Noninvasive Ventilation
After reading this chapter you will be able to:
 Identify the goals and benefits of noninvasive ventilation (NIV).
Identify the goals and benefits of noninvasive ventilation (NIV).
 Discuss indications for NIV and the relative strength of the supporting evidence for each indication.
Discuss indications for NIV and the relative strength of the supporting evidence for each indication.
 List the selection and exclusion criteria for successful NIV.
List the selection and exclusion criteria for successful NIV.
 List the factors that predict successful NIV.
List the factors that predict successful NIV.
 Identify the types of patient interfaces available for NIV and how to choose an appropriate interface for a patient.
Identify the types of patient interfaces available for NIV and how to choose an appropriate interface for a patient.
 Discuss the types of mechanical ventilators and ventilation modes used to provide NIV.
Discuss the types of mechanical ventilators and ventilation modes used to provide NIV.
 Describe the role of the respiratory therapist during the initial application of NIV.
Describe the role of the respiratory therapist during the initial application of NIV.
 Describe the ongoing ventilator management of NIV in the acute care setting.
Describe the ongoing ventilator management of NIV in the acute care setting.
 List potential complications associated with NIV, and suggest possible solutions.
List potential complications associated with NIV, and suggest possible solutions.
Interest in NIV has increased in recent years with the publication of findings from clinical trials using NIV in the management of respiratory failure. At the same time, technologically advanced noninvasive ventilators were introduced. Most intensive care unit (ICU) ventilators now include a specific noninvasive mode of ventilation. Improvements in the design of patient interfaces have made them more comfortable so that patients can tolerate NIV for longer periods. The net effect of these developments is that clinicians are using NIV more often than ever before in both acute and long-term care settings.1 This chapter reviews the evidence supporting the use of NIV to manage various disease processes and makes recommendations on the types of patients and specific techniques for its successful application.
History and Development of Noninvasive Ventilators
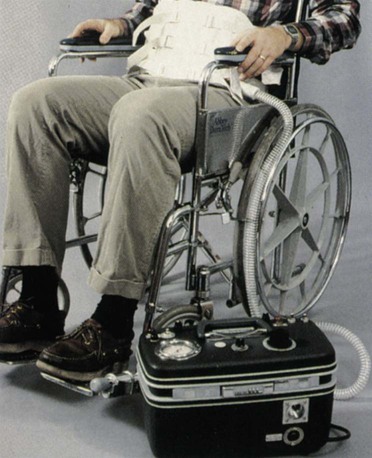
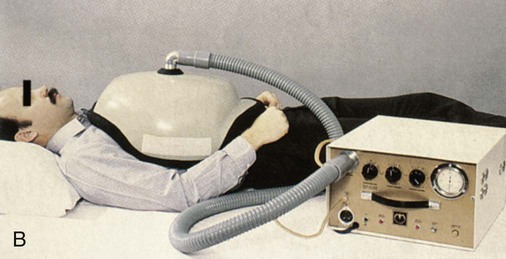
First described in the 1930s, the pneumobelt consists of a rubber bladder that is strapped around the abdomen and connected to a positive pressure ventilator (Figure 45-1).2 The pneumobelt is properly positioned above the pelvic arch and below the umbilicus. When inflated, the rubber bladder compresses the abdomen, pushes the diaphragm upward, and assists exhalation. On deflation of the rubber bladder, the abdominal contents and diaphragm move down, facilitating inspiration.2 Because this device requires gravity to be effective, the patient must be sitting at an angle of at least 30 degrees.3 Some abdominal mass is necessary for the pneumobelt to be effective. It does not work well in thin patients. Some patients with neuromuscular or neurologic disease and long-term dependence on a ventilator prefer to use a pneumobelt when they are out of bed and in a wheelchair.4
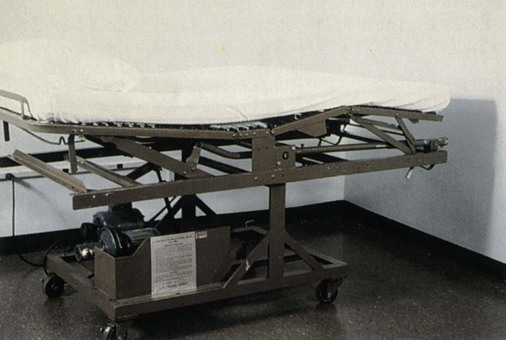
The rocking bed (Figure 45-2) periodically rocks from the Trendelenburg position to reverse Trendelenburg position, using gravity to produce exhalation and inspiration. Rocking beds were used in the 1950s to wean patients from negative pressure ventilators and to provide long-term ventilatory support to patients following recovery from polio.2 A major problem with the rocking bed is motion sickness, which prevents some patients from tolerating the device despite adequate ventilation.
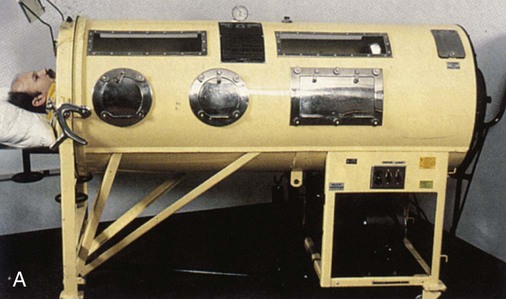
A negative pressure ventilator generates negative pressure within a chamber that surrounds the thorax. The chest wall expands owing to the decrease in pressure in the chamber, and intraalveolar pressure becomes negative, causing air to flow into the lung during inspiration. When the negative pressure is released, the lungs and chest wall return to their normal size owing to elastic recoil, resulting in passive exhalation. The first electrically powered negative pressure ventilator, known as the iron lung (Figure 45-3), surrounded the entire body from the neck down. Negative pressure ventilators were widely used from the late 1920s through the 1960s during the polio epidemic.2 Other designs were developed during that time, including the chest cuirass, which enclosed only the chest, the Porta-Lung, and the poncho wrap.2 Effective negative pressure ventilation can be challenging to achieve if the device has air leaks or does not fit properly. Negative pressure ventilators that enclose the body limit caregiver access to the patient. In addition, collapse of the upper airway can occur during inspiration when excessive negative pressure is applied. With the development of positive pressure ventilators and the expanded use of NPPV, interest in negative pressure ventilation has greatly diminished.2 Negative pressure ventilators are seldom seen in hospitals but continue to be used successfully in the home by patients with chronic respiratory failure from neuromuscular diseases, such as polio.
The first reported use of NPPV was in 1780, when Chaussier used a bag and face mask during resuscitation.1 However, widespread clinical use of NPPV did not begin until much later, with the introduction of intermittent positive pressure breathing in 1947.1,2 Intermittent positive pressure breathing was primarily used to deliver aerosolized medications. The use of intermittent positive pressure breathing declined significantly in the mid-1980s2 after no benefit was found compared with aerosol medication delivery with a small volume nebulizer in a randomized, controlled trial involving the treatment of patients with chronic obstructive pulmonary disease (COPD).5 Around this time, nasal mask CPAP was suggested as a therapy for obstructive sleep apnea.6 Nasal masks also were used nocturnally in conjunction with positive pressure ventilators to provide rest for the respiratory muscles in patients with neuromuscular disorders.7 In 1989, NPPV was used successfully to support 8 of 10 patients with acute respiratory failure (ARF).8 Those positive findings renewed the interest in NIV that continues today. NIV has been used to treat a wide variety of clinical conditions in numerous trials during the past 20 years. The next section provides background and discussion focusing on the most relevant studies. Study findings are evaluated, and evidence-based recommendations for NIV are provided for each indication.
Indications for Noninvasive Ventilation
Goals and Benefits of Using Noninvasive Ventilation
ARF causes impairment in gas exchange that is often severe enough for patients to require additional ventilatory support. The standard treatment for severe ARF is endotracheal intubation and mechanical ventilation. The primary goals of NIV are to improve gas exchange and avoid endotracheal intubation. If NIV is applied successfully, significant complications associated with intubation can be prevented. The goals and potential benefits of using NIV in the acute care setting include improving survival, decreasing the length of mechanical ventilation, decreasing the length of hospitalization, and decreasing the incidence of ventilator-associated pneumonia. In the long-term care setting, major goals are improving the patient’s quality of life and relieving symptoms of hypoventilation. The goals of NIV in acute and long-term care settings are listed in Box 45-1.
