CHAPTER 14 Neurotology
The otolaryngologic subspecialty of otology, neurotology, and cranial base surgery has a focus on the anatomy, physiology, and abnormalities of the three cranial nerves that traverse the temporal bone—the cochlear, vestibular, and facial nerves—as well as specific tests of their functions.1 Surgical management of intractable vertigo is reviewed in Chapter 92; however, the indications for vestibular neurectomy are briefly introduced in this chapter in the context of vestibular assessment and discussion of various vestibular disorders. Facial nerve injury secondary to temporal bone fracture is reviewed in Chapters 338 and 339 and is included in this chapter only in the context of the cochlear and vestibular nerves, although more extensive reviews are offered elsewhere.2–4
Anatomy of the Inner Ear
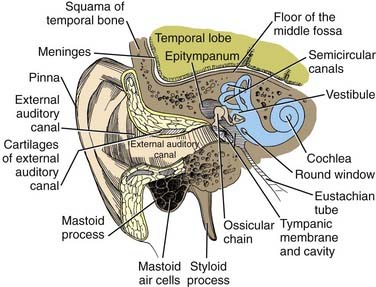
In postnatal infants and adults, the vestibulocochlear labyrinth consists of a continuous complex of bony spaces encased in the otic capsule and within the petrous bone, called the bony labyrinth (Figs. 14-1 to 14-4). It is filled with perilymph (biochemically similar to cerebrospinal fluid), and suspended within it is an almost true, miniature replica of the bony spaces—the membranous labyrinth. The labyrinth is filled with endolymph (see Fig. 14-2). The cochlea is located anteriorly and is divided from the vestibular labyrinth by the vestibular, cochlear, and facial nerves within the internal auditory canal (IAC). The vestibular portion of the labyrinth lies posterior to the cochlea and the IAC (see Figs. 14-3 and 14-4).
The Cochlear System
The basal turn of the cochlea forms a distinct bony promontory on the medial wall of the mesotympanum (i.e., the middle ear) and is the only portion of the cochlea that is visible during middle ear operations. The remaining turns of the cochlea are enclosed within the petrous bone. At the posterior aspect of the promontory are two fibrous barriers to communication with the inner ear: the oval window superiorly, which faces laterally and houses the footplate of the stapes, and the round window inferiorly, which faces posteriorly and is contained within the bony round window niche (see Figs. 14-1 and 14-2).
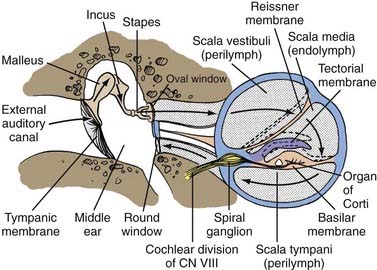
A transverse section through the cochlea shows that it is composed of three compartments: the scala vestibuli, scala media, and scala tympani (see Figs. 14-2 and 14-4). The scala media, which contains endolymph, is separated from the scala vestibuli and tympani by Reissner’s membrane and the basilar membrane, respectively. The scala vestibuli and scala tympani contain perilymph, and these two compartments communicate with each other at the cochlear apex through an opening at the tip of the basilar membrane called the helicotrema. At the base of the upper compartment, called the scala vestibuli, is the oval window, and at the base of the lower compartment, called the scala tympani, is the round window.
The basilar membrane has a complex structure resting on it that is best appreciated in cross section. The basilar membrane and the thin sloping membrane, called Reissner’s membrane, form a tube that ends blindly and is sealed at the helicotrema. This is the scala media, or cochlear duct, and it contains endolymph (see Figs. 14-2 and 14-4). Extending along the entire basilar membrane and spiraling with the cochlea is the organ of Corti. It contains the structurally complex sensory epithelium innervated by the cochlear nerve. As viewed from above, the basilar membrane is widest near the helicotrema and narrowest at the base. Maximal high-frequency vibration of the basilar membrane occurs at the base, and maximal low-frequency vibration occurs at the apex, thereby resulting in hair cell transduction of high frequencies at the base and low frequencies at the apex.
Physiology of Hearing
For an understanding of the various types of hearing loss, it is important to review the mechanism of transmission of sound to the neural receptors (see Fig. 14-2). Air vibrations impinge on the tympanic membrane and cause it and the malleus to vibrate. This physical vibration is transmitted through the incus to the stapes and by way of its footplate to the fluids of the labyrinth. The fluid vibrations produce a stimulus along the basilar membrane that activates the organ of Corti. Impulses are transmitted through the cochlear nerve endings to the cochlear nuclei in the pontomedullary junction. The impulses then continue to the auditory areas of the cortex.
Transformer Mechanism of the Tympanum
Because fluid is incompressible, the round window membrane acts as a compensating membrane to accommodate the vibrations of the stapes footplate (see Fig. 14-2). If sound vibrations were to reach the oval and round windows at the same time, a certain amount of cancellation of the sound would take place. This does not normally take place because of the phase difference between the windows, which is facilitated by several factors. The intact tympanic membrane protects the round window from direct sound impingement; the tympanic membrane is connected to the oval window through the ossicular chain, thus making direct transmission by this route faster; and the round window membrane faces backward, at right angles to the plane of the tympanic membrane, and is recessed within the niche. These factors delay the impingement of sound onto the round window membrane, which produces the phase difference.
Transmission in the Labyrinth
Cochlear signal transduction of high frequencies occurs near the basal portion, and transduction of low frequencies occurs near the apical regions of the basilar membrane and its organ of Corti. Detailed mechanisms of sound transduction and perception are beyond the scope of this chapter but can be found elsewhere.1
Measures of Auditory System Function
Subjective Measures of Hearing
Pure-Tone Audiometry
Air Conduction
Pure-tone threshold hearing sensitivity has developed to be the subjective procedure by which auditory sensitivity is determined. In the United States, the American National Standards Institute (ANSI) has established standards for the calibration of clinical audiometers. The output sound pressure level for standard circumaural or inserted earphones, or both, is specified when measured in a standard coupler, referred to as an artificial ear. The artificial ear simulates the impedance characteristics of the average human ear at the plane of the tympanic membrane. The decibel levels used in audiometers for the normal threshold for air conduction can be found elsewhere.5
Bone Conduction
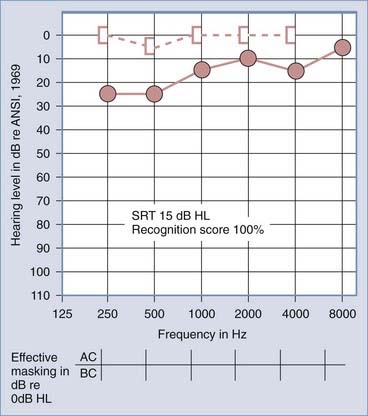
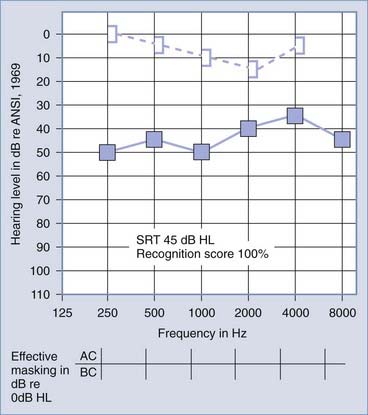
The primary audiologic tests used to distinguish conductive from sensorineural hearing loss are the comparative measures of air and bone conduction thresholds. The procedure for measuring bone conduction thresholds is similar to that for measuring air conduction thresholds, except that a vibrotactile stimulator transduces the signal, usually coupled to the mastoid of the ear under test. The diagnostic utility of the difference between air and bone conduction sensitivity is based primarily on two assumptions: that the air conduction threshold measures the function of the total auditory system, both conductive and sensorineural components, and that the threshold for bone conduction is primarily a measure of the integrity of the sensorineural auditory system and is not significantly influenced by the functional status of the external or middle ear. It has been demonstrated, however, that the external ear and middle ear do provide minor, but important contributions to the bone conduction threshold in the normal auditory system.6 Consequently, some conductive disorders do cause minor, but significant alterations in bone conduction sensitivity because of changes in the contribution of the middle ear to the bone-conducted signal reaching the cochlea. Despite this limitation, the difference between air and bone conduction pure-tone thresholds provides the most definitive indication of the effect of disorders in the external and middle ear on threshold sensitivity. A thorough review of the clinical principles of bone conduction testing is provided by Dirks.7 Examples of conductive and sensorineural hearing loss can be seen in Figures 14-5 and 14-6, respectively. Notice that in conductive hearing loss (see Fig. 14-5), hearing sensitivity by air conduction deviates from normal hearing (0-dB HL) but bone conduction sensitivity is within the normal limits. In sensorineural hearing loss (see Fig. 14-6), hearing sensitivity by both air conduction and bone conduction deviates equally from normal hearing (0-dB HL). Combinations of sensorineural and conductive hearing loss are called mixed hearing loss.
Masking
When a patient has a substantial difference in hearing sensitivity between the two ears, it is necessary to rule out the potential participation of the better hearing ear when testing the poorer hearing ear. Masking is defined by ANSI as the amount by which the threshold of audibility of a sound is raised by the presence of another (masking) sound.8 As early as 1940, Fletcher observed that a restricted bandwidth of frequencies contained within a broadband noise was sufficient to effectively mask a pure-tone threshold.9 Most clinical audiometers contain narrow bands of noise that encompass the critical band of frequencies necessary to mask frequency-specific stimuli.
