Chapter 70B Nonhepatic surgery in the cirrhotic patient
Overview
Chronic liver disease and cirrhosis were the twelfth leading cause of death in the United States and resulted in more than 30,000 deaths in 2009 (Kochanek et al, 2011). Chronic viral infection and alcohol abuse account for the majority of the disease burden globally, but the incidence of obesity-associated nonalcoholic fatty liver disease accounts for an ever-increasing proportion of cases, especially in Western societies. Furthermore, clinicians continue to gain knowledge and skills to care for patients with cirrhosis in the end stages of their disease. These facts explain why a significant increase has been seen in the number of patients with comorbid liver disease and cirrhosis encountered in both general and specialty surgical practice.
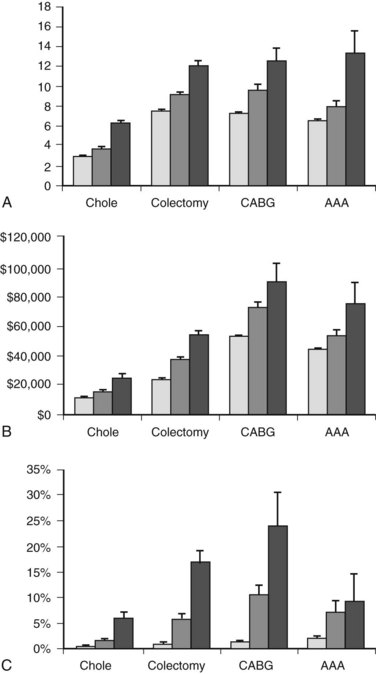
Cirrhosis can have dramatic effects on multiple organ systems, making surgery on the cirrhotic patient a complex and difficult undertaking. A recent, large, population-based study demonstrated that people with cirrhosis, in particular those with portal hypertension, have significantly worse outcomes after elective operations than those without (Fig. 70B.1; Csikesz et al, 2009). The mere act of opening the abdominal wall in a cirrhotic patient with portal hypertension causes collateral blood vessels to dilate and may lead to systemic hypotension and hepatic decompensation secondary to ischemia (Haskal et al, 1994; Norton et al, 2003).
Evaluation and Stratification of Liver Disease
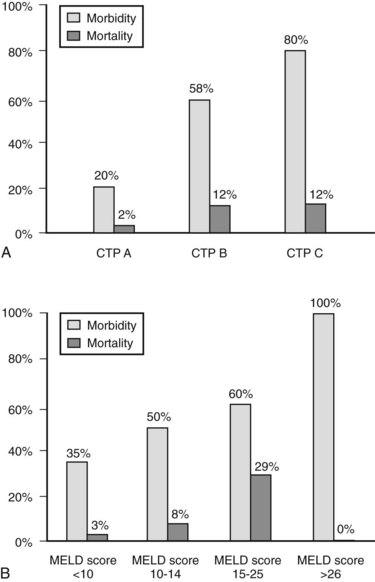
The decision regarding whether a cirrhotic patient is medically fit to undergo an operation can be difficult to make. Many factors must be considered, but the most important are the magnitude and necessity of the proposed operation, the nonhepatic comorbidities of the patient, and the severity of the liver disease. Numerous factors have been correlated with poor outcome in patients with cirrhosis—including low albumin levels, blood transfusion requirements, abnormal coagulation, and ascites—and various scoring systems to gauge these have evolved. The first, most widely used scoring system is the Child-Turcotte-Pugh (CTP) system, which incorporates several objective and subjective variables to stratify severity of liver disease. Historically, patients with CTP scores of A, B, or C have been shown to have operative mortality rates of 10%, 30%, and 80%, respectively (Garrison et al, 1984; Mansour et al, 1997).
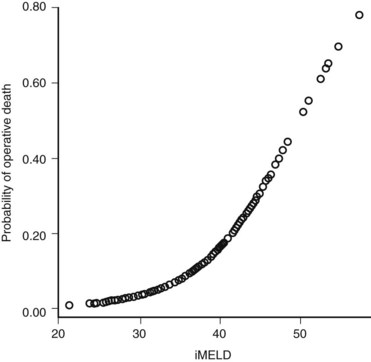
The Model for End-Stage Liver Disease (MELD) score was developed to predict death after transjugular intrahepatic portosystemic shunt (TIPS) in an effort to determine who was likely to progress toward needing liver transplantation. In an effort to improve on the tripartite CTP system, researchers have recently evaluated to ability of MELD to predict morbidity and death after nonshunt abdominal surgery (Befeler et al, 2005; Farnsworth et al, 2004). As with CTP, MELD correlates with risk of postoperative death (Figs. 70B.1 and 70B.3). Several reports have found that MELD is superior to CTP in predicting postoperative problems, although these improvements generally are modest (Perkins et al, 2004). Interestingly, the survival rates from the more recent series indicate a fairly dramatic improvement in survival compared with older series (Fig. 70B.3).
In addition to factors considered in MELD and CTP scoring, other factors have been identified for risk stratification and prediction in cirrhotic patients undergoing major operations. These include elevated creatinine level, chronic obstructive pulmonary disease (COPD), male gender, and an American Society of Anesthesiologists (ASA) class of IV or V (Ziser et al, 1999). Teh and colleagues (2007) demonstrated ASA classification as a useful marker to further stratify the comorbid illness in cirrhotic patients preoperatively. This case-control study of cirrhotic patients who underwent major nontransplant operations identified MELD score, ASA class, and age as predictors of perioperative death. An ASA class of IV was equivalent to the addition of 5.5 MELD points in added risk, and age greater than 70 years was equivalent to three additional MELD points. A single point increase in MELD score was associated with a 15% increase in perioperative death. Emergency surgery predicted in-hospital death, although patients undergoing emergency operations had a higher median MELD score. ASA class V was the strongest predictor of 7-day mortality, and MELD score was the most robust predictor beyond 7 days. The median survival of patients in this series was 4.8 years for a MELD score of 0 to 7, 3.4 years for a score of 8 to 11, 1.6 years for 12 to 15, 64 days for 16 to 20, 23 days for 21 to 25, and 14 days for 26 or greater.
Perioperative Management and Optimization
Most cirrhotic patients have protein-energy malnutrition, placing them at an increased risk for developing a variety of postoperative complications, such as wound dehiscence, infections, reaccumulation of ascites, and death (Merli et al, 2002). Nutritional status should be evaluated preoperatively; when found to be deficient, efforts should be undertaken to improve it (see Chapter 24). Nasoenteric or parenteral feedings that use low-sodium formulas supplemented with trace elements and branched-chain amino acids are an option for patients who are unable to adequately nourish themselves.