The primary indication for NIV is hypercapnic respiratory failure secondary to COPD exacerbation. Although patients with acute hypercapnic respiratory failure are the most likely to benefit from the use of NIV, it is also indicated for selected patients with hypoxemic respiratory failure or with respiratory failure resulting from numerous other conditions (Box 45-2).2,9
Acute Care Indications
Hypercapnic Respiratory Failure
Chronic Obstructive Pulmonary Disease
Evidence from numerous randomized, controlled trials strongly supports the use of NIV in the management of hypercapnic respiratory failure secondary to COPD exacerbation.10–15 Only one randomized, controlled trial that compared NIV with standard therapy in patients with an acute COPD exacerbation failed to show significant improvement in outcome.16 In this study, no patients in either the treatment or the control group required intubation, and all of the patients survived. The average arterial pH was higher than in other studies,11,12 and the resulting respiratory acidosis was less severe. This study has been criticized because the study patients, with only mild hypercapnic respiratory failure, were not sick enough to require NIV.13 The results of four trials show that patients with COPD and ARF require intubation less often with NIV than with standard medical treatment.10–13 Other studies have shown a reduction in hospital mortality,11,13–15 reduced length of hospital stay,11,12 and significantly fewer complications compared with standard therapy.11
Based on strong evidence from the aforementioned investigations, NIV should be considered the standard of care for treatment of an acute COPD exacerbation. NIV should be available as first-line therapy in all institutions treating patients with COPD.17
Asthma
NIV has been used successfully in the management of ARF caused by severe asthma, but the supporting evidence is weak. Meduri and colleagues18 reported positive results in an uncontrolled study using NIV in the care of 17 patients with status asthmaticus. The initial average arterial partial pressure of carbon dioxide (PaCO2) was 65 mm Hg, and pH was 7.25. Within 2 hours after initiation of NIV, gas exchange (both ventilation and oxygenation) had markedly improved. Arterial blood gas measurements during this initial period showed significantly deceased PaCO2, increased pH, and increased PaO2. Respiratory rate also was significantly reduced from an average of 29 breaths/min to 22 breaths/min. These physiologic changes were achieved without using excessively high airway pressures. The average inspiratory pressure used in the study was 18 cm H2O, and the maximum inspiratory pressure was 25 cm H2O.18 Only two of the patients in the study required intubation.
Several other studies have reported using NIV successfully to manage ARF in patients with asthma,19–21 but this indication remains controversial. Without a randomized, controlled trial focusing on this patient population, routine management with NIV cannot be recommended in asthmatic patients. Frequently, patients with severe asthma are unable to tolerate the tight-fitting mask necessary to provide NIV. Effective NIV can be difficult to achieve in patients with severe asthma and high airways resistance. They may require ventilating pressures that exceed 20 to 25 cm H2O, increasing the likelihood of failure. If patients with severe asthma receive a trial of NIV, they must be monitored closely. Significant improvement in the symptoms of respiratory failure should be evident within 1 to 2 hours, and if improvement is not evident, intubation should proceed without delay.
Facilitation of Weaning in Chronic Obstructive Pulmonary Disease
The use of NIV to facilitate weaning from mechanical ventilation has been primarily evaluated in patients with COPD. In two similar studies, patients who failed weaning trials were electively extubated to NIV. In one study, NIV reduced weaning time, length of ICU stay, nosocomial pneumonia rate, and 60-day mortality compared with conventional weaning with invasive pressure support ventilation (PSV).15 A second study showed no benefit from NIV, but this study was criticized because patients with COPD did not receive NIV before intubation, which is inconsistent with the current standard of care. In a third study, Ferrer and colleagues22 randomly assigned only patients who failed 3 consecutive days of spontaneous breathing trials and found that NIV was associated with faster weaning. A prospective case series of difficult-to-wean patients with COPD showed that both NIV and invasive PSV significantly reduced work of breathing and improved ventilation compared with a T-piece trial.23 This study also showed that NIV was associated with greater respiratory pump efficiency and lower dyspnea scores than invasive PSV in this patient population.23
Acute Cardiogenic Pulmonary Edema
In 1991, mask CPAP was shown to improve hypoxemia and reduce the need for intubation in patients with severe cardiogenic pulmonary edema.24 Similar findings in other randomized controlled trials provided strong evidence that both CPAP25,26 and NPPV27–29 improve outcomes in these patients compared with simple oxygen (O2) therapy. NPPV seems to be superior to CPAP only if hypercapnia is present.30–35 Mask CPAP of 8 to 12 cm H2O and 100% O2 is first-line therapy to treat hypoxemia associated with severe cardiogenic pulmonary edema. NPPV should be reserved only for patients who cannot adequately ventilate without assistance. Extra caution is recommended for patients who present with cardiac ischemia, hemodynamic instability, arrhythmias, or depressed mental status. Patients with these risk factors should be intubated and invasively ventilated.31
Pneumonia
Jolliet and associates36 studied NIV in 24 patients with severe community-acquired pneumonia. None of the patients in the study had chronic lung disease. Although oxygenation initially improved with NIV, two-thirds of the patients went on to require intubation, and one-third died. A randomized, controlled trial of NIV in patients with severe community-acquired pneumonia showed significant decreases in intubation rate, number of days in the ICU, and 2-month mortality when patients received NIV. Subgroup analysis determined that these positive findings occurred only in patients with underlying COPD.37 Further studies are needed to validate these findings. The current recommendation for the use of NIV in pneumonia is to limit its routine use to patients who also have COPD.37
Acute Lung Injury and Acute Respiratory Distress Syndrome
Several studies have reported failure rates greater than 50% when NIV is used to treat acute lung injury (ALI) and acute respiratory distress syndrome (ARDS).38–40 Patients with risk factors such as hemodynamic instability, metabolic acidosis, or profound hypoxemia are more likely to fail NIV.38 Survey data from centers in the United States and Europe with extensive experience using NIV indicate that more than 60% of patients with hypoxemic respiratory failure required intubation, with greater than 60% mortality.40 More recently, positive findings were reported in a prospective multicenter survey that used NIV as the first-line intervention in selected patients with ALI/ARDS.41 Results showed that 54% avoided intubation with improved outcome. Failure was predicted if PaO2/FiO2 ratio was less than 175 at 1 hour after initiation of NIV.41 Three randomized controlled trials in patients with ALI/ARDS found that NIV decreased intubation rate and mortality, resulting in improved outcome.40,42,43 However, a meta-analysis of these trials indicated that NIV did not decrease mortality despite a reduction in intubation rate,44 and this analysis was consistent with other reports.45
Another randomized controlled trial46 in patients with hypoxemic respiratory failure but no hypercapnia showed that mask CPAP significantly improved PaO2/FiO2 ratio within the first hour but failed to reduce the intubation rate, length of ICU stay, or hospital mortality. In this study, many patients who failed CPAP sustained cardiac arrest during intubation.46 It is thought that clinicians did not readily accept the failure of NIV to correct hypoxemia in these patients, resulting in an unacceptable delay before intubation that contributed to the poor outcome in the failed CPAP group.
The evidence to date does not support routine NIV use in patients with ALI/ARDS, but randomized controlled trials focusing exclusively on ALI/ARDS are needed.9 The data suggest that a closely monitored trial of NIV in carefully selected patients may be appropriate. If NIV does not markedly improve hypoxemia within 1 hour, patients should be intubated.
Respiratory Failure in Immunosuppressed Patients
The risk of developing nosocomial infections, including ventilator-associated pneumonia, is decreased when NIV is used compared with intubation and invasive mechanical ventilation. Randomized controlled trials involving immunosuppressed patients47 and patients awaiting solid organ transplantation48 who developed hypoxemic respiratory failure found decreased intubation rates and mortality with NIV compared with standard therapy. A nonrandomized trial of NIV in patients with AIDS and Pneumocystis carinii pneumonia showed similar results.49 Despite the small numbers of patients in these single-center trials, NIV is accepted as first-line therapy in immunosuppressed patients because it avoids the risk of infection50–52 associated with intubation in a setting where an infection can have devastating consequences.
Palliative Care and Do-Not-Intubate Orders
The use of NIV in patients with do-not-intubate (DNI) orders and end-stage disease remains controversial. Patients with DNI orders may receive NIV either for supportive treatment of a nonterminal, reversible event or for palliative care. There is evidence that NIV in patients with end-stage disease provides an effective method of support with some relief from associated symptoms.53 Two more recent case series54,55 support the use of NIV in the management of ARF in patients with DNI orders. Schettino and colleagues54 and Levy and coworkers55 showed that more than 65% of patients with COPD or cardiogenic pulmonary edema and DNI status were successfully managed with NIV. For palliative care application, the expectation is that NIV should make the process of dying more comfortable. If the patient is not more comfortable with NIV, it should be discontinued.
