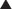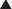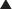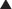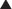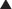Chapter 21 Neurology and the Neuromuscular System
Acetaminophen
MOA (Mechanism of Action) (Figure 21-1)
 Acetaminophen is neither a narcotic nor a nonsteroidal antiinflammatory drug (NSAID). It is in a drug class of its own called aniline analgesics.
Acetaminophen is neither a narcotic nor a nonsteroidal antiinflammatory drug (NSAID). It is in a drug class of its own called aniline analgesics. Acetaminophen has analgesic and antipyretic effects similar to those of aspirin and NSAIDs and most likely exerts its effects through the inhibition of cyclooxygenase (COX).
Acetaminophen has analgesic and antipyretic effects similar to those of aspirin and NSAIDs and most likely exerts its effects through the inhibition of cyclooxygenase (COX). COX catalyzes the formation of prostaglandins (PGs) and other mediators that are important in the processing and signaling of pain and control of the thermoregulatory center in the brain.
COX catalyzes the formation of prostaglandins (PGs) and other mediators that are important in the processing and signaling of pain and control of the thermoregulatory center in the brain. In contrast to NSAIDs and aspirin, acetaminophen is not an antiplatelet agent, nor does it possess antiinflammatory properties; therefore there are differences in the mechanism of action compared with aspirin or NSAIDs:
In contrast to NSAIDs and aspirin, acetaminophen is not an antiplatelet agent, nor does it possess antiinflammatory properties; therefore there are differences in the mechanism of action compared with aspirin or NSAIDs:
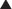 Tissue selectivity: Acetaminophen demonstrates variable COX inhibition in different tissues. Of primary importance, it inhibits prostaglandin E2 (PGE2) production in the central nervous system (CNS), which is probably the primary mediator of its analgesic and antipyretic properties.
Tissue selectivity: Acetaminophen demonstrates variable COX inhibition in different tissues. Of primary importance, it inhibits prostaglandin E2 (PGE2) production in the central nervous system (CNS), which is probably the primary mediator of its analgesic and antipyretic properties. COX site binding: COX possesses two different catalytic sites: a COX site and a peroxidase site. Acetylsalicylic acid (ASA) and NSAIDs inhibit the COX site, whereas acetaminophen inhibits the peroxidase site.
COX site binding: COX possesses two different catalytic sites: a COX site and a peroxidase site. Acetylsalicylic acid (ASA) and NSAIDs inhibit the COX site, whereas acetaminophen inhibits the peroxidase site. Inhibition by hydroperoxide: Hydroperoxide is produced by macrophages, which are important inflammatory cells; the hydroperoxide at the sites of inflammation displaces acetaminophen from the peroxidase site and thus dramatically limits the potential antiinflammatory action of acetaminophen. Furthermore, platelet 12-lipoxygenase produces hydroperoxide in platelets, which is likely the explanation for lack of antiplatelet effect by acetaminophen.
Inhibition by hydroperoxide: Hydroperoxide is produced by macrophages, which are important inflammatory cells; the hydroperoxide at the sites of inflammation displaces acetaminophen from the peroxidase site and thus dramatically limits the potential antiinflammatory action of acetaminophen. Furthermore, platelet 12-lipoxygenase produces hydroperoxide in platelets, which is likely the explanation for lack of antiplatelet effect by acetaminophen.Important Notes
 Therapeutic doses of acetaminophen have no effect on the cardiovascular and respiratory systems or platelet function and do not produce gastric irritation, erosion, or bleeding.
Therapeutic doses of acetaminophen have no effect on the cardiovascular and respiratory systems or platelet function and do not produce gastric irritation, erosion, or bleeding.Overdose
 Acetaminophen exposure is the most commonly reported drug exposure reported to U.S. poison control centers.
Acetaminophen exposure is the most commonly reported drug exposure reported to U.S. poison control centers. The therapeutic index is about 10: the toxic dose is 10 times greater than the therapeutic dose (15 mg/kg versus 150 mg/kg). For example, if you weigh 100 kg, then an upper limit of a therapeutic dose would theoretically be 1500 mg and a toxic dose would be 15,000 mg. To put this in perspective, tablets and suppositories are usually in the range of 325 to 650 mg each. A U.S. Food and Drug Administration (FDA) panel of experts in 2009 recommended that the maximum single dose of acetaminophen in adults be lowered from 1000 to 650 mg.