Coagulopathy secondary to impaired hepatic synthesis of clotting factors and decreased vitamin K stores as a result of malnutrition and poor intestinal absorption may be present. Thrombocytopenia is also common in these patients secondary to splenic sequestration and bone marrow suppression. Preoperative transfusions of fresh frozen plasma and platelets lower the risk of bleeding. In the operating room, careful tissue handling and the maintenance of a low central venous pressure are other factors that can help to minimize blood loss (Alkozai et al, 2009). If significant bleeding arises, cryoprecipitate, diamino-8-d-arginine vasopressin, and recombinant factor VIIa are additional options to reverse coagulopathy and control hemorrhage (O’Leary et al, 2009).
In the perioperative period, altered mental status in a cirrhotic patient should be thoroughly evaluated. Only after all other potential causes have been ruled out can hepatic encephalopathy be diagnosed. With the acute onset of encephalopathy, protein intake may be briefly withheld. Lactulose should be administered and titrated for three soft bowel movements a day. Oral antibiotics, such as metronidazole or neomycin, may also be used to decrease the intestinal bacterial load (Muilenburg et al, 2009). Surgery should be postponed if encephalopathy arises preoperatively. Postoperatively, the administration of narcotics and other sedating medications should be kept to a minimum. There is no role for prophylactic lactulose therapy for all cirrhotic patients undergoing surgery (O’Leary et al, 2009).
Perioperative fluid management in cirrhotic patients can be very difficult. Patients with cirrhosis should be adequately resuscitated to avoid hypotension and hepatic ischemia, but fluids should also be given judiciously to minimize the accumulation of ascites postoperatively. One center that performs a large volume of surgery on patients with end-stage liver disease limits the infusion of crystalloid solutions in the perioperative period (Telem et al, 2010b). Instead, all patients with advanced liver disease are placed on a postoperative sodium-poor albumin drip until they are able to resume oral intake.
Ascites increases the risk of postoperative renal failure, infections, and wound dehiscence. Even if ascites is drained at the time of surgery, it rapidly reaccumulates in the postoperative period. Medical therapy, which includes salt restriction and diuretics, is typically considered the first line of treatment. TIPS, however, may be considered a first-line treatment for refractory ascites and may be placed before elective surgery to help control ascites throughout the entire perioperative period (Azoulay et al, 2001; Gines et al, 2004; Schlenker et al, 2009). Moreover, TIPS may also reduce the risk of significant perioperative bleeding. In one of the largest series on the use of TIPS in patients undergoing major extrahepatic surgery, 25 cirrhotic patients with a mean MELD score of 15 ± 7.6 underwent TIPS at a median of 20 days before major abdominal and cardiothoracic operations. Blood transfusions were relatively minimal in the series, with a median of 3 units for abdominal operations (range, 0 to 21) and 4 units for cardiothoracic operations (range, 0 to 20). With a median follow-up of 33 months, actuarial 1-year patient survival was 74%, and the three postoperative deaths in the series occurred in patients with MELD scores higher than 24, all of whom underwent emergent surgery (Kim et al, 2009). Studies that compare outcomes after surgery with preoperative TIPS placement versus those with no TIPS, however, are lacking. Also, the optimal time interval between TIPS placement and elective surgery is unclear, but it is common for a period of several weeks to be required to see a clinical improvement in ascitic volume after TIPS; therefore shunts should probably be placed several weeks prior to planned operations.
Specific Procedures
Cholecystectomy
The incidence of gallstones in patients with cirrhosis is twice that of the general population as a result of increased intravascular hemolysis and decreased gallbladder motility and emptying (Bouchier, 1969; Conte et al, 1991; Schwesinger et al, 1985). In the 1980s, rates of major morbidity and mortality in patients with cirrhosis undergoing cholecystectomy were as high as 35% and 25%, respectively (Aranha et al, 1982; Cryer et al, 1985; Garrison et al, 1984; Schwartz, 1981). Postoperative death occurred secondary to blood loss, sepsis, and liver failure. Almost invariably, these procedures were performed as open laparotomy. At that time, the presence of cirrhosis was considered a contraindication for the laparoscopic approach, based on the assumption that it would lead to increased rates bleeding and liver failure (Yerdel et al, 1993). However, as greater experience in laparoscopic surgery has been gained, this point of view has changed, and now many studies demonstrate favorable morbidity and minimal to no mortality in patients with early cirrhosis (CTP class A and B) undergoing laparoscopic cholecystectomy (Cucinotta et al, 2003; Fernandes et al, 2000; Ji et al, 2004; Morino et al, 2000; Poggio et al, 2000; Yeh et al, 2002). A recent meta-analysis comparing laparoscopic cholecystectomy in cirrhotic and noncirrhotic patients demonstrated significantly higher rates of morbidity (21% vs. 8%, respectively; P < .001), intraoperative bleeding (26% vs. 3%; P < .001), and open conversion (7% vs. 4%; P < .05), but no difference was reported in terms of wound infection and mortality rates between the two (Puggioni & Wong, 2003).
Laparoscopic cholecystectomy offers improved visualization for meticulous dissection and avoids the need for a large subcostal or upper midline incision. It is associated with less operative blood loss, shorter operative time, and decreased length of hospital stay versus the open procedure in cirrhotic patients (Puggioni & Wong, 2003). Furthermore, laparoscopic cholecystectomy leads to fewer adhesions in the right upper quadrant, which are associated with increased bleeding complications and longer operative times in patients who ultimately undergo liver transplant.
Some specific technical considerations must be considered when performing laparoscopic cholecystectomy in a cirrhotic patient. In placing the umbilical port, particular care must be taken to avoid venous collaterals and the umbilical vein. One approach may be to place one of the other ports first, such as the subxiphoid port, in order to then place the umbilical port under direct visualization. Transillumination of the abdominal wall helps the surgeon to avoid these and other venous collaterals during trocar placement. Furthermore, the subxiphoid port should be placed off midline to avoid the falciform ligament and the recanalized umbilical vein. Careful traction on the gallbladder helps to avoid undue bleeding as it is dissected off the liver. Instruments such as the Harmonic Scalpel (Ethicon, Somerville, NJ) or LigaSure (Covidien, Mansfield, MA) may also be used to help control bleeding during the dissection (Curro et al, 2005; El-Awadi et al, 2009; Morino et al, 2000; Schiff et al, 2005; Tuech et al, 2002). A subtotal or supracystic cholecystectomy, leaving part of the gallbladder wall attached to the liver bed and ligating the cystic duct from within the gallbladder with a purse-string suture, is an option in patients with large pericholecystic venous collaterals (Bornman & Terblanche, 1985; see Chapter 33). Trocars should also be removed under direct visualization to ensure adequate abdominal wall hemostasis at the end of the procedure. Finally, if anatomy is unclear or any doubt exists as to the safety of continuing laparoscopically, the procedure should be converted to open.