The primary controversy over the use of NIV in patients with DNI orders involves patient consent. Before consent is obtained, clinicians should take the time to discuss wishes for life-sustaining treatment with the patient and family and explain what NIV is and what it is intended to accomplish.56 NIV is appropriate and can be beneficial in the care of patients with DNI orders if a patient understands that NIV is a form of life support and that its goal is either to reverse an acute disease process or to provide a comfort measure at the end of life.56
Postoperative Respiratory Failure
In one study, prophylactic use of NIV by obese patients who underwent gastroplasty markedly improved pulse oximetry oxygen saturation (SpO2) and forced vital capacity (FVC), which allowed faster recovery of preoperative pulmonary function.57 Two studies compared postoperative use of noninvasive CPAP with simple O2 therapy. Squadrone and associates58 found that patients receiving CPAP had lower intubation, pneumonia, overall infection, and sepsis rates after major abdominal surgery. Kindgen-Milles and coworkers59 showed fewer pulmonary complications and a shorter hospital length of stay when patients received CPAP after thoracoabdominal aortic aneurysm repair. Although the results of these studies are encouraging, additional randomized trials are needed to identify specific postoperative populations that would benefit from NPPV or CPAP. At the present time, there is insufficient evidence to support routine postoperative use of NIV.
Prevention of Reintubation in High-Risk Patients
Reintubation has been associated with increased mortality, longer hospital stay, and a greater need for long-term care than in patients who are initially successfully extubated.60 In more recent studies, Nava and colleagues61 and Ferrer and coworkers62 randomly assigned patients at risk for reintubation to NIV or standard care; both studies showed lower reintubation rates with NIV. Patients with hypercapnia gained the most benefit from NIV. Risk factors associated with extubation failure included a diagnosis of COPD or congestive heart failure, age older than 65 years, ineffective cough and excessive secretions, upper airway obstruction, history of one or more weaning failures, one or more comorbid conditions, and Acute Physiology and Chronic Health Evaluation (APACHE) II score greater than 12 on the day of extubation.
Postextubation Respiratory Failure
The use of NIV to manage postextubation hypoxemic respiratory failure requires a cautious approach. Two randomized controlled trials63,64 indicated no benefit or worse outcome when NIV was used to manage hypoxemic ARF. Keenan and associates63 compared NIV and standard therapy in patients who developed respiratory failure during the first 2 days after extubation and observed a 70% reintubation rate in both groups. Esteban and colleagues64 randomly assigned patients to standard therapy or NIV at the first sign of postextubation respiratory distress. Both groups had a 50% reintubation rate, but the group managed with NIV had higher mortality. This finding was attributed to the delay in reintubation that occurred in the NIV group. Relatively few patients with COPD were included in these two studies. The use of NIV to treat postextubation ARF generally should be reserved for patients with COPD and hypercapnic respiratory failure or patients with congestive heart failure. If hypoxemia does not significantly improve with NIV, these patients should be reintubated without delay.
Long-Term Care Indications
Nocturnal Hypoventilation
Nocturnal hypoventilation is common with neuromuscular diseases, severe kyphoscoliosis, COPD, obesity, and central and obstructive sleep apnea.65 Patients with these disorders are able to breathe spontaneously without assistance but typically have symptoms related to hypoventilation and sleep-disordered breathing. These symptoms may include excessive sleepiness during daytime hours; fatigue; morning headaches; and cognitive dysfunction, such as difficulty concentrating.66
Normally, the onset of sleep is characterized by a slight increase in PaCO2 followed by a further increase in hypercapnia during rapid eye movement (REM) sleep. It is thought that the increased work of breathing associated with obesity and COPD or the muscle weakness caused by neuromuscular diseases results in greater levels of hypercapnia. Some of these patients are hyporesponsive to carbon dioxide (CO2), which contributes to even more CO2 retention. In response, the kidneys attempt to compensate by retaining bicarbonate, reducing respiratory drive further. This vicious cycle progressively worsens, leading to pulmonary hypertension, cor pulmonale, CO2 narcosis, and eventually death.65 There is strong evidence that the cycle can be stopped if breathing is assisted by NIV for 4 hours per night for 1 to 3 months.65 One study suggested that NIV use during the daytime hours promotes similar improvement in gas exchange during periods of unassisted ventilation as seen when NIV is used at night.67
Three mechanisms have been proposed to explain the positive effects of NIV on nocturnal hypoventilation. First, it was thought that NIV rests fatigued respiratory muscles, improving their performance during the day.68,69 Common sense supports this hypothesis, but few studies have been able to show significant or sustained improvement in muscle strength after using NIV.70 Second, NIV reduces PaCO2 and may reset the central ventilatory controller to a lower baseline PaCO2. Current evidence suggests that NIV is effective because it prevents nocturnal hypoventilation and preserves ventilatory response to increases in CO2 in patients with COPD or restrictive thoracic diseases.67,71–73 Third, the improvements in lung compliance, lung volume, and dead space that result from NIV may be beneficial.66
Restrictive Thoracic Diseases
Restrictive thoracic diseases successfully managed with NIV include postpolio syndrome, neuromuscular diseases, chest wall deformities, spinal cord injuries, and severe kyphoscoliosis.66 Patients with severe kyphoscoliosis showed improved nighttime and daytime gas exchange, fewer symptoms of hypoventilation, and increased spontaneous VT and FVC after using NIV.74 A study of eight patients with Pompe disease found that NIV corrected arterial blood gas abnormalities and resolved cor pulmonale 3 to 6 months after starting NIV.75 Disease progression was not slowed by NIV. Diaphragmatic weakness is characteristic of this disease, in contrast to most other neuromuscular diseases. After starting NIV, pulmonary function tests improved slightly, but the improvement was not sustained for a long period.75 Patients with Duchenne muscular dystrophy, a rapidly progressive neuromuscular disorder, were randomly assigned to either prophylactic nocturnal NIV or conventional treatment.76 In this study, nocturnal NIV failed to slow progression of the disease and was associated with a higher mortality.76
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a neurodegenerative disease that affects motor neurons, resulting in progressive muscle weakness and paralysis. Mean survival time is 3 to 5 years after diagnosis. All patients with ALS eventually need full ventilatory support to survive. NIV is probably effective in prolonging the lives of patients with ALS.77
In a randomized controlled trial, Bourke and associates78 found that patients with ALS who used NIV gained a median survival benefit of 205 days but only in the absence of bulbar dysfunction. Evidence suggests that using NIV slows the rate of lung function decline as indicated by FVC measurement.78,79 Lung function decline occurs more slowly and survival benefit increases when NIV is used for more than 4 hours per day.79 Several factors influencing compliance with NIV of patients with ALS have been identified. Early intervention80 and orthopnea81 correlated with better tolerance of NIV, whereas bulbar involvement78,82 and the presence of cognitive or executive dysfunction83 negatively affected compliance with NIV.
In contrast to other restrictive thoracic diseases, there may be a survival benefit when NIV is initiated earlier in the course of ALS. In one study, patients experiencing more than 15 episodes of nocturnal O2 desaturation per hour were started on NIV.80 This “early” intervention resulted in survival lasting 11 months longer and suggested that NIV may also provide some benefit in patients with bulbar involvement.80 More studies are needed to assess the effects of early NIV initiation.
Clinicians in the acute care setting have conflicting feelings about initiating NIV in patients with ALS, often voicing concerns about the patient’s quality of life and eventual dependence on NIV. Four studies found that NIV had a positive effect on patients’ quality of life.78,81,84,85 Positive changes associated with NIV included relief from dyspnea, increased energy and vitality, better concentration, less physical fatigue, and fewer symptoms of hypoventilation.85 There was no difference in the perceived quality of life of patients using NIV compared with patients who received invasive mechanical ventilation through a tracheostomy.86 However, invasive ventilation through a tracheostomy tube may have a negative effect on caregivers’ quality of life. Most patients were comfortable with their decisions involving assisted ventilation with 94% of patients receiving NIV and 81% of patients with a tracheostomy indicating that they would choose ventilation again.86
There is good evidence that using NIV lengthens survival and slows the decline of lung function of patients with ALS. In its evidence-based Practice Parameters, the American Academy of Neurology recommended considering NIV for all patients with ALS and respiratory failure.77 NIV is typically started when pulmonary function declines significantly (FVC < 50%). Although the evidence supporting early NIV initiation is weak, starting NIV at the first sign of nocturnal hypoventilation may be considered to improve compliance with NIV.