In some circumstances of severe bilateral conductive hearing loss, it may be impossible to obtain a threshold for bone conduction (or possibly air conduction) without overmasking. Fortunately, as described later in this section, acoustic immittance studies can be performed without regard to “masking dilemmas” and can give additional diagnostic information on the functional status of the middle ear. Studebaker has described the rules for clinical masking in detail.10
Speech Audiometry
Speech Recognition Threshold
Traditionally, the SRT is measured with the use of spondaic words, that is, two-syllable words in which equal stress is placed on each syllable, such as hot-dog, baseball, cowboy, and sidewalk. No standardized method for presentation of the words has been accepted, although practical means for standardization have been suggested.11 The SRT is reported as the decibel HL below which the patient cannot successfully recognize the two-syllable words. It is expected that the SRT will approximately equal the average hearing loss for pure tones in the midfrequency region (500 to 2000 Hz), regardless of the type of hearing loss (i.e., conductive or sensorineural). The SRT has little differential diagnostic significance, except in cases of pseudohypacusis, but it is used to provide a descriptive measure of hearing loss for speech and to confirm the pure-tone air conduction sensitivity measures.
Speech Recognition Measures
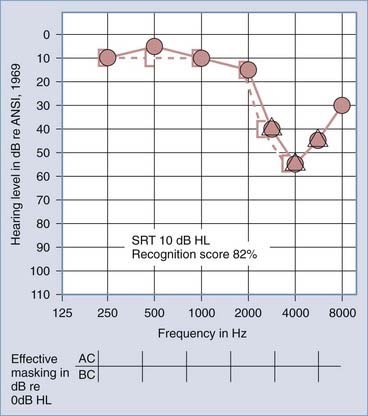
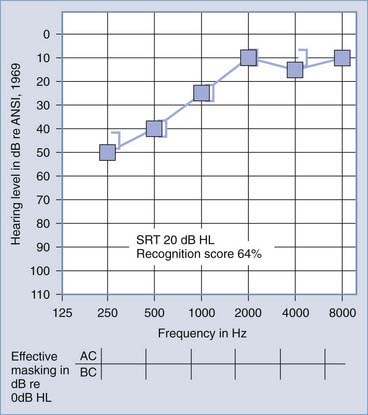
Figures 14-7 and 14-8 provide examples of the effects of conductive and sensorineural hearing loss on the SRT and speech recognition scores of two patients. Figure 14-7 is an example of conductive hearing loss secondary to otosclerosis. Notice the elevated pure-tone air conduction threshold and SRT, together with normal bone conduction sensitivity and excellent speech recognition score. Figure 14-8 is an example of sensorineural hearing loss secondary to Meniere’s disease, which is discussed later. Notice the low-frequency sensorineural hearing loss, the mildly elevated SRT, and the diminished speech recognition score (64%). This example reveals the potential effect of a cochlear lesion site on speech recognition ability. Early in the course of Meniere’s disease, it is unusual for speech discrimination ability to be abnormal. Review of Figures 14-5 and 14-6 reveals other examples in which conductive hearing loss from chronic otitis media (see Fig. 14-5) and sensorineural hearing loss from industrial noise trauma (see Fig. 14-6) affect speech and pure-tone results.
Objective Measures of Auditory System Function
Immittance Studies
Among the most significant advancements in the differential diagnosis of middle ear impairments and one that provides definitive information on cranial nerve VIII and low-brainstem function is measurement of acoustic immittance.12 This procedure requires no active participation by the patient but provides objective evidence of middle ear function and a means of testing the integrity of the acoustic reflex arc. The two procedures included in immittance studies are tympanometry and acoustic reflex measures.
Acoustic Reflex
The acoustic reflex refers to the reflexive contraction of the stapedius muscle on delivery of an acoustic stimulus. The stapedius muscle contracts reflexively and bilaterally on presentation of an acoustic stimulus.13 The muscle contraction results in a concomitant increase in impedance in the middle ear when measured at the plane of the tympanic membrane. Unfortunately, when middle ear pathologies introduce changes in middle ear impedance, it is not possible to measure evidence of further changes in impedance that might be introduced by contraction of the stapedius muscle. Only when tympanometry reveals the middle ear system to be functioning normally is it possible to test the integrity of the acoustic reflex arc.
Constant air pressure is maintained in the external auditory canal, and impedance or admittance is monitored over time. The intensity of a reflex-inducing acoustic stimulus is increased until a change in impedance or admittance is observed. The lowest intensity at which the reflex-inducing acoustic stimulus results in a change in acoustic impedance or admittance is specified as the acoustic reflex threshold. Typically, lesions in cochlear sites produce a change in the threshold of the acoustic reflex only for wide-band noise stimuli, not for pure-tone stimuli, until the hearing loss exceeds approximately the 60-dB HL. When the hearing loss is of cochlear origin and the loss exceeds 60 dB, there may be an increase in the threshold of the acoustic reflex even for pure-tone stimuli. The acoustic reflex threshold measure can be used in cases of sensorineural hearing loss to provide differential diagnostic information on the site of the sensorineural hearing loss.14
In patients with a retrocochlear site of the lesion (cranial nerve VIII and low brainstem), the acoustic reflex may be elevated or absent. An abnormally elevated or absent acoustic reflex in the presence of hearing loss at less than a 60-dB HL is audiologic evidence supporting a retrocochlear site of the lesion.15,16
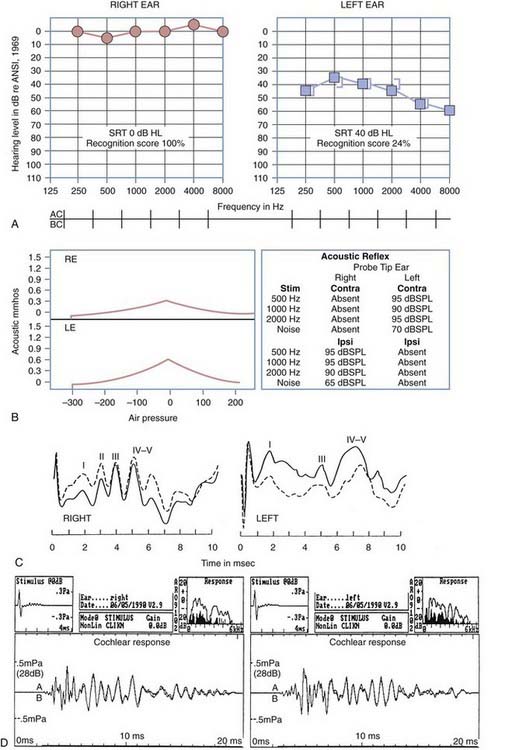
Figure 14-9 is an example of audiometric data from a patient with a left acoustic neuroma within the cerebellopontine angle. The pure-tone results reveal mild, left ear sensorineural hearing loss with a very poor speech recognition score (24%). The tympanograms were normal bilaterally, but there was no acoustic reflex identifiable with acoustic stimulation of the left ear. When the measuring tip was in the right ear, evidence of stapedius muscle contraction was observed only with ipsilateral stimulation. When the measuring tip was in the left ear, the stapedius muscle contracted only when the acoustic stimulus was presented contralaterally. As evidenced by acoustic reflex measures, this is the classic audiometric result seen in a patient with a left acoustic neuroma.
Auditory Brainstem Evoked Response Measures
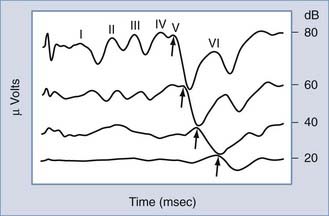
Possibly the most powerful audiologic test available today in differentiating between cochlear and retrocochlear lesions is measurement of the auditory brainstem evoked response (ABR). The ABR is one of several clinically useful evoked auditory potentials and the one most often applied in site-of-lesion testing.17 The ABR is typically evoked with a short-duration pulse delivered to the ear at a predetermined intensity. At high-intensity levels, the acoustic stimulus evokes as many as five amplitude peaks. The peaks were first identified and categorized by Jewett and Williston.18 Three of these peaks (waves I, III, and V) are the major peaks and are generally accepted as corresponding to firing of the first-order neurons of cranial nerve VIII (wave I), the superior olivary complex (wave III), and the inferior colliculus (wave V). These three major waves are present at approximately 2-msec intervals (in normal-hearing children and adults) after the onset of the acoustic stimulus at high-intensity levels (Fig. 14-10). As revealed in Figure 14-10, as the intensity of the stimulus is decreased, the amplitude of all peaks decreases, the latency of each peak increases, and the replicability of the early waves (I and III) decreases. Only wave V is identifiable at threshold levels.
Starr and Achor were among the earliest to use ABR results to describe a diverse set of patients with neurological disorders.19 Patients with cortical problems had normal ABR values, whereas patients with acoustic nerve and low-brainstem disorders had abnormal ABR results. This early evidence has been corroborated by numerous other reports in which ABR values were abnormal in patients with cranial nerve VIII and low-brainstem disorders.20,21
Auditory Neuropathy
Results from auditory evoked potential recordings combined with otoacoustic emission (OAE) testing provide objective measures that can identify patients with auditory neuropathy. The term auditory neuropathy, coined by Starr and colleagues,22 has been used to describe a group of patients with abnormal neural function demonstrated by absent or abnormal ABR results and absent middle ear reflexes but with normal outer hair cell function determined by normal OAE testing and cochlear microphonics. These patients also show evidence of poor speech discrimination, particularly in the presence of noise. Pure-tone thresholds vary widely in severity from normal to severe to profound and may be asymmetric or have a variety of configurations. Doyle and coworkers described the audiometric and electrophysiologic findings associated with auditory neuropathy.23 The results obtained after performing ABR and OAE testing suggest an abnormality of the auditory system at the level of the inner hair cells, at the synapse between hair cells and the cochlear nerve, at the level of the cochlear nerve itself, or a combination of these sites.
The ABR results in patients with cochlear hearing loss typically reveal normal ABR replicability and latencies at high-intensity levels, but with an elevated ABR “threshold” (a function of the elevated hearing thresholds secondary to cochlear pathology). In patients with a retrocochlear site of a lesion, the site of the auditory lesion affects the results. If a space-occupying lesion is present in the brainstem but after the first-order neurons of cranial nerve VIII, wave I may be normal, but all subsequent waves may be absent or significantly delayed in latency. If the lesion affects function of the first-order neurons of cranial nerve VIII, there may be no replicable waveforms evoked by the acoustic stimulus. Figure 14-9 presents the ABR results in a patient with an acoustic neuroma within the left cerebellopontine angle. The ABR result at a 75-dB normalized HL in the right ear reveals a replicable waveform with normal absolute and interwave latencies. The response from the left ear reveals poor replicability of waves III and V, increased latency of waves III and V, and consequently, abnormal I-III and I-V interwave latencies.