The therapeutic index is about 10: the toxic dose is 10 times greater than the therapeutic dose (15 mg/kg versus 150 mg/kg). For example, if you weigh 100 kg, then an upper limit of a therapeutic dose would theoretically be 1500 mg and a toxic dose would be 15,000 mg. To put this in perspective, tablets and suppositories are usually in the range of 325 to 650 mg each. A U.S. Food and Drug Administration (FDA) panel of experts in 2009 recommended that the maximum single dose of acetaminophen in adults be lowered from 1000 to 650 mg. Inadvertent co-ingestion of acetaminophen with other medications that also contain acetaminophen (such as cold remedies) is a cause of accidental overdose. Overdose can also occur with repeated subtoxic doses that, when combined over time, become toxic.
Inadvertent co-ingestion of acetaminophen with other medications that also contain acetaminophen (such as cold remedies) is a cause of accidental overdose. Overdose can also occur with repeated subtoxic doses that, when combined over time, become toxic. Because of hepatic metabolism, the liver is the predominant organ that is initially injured, and fatal hepatic necrosis can occur in severe and untreated overdoses. In less severe conditions, significant liver injury and dysfunction can occur, resulting in an increase in transaminases (aspartate transaminase [AST], alanine transaminase [ALT]) and abnormalities in coagulation because of abnormal production of hepatic coagulation proteins.
Because of hepatic metabolism, the liver is the predominant organ that is initially injured, and fatal hepatic necrosis can occur in severe and untreated overdoses. In less severe conditions, significant liver injury and dysfunction can occur, resulting in an increase in transaminases (aspartate transaminase [AST], alanine transaminase [ALT]) and abnormalities in coagulation because of abnormal production of hepatic coagulation proteins. The mechanism of injury is as follows:
The mechanism of injury is as follows:
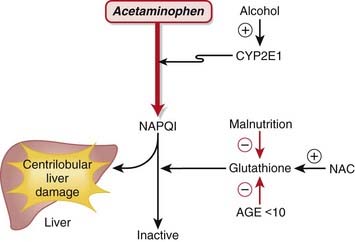
 Acetaminophen is metabolized in the liver via CYP2E1 to N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is highly reactive, electrophilic, and toxic.
Acetaminophen is metabolized in the liver via CYP2E1 to N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is highly reactive, electrophilic, and toxic. Treatment is specifically targeted at replenishing GSH stores. This is accomplished through the administration of a GSH precursor called N-acetylcysteine (NAC).
Treatment is specifically targeted at replenishing GSH stores. This is accomplished through the administration of a GSH precursor called N-acetylcysteine (NAC).Evidence
Analgesia
Acetaminophen versus Placebo for Treatment of Osteoarthritis
 A Cochrane review in 2005 (seven studies) demonstrated that acetaminophen was superior to placebo in five of the seven randomized controlled trials (RCTs). A pooled analysis demonstrated a statistically significant but minimal difference that is of questionable clinical significance. The relative percent improvement in pain score from baseline was 5%, with an absolute change of 4 points on a 0-to-100 scale.
A Cochrane review in 2005 (seven studies) demonstrated that acetaminophen was superior to placebo in five of the seven randomized controlled trials (RCTs). A pooled analysis demonstrated a statistically significant but minimal difference that is of questionable clinical significance. The relative percent improvement in pain score from baseline was 5%, with an absolute change of 4 points on a 0-to-100 scale.Acetaminophen versus Nonsteroidal Antiinflammatory Drugs for Treatment of Osteoarthritis
 The same Cochrane review in 2005 (10 studies) demonstrated that acetaminophen was less effective overall than NSAIDs in terms of pain reduction, global assessments, and improvements in functional status. Patients taking traditional NSAIDS were more likely to experience an adverse GI event (relative risk [RR] 1.47). However, the median trial duration was only 6 weeks, which is too short to adequately assess adverse outcomes.
The same Cochrane review in 2005 (10 studies) demonstrated that acetaminophen was less effective overall than NSAIDs in terms of pain reduction, global assessments, and improvements in functional status. Patients taking traditional NSAIDS were more likely to experience an adverse GI event (relative risk [RR] 1.47). However, the median trial duration was only 6 weeks, which is too short to adequately assess adverse outcomes.Acetaminophen plus Codeine versus Placebo
 A Cochrane review in 2008 (26 studies, N = 2295 patients) of postoperative patients demonstrated significant differences for obtaining at least 50% pain relief over 4 to 6 hours, with a number needed to treat (NNT) of 2.2 for high doses (800 to 1000 mg acetaminophen plus 60 mg codeine) and smaller effect sizes for medium and smaller doses (as low as 325 mg acetaminophen with 30 mg codeine).
