Chapter 8 Nonmotor problems in Parkinson disease
Introduction
While the motor symptoms of Parkinson disease (PD) dominate the clinical picture – and even define the parkinsonian syndrome – many patients with PD have other complaints that have been classified as nonmotor (Chaudhuri et al., 2006a), and a scale has been developed to quantify them (Chaudhuri et al., 2006b). Also, there is an ongoing modification process to update the Unified Parkinson’s Disease Rating Scale (UPDRS) to include more nonmotor features of PD (Goetz et al., 2007). These include fatigue, depression, anxiety, sleep disturbances, constipation, bladder and other autonomic disturbances (sexual, gastrointestinal), and sensory complaints. Sensory symptoms, including pain, may occur. Orthostatic hypotension can lead to syncope. Behavioral and mental alterations include changes in mood, lack of motivation or apathy, slowness in thinking (bradyphrenia), and a declining cognitive capacity; and these are frequent causes for concern. In one survey of nondemented PD patients, nonmotor symptoms (NMS) were found to occur in the majority of patients (Table 8.1). Genetic as well as sporadic forms of PD have NMS (Kasten et al., 2010).There is even the suggestion that presentation of nonmotor symptoms that commonly occur in PD patients but without any of the cardinal signs of PD may be considered part of the same disease spectrum (Langston, 2006). Lang (2011) uses the term premotor for nonmotor symptoms that are part of PD and precede the motor symptoms. This is based on the Braak hypothesis discussed in Chapter 5. Braak and his colleagues are now estimating that the disease (pathology of Lewy neurites) begins in the olfactory and autonomic system by approximately 20 years before the onset of the motor symptoms of PD, and that many nonmotor symptoms appear (Hawkes et al., 2010).
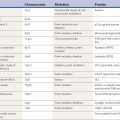
Table 8.1 Frequency of nonmotor symptoms in Parkinson disease
| Symptom | Frequency |
|---|---|
| Depression | 36% |
| Anxiety | 33% |
| Fatigue | 40% |
| Sleep disturbances | 47% |
| Sensory symptoms | 63% |
| No nonmotor symptoms | 12% |
Data from Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord 2001;16:507–510.
A large collaborative Italian study of more 1072 patients with PD found that 98.6% of patients with PD reported the presence of NMS (Barone et al., 2009). The most common were: fatigue (58%), anxiety (56%), leg pain (38%), insomnia (37%), urinary urgency and nocturia (35%), drooling of saliva (31%), and difficulties in maintaining concentration (31%). The mean number of NMS per patient was 7.8 (range, 0–32). NMS in the psychiatric domain were the most frequent (67%). Frequency of NMS increased along with the disease duration and severity.
Always ask the patient what problems bother him/her; many times a nonmotor symptom is the most troublesome. Helping patients with PD to cope with these difficulties is just as important as manipulating therapy to provide control of their motor symptoms. In addition, antiparkinsonian drugs commonly induce unwanted nonmotor effects and aggravate such complaints. And so-called sensory “offs” are often underappreciated but are usually a greater source of discomfort than are motor “offs.” In this chapter, we cover nonmotor problems inherent to the disease and also those induced by medications to treat the disease. Nonmotor symptoms can become disabling with the increasing duration of the disease (see Tables 6.7 and Table 6.8).
Nonmotor symptoms not uncommonly can be the presenting complaint in PD. In pathologically proven PD 91/433 (21%) of patients presented with nonmotor symptoms, of which the most frequent were pain (53%), urinary dysfunction (16.5%), and anxiety, or depression (12%) (O’Sullivan et al., 2008). Presenting with nonmotor symptoms was associated with a delayed diagnosis of PD. These patients were more likely to be misdiagnosed initially and were more likely to have been referred to orthopedic surgeons or rheumatologists than neurologists. Comparing newly diagnosed patients with a control population, Miller and colleagues (2011) found a statistically significant higher number of autonomic and sensory symptoms in the PD group, especially olfaction, urinary, drooling, constipation, and sensory complaints.
With continuing PD, nonmotor symptoms become more common and can become the major troublesome symptom (Hely et al., 2005, 2008). Also medications to treat the motor symptoms of PD can cause nonmotor symptoms, including impulse control problems, confusion, hallucinations, and paranoid psychosis. Behavioral, mood, and cognitive problems can develop as complications of surgery for PD, such as deep brain stimulation (Voon et al., 2006c), and these are covered in Chapter 7.
Nonmotor symptoms as part of PD fit the pattern of the location of Lewy neurites described by Braak and his colleagues (see review by Braak et al., 2006), and presented in detail in Chapter 6. The Braak hypothesis as an early feature of PD is not uniformly accepted (Burke et al., 2008). As more parkinsonologists are focusing on nonmotor symptoms, there are more publications. Three recent reviews on this topic are recommended: Simuni and Sethi (2008), Lim et al. (2009), Chaudhuri and Schapira (2009), Lang (2011).
Two prominent risk factors for mortality in PD are the nonmotor symptoms of psychosis and dementia (Forsaa et al., 2010).
Sensory symptoms
Pain
Many textbooks do not list pain and the other sensory complaints in Table 8.2 as a part of PD, and they are often not considered symptoms of the disease, but they can be (Snider et al., 1976; Koller, 1984; Goetz et al., 1986; Quinn et al., 1986; Ford et al., 1996; Ford, 2010). A constant, boring pain in the initially affected limb may be the first complaint. Aching in the shoulder and arm is a common earlier symptom in PD and is often incorrectly attributed to bursitis or a frozen shoulder. When pain occurs in the hip or leg, it is often attributed to arthritis, whereas this could be a symptom of PD. That such pain is due to the PD is indicated by its relief with antiparkinsonian medication. Once adequate dosing is achieved, whether or not mobility is restored, such pain commonly abates. Of course, patients with PD may also have coincidental joint disease, so if the pain persists, patients require appropriate investigation.
Another initial complaint, particularly in younger patients, may be painful dystonic foot cramps, especially on walking. Rarely, similar painful cramp may occur in the hands. An extended big toe or curling of small toes may be seen with the cramping. In recent surveys, about two-thirds of patients experience chronic pain (Defazio et al., 2008; Nègre-Pagès et al., 2008).
When patients with PD develop fluctuations and dyskinesias, pain may become a major feature. “Off-period” dystonia often is painful. This may manifest as early morning painful cramps, particularly affecting the feet (Melamed, 1979). Similar painful dystonic cramps may emerge during “off” periods during the day, and can be very distressing (Ilson et al., 1984). Some patients may experience more generalized excruciating pain during “off” periods, often a deep-seated aching, but sometimes with a more superficial burning quality. Again, such pains disappear when the patient is switched “on” by appropriate medication to regain mobility. “Off-period” pain may be an indication for the use of rapidly-acting water-soluble preparations of levodopa or apomorphine rescue injections.
Burning, numbness, and paresthesia
Other specific sensory symptoms, such as burning, numbness, and paresthesia, are less common in PD. However, some patients may describe rather nonspecific paresthesia in the affected limbs, but objective sensory signs are not evident (Snider et al., 1976; Koller, 1984). A rare patient may have sensory complaints from levodopa therapy, unaccompanied by dystonia. Electroconvulsive therapy (ECT) can be effective in alleviating the problem. If parkinsonian pain occurs during an “off” or due to parkinsonism, one should increase medications to avoid “offs.” (Sensory “offs” are considered later in this chapter.) If pain occurs during peak-dose dystonia, one needs to lower the dose. If pain is secondary to levodopa or dopamine agonists, one needs to reduce or eliminate the causal agent. Occasionally, the ergot dopamine agonists, bromocriptine and pergolide, cause a burning pain with inflammatory skin on parts of the body, known as St Anthony’s fire. If this occurs, the agonist needs to be discontinued.
Akathisia
A more common sensory symptom is akathisia or a sense of inner restlessness. This sometimes is focused on the legs with uncomfortable paresthesias and the need to move them to gain relief, in which case it may be termed a true restless legs syndrome (Lang, 1987). More often, there is a sense of generalized inner restless discomfort, demanding walking for relief, when akathisia is the more appropriate description (Lang and Johnson, 1987). Akathisia may be a presenting feature of PD. The symptoms of both restless legs and akathisia may respond to dopamine replacement therapy. Akathisia may also occur during the “off” period (Lang, 1994). It may be difficult for the patient and the clinician to distinguish between akathisia and restless legs syndrome in some patients.
Akathisia probably occurs more often in PD than is commonly recognized. It can be a sensory complaint of the disease itself and also an adverse effect from levodopa. Lang and Johnson (1987) asked patients with PD specifically for complaints of restlessness and found that 86% did have this subjective complaint. Most patients with PD who complained of an inner feeling of restlessness did not overtly manifest any signs such as moving about. From Lang and Johnson’s study it was not clear whether akathisia represented an adverse effect from levodopa or was a feature of the disease. In most of their patients it appeared only after the introduction of antiparkinsonian drugs, but a small number had this symptom early in the course of PD, prior to receiving any medication. It is likely that levodopa and other antiparkinsonian agents may also contribute to this complaint, for it has occurred in patients with primary torsion dystonia after starting these drugs.
Restless legs syndrome
Restless legs syndrome (RLS) is encountered fairly often in patients with PD. In one study, 24% of patients with PD had RLS (Peralta et al., 2009). The symptoms are described in Chapter 23. Briefly, it consists of unpleasant crawling sensations in the legs, particularly when sitting and relaxing in the evening, and disappears on walking. Whether RLS is an epiphenomenon of PD, because both respond to dopaminergics, is not clear. Like PD, dopamine transporter binding is reduced in the striatum in sporadic RLS (Early et al., 2011). Sporadic and familial RLS respond to dopamine agonists and levodopa, but these drugs can cause augmentation, a worsening of the restless legs symptoms – more severe unpleasant sensations, occurring earlier in the day, and spread to involve other body parts. This raises the possibility that some cases of RLS in patients with PD may be the result of dopaminergic medications used to treat PD. Fortunately, opioids are effective in treating RLS and periodic movements in sleep, whether in patients with PD or in sporadic and familial RLS (Hening et al., 1986; Kavey et al., 1988), and these can be used safely in PD. Propoxyphene 65 mg late in the day before the onset of symptoms is usually effective. Start with a half-tablet, and titrate up to two tablets if necessary. Other opioids like oxycodone (Walters et al., 1993), tramadol (Lauerma and Markkula, 1999) and methadone (Silver et al., 2011) are effective without the augmentation problem.
Hyposmia
Decreased sense of smell is not a complaint that patients usually make, but if olfaction is tested, decreased olfaction is detected in most patients with PD. In one study, 45% of patients were functionally anosmic, 51.7% were hyposmic, and only 3.3% were normosmic (Haehner et al., 2009). This indicates that 96.7% of PD patients present with significant olfactory loss when compared to young normosmic subjects. This figure falls to 74.5%, however, when adjusted to age-related norms.
Hyposmsia often precedes the onset of motor symptoms (Ponsen et al., 2004; Haehner et al., 2007), and is now being studied to determine if it can predict future PD. Hyposmia is more predictive than is executive dysfunction (Ponsen et al., 2009). One study indicates that it can predict PD in men up to 4 years before onset of motor features (Ross et al., 2008), and an autopsy study showed that those with the greatest reduction of smell were more likely to have incidental Lewy bodies at autopsy (Ross et al., 2006). One problem as a predictive test is that hyposmia is not selective; it is decreased in other neurodegenerative disorders, including corticobasal degeneration (Pardini et al., 2009). Hyposmia has been associated with striatal dopamine deficiency (Wong et al., 2010), but showed a better correlation with decreased cholinergic activity in the cortical and limbic areas (Bohnen et al., 2010).
Autonomic dysfunctions: bladder and sexual problems
The listing in Table 8.1 does not include autonomic symptoms, but PD patients also complain more about these, such as gastrointestinal, urinary, cardiovascular, thermoregulatory, and sexual dysfunction, than a control population, with the greatest differences in the gastrointestinal and urinary domain (Verbaan et al., 2008). These symptoms were found to increase with age, disease severity, and medication use.
The autonomic dysfunctions in patients with PD can be segregated into urogenital problems and those that affect other functions, such as blood pressure, the gastrointestinal tract, and skin (Table 8.3). In this section, we discuss bladder problems in patients with PD.
The prevalence of bladder symptoms in PD is high; the most common complaint is nocturia followed by frequency and urgency (Fitzmaurice et al., 1985; Winge and Fowler, 2006). Of course PD patients are usually of an age at which prostatic problems in the male and stress incontinence in the female can occur anyway. But PD itself affects bladder control, owing to detrusor hyperreflexia. As a result, premature uninhibited bladder contractions cause frequency and urgency, which can be particularly troublesome at night and during “off” periods. Araki and Kuno (2000) assessed voiding dysfunction in 203 consecutive PD patients and found that 27% had symptomatic voiding dysfunction. Its severity correlated with the severity of PD and not with disease duration, age, or gender.