Chronic Obstructive Pulmonary Disease in Patients Needing Long-Term Care
There are two proposed hypotheses to explain how patients with severe COPD benefit from the use of NIV.66 First, positive inspiratory pressure improves gas exchange and may unload the respiratory muscles, allowing them to recover, gain strength, and reduce fatigue resulting in improved quality of life. Second, NIV should decrease the symptoms of nocturnal hypoventilation and sleep-disordered breathing, improving sleep quality and daytime gas exchange.66
The use of NIV in the management of stable COPD is controversial. Four studies investigated the use of nocturnal NIV at varied levels of pressure support in stable, hypercapnic patients with COPD.87–90 Only one of the studies showed improvement in gas exchange and quality of life.89 The major criticism of this study was that its sample size was too small to draw any meaningful conclusions. A meta-analysis of the four trials found that NIV did not significantly improve gas exchange, lung function, or sleep quality when used nocturnally by patients with COPD.70 In contrast, positive findings were reported in an Italian multicenter trial.91 This study included 90 patients with COPD who were randomly assigned to nocturnal NIV with O2 or O2 therapy alone. After 2 years, the NIV group had lower PaCO2, better quality of life, and fewer annual hospital days per patient.91 A small retrospective study reported that when NIV was started on a group of patients who required frequent hospitalizations, the number of annual hospital days decreased significantly from 78 to 25 per patient.92 Compliance with NIV therapy has been problematic in many studies involving patients with COPD.93
The role of NIV in the management of patients with stable, severe COPD has not been clearly established and remains controversial. A 1999 consensus conference report on indications for NIV recommended starting NIV in patients with stable COPD who have symptoms of hypoventilation plus PaCO2 of at least 55 mm Hg or PaCO2 of 50 to 54 mm Hg with nocturnal desaturation, or at least two hospitalizations for hypercapnic respiratory failure within the previous year.66
Obesity-Hypoventilation Syndrome
Obesity-hypoventilation syndrome (OHS) is defined as chronic daytime hypoventilation (PaCO2 > 45 mm Hg) associated with obesity (body mass index >30 kg/m2) when no other known cause for hypoventilation is present. Approximately 0.5% of women and 1% of men in the general population are estimated to have OHS.94 Evidence suggests that OHS is a common yet underdiagnosed condition in extremely obese patients.95 Obese patients consume many health care resources before a diagnosis of OHS is made,96 which is a cause for concern as obesity becomes more prevalent in the United States. In a study of hospitalized obese patients, Nowbar and colleagues97 found that hypercapnia with no other reason for hypoventilation was present in approximately one-third of patients with body mass index greater than 35 kg/m2 and almost one-half of patients with body mass index greater than 50 kg/m2.
Several studies showed improved daytime gas exchange and relief of symptoms associated with nocturnal hypoventilation within 1 to 4 months of initiation of NIV.97–99 A randomized trial of CPAP versus NPPV in patients with OHS without severe nocturnal desaturations found that both modes were equally effective in decreasing daytime PaCO2.100 At the present time, nocturnal NPPV is recommended for OHS when nasal CPAP and other first-line therapies fail to alleviate the hypoventilation.66,101
Selecting Appropriate Patients for Noninvasive Ventilation
Acute Care Setting
The success or failure of NIV depends to a large degree on the clinician’s clinical judgment in choosing appropriate patients. The primary selection criterion is the need for ventilatory assistance resulting from ARF. Patients who are unable to ventilate adequately on their own typically show signs and symptoms of respiratory distress, including use of accessory muscles, paradoxical breathing, tachypnea, and dyspnea.2,9 The feeling of dyspnea should be worse than usual for patients with COPD. In addition, these patients are unable to maintain normal gas exchange and usually develop respiratory acidosis or severe hypoxemia (Box 45-3).2,9
NIV exclusion criteria include apnea, hemodynamic or cardiac instability, lack of cooperation by the patient, conditions that preclude use of a noninvasive interface, copious amounts of secretions, and high risk of aspiration (Box 45-4).2,9 Two studies of hypercapnic ARF in patients with COPD showed that NIV could be successful in patients with decreased level of consciousness or hypercapnic coma.102,103 Decreased level of consciousness should not be considered an exclusion criterion for NIV in patients with COPD, but caution and monitoring are recommended when NIV is applied to these patients. The patient should be under constant observation until consciousness is regained. If the selection criteria are met and there are no contraindications, NIV should be considered.
It is important to consider the cause of ARF because the efficacy of NIV varies depending on the underlying condition being treated. In the acute care setting, most evidence supports the use of NIV in patients with COPD exacerbations or acute cardiogenic pulmonary edema. There is less evidence supporting NIV for the other indications discussed earlier; however, it is being used more frequently for patients with DNI orders, to facilitate extubation of high-risk patients, and as a means of preventing extubation failure and reintubation. Several studies identified potential predictors of success during NIV (Box 45-5). Patients with COPD and acute-on-chronic respiratory failure with comorbid conditions such as pneumonia are less likely to be managed successfully with NIV.104 Early initiation of NIV is encouraged in patients with ARF because severe hypercapnia and acidosis are predictors of NIV failure.104 Significant improvements in PaCO2 and pH after 30 to 120 minutes of NIV is predictive of success.105–107 In some cases, clinical judgment precludes an NIV trial based on the severity of the respiratory failure or other factors that increase the likelihood of failure to respond to NIV. However, in many situations, a reasonable plan would be a trial of NIV for 1 to 2 hours, with periodic reassessment and plans to intubate if the patient’s condition does not significantly improve. Successful application of NIV includes short-term goals of improving gas exchange and preventing endotracheal intubation and long-term goals of improved outcome, decreased length of stay, and decreased mortality.
Long-Term Care Setting
The current recommended selection guidelines for NIV in restrictive thoracic disease may be separated into two parts. First, patients should have symptoms of chronic hypoventilation and lack of sleep quality. Second, patients should meet one of the following measurable parameters: PaCO2 45 mm Hg or greater, nocturnal O2 saturation less than 88% for 5 minutes, maximal inspiratory pressure less than 60 cm H2O, or FVC less than 50% of predicted.66 Although a decline in pulmonary function has been associated with CO2 retention, more evidence is needed to support the use of a declining maximal inspiratory pressure or FVC as an indication for NIV.2
Recommendations for use of NIV in the management of nocturnal hypoventilation caused by disorders other than restrictive lung disease and COPD include documentation of a disorder that causes hypoventilation and failure of the disorder to respond to first-line therapy. First-line therapy includes weight loss, O2 therapy, respiratory stimulants, and CPAP. NIV is recommended as the initial therapy for moderate to severe cases of nocturnal hypoventilation.66
Patients with COPD and signs and symptoms of chronic hypoventilation and poor quality of sleep should receive optimal medical treatment before NIV is recommended.66 If symptoms remain despite optimal management, the presence of one of the following selection criteria indicates the need for NPPV: PaCO2 55 mm Hg or greater or PaCO2 50 to 54 mm Hg with recurrent hospitalizations or nocturnal desaturation.66 Recurrent hospitalization is defined as two or more hospitalizations for hypercapnic respiratory failure in a 12-month period. Nocturnal desaturation is defined by a pulse oximeter reading of less than 89% for 5 minutes with administration of at least 2 L/min of O2.62
In long-term care settings, a follow-up examination is suggested 1 month or so after starting NIV to help the patient acclimate to the device. A 2-month follow-up is recommended to determine compliance with NIV and to assess benefit.66
Exclusion Criteria for Noninvasive Ventilation in a Long-Term Care Setting
Relative contraindications for the use of NIV for restrictive thoracic disease, nocturnal hypoventilation, and chronic COPD include an unsupportive family, copious amounts of secretions, uncooperative behavior on the part of the patient, high risk of aspiration, and any anatomic abnormality that interferes with gas delivery.2
Equipment Used For Noninvasive Ventilation
Patient Interfaces
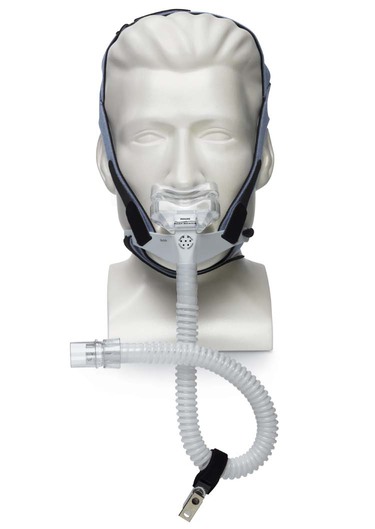
Nasal and Oronasal Masks
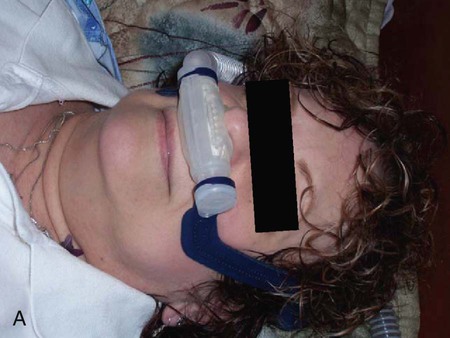
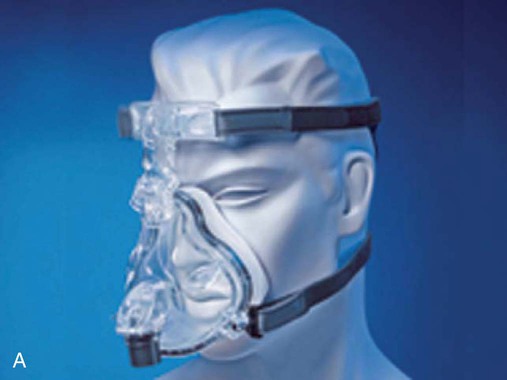
NIV masks incorporate straps and headgear to maintain and stabilize the mask’s position on the face (Figure 45-4). It is important to avoid tightening the straps more than necessary. A perfect seal between the mask and the face is not required because ventilators used for NIV are designed to function properly in the presence of small air leaks. Pulling the straps excessively tight is likely to result in pressure-related damage to the skin on the bridge of the nose or sometimes on the cheeks. Clinicians must be vigilant for early signs of skin damage and take steps to minimize damage and prevent development of pressure ulcers. An area of reddened skin that persists after removal of the mask is usually the first sign of pressure-related skin damage. A liquid skin barrier and a hydrocolloid patch may be applied to protect the reddened area.