Electrically Evoked Auditory Potentials with Cochlear Implant Users
Electrically evoked auditory potentials have been studied for a number of purposes, including assessment of neural integrity, evaluation of cochlear implant function, and estimation of the psychophysical measures needed to program the cochlear implant speech processor, as well as an indication of performance after cochlear implantation.24–26 Preimplantation electrical recordings have included placement of a transtympanic needle electrode on the promontory and subsequent delivery of electrical current. In general, these results have been variable, and there has not been a clear relationship between these measures and postimplantation performance.27 Electrical auditory brainstem response (EABR) has been reliably recorded through a variety of cochlear implant devices and arrays. In general, four peaks can be identified, with the most robust being wave V. Wave I is usually obliterated by the stimulus artifact that occurs at the beginning of the recording. The EABR is affected by stimulus intensity, with a decrease in wave V amplitude and a slight increase in latency as the electrical current is decreased. On average, the latency of wave V is approximately 1.0 to 1.5 msec earlier than after the corresponding acoustic signal. Because the age at cochlear implantation has steadily decreased (now as young as 6 months of age), there is increased interest in the use of EABR to determine the threshold for each electrode and thereby assist in programming and fitting of the external speech processor.28 Several studies have compared the EABR wave V thresholds and behavior levels used in fitting of the speech processor. EABR wave V thresholds generally fall within the dynamic range for a given tested electrode and represent levels that are at least audible.24 Thresholds, amplitudes, and waveform morphologies differ across subjects and within individuals for different electrodes.24,25 The variations may be caused by differences in the population and pattern of surviving primary afferent neurons and dendrites within and across subjects.
Otoacoustic Emission Measures
Kemp was the first to report the presence of audiofrequency energy in the ear canal of subjects with normal hearing who were stimulated with a short-duration, broadband acoustic signal.29 Kemp identified these “emissions” as energy leakage from normal stimulation of cochlear structures. Since the early reports, evidence has accumulated confirming that such acoustic energy leakage is a biochemical property of the healthy, functioning cochlea.
Since identification of the phenomenon in 1978, research interests have focused on sources of the OAE30 and its clinical applications.31 OAE has been determined to be a product of the outer hair cells of the cochlea. Clinical evidence suggests that patients with thresholds of greater than 30-dB HL because of a cochlear-site lesion have no identifiable OAE to transient auditory stimulation. Any middle ear lesion typically precludes measurement of OAE. However, in the case of a retrocochlear lesion, when there has been no retrograde degeneration of the outer hair cells, normal OAE can be evoked in the presence of significant sensorineural hearing loss.31 OAE represents the first available auditory function test with which it may be possible to differentiate neural from cochlear sites of a lesion when the potential exists for each site to be implicated in sensorineural loss. As a consequence, OAE results may contribute information leading to more informed decisions by surgeons who need to address the potential for “hearing preservation” after excision of space-occupying lesions affecting cranial nerve VIII.
Figure 14-9D includes results from OAE measurements for the patient with an acoustic neuroma within the cerebellopontine angle presented earlier in this chapter. The results were obtained with a click delivered at an 80-dB sound pressure level. Results of the average OAE can be analyzed in terms of the replicability of the emission as a function of time after delivery of the emission-evoking stimulus (see Fig. 14-9D, lower section). The OAE response can be collapsed over time, and Fourier analysis of the energy provides a measure of the relative amplitude of the energy in the OAE as a function of the frequency (see Fig. 14-9D, upper right section). Noise in the external ear canal is represented by the dark spectrum; OAE energy is represented by the light spectrum. Despite left ear sensorineural hearing loss exceeding a 35-dB HL throughout the frequency range of 250 to 8000 Hz, there is OAE energy throughout the frequency range of 1000 to 4000 Hz. This observation reveals that the outer hair cells of the cochlea are functioning normally and that the source of the sensorineural hearing loss is neural, not cochlear. Postoperative measurements of OAE can reveal any changes in cochlear function caused by surgical intervention to remove the acoustic neuroma.
The Vestibular System
Anatomy
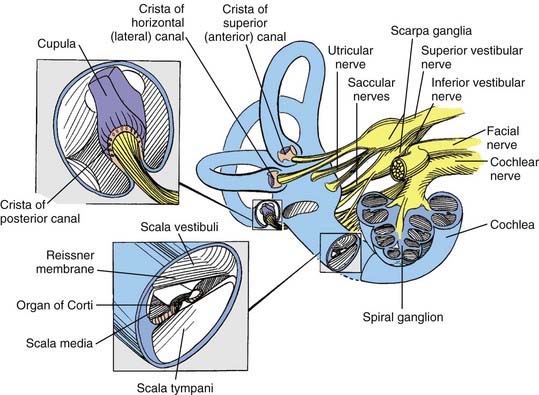
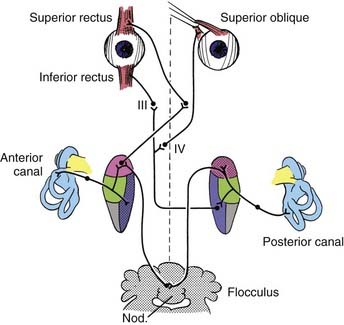
The bony vestibular labyrinth consists of an expansion that is continuous with the basal turn of the cochlea, called the vestibule, and three semicircular bony canals oriented at right angles to one another, each representing a dimension in space: a horizontal (lateral) canal, a superior (anterior) canal, and a posterior canal (Fig. 14-11; see also Fig. 14-4). Each canal originates from the lateral aspect of the vestibule and has its return insertion into the vestibule at a more medial location. The horizontal semicircular canal has its own insertion, but the vertical canals merge into a common crus before joining the vestibule (see Fig. 14-4). Near the origin of each canal is a bony expansion known as the ampulla.
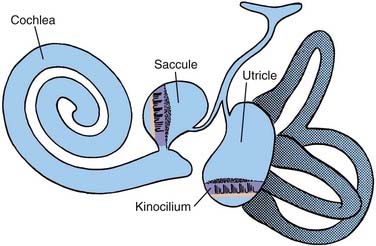
Within the bony vestibular labyrinth is a hollow, membranous replica of the system, approximately a fourth of its size. Each of the three endolymph-filled semicircular ducts is distended at the level of the bony ampulla. The distended area contains a crista ampullaris, whose neuroepithelia are responsible for transducing changes in angular acceleration (Fig. 14-12; see also Fig. 14-11). Within the vestibule proper are two bag-like distentions, the utricle and the saccule, collectively called the otolith organs (Figs. 14-13 and 14-14). Each otolith organ contains a mass of neuroepithelia coupled by a gelatinous matrix to calcium carbonate crystals or otoliths. Displacement of the otolithic membrane by gravitational forces deflects the hair cell stereocilia toward or away from the kinocilium, thereby exciting or inhibiting the hair cell, respectively (Figs. 14-15 and 14-16). The membranous labyrinth is filled with endolymph and surrounded by perilymph. Each membranous ampulla contains within it a flame-shaped, gelatinous structure called the cupula, which extends from the neuroepithelium of the crista ampullaris to the limit of the membranous labyrinth (see Fig. 14-4). The cupula couples the hair cell stereocilia and kinocilia to the endolymph.
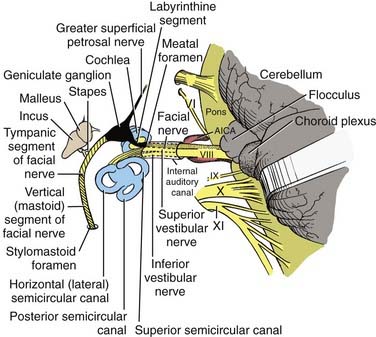
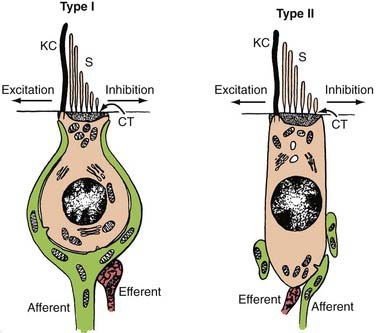
The type I and type II vestibular hair cells within the five vestibular end-organs on each side of the head are innervated by afferent dendrites that perforate the fundus of the IAC to form the superior and inferior vestibular nerves. The primary afferent neuron somata are located in Scarpa’s ganglion within the IAC (see Figs. 14-3 and 14-4). Medial to the somata, the axons converge to form a single vestibular nerve. This nerve, together with the cochlear nerve, enters the pons, where its fibers synapse within the four principal vestibular nuclei in the floor of the fourth ventricle: the superior (Bechterew) nucleus, the lateral (Deiters) nucleus, the medial (Schwalbe) nucleus, and the inferior (descending or spinal or Roller) nucleus.
Blood is supplied to the membranous labyrinth through very thin labyrinthine branches of the anterior inferior cerebellar artery (AICA) and the basilar artery. The labyrinthine artery arising from the AICA divides into two terminal branches, the cochlear and vestibular rami. The vessel arising from the basilar artery system supplies the endolymphatic sac (more information is provided by Wackym and Linthicum32).
The contents of the IAC are diagnostically valuable, and the relationships of the structures to each other are of paramount importance for proper orientation in performing the middle cranial fossa, retrolabyrinthine, or translabyrinthine approaches to the IAC.3,33–35 These features are illustrated in Figures 14-3 and 14-4.