A Cochrane review in 2008 (26 studies, N = 2295 patients) of postoperative patients demonstrated significant differences for obtaining at least 50% pain relief over 4 to 6 hours, with a number needed to treat (NNT) of 2.2 for high doses (800 to 1000 mg acetaminophen plus 60 mg codeine) and smaller effect sizes for medium and smaller doses (as low as 325 mg acetaminophen with 30 mg codeine).Acetaminophen Plus Codeine versus Acetaminophen Alone
 A Cochrane review in 2008 (14 studies, N = 926 patients) of postoperative patients demonstrated that addition of codeine increased the proportion of participants achieving at least 50% pain relief over 4 to 6 hours by 10% to 15% and reduced the proportion of patients needing rescue medication by about 15%.
A Cochrane review in 2008 (14 studies, N = 926 patients) of postoperative patients demonstrated that addition of codeine increased the proportion of participants achieving at least 50% pain relief over 4 to 6 hours by 10% to 15% and reduced the proportion of patients needing rescue medication by about 15%.Opioids
MOA (Mechanism of Action)
 The pain pathways in the body are very complex and only briefly summarized here. In short, opioids reduce the signaling and processing of pain pathways through a variety of receptor types, receptor locations, and complex interactions.
The pain pathways in the body are very complex and only briefly summarized here. In short, opioids reduce the signaling and processing of pain pathways through a variety of receptor types, receptor locations, and complex interactions. Exogenous opioids also bind multiple opioid receptors (Table 21-1). Opioid receptors are present in both the brain and the spinal cord, specifically:
Exogenous opioids also bind multiple opioid receptors (Table 21-1). Opioid receptors are present in both the brain and the spinal cord, specifically:
 The descending pathways in the spinal cord decrease the pain processing that occurs in the spinal cord.
The descending pathways in the spinal cord decrease the pain processing that occurs in the spinal cord.| Receptor | Action |
|---|---|
| Mu (µ) |
Pharmacokinetics (Table 21-2)
 For equivalency, note that:
For equivalency, note that:
 Routes of administration:
Routes of administration:
 Important metabolites:
Important metabolites:
 Most opioids are metabolized by CYP3A4 and renally eliminated (except where the following information states otherwise).
Most opioids are metabolized by CYP3A4 and renally eliminated (except where the following information states otherwise). Morphine → morphine-3-glucuronide (90%), morphine-6-glucuronide (10%).
Morphine → morphine-3-glucuronide (90%), morphine-6-glucuronide (10%).
• The glucuronides (being water soluble) are eliminated by the kidneys; renal disease can prolong and increase the effects of morphine.
• The glucuronides are water soluble; thus they do not readily cross the blood-brain barrier, but with high concentrations of drug, brain levels will increase.
Contraindications
Side Effects
 Respiratory depression: All narcotics are powerful respiratory depressants. They can cause hypoventilation, hypoxia, and apnea. The effect is dose dependent. Low-efficacy opioids (codeine) are less likely to result in profound respiratory depression.
Respiratory depression: All narcotics are powerful respiratory depressants. They can cause hypoventilation, hypoxia, and apnea. The effect is dose dependent. Low-efficacy opioids (codeine) are less likely to result in profound respiratory depression. Nausea and vomiting: Some patients are exquisitely sensitive. This is a very common side effect. The mechanism involves stimulation of the chemoreceptor trigger zone, the area of the brain responsible for vomiting.
Nausea and vomiting: Some patients are exquisitely sensitive. This is a very common side effect. The mechanism involves stimulation of the chemoreceptor trigger zone, the area of the brain responsible for vomiting. Pruritus (itching): This effect is not mediated by histamine (the most common mediator for itchy skin) but is rather a centrally (in the brain) mediated effect. Patients will often scratch their nose about 45 seconds after receiving intravenous fentanyl. For some patients the itching can be very uncomfortable and distressing and could mandate changing to a different opioid or stopping opioids completely.
Pruritus (itching): This effect is not mediated by histamine (the most common mediator for itchy skin) but is rather a centrally (in the brain) mediated effect. Patients will often scratch their nose about 45 seconds after receiving intravenous fentanyl. For some patients the itching can be very uncomfortable and distressing and could mandate changing to a different opioid or stopping opioids completely. Constipation: This effect can be extremely severe. Codeine even in low doses can produce profound constipation. Mu receptors in the bowel are responsible for this side effect.
Constipation: This effect can be extremely severe. Codeine even in low doses can produce profound constipation. Mu receptors in the bowel are responsible for this side effect.Important Notes
 The cardinal signs of opioid withdrawal include rhinorrhea, lacrimation, yawning, chills, piloerection (goose bumps), hyperventilation, hyperthermia, mydriasis, muscular aches, vomiting, diarrhea, anxiety, and hostility.