A key factor in patient tolerance and NIV efficacy is the choice of an appropriately sized mask. Most masks include sizing templates that can be used before removing the mask from its packaging (Figure 45-5). Nasal masks should be sized so that the cushion starts one-third of the way down from the top of the bridge of the nose and fits closely around the lateral aspects of the nose and rests above the upper lip, just under the nose. Full-face masks fit similarly except the bottom of the mask rests in the depression above the chin and just below the lower lip. Mask sizes range from extra small to large, but for many individuals, a small or medium-small mask is a good fit. Ill-fitting masks can allow air leaks into the eyes, leading to poor tolerance. Small leaks around the mouth are generally less problematic because most ventilators used for NIV are designed to function with a baseline leak.
Nasal Pillows
Nasal pillows (Figure 45-6) are round, soft cushions that fit directly into the nares. Specially designed headgear holds the prongs in place. This interface is used most often during nasal CPAP by patients with chronic disease who do not tolerate a nasal mask. Nasal pillows are often a good option for patients with skin necrosis on the bridge of the nose or to deliver nocturnal CPAP to patients who prefer to sleep on their side. A newer design is the wedge-shaped mini-nasal mask that covers only the end of the nose. Another is the hybrid mask (Figure 45-7), which covers the mouth with a small mask and seals the nares with nasal pillows connected to the top. This interface allows ventilation with fewer leaks, similar to an oronasal mask that does not have contact with the skin on the bridge of the nose. Besides the advantage of decreased risk of tissue damage, this design allows patients to wear glasses during NIV.
Face Masks
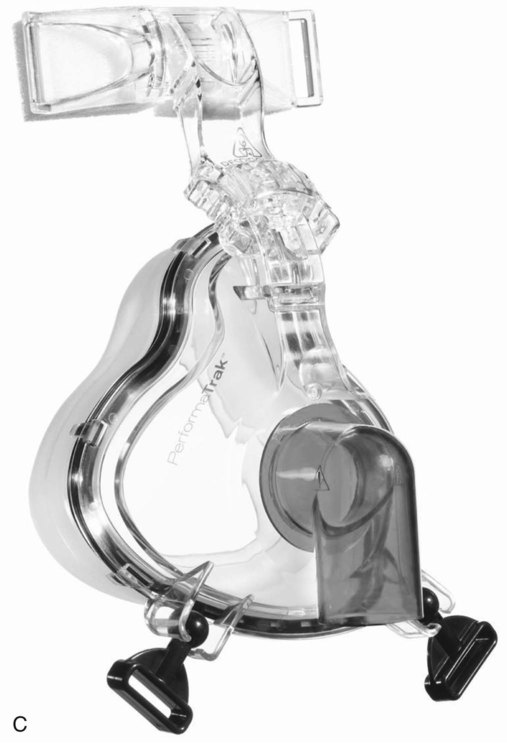
A variation of the full-face mask is the total face mask, which surrounds the entire face (Figure 45-8). A soft, flexible layer around the edge of this mask forms a seal and prevents leaking when the mask is pressurized. The total face mask comes in one size, which allows quick application in the emergency department or critical care unit. Because it does not obstruct the patient’s vision, this mask may help patients who feel claustrophobic when wearing other full-face or nasal masks. However, it has a large dead space and can interfere with triggering and cycling.
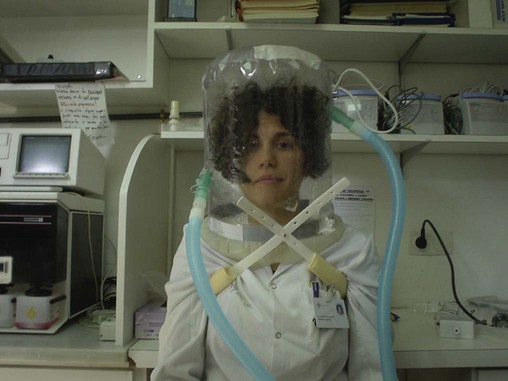
The helmet is an interface that is unavailable in the United States at the present time (Figure 45-9). This interface surrounds the entire head like a plastic bubble. The only point where the patient experiences stress is in the axillary area where the two straps holding the helmet in place cross. Numerous more recent studies discussed the effectiveness of the helmet during CPAP and NPPV.108–112 Results indicated that the helmet is more effective for CPAP than NPPV.111 However, CPAP must be applied with a high continuous flow to prevent CO2 from accumulating inside the helmet.112 If CPAP is provided by a ventilator, the patient is likely to rebreathe CO2 because of the large capacitance of the helmet.112 During NIV with the helmet, CO2 is not eliminated as effectively compared with full-face masks, and triggering and cycling the ventilator can be adversely affected.111
Face masks are specifically designed for use with either ICU or noninvasive ventilators (Figure 45-10). Face masks for noninvasive ventilators have entrainment valves that prevent asphyxia if the ventilator fails or the tubing becomes disconnected. Full-face masks designed for ICU ventilators do not have this feature. In addition, noninvasive ventilators using a single-limb circuit require a leak port either in the tubing or in the mask itself. Masks with leak ports should be used only with noninvasive ventilators because the leak interferes with the function of ICU ventilators.
Few data are available in the literature to help guide the choice of an interface for NIV. One study compared 30 minutes of full-face mask, nasal mask, and nasal pillows applied in random order to hypercapnic patients.113 The investigators reported that the full-face mask and nasal pillows improved ventilation more than the nasal mask but that the nasal mask was better tolerated.113 Use of a full-face mask also was associated with a significant increase in VT compared with the nasal mask.113 Similar findings were reported in a more recent study of 90 patients with hypercapnic ARF randomly assigned to full-face mask or nasal mask.114 Both studies support the belief that full-face masks may be more effective for patients in the acute care setting.113,114 There is no perfect NIV interface that meets the needs of every patient. Success is more likely if the interface can be tolerated for long periods and only a small air leak is present. A full-face mask is the interface of choice for patients requiring NIV for ARF. For patients who cannot tolerate a full-face mask, a nasal mask should be tried before accepting failure.
Types of Mechanical Ventilators and Modes of Ventilation
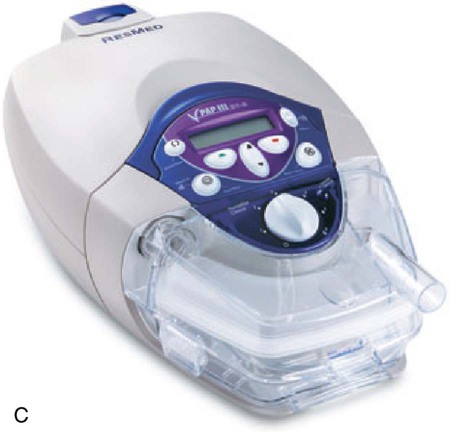
Noninvasive Ventilators
Most noninvasive ventilators are electrically powered, blower-driven, and microprocessor-controlled (Figure 45-11). These devices deliver a continuous but variable flow of gas to the patient through a single-limb circuit without an exhalation valve. To function properly, noninvasive ventilators must have a continuous air leak through one or more small ports either in the ventilator circuit or in the patient interface. The ports also provide the outlet through which the patient’s exhaled gas is vented from the circuit. The main advantage of noninvasive ventilators over other types of ventilators is the ability to trigger and cycle appropriately when small to moderate air leaks are present.