Physiology
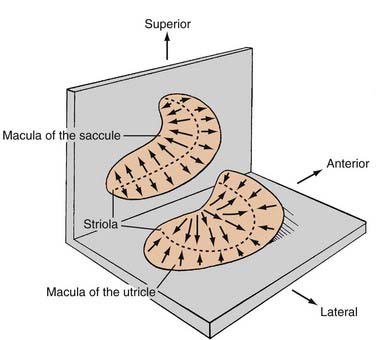
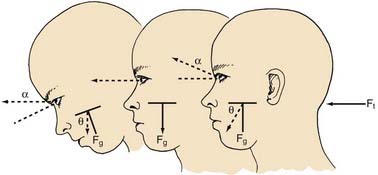
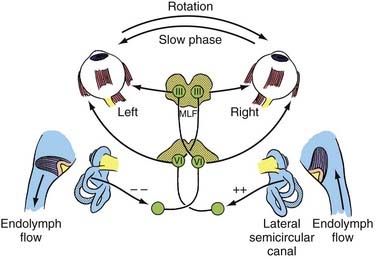
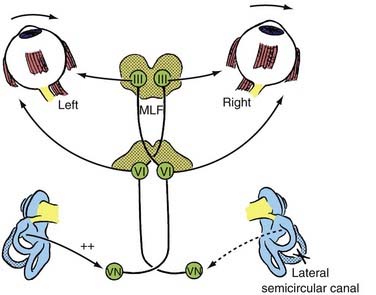
The peripheral vestibular system represents a unique neurosensory system. At rest, the type I and type II vestibular hair cells and their primary afferent neurons have a relatively constant and symmetrical resting discharge rate of approximately 80 spikes per second (Fig. 14-17). This discharge rate increases if the stereocilia are deflected toward the kinocilium of each type I or type II vestibular hair cell, and it decreases if they are deflected away from the kinocilium (see Fig. 14-16). Transduction of accelerated motion is brought about by movement of the endolymph, which is coupled to the stereocilia and kinocilia of the neuroepithelium. All the kinocilia are oriented in the same direction relative to the long axis of each crista, and flow of endolymph in one direction results in the same discharge characteristics for all the hair cells in each individual end-organ. A further level of redundancy exists in the push-pull organization between both sets of vestibular apparatus (see Fig. 14-11). For example, with rotation to the right in the horizontal plane, there is relative flow of endolymph to the left (Fig. 14-18). The resting discharge rate from the right horizontal crista ampullaris is greatly increased as the cupula is deflected toward the vestibule (i.e., ampullipetal displacement), whereas the discharge rate from the left side decreases an equal amount as the cupula of the left horizontal crista ampullaris is deflected away from the vestibule (i.e., ampullifugal displacement) (Fig. 14-18). The vestibulo-ocular reflex (VOR) arc results in compensatory eye movements by stimulation of the medial and lateral recti muscles to maintain gaze (Fig. 14-18). The relationship between the vestibular receptors and eye movement is exploited in our objective testing of vestibular function, as described later in this chapter. Normally, this bilateral system is constantly at work, receiving signals and passing them on to regulate posture and movement of the body, limbs, and eyes. Under normal circumstances, the vestibular signals produced by each side are equal and opposite in magnitude bilaterally (Fig. 14-18). The paired otolithic organs function by similar mechanisms, except that type I and type II vestibular hair cells are coupled to gravitational force through the otolithic membrane and their overlying otoconia (see Figs. 14-13 to 14-15) and the kinocilia are polarized relative to a region called the striola (see Fig. 14-14). Consequently, conscious perception of this normal vestibular activity does not occur. However, if there is an imbalance in the relative increase and decrease in afferent firing between sides, patients experience vertigo or impairment of gravitational perception (Fig. 14-19).
When there is unilateral loss of vestibular function, as illustrated by ablation of the right horizontal semicircular canal shown in Figure 14-19, a relative increase in afferent discharge firing from the left horizontal crista is transmitted to the vestibular nuclei. There will be a slow tonic deviation of the eyes to the right and a rapid compensatory movement of the eyes to the left. The vestibular stimulus produces a relatively slow movement of the eyes, and the central stimulus produces a rapid return. This is the basis for vestibular nystagmus, which may be defined as an abnormal spontaneous or an induced involuntary, sustained-rhythmic, coupled movement of the eyes consisting of a slow (vestibular) phase in one direction followed by a fast (central) return in the opposite direction. Unfortunately, the direction of the nystagmus has erroneously been established according to the fast component, which may lead to some confusion when interpreting eye movements.
Tests of Vestibular Function
Spontaneous Nystagmus
Observation of spontaneous eye movements is facilitated by the use of Frenzel lenses. These glasses have 14- to 20-diopter lenses that eliminate visual fixation and magnify the patient’s eyes, thereby resulting in easier visualization of the eyes. Small bulbs that further facilitate objective assessment of eye movements illuminate these goggle-like glasses. The pattern and character of the spontaneous nystagmus have both diagnostic and localizing value, but these issues are beyond the scope of this chapter. More information may be obtained in books by Baloh and Honrubia36 and by Leigh and Zee.37
Labyrinthine Fistula Test
The fistula test is performed by compressing the air in the external auditory canal with a pneumatic otoscope. A positive result leads to transient conjugate deviation of the eyes toward the opposite side. Negative pressure causes deviation to the side of the affected ear. In the case of a perforated tympanic membrane in a patient with vertigo, the labyrinthine fistula test should always be performed. A positive fistula test result in a patient with chronic suppurative otitis media implies the presence of otic capsule erosion down to the endosteum of the labyrinthine cavity. A positive fistula test with an intact tympanic membrane may be encountered in patients who have undergone fenestration or stapedectomy operations or in patients with Meniere’s disease, traumatic or spontaneous perilymph fistulas, or otosyphilis.38,39 For the test to be positive, the involved vestibule must be viable. A rare clinical manifestation of a labyrinthine fistula (also seen in severe endolymphatic hydrops) is the Tullio phenomenon. In such cases, the patient experiences rotatory or otolithic dysfunction with the sudden application of a loud sound to the affected ear.
Positional Tests
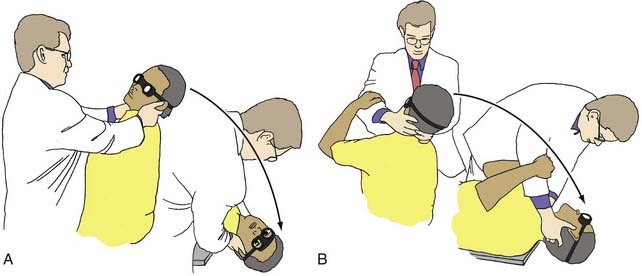
Positional tests should be done with the eyes open while wearing Frenzel lenses so that the eyes may be observed for nystagmus, or they may be performed with electro-oculography for objective measurement of eye movement. In the Hallpike maneuver (Fig. 14-20), the patient’s head is placed in the following positions. The subject is seated with the feet on an examining table so that if supine, the head would hang over the edge of the table. The head is turned toward the examiner, who holds it between both hands. The subject is then suddenly placed in the supine position so that the head hangs slightly over the edge of the table and remains rotated toward the examiner. The examiner bends down with the subject and watches the eyes for positional nystagmus (Fig. 14-20) for at least 20 seconds. After the nystagmus has ceased, the patient is suddenly repositioned to sit upright, and the eyes are again observed for nystagmus. The test is repeated with the head turned in the opposite lateral direction.
Two types of abnormal responses may be seen. Static positional nystagmus is a type of positional nystagmus that remains as long as the position is held, although it may fluctuate in frequency and amplitude. It may be in the same direction in all positions or change directions in different positions. Direction-changing or direction-fixed static positional nystagmus can be associated with peripheral or central disorders.36 In testing for benign paroxysmal positional nystagmus, after the head is placed in the head-hanging left, center, or right position, there is a latent period of 5 to 10 seconds. The characteristic nystagmus is rotary (i.e., torsional) in the ipsilateral eye (i.e., the side turned toward the examiner) and vertical in the contralateral eye (i.e., the side opposite the examiner). The neurology of these eye movements has been well characterized and is illustrated in Figure 14-21. The vertigo and nystagmus usually have an intense onset and attenuate in approximately 20 to 30 seconds. The nystagmus is always accompanied by vertigo, and its direction does not change. On repeating the test, the subsequent responses progressively fatigue, and they may not appear at all after two or three repetitions of the Hallpike maneuver. The pathophysiology is discussed later in the differential diagnosis of vertigo.
Objective Measurement of Vestibular Function
The two most widely applied methods for objectively studying vestibular function are electronystagmography (ENG) and rotatory testing.1,36 Investigational methods such as posturography, stimulation of the vestibular nerve with a galvanic current, otolith function tests, and high-frequency vestibular autorotation tests are beyond the scope of this chapter but are considered in reviews by Baloh and Honrubia.36
Electronystagmography
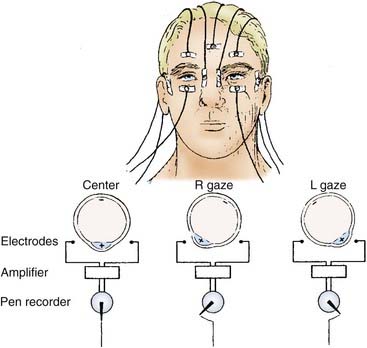
ENG is an objective method of monitoring eye movements. It has been used widely for the diagnostic evaluation of patients with vertigo, dizziness, or unsteadiness. ENG depends on the fact that there are steady direct current potentials (i.e., corneal-retinal potentials) between the cornea and pigmented retina of each eye. These potentials create an electric field in the front of the head that rotates as the eyes rotate in their orbits. Rotation of this electric field produces a change in the voltage between electrodes attached to the skin on either side of the eyes. Horizontal eye position is monitored by electrodes placed on the patient’s temples. Vertical eye position is monitored by electrodes placed above and below each of the patient’s eyes (Fig. 14-22).