The cardinal signs of opioid withdrawal include rhinorrhea, lacrimation, yawning, chills, piloerection (goose bumps), hyperventilation, hyperthermia, mydriasis, muscular aches, vomiting, diarrhea, anxiety, and hostility. A new antagonist called methylnaltrexone is a methylated version of naltrexone. It is special because it does not cross the blood-brain barrier. Therefore it has the ability to antagonize all the peripherally mediated side effects of narcotics (mostly constipation) without reducing the analgesic effects (which are mediated within the CNS). The most common indication for methylnaltrexone is treatment of constipation.
A new antagonist called methylnaltrexone is a methylated version of naltrexone. It is special because it does not cross the blood-brain barrier. Therefore it has the ability to antagonize all the peripherally mediated side effects of narcotics (mostly constipation) without reducing the analgesic effects (which are mediated within the CNS). The most common indication for methylnaltrexone is treatment of constipation. Loperamide is an antidiarrheal opioid. It is absorbed very poorly from the gastrointestinal tract and therefore, when given orally, acts on mu receptors in the stomach, intestine, and colon only, reducing motility.
Loperamide is an antidiarrheal opioid. It is absorbed very poorly from the gastrointestinal tract and therefore, when given orally, acts on mu receptors in the stomach, intestine, and colon only, reducing motility. Methadone is most commonly used to control withdrawal symptoms in patients who are recovering from narcotic addiction (usually heroin) because it has a long half-life. Furthermore, methadone is also an N-methyl-d-aspartate (NMDA) receptor antagonist, and this property has been implicated in a role of preventing tolerance to methadone. NMDA antagonists are also analgesics. Methadone also has monoamine oxidase inhibitor (MAOI) properties. MAOIs are antidepressants.
Methadone is most commonly used to control withdrawal symptoms in patients who are recovering from narcotic addiction (usually heroin) because it has a long half-life. Furthermore, methadone is also an N-methyl-d-aspartate (NMDA) receptor antagonist, and this property has been implicated in a role of preventing tolerance to methadone. NMDA antagonists are also analgesics. Methadone also has monoamine oxidase inhibitor (MAOI) properties. MAOIs are antidepressants. Tramadol is a very weak mu agonist whose mechanism of action is predominantly based on blockade of serotonin reuptake. It does not cause respiratory depression. It can cause seizures, as can meperidine, which is in the same chemical class.
Tramadol is a very weak mu agonist whose mechanism of action is predominantly based on blockade of serotonin reuptake. It does not cause respiratory depression. It can cause seizures, as can meperidine, which is in the same chemical class. The partial agonists all have kappa receptor activity and only limited mu receptor activity. This is significant because the mu receptor mediates analgesia, so partial agonists are less effective analgesics than full agonists.
The partial agonists all have kappa receptor activity and only limited mu receptor activity. This is significant because the mu receptor mediates analgesia, so partial agonists are less effective analgesics than full agonists.
 Opioid antagonists can precipitate severe withdrawal in patients who are physically dependent on opioids. They should be administered carefully.
Opioid antagonists can precipitate severe withdrawal in patients who are physically dependent on opioids. They should be administered carefully. Tolerance: Opioid receptor down-regulation occurs with chronic administration of opioids. The result is that higher and higher doses are required to achieve the same effect. Neuromodulation is another term for tolerance.
Tolerance: Opioid receptor down-regulation occurs with chronic administration of opioids. The result is that higher and higher doses are required to achieve the same effect. Neuromodulation is another term for tolerance. Although respiratory depression is a side effect of opioids, the same mechanism is responsible for reducing the ventilatory drive in patients who are short of breath; therefore opioids can reduce the unpleasant sensation of breathlessness and can be effective at relieving discomfort related to breathing in selected patients.
Although respiratory depression is a side effect of opioids, the same mechanism is responsible for reducing the ventilatory drive in patients who are short of breath; therefore opioids can reduce the unpleasant sensation of breathlessness and can be effective at relieving discomfort related to breathing in selected patients.Evidence
Opioids versus Nonsteroidal Antiinflammatory Drugs for Treatment of Renal Colic
 A systematic review in 2004 (20 trials, 1613 participants) found that both NSAIDs and opioids led to clinically important reductions in patient-reported pain scores. Pooled analysis of six trials showed a greater reduction in pain scores for patients treated with NSAIDs than with opioids. Patients treated with NSAIDs were significantly less likely to require rescue analgesia (RR 0.75). Most trials showed a higher incidence of adverse events in patients treated with opioids. Compared with patients treated with opioids, those treated with NSAIDs had significantly less vomiting (0.35).