Ideally, noninvasive ventilators should have an internal blending device, capable of providing FiO2 ranging from 0.21 to about 1. Bleeding O2 into the ventilator circuit or the interface results in maximum FiO2 of about 0.5,115 which is prone to wide fluctuations from variations in flow rates, patient effort, and leaks. Low baseline pressure settings have been associated with significant rebreathing of CO2 in single-limb circuits.116,117 These reports suggest the use of 3 to 5 cm H2O PEEP or the use of a nonrebreathing valve to prevent rebreathing of CO2.117 For this reason, the expiratory pressure can be set no lower than 3 or 4 cm H2O on many noninvasive ventilators. Newer noninvasive ventilators for use in acute care usually include an internal battery; include graphics monitoring; and display calculated volumes that estimate leak, VT, and minute ventilation. Graphics are very useful when adjusting NIV settings to enhance patient-ventilator synchrony.
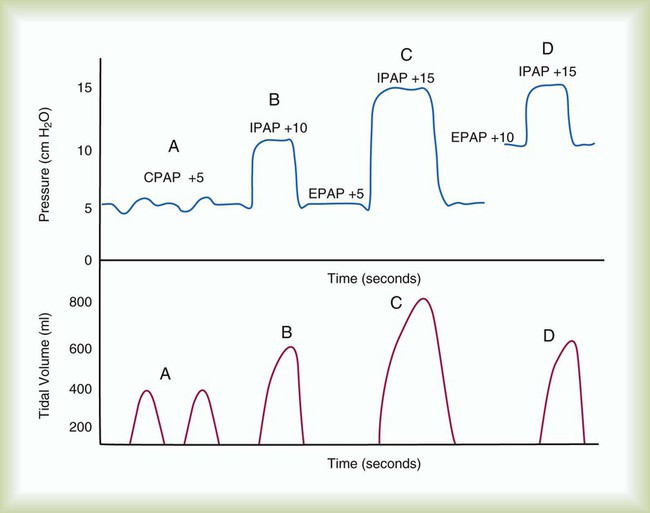
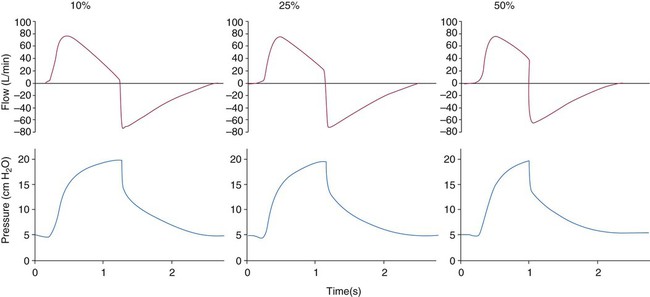
Modes available on noninvasive ventilators usually include CPAP, spontaneous (pressure support), and timed (pressure assist/control). With CPAP, the ventilator delivers no additional pressure to alleviate the work of breathing when the patient inspires. The patient simply breathes at an elevated baseline. With pressure support and spontaneous modes, inspiration is patient-triggered. With pressure assist/control, inspiration is either patient-triggered or time-triggered. Similar to PSV, inspiratory positive airway pressure (IPAP), equal to peak airway pressure, is typically pressure-limited and flow-cycled or time-cycled (Figure 45-12).
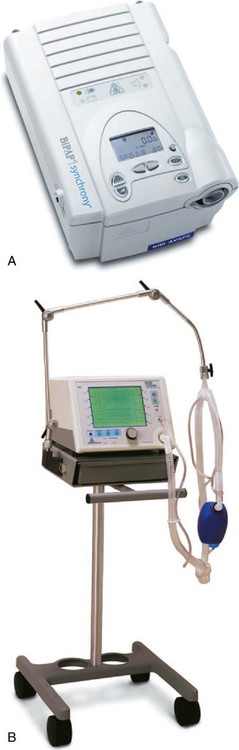
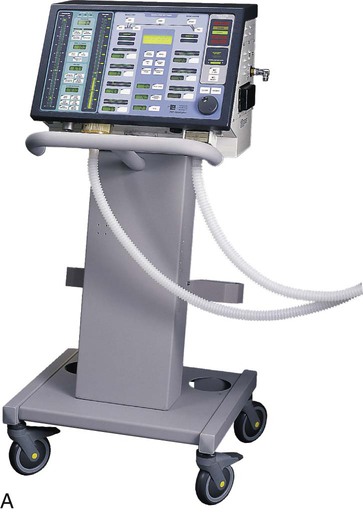
Critical Care Ventilators
Critical care ventilators can be used to deliver NIV effectively (Figure 45-13). The choices of modes and settings are important determinants of patient tolerance, but these choices often depend on the functionality of the specific ventilator to be used. For this reason, it is important for the RT to understand the actual change in the operation of the ventilator that occurs in the NIV mode.
To deliver NIV, ICU ventilators can be set in PSV mode, which is a patient-triggered, pressure-limited, flow-cycled mode. During inspiration, a high gas flow is delivered until the preset pressure limit is achieved. At that point, the flow begins to decrease until a predetermined level of flow is reached, cycling the breath to exhalation. Depending on the specific ventilator used, this flow level can be a fixed flow rate (e.g., 5 L/min) or a percentage of the peak flow (e.g., 25%). The flow-cycling mechanism of PSV can cause problems during NIV if air leaks are present because the flow may not decrease to the level necessary to cycle the breath to expiration. In this case, the patient may have to exhale actively, using abdominal muscles to increase airway pressure to cycle the ventilator to exhalation using secondary criteria.118,119 This action increases work of breathing and can lead to failure of NIV.
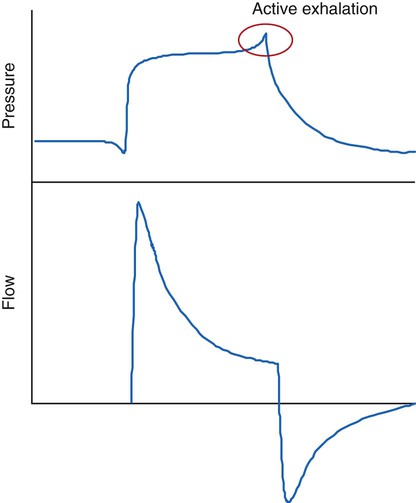
Active exhalation can be recognized on the ventilator graphics by a spike in airway pressure at the end of the breath (Figure 45-14). There are two ways to correct this problem so that the patient can exhale passively again. First, inspiration can be time-cycled instead of flow-cycled by decreasing the maximum inspiratory time setting; if a noninvasive mode is unavailable, this can be accomplished by changing the mode to pressure assist/control. Time-cycled (instead of flow-cycled), pressure-limited ventilation markedly improves patient-ventilator synchrony and patient comfort and compliance with therapy in the presence of air leaks.120
The second option is to adjust the termination criterion121 by changing the percentage of the peak inspiratory flow that terminates the breath. Most current generation ICU ventilators allow the termination criterion setting to range from 5% to 10% to 60% to 80% or more of the peak flow rate (Figure 45-15). To prevent active exhalation, the percentage is simply increased until the spike disappears from the pressure waveform. Proper adjustment of the termination criterion can markedly improve patient-ventilator synchrony during NIV.
Several studies have shown no difference in success or gas exchange between volume-controlled and pressure-controlled modes.113,122,123 In two of these studies, patients preferred PSV to volume assist/control.122,123 The current recommendation by an international consensus conference on NIV in ARF is as follows: “Choice of mode should be based on local expertise and familiarity, tailored to the etiology and severity of the pathophysiological process responsible for ARF.”124 Most RTs are familiar with using pressure-targeted ventilation for NIV because this mode is commonly used on noninvasive ventilators. In severe ARF, using pressure ventilation makes sense because leak compensation is provided, triggering and cycling can be adjusted to suit patient needs, and minute ventilation and end expiratory pressures are preserved. Volume-controlled modes are not recommended for NIV. Inability to compensate for the decrease in minute ventilation and loss of pressure caused by large leaks are major problems when volume targeted modes are used for NIV. Air leaks during volume-controlled ventilation can result in hypoventilation secondary to loss of the set VT.