Prostatic outflow obstruction can add to the problem in the male. Incontinence not explained by immobility when taken by the urge to micturate or by retention with overflow is not, however, a part of PD. True neurogenic incontinence in someone with parkinsonism suggests a diagnosis of multiple system atrophy (MSA) (Stocchi et al., 1997). In this case, sphincter electromyographic studies usually reveal signs of denervation due to involvement of Onuf’s nucleus in the sacral spinal cord, which does not occur in PD.
Impotence in the male patient with PD causes distress to both partners (as do immobility and other problems in the female). PD itself does not normally cause impotence, although this is a common early complaint in MSA. Loss of libido and failure to gain or sustain erections may have some other cause in this age group, be it psychological, vascular, hormonal or neurogenic, and appropriate investigation is warranted. Some antidepressant drugs, monoamine oxidase inhibitors, and antihypertensive medications can impair sexual performance. Failure of erection can be overcome by a variety of intrapenile or oral medications such as sildenafil (Viagra) (Zesiewicz et al., 2000). Sildenafil can be efficacious in the treatment of erectile dysfunction in both PD and MSA; however, it can unmask or exacerbate hypotension in MSA (Hussain et al., 2001). Parkinsonian symptoms are not affected, but a side benefit of reduced dyskinesias has been reported (Swope, 2000). Hypersexuality, particularly in the male, is a rare and unacceptable side effect of dopamine replacement therapy in PD, both levodopa and dopamine agonists, and usually requires reduction of antiparkinsonian medication.
Levodopa itself can affect the bladder (Brusa et al., 2007). In dopa-naive PD patients challenged with carbidopa/levodopa 50/200 mg, bladder overactivity (neurogenic overactive detrusor contractions) threshold and bladder capacity significantly worsened (32% and 22% of worsening, respectively). But when the same patients were rechallanged after being on levodopa therapy for 2 months, there was improvement of bladder function. Compared to the values obtained earlier, bladder activity and capacity improved 93% and 33%, respectively. Furthermore the sensation of bladder filling had a 120% improvement.
Other autonomic symptoms
Lewy body degeneration affects the autonomic nervous system in PD. Both sympathetic ganglion neurons and parasympathetic myenteric and cardiac plexi can be involved (Qualman et al., 1984; Kupsky et al., 1987; Wakabayashi et al., 1988). The postganglionic sympathetic nerves to the heart degenerate early and in a centripetal manner, with synuclein accumulation, not only in PD but also in persons with incidental Lewy bodies (Orimo et al., 2008). The loss of these sympathetic neurons is reflected in the reduced cardiac uptake of 123I-meta-iodobenzylguanidine (MIBG), a physiologic analog of norepinephrine, in patients with PD and dementia with Lewy bodies (Oka et al., 2007a). In contrast, postganglionic sympathetic fibers remain intact in MSA, and so MIBG uptake is normal in MSA. Central autonomic nuclei, such as those of the hypothalamus and dorsal motor nucleus of the vagus, can also be affected in Lewy body degeneration (Eadie, 1963).
Orthostasis: Control of blood pressure may be compromised by sympathetic failure with impaired vasoconstriction and inadequate intravascular volume. Faintness on standing (pre-syncope) and frank loss of consciousness on standing (postural syncope) can occur owing to orthostatic hypotension (OH). OH can also cause posturally induced fatigue and weakness, blurring of vision, and “coat-hanger” neck and shoulder aching. Hypotension also may occur postprandially due to gastrointestinal vasodilatation. Levodopa, dopamine agonists, and selegiline (Churchyard et al., 1999) may aggravate postural hypotension. Oka and colleagues (2007b) compared PD patients with and without OH and found a greater association with male gender, older age, longer disease duration, posture and gait instability phenotype, low Mini-Mental State Examination (MMSE) scores, and visual hallucinations. Cardiac 123I-MIBG uptakes were lower in patients with OH.
Prominent early symptoms of postural hypotension are, of course, one of the hallmarks of MSA, so such complaints may raise concern over the diagnosis of PD. The severity of postural hypotension in PD rarely is as severe as that seen in MSA. Nevertheless, treatment might be required. A selective peripheral dopamine antagonist such as domperidone sometimes helps, as does increasing fluid and salt intake, with head-up tilt at night which reduces nocturnal polyuria. Intranasal DDAVP (desmopressin) (5–40 µg) at night also reduces nocturnal polyuria, but can cause hyponatremia. However, a small dose of fludrocortisone (0.1–0.5 mg) (to promote salt retention), or midodrine (ProAmatine) (a selective α-agonist) (2.5–5 mg three times a day), might be required to maintain adequate blood pressure. Pyridostigmine was found to improve orthostatic hypotension, probably due to enhanced sympathetic ganglionic neurotransmission and a vagal shift in cardiac sympathovagal balance (Singer et al., 2006).
Gastrointestinal problems cause significant disability in PD (Edwards et al., 1991, 1992). Dysphagia is due mainly to poor masticatory and oropharyngeal muscular control making it difficult to chew and propel the bolus of food into the pharynx and esophagus (Bushman et al., 1989; Edwards et al., 1994). Soft food is easier to eat, and antiparkinsonian medication improves swallowing.
Parasympathetic failure may contribute to gastrointestinal problems in PD, causing delay in esophageal and gastric motility. A sense of bloating, indigestion, and gastric reflux are common in PD (Edwards et al., 1992). Many factors contribute to delayed gastric emptying, including immobility, parasympathetic failure, constipation, and antiparkinsonian drugs (both anticholinergic and dopamine agonists). Levodopa is absorbed in the upper small bowel, so gastric stasis may slow or prevent levodopa assimilation, leading to “delayed-ons” and “no-ons” (dose failures) after single oral doses (either there is an excessive interval before the drug works, or it does not work at all).
Constipation is another frequent complaint in PD (Edwards et al., 1992, 1994; Kaye et al., 2006), and is multifactorial. Again, immobility, drugs, reduced fluid and food intake, and parasympathetic involvement prolonging colonic transit time may all contribute. In addition, malfunction of the striated muscles of the pelvic floor due to the PD itself can make evacuation of the bowels difficult (Mathers et al., 1988, 1989). Constipation may exacerbate gastric stasis. Anticholinergic drugs should be stopped and physical exercise should be increased. The role of levodopa in causing or treating constipation is uncertain. This drug usually does not relieve the problem, and some patients believe that it worsens the problem. Constipation is ameliorated by adequate fluid intake, fruit, vegetables, fiber, and lactulose (10–20 g/day) or other mild laxatives. The following “rancho recipe” provided by Dr Cheryl Waters has been found useful for many patients: Mix together one cup each of bran, applesauce, and prune juice; take two tablespoons every morning; the mixture can be refrigerated for one week, then should be discarded. Polyethylene glycol powder (marketed as MiraLax) can be effective to overcome constipation; the usual dose is 17 g/day dissolved in a glass of water at bedtime. Refractory constipation may be helped by apomorphine injections to assist defecation (Edwards et al., 1993; Merello and Leiguarda, 1994).
Excessive sweating can be a problem, particularly in the form of sudden drenching sweats (sweating crises). These seem to occur as part of an “off” phenomenon (Sage and Mark, 1995; Swinn et al., 2003; Pursiainen et al., 2007). Sweating can cause physical, social, and emotional impairment.
Excessive salivation (sialorrhea) is due more to failure to swallow saliva frequently than to overproduction (Bateson et al., 1973). Drooling of saliva can be helped by chewing gum (which also helps those with dry mouth) or by using peripherally-acting anticholinergic drugs, which are quaternary ammonium compounds that do not cross the blood–brain barrier. Two such compounds are glycopyrrolate and propantheline. The former was tested in a controlled clinical trial and found to be effective and safe therapy for sialorrhea in PD (Arbouw et al., 2010). If these are unsuccessful, intraparotid injections of botulinum toxin B can sometimes be effective in reducing salivary secretions and drooling (Lipp et al., 2003; Racette et al., 2003; Ondo et al., 2004). Chewing gum has also been found useful to increase swallow frequency, and it decreases latency of swallowing in PD (South et al., 2010), which are common problems in advanced PD and contribute to weight loss in PD.
Rhinorrhea is not infrequent in patients with PD and has been reported to occur in almost 50% (Friedman et al., 2008). Patients with PD with rhinorrhea were older and had a higher Hoehn and Yahr stage. Duration of disease was not different between those with and without rhinorrhea. Most patients with rhinorrhea reported that it worsened with eating.
Respiratory distress
Respiratory distress such as dyspnea can occur as a symptom of PD in some patients, including during the “off” period in some (Ilson et al., 1983). It can also occur as a complication of dystonia, usually peak-dose dystonia (Braun et al., 1983), and with some dopamine agonists, particularly pergolide. Removing the offending drug is required. “Off” period dyspnea is difficult to treat, other than attempting to keep the patient “on.” Despite the sensation of dyspnea, oxygen saturation is not affected because the patient will have a voluntary sigh or transient deep breathing when feeling short of breath. Some forms of parkinsonism-plus syndromes may have an accompanying apnea that is life-threatening, such as postencephalitic parkinsonism (Strieder et al., 1967; Efthimiou et al., 1987), frontotemporal dementia (Lynch et al., 1994), MSA (Chester et al., 1988; Salazar-Grueso et al., 1988), Joseph disease (SCA3) (Kitamura et al., 1989), and other familial parkinsonian syndromes (Perry et al., 1990).
Difficulties at night and daytime sleepiness
Table 8.4 lists some of the sleep problems seen in PD. Patients often have troubled nights for many reasons (Factor et al., 1990; Askenasy, 1993; Van Hilten et al., 1994; Bliwise et al., 1995). The most common problem is difficulty with sleep maintenance (so-called sleep fragmentation). Frequent awakenings may be caused by tremor reappearing in the lighter stages of sleep, difficulty in turning in bed due to nocturnal akinesia as the effects of daytime administration of dopaminergic drugs wear off at night, and nocturia. In addition, periodic leg movements in sleep (sometimes associated with restless legs), fragmentary nocturnal myoclonus, sleep apnea, REM sleep behavioral disorders (intense dream-like motor and behavioral problems), and parasomnias (nocturnal hallucinations and nocturnal wandering with disruptive behavior) may all disrupt sleep in PD. Reversal of sleep rhythm with sundowning also is common in PD (Bliwise et al., 1995). Many of these conditions probably occur more frequently in PD than in other aged populations. As a result, PD patients and their caregivers face disrupted nights, which lead to poor quality of life and worse parkinsonism the next day. In fact, along with depression, poor sleep is a major factor in a PD patient’s assessment of having a poor quality of life (QoL) (Karlsen et al., 1999b). Rating scales to assess severity of sleep disturbances have been developed (Trenkwalder et al., 2011). A good night’s sleep reduces the severity of daytime parkinsonism, and many patients comment on sleep benefit, describing better mobility the morning after a restful night. Indeed, patients with marked sleep benefit might not require antiparkinsonian medication for some hours after they awaken, and some PD patients find that a daytime nap “charges the batteries.” These may be the young-onset PD patients with mutations in the parkin gene for they typically show sleep benefit (Elibol et al., 2000).
Another cause of disturbed sleep is the return of parkinsonian symptoms during the night after the last dose of medication has worn off. Nocturnal tremor and akinesia due to the PD and nocturia in the elderly can cause arousals. Depression, which is common in PD, can cause insomnia and is a major factor associated with nighttime sleep problems (Verbaan et al., 2008). Drugs given to treat PD symptoms can interfere with sleep.
PD pathology can also be associated with REM sleep behavior disorder (RBD) and parasomnias, especially in patients with incipient or frank dementia. RBD, described initially by Schenck and colleagues (1986), is a condition in which there is lack of somatic muscle atonia, thus enabling such individuals to move while they dream (acting out their dreams). The animal model of REM sleep without atonia indicates that lesions to the perilocus coeruleus disrupt the excitatory connection to the nucleus reticularis magnocellularis in the descending medullary reticular formation and disable the hyperpolarization of the alpha spinal motoneurons (Ferini-Strambi and Zucconi, 2000). The development of RBD may be an early marker for the later onset of PD (Schenck et al., 1996; Tan et al., 1996; Postuma et al., 2006).
RBD may precede or develop after PD. RBD is suspected of arising in the pons–medulla area, and therefore the Braak scheme would have RBD occurring before PD, if RBD is a component of PD. In the survey of their PD patients, Scaglione and colleagues (2005) found that only 33% had RBD. Of these, PD preceded RBD in 73%, an average of 8 years before onset of RBD. In another study, RBD preceded PD in only 22% (De Cock et al., 2007). Postuma and colleagues (2009) followed patients with idiopathic RBD and found that the risk for developing any neurodegenerative disease (PD, dementia with Lewy bodies, MSA, or Alzheimer disease) is 17.7% by 5 years, 40.6% by 10 years, and 52.4% by 12 years. Only 14 out of 93 developed PD. With longer follow-up, approximately 50% of idiopathic RBD cases will develop PD and the more severe the loss of atonia on baseline polysomnograms, the better the prediction of the development of PD (Postuma et al., 2010).
RBD is usually successfully treated with a bedtime dose of clonazepam (Schenck et al., 1987); 0.5 mg is often sufficient, but sometimes a higher dosage is required to obtain complete relief.
The treatment of sleep disorders in PD is important. Attention to sleep hygiene by avoiding alcohol, caffeine, and nicotine, and excessive fluid intake at night is helpful. Deprenyl (selegiline), which is metabolized to methamphetamine and amphetamine, should not be given at night. Treatment of depression might be required. A sedative antidepressant, such as amitriptyline (10–25 mg at night), mirtazapine, or trazodone, can be very useful, not only to induce and maintain sleep, but also to reduce urinary frequency. A dose of a long-acting levodopa preparation last thing at night may improve nocturnal akinesia (Laihnen et al., 1987; Lees, 1987). However, levodopa given at night may provoke excessive dreaming and disrupted sleep in some patients (Nausieda et al., 1982). A benzodiazepine, especially clonazepam, may lessen REM sleep behavior disorders. A low dose of 0.5 mg at bedtime is usually effective. Propoxyphene is useful for periodic leg movements of sleep and restless legs (Hening et al., 1986). A small bedtime dose of clozapine (Rabey et al., 1995) or quetiapine may be very effective in improving sleep. For patients who have no problem falling asleep, but awaken in 2–3 hours, the short-acting hypnotic zolpidem is useful when taken after the awakening. It helps the patient get back to sleep quickly and still be refreshed in the morning.
Excessive daytime sleepiness (EDS) occurs in about 15% of patients with PD, and is associated with more severe PD and patients with cognitive decline (Tandberg et al., 1999). In one study, EDS was found in 50% of patients (Shpirer et al., 2006). EDS is determined by short sleep latency and sleep-onset REM periods. When studied with tests determining these two criteria, PD patients with EDS were found to correlate not with variables related to disease severity or to total sleep time or sleep stage percentages, but rather those related to primary impairments of waking arousal and REM-sleep expression (Rye et al., 2000). Dopamine agonists are more likely than levodopa to be associated with EDS (Ondo et al., 2001; Gjerstad et al., 2006). The antisoporific agent modafinil can sometimes be beneficial in overcoming EDS in patients with PD (Happe et al., 2001). Oxybate has been reported to reduce EDS (Ondo et al., 2008).
Sleep attacks
Falling asleep while driving and without warning is a serious problem that has been encountered with dopaminergic agents; it seems more likely to occur with pramipexole and ropinirole, but is not limited to just these drugs (Frucht et al., 1999; Ferreira et al., 2000; Hoehn, 2000; Schapira, 2000). The decision about which dopamine agonist to place a patient on was discussed in Chapter 6. Once sleep attacks have occurred, the patient should not drive, except on short trips, or the medication should be changed. Fortunately, modafinil has been reported to be helpful in preventing sleep attacks (Hauser et al., 2000). A review of the literature (Homann et al., 2002) showed that sleep attacks have been reported with all dopaminergic medications, including levodopa, the greatest number being associated with pramipexole and ropinirole. Unfortunately, not all reports refer strictly to sudden attacks without warning; some reports refer to falling asleep from drowsiness, so the interpretation is open to uncertainty.
Sleep and deep brain stimulation of the pedunculopontine (PPN) area
One consequence of the experimental deep brain stimulation in the PPN in an attempt to treat parkinsonian gait disorders has been the effect this procedure has had on sleep. It was found that low-frequency stimulation of the PPN area increased alertness, whereas high-frequency stimulation induced non-rapid eye movement sleep (Arnulf et al., 2010).
Fatigue
Although fatigue can be a symptom of sleepiness or depression, it is also a symptom that may be unassociated with these states. The clinician should probe the patient to distinguish between fatigue and sleepiness. In patients with PD, fatigue is often a complaint during the earliest phase of the disease, before motor symptoms, such as stiffness and slowness, become prominent. As these other features of PD develop, with their important contribution to disability, these become more of a complaint than does fatigue. But when patients are specifically asked, fatigue remains a common feature in PD. In a community study of elderly people in Norway, 44% of PD patients and 18% of healthy controls reported fatigue (Karlsen et al., 1999a). In a Japanese study involving 361 PD patients, fatigue was present in 41.8% and depression was not a contributing factor (Okuma et al., 2009). In newly diagnosed patients with PD, fatigue was discerned in 36% and was less likely to worsen if the patient received levodopa compared to a placebo (Schifitto et al., 2008). Treating depression and daytime sleepiness would be helpful, but when fatigue is an independent symptom, no treatment has been found to be satisfactory. Despite the claimed benefit of amantadine in treating fatigue in multiple sclerosis, neither this drug nor the monoamine oxidase inhibitor selegiline has been found particularly beneficial in treating fatigue in PD. Neither has modafinil in a small controlled trial (Lou et al., 2009). Methylphenidate 30 mg/day has been reported to reduce fatigue in a controlled clinical trial (Mendonça et al., 2007) and in fatigue in patients with prostate cancer (Roth et al., 2010). Oxybate at bedtime has also been reported to help (Ondo et al., 2008). Friedman and colleagues have published a thorough review of fatigue in PD (Friedman et al., 2007).
Depression, anxiety, and change in personality
Loss of motivation
It is common in patients with PD to have a change in personality (Table 8.5), and such change may precede motor symptoms, but usually develops and worsens over the course of PD. Executives with decision-making tasks might find such duties so difficult that they might not be able to continue in their work. Passivity, dependency, and lack of motivation are often more troublesome for the spouse than for the patient. Lack of motivation, as it becomes more severe, becomes apathy; and when apathy becomes more severe, it is abulia. Abulia is a severe form of both mental and motor apathy, with not only a loss of initiative and drive but also a general restriction of activities, including the reticence to speak. Abulia is a recognized clinical syndrome due to caudate and prefrontal dysfunction, so it might well feature in the overall symptomatology of PD. In its milder form (i.e., apathy), the loss of initiative, both mental and motor, is often commented on by the spouse or close relatives, who perceive a change in personality. Spouses particularly complain about the patient’s lack of desire to socialize with friends and communicate freely. When the spouse wants to go out, dine, or meet with friends, the apathetic patient just wants to sit at home and not participate in these activities. Such alterations in activity may be due to depression, but not infrequently, there is no change in mood. A direct test for apathy and depression in patients with PD and dystonia (control group) found no apathy in the absence of depression in the dystonic population, whereas apathy in the absence of depression was frequent in PD (29%) (Kirsch-Darrow et al., 2006). Evaluation in 175 untreated PD patients found 37% with depression, 27% with apathy, 18% with sleep disturbance, and 17% with anxiety (Aarsland et al., 2009b). Apathy does not respond to dopamine replacement therapy in the way the motor problems of PD do, nor to antidepressants, unless depression is present and is itself the cause of the apathy.
In one survey of 164 patients with PD, 52 (32%) met diagnostic criteria for apathy (Starkstein et al., 2009). Of these, 83% had comorbid depression and 56% had dementia. Forty of the 164 PD patients had neither depression nor dementia; only 5 (13%) of the 40 had apathy. One study found that apathy appears to be a predictive factor for dementia and cognitive decline over time (Dujardin et al., 2009).
Depression
Depression is common in PD, with at least one-third of patients exhibiting significant depressive symptoms in cross-sectional surveys (Brown et al., 1988; Dooneief et al., 1992). Anguenot and colleagues (2002) reported a higher number of one-half of PD patients having depression. The Geriatric Depression Scale was administered to subjects enrolled in clinical trials for early PD, and 28% were found to have some depression (Ravina et al., 2007). Forty percent did not require treatment. However, depression was a predictor of more impairment in activities of daily living and increased need for symptomatic therapy of PD. In another study, all patients with early PD were found to have executive dysfunction, and those with major depression also had episodic/working memory and language deficits (Stefanova et al., 2006).
However, it is often difficult to distinguish true depression from the apathy (abulia) associated with PD, especially in the presence of the characteristic expressionless face, bowed posture, and slowed movement, which resemble the psychomotor retardation of a primary depressive illness. The critical factor is whether the patient has a true disturbance of mood (dysphoria), with low spirits, loss of interest, bleak outlook, typical depressive sleep disturbance, paranoid ruminations, and sometimes suicidal thoughts. Schrag and colleagues (2007) evaluated a variety of depression rating scales and found many of them satisfactory for use and conclude that it is not necessary to develop new ones.
The reasons for depression in PD are debated. On the one hand, depressive symptoms are not surprising in vulnerable individuals who are faced with the disabilities and handicaps imposed by PD, with reduced activities and independence, and the prospects of a chronic incurable condition. Such reactive depression certainly contributes to the problem. But one study suggests that depression in PD is more strongly influenced by the patients’ perceptions of handicap than by actual disability (Schrag et al., 2001). However, there is also the probability that the pathology of PD in itself might predispose to depression, especially that involving serotonergic and noradrenergic systems, which have been implicated in the neurochemical basis of primary depressive illnesses. The substantia nigra, itself, is implicated by the report that deep brain stimulation of this structure in a patient with PD induced acute severe depression (Bejjani et al., 1999).
The recognition and treatment of depression in PD is important because depression has a major impact on the overall disability imposed by the illness. In fact, depression carries a hazard ratio of 2.66 for increased mortality in PD (Hughes et al., 2004). Most antidepressant drugs can be used safely in PD. However, nonselective monoamine oxidase inhibitors are contraindicated in patients who are taking levodopa because of potential pressor reactions. There also have been concerns over the use of selective serotonin reuptake inhibitors (SSRIs), which in a few cases have been reported to interact with levodopa to induce the “serotonin” syndrome (confusion, myoclonus, rigidity, and restlessness) and to worsen PD symptoms. Despite these worries, many depressed patients with PD have been treated safely and successfully with SSRIs, for example fluoxetine or paroxetine (20–40 mg daily). By enhancing serotonergic “tone” and thereby potentially inhibiting dopaminergic neurons in the substantia nigra, there is the potential, especially in the absence of dopaminergic drug treatment, that SSRIs may increase parkinsonian symptoms, but such events are rare (Jansen Steur, 1993; Jimenez-Jimenez et al., 1994; Richard et al., 1999; Ceravolo et al., 2000; Tesei et al., 2000). The traditional tricyclic antidepressants can also be employed, although their sedative and anticholinergic properties may be detrimental in elderly patients. A small double-blind trial comparing paroxetine, nortriptyline, and placebo in 52 patients with PD and depression was carried out over 8 weeks and showed that nortriptyline was superior to placebo but paroxetine was not (Menza et al., 2009). A clinical trial testing the efficacy of pramipexole found the drug to be superior to placebo in treating the depression of PD patients (Barone et al., 2010). A trial of the norepinephrine uptake inhibitor, atomoxetine, found it to be ineffective in treating depression in PD (Weintraub et al., 2010b).
If a severely depressed patient with PD fails to respond to antidepressant drug treatment, electroconvulsive therapy (ECT) can be used (Douyon et al., 1989). Indeed, ECT in itself can temporarily improve mobility in PD. Tranylcypromine, a noncompetitive inhibitor of types A and B monoamine oxidase, is an effective antidepressant (Fahn and Chouinard, 1998), but cannot be give in the presence of levodopa because of the likelihood of hypertension.
Anxiety
Anxiety and panic can be major difficulties in PD (Stein et al., 1990). Many patients, even early in the illness, complain of loss of confidence. In particular, they fear social occasions and public display at work, and they tend to withdraw from outside life. In part, this is due to anxiety over their friends and acquaintances perceiving that they have PD, and to their loss of mobility and their nonverbal emotional responses to social interactions. However, some also develop a generalized and disturbing anxiety state, which might require psychotherapy and anxiolytic drug treatment. Those in the more advanced stages of the illness may experience profound anxiety and even terror during “off” periods (Nissenbaum et al., 1987).
Cognitive problems
As listed in Table 8.6, slowness of thinking (bradyphrenia) and trouble finding words (“tip of the tongue phenomenon”) are common in patients with PD. (Dementia is discussed in the next section.) If these defects are specifically looked for, about two-thirds of patients with early PD will show abnormalities of cognitive function on formal neuropsychological testing (Lees and Smith, 1983; Brown and Marsden, 1990; Levin et al., 1989; Cooper et al., 1991). In particular, such patients are weak in performance of tests that are sensitive to frontal lobe dysfunction (executive function), such as verbal fluency, the Wisconsin card sorting test, the Tower of London test and its variants, and tests of working memory. Poor performance on tests such as these suggests abnormalities of frontal lobe executive functions, which may be due to defective input from nonmotor basal ganglia regions (via thalamus) into prefrontal cerebral cortical areas. Thus, some patients with early PD may exhibit a frontostriatal cognitive syndrome, which is sometimes rather inaccurately described as a subcortical dementia, in the absence of any major defects in language, episodic memory, or visuospatial functions. The pathologic substrate of such a frontostriatal cognitive syndrome in PD is debatable. Dopamine deficiency in the nonmotor regions of the striatum, especially the caudate nucleus which receives from and projects to prefrontal cerebral cortex, loss of dopamine projections from the midbrain ventral tegmental area to the frontal lobes, loss of cortical cholinergic projections from the substantia innominata, loss of cortical noradrenergic projections from the locus coeruleus, and cortical Lewy body degeneration may all contribute.
The Montreal Cognitive Assessment (MoCA) was found to be a useful screening tool for cognitive dysfunction in PD and more sensitive than the MMSE (Gill et al., 2008; Hoops et al., 2009). Using more formal neuropsychological testing in newly diagnosed PD patients and controls, Aarsland and colleagues (2009) found that 18.9% of patients had mild cognitive impairment, with a relative risk of 2.1. Ultimately most patients with PD have cognition problems and eventually dementia. In one study, 13 out of 126 (10%) newly diagnosed cases of PD had developed dementia at a mean of 3.5 years from diagnosis, corresponding to an annual dementia incidence of 30.0 per 1000 person-years; rapid development of dementia was associated with the postural instability and gait disorder (PIGD) form of PD (Williams-Gray et al., 2007). Hely and colleagues (2005) reported cognitive decline in 84% and dementia in 48% of patients with PD for 15 years. By 20 years, 83% had dementia (Hely et al., 2008). The Hely results are presented in Tables 6.7 and Table 6.8. Aarsland and colleagues (2003), following patients for 8 years, found that by that time span, 78% had cognitive decline. In another Norwegian study, in which PD patients were followed for 12 years, 60% had developed dementia, with dementia steadily increasing with age, reaching to 80–90% by age 90 years (Buter et al., 2008).
One approach to determine the pathoanatomic correlates with cognitive decline is to measure the functional networks detected by fluorodeoxyglucose positron emission tomography (FDG PET) scanning. Whereas the motor manifestations of PD have been linked to an abnormal covariance pattern involving basal ganglia thalamocortical pathways, a covariance pattern that correlated with impaired memory and executive functioning was found to be metabolic reductions in frontal and parietal association areas and relative increases in the cerebellar vermis and dentate nuclei (Huang et al., 2007). This network of PD cognitive pattern (PDCP) increased stepwise with worsening cognitive impairment (Huang et al., 2008).
Bradyphrenia or slowness of thought probably is a real component of PD in many patients. For example, patients might comment on a slowing of mental processing and memory retrieval, and examination of neuropsychological test performance might show delay in deciding on choices, although the final decisions are correct. Finding the right word, or the answer to a question may be slow. The “tip of the tongue” phenomenon refers to the patient’s knowing the word he or she wants, but not being able to come up with it and say it at that moment, a problem that is often encountered in PD (Matison et al., 1982). Some patients with PD spontaneously volunteer the observation that they have less ability to deal with mental problems, particularly multiple tasks at the same time. However, the extent to which depression might contribute to such problems is controversial. The general concept of bradyphrenia could be considered in the wider issue of abulia.
The overall impression of cognitive dysfunction in nondemented patients with PD may be summarized as follows: (1) Many, but not all, patients with early PD exhibit subtle frontostriatal cognitive impairments, but to begin with, these do not necessarily intrude into everyday life. (2) However, a substantial number of patients with early PD do complain of some cognitive change, in particular a slowing of thought, a difficulty or delay in memory retrieval, and problems in handling multiple tasks. (3) Such cognitive impairments may be due to depression, when there is a change in mood, but often there is no dysphoria. One multicenter survey of nondemented PD patients revealed that 26% had mild cognitive impairment (Aarsland et al., 2010).
Whether these cognitive difficulties are the precursor to frank dementia is uncertain, but obviously, they raise concern. In practice, it is likely that a minority of patients with such complaints in the early stages of PD will progress to frank dementia. Risk factors for developing dementia are low serum epidermal growth factor (Chen-Plotkin et al., 2011), low CSF amyloid-beta (Siderowf et al., 2010), and severity of olfactory impairment (Stephenson et al., 2010).
Dementia and confusion
Unfortunately, a sizable proportion of patients with PD eventually develop a multifocal, pervasive dementia. This typically occurs in elderly patients. Cross-sectional studies suggested that in those with PD aged over 65 years some 20% will be demented, compared to some 10% of non-PD individuals in this age group (Brown and Marsden, 1984). Initial prospective longitudinal studies raised that up to 40% becoming demented as they age (Biggins et al., 1992; Mayeux et al., 1990; Hughes et al., 2000). Aarsland and colleagues (2001) found the incidence rate for dementia to be 95.3 per 1000 person-years, which is a six-fold greater risk than that in non-PD individuals. Following PD patients over an 8-year period, Aarsland and colleagues (2003) found that 78.2% developed dementia, and that hallucinations and akinetic-dominant or mixed tremor/akinetic PD were high risk factors for dementia. Another study also found that dementia was more likely to occur in the PIGD (postural instability gait disorder) category of patient than in the type presenting with tremor (Burn et al., 2006a). In fact, in studying the incidence of dementia, Alves and colleagues (2006) found that the tremor-dominant subtype did not develop dementia until those patients converted to the PIGD subtype. Other correlations for developing dementia are older age, greater severity and longer duration of PD, and male gender. However, looked at from the opposite perspective, most patients with PD, particularly the young, do not have dementia. Aarsland and colleagues (2004) followed the MMSE and found that the mean annual decline for PD patients was 1 point. However, a marked variation was found. In patients with PD and dementia, the mean annual decline was 2.3, which was similar to the decline observed in patients with Alzheimer disease. The Montreal Cognitive Assessment was found to be more sensitive as a screening tool than the MMSE, but a positive screen using either instrument requires additional assessment due to suboptimal specificity at the recommended screening cutoff point (Hoops et al., 2009).
It is bad enough to develop the debilitating and disabling problem of dementia, but what compounds this development is a lesser response to levodopa therapy in controlling motor symptoms (Alty et al., 2009). In patients without dementia the “off” phase motor function worsened at a yearly rate of 2.2% of the maximum disability score, and the magnitude of the levodopa response was well preserved as the disease progressed. Typically, patients who developed motor fluctuations maintained better “on” phase motor function than non-fluctuators (P = 0.01). However, dementia was associated with worse “on” and “off” motor disability scores (P < 0.001), and a smaller levodopa response magnitude after 14 years (P = 0.008).
The Movement Disorder Society’s Task Force on Dementia developed a set of criteria for diagnosing dementia in patients with PD (Emre et al., 2007). PD dementia (PDD) is characterized by impairment in attention, memory, executive and visuospatial functions, and behavioral symptoms such as affective changes, hallucinations, and apathy are frequent. The Task Force proposed clinical diagnostic criteria for “probable” and “possible” PDD (Emre et al., 2007). It also proposed guidelines for assessing such patients (Dubois et al., 2007). Level 1 is for general clinicians; and Level 2 tests are for more sophisticated evaluations and even for clinical trials.
Concurrent Alzheimer disease, probably coincidental, obviously accounts for some of the dementia in PD (Boller et al., 1980). However, in recent years with the use of antisynuclein antibody stains to detect Lewy bodies, it has become apparent that widespread Lewy body degeneration is another common cause of dementia (Byrne et al., 1989), some say second in frequency to Alzheimer disease. In those with prior PD, this may follow the progression and spread of Lewy neurite distribution observed by Braak and colleagues (2003). PD dementia (PDD) is now the term applied to those whose PD symptoms began at least one year prior to the onset of dementia. It is called dementia with Lewy bodies (DLB) (McKeith et al., 1996) when the dementia precedes or occurs within 1 year after onset of parkinsonian motor features (Lippa et al., 2007). While there is a clear relationship between the duration of PD prior to the onset of dementia and key neuropathologic and neurochemical characteristics, there is a gradation of these differences across the dementia with Lewy bodies/Parkinson disease dementia spectrum and the findings do not support an arbitrary cutoff between the two disorders (Ballard et al., 2006).
DLB may coexist with the pathologic changes of Alzheimer disease, particularly with amyloid plaques rather than neurofibrillary tangles. One hypothesis is that the two pathologies, Lewy body degeneration in cerebral cortex and the cholinergic substantia innominata, and Alzheimer disease change, coexist by chance but summate to cause dementia. Another hypothesis is that the one predisposes to the other, and it is intriguing that α-synuclein is a component of both plaques (the non-amyloid component) and Lewy bodies. Whatever the pathogenic mechanism, this combination (which some have called the Lewy body variant of Alzheimer disease) is a common cause of dementia in PD. Pure dementia with Lewy bodies probably is less common. But it has been shown pathologically that cortical Lewy bodies can be associated with cognitive impairment independent of Alzheimer disease type pathology (Mattila et al., 2000).
Thus, there are at least three common substrates for dementia in PD: Alzheimer disease, Alzheimer disease with Lewy bodies, and diffuse Lewy body disease (also called dementia with Lewy bodies) (DLB). A fourth possibility is dementia with just the standard pathology of PD (PDD), typically with Lewy bodies in the cerebral cortex. Whether such dementia in patients with PD occurs without the spread of Lewy bodies into the cortex is uncertain. In addition, all the other causes of dementia may occur in patients with PD, including cerebrovascular disease, rarer degenerations such as frontotemporal dementia and Pick disease, cerebral tumors and other intracranial mass lesions, hydrocephalus, and metabolic, endocrine, and vitamin abnormalities. In one pathologic study, the Lewy body score was significantly associated with the rate of cognitive decline (Aarsland et al., 2005).
Parkinson disease dementia (PDD) shares identical clinical features with dementia with Lewy bodies (DLB); both entities can be distinguished from Alzheimer disease (Galvin et al., 2006). A personality trait that distinguishes DLB/PDD from Alzheimer disease is passivity (diminished emotional responsiveness, relinquished hobbies, growing apathy, and purposeless hyperactivity) (Galvin et al., 2007). Neuroimaging with Pittsburgh Compound B (PIB) ligand with PET can reveal the presence of fibrillar Abeta amyloid, which is present in patients with PDD (Brooks, 2009; Burack et al., 2010). This is present in the absence of Alzheimer disease at autopsy.
Accordingly, the first step in assessment of a patient with PD who has dementia is to undertake the usual investigations for known causes, especially those that are treatable. Depression causing a pseudo-dementia also must be carefully assessed. Anticholinergic drugs in PD appear to hasten cognitive decline (Ehrt et al., 2010), and withdrawal of such agents could improve cognitive function.
Having excluded such symptomatic causes of dementia, the clinical picture may give important clues as to the underlying degenerative condition causing the dementia. Prominent early memory difficulties with language, praxis, and visuospatial problems pointing to temporo-parietal problems suggest Alzheimer disease. A variable, fluctuating course with prominent hallucinations (especially visual), confusion, and an unusual susceptibility to neuroleptics could indicate dementia with Lewy bodies (McKeith et al., 1996, 1999), i.e., diffuse Lewy body disease. Prominent behavioral, speech and memory difficulties could point to frontotemporal dementia or Pick disease.
FDG PET scans were correlated with the dementia score on the UPDRS. A correlation was found with left limbic structures such as the cingulate gyrus, parahippocampal gyrus, and medial frontal gyrus (Wu et al., 2000).
Whatever the pathologic substrate of dementia in PD, the combination poses formidable management problems. These are similar to those discussed in the section entitled “Psychosis; hallucinations, and paranoia.” The difficulty is to maintain mobility with adequate doses of antiparkinsonian medication without exacerbating the mental and behavioral problems. Behavioral disturbances, including verbal and physical aggression, wandering, agitation, inappropriate sexual behavior, uncooperativeness, and urinary incontinence, cause major difficulties. General structured care in a familiar environment is essential. Judicious use of daycare facilities and home assistants may be necessary. Drug therapy should be simplified, removing selegiline, anticholinergic agents, amantadine, and dopamine agonists. Depression might require specific treatment, preferably avoiding antidepressants with marked anticholinergic properties. Nocturnal sedation might require quetiapine, which provides both sedation and antihallucinatory effects. Clozapine does the same but requires weekly ascertainment for neutropenia. Other bedtime hypnotics, such as benzodiazepines and zolpidem, can be effective. Donepezil (Aricept), which provides modest benefit in Alzheimer disease, has been found also to provide modest benefit in cognition in those with PD who are demented without aggravating the motoric symptoms of PD (Aarsland et al., 2002; Ravina et al., 2005). Rivastigmine and other centrally active cholinesterase inhibitors were found to provide some improvement in apathy, anxiety, delusions, and hallucinations in patients with DLB (McKeith et al., 2000) and can improve dementia (Giladi et al., 2003). In a large multicenter, controlled clinical trial, rivastigmine was found to provide moderate improvement in dementia associated with PD (Emre et al., 2004). Memantine was found to be superior to a placebo in a couple of small studies (Aarsland et al., 2009a; Leroi et al., 2009). An evidence-based review of treatment of PD dementia and psychosis was conducted by the American Academy of Neurology (Miyasaki et al., 2006).
Dementia, with or without a frank confusional state, is the commonest cause of final nursing home placement in those with PD, and shortens life expectancy (Goetz and Stebbins, 1993).
Compulsive behaviors
A large multicenter, cross-sectional evaluation of impulse control disorders (ICDs) assessed 3090 patients with PD (Weintraub et al., 2010a). The point prevalence estimates of four ICDs were 5.0% with pathological gambling, 3.5% with hypersexuality, 5.7% with compulsive buying, and 4.3% with binge-eating. ICDs were more common in patients treated with a dopamine agonist than in patients not taking a dopamine agonist (17.1% vs. 6.9%; odds ratio, 2.72; P < 0.001). ICD frequency was similar for pramipexole and ropinirole (17.7% vs. 15.5%). ICDs have become a problem, particularly for young individuals on a dopamine agonist. A screening questionnaire for ICDs has been validated (Weintraub et al., 2009).
In the category of compulsive behaviors, the most newsworthy has been pathologic gambling (Driver-Dunckley et al., 2003), which is now being more widely recognized. It is possibly related to dopaminergic stimulation in the mesolimbic system (Gschwandtner et al., 2001). Treatment of PD with a dopamine agonist increases the lifetime risk of a PD patient to have pathologic gambling from 3.4% to 7.2% (Voon et al., 2006a). In a PD clinic in Scotland, pathologic gambling occurred in 8% of patients on dopamine agonists (Grosset et al., 2006b). Patients with a younger age at PD onset, higher novelty seeking traits, and a personal or family history of alcohol use disorders were found to have a greater risk for pathologic gambling with dopamine agonists (Voon et al., 2007). Pathologic gambling is not limited to patients with PD on dopamine agonists. It has also been seen in patients with restless legs syndrome treated with these drugs (Tippmann-Peikert et al., 2007). In a PET study of PD patients during gambling, those with pathologic gambling had greater decreases in raclopride binding in the ventral striatum during gambling (13.9%) than control patients (8.1%), likely reflecting greater dopaminergic release (Steeves et al., 2009).
In a survey of other reward-seeking behaviors in 297 patients with PD, pathologic hypersexuality lifetime prevalence was 2.4%, and compulsive shopping was 0.7% (Voon et al., 2006b); combined with pathologic gambling data, the lifetime prevalence of these behaviors was found to be 6.1% and increased to 13.7% in patients on dopamine agonists. In another survey of 272 patients with PD, these compulsive behaviors were found to have occurred in 6.6% (Weintraub et al., 2006). Dopamine agonists and a history of ICD symptoms prior to PD onset were found to be risk factors.
Compulsive eating with weight gain has also been reported with dopamine agonist therapy, especially pramipexole (Kumru et al., 2006; Nirenberg and Waters, 2006). This is in contrast to the typical weight loss often seen in patients with PD. In one analysis, patients with PD were found to have lost a mean of 7.7% of body weight over a mean of 13.1 years with PD (Uc et al., 2006).
The term punding has been used to describe an abnormal motor behavior in which there is an intense fascination with repetitive handling and examining of mechanical objects, such as picking at oneself, taking apart watches and radios, or sorting and arranging of common objects, such as lining up pebbles, rocks, or other small objects. Punding has been reported with levodopa (Fernandez and Friedman, 1999) and dopamine agonists (McKeon et al., 2007), including for the treatment of restless legs syndrome (Evans and Stegeman, 2009).
A common form is repetitive cleaning/rearranging/ordering behaviors, which can be disabling. These have associated features of hypomania, occur during motor “on” periods, and often occur nocturnally (Kurlan, 2004). The repetitive behavior responds poorly to serotonin reuptake inhibitors, but may benefit from atypical antipsychotics (Kurlan, 2004). Punding, a term that was first used in amphetamine abusers, is considered a dopamine dysregulation disorder (Evans et al., 2004). This behavior would appear to be a form of compulsive disorder. In this age of technology, excessive use of the computer is a particular form of repetitive behavior reported to be caused by levodopa therapy (Fasano et al., 2006). Punding incidence is lower than other compulsive behaviors (Miyasaki et al., 2007).
In a small cross-over study, amantadine was found to reduce pathologic gambling (Thomas et al., 2010), however two reviews of databases showed that pathologic gambling and other ICDs are more often associated with patients on amantadine (Weintraub et al., 2010c; Lee et al., 2011).
Psychosis: hallucinations and paranoia
Criteria for diagnosing psychosis in PD and differentiating it from other causes of psychosis were established by an NIH working group (Ravina et al., 2007). These criteria were in the style of the Diagnostic and Statistical Manual of Mental Disorders IV-TR (Table 8.7).
Table 8.7 Proposed diagnostic criteria for PD-associated psychosis
| Characteristic symptoms |
| Presence of at least one of the following symptoms (Criterion A) (specify which of the symptoms fulfill the criteria): |
| Illusions |
| False sense of presence |
| Hallucinations |
| Delusions |
| Primary diagnosis |
| UK brain bank criteria for PD |
| Chronology of the onset of symptoms of psychosis |
| The symptoms in Criterion A occur after the onset of PD |
| Duration |
| The symptom(s) in Criterion A are recurrent or continuous for 1 month |
| Exclusion of other causes |
| The symptoms in Criterion A are not better accounted for by another cause of parkinsonism such as dementia with Lewy bodies, psychiatric disorders such as schizophrenia, schizoaffective disorder, delusional disorder, or mood disorder with psychotic features, or a general medical condition including delirium |
| Associated features (specify if associated): |
| With/without insight |
| With/without dementia |
| With/without treatment for PD (specify drug, surgical, other) |
From Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord 2007;22(8):1061–1068.
Hallucinations occur in a significant proportion of those with PD, especially in elderly patients. In a community study in Norway, 10% of PD patients had hallucinations with insight retained, and another 6% had more severe hallucinations or delusions (Aarsland et al., 1999). Forsaa and colleagues (2010b) followed Norwegian PD patients over 12 years, and found that 60% developed hallucinations or delusions, with an incidence rate of 79.7 per 1000 person-years. Risk factors were higher age at onset, dopaminergic dose, and RBD at baseline.
Isolated visual hallucinations are fairly common (Naimark et al., 1996; Sanchez-Ramos et al., 1996). Auditory hallucinations are very uncommon (Inzelberg et al., 1998). Visual hallucinations often take the form of familiar humans or animals, which the patients know are false (pseudo-hallucinations). Even when the hallucinations do not disturb the patient because the visual images are friendly and not frightening (benign hallucinations), these milder forms can worsen to a more malignant type of hallucination (Goetz et al., 2006). Such hallucinations may progress to a delusional paranoid state (often concerning infidelity) or a frank confusional state with impairment of attentiveness and disorientation. FDG PET reveals that PD patients with visual hallucinations have a reduced metabolic rate in the occipitotemporoparietal regions, sparing the occipital pole (Boecker et al., 2007).
When such symptoms occur, although antiparkinsonian drug therapy is the most likely cause, it is wise first to search for some intercurrent illness, such as a stroke or intracranial mass lesion, a chest or urinary infection, disturbance of electrolytes, renal or hepatic dysfunction, anemia, or endocrine dysfunction. Psychosis due to antiparkinsonian medications can usually be counteracted by atypical antipsychotics, drugs that usually do not aggravate parkinsonism at a dosage that has a therapeutic benefit in treating the psychosis, namely quetiapine (Seroquel) and clozapine (Clozaril). Quetiapine appears to be effective only for milder psychosis, such as hallucinations, but not in more severe forms of psychosis, as demonstrated by its failure in controlled trials (Ondo et al., 2005; Kurlan et al., 2007; Rabey et al., 2007). Quetiapine had a similar clinical benefit to clozapine in one study (Merims et al., 2006). Because quetiapine does not cause agranulocytosis and does not require blood count monitoring (Fernandez et al., 1999; Juncos et al., 2004) as does clozapine, it is practical to start therapy with quetiapine if the hallucinations are mild. A dose of 25–50 mg at night can often control confusion and psychosis without worsening the parkinsonism. Because of the potential for drowsiness, it is best initially to use a small dose of such drugs at night, thereafter gradually increasing the dose to that required to control the confusion without worsening the parkinsonism. The benefit of aiding sleep at night is an advantage, but try to avoid a dose that might extend the drowsiness to daytime. Quetiapine can be utilized as a hypnotic in older patients with PD to take advantage of suppressing the development of hallucinations in this susceptible population.
If quetiapine is ineffective or produces too much daytime drowsiness or other adverse effects, including worsening of parkinsonism, clozapine (Clozaril) should be tried next, starting with 12.5 mg at night to avoid daytime drowsiness and increasing the dose until benefit or adverse effects are encountered. It is more effective than quetiapine, but its use requires regular monitoring of blood counts to prevent the 1–2% risk of agranulocytosis (Scholz and Dichgans, 1985; Friedman and Lannon, 1990; Pfeiffer et al., 1990; Wolters et al., 1990; Kahn et al., 1991; Factor and Brown, 1992; Greene et al., 1993; Pinter and Helscher, 1993; Factor et al., 1994; Diederich et al., 1995; Rabey et al., 1995; Factor and Friedman, 1997; Ruggieri et al., 1997; Friedman et al., 1999; Pollak et al., 2004). In one open-label comparative trial, quetiapine and clozapine appeared equally effective; interestingly both also reduced the severity of dyskinesias as well as controlling psychosis (Morgante et al., 2004).
Other so-called atypical antipsychotics appear to be so designated for marketing purposes. Olanzapine can be effective, but easily increases parkinsonism and so needs to be used in small doses to avoid a worsening of parkinsonism (Wolters et al., 1996; Friedman, 1998; Jimenez-Jimenez et al., 1998; Goetz et al., 2000); therefore, it is relegated to third choice. Risperidone more closely resembles a typical, rather than an atypical antipsychotic, and worsens PD. Aripiprazole has also worsened parkinsonism (Fernandez et al., 2004). A more thorough discussion of atypical antipsychotics is found in Chapter 19. Molindone, pimozide, or other relatively weak antipsychotics might also be considered.
An alternative to atypical antipsychotics are the centrally active anticholinesterase drugs that are used in the treatment of dementia. They have been reported to have similar efficacy on psychosis as quetiapine (Van Laar et al., 2001; Bergman and Lerner 2002; Reading et al., 2002; Burn et al., 2006b).
If psychosis continues without adequate benefit from the antipsychotics, selegiline, anticholinergics, and amantadine should be withdrawn. The need for anxiolytics and antidepressants should be reconsidered. If the symptoms persist, dopamine agonists should be reduced or stopped. If necessary, the dose of levodopa should be tapered. However, more often than not, as drugs are reduced to improve the mental state, mobility deteriorates. A brittle balance is reached at which the patient either is mobile but confused, paranoid or hallucinating, or is mentally clear but immobile. More dopamine replacement therapy is required to maintain mobility, but this causes a recurrence of the confusion, and it is very difficult to achieve a compromise. In this situation, a limited drug holiday, withdrawing dopaminergic drugs for 1–2 days each week, might help to dispel psychotoxicity, allowing a reasonable dose of medication to maintain mobility on other days. Sustained withdrawal of levodopa provokes unacceptable parkinsonism and, sometimes, particularly if the levodopa is withdrawn suddenly, a “neuroleptic” malignant syndrome (Friedman et al., 1985; Hirschorn and Greenberg, 1988). When it comes to balancing drug-induced psychosis and parkinsonism, keep in mind that an intact mental function is more important than an intact motor function.
The serotonin 5HT3-receptor antagonist ondansetron blocks nausea and vomiting due to anticancer drugs. It has been reported to reduce hallucinations, paranoia, and confusion in PD (Zoldan et al., 1995). Fifteen out of the 16 patients treated with 12–25 mg/day for up to 8 weeks showed moderate to marked improvement of visual hallucinations and paranoid delusions. There was no worsening of the parkinsonism. However, this was not replicated by Eichhorn and colleagues (1996), who failed to find benefit in most patients, and found waning of benefit in the few for whom the drug was initially beneficial.
Quality of life
Measuring quality of life (QoL) in 124 patients with PD, Schrag and colleagues (2000) showed that it significantly deteriorated with increasing disease severity, as measured by the PDQ-39, the EQ-5D, and the physical summary of the SF 36. The greatest impairment was seen in the areas related to physical and social functioning, whereas reports of pain and poor emotional adjustment had similar prevalence in patients with PD and the general population. The impairment of QoL was seen in all age groups and was similar for men and women, but the differences between patients with PD and the general population were most marked in the younger patient groups.
From the QoL survey of patients with PD conducted in Norway by Karlsen and her colleagues (1999b), the three most important factors impacting QoL were depression, sleep disorders, and a sense of low degree of independence. The first two were addressed in this chapter, and their prominence in QoL indicates the importance of the nonmotor symptoms of PD. Independence would be affected by gait and balance disorders predominantly. Sexuality is part of quality of life, and patients with PD are more dissatisfied with their sexual functioning and relationship than controls (Jacobs et al., 2000).
Falls and fractures also impact QoL. Compared to an equal number of age- and sex-matched non-PD subjects, PD patients were found to be at a 2.2-fold increased risk of fractures generally and a 3.2-fold greater risk of hip fractures specifically (Melton et al., 2006). Adjusting for age, the independent predictors of overall fracture risk in the PD subjects were female gender and dementia, both with hazard ratios of 1.6. Interestingly, chronic depression was associated with a reduced risk (hazard ratio 0.4). Perhaps depressed patients are more cautions in walking. Hip fractures were predicted by dementia (hazard ratio 2.2). Sato and colleagues (2006, 2007) showed that the incidence of fractures could be reduced by treating with risedronate, ergocalciferol (Sato et al., 2007), and lendronate and vitamin D2 (Sato et al., 2006).
Patients with PD tend to forget to take their medication or are late in the timing of a dose, which usually affects the patients with wearing-off. Erratic intake was shown to be an emerging and more common problem, which is likely to affect motor control and QoL adversely (Grosset et al., 2005). Electronic monitoring of tablet administration provides the most accurate methodology for determining accurate compliance (Grosset et al., 2006a). Improved QoL might be better achieved with better compliance of timing of medication in those who are fluctuators.
Aarsland D., Andersen K., Larsen J.P., et al. Prevalence and characteristics of dementia in Parkinson disease – an 8-year prospective study. Arch Neurol. 2003;60(3):387-392.
Aarsland D., Andersen K., Larsen J.P., et al. Risk of dementia in Parkinson’s disease – a community-based, prospective study. Neurology. 2001;56:730-736.
Aarsland D., Andersen K., Larsen J.P., et al. The rate of cognitive decline in Parkinson disease. Arch Neurol. 2004;61(12):1906-1911.
Aarsland D., Ballard C., Walker Z., et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
Aarsland D., Brønnick K., Alves G., et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80(8):928-930.
Aarsland D., Brønnick K., Larsen J.P., et al. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121-1126.
Aarsland D., Brønnick K., Williams-Gray C., et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062-1069.
Aarsland D., Laake K., Larsen J.P., Janvin C. Donepezil for cognitive impairment in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72(6):708-712.
Aarsland D., Larsen J.P., Cummings J.L., Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease – a community-based study. Arch Neurol. 1999;56:595-601.
Aarsland D., Perry R., Brown A., et al. Neuropathology of dementia in Parkinson’s disease: a prospective, community-based study. Ann Neurol. 2005;58(5):773-776.
Alty J.E., Clissold B.G., McColl C.D., et al. Longitudinal study of the levodopa motor response in Parkinson’s disease: relationship between cognitive decline and motor function. Mov Disord. 2009;24(16):2337-2343.
Alves G., Larsen J.P., Emre M., et al. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21(8):1123-1130.
Anguenot A., Loll P.Y., Neau J.P., et al. Depression and Parkinson’s disease: a study of 135 parkinsonian patients. Can J Neurol Sci. 2002;29(2):139-146.
Araki I., Kuno S. Assessment of voiding dysfunction in Parkinson’s disease by the international prostate symptom score. J Neurol Neurosurg Psychiatry. 2000;68:429-433.
Arbouw M.E., Movig K.L., Koopmann M., et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology. 2010;74(15):1203-1207.
Arnulf I., Ferraye M., Fraix V., et al. Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann Neurol. 2010;67(4):546-549.
Askenasy J.J.M. Sleep in Parkinson’s disease. Acta Neurol Scand. 1993;87(Suppl 3):167-170.
Ballard C., Ziabreva I., Perry R., et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
Barone P., Antonini A., Colosimo C., et alPRIAMO study group. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641-1649.
Barone P., Poewe W., Albrecht S., et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(6):573-580.
Bateson M.C., Gibberd F.B., Wilson R.S.E. Salivary symptoms in Parkinson’s disease. Arch Neurol. 1973;29:274-275.
Bejjani B.P., Damier P., Arnulf I., et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476-1480.
Bergman J., Lerner V. Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson’s disease. Clin Neuropharmacol. 2002;25(2):107-110.
Biggins C.A., Boyd J.L., Harrop F.M., et al. A controlled, longitudinal study of dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55:566-571.
Bliwise D.L., Watts R.L., Watts N., et al. Disruptive nocturnal behavior in Parkinson’s disease and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 1995;8:107-110.
Boecker H., Ceballos-Baumann A.O., Volk D., et al. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol. 2007;64(7):984-988.
Bohnen N.I., Müller M.L., Kotagal V., et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain. 2010;133(Pt 6):1747-1754.
Boller F., Mizutani T., Roessmann U., Gambetti P. Parkinson disease, dementia, and Alzheimer disease: clinicopathological correlations. Ann Neurol. 1980;7:329-335.
Braak H., Bohl J.R., Muller C.M., et al. Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042-2051.
Braak H., Del Tredici K., Rub U., et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211.
Braun A.R., Tanner C.M., Goetz C.G., Klawans H.L. Respiratory distress due to pharyngeal dystonia: A side effect of chronic dopamine agonism. Neurology. 1983;33(Suppl 2):220.
Brooks D.J. Imaging amyloid in Parkinson’s disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24(Suppl 2):S742-S747.
Brown R.G., Marsden C.D. How common is dementia in Parkinson’s disease? Lancet. 1984;2:1262-1265.
Brown R.G., Marsden C.D. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci. 1990;13:21-29.
Brown R.G., MacCarthy B., Gotham A.M., et al. Depression and disability in Parkinson’s disease: a follow-up of 132 cases. Psychol Med. 1988;18:49-55.
Brusa L., Petta F., Pisani A., et al. Acute vs chronic effects of l-dopa on bladder function in patients with mild Parkinson disease. Neurology. 2007;68(18):1455-1459.
Burack M.A., Hartlein J., Flores H.P., et al. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74(1):77-84.
Burke R.E., Dauer W.T., Vonsattel J.P. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64(5):485-491.
Burn D.J., Rowan E.N., Allan L.M., et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77(5):585-589.
Burn D., Emre M., McKeith I., et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
Bushman M., Dobmeyer S.M., Leeker L., Perlmutter J.S. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309-1314.
Buter T.C., van den Hout A., Matthews F.E., et al. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70(13):1017-1022.
Byrne E.J., Lennox G., Lowe J., Godwin-Austen R.B. Diffuse Lewy body disease: Clinical features in 15 cases. J Neurol Neurosurg Psychiatry. 1989;52:709-717.
Ceravolo R., Nuti A., Piccinni A., et al. Paroxetine in Parkinson’s disease: Effects on motor and depressive symptoms. Neurology. 2000;55:1216-1218.
Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235-245.
Chaudhuri K.R., Martinez-Martin P., Schapira A.H., et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21(7):916-923.
Chaudhuri K.R., Schapira A.H. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464-474.
Chen-Plotkin A.S., Hu W.T., Siderowf A., et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69(4):655-663.
Chester C.S., Gottfried S.B., Cameron D.I., Strohl K.P. Pathophysiological findings in a patient with Shy-Drager and alveolar hypoventilation syndromes. Chest. 1988;94:212-214.
Churchyard A., Mathias C.J., Phil D., Lees A.J. Selegiline induced postural hypotension in Parkinson’s disease: a longitudinal study on the effects of drug withdrawal. Mov Disord. 1999;14:246-251.
Cooper J.A., Sagar H.J., Jordan N., et al. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095-2122.
De Cock V.C., Vidailhet M., Leu S., et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain. 2007;130(Pt 2):450-456.
Defazio G., Berardelli A., Fabbrini G., et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol. 2008;65(9):1191-1194.
Diederich N., Keipes M., Grass M., Metz H. Use of clozapine for psychiatric complications of Parkinson’s disease. Rev Neurol. 1995;151:251-257.
Dooneief G., Chen J., Mirabello E., et al. An estimate of the incidence of depression in idiopathic Parkinson’s disease. Arch Neurol. 1992;49:305-307.
Douyon R., Serby M., Klutchko B., Rotrosen J. ECT and Parkinson’s disease revisited: a “naturalistic” study. Am J Psychiatry. 1989;146:1451-1455.
Driver-Dunckley E., Samanta J., Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61(3):422-423.
Dubois B., Burn D., Goetz C., et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
Dujardin K., Sockeel P., Delliaux M., et al. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov Disord. 2009;24(16):2391-2397.
Eadie M.J. The pathology of certain medullary nuclei in parkinsonism. Aust Ann Med. 1963;86:781-792.
Earley C.J., Kuwabara H., Wong D.F., et al. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep. 2011;34(3):341-347.
Edwards L.L., Pfeiffer R.F., Quigley E.M., et al. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord. 1991;6:151-156.
Edwards L.L., Quigley E.M., Harned R.K., et al. Defecatory function in Parkinson’s disease: response to apomorphine. Ann Neurol. 1993;33:490-493.
Edwards L.L., Quigley E.M., Harned R.K., et al. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89:15-25.
Edwards L.L., Quigley E.M., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease – frequency and pathophysiology. Neurology. 1992;42:726-732.
Efthimiou J., Ellis S.J., Hardie R.J., Stern G.M. Sleep apnea in idiopathic and postencephalitic parkinsonism. Adv Neurol. 1987;45:275-276.
Ehrt U., Broich K., Larsen J.P., et al. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry. 2010;81(2):160-165.
Eichhorn T.E., Brunt E., Oertel W.H. Ondansetron treatment of L-dopa-induced psychosis. Neurology. 1996;47:1608-1609.
Elibol B., Hattori N., Atac F.B., et al. Distinguishing clinical features in patients with parkin mutations. Neurology. 2000;54(Suppl 3):A444.
Emre M., Aarsland D., Albanese A., et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509-2518.
Emre M., Aarsland D., Brown R., et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689-1707.
Evans A.H., Katzenschlager R., Paviour D., et al. Punding in Parkinson’s disease: Its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19(4):397-405.
Evans A.H., Stegeman J.R. Punding in patients on dopamine agonists for restless leg syndrome. Mov Disord. 2009;24(1):140-141.
Factor S.A., Brown D. Clozapine prevents recurrence of psychosis in Parkinson’s disease. Mov Disord. 1992;7:125-131.
Factor S.A., Brown D., Molho E.S., Podskalny G.D. Clozapine: A 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44:544-546.
Factor S.A., Friedman J.H. The emerging role of clozapine in the treatment of movement disorders. Mov Disord. 1997;12:483-496.
Factor S.A., McAlarney T., Sanchez-Ramos J.R., Weiner W.J. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 1990;4:280-285.
Fahn S., Chouinard S. Experience with tranylcypromine in early Parkinson’s disease. J Neural Transm. 1998;52(Suppl):49-61.
Fasano A., Elia A.E., Soleti F., et al. Punding and computer addiction in Parkinson’s disease. Mov Disord. 2006;21(8):1217-1218.
Ferini-Strambi L., Zucconi M. REM sleep behavior disorder. Clin Neurophysiol. 2000;111:S136-S140.
Fernandez H.H., Friedman J.H. Punding on l-dopa. Mov Disord. 1999;14:836-838.
Fernandez H.H., Friedman J.H., Jacques C., Rosenfeld M. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14:484-487.
Fernandez H.H., Trieschmann M.E., Friedman J.H. Aripiprazole for drug-induced psychosis in Parkinson disease: Preliminary experience. Clin Neuropharmacol. 2004;27(1):4-5.
Ferreira J.J., Galitzky M., Montastruc J.L., Rascol O. Sleep attacks and Parkinson’s disease treatment. Lancet. 2000;355:1333-1334.
Fitzmaurice H., Fowler C.J., Rickards D., et al. Micturition disturbance in Parkinson’s disease. Br J Urol. 1985;57:652-656.
Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S98-103.
Ford B., Louis E.D., Greene P., Fahn S. Oral and genital pain syndromes in Parkinson’s disease. Mov Disord. 1996;11:421-426.
Forsaa E.B., Larsen J.P., Wentzel-Larsen T., Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75(14):1270-1276.
Forsaa E.B., Larsen J.P., Wentzel-Larsen T., et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996-1001.
Friedman J. Olanzapine in the treatment of dopaminomimetic psychosis in patients with Parkinson’s disease. Neurology. 1998;50:1195-1196.
Friedman J.H., Amick M.M., Chou K.L. Rhinorrhea and olfaction in Parkinson disease. Neurology. 2008;70(6):487-489.
Friedman J.H., Brown R.G., Comella C., et alWorking Group on Fatigue in Parkinson’s Disease. Fatigue in Parkinson’s disease: a review. Mov Disord. 2007;22(3):297-308.
Friedman J.H., Feinberg S.S., Feldman R.G. A neuroleptic malignantlike syndrome due to levodopa therapy withdrawal. JAMA. 1985;254:2792-2795.
Friedman J.H., Lannon M.C. Clozapine in idiopathic Parkinson’s disease. Neurology. 1990;40:1151-1152.
Friedman J., Lannon M., Comella C., et al. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med. 1999;340:757-763.
Frucht S., Rogers J.D., Greene P.E., et al. Falling asleep at the wheel: Motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52:1908-1910.
Galvin J.E., Malcom H., Johnson D., Morris J.C. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. 2007;68(22):1895-1901.
Galvin J.E., Pollack J., Morris J.C. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67(9):1605-1611.
Giladi N., Shabtai H., Gurevich T., et al. Rivastigmine (Exelon) for dementia in patients with Parkinson’s disease. Acta Neurol Scand. 2003;108(5):368-373.
Gill D.J., Freshman A., Blender J.A., Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043-1046.
Gjerstad M.D., Alves G., Wentzel-Larsen T., et al. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006;67(5):853-858.
Goetz C.G., Blasucci L.M., Leurgans S., Pappert E.J. Olanzapine and clozapine – comparative effects on motor function in hallucinating PD patients. Neurology. 2000;55:789-794.
Goetz C.G., Fan W., Leurgans S., et al. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol. 2006;63(5):713-716.
Goetz C.G., Stebbins G.T. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology. 1993;43:2227-2229.
Goetz C.G., Tanner C.M., Levy M., et al. Pain in Parkinson’s disease. Mov Disord. 1986;1:45-49.
Goetz C.G., Tilley B.C., Schaftman S., et al. Clinimetric testing of the new UPDRS (MDS-UPDRS) vs original version. Mov Disord. 2007;22(Suppl 16):S6.
Greene P., Cote L., Fahn S. Treatment of drug-induced psychosis in Parkinson’s disease with clozapine. Adv Neurol. 1993;60:703-706.
Grosset K.A., Bone I., Reid J.L., Grosset D. Measuring therapy adherence in Parkinson’s disease: a comparison of methods. J Neurol Neurosurg Psychiatry. 2006;77(2):249-251.
Grosset K.A., Macphee G., Pal G., et al. Problematic gambling on dopamine agonists: Not such a rarity. Mov Disord. 2006;21(12):2206-2208.
Grosset K.A., Reid J.L., Grosset D.G. Medicine-taking behavior: implications of suboptimal compliance in Parkinson’s disease. Mov Disord. 2005;20(11):1397-1404.
Gschwandtner U., Aston J., Renaud S., Fuhr P. Pathologic gambling in patients with Parkinson’s disease. Clin Neuropharmacol. 2001;24:170-172.
Haehner A., Boesveldt S., Berendse H.W., et al. Prevalence of smell loss in Parkinson’s disease – a multicenter study. Parkinsonism Relat Disord. 2009;15(7):490-494.
Haehner A., Hummel T., Hummel C., et al. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 2007;22(6):839-842.
Happe S., Pirker W., Sauter C., et al. Successful treatment of excessive daytime sleepiness in Parkinson’s disease with modafinil. J Neurol. 2001;248:632-634.
Hauser R.A., Wahba M.N., Zesiewicz T.A., Anderson W.M. Modafinil treatment of pramipexole-associated somnolence. Mov Disord. 2000;15:1269-1271.
Hawkes C.H., Del Tredici K., Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(2):79-84.
Hely M.A., Morris J.G.L., Reid W.G.J., Trafficante R. Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20(2):190-199.
Hely M.A., Reid W.G., Adena M.A., et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837-844. 30
Hening W., Walters A., Kavey N., et al. Dyskinesias while awake and periodic movements in sleep in restless legs syndrome: Treatment with opioids. Neurology. 1986;36:1363-1366.
Hirschorn K.A., Greenberg H.S. Successful treatment of levodopa-induced myoclonus and levodopa withdrawal-induced neuroleptic malignant syndrome: A case report. Clin Neuropharmacol. 1988;2:278-281.
Hoehn M.M. Falling asleep at the wheel: Motor vehicle mishaps in people taking pramipexole and ropinirole. Neurology. 2000;54:275.
Homann C.N., Wenzel K., Suppan K., et al. Sleep attacks in patients taking dopamine agonists: review. Br Med J. 2002;324(7352):1483-1487.
Hoops S., Nazem S., Siderowf A.D., et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738-1745.
Huang C., Mattis P., Perrine K., et al. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 Pt 2):1470-1477.
Huang C., Mattis P., Tang C., et al. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34(2):714-723.
Hughes T.A., Ross H.F., Musa S., et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology. 2000;54:1596-1602.
Hughes T.A., Ross H.F., Mindham R.H.S., Spokes E.G.S. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118-123.
Hussain I.F., Brady C.M., Swinn M.J., et al. Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson’s disease or multiple system atrophy with observations. J Neurol Neurosurg Psychiatry. 2001;71:371-374.
Ilson J., Braun N., Fahn S. Respiratory fluctuations in Parkinson’s disease. Neurology. 1983;33(Suppl 2):113.
Ilson J., Fahn S., Cote L. Painful dystonic spasms in Parkinson’s disease. Adv Neurol. 1984;40:395-398.
Inzelberg R., Kipervasser S., Korczyn A.D. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533-535.
Jacobs H., Vieregge A., Vieregge P. Sexuality in young patients with Parkinson’s disease: a population based comparison with healthy controls. J Neurol Neurosurg Psychiatry. 2000;69:550-552.
Jansen Steur E.N.H. Increase of Parkinson disability after fluoxetine medication. Neurology. 1993;43:211-213.
Jimenez-Jimenez F.J., Tallon-Barranco A., Orti-Pareja M., et al. Olanzapine can worsen parkinsonism. Neurology. 1998;50:1183-1184.
Jimenez-Jimenez F.J., Tejeiro J., Martinez-Junquero G., et al. Parkinsonism exacerbated by paroxetine. Neurology. 1994;44:2406.
Juncos J.L., Roberts V.J., Evatt M.L., et al. Quetiapine improves psychotic symptoms and cognition in Parkinson’s disease. Mov Disord. 2004;19(1):29-35.
Kahn N., Freeman A., Juncos J.L., et al. Clozapine is beneficial for psychosis in Parkinson’s disease. Neurology. 1991;41:1699-1700.
Karlsen K., Larsen J.P., Tandberg E., Jorgensen K. Fatigue in patients with Parkinson’s disease. Mov Disord. 1999;14:237-241.
Karlsen K.H., Larsen J.P., Tandberg E., Maeland J.G. Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;66:431-435.
Kasten M., Kertelge L., Brüggemann N., et al. Nonmotor symptoms in genetic Parkinson disease. Arch Neurol. 2010;67(6):670-676.
Kavey N., Walters A.S., Hening W., Gidro-Frank S. Opioid treatment of periodic movements in sleep in patients without restless legs. Neuropeptides. 1988;11(4):181-184.
Kaye J., Gage H., Kimber A., et al. Excess burden of constipation in Parkinson’s disease: a pilot study. Mov Disord. 2006;21(8):1270-1273.
Kirsch-Darrow L., Fernandez H.H., Marsiske M., et al. Dissociating apathy and depression in Parkinson disease. Neurology. 2006;67(1):33-38.
Kitamura J., Kubuki Y., Tsuruta K., et al. A new family with Joseph disease in Japan. Homovanillic acid, magnetic resonance, and sleep apnea studies. Arch Neurol. 1989;46:425-428.
Koller W.C. Sensory symptoms in Parkinson’s disease. Neurology. 1984;34:957-959.
Kumru H., Santamaria J., Valldeoriola F., et al. Increase in body weight after pramipexole treatment in Parkinson’s disease. Mov Disord. 2006;21(11):1972-1974.
Kupsky W.J., Grimes M.M., Sweeting J., et al. Parkinson’s disease and megacolon: Concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253-1255.
Kurlan R. Disabling repetitive behaviors in Parkinson’s disease. Mov Disord. 2004;19(4):433-437.
Kurlan R., Cummings J., Raman R., Thal L., Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356-1363.
Laihnen A., Alihanka J., Raitasuo, et al. Sleep movements and associated autonomic nervous activities in patients with Parkinson’s disease. Acta Neurol Scand. 1987;76:64-68.
Lang A.E. Restless legs syndrome and Parkinson’s disease: insights into pathophysiology. Clin Neuropharmacol. 1987;10:476-478.
Lang A.E. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9:188-192.
Lang A.E. A critical appraisal of the premotor symptoms of Parkinson’s disease: Potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord. 2011;26(5):775-783.
Lang A.E., Johnson K. Akathisia in idiopathic Parkinson’s disease. Neurology. 1987;37:477-481.
Langston J.W. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
Lee J.Y., Kim H.J., Jeon B.S. Is pathological gambling in Parkinson’s disease reduced by amantadine? Ann Neurol. 2011;69(1):213-214.
Lees A.J. A sustained-release formulation of L-dopa (Madopar HBS) in the treatment of nocturnal and early-morning disabilities in Parkinson’s disease. Eur Neurol. 1987;27(suppl 1):126-134.
Lees A.J., Smith E. Cognitive defects in the early stages of Parkinson’s disease. Brain. 1983;106:257-270.
Leroi I., Overshott R., Byrne E.J., et al. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov Disord. 2009;24(8):1217-1221.
Levin B.E., Llabre M.M., Weiner W.J. Cognitive impairment associated with early Parkinson’s disease. Neurology. 1989;39:557-561.
Lim S.Y., Fox S.H., Lang A.E. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66(2):167-172.
Lipp A., Trottenberg T., Schink T., et al. A randomized trial of botulinum toxin A for treatment of drooling. Neurology. 2003;61(9):1279-1281.
Lippa C.F., Duda J.E., Grossman M., et alDLB/PDD Working Group. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812-819.
Lou J.S., Dimitrova D.M., Park B.S., et al. Using modafinil to treat fatigue in Parkinson disease: a double-blind, placebo-controlled pilot study. Clin Neuropharmacol. 2009;32(6):305-310.
Lynch T., Sano M., Marder K.S., et al. Clinical characteristics of a family with chromosome 17-linked disinhibition dementia parkinsonism amyotrophy complex. Neurology. 1994;44:1878-1884.
Mathers S.E., Kempster P.A., Law P.J., et al. Anal sphincter dysfunction in Parkinson’s disease. Arch Neurol. 1989;46:1061-1064.
Mathers S.E., Kempster P.A., Swash M., Lees A.J. Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon. J Neurol Neurosurg Psychiatry. 1988;51:1503-1507.
Matison R., Mayeux R., Rosen J., Fahn S. ‘Tip-of-the-tongue’ phenomenon in Parkinson disease. Neurology. 1982;32:567-570.
Mattila P.M., Rinne J.O., Helenius H., et al. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100:285-290.
Mayeux R., Chen J., Mirabello E., et al. An estimate of the incidence of dementia in idiopathic Parkinson’s disease. Neurology. 1990;40:1513-1517.
McKeith I., DelSer T., Spano P., et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031-2036.
McKeith I.G., Galasko D., Kosaka K., et al. Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies: report of the consortium on DLB International Workshop. Neurology. 1996;47:1113-1124.
McKeith I.G., Perry E.K., Perry R.H. Report of the second dementia with Lewy body international workshop – diagnosis and treatment. Neurology. 1999;53:902-905.
McKeon A., Josephs K.A., Klos K.J., et al. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2007;13(8):516-519.
Melamed E. Early-morning dystonia: A late side effect of long-term levodopa therapy in Parkinson’s disease. Arch Neurol. 1979;36:308-310.
Melton L.J.3rd, Leibson C.L., Achenbach S.J., et al. Fracture risk after the diagnosis of Parkinson’s disease: Influence of concomitant dementia. Mov Disord. 2006;21(9):1361-1367.
Mendonça D.A., Menezes K., Jog M.S. Methylphenidate improves fatigue scores in Parkinson disease: a randomized controlled trial. Mov Disord. 2007;22(14):2070-2076.
Menza M., Dobkin R.D., Marin H., et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72(10):886-892.
Merello M., Leiguarda R. Adynamic bowel syndrome in Parkinson’s disease with dramatic response to apomorphine. Clin Neuropharmacol. 1994;17:574-577.
Merims D., Balas M., Peretz C., et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson’s disease psychosis. Clin Neuropharmacol. 2006;29(6):331-337.
Miyasaki J.M., Al Hassan K., Lang A.E., Voon V. Punding prevalence in Parkinson’s disease. Mov Disord. 2007;22(8):1179-1181.
Miyasaki J.M., Shannon K., Voon V., et alQuality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):996-1002.
Morgante L., Epifanio A., Spina E., et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27(4):153-156.
Müller B., Larsen J.P., Wentzel-Larsen T., et al. Autonomic and sensory symptoms and signs in incident, untreated Parkinson’s disease: frequent but mild. Mov Disord. 2011;26(1):65-72.
Naimark D., Jackson E., Rockwell E., Jeste D.V. Psychotic symptoms in Parkinson’s disease patients with dementia. J Am Geriatr Soc. 1996;44:296-299.
Nausieda P.A., Weiner W.J., Kaplan L.R., et al. Sleep disruption in the course of chronic levodopa therapy: an early feature of the levodopa psychosis. Clin Neuropharmacol. 1982;5:183-194.
Nègre-Pagès L., Regragui W., Bouhassira D., et alDoPaMiP Study Group. Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23(10):1361-1369.
Nirenberg M.J., Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21(4):524-529.
Nissenbaum H., Quinn N.P., Brown R.G., et al. Mood swings associated with the ‘on-off’ phenomenon in Parkinson’s disease. Psychol Med. 1987;17:899-904.
Oka H., Yoshioka M., Morita M., et al. Reduced cardiac 123I-MIBG uptake reflects cardiac sympathetic dysfunction in Lewy body disease. Neurology. 2007;69(14):1460-1465.
Oka H., Yoshioka M., Onouchi K., et al. Characteristics of orthostatic hypotension in Parkinson’s disease. Brain. 2007;130(Pt 9):2425-2432.
Okuma Y., Kamei S., Morita A., et al. Fatigue in Japanese patients with Parkinson’s disease: a study using Parkinson fatigue scale. Mov Disord. 2009;24(13):1977-1983.
Ondo W.G., Hunter C., Moore W. A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson’s disease. Neurology. 2004;62(1):37-40.
Ondo W.G., Perkins T., Swick T., et al. Sodium oxybate for excessive daytime sleepiness in Parkinson disease: an open-label polysomnographic study. Arch Neurol. 2008;65(10):1337-1340.
Ondo W.G., Tintner R., Dat Voung K., et al. Doubleblind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord. 2005;20:958-963.
Ondo W.G., Vuong K.D., Khan H., et al. Daytime sleepiness and other sleep disorders in Parkinson’s disease. Neurology. 2001;57:1392-1396.
Orimo S., Uchihara T., Nakamura A., et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131(Pt 3):642-650.
O’Sullivan S.S., Williams D.R., Gallagher D.A., et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord. 2008;23(1):101-106.
Pardini M., Huey E.D., Cavanagh A.L., Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66(1):92-96.
Peralta C.M., Frauscher B., Seppi K., et al. Restless legs syndrome in Parkinson’s disease. Mov Disord. 2009;24(14):2076-2080.
Perry T.L., Wright J.M., Berry K., et al. Dominantly inherited apathy, central hypoventilation, and Parkinson’s syndrome: clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology. 1990;40:1882-1887.
Pfeiffer R.F., Kang J., Graber B., et al. Clozapine for psychosis in Parkinson’s disease. Mov Disord. 1990;5:239-242.
Pinter M.M., Helscher R.J. Therapeutic effect of clozapine in psychotic decompensation in idiopathic Parkinson’s disease. J Neural Transm-Parkinsons Dis Dement Sect. 1993;5:135-146.
Pollak P., Tison F., Rascol O., et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
Ponsen M.M., Stoffers D., Booij J., et al. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56(2):173-181.
Ponsen M.M., Stoffers D., Twisk J.W., et al. Hyposmia and executive dysfunction as predictors of future Parkinson’s disease: a prospective study. Mov Disord. 2009;24(7):1060-1065.
Postuma R.B., Gagnon J.F., Vendette M., et al. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296-1300.
Postuma R.B., Gagnon J.F., Rompré S., Montplaisir J.Y. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74(3):239-244.
Postuma R.B., Lang A.E., Massicotte-Marquez J., Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66(6):845-851.
Pursiainen V., Haapaniemi T.H., Korpelainen J.T., et al. Sweating in Parkinsonian patients with wearing-off. Mov Disord. 2007;22(6):828-882.
Qualman S.J., Haupt H.M., Yang P., Hamilton S.J. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87:848-856.
Quinn N.P., Koller W.C., Lang A.E., Marsden C.D. Painful Parkinson’s disease. Lancet. 1986;1:1366-1369.
Rabey J.M., Prokhorov T., Miniovitz A., et al. Effect of quetiapine in psychotic Parkinson’s disease patients: a double-blind labeled study of 3 months’ duration. Mov Disord. 2007;22(3):313-318.
Rabey J.M., Treves T.A., Neufeld M.Y., et al. Low-dose clozapine in the treatment of levodopa-induced mental disturbances in Parkinson’s disease. Neurology. 1995;45:432-434.
Racette B.A., Good L., Sagitto S., Perlmutter J.S. Botulinum toxin B reduces sialorrhea in parkinsonism. Mov Disord. 2003;18(9):1059-1061.
Ravina B., Marder K., Fernandez H.H., et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061-1068.
Ravina B., Putt M., Siderowf A., et al. Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry. 2005;76(7):934-939.
Reading P.J., Luce A.K., McKeith I.G. Rivastigmine in the treatment of parkinsonian hallucinosis. Neurology. 2002;58:A380.
Richard I.H., Maughn A., Kurlan R. Do serotonin reuptake inhibitor antidepressants worsen Parkinson’s disease? A retrospective case series. Mov Disord. 1999;14:155-157.
Ross G.W., Abbott R.D., Petrovitch H., et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21(12):2062-2067.
Ross G.W., Petrovitch H., Abbott R.D., et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63(2):167-173.
Roth A.J., Nelson C., Rosenfeld B., et al. Methylphenidate for fatigue in ambulatory men with prostate cancer. Cancer. 2010;116(21):5102-5110.
Ruggieri S., Depandis M.F., Bonamartini A., et al. Low dose of clozapine in the treatment of dopaminergic psychosis in Parkinson’s disease. Clin Neuropharmacol. 1997;20:204-209.
Rye D.B., Bliwise D.L., Dihenia B., Gurecki P. Daytime sleepiness in Parkinson’s disease. J Sleep Res. 2000;9:63-69.
Sage J.I., Mark M.H. Drenching sweats as an off phenomenon in Parkinson’s disease: Treatment and relation to plasma levodopa profile. Ann Neurol. 1995;37:120-122.
Salazar-Grueso E.F., Rosenberg R.S., Roos R.P. Sleep apnea in olivopontocerebellar degeneration: treatment with trazodone. Ann Neurol. 1988;23:399-401.
Sanchez-Ramos J.R., Ortoll R., Paulson G.W. Visual hallucinations associated with Parkinson’s disease. Arch Neurol. 1996;53:1265-1268.
Sato Y., Honda Y., Iwamoto J. Risedronate and ergocalciferol prevent hip fracture in elderly men with Parkinson disease. Neurology. 2007;68(12):911-915.
Sato Y., Iwamoto J., Kanoko T., Satoh K. Alendronate and vitamin D2 for prevention of hip fracture in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2006;21(7):924-929.
Scaglione C., Vignatelli L., Plazzi G., et alBologna, Genova, Parma and Pisa Universities group for the study of REM Sleep Behavior Disorder in Parkinson’s Disease. REM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based study. Neurol Sci. 2005;25(6):316-321.
Schapira A.H.V. Sleep attacks (sleep episodes) with pergolide. Lancet. 2000;355:1332-1333.
Schenck C.H., Bundlie S.R., Ettinger M.G., Mahowald M.W. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293-308.
Schenck C.H., Bundlie S.R., Mahowald M.W. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46(2):388-393. Erratum in: Neurology 1996; 46(6):1787
Schenck C.H., Bundlie S.R., Patterson A.L., Mahowald M.W. Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA. 1987;257:1786-1789.
Schifitto G., Friedman J.H., Oakes D., et alParkinson Study Group ELLDOPA Investigators. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. 2008;71(7):481-485.
Scholz E., Dichgans J. Treatment of drug-induced exogenous psychosis in parkinsonism with clozapine and fluperlapine. Eur Arch Psychiatry Neurol Sci. 1985;235:60-64.
Schrag A., Barone P., Brown R.G., et al. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22(8):1077-1092.
Schrag A., Jahanshahi M., Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15:1112-1118.
Schrag A., Jahanshahi M., Quinn N.P. What contributes to depression in Parkinson’s disease? Psychol Med. 2001;31:65-73.
Shpirer I., Miniovitz A., Klein C., et al. Excessive daytime sleepiness in patients with Parkinson’s disease: a polysomnography study. Mov Disord. 2006;21(9):1432-1438.
Shulman L.M., Taback R.L., Bean J., Weiner W.J. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16:507-510.
Siderowf A., Xie S.X., Hurtig H., et al. CSF amyloid β 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055-1061.
Silver N., Allen R.P., Senerth J., Earley C.J. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med. 2011;12(5):440-444.
Simuni T., Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol. 2008;64(Suppl 2):S65-S80.
Singer W., Opfer-Gehrking T.L., Nickander K.K., et al. Acetylcholinesterase inhibition in patients with orthostatic intolerance. J Clin Neurophysiol. 2006;23(5):476-481.
Snider S.R., Fahn S., Isgreen W.P., Cote L.J. Primary sensory symptoms in parkinsonism. Neurology. 1976;26:423-429.
South A.R., Somers S.M., Jog M.S. Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology. 2010;74(15):1198-1202.
Starkstein S.E., Merello M., Jorge R., et al. The syndromal validity and nosological position of apathy in Parkinson’s disease. Mov Disord. 2009;24(8):1211-1216.
Steeves T.D., Miyasaki J., Zurowski M., et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132(Pt 5):1376-1385.
Stefanova E., Potrebic A., Ziropadja L., et al. Depression predicts the pattern of cognitive impairment in early Parkinson’s disease. J Neurol Sci. 2006;248(1–2):131-137.
Stein M.B., Heuser I.J., Juncos J.L., Uhde T.W. Anxiety disorders in patients with Parkinson’s disease. Am J Psychiatry. 1990;147:217-220.
Stephenson R., Houghton D., Sundarararjan S., et al. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson’s disease. Mov Disord. 2010;25(13):2099-2104.
Stocchi F., Carbone A., Inghilleri M., et al. Urodynamic and neurophysiological evaluation in Parkinson’s disease and multiple system atrophy. J Neurol Neurosurg Psychiatry. 1997;62:507-511.
Strieder D.J., Baker W.G., Baringer J.R., Kazemi H. Chronic hypoventilation of central origin. A case with encephalitis lethargica and Parkinson’s syndrome. Am Rev Respir Dis. 1967;96:501-507.
Swinn L., Schrag A., Viswanathan R., et al. Sweating dysfunction in Parkinson’s disease. Mov Disord. 2003;18(12):1459-1463.
Swope D.M. Preliminary report: Use of sildenafil to treat dyskinesias in patients with Parkinson’s disease. Neurology. 2000;54(Suppl 3):A90-A91.
Tan A., Salgado M., Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa. Mov Disord. 1996;11:214-216.
Tandberg E., Larsen J.P., Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson’s disease: A community-based study. Mov Disord. 1999;14:922-927.
Tesei S., Antonini A., Canesi M., et al. Tolerability of paroxetine in Parkinson’s disease: A prospective study. Mov Disord. 2000;15:986-989.
Thomas A., Bonanni L., Gambi F. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68(3):400-404.
Tippmann-Peikert M., Park J.G., Boeve B.F., et al. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68(4):301-303.
Trenkwalder C., Kohnen R., Högl B., et al. Parkinson’s disease sleep scale-validation of the revised version PDSS-2. Mov Disord. 2011;26(4):644-652.
Uc E.Y., Struck L.K., Rodnitzky R.L., et al. Predictors of weight loss in Parkinson’s disease. Mov Disord. 2006;21(7):930-936.
Van Hilten B., Hoff J.I., Middelkoop H.A.M., et al. Sleep disruption in Parkinson’s disease – assessment by continuous activity monitoring. Arch Neurol. 1994;51:922-928.
Van Laar T., de Vries J.J., Nakhosteen A., Leenders K.L. Rivastigmine as anti-psychotic in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2001;7(Suppl):S73.
Verbaan D., Marinus J., Visser M., et al. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69(4):333-341.
Verbaan D., van Rooden S.M., Visser M., et al. Nighttime sleep problems and daytime sleepiness in Parkinson’s disease. Mov Disord. 2008;23(1):35-41.
Voon V., Hassan K., Zurowski M., et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66(11):1750-1752.
Voon V., Hassan K., Zurowski M., et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254-1257.
Voon V., Kubu C., Krack P., et al. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(Suppl 14):S305-S327.
Voon V., Thomsen T., Miyasaki J.M., et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007;64(2):212-216.
Wakabayashi K., Takahashi H., Takeda E., et al. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217-221.
Walters A.S., Wagner M.L., Hening W.A., et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327-332.
Weintraub D., Hoops S., Shea J.A., et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461-1467.
Weintraub D., Koester J., Potenza M.N., et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
Weintraub D., Mavandadi S., Mamikonyan E., et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75(5):448-455.
Weintraub D., Siderowf A.D., Potenza M.N., et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63(7):969-973.
Weintraub D., Sohr M., Potenza M.N., et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010;68(6):963-968.
Williams-Gray C.H., Foltynie T., Brayne C.E., et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787-1798.
Winge K., Fowler C.J. Bladder dysfunction in Parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21(6):737-745.
Wolters E.C., Hurwitz T.A., Mak E., et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology. 1990;40:832-834.
Wolters E.C., Jansen E.N.H., Tuynman-Qua H.G., Bergmans P.L.M. Olanzapine in the treatment of dopaminomimetic psychosis in patients with Parkinson’s disease. Neurology. 1996;47:1085-1087.
Wong K.K., Muller M.L., Kuwabara H., et al. Olfactory loss and nigrostriatal dopaminergic denervation in the elderly. Neurosci Lett. 2010;484(3):163-167.
Wu J.C., Iacono R., Ayman M., et al. Correlation of intellectual impairment in Parkinson’s disease with FDG PET scan. Neuroreport. 2000;11:2139-2144.
Zesiewicz T.A., Helal M., Hauser R.A. Sildenafil citrate (Viagra) for the treatment of erectile dysfunction in men with Parkinson’s disease. Mov Disord. 2000;15:305-308.
Zoldan J., Friedberg C., Livneh M., Melamed E. Psychosis in advanced Parkinson’s disease: Treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology. 1995;45:1305-1308.