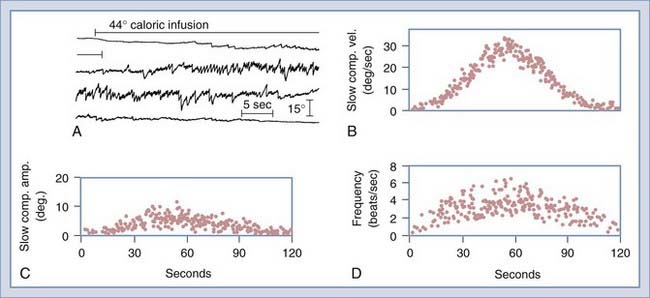
The ENG test battery generally consists of seven tests. Four of the tests primarily assess vestibular function, although they sometimes reveal nonvestibular eye movement abnormalities as well: the bithermal caloric test, designed primarily to detect unilateral lesions of the labyrinth or vestibular nerve; the gaze test, designed to detect nystagmus induced by eccentric gaze; the positional test, designed to determine whether different head positions induce or modify the nystagmus; and the Hallpike maneuver, designed to provoke a nystagmus response in patients with benign positional vertigo. The remaining three tests assess eye movement function independent of the peripheral vestibular system: the saccade test, designed to detect disorders of the saccadic eye movement control system, and the tracking test and optokinetic test, both designed to detect disorders of the pursuit eye movement control system. Saccadic and pursuit eye movements are routinely tested because abnormalities are occasionally detected in patients complaining of balance difficulty. ENG has been computerized for storage, retrieval, and analysis of eye movement data. Computed ENG allows rapid and sophisticated analysis of saccade, tracking, and caloric test data (Fig. 14-23). Videonystagmography (VNG) uses infrared illuminated goggles with digital video capture systems to record and analyze eye movements, and this technology has largely supplanted ENG recording.
Gaze Test
The gaze test detects many types of nystagmus of vestibular and nonvestibular origin. For example, upbeat nystagmus occurs most commonly as a result of medullary lesions involving the vertical vestibular pathways.40 Other types of central nystagmus seen in the gaze test are described by Leigh and Zee.37 The gaze test may also detect spontaneous nystagmus caused by a unilateral vestibular lesion, although spontaneous nystagmus is better appreciated during the positional test without visual fixation.
Saccade Test
Patients may show abnormalities in any of these three measures. Abnormally slow saccades bilaterally are characteristic of many central degenerative and metabolic diseases. Patients may also show abnormalities in saccade accuracy and make saccades that are too small or too large, indicative of a lesion of the cerebellar vermis. Abnormally long saccade latencies may also be associated with lesions of the frontoparietal cortex. A thorough review of saccade abnormalities and their localizing value can be found in the textbook authored by Leigh and Zee.37
Pursuit Tests
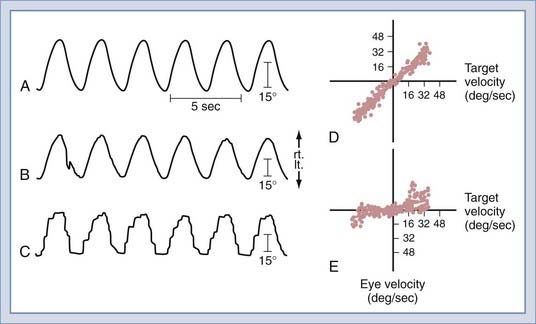
Two tests of pursuit—smooth pursuit and the optokinetic test—are commonly performed in the VNG/ENG examination. The tracking test is often performed by monitoring the patient’s horizontal eye movements as the eyes follow a computer-controlled visual target moving in the horizontal plane. The target moves back and forth, following a sinusoidal waveform at frequencies from 0.2 to 0.7 Hz. After testing, the computer deletes invalid eye movement data and interpolated saccades, differentiates the eye position signal, calculates the gain of eye velocity with respect to target velocity separately for rightward and leftward tracking at each target frequency, and plots these data (Fig. 14-24). Normal individuals are able to follow the target smoothly in both directions at all target frequencies.
Rotation Tests
Quantitative rotational testing of vestibular function is based on the VOR (i.e., receptor–end-organ–brainstem–vestibular second-order neuron–oculomotor neuron) because it is the easiest reflex to stimulate and record. The purpose of the VOR is to produce eye movements that compensate for head movements. Rotational testing usually involves positioning the patient so that the rotational axis is vertical and passes through the center of the head, with stimulation of only the horizontal semicircular canals. Horizontal eye movements are monitored with electro-oculography or infrared video eye movement recording, as in VNG/ENG testing (see Fig. 14-22). Rotational testing of the horizontal semicircular canal offers several advantages over caloric testing. Multiple, graded stimuli can be applied in a relatively short period, it is well tolerated by patients, and in contrast to caloric testing, a rotational stimulus to the horizontal semicircular canals is unrelated to the physical characteristics of the external auditory canal or middle ear. All aspects of rotational testing, including stimulus generation, response measurement, and data analysis, are computer controlled.
The most frequently performed rotational test uses a sinusoidal stimulus. The patient is seated in a chair mounted on a servocontrolled torque motor enclosed within a light-proof, sound-attenuated booth. The horizontal semicircular canals are in the plane of rotation, and horizontal eye movements are monitored by electro-oculography. The patient is tested in total darkness with the eyes open while performing mental arithmetic to maintain mental alertness. Sinusoidal oscillation around the vertical axis is performed at several different frequencies. The precise test protocol varies among laboratories, but the oscillation frequencies commonly used are 0.0125 to 1.6 Hz, with peak angular velocities of 15 to 120 degrees per second.36 The patient undergoes multiple cycles of oscillation at each frequency. Rotational stimuli are ideally suited for testing patients with bilateral peripheral lesions because both labyrinths are stimulated simultaneously and the degree of remaining function is accurately quantified (Fig. 14-25).
Visual-Vestibular Interaction
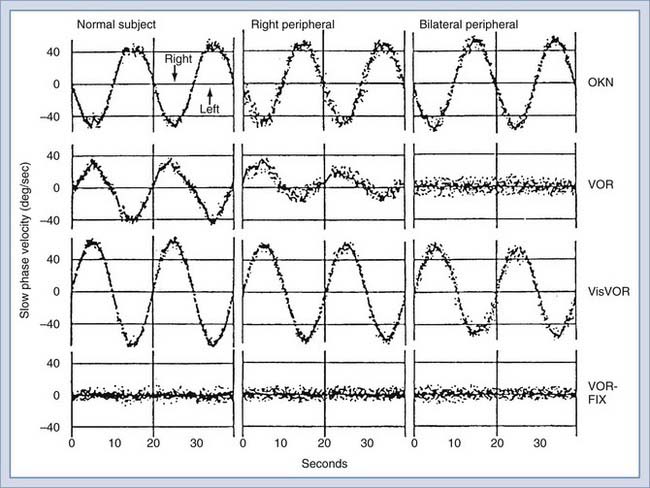
By presenting various visual stimuli during rotational stimulation, further quantitative information is obtained about peripheral and central vestibular function (Fig. 14-25). Visual-vestibular interaction is typically tested by rotating the subject while the surrounding optokinetic drum is stationary to produce a synergistic interaction of the visual and vestibular systems (i.e., visuo-vestibulo-ocular reflex [VisVOR]) or while the drum and chair are coupled so that they move together to produce an antagonistic interaction between the visual and vestibular systems (i.e., fixation suppression of the VOR [VOR-FIX]).
Patients with peripheral vestibular lesions have decreased or asymmetric (or both) VOR gain (i.e., peak slow component velocity/peak chair velocity), but visual-vestibular responses are usually normal at low stimulus frequencies and velocities (Fig. 14-25, center and right). Even with bilateral complete loss of peripheral vestibular function, the visual-motor system can provide good ocular stability. Figure 14-25 shows complete loss of the VOR with a bilateral peripheral vestibular lesion, but VOR-FIX, VisVOR, and OKN are preserved. Patients with central nervous system lesions have abnormal patterns of visual-vestibular interaction. More information on this topic is available in a review by Baloh and Honrubia.36
Differential Diagnosis of Vertigo
In attempting to establish the diagnosis of vertigo, it is essential to obtain an accurate and detailed history. In most instances this proves to be the most significant aspect of the evaluation and typically gives a good indication of the diagnosis. In obtaining the history, the patient should be encouraged to describe the symptoms (Tables 14-1 and 14-2). The word dizzy should not necessarily be accepted without a detailed description. Vertigo is most broadly defined as a hallucination of motion of any kind and in any direction. The definition of vertigo does not include light-headedness, nausea, a feeling of faintness, or a feeling that the subject may fall.
TABLE 14-2 Mechanisms of Various Types of Dizziness
Differentiating between Peripheral and Central Lesions
Common causes of dizziness are outlined in Table 14-1. The mechanisms of these various types of dizziness are described in Table 14-2. Tables 14-3 and 14-4 list the common peripheral and central causes of vertigo.
TABLE 14-4 Common Central Causes of Vertigo
Vertigo of Peripheral Origin
The following entities, which may be considered in the differential diagnosis of vertigo of peripheral origin, are discussed in the order of their frequency of occurrence (see Table 14-3).