In the long-term care setting, volume ventilation is sometimes used for patients with neuromuscular weakness.3 Volume ventilation may allow a patient to stack breaths, increasing lung volume to near inspiratory capacity and resulting in improved cough peak flow. High cough peak flow should enhance secretion clearance. In patients with limited muscle strength, pressure-controlled ventilation does not allow breath stacking because inspiratory pressure is held constant, in contrast to volume ventilation, in which delivered VT is constant. However, there is no proven advantage of one mode versus another in the long-term care setting.2 Enhanced secretion clearance is easily provided using a mechanical cough assist device.
Portable Home Care or Transport Ventilators
Most portable home care ventilators (Figure 45-16) are electrically powered and microprocessor-controlled. These devices can operate from alternating current (AC) or, if equipped with internal or external batteries, direct current (DC) power sources. The batteries usually can provide power for several hours. Batteries add a measure of safety in areas where AC power outages occur regularly. They also allow the patient to be more mobile, which may improve their quality of life. These ventilators use a single-limb or double-limb ventilator circuit with an exhalation valve that prevents rebreathing of CO2. Updated technology has improved the performance characteristics of these ventilators to a level comparable to critical care ventilators with the added advantages of small size, light weight, and battery power that allow portability. Most newer models incorporate an NIV mode with the ability to compensate for leaks. Some models with adjustable bias flow settings improve the ability of the ventilator to sense patient triggering. Similar to critical care ventilators, additional features, such as variable termination criteria and maximum inspiratory time, are available on some of these devices to enhance breath termination in PSV.
Portable home care ventilators are currently recommended for patients who need continuous ventilatory support or high ventilating pressures, such as patients with severe chest wall deformities or obesity or for patients who want greater mobility.2 For acute care, these ventilators could easily be used to initiate NIV in nontraditional locations and allow transport to an emergency department or ICU without the need to interrupt ventilation.
Heated Humidifiers
An increase in nasal resistance and congestion has been reported with CPAP in patients with mouth leaks.125,126 The addition of heated humidity relieves nasal resistance and congestion. Cold passover humidification does not provide relief.125 The use of heated humidity during nasal CPAP in patients with sleep apnea and nasal symptoms (sneezing, nasal draining, nasal and oral dryness, and nasal obstruction) has significantly improved patients’ compliance with this therapy.127,128 It seems likely that the same effect would occur in NPPV. To avoid the negative effect on patient compliance caused by this common complaint and the accumulation of dried retained secretions in the back of the oral pharynx, humidified gas, heated to a temperature that is comfortable to the patient (usually about 30° C), should be the standard when using NIV.
Management of Noninvasive Ventilation
Initial Application of Noninvasive Ventilation
The patient should be seated in a chair or bed at an angle of 30 degrees or greater (Box 45-6).2 When the mask is applied for the first time, the ventilating pressures should be set as low as possible. It is difficult for patients to acclimate to high airway pressures, and a strategy of starting with low pressures can help patients adjust more readily. As the patient becomes accustomed to the sensation of NIV, pressures can be adjusted in small increments until exhaled VT is 4 to 6 ml/kg predicted body weight or respiratory distress improves. When applying the mask for the first time, the RT should hold the mask in place or allow the patient to hold it, if he or she is able to do so. Holding the mask allows rapid removal of the mask if the patient begins to panic or needs to communicate. The mask should not be strapped on until the patient is comfortable with the application of NIV and the RT has adjusted pressures to provide proper ventilation.
The actual final airway pressures required to support the patient during NIV are difficult or impossible to determine before NIV has been started. Some patients may need high pressures; others need low pressures—it is impossible to know until the process is begun. However, most patients require PEEP levels of 5 to 8 cm H2O and ventilating pressure of 8 to 12 cm H2O. Peak airway pressures greater than 20 cm H2O are rarely needed. To avoid gastric distention, ventilating pressure should be less than the normal gastric opening pressures of 20 to 25 cm H2O.129 If the patient has a nasogastric tube in place, the probability of gastric distention increases dramatically, as does the probability of NIV failure.
Clinical Assessment Criteria to Identify Success or Failure of Noninvasive Ventilation
Successful application of NIV is easy to define—gas exchange improves, PaCO2 decreases, pH normalizes, and PaO2 and SpO2 increase. Along with improvement of these measured values, the patient’s clinical presentation improves; respiratory rate decreases, VT increases, accessory muscle use decreases or is eliminated, and pulse rate and blood pressure normalize. If the patient’s clinical status improves within 1 to 2 hours, he or she may be on the way to successful application of NIV. NIV should be continued, but the patient needs to be monitored carefully. If clinical status and gas exchange have not improved after 1 to 2 hours of NIV, intubation should be considered; this is especially true if the indication for NIV was hypoxemic respiratory failure. Various reports indicate increased risk of cardiac arrest in patients with hypoxemic respiratory failure who do not improve after a trial of NIV for 1 to 2 hours.46
Adjusting Noninvasive Ventilator Settings
If the assist/control mode is used for NIV, the rate should be set at a level below the patient’s spontaneous rate, allowing the patient to trigger the ventilator as needed. Setting the rate in this manner provides a backup rate for safety if apnea occurs and avoids overventilating the patient. If the patient’s disease process limits the ability to trigger or breathe spontaneously, as in some neuromuscular disorders, the set rate has a direct relationship to minute ventilation and an inverse relationship to PaCO2. These relationships may not always be the case in clinical practice and in a spontaneously breathing patient. Table 45-1 summarizes ventilator adjustments during NIV.
TABLE 45-1
Expected Results of Changing Noninvasive Ventilator Settings
| Setting | Adjustment | Anticipated Result |
| IPAP | ↑ | ↑ VT, ↑ minute ventilation, ↓ PaCO2 |
| ↓ | ↓ VT, ↓ minute ventilation, ↑ PaCO2 | |
| EPAP | ↑ | ↑ FRC, ↑ PaO2, ↓ VT |
| If intrinsic PEEP is present, fewer missed trigger attempts and improved patient-ventilator synchrony | ||
| ↓ | ↓ FRC, ↓ PaO2, ↑ VT, ↓ PaCO2 | |
| Possible rebreathing of CO2 if EPAP < 4 cm H2O | ||
| FiO2 | ↑ | ↑ PaO2; if bleeding O2 into circuit, maximum expected FiO2 is approximately 0.5; increasing O2 flow >15 L/min may adversely affect triggering |
| ↓ | ↓ PaO2 | |
| Rate control* | ↑ | ↑ minute volume in timed modes, ↓ PaCO2 |
| ↓ | ↓ minute volume in timed modes, ↑ PaCO2 |
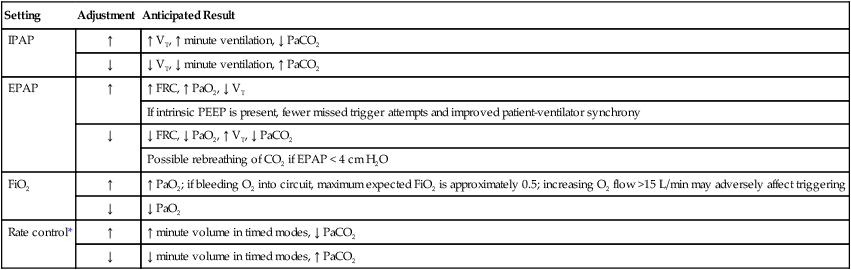
FRC, Functional residual capacity.
*Rate control is generally set at 8 to 10 as a backup rate and not changed in spontaneous/timed mode.
Aerosolized Medication Delivery
For patients on intermittent NIV, aerosol medications are often administered while the patient is off the ventilator. Removal of assisted ventilation from patients in ARF makes little sense and is unnecessary. Aerosol therapy can be delivered in the usual manner through an ICU ventilator used for NIV. With a noninvasive ventilator, positioning the nebulizer between the exhalation port and mask maximizes drug delivery.130 If the exhalation port is located in the mask or any other position distal to the nebulizer, much of the drug is lost via the exhalation port during both inspiration and expiration. A metered dose inhaler (MDI) can be administered by placing an adapter or collapsible holding chamber in the same position in the circuit. Drug delivery should improve because the MDI is actuated only during inspiration. However, dosing can safely be doubled and should be adjusted based on patient response to compensate for loss through leaks in the system and other inefficiencies.