In patients with advanced cirrhosis (CTP class C), cholecystectomy in general is associated with very poor outcomes. Despite the lack of controlled trials, the literature supports the use of alternatives to cholecystectomy, such as a percutaneous cholecystostomy, in this high-risk group (Cucinotta et al, 2003; Curro et al, 2005; Delis et al, 2010; Yeh et al, 2002). An endoscopically placed cystic duct stent is another option proposed by some (Gaglio et al, 1996; Shrestha et al, 1996, 1999).
In general, the CTP scoring system is used in the literature to stratify patients suitable for cholecystectomy. Although some evidence suggests that the MELD classification system may be more accurate in predicting postoperative complications, these data are inconsistent, and cutoff values vary among studies (Delis et al, 2010; Perkins et al, 2004). Therefore, until the MELD system has been further validated, CTP should be used for stratification. In CTP classes A and B patients with symptomatic cholelithiasis and/or acute cholecystitis, laparoscopic cholecystectomy should be performed by surgeons experienced in managing cirrhosis. In patients with class C cirrhosis, surgery should be deferred until liver disease is under better control, and alternate interventions, such as percutaneous cholecystostomy or endoscopic stenting, should be used.
Herniorrhaphy
Umbilical hernias occur in up to 20% of patients with cirrhosis. The pathogenesis of umbilical hernias in the setting of cirrhosis is multifactorial; ascites causes increased intraabdominal pressure, poor nutritional status leads to decreased abdominal muscle mass and fascial strength, and umbilical vein dilation results in enlargement of the preexisting supraumbilical fascial opening (Belghiti & Durand, 1997). In addition to the risks of bowel incarceration and strangulation that affect all patients with umbilical hernias, additional unique risks accompany having an umbilical hernia and cirrhosis. Skin ulceration over the hernia may be associated with the leakage of ascites, sac rupture, bacterial peritonitis, and even evisceration, with very high attendant rates of morbidity and mortality. Although rare, spontaneous umbilical rupture is associated with up to a 60% perioperative mortality rate and has been shown to correlate independently with an adverse outcome after emergent repair (Telem et al, 2010a).
Historically, mortality rates for umbilical hernia repair (UHR) in patients with cirrhosis were prohibitively high, and the prevailing opinion among surgeons was that uncomplicated hernias should be left untreated (Baron, 1960; O’Hara et al, 1975). UHR was only undertaken when complications, such as incarceration, strangulation, skin breakdown or impending rupture, or overt rupture, necessitated immediate intervention. In the present era, however, outcomes appear to be significantly improved for elective and even complicated UHR in patients with end-stage liver disease. Most series published in the literature since the early 1980s have reported little to no perioperative deaths and relatively low morbidity, although most of them have been relatively small retrospective studies (Belghiti et al, 1990; de la Pena et al, 2000; Ozden et al, 1998; Pescovitz, 1984; Runyon & Juler, 1985).
Marsman and colleagues (2007) looked at outcomes of elective UHR versus conservative management in patients with umbilical hernias, cirrhosis, and ascites. Seventeen patients with a median MELD score of 23 (range, 18 to 26) underwent elective UHR, and another 13 patients, also with a median MELD score of 23 (range, 18 to 27), were observed. In the elective UHR group, 3 patients (18%) had local wound complications, but there were no instances of hepatic decompensation or perioperative death. In contrast, in the group that was observed, 10 patients (77%) developed complications, including nine cases of incarceration and one spontaneous rupture with evisceration; these led to emergency UHR in six patients, and there were two perioperative deaths (15%). Although this was a retrospective study, and it is likely that some selection bias existed between the two groups, it is one of the few studies to compare early UHR versus watchful waiting in patients with comparable and relatively high MELD scores.
Another recent report, a retrospective review of the Veterans’ Affairs National Surgical Quality Improvement Program (VA NSQIP) database, analyzed the results of UHR in 127 cirrhotic and 1294 noncirrhotic patients (Gray et al, 2008). It determined that UHR performed electively in patients with cirrhosis was associated with an outcome similar to that in patients without cirrhosis. When UHR was performed emergently, however, patients with cirrhosis had significantly worse outcomes than those without. One drawback of this study was that the authors were not able to classify the degree of hepatic impairment among the patients with cirrhosis.
In patients with ascites, UHR has been combined with peritoneovenous (PV) shunting, closed-system suction drainage, or temporary peritoneal dialysis catheters to help manage ascites as the repairs heal (Ammar, 2010; Belghiti et al, 1990; Leonetti et al, 1984; O’Connor et al, 1984). A meta-analysis compared UHR with concomitant interventions to control ascites, namely PV shunting, versus repair alone and determined that uncontrolled ascites was associated with a relative risk ratio of 8.51 (95% confidence interval, 2.69 to 26.9) for hernia recurrence (McKay et al, 2009). Although PV shunting cannot be recommended in the current era because of the associated complications and high incidence of shunt occlusion, TIPS has been used more recently to successfully reduce ascites in the period surrounding UHR (Fagan et al, 2004; Inturri et al, 1996; Telem et al, 2010a). No controlled studies have evaluated the perioperative use of TIPS in UHR in patients with ascites, and definitive recommendations regarding its use cannot be made. In addition, TIPS may lead to new onset of encephalopathy or worsening of existing encephalopathic symptoms. Nonetheless, it is an option to manage ascites in patients who are deemed suitable candidates.