Benign Paroxysmal Positional Vertigo
Benign paroxysmal positional vertigo (i.e., benign positional vertigo) is the single most common cause of vertigo of peripheral origin. This condition can result from several different insults to the inner ear (e.g., viral infection, head injury). The vertigo is brought on by turning the head into a particular position, but the symptoms last only several seconds. In most cases this is the only symptom, and it is usually inconsistent. An observant patient may volunteer that repeated movements of the head into the critical position bring on vertigo of progressively lesser severity or that this may lead to a symptom-free period lasting several hours. In many instances, careful questioning may elicit a history of a recent mild head injury. The results of routine clinical and audiometric examination are generally normal. The only vestibular test result that may be abnormal and that is characteristic of this entity is the positional test. A typical positive response on suddenly placing the patient’s head in the critical position via the Hallpike maneuver (see Fig. 14-20) is a latent period of 5 to 10 seconds, followed by gross vertigo and nystagmus that attenuate rapidly and cease after about 20 seconds. The nystagmus persists in the same direction when the test is repeated or if the head position is changed. The nystagmus is highly characteristic with rotary (torsional) movement of the ipsilateral eye (side turned toward the examiner) and vertical movement of the contralateral eye (side opposite the examiner) (see Fig. 14-21). Repeating the test may produce a similar response but of lesser severity and shorter duration until, after the second or third time, it may not be reproducible; however, it may be induced again after 1 or 2 hours. This tendency for the response to fatigue is characteristic of the disease. The condition is probably caused by mild injury to the otolith organ of the utricle in the ear to which the side of the head is turned in the critical position. The symptoms generally subside spontaneously after about a year without treatment, within 2 months with Cawthorne exercises, or immediately after canalith-repositioning maneuvers.41,42
Meniere’s Disease
Meniere’s disease (i.e., Meniere’s syndrome or endolymphatic hydrops) is an idiopathic condition of the membranous labyrinth that is characterized by spontaneous bouts of prolonged vertigo, fluctuating hearing loss, and tinnitus. Histologically, there is excessive endolymph within the scala media.43–45 The disease affects primarily adults between the ages of 30 and 60 years and is somewhat more common in women. The condition is unilateral in approximately 80% of patients, and when it is bilateral, the second ear generally becomes affected within 3 years of the first episode. Bilateral involvement has been reported in 16% to 50% of patients.46 By far the most common pattern is involvement of both the vestibular and the cochlear labyrinths; however, in rare instances, the disease may produce vestibular symptoms alone or episodes of fluctuating hearing loss alone. In these uncommon manifestations, the other portion of the labyrinth eventually becomes symptomatic as the disease progresses. The vertiginous episodes last from 20 minutes to several hours. A rare subset of patients with Meniere’s disease experience vestibular otolith dysfunction, called an otolithic crisis of Tumarkin. In most of these patients a sudden disorientation relative to the ground develops, but some patients have the illusion of being violently pushed to the ground.
The pattern of occurrence of the spells varies. Some patients exhibit long remissions between attacks, but others have a clustering of attacks punctuated by long symptom-free periods. A classic spell is usually preceded by an aura consisting of pressure on the affected side of the head, tinnitus, and hearing loss. The spell then comes on suddenly, with severe whirling vertigo accompanied by diaphoresis and nausea. In the early stages of the disease, the nausea may progress to vomiting. Head motion usually accentuates the symptoms. The hearing loss can also be characterized by a distortion of sound and by poor speech perception later in the course of the disease (see Fig. 14-8). This distortion may also be manifested between spells, especially when the volume of sound is raised. Loud sounds may be intolerably painful (i.e., recruitment or hyperacusis). In the intervals between spells, the hearing loss may fluctuate considerably, especially in the early stages of the disease before much destruction of the cochlear labyrinth has taken place. Most patients are controlled medically with a low-sodium diet (1000 to 1500 mg/day), with or without a diuretic. Less than 10% of patients fail medical therapy and are candidates for a vestibular denervation procedure (i.e., labyrinthectomy or vestibular neurectomy; see Chapter 92).42 The cause of Meniere’s disease remains unknown.43–47
The results of a general physical examination are usually normal, and the diagnosis has to depend on an accurate history and on the results of audiometric and vestibular tests. Pure-tone audiometry typically shows sensorineural loss in the affected ear, as illustrated by a flat or rising curve (see Fig. 14-8). Fluctuation in hearing level is elicited on repeated testing at various time intervals. Speech audiometry invariably demonstrates a drop in discrimination out of proportion to the level of the sensorineural loss. Audiograms performed before and after the ingestion of 1.2 mL of glycerol per kilogram of body weight often show improvement in the speech reception threshold and word discrimination after glycerol administration in patients with Meniere’s disease; however, this test is rarely performed because of the discomfort that many patients experience. Tuning fork and whisper tests can corroborate the sensorineural nature of the loss and demonstrate the discrimination problem and recruitment.
Secondary Endolymphatic Hydrops
Late congenital syphilis may closely mimic Meniere’s disease. Unlike the 16% to 50% incidence of bilaterality in Meniere’s disease, syphilis generally affects both ears. Syphilitic hearing loss also has an earlier age of onset and may produce a positive fistula test result in the presence of an intact tympanic membrane. Large doses of systemic steroids can evoke a good transient improvement in hearing.38,48
Vestibular Neuronitis
This condition is characterized by a sudden onset of sustained and severe vertigo, made worse with head movements. Vestibular neuronitis usually affects subjects in early to middle age and is generally unilateral. It has various degrees of severity. Thought to be caused by a viral infection of Scarpa’s ganglion, the condition is often preceded or accompanied by an obvious viral infection and may affect several individuals in a community at the same time—hence the term endemic vertigo or endemic labyrinthitis. The most severe period of the attack usually lasts from 1 to 3 weeks and is characterized by an improvement in the symptoms from day to day, although they may be made worse by sudden movements of the head (see Fig. 14-19). There is no accompanying deafness or tinnitus, and the central nervous system is normal. Spontaneous nystagmus has its fast component toward the opposite side (i.e., destructive nystagmus), and the caloric response in the affected ear is reduced or absent.48,49
Vestibular neuronitis is frequently called acute viral labyrinthitis and labyrinthine apoplexy, particularly by emergency department physicians. Viral and bacterial causes of labyrinthitis are rare. In addition to acute vestibular dysfunction, these patients experience sudden, profound hearing loss in the affected ear. Although the vertigo subsides after 3 weeks, in certain instances it may still be provoked by sudden movements of the head. As a general rule, compensation after 3 weeks is normally the case, with complete recovery from any symptoms, especially in younger patients. During the active period of disease, patients should be treated symptomatically with pharmacotherapy.48,49 Although some patients have complete resolution, others may be left with permanent hypofunction detectable by the caloric test in the affected ear, but this deficit is invariably asymptomatic after central vestibular compensation.
Latent virus (e.g., herpes simplex virus) is thought to remain dormant within the vestibular primary afferent neurons of the ganglion of Scarpa. Although this evidence has been circumstantial in nature, demonstration of varicella-zoster viral DNA in archival temporal bone sections of the spiral and geniculate ganglia of patients with Ramsay Hunt syndrome (i.e., herpes zoster oticus) suggests that similar studies of Scarpa’s ganglia neurons in patients with vestibular neuronitis can be completed.50,51
Posttraumatic Vertigo
Trauma to the temporal bone, common even with minor head injury,52 can result in peripheral vestibular dysfunction, such as temporal bone fracture, labyrinthine concussion, posttraumatic positional vertigo, and perilymph fistulas. Central vestibular dysfunction, such as dizziness secondary to brainstem trauma or postconcussion syndrome, is also common.
Drug-Induced Ototoxicity
Peripheral vestibular dysfunction after toxic injury to the inner ear is produced by a group of ototoxic drugs that consist primarily of various antibiotic, chemotherapeutic, and diuretic agents. Damage from heavy metals or from quinine is now seldom seen. Patients with ototoxic injury rarely experience vertigo because both labyrinths are involved equally; however, disequilibrium and ataxia are common, especially if there are impairments of proprioception or vision.36,42
Vertigo of Central Origin
When confronted by a patient suffering from vertigo, it is important to exclude the possibility of a central nervous system disorder despite the fact that many vertigo cases are probably caused by a lesion of the vestibular end-organs or the vestibular nerve. Although only a few of the peripheral causes of vertigo may be dangerous to health, most of the factors responsible for central vertigo may be associated with serious disease and could be life-threatening. In addition to the common central causes of vertigo (see Table 14-4), there are many other conditions, such as systemic metabolic disorders,36 craniovertebral junction disorders,53 and focal seizure disorders,36 that may cause dizziness or vertigo. Even though vertigo may be a prominent symptom in these patients, there are often other findings that indicate a lesion within the central nervous system. The complaint of vertigo may well be overlooked. Spontaneous nystagmus as a result of involvement of the central vestibular pathways in the brainstem may persist indefinitely, even though the vertigo may not be in proportion to the nystagmus or the vertigo may not be a symptom any longer.
Multiple Sclerosis
Vertigo is the initial symptom in approximately 5% of patients with multiple sclerosis and is reported sometime during the course of the disease in up to 50% of patients.54,55 The duration of the vertigo is usually several hours to several days. Hearing loss occurs in approximately 10% of patients, and the loss can be acute (hours to days), subacute (over a period of months), or slowly progressive. Partial or complete remission after the onset of hearing loss is common. Plaque involving the vestibular and cochlear nerve root entry zones is commonly seen on magnetic resonance imaging and can explain the frequent findings of unilateral caloric hypoexcitability and hearing loss. Diplopia, weakness, numbness, and ataxia are also common early in the disease process.
Migraine
Migraine has long been considered a vascular disorder, with vasodilation being responsible for the headache and vasoconstriction being responsible for the neurological symptoms. Basilar artery migraine produces symptoms related to areas of the central nervous system supplied by the posterior circulation. Approximately 30% to 40% of patients with classic migraine experience true vertigo. Other patients can have vertigo without concomitant headache, referred to as a migraine equivalent.48,56–58 Interestingly, only 10% to 30% of patients with migraine-associated vertigo will experience a concomitant headache. This common disorder can be managed medically with antiepileptic medications (e.g., topiramate [Topamax], 25 to 50 mg at bedtime), tricyclic antidepressants (e.g., nortriptyline [Pamelor], 10 to 150 mg at bedtime), calcium channel blockers (e.g., verapamil [Verelan PM], 120 mg at bedtime), or beta blockers (e.g., propranolol, 0.5 mg/kg twice daily in a divided dose).48
Vertebrobasilar Insufficiency
Approximately a third of transient ischemic attacks (TIAs) involve the territories of the vertebrobasilar system, and they are a common cause of vertigo in patients older than 50 years. These patients often manifest short-lived symptoms such as vertigo, diplopia, dysarthria, bilateral limb weakness, gait ataxia, variable and often bilateral sensory disturbance, and memory loss.59 Common precipitating maneuvers include looking up and reaching for an item on a shelf, looking over one shoulder while backing up a vehicle, and therapeutic neck manipulations. Antiplatelet therapy has been demonstrated to be effective in reducing the incidence of stroke after TIA. This effect has most clearly been established for aspirin, which can reduce the risk for stroke after TIA by half. Although most previous studies have used doses of 1200 mg daily, it is likely that 325 mg/day is equally as effective.60,61 Ticlopidine (Ticlid) is another platelet aggregate inhibitor that has been shown to have favorable effects on the risk for stroke after TIA, particularly in patients with vertebrobasilar insufficiency. This agent may be more effective than aspirin in that it reduces the risk for stroke by 48% relative to aspirin during the first year after TIA. However, ticlopidine is associated with a risk for neutropenia, which may be life-threatening, and it should be reserved for patients who are intolerant of aspirin therapy. All patients receiving ticlopidine therapy should have a complete blood cell count and white blood cell differential determination made at least once every 2 weeks through the first 3 months of therapy. The neutropenia reverses within 1 to 3 weeks after discontinuing treatment.
Tumors of the Posterior Cranial Fossa
Tumors of the posterior cranial fossa can give rise to marked and persistent disequilibrium, ataxia, or vertigo. However, acoustic neuromas (i.e., vestibular schwannomas), which are by far the most common neoplasms of the posterior fossa, result in vertigo in less than 20% of patients, but up to 50% experience imbalance (see Chapter 133). Other cerebellopontine angle tumors are also seen, including meningiomas, epidermoid cysts, cholesterol granulomas, and neurofibromas. Cerebellar gliomas in adults may be relatively silent until brainstem compression or obstruction to flow of cerebrospinal fluid occurs. In children, the possibility of a cerebellar glioma should be considered, and in later life, secondary neoplastic deposits, especially from bronchial or breast carcinoma, should be taken into account.
Indications for Vestibular Neurectomy
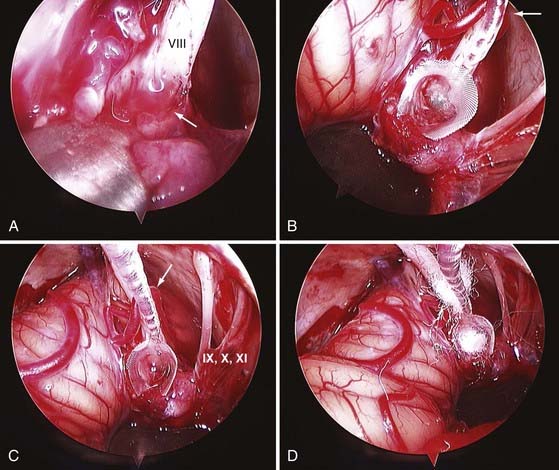
The rationale for unilateral deafferentation of the vestibular labyrinth is that the central nervous system is better able to compensate for complete loss of vestibular function than for fluctuating partial loss. The single most common peripheral vestibular disorder requiring vestibular neurectomy is Meniere’s disease, although most of these patients are successfully managed with medical therapy.42,48 Rarely, recurrent vestibular neuronitis or traumatic labyrinthitis requires unilateral ablation of vestibular function to achieve compensation. Vestibular neurectomy has the advantage of preserving hearing in patients with useful cochlear function. A survey of the American Otological Society and the American Neurotology Society in 1990 indicated that almost 3000 vestibular neurectomies had been performed in the United States since the introduction of the operating microscope.62 The various surgical approaches are discussed elsewhere42,63 and in Chapter 92. The technique of endoscopically assisted vestibular neurectomy has been described.64,65
Indications for Cochlear Implantation
Candidacy criteria for adults include severe or profound bilateral sensorineural hearing loss, limited benefit from hearing aids, no medical contraindications, appropriate expectations, and family support.66 Adults may have been deafened prelingually (before the age of 5 years) or postlingually (after the age of 6 years). Prelingually deafened adults perform at a level much lower than postlingually deafened adults who receive cochlear implants. A cochlear implant should be considered for hearing-impaired adults who do not perform as well with hearing aids as the average cochlear implant user does. Candidacy criteria for children include age 12 months and older, severe to profound bilateral sensorineural hearing loss, limited benefit from hearing aids, no medical contraindications,67 family support and appropriate expectations, and an educational program that emphasizes auditory skill development. A cochlear implant should be considered for children who do not have aided hearing levels in the speech range or who have reached a plateau in the development of their auditory skills. All children with severe to profound hearing loss should be monitored closely in their ability to detect and understand conversational speech when presented at average loudness levels. Currently, individuals receive bilateral cochlear implants if each ear has hearing poor enough to warrant implantation.
For individuals who do not have an intact cochlear nerve and have bilateral deafness (or the expectation of bilateral deafness developing), auditory brainstem implants (ABIs) represent an option for hearing rehabilitation. These U.S. Food and Drug Administration–approved auditory prostheses use the same speech processors, but they have electrode arrays that are designed to be placed directly on the brainstem cochlear nuclei (Fig. 14-26).68 Current selection criteria include patients with neurofibromatosis type 2 (bilateral acoustic neuromas) and those with only one hearing ear containing a sporadic acoustic neuroma; however, patients with temporal bone fractures resulting in destruction of the cochlear nerve or children with cochlear nerve aplasia have received ABIs.
Baloh RW, Honrubia V. Clinical Neurophysiology of the Vestibular System, 3rd ed. New York: Oxford University Press; 2001.
Baloh RW, Lopez I, Ishiyama A, et al. Vestibular neuritis: clinical-pathologic correlation. Otolaryngol Head Neck Surg. 1996;114:586-592.
Borg E. On the neuronal organization of the acoustic middle ear reflex: a physiological and anatomical study. Brain Res. 1973;49:101-123.
Buss E, Pillsbury HC, Buchman CA, et al. Multi-center U.S. bilateral MED-EL cochlear implantation study: speech perception over the first year of use. Ear Hearing. 2008;29:20-32.
Doyle KJ, Sininger Y, Starr A. Auditory neuropathy in childhood. Laryngoscope. 1998;108:1374-1377.
Friedland DR, Wackym PA. A critical appraisal of spontaneous perilymphatic fistulas of the inner ear. Am J Otol. 1999;20:261-276.
Friedland DR, Wackym PA. Evaluation of surgical approaches to endoscopic auditory brainstem implantation. Laryngoscope. 1999;109:175-180.
Kraus N, Micco AG, Koch DB, et al. The mismatch negativity cortical evoked potential elicited by speech in cochlear-implant users. Hear Res. 1993;65:118-124.
Leigh RJ, Zee DS. The Neurology of Eye Movements, 3rd ed. New York: Oxford University Press; 1999.
Olsen W, Noffsinger PD, Kurdziel S. Acoustic reflex and reflex decay occurrence in patients with cochlear and VIII nerve lesions. Arch Otolaryngol. 1975;101:622-625.
Parker W. Migraine and the vestibular system in adults. Am J Otol. 1991;12:25-34.
Parnes LS, Price-Jones RG. Particle repositioning maneuver for benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1993;102:325-331.
Runge-Samuelson CL, Drake SE, Wackym PA. Quantitative analysis of electrically evoked auditory brainstem responses in implanted children with auditory neuropathy/dyssynchrony. Otol Neurotol. 2008;29:174-178.
Shepard NT, Telian SA, Smith-Wheelock M. Balance disorders in multiple sclerosis: assessment and rehabilitation. Semin Hear. 1990;11:292-305.
Snow JBJr, Wackym PA, editors. Ballenger’s Otorhinolaryngology Head and Neck Surgery. 17th ed. Shelton, CT: People’s Medical Publishing House. 2009:1-1209.
Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain. 1996;119:741-753.
Wackym PA. Cochlear and brainstem implantation. guest ed. Oper Tech Otolaryngol Head Neck Surg. 2005;16;:73-163.
Wackym PA. Molecular temporal bone pathology. Laryngoscope. 1997;107:1056-1075.
Wackym PA, King WA, Baker F, et al. Endoscope-assisted vestibular neurectomy. Laryngoscope. 1998;108:1787-1793.
. Minimally Invasive Surgery of the Head, Neck, and Cranial Base. DH Rice, SD, Wackym Schaefer. Philadelphia: Lippincott Williams & Wilkins. 2002:1-559.
1 Snow JBJr, Wackym PA, editors. Ballenger’s Otorhinolaryngology Head and Neck Surgery. 17th ed. Shelton, CT: People’s Medical Publishing House. 2009:1-1209.
2 Wackym PA, Rhee JS. Facial paralysis. In: Snow JBJr, Wackym PA, editors. Ballenger’s Otorhinolaryngology Head and Neck Surgery. 17th ed. Shelton, CT: People’s Medical Publishing House; 2009:395-412.
3 Gantz BJ, Wackym PA. Facial nerve abnormalities. In: Bumstead R, Smith J, editors. Pediatric Facial Plastic and Reconstructive Surgery. New York: Raven Press; 1993:337-347.
4 May M, Schaitkin BM, editors. The Facial Nerve. 2nd ed. New York: Thieme Medical. 2000:1-877.
5 American National Standards Institute. Specifications for Audiometers, ANSI S3.6. New York: American National Standards Institute; 1989.
6 Tonndorf J. Bone conduction: studies in experimental animals. Acta Otolaryngol Suppl. 1966;213:1-132.
7 Dirks D. Bone conduction testing. In: Katz J, editor. Handbook of Clinical Audiology. 3rd ed. Baltimore: Williams & Wilkins; 1985:202-223.
8 American National Standards Institute. American National Standard Psychoacoustical Terminology, ANSI S3.20. New York: American National Standards Institute; 1973.
9 Fletcher H. Auditory patterns. J Acoust Soc Am. 1940;12:47-65.
10 Studebaker G. Clinical masking. In: Rintelmann W, editor. Hearing Assessment. Baltimore: University Park Press; 1979:51-100.