A systematic review in 2004 (20 trials, 1613 participants) found that both NSAIDs and opioids led to clinically important reductions in patient-reported pain scores. Pooled analysis of six trials showed a greater reduction in pain scores for patients treated with NSAIDs than with opioids. Patients treated with NSAIDs were significantly less likely to require rescue analgesia (RR 0.75). Most trials showed a higher incidence of adverse events in patients treated with opioids. Compared with patients treated with opioids, those treated with NSAIDs had significantly less vomiting (0.35).Opioids for Treatment of Chronic Back Pain
 A systematic review in 2007 examined multiple questions pertaining to opioid treatment of chronic low back pain. 11 studies showed that there was significant variation in how opioids are prescribed for chronic low back pain. A meta-analysis of the four studies assessing the efficacy of opioids compared with placebo or a nonopioid control did not show reduced pain with opioids. A meta-analysis of the five studies directly comparing the efficacy of different opioids demonstrated a nonsignificant reduction in pain from baseline.
A systematic review in 2007 examined multiple questions pertaining to opioid treatment of chronic low back pain. 11 studies showed that there was significant variation in how opioids are prescribed for chronic low back pain. A meta-analysis of the four studies assessing the efficacy of opioids compared with placebo or a nonopioid control did not show reduced pain with opioids. A meta-analysis of the five studies directly comparing the efficacy of different opioids demonstrated a nonsignificant reduction in pain from baseline.
 With respect to risk of addiction, the prevalence of lifetime substance use disorders ranged from 36% to 56%; the prevalence of current substance use disorders was as high as 43%; and aberrant medication-taking behaviors (“drug seeking”) ranged from 5% to 24%. The authors found that the study was limited by retrieval and publication biases and poor study quality. No trial evaluating the efficacy of opioids was longer than 16 weeks.
With respect to risk of addiction, the prevalence of lifetime substance use disorders ranged from 36% to 56%; the prevalence of current substance use disorders was as high as 43%; and aberrant medication-taking behaviors (“drug seeking”) ranged from 5% to 24%. The authors found that the study was limited by retrieval and publication biases and poor study quality. No trial evaluating the efficacy of opioids was longer than 16 weeks.FYI
 The term opioid refers broadly to all compounds related to opium, although only morphine and codeine are actually produced by the opium poppy, Papaver somniferum.
The term opioid refers broadly to all compounds related to opium, although only morphine and codeine are actually produced by the opium poppy, Papaver somniferum. The name morphine is from Morpheus, the Greek god of dreams. Morphine was first isolated in 1803 by Sertürner.
The name morphine is from Morpheus, the Greek god of dreams. Morphine was first isolated in 1803 by Sertürner. The word narcotic is derived from the Greek word meaning stupor. The term narcotic is often used in a legal context to refer to a variety of illegal substances; the term in medical vocabulary refers only to opioids.
The word narcotic is derived from the Greek word meaning stupor. The term narcotic is often used in a legal context to refer to a variety of illegal substances; the term in medical vocabulary refers only to opioids. Etorphine is an opioid with about 2000 times the potency of morphine. It is used as a large animal immobilizer (for veterinary use). One drop on the skin of a human would be fatal because of the profound respiratory depression. Carfentanil, another veterinary opioid, is 10,000 times more potent than morphine (and 10 times more potent than sufentanil, the most potent opioid for humans). Nonhuman mammals do not experience the same degree of respiratory depression as humans do, and so these drugs are suitable as immobilizing agents in large animals.
Etorphine is an opioid with about 2000 times the potency of morphine. It is used as a large animal immobilizer (for veterinary use). One drop on the skin of a human would be fatal because of the profound respiratory depression. Carfentanil, another veterinary opioid, is 10,000 times more potent than morphine (and 10 times more potent than sufentanil, the most potent opioid for humans). Nonhuman mammals do not experience the same degree of respiratory depression as humans do, and so these drugs are suitable as immobilizing agents in large animals.α2 Agonists
MOA (Mechanism of Action)
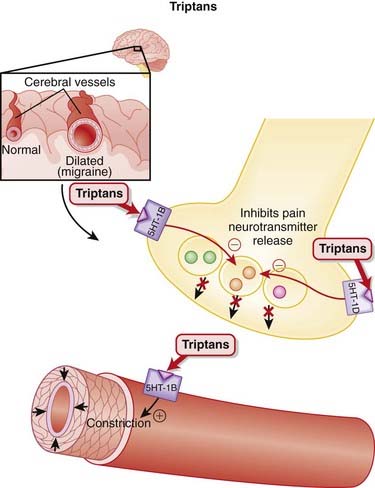
 Glaucoma is characterized by increased intraocular pressure (IOP). Strategies to reduce intraocular pressure include reducing the production and secretion of aqueous humor and facilitating its drainage.
Glaucoma is characterized by increased intraocular pressure (IOP). Strategies to reduce intraocular pressure include reducing the production and secretion of aqueous humor and facilitating its drainage. The exact mechanism by which α2 agonists reduce IOP has not been established, but is likely multifactorial, employing several strategies:
The exact mechanism by which α2 agonists reduce IOP has not been established, but is likely multifactorial, employing several strategies:
 Through a secondary mechanism, brimonidine may also have a neuroprotective role, mitigating the damage to the optic nerve caused by the elevated IOP.