Whether nonabsorbable mesh should be used for the repair in cirrhotic patients is another issue of debate. Traditionally, the use of foreign material has been avoided in the repair of complicated hernias. However, some evidence suggests that nonabsorbable mesh may be used successfully, even when ascites is present (Ozden et al, 1998). A recent study prospectively randomized 80 CTP class A and B patients with cirrhosis and an umbilical hernia to either primary tissue repair with sutures or mesh repair with polypropylene onlay (Ammar, 2010). Surgical site infection occurred at a higher rate in the mesh group (16%) versus suture repair (9%), but the difference was not significant. Furthermore, all infections were successfully managed conservatively and did not require mesh removal. At a minimum of 6 months of follow-up, hernias recurred in 14% of patients in the suture repair group and in only 3% of patients in the mesh repair group (P < .05). Again, the dataset is small, but it does appear that mesh onlay may be used to lower the risk of recurrence in patients with cirrhosis who have umbilical hernias, in particular those with well-compensated disease.
In terms of inguinal hernia repair, results in this patient population are similar to those for umbilical herniorrhaphy (Inagaki et al, 2009; Lawson et al, 2009; Patti et al, 2008). Outcomes after elective repair do not vary between cirrhotic and noncirrhotic patients, although outcomes after emergent repair of an incarcerated or strangulated inguinal hernia are significantly worse in patients with cirrhosis (Carbonell et al, 2005). It appears that cirrhotic patients may safely undergo inguinal hernia repair with mesh; local wound complications, if they do arise, are usually easily treated (Inagaki et al, 2009; Lawson et al, 2009). In short, it is reasonable, and the data would suggest even advisable, to repair hernias electively in patients with cirrhosis. However, as with any elective operation, patients should be well compensated and medically optimized, and ascites should be controlled.
Colectomy
Compared with cholecystectomy and herniorrhaphy, considerably less data are available on colon resection in patients with chronic liver disease and cirrhosis. As in patients without cirrhosis, indications for colon resection include colon cancer, diverticular disease, and colonic ischemia. Colorectal surgery, which is associated with relatively high rates of morbidity and mortality when performed on the general population, is particularly high risk for those with cirrhosis. In fact, the mortality rate for patients with cirrhosis undergoing colectomy is around 20% to 25% (Metcalf et al, 1987; Meunier et al, 2008; Nguyen et al, 2009). Emergent colectomy is associated with an even higher mortality rate: up to 36% in patients with cirrhosis who have portal hypertension and 21% in those with portal hypertension who did not have cirrhosis (Nguyen et al, 2009). It is preferable to perform colorectal surgery in a cirrhotic patient on an elective basis whenever possible.
In a recent, large, population-based study, extraintestinal complications, such as pulmonary and wound complications, were increased in patients with cirrhosis and portal hypertension in particular. On multivariate analysis, total colectomy, malnutrition, and comorbid cardiac, pulmonary, and renal disease were the most significant predictors of postoperative morbidity (Nguyen et al, 2009). In another recent, retrospective, single-center study that included 41 cirrhotic patients undergoing colorectal surgery (CTP class A, 40%; class B, 49%; class C, 12%), the presence of preoperative ascites was significantly linked to postoperative morbidity (Meunier et al, 2008). When colectomy is performed on an elective basis, every effort should be made to optimize comorbid conditions and nutrition and to control ascites before surgery.
When feasible, the laparoscopic approach for colectomy may be advantageous in this patient population. Laparoscopy has significantly improved outcomes for gallbladder surgery in patients with cirrhosis, in particular in patients with well-compensated disease. Along these lines, a report on laparoscopic-assisted colectomy in 17 patients with compensated cirrhosis (CTP class A, 71%; class B, 29%) was notable for relatively minimal blood loss (mean, 245 mL; range, 100 to 250 mL), a 29% morbidity rate, and no operative deaths (Martinez et al, 2004). The study was small, and the group of patients was highly selected. Nonetheless, this research does offer some indication that laparoscopic colectomy, when performed at centers experienced in advanced laparoscopic surgery, may lead to improved outcomes in CTP class A and B patients versus the open approach.
Bariatric Surgery
Because of the high incidence of nonalcoholic fatty liver disease (NAFLD) and its association with the metabolic syndrome, chronic liver disease is not infrequent among patients being considered for bariatric procedures. The finding of overt cirrhosis is estimated to occur in 1.4% of patients undergoing these operations (Dallal et al, 2004). The majority of these patients are diagnosed with cirrhosis intraoperatively, and surgery is abandoned. Few reports exist as to the safety and efficacy of bariatric procedures in known cirrhotic patients. In the largest series to date, which includes 30 patients analyzed retrospectively, 27 were diagnosed intraoperatively (Dallal et al, 2004). All patients were classified as CTP class A, and there were no perioperative deaths.
Given the paucity of data, the decision of whether to proceed with operation in patients with well-compensated cirrhosis is unclear. Certainly, patients with more advanced disease and/or portal hypertension are at significant mortality risk. However, several reports document the reversal of even advanced liver disease after surgical weight loss (Dixon et al, 2004; Kral et al, 2004). Therefore it appears that bariatric surgery may be reasonable in patients with early fibrosis and possibly in those with well-compensated cirrhosis. As outlined earlier, the risk inherent to the procedure, severity of liver disease, and comorbid conditions must be considered individually along with the potential benefit of surgical weight loss.
The choice of weight loss procedure in those deemed candidates is also unclear (Baltasar, 2006; Sarr, 2006). Despite the histologic improvement in liver disease seen with biliopancreatic diversion, several case reports of hepatic failure after this procedure exist (Castillo et al, 2001; Grimm et al, 1992; Kral et al, 2004). The optimal procedure regarding weight loss remains under intense debate in the bariatric surgical community and is beyond the scope of this chapter. However, the suggestion has been made that sleeve gastrectomy may be the procedure of choice in patients with cirrhosis because of minimal bleeding complications, protection from malabsorption associated with other bariatric procedures, and potential improvement in transplant candidacy (Baltasar, 2006; Takata et al, 2008). No studies evaluating the use of adjustable gastric bands in cirrhotic patients are available.
Cardiac Procedures
Cardiovascular risk factors occur at greater rates among those with cirrhosis than among the general population (Hessheimer et al, 2010). Furthermore, although an uncommon cause, congestive heart failure may actually lead to hepatic congestion, fibrosis, and ultimately cirrhosis. Because of these and other factors, cardiac surgery is becoming increasingly more frequent in patients with end-stage liver disease, in particular those awaiting liver transplantation (Thielmann et al, 2010). Cardiac surgery, however, is extremely risky in patients with cirrhosis. Mortality rates are as high as 50% to 80% in patients with advanced liver disease undergoing cardiac surgery with cardiopulmonary bypass (CPB) because of high rates of infection, gastrointestinal complications, and bleeding (Bizouarn et al, 1999; Kaplan et al, 2002; Klemperer et al, 1998).