11 Olsen W, Matkin N. Speech audiometry. In: Rintelmann W, editor. Hearing Assessment. Baltimore: University Park Press; 1979:133-174.
12 Jerger J, Hayes D. Diagnostic applications of impedance audiometry: middle ear disorder, sensorineural disorder. In: Jerger J, Northern J, editors. Clinical Impedance Audiometry. Acton, MA: American Electromedics; 1980:139-152.
13 Borg E. On the neuronal organization of the acoustic middle ear reflex: a physiological and anatomical study. Brain Res. 1973;49:101-123.
14 Wilson R, Margolis R. Acoustic reflex measurement. In: Rintelmann W, editor. Hearing Assessment. 2nd ed. Austin, TX: Pro-Ed; 1990:247-319.
15 Olsen W, Noffsinger PD, Kurdziel S. Acoustic reflex and reflex decay occurrence in patients with cochlear and VIII nerve lesions. Arch Otolaryngol. 1975;101:622-625.
16 Sheehy J, Inzer B. Acoustic reflex test in neuro-otologic diagnosis: a review of 24 cases of acoustic tumors. Arch Otolaryngol. 1976;102:647-653.
17 Selters WA, Brackmann DE. Acoustic tumor detection with brainstem electric response audiometry. Arch Otolaryngol. 1977;103:181-187.
18 Jewett D, Williston J. Auditory-evoked far fields averaged from the scalp of humans. Brain. 1971;94:681-696.
19 Starr A, Achor LJ. Auditory brainstem responses in neurological disease. Arch Neurol. 1975;32:761-768.
20 Eggermont J, Don M, Brackmann D. Electrocochleography and auditory brainstem response in humans. Ann Otol Rhinol Laryngol Suppl. 1980;89:1-19.
21 Musiek F. ABR in eighth-nerve and brainstem disorders. Am J Otol. 1982;3:243-248.
22 Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain. 1996;119:741-753.
23 Doyle KJ, Sininger Y, Starr A. Auditory neuropathy in childhood. Laryngoscope. 1998;108:1374-1377.
24 Runge-Samuelson CL, Drake SE, Wackym PA. Quantitative analysis of electrically evoked auditory brainstem responses in implanted children with auditory neuropathy/dyssynchrony. Otol Neurotol. 2008;29:174-178.
25 Wackym PA, Firszt JB, Gaggl W, et al. Electrophysiological effects of placing cochlear implant electrodes in a perimodiolar position in young children. Laryngoscope. 2004;114:71-76.
26 Kraus N, Micco AG, Koch DB, et al. The mismatch negativity cortical evoked potential elicited by speech in cochlear-implant users. Hear Res. 1993;65:118-124.
27 Abbas PJ. Electrophysiology. In: Tyler RS, editor. Cochlear Implants: Audiological Foundations. San Diego, CA: Singular Publishing; 1993:317-355.
28 Wackym PA. Cochlear and brainstem implantation. guest ed. Oper Tech Otolaryngol Head Neck Surg. 2005;16;:73-163.
29 Kemp D. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386-1391.
30 Kemp D, Chum R. Observations on the generator mechanism of stimulus frequency acoustic emission-two tone suppression. In: van den Brink G, Bilsen F, editors. Psychophysical, Physiological and Behavioral Studies in Hearing. Delft, Netherlands: Delft University; 1983:34-42.
31 Robinette M. Clinical observations with transient evoked otoacoustic emissions with adults. Semin Hear. 1992;13:23-36.
32 Wackym PA, Linthicum FHJr. Diabetes mellitus and hearing loss: clinical and histopathological relationships. Am J Otol. 1986;7:176-182.
33 Wackym PA, Andrews JC. Middle cranial fossa approach. In: Samii M, Cheatham M, Becker DP, editors. Atlas of Cranial Base Surgery. Philadelphia: WB Saunders; 1995:26-31.
34 Andrews JC, Wackym PA, Canalis RF. Translabyrinthine approach to the cerebellopontine angle and internal auditory canal. In: Samii M, Cheatham M, Becker DP, editors. Atlas of Cranial Base Surgery. Philadelphia: WB Saunders; 1995:18-23.
35 Wackym PA, Rice DH, Schaefer SD, editors. Minimally Invasive Surgery of the Head, Neck, and Cranial Base. Philadelphia: Lippincott Williams & Wilkins. 2002:1-559.
36 Baloh RW, Honrubia V. Clinical Neurophysiology of the Vestibular System, 3rd ed. New York: Oxford University Press; 2001.
37 Leigh RJ, Zee DS. The Neurology of Eye Movements, 3rd ed. New York: Oxford University Press; 1999.
38 Smith ME, Canalis RF. Otologic manifestations of AIDS: the otosyphilis connection. Laryngoscope. 1989;99:365-372.
39 Friedland DR, Wackym PA. A critical appraisal of spontaneous perilymphatic fistulas of the inner ear. Am J Otol. 1999;20:261-276.
40 Fisher A, Gresty M, Chambers BR, et al. Primary position upbeating nystagmus: a variety of central positional nystagmus. Brain. 1983;106:949-964.
41 Parnes LS, Price-Jones RG. Particle repositioning maneuver for benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1993;102:325-331.
42 Wackym PA. Therapy: surgical alternatives. In: Goebel JA, editor. Practical Management of the Dizzy Patient. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:365-377.
43 Wackym PA, Linthicum FH, Ward PH, et al. Re-evaluation of the role of the human endolymphatic sac in Ménière’s disease. Otolaryngol Head Neck Surg. 1990;102:732-744.
44 Wackym PA, Schuknecht HF, Ward PH, et al. Blinded, controlled study of endolymphatic duct and sac fibrosis in Ménière’s disease. In: Filipo R, Barbara M, editors. Ménière’s Disease: Perspectives in the 90’s. Amsterdam: Kugler Publications; 1994:209-215.
45 Wackym PA, Sando I. Molecular and cellular pathology of Ménière’s disease. Otolaryngol Clin North Am. 1997;30:947-960.
46 Wackym PA. Ménière’s disease. Am Speech Hear Assoc Rep. 1992;21:19-23.
47 Wackym PA, Storper IS, Fu Y-S, et al. Differential diagnosis of virus-like particles in the human inner ear. Am J Otol. 1992;13:431-437.
48 Wackym PA, Schumacher-Monfre TS. Medical management of vestibular disorders. In: Bailey BJ, Johnson JT, editors. Head and Neck Surgery-Otolaryngology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:2317-2332.
49 Baloh RW, Lopez I, Ishiyama A, et al. Vestibular neuritis: clinical-pathologic correlation. Otolaryngol Head Neck Surg. 1996;114:586-592.
50 Wackym PA, Popper P, Kerner MM, et al. Varicella-zoster DNA in temporal bones of patients with Ramsay Hunt syndrome. Lancet. 1993;342:1555.
51 Wackym PA. Molecular temporal bone pathology. Laryngoscope. 1997;107:1056-1075.
52 Feuerman T, Wackym PA, Gade GF, et al. Value of skull radiography, head computed tomographic scanning, and admission for observation in cases of minor head injury. Neurosurgery. 1988;22:449-453.
53 Wackym PA, Friedland DR. Abnormalities of the craniovertebral junction. In: Jackler RK, Brackmann DE, editors. Neurotology. 2nd ed. St. Louis: CV Mosby; 2005:1136-1163.
54 Ward PH, Cannon D, Lindsay JR. The vestibular system in multiple sclerosis: a clinical-histopathological study. Laryngoscope. 1965;75:1031-1047.
55 Shepard NT, Telian SA, Smith-Wheelock M. Balance disorders in multiple sclerosis: assessment and rehabilitation. Semin Hear. 1990;11:292-305.
56 Harker LA, Rassekh CH. Migraine equivalent as a cause of episodic vertigo. Laryngoscope. 1988;98:160-164.
57 Olsson JE. Neurotologic findings in basilar migraine. Laryngoscope. 1991;101(suppl 52):1-41.
58 Parker W. Migraine and the vestibular system in adults. Am J Otol. 1991;12:25-34.
59 Oas JG, Baloh RW. Vertigo and the anterior inferior cerebellar artery syndrome. Neurology. 1992;42:2274-2279.
60 Wade J. Transient ischemic attacks. Practitioner. 1989;233:1089-1092.
61 Sivenius J, Riekkinen PJ, Smets P, et al. The European Stroke Prevention Study (ESPS): results by arterial distribution. Ann Neurol. 1991;29:596-600.
62 Silverstein H, Wanamaker H, Flanzer J, et al. Vestibular neurectomy in the United States—1990. Am J Otol. 1992;13:23-30.
63 Wackym PA, Kurpad SN, King WA. Functional surgery of the cerebellopontine angle. In: Bambakidis NC, Megerian CA, editors. Surgery of the Cerebellopontine Angle. Shelton, CT: People’s Medical Publishing House; 2009:83-105.
64 Wackym PA, King WA, Baker F, et al. Endoscope-assisted vestibular neurectomy. Laryngoscope. 1998;108:1787-1793.
65 Wackym PA, King WA, Meyer GA, et al. Endoscope-assisted surgery of the trigeminal, facial, cochlear or vestibular nerve. In: Wackym PA, Rice DH, Schaefer SD, editors. Minimally Invasive Surgery of the Head, Neck, and Cranial Base. Philadelphia: Lippincott Williams & Wilkins; 2002:101-116.
66 Buss E, Pillsbury HC, Buchman CA, et al. Multi-center U.S. bilateral MED-EL cochlear implantation study: speech perception over the first year of use. Ear Hearing. 2008;29:20-32.
67 Friedland DR, Rhee JS, Zito-Finn M, et al. Cochlear implantation for auditory rehabilitation in Camurati-Engelmann disease (hereditary diaphyseal dysplasia). Ann Otol Rhinol Laryngol. 2000;109:160-162.
68 Friedland DR, Wackym PA. Evaluation of surgical approaches to endoscopic auditory brainstem implantation. Laryngoscope. 1999;109:175-180.