Safe Delivery of Noninvasive Ventilation
Monitoring During Noninvasive Ventilation
In acute care or long-term care applications, clinicians must not lose sight of the goals of NIV.2 The RT should confirm ventilator function and assess the patient on a regular basis for leaks, accessory muscle use, ventilator synchrony, comfort, and changes in vital signs and gas exchange. In the acute care setting, respiratory rate, heart rate, and gas exchange should improve within 1 to 2 hours after initiation of NIV. If there is no improvement after 30 to 60 minutes on optimal settings, intubation should be considered. At a minimum, SpO2 must be continuously monitored. In the acute care setting, continuous monitoring of heart rate and blood pressure is the safest practice. A current consensus statement recommends a higher level of monitoring for patients with acute hypoxemia, worsening condition, involvement of nonrespiratory organ systems, or persistent acidosis.124 If the patient cannot sustain ventilation independent of NIV for at least 1 hour, the same level of monitoring as any intubated patient should occur.
In the long-term care setting, improvement in gas exchange may require weeks to several months depending on daily use and compliance with the prescribed therapy.2 Clinicians providing follow-up treatment in the long-term care setting should determine usage with the elapsed time indicator on the ventilator. The patient should be assessed for complications, symptoms of hypoventilation and poor sleep quality, patient-ventilator synchrony, and other factors that affect compliance.
Patient Location
NIV can be initiated in any acute care location, including the emergency department, ICU, or general care floor.124 After NIV is initiated, patients should be transferred to an ICU or other inpatient location with continuous monitoring capabilities, skilled staff, and access to endotracheal intubation if needed.2,124 Hypercapnic patients with COPD and a pH of 7.30 or greater124 and patients who can sustain ventilation without NIV for at least 1 hour can be managed safely on a general care floor.56 It is important that staff members be adequately trained before caring for patients on NIV. Another consensus conference recommendation calls for one-to-one monitoring of NIV patients for the first few hours by a trained, experienced RT, nurse, or physician.124
Complications of Noninvasive Ventilation
The reported failure rate of NIV ranges from 7% to 70%.2 Serious complications such as aspiration or pneumothorax occur less than 5% of the time. The undesired side effects of NIV are less serious, although more common. These side effects can be grouped into categories related to the noninvasive interface and to gas flow and airway pressures. Table 45-2 lists the complications, frequency of occurrence, and suggested remedies.
TABLE 45-2
Side Effects and Complications of Noninvasive Ventilation
| Incidence* | Possible Solutions | |
| Interface-Related Side Effects | ||
| Discomfort | Common | Loosen straps |
| Refit, reposition, or change interface | ||
| Erythema | Common | Apply skin barrier or hydrocolloid dressing or both |
| Loosen straps, adjust forehead support, or add spacer | ||
| Alternate the use of 2 masks | ||
| Claustrophobia | Infrequent | Change interface |
| Consider anxiolytic | ||
| Pressure ulcer | Infrequent | Apply hydrocolloid dressing |
| Change interface | ||
| Skin rash | Infrequent | Apply topical steroid or antibiotic |
| Air Pressure–Related or Flow-Related Side Effects | ||
| Nasal congestion | Common | Administer inhaled corticosteroid or decongestant |
| Nasal dryness | Common | Avoid by using heated humidification with NIV |
| Administer saline nasal spray | ||
| Sinus or ear pain | Common | Decrease ventilating pressure |
| Eye irritation | Common | Refit, reposition, or change interface |
| Gastric distention | Infrequent | Administer simethicone |
| Lower pressures | ||
| Serious Complications | ||
| Aspiration | Rare | Avoid through careful patient selection |
| Stop NIV, if status will allow | ||
| Pneumothorax | Rare | Decrease ventilating pressure |
| Place chest tube, if indicated | ||
| Hypotension | Rare | Decrease inflation pressure |
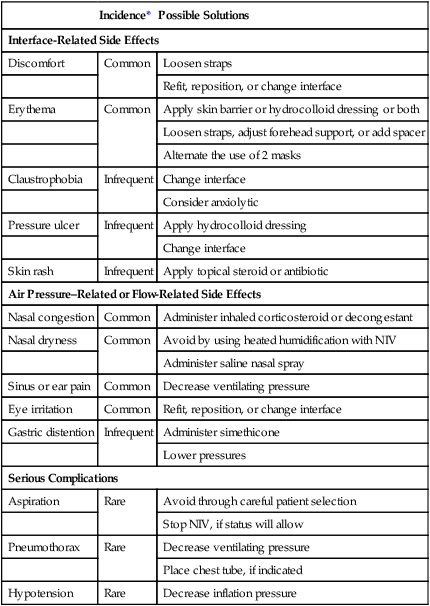
*Common—occurs in 30% to 50% of patients; infrequent—occurs in approximately 5% to 20% of patients; rare—occurs in <5% of patients.
From Mehta S, Hill NS: Noninvasive ventilation. Am J Respir Crit Care Med 163:540, 2001.
Air leaks can cause several problems of various levels of concern, ranging from eye irritation and dry mouth to inability to trigger inspiration. Small air leaks should be expected during NIV. Large air leaks should be addressed immediately before they lead to patient-ventilator asynchrony or worsening gas exchange.3 Air leaks often can be avoided by selecting an appropriately sized mask. If excessive airflow is leaking through the mouth, changing to a full-face mask should be helpful. If the problem persists, one can reposition the mask, add a forehead spacer, and readjust strap tension. Sometimes, ventilator settings must be adjusted if ventilation is adversely affected by the leak. Using a ventilator with leak compensation should resolve problems with leaks. If that option is unavailable, one can consider changing the termination criteria, using a time-cycled mode and adjusting the trigger sensitivity.
Mask-related side effects are the most common problems. Mask discomfort may be reported by 50%2 of patients receiving NIV, and excessive discomfort decreases patient tolerance for NIV. Switching to a correctly sized mask or loosening the straps slightly is often all that it takes to resolve this issue. Skin damage is a problem that is only going to get worse if not addressed immediately for patients who need to continue using NIV. RTs should keep a watchful eye on reddened areas, usually on the bridge of the nose. A liquid skin barrier should be applied to protect the area. Applying “artificial skin” or a hydrocolloid dressing or patch is a good idea to provide further protection. These patches should also be used if a pressure ulcer forms. Taking steps to minimize pressure such as loosening the straps and adding a forehead spacer are important. Other strategies to reduce the risk of pressure ulcers include alternating use of two or more different masks and removing the mask every 4 to 6 hours for a few minutes if the patient can tolerate being off the ventilator without increased respiratory distress.
Complications related to air pressure and flow include nasal congestion, upper airway dryness, sinus and ear pain, eye irritation, and gastric insufflation.2 Usually, nasal congestion and upper airway dryness can be prevented by using heated humidity whenever NIV is initiated. Decongestants and saline spray are sometimes needed to relieve symptoms of congestion. Sinus and ear pain may be related to high inspiratory pressure; use of the lowest effective inspiratory pressure may prevent or alleviate this problem.
Clinically significant gastric insufflation is a rare occurrence in patients using NIV.2 Use of the lowest effective pressure may prevent gastric insufflation. Routine use of a nasogastric tube is not recommended. A nasogastric tube increases the risk of gastric insufflation, adversely affects mask fit, and usually causes a large air leak, increasing the likelihood of NIV failure.
Time and Costs Associated With Noninvasive Ventilation
The cost-effectiveness of NIV is linked to appropriate patient selection, familiarity of staff members with NIV, and the success or failure of NIV in preventing endotracheal intubation.131,132 Staff time is one of the most valuable and expensive resources in hospitals. Time required by nurses and physicians during the first 48 hours of NIV is similar to the time required for invasive mechanical ventilation, but the time required by RTs for NIV is considerably greater than for invasive mechanical ventilation.133 However, another study showed that the time required by RTs was significantly greater for the first 8 hours but significantly lower during the next 8 hours.10 The increased time requirement associated with starting NIV is due to mask fitting, gradual upward adjustment of ventilator settings, and remaining at the bedside to provide coaching and encouragement to patients as they acclimate to NIV. After the patient begins to improve, the time required to maintain NIV should decrease to a level similar to invasive ventilation.




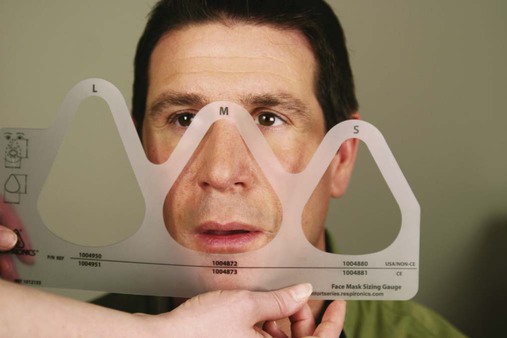
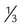 of the way from the top of the bridge of the nose and in the indentation between the chin and the lower lip.
of the way from the top of the bridge of the nose and in the indentation between the chin and the lower lip.