Outcomes for cirrhotic patients undergoing cardiac surgery have variably been associated with preoperative serum cholinesterase, serum bilirubin, and platelet count (An et al, 2007; Filsoufi et al, 2007; Hirata et al, 1999; Morisaki et al, 2010; Reinhartz et al, 1998). More consistently, however, outcomes have been linked to the preoperative CTP or MELD score. CTP class B and C and/or a MELD score greater than 13 to 15 have been shown to correlate with a significantly increased risk for postoperative complications and death in cirrhotic patients undergoing cardiac procedures, in particular those involving CPB (Ailawadi et al, 2009; An et al, 2007; Filsoufi et al, 2007; Hayashida et al, 2004; Klemperer et al, 1998; Morisaki et al, 2010; Thielmann et al, 2010).
CPB induces the production of inflammatory cytokines and other vasoactive substances and leads to further derangements in hemodynamics, coagulation, and immune function. Advanced cirrhosis (CTP class B and C) is considered a contraindication for CPB (An et al, 2007; Filsoufi et al, 2007; Hayashida et al, 2004). A few recent reports, however, have described successful on-pump valve procedures in patients with advanced liver disease (Iino et al, 2008; Nemati et al, 2008; Takahashi et al, 2006). By preoperative optimization of hepatic status, aggressive administration of platelets and fresh frozen plasma throughout the perioperative period, and dilutional ultrafiltration (DUF) during CPB, these investigators were able to curtail the negative effects of CPB in patients with decompensated disease. DUF in particular helps to minimize hemodilution and remove inflammatory mediators produced during CPB (Iino et al, 2008). Another option that has shown modest success in patients with moderate to severe cirrhosis in need of coronary artery bypass (CAB) is off-pump surgery (Ben Ari et al, 2006; Hayashida et al, 2004; Murashita et al, 2009). Off-pump CAB may reduce bleeding and transfusion requirements. Further investigations, however, are required to determine the true applicability of these techniques in this patient population.
Thoracic Procedures
The literature on thoracic surgery in patients with cirrhosis primarily describes thoracoscopic procedures for the treatment of refractory hepatic hydrothorax, a complication that occurs in approximately 5% of cirrhotic patients (Alberts et al, 1991). It is a symptomatic transudative pleural effusion that is believed to arise secondary to tiny defects in the diaphragm that allow ascites to flow from the high-pressure peritoneal cavity into the low-pressure pleural space (Huang et al, 2005; Nakamura et al, 1996; Zenda et al, 1998). Hepatic hydrothorax may result in significant respiratory compromise, and initial treatments include sodium restriction, diuretics, TIPS, and frequent thoracenteses. When these fail, video-assisted thoracoscopic surgery (VATS) with closure of obvious diaphragmatic defects and/or mechanical or chemical pleurodesis are options that may allow prolonged symptomatic relief (Assouad et al, 2003; Ferrante et al, 2002; Mouroux et al, 1996; Northup et al, 2009). Potential complications include empyema and persistent leakage of high volumes of ascites from the chest tube site. Nonetheless, the procedure is relatively well tolerated and is associated with success rates of up to 80%, even in patients with advanced cirrhosis (Cerfolio & Bryant, 2006).
Cirrhosis is present in approximately 4% to 7% of patients undergoing esophagectomy in some series (Fekete et al, 1987; Lu et al, 2005; Tachibana et al, 2000). Actual rates of cirrhosis in patients with esophageal neoplasms are likely higher than reported, based on the fact that alcohol plays an important role in the pathogenesis of both diseases; stage for stage, many more cirrhotics than noncirrhotics would be deemed unsuitable for surgery. Unlike gastric resection, esophagectomy in cirrhotic patients is actually associated with relatively few deaths as a result of postoperative liver failure, in theory because of the radical gastroesophageal devascularization and postoperative anorexia, which may lead to a significant reduction in alcohol consumption (Tachibana et al, 2000). Nonetheless, esophagectomy in cirrhotic patients is associated with much higher rates of surgical morbidity and mortality compared with noncirrhotics, in the range of 31% to 87% and 17% to 26%, respectively (Fekete et al, 1987; Lu et al, 2005; Tachibana et al, 2000). Perioperative bleeding, ascitic effusions, pneumonia, and sepsis are the most common complications and cause the majority of postoperative deaths. Significant preoperative coagulopathy, hyperbilirubinemia (>3 mg/dL), weight loss (>10% to 15% of body weight), and CTP classes B and C are associated with the highest rates of postoperative death and may be considered contraindications for surgery. For patients surviving the immediate postoperative period, however, overall survival appears to be similar to that of noncirrhotic patients undergoing the same procedures (Tachibana et al, 2000). Esophageal resection may play some role in well-selected patients with mild underlying liver disease.
Only four published reports from two centers have reported on pulmonary resection for non–small-cell lung cancer in patients with cirrhosis, the great majority of whom were CTP class A (Iwasaki et al, 2006; Iwata et al, 2007a, 2007b, 2008). Surgical morbidity rates were approximately 19% to 27%, and mortality rates were 5% to 13%. The most common complications were infection, bleeding, and acute liver failure. Based on the retrospective nature of the studies and the small number of patients they included, the authors were unable to conclude whether these operations had any impact on life expectancy in this patient population.
Trauma
Cirrhotic patients represent approximately 1% to 1.5% of the trauma population and an even greater proportion of those sustaining blunt trauma (Georgiou et al, 2009; Wutzler et al, 2009). In these patients, falls are the most common cause of injury (43%), followed by assault (Chen et al, 2007; Georgiou et al, 2009). Complications occur in up to 10% of patients and primarily include acute respiratory distress syndrome, trauma-associated coagulopathy, pneumonia, and sepsis (Christmas et al, 2005; Georgiou et al, 2009). In-hospital mortality rates are high, in the range of 22% to 33%. The CTP classification appears to be associated with a stepwise increase in the risk of death: up to 15% in class A, 37% in class B, and 76% in class C (Christmas et al, 2005).
Cirrhotic trauma patients are significantly more likely than those without cirrhosis to undergo laparotomy, and splenectomy is one of the most common operations performed (Christmas et al, 2005; Georgiou et al, 2009). This is not surprising given the prevalence of portal hypertension and splenomegaly among these patients. Among cirrhotic patients, nonoperative management of splenic injury fails in up to 92% of patients (Fang et al, 2003). On the other hand, multivariate analyses from several studies have demonstrated that after laparotomy, 40% to 55% of cirrhotic patients ultimately die in the hospital, versus only 15% to 24% of those who avoid laparotomy (Christmas et al, 2005; Demetriades et al, 2004; Georgiou et al, 2009).
Obviously, management of these patients is highly complex. The American College of Surgeons recommends that trauma patients with a history of cirrhosis be transferred to a trauma center, if they are not at one already (Wahlstrom et al, 2000), and should be very carefully resuscitated and monitored in an intensive care setting. Bleeding complications are common, and coagulopathy and hypothermia should be aggressively corrected. Because these patients are almost routinely malnourished and hypoalbuminemia has been significantly linked with poor outcomes, nutritional support with solutions rich in branched-chain amino acids should be started early (Chiarla et al, 1997). It is imperative to promptly diagnose injuries in these patients, although interventions should be undertaken judiciously. Early consultation with hepatology and/or hepatobililary surgery, if possible, is strongly advised.
Ailawadi G, et al. Model for end-stage liver disease predicts mortality for tricuspid valve surgery. Ann Thorac Surg. 2009;87(5):1460-1467.
Alberts WM, et al. Hepatic hydrothorax: cause and management. Arch Intern Med. 1991;151(12):2383-2388.
Alkozai EM, Lisman T, Porte RJ. Bleeding in liver surgery: prevention and treatment. Clin Liver Dis. 2009;13(1):145-154.
Ammar SA. Management of complicated umbilical hernias in cirrhotic patients using permanent mesh: randomized clinical trial. Hernia. 2010;14(1):35-38.
An Y, Xiao YB, Zhong QJ. Open-heart surgery in patients with liver cirrhosis. Eur J Cardiothorac Surg. 2007;31(6):1094-1098.
Aranha GV, Sontag SJ, Greenlee HB. Cholecystectomy in cirrhotic patients: a formidable operation. Am J Surg. 1982;143(1):55-60.
Assouad J, et al. Recurrent pleural effusion complicating liver cirrhosis. Ann Thorac Surg. 2003;75(3):986-989.
Azoulay D, et al. Neoadjuvant transjugular intrahepatic portosystemic shunt: a solution for extrahepatic abdominal operation in cirrhotic patients with severe portal hypertension. J Am Coll Surg. 2001;193(1):46-51.
Baltasar A. Liver cirrhosis and bariatric operations. Surg Obes Relat Dis. 2006;2(5):580. author reply 581
Baron HC. Umbilical hernia secondary to cirrhosis of the liver: complications of surgical correction. N Engl J Med. 1960;263:824-828.
Befeler AS, et al. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140(7):650-654.
Belghiti J, Durand F. Abdominal wall hernias in the setting of cirrhosis. Semin Liver Dis. 1997;17(3):219-226.
Belghiti J, et al. Herniorrhaphy and concomitant peritoneovenous shunting in cirrhotic patients with umbilical hernia. World J Surg. 1990;14(2):242-246.
Ben Ari A, et al. Off-pump coronary artery bypass grafting in a patient with Child class C liver cirrhosis awaiting liver transplantation. Br J Anaesth. 2006;97(4):468-472.
Bizouarn P, et al. Early and late outcome after elective cardiac surgery in patients with cirrhosis. Ann Thorac Surg. 1999;67(5):1334-1338.
Bornman PC, Terblanche J. Subtotal cholecystectomy: for the difficult gallbladder in portal hypertension and cholecystitis. Surgery. 1985;98(1):1-6.
Bouchier IA. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. 1969;10(9):705-710.
Carbonell AM, Wolfe LG, DeMaria EJ. Poor outcomes in cirrhosis-associated hernia repair: a nationwide cohort study of 32,033 patients. Hernia. 2005;9(4):353-357.
Castillo J, et al. Liver transplantation in a case of steatohepatitis and subacute hepatic failure after biliopancreatic diversion for morbid obesity. Obes Surg. 2001;11(5):640-642.
Cerfolio RJ, Bryant AS. Efficacy of video-assisted thoracoscopic surgery with talc pleurodesis for porous diaphragm syndrome in patients with refractory hepatic hydrothorax. Ann Thorac Surg. 2006;82(2):457-459.
Chen ZB, et al. Pre-existing cirrhosis is associated with increased mortality of traumatic patients: analysis of cases from a trauma center in east China. World J Gastroenterol. 2007;13(42):5654-5658.
Chiarla C, et al. The branched-chain amino acids. Minerva Gastroenterol Dietol. 1997;43(4):189-196.
Christmas AB, et al. Cirrhosis and trauma: a deadly duo. Am Surg. 2005;71(12):996-1000.
Conte D, et al. Cholelithiasis in cirrhosis: analysis of 500 cases. Am J Gastroenterol. 1991;86(11):1629-1632.
Cryer HM, Howard DA, Garrison RN. Liver cirrhosis and biliary surgery: assessment of risk. South Med J. 1985;78(2):138-141.
Csikesz NG, et al. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009;208(1):96-103.
Cucinotta E, Lazzara S, Melita G. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2003;17(12):1958-1960.
Curro G, et al. Laparoscopic cholecystectomy in Child-Pugh class C cirrhotic patients. JSLS. 2005;9(3):311-315.
Dallal RM, et al. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg. 2004;14(1):47-53.
de la Pena CG, et al. Umbilical herniorrhaphy in cirrhotic patients: a safe approach. Eur J Surg. 2000;166(5):415-416.
Delis S, et al. Laparoscopic cholecystectomy in cirrhotic patients: the value of MELD score and Child-Pugh classification in predicting outcome. Surg Endosc. 2010;24(2):407-412.
Demetriades D, et al. Liver cirrhosis in patients undergoing laparotomy for trauma: effect on outcomes. J Am Coll Surg. 2004;199(4):538-542.
Dixon JB, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39(6):1647-1654.
El-Awadi S, et al. Laparoscopic versus open cholecystectomy in cirrhotic patients: a prospective randomized study. Int J Surg. 2009;7(1):66-69.
Fagan SP, Awad SS, Berger DH. Management of complicated umbilical hernias in patients with end-stage liver disease and refractory ascites. Surgery. 2004;135(6):679-682.
Fang JF, et al. Liver cirrhosis: an unfavorable factor for nonoperative management of blunt splenic injury. J Trauma. 2003;54(6):1131-1136.
Farnsworth N, et al. Child-Turcotte-Pugh versus MELD score as a predictor of outcome after elective and emergent surgery in cirrhotic patients. Am J Surg. 2004;188(5):580-583.
Fekete F, et al. Results of esophagogastrectomy for carcinoma in cirrhotic patients: a series of 23 consecutive patients. Ann Surg. 1987;206(1):74-78.
Fernandes NF, et al. Laparoscopic cholecystectomy and cirrhosis: a case-control study of outcomes. Liver Transpl. 2000;6(3):340-344.
Ferrante D, et al. Video-assisted thoracoscopic surgery with talc pleurodesis in the management of symptomatic hepatic hydrothorax. Am J Gastroenterol. 2002;97(12):3172-3175.
Filsoufi F, et al. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl. 2007;13(7):990-995.
Gaglio PJ, Buniak B, Leevy CB. Primary endoscopic retrograde cholecystoendoprosthesis: a nonsurgical modality for symptomatic cholelithiasis in cirrhotic patients. Gastrointest Endosc. 1996;44(3):339-342.
Garrison RN, et al. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199(6):648-655.
Georgiou C, et al. Cirrhosis and trauma are a lethal combination. World J Surg. 2009;33(5):1087-1092.
Gines P, et al. Management of cirrhosis and ascites. N Engl J Med. 2004;350(16):1646-1654.
Gray SH, et al. Umbilical herniorrhapy in cirrhosis: improved outcomes with elective repair. J Gastrointest Surg. 2008;12(4):675-681.
Grimm IS, Schindler W, Haluszka O. Steatohepatitis and fatal hepatic failure after biliopancreatic diversion. Am J Gastroenterol. 1992;87(6):775-779.
Haskal ZJ, et al. Intestinal varices: treatment with the transjugular intrahepatic portosystemic shunt. Radiology. 1994;191(1):183-187.
Hayashida N, et al. Clinical outcome after cardiac operations in patients with cirrhosis. Ann Thorac Surg. 2004;77(2):500-505.
Hessheimer AJ, et al. Metabolic risk factors are a major comorbidity in patients with cirrhosis independent of the presence of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22(10):1239-1244.
Hirata N, Sawa Y, Matsuda H. Predictive value of preoperative serum cholinesterase concentration in patients with liver dysfunction undergoing cardiac surgery. J Card Surg. 1999;14(3):172-177.
Huang PM, et al. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg. 2005;130(1):141-145.
Iino K, et al. Successful aortic valve replacement using dilutional ultrafiltration during cardiopulmonary bypass in a patient with Child-Pugh class C cirrhosis. Interact Cardiovasc Thorac Surg. 2008;7(2):331-332.
Inagaki M, et al. “Tension-free” herniorrhaphy for groin hernias in patients with cirrhosis: report of four cases. Surg Today. 2009;39(6):540-543.
Inturri P, Graziotto A, Rossaro L. Treatment of ascites: old and new remedies. Dig Dis. 1996;14(3):145-156.
Iwasaki A, et al. Lung cancer surgery in patients with liver cirrhosis. Ann Thorac Surg. 2006;82(3):1027-1032.
Iwata T, et al. Long-term outcome of surgical treatment for non–small cell lung cancer with comorbid liver cirrhosis. Ann Thorac Surg. 2007;84(6):1810-1817.
Iwata T, et al. Factors predicting early postoperative liver cirrhosis–related complications after lung cancer surgery in patients with liver cirrhosis. Interact Cardiovasc Thorac Surg. 2007;6(6):720-730.
Iwata T, et al. Pulmonary resection for non–small cell lung cancer in patients with hepatocellular carcinoma. World J Surg. 2008;32(10):2204-2212.
Ji W, et al. Application of laparoscopic cholecystectomy in patients with cirrhotic portal hypertension. Hepatobiliary Pancreat Dis Int. 2004;3(2):270-274.
Kaplan M, et al. Cardiac operations for patients with chronic liver disease. Heart Surg Forum. 2002;5(1):60-65.
Kim JJ, et al. Cirrhotic patients with a transjugular intrahepatic portosystemic shunt undergoing major extrahepatic surgery. J Clin Gastroenterol. 2009;43(6):574-579.
Klemperer JD, et al. Cardiac operations in patients with cirrhosis. Ann Thorac Surg. 1998;65(1):85-87.
Kochanek KD, et al. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59(4):1-51.
Kral JG, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135(1):48-58.
Lawson EH, et al. Groin herniorrhaphy in patients with cirrhosis and after liver transplantation. Am Surg. 2009;75(10):962-965.
Leonetti JP, et al. Umbilical herniorrhaphy in cirrhotic patients. Arch Surg. 1984;119(4):442-445.
Lu MS, et al. Is it safe to perform esophagectomy in esophageal cancer patients combined with liver cirrhosis? Interact Cardiovasc Thorac Surg. 2005;4(5):423-425.
Mansour A, et al. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122(4):730-735.
Marsman HA, et al. Management in patients with liver cirrhosis and an umbilical hernia. Surgery. 2007;142(3):372-375.
Martinez JL, et al. Laparoscopic-assisted colectomy in patients with liver cirrhosis. Surg Endosc. 2004;18(7):1071-1074.
McKay A, et al. Umbilical hernia repair in the presence of cirrhosis and ascites: results of a survey and review of the literature. Hernia. 2009;13(5):461-468.
Merli M, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18(11-12):978-986.
Metcalf AM, et al. The surgical risk of colectomy in patients with cirrhosis. Dis Colon Rectum. 1987;30(7):529-531.
Meunier K, et al. Colorectal surgery in cirrhotic patients: assessment of operative morbidity and mortality. Dis Colon Rectum. 2008;51(8):1225-1231.
Morino M, et al. Laparoscopic cholecystectomy in cirrhosis: contraindication or privileged indication? Surg Laparosc Endosc Percutan Tech. 2000;10(6):360-363.
Morisaki A, et al. Risk factor analysis in patients with liver cirrhosis undergoing cardiovascular operations. Ann Thorac Surg. 2010;89(3):811-817.
Mouroux J, et al. Management of pleural effusion of cirrhotic origin. Chest. 1996;109(4):1093-1096.
Muilenburg DJ, et al. Surgery in the patient with liver disease. Med Clin North Am. 2009;93(5):1065-1081.
Murashita T, et al. Preoperative evaluation of patients with liver cirrhosis undergoing open heart surgery. Gen Thorac Cardiovasc Surg. 2009;57(6):293-297.
Nakamura A, et al. Peritoneal–pleural communications in hepatic hydrothorax demonstrated by thoracoscopy. Chest. 1996;109(2):579-581.
Nemati MH, Astaneh B, Zamirian M. Aortic valve replacement in a patient with liver cirrhosis and coagulopathy. Gen Thorac Cardiovasc Surg. 2008;56(8):430-433.
Nguyen GC, Correia AJ, Thuluvath PJ. The impact of cirrhosis and portal hypertension on mortality following colorectal surgery: a nationwide, population-based study. Dis Colon Rectum. 2009;52(8):1367-1374.
Northup PG, et al. Mechanical pleurodesis aided by peritoneal drainage: procedure for hepatic hydrothorax. Ann Thorac Surg. 2009;87(1):245-250.
Norton SA, et al. The role of preoperative TIPSS to facilitate curative gastric surgery. Cardiovasc Intervent Radiol. 2003;26(4):398-399.
O’Connor M, Allen JI, Schwartz ML. Peritoneovenous shunt therapy for leaking ascites in the cirrhotic patient. Ann Surg. 1984;200(1):66-69.
O’Hara ET, et al. Management of umbilical hernias associated with hepatic cirrhosis and ascites. Ann Surg. 1975;181(1):85-87.
O’Leary JG, Yachimski PS, Friedman LS. Surgery in the patient with liver disease. Clin Liver Dis. 2009;13(2):211-231.
Ozden I, et al. Elective repair of abdominal wall hernias in decompensated cirrhosis. Hepatogastroenterology. 1998;45(23):1516-1518.
Patti R, et al. Inguinal hernioplasty improves the quality of life in patients with cirrhosis. Am J Surg. 2008;196(3):373-378.
Perkins L, Jeffries M, Patel T. Utility of preoperative scores for predicting morbidity after cholecystectomy in patients with cirrhosis. Clin Gastroenterol Hepatol. 2004;2(12):1123-1128.
Pescovitz MD. Umbilical hernia repair in patients with cirrhosis: no evidence for increased incidence of variceal bleeding. Ann Surg. 1984;199(3):325-327.
Poggio JL, et al. A comparison of laparoscopic and open cholecystectomy in patients with compensated cirrhosis and symptomatic gallstone disease. Surgery. 2000;127(4):405-411.
Puggioni A, Wong LL. A meta-analysis of laparoscopic cholecystectomy in patients with cirrhosis. J Am Coll Surg. 2003;197(6):921-926.
Reinhartz O, et al. Importance of preoperative liver function as a predictor of survival in patients supported with Thoratec ventricular assist devices as a bridge to transplantation. J Thorac Cardiovasc Surg. 1998;116(4):633-640.
Runyon BA, Juler GL. Natural history of repaired umbilical hernias in patients with and without ascites. Am J Gastroenterol. 1985;80(1):38-39.
Sarr MG. Is a bariatric procedure appropriate in patients with portal hypertension secondary to cirrhosis? Surg Obes Relat Dis. 2006;2(3):405-406.
Schiff J, et al. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2005;19(9):1278-1281.
Schlenker C, Johnson S, Trotter JF. Preoperative transjugular intrahepatic portosystemic shunt (TIPS) for cirrhotic patients undergoing abdominal and pelvic surgeries. Surg Endosc. 2009;23(7):1594-1598.
Schwartz SI. Biliary tract surgery and cirrhosis: a critical combination. Surgery. 1981;90(4):577-583.
Schwesinger WH, et al. Cirrhosis and alcoholism as pathogenetic factors in pigment gallstone formation. Ann Surg. 1985;201(3):319-322.
Shrestha R, Trouillot TE, Everson GT. Endoscopic stenting of the gallbladder for symptomatic gallbladder disease in patients with end-stage liver disease awaiting orthotopic liver transplantation. Liver Transpl Surg. 1999;5(4):275-281.
Shrestha R, et al. Endoscopic stenting of gallbladder for symptomatic cholelithiasis in patients with end-stage liver disease awaiting orthotopic liver transplantation. Am J Gastroenterol. 1996;91(3):595-598.
Tachibana M, et al. Esophageal cancer with cirrhosis of the liver: results of esophagectomy in 18 consecutive patients. Ann Surg Oncol. 2000;7(10):758-763.
Takahashi M, et al. Successful aortic valve replacement for infective endocarditis in a patient with severe liver cirrhosis. Ann Thorac Cardiovasc Surg. 2006;12(4):287-289.
Takata MC, et al. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4(2):159-164. discussion 164-165
Teh SH, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132(4):1261-1269.
Telem DA, Schiano T, Divino CM. Complicated hernia presentation in patients with advanced cirrhosis and refractory ascites: management and outcome. Surgery. 2010;148(3):538-543.
Telem DA, et al. Factors that predict outcome of abdominal operations in patients with advanced cirrhosis. Clin Gastroenterol Hepatol. 2010;8(5):451-457.
Thielmann M, et al. Risk prediction and outcomes in patients with liver cirrhosis undergoing open-heart surgery. Eur J Cardiothorac Surg. 2010;38(5):592-599.
Tuech JJ, et al. Laparoscopic cholecystectomy in cirrhotic patients. Surg Laparosc Endosc Percutan Tech. 2002;12(4):227-231.
Wahlstrom K, et al. Trauma in cirrhotics: survival and hospital sequelae in patients requiring abdominal exploration. Am Surg. 2000;66(11):1071-1076.
Wutzler S, et al. Association of preexisting medical conditions with in-hospital mortality in multiple-trauma patients. J Am Coll Surg. 2009;209(1):75-81.
Yeh CN, Chen MF, Jan YY. Laparoscopic cholecystectomy in 226 cirrhotic patients: experience of a single center in Taiwan. Surg Endosc. 2002;16(11):1583-1587.
Yerdel MA, et al. Laparoscopic cholecystectomy in cirrhotic patients: expanding indications. Surg Laparosc Endosc. 1993;3(3):180-183.
Zenda T, et al. Detection of diaphragmatic defect as the cause of severe hepatic hydrothorax with magnetic resonance imaging. Am J Gastroenterol. 1998;93(11):2288-2289.
Ziser A, et al. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999;90(1):42-53.