Through a secondary mechanism, brimonidine may also have a neuroprotective role, mitigating the damage to the optic nerve caused by the elevated IOP. The antihypertensive effect of α2 agonists is a result of inhibition of presynaptic release of vasoconstrictors such as norepinephrine. Recall that the α2 receptor is an autoreceptor (see Chapter 3). An autoreceptor is a receptor that when stimulated by an agonist, reduces release of transmitter into the synaptic cleft.
The antihypertensive effect of α2 agonists is a result of inhibition of presynaptic release of vasoconstrictors such as norepinephrine. Recall that the α2 receptor is an autoreceptor (see Chapter 3). An autoreceptor is a receptor that when stimulated by an agonist, reduces release of transmitter into the synaptic cleft.Pharmacokinetics
Contraindications
Side Effects
Important Notes
Evidence
In Primary Open Angle Glaucoma and Ocular Hypertension
 A 2007 Cochrane review of all medical interventions for glaucoma and ocular hypertension found three trials comparing brimonidine to timolol. There were no differences in visual field progression (glaucoma) or visual field defects (ocular hypertension) within 1 year. Timolol was better tolerated than brimonidine, as measured by the incidence of dropouts from drug-related adverse events (OR 0.21). There were no trials comparing brimonidine with placebo.
A 2007 Cochrane review of all medical interventions for glaucoma and ocular hypertension found three trials comparing brimonidine to timolol. There were no differences in visual field progression (glaucoma) or visual field defects (ocular hypertension) within 1 year. Timolol was better tolerated than brimonidine, as measured by the incidence of dropouts from drug-related adverse events (OR 0.21). There were no trials comparing brimonidine with placebo.Inhaled Anesthetics
MOA (Mechanism of Action) (Figure 21-2)
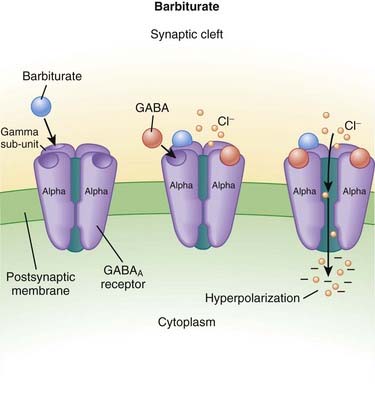
 The primary site of action in causing CNS depression is most likely the γ-aminobutyric acid A (GABAA) receptor. Inhaled anesthetics activate these receptors.
The primary site of action in causing CNS depression is most likely the γ-aminobutyric acid A (GABAA) receptor. Inhaled anesthetics activate these receptors. The GABAA receptor is linked to a chloride channel; this is important because one way to turn off a neuron is to hyperpolarize it so that it cannot be depolarized enough to trigger an action potential. Opening a chloride channel will allow the negatively charged chloride ion to enter the cell and will reduce the electrical charge inside the cell (thereby hyperpolarizing it) and effectively render the neuron unresponsive to incoming stimuli that would otherwise depolarize the cell.
The GABAA receptor is linked to a chloride channel; this is important because one way to turn off a neuron is to hyperpolarize it so that it cannot be depolarized enough to trigger an action potential. Opening a chloride channel will allow the negatively charged chloride ion to enter the cell and will reduce the electrical charge inside the cell (thereby hyperpolarizing it) and effectively render the neuron unresponsive to incoming stimuli that would otherwise depolarize the cell.Pharmacokinetics
 As their name suggests, these drugs are administered only via inhalation. They are supplied in liquid form and then vaporized using very precise vaporizers that are part of the anesthetic machine, the anesthetic oxygen and air mixture is combined with calculated doses of the inhaled anesthetic.
As their name suggests, these drugs are administered only via inhalation. They are supplied in liquid form and then vaporized using very precise vaporizers that are part of the anesthetic machine, the anesthetic oxygen and air mixture is combined with calculated doses of the inhaled anesthetic. Very small amounts of the drug are metabolized. For example, desflurane undergoes <0.02% metabolism, which is clinically insignificant. Older drugs had higher rates of metabolism, with halothane as high as 40%.
Very small amounts of the drug are metabolized. For example, desflurane undergoes <0.02% metabolism, which is clinically insignificant. Older drugs had higher rates of metabolism, with halothane as high as 40%. Inhaled anesthetics move through the body by dissolving in the blood and distributing into tissues; the important tissue interfaces include the following:
Inhaled anesthetics move through the body by dissolving in the blood and distributing into tissues; the important tissue interfaces include the following:
 Blood ∂ fat (and other vessel-poor tissues)
Blood ∂ fat (and other vessel-poor tissues)
• When inhaled anesthetics are administered, the drug must enter the lungs, then the blood, then the brain.
• For inhaled anesthetics to be eliminated, drug must exit the brain and other tissues, be carried to the lungs, and then exhaled.
• Vessel-poor tissues are slow to take up and release drug. Therefore vessel-poor tissues can act as a sink and slowly absorb drug at the early parts of an anesthetic procedure (lowering the drug levels) but then at the end of a long anesthetic procedure can release drug, prolonging elimination of the drug.
 The solubility of an inhaled anesthetic affects the speed at which it is taken up by the body and exerts its action (speed of onset). The key point is that the partial pressure is the measure of the drug’s active form. If a drug has a high solubility, then a lot of drug needs to be absorbed into blood and tissues before the partial pressure starts to rise; this impedes the onset of action of the drug. If the solubility is low, then the partial pressure will rise quickly. Conversely, eliminating the drug follows the same rules, and high solubility correlates with slower elimination because more drug had to be dissolved into the body initially to achieve the desired partial pressure.
The solubility of an inhaled anesthetic affects the speed at which it is taken up by the body and exerts its action (speed of onset). The key point is that the partial pressure is the measure of the drug’s active form. If a drug has a high solubility, then a lot of drug needs to be absorbed into blood and tissues before the partial pressure starts to rise; this impedes the onset of action of the drug. If the solubility is low, then the partial pressure will rise quickly. Conversely, eliminating the drug follows the same rules, and high solubility correlates with slower elimination because more drug had to be dissolved into the body initially to achieve the desired partial pressure.
• Solubility is described for the blood and is called the blood/gas coefficient. The smaller the number, the less soluble the drug is in blood and therefore the faster it can change its partial pressure (because only a small amount of drug actually needs to be dissolved into the blood). The agents are ranked from fastest to slowest (lowest to highest solubility coefficient) as follows:
Side Effects
 Respiratory depression: The respiratory drive in response to carbon dioxide is blunted, and blood CO2 levels rise. The tidal volume is reduced, but the respiratory rate is actually increased (the reverse occurs with opioid respiratory depression).
Respiratory depression: The respiratory drive in response to carbon dioxide is blunted, and blood CO2 levels rise. The tidal volume is reduced, but the respiratory rate is actually increased (the reverse occurs with opioid respiratory depression).Important Notes
 The potency of inhaled anesthetics is measured by the minimum anesthetic concentration (MAC), which is strictly defined as the dose of inhaled anesthetic required to prevent movement in 50% of the population in response to a surgical stimulus when no other drugs are administered. For example, the MAC of desflurane is 6%. Note that this definition is not the same as the dose required to keep someone asleep. Drugs such as opioids decrease the MAC requirements of a drug and are called MAC-sparing agents.
The potency of inhaled anesthetics is measured by the minimum anesthetic concentration (MAC), which is strictly defined as the dose of inhaled anesthetic required to prevent movement in 50% of the population in response to a surgical stimulus when no other drugs are administered. For example, the MAC of desflurane is 6%. Note that this definition is not the same as the dose required to keep someone asleep. Drugs such as opioids decrease the MAC requirements of a drug and are called MAC-sparing agents. Inhaled anesthetics cause hypotension. The primary mechanism is through vasodilation, although older agents (e.g., halothane) produced cardiac depression (decreased contractility and decreased heart rate), which caused hypotension.
Inhaled anesthetics cause hypotension. The primary mechanism is through vasodilation, although older agents (e.g., halothane) produced cardiac depression (decreased contractility and decreased heart rate), which caused hypotension.
 In addition to being vascular smooth muscle relaxants, inhaled anesthetics also relax bronchial smooth muscle and through this mechanism can help to relieve bronchospasm in rare cases of life-threatening refractory status asthmaticus.
In addition to being vascular smooth muscle relaxants, inhaled anesthetics also relax bronchial smooth muscle and through this mechanism can help to relieve bronchospasm in rare cases of life-threatening refractory status asthmaticus. MH is a rare condition that is precipitated by inhaled anesthetics or succinylcholine (a paralyzing agent). It is caused by a channelopathy, which is a mutation in an ion channel in muscle. It is a life-threatening condition that occurs as a result of pathologically high levels of skeletal muscle contraction from increased intracellular calcium that occurs in the absence of neuromuscular stimulation. Because of the muscular contraction, the following effects occur:
MH is a rare condition that is precipitated by inhaled anesthetics or succinylcholine (a paralyzing agent). It is caused by a channelopathy, which is a mutation in an ion channel in muscle. It is a life-threatening condition that occurs as a result of pathologically high levels of skeletal muscle contraction from increased intracellular calcium that occurs in the absence of neuromuscular stimulation. Because of the muscular contraction, the following effects occur:
 MH is treated with dantrolene, a drug that reduces intracellular calcium through binding the ryanodine receptor, which mediates calcium release from the sarcoplasmic reticulum.
MH is treated with dantrolene, a drug that reduces intracellular calcium through binding the ryanodine receptor, which mediates calcium release from the sarcoplasmic reticulum.Advanced
 Nitrous oxide has a MAC value of 104%. Therefore, it is not potent enough to be a solo anesthetic agent, because delivering a mixture of 100% nitrous oxide would mean that no oxygen could be delivered and the patient would die of asphyxiation. The greatest concentration that is administered is about 60% to 70%—a MAC value of 0.6 or 0.7. For this reason, nitrous is used only as an adjuvant drug for general anesthesia and is added to one of the other volatile anesthetics.
Nitrous oxide has a MAC value of 104%. Therefore, it is not potent enough to be a solo anesthetic agent, because delivering a mixture of 100% nitrous oxide would mean that no oxygen could be delivered and the patient would die of asphyxiation. The greatest concentration that is administered is about 60% to 70%—a MAC value of 0.6 or 0.7. For this reason, nitrous is used only as an adjuvant drug for general anesthesia and is added to one of the other volatile anesthetics.
FYI
 Ether was the first inhaled anesthetic. Most inhaled anesthetics (not including nitrous oxide or xenon) are fluorinated (fluorine is a halogen, thus the name “halogenated”) derivatives of ether:
Ether was the first inhaled anesthetic. Most inhaled anesthetics (not including nitrous oxide or xenon) are fluorinated (fluorine is a halogen, thus the name “halogenated”) derivatives of ether:
Intravenous Anesthetics
MOA (Mechanism of Action)
γ-Aminobutyric Acid (GABA) (Figure 21-3)
 GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
Pharmacokinetics
 Intravenous anesthetics work within one arm to brain circulation time; that is, if the drug is administered through an intravenous line in the arm, it is the time required to circulate back to the heart and then up to the brain (usually less than 1 minute).
Intravenous anesthetics work within one arm to brain circulation time; that is, if the drug is administered through an intravenous line in the arm, it is the time required to circulate back to the heart and then up to the brain (usually less than 1 minute). Compartmental distribution is a very important factor for intravenous anesthetics. When the drug is administered into the blood, it rapidly is taken up by the brain (inducing its effect), but very quickly the drug is also taken up by the vessel-rich organs (liver, muscle, kidney, heart), and this uptake quickly reduces the levels in the blood and therefore the brain in a matter of minutes. Therefore when the drug is given as a single bolus, the termination of action of the drug is primarily a result of redistribution of the drug and not metabolism or elimination of the drug. When the drug is given as an infusion, metabolism and elimination become important because all compartments would be equilibrated.
Compartmental distribution is a very important factor for intravenous anesthetics. When the drug is administered into the blood, it rapidly is taken up by the brain (inducing its effect), but very quickly the drug is also taken up by the vessel-rich organs (liver, muscle, kidney, heart), and this uptake quickly reduces the levels in the blood and therefore the brain in a matter of minutes. Therefore when the drug is given as a single bolus, the termination of action of the drug is primarily a result of redistribution of the drug and not metabolism or elimination of the drug. When the drug is given as an infusion, metabolism and elimination become important because all compartments would be equilibrated.Side Effects
 Respiratory depression: The respiratory center is depressed with GABA agonists. It is typical for a patient who is administered a dose large enough to induce unconsciousness to develop apnea (to completely stop breathing). For this reason, administration of intravenous anesthetics (regardless of dose) must always be performed in the presence of healthcare workers who are skilled in respiratory resuscitation and who have respiratory resuscitation equipment immediately available.
Respiratory depression: The respiratory center is depressed with GABA agonists. It is typical for a patient who is administered a dose large enough to induce unconsciousness to develop apnea (to completely stop breathing). For this reason, administration of intravenous anesthetics (regardless of dose) must always be performed in the presence of healthcare workers who are skilled in respiratory resuscitation and who have respiratory resuscitation equipment immediately available. Hypotension: Intravenous anesthetics, especially propofol, are myocardial depressants. They decrease contractility and often also reduce sympathetic nervous system activity.
Hypotension: Intravenous anesthetics, especially propofol, are myocardial depressants. They decrease contractility and often also reduce sympathetic nervous system activity.Important Notes
 Dosage: Some general concepts should be applied when choosing the dose of intravenous anesthetic. Administering a larger-than-required dose virtually guarantees significant hypotension:
Dosage: