 Nov;36(11):1357-1366.
35 van de Berg PJ, van Leeuwen EM, ten Berge IJ, van Lier R. Cytotoxic human CD4(+) T cells. Curr Opin Immunol. 2008 Jun;20(3):339-343.
36 Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008 May;7(5):370-375.
37 Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41(2):87-102.
38 Andoniou CE, Coudert JD, Degli-Esposti MA. Killers and beyond: NK-cell-mediated control of immune responses. Eur J Immunol. 2008 Nov;38(11):2938-2942.
39 Dent AL, Kaplan MH. T cell regulation of hematopoiesis. Front Biosci. 2008;13:6229-6236.
40 White JG. Platelet structural physiology: the ultrastructure of adhesion, secretion, and aggregation in arterial thrombosis. Cardiovasc Clin. 1987;18(1):13-33.
41 Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008 Feb 1;111(3):981-986.
42 Stevens RF, Alexander MK. A sex difference in the platelet count. Br J Haematol. 1977 Oct;37(2):295-300.
43 Bain BJ. Platelet count and platelet size in males and females. Scand J Haematol. 1985 Jul;35(1):77-79.
44 Bain BJ, Seed M. Platelet count and platelet size in healthy Africans and West Indians. Clin Lab Haematol. 1986;8(1):43-48.
45 Perry D, Lowndes K. Blood disorders specific to pregnancy. Warrell D, Cox T, Firth J, editors. 3rd ed. Oxford Textbook of Medicine: Oxford University Press; 2010. 2173–80
46 Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003 Apr;29(2):125-130.
47 Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114(5–6):409-414.
48 Bain BJ, editor. Blood cells. A practical guide, 4th ed, Oxford: Blackwell Publishing, 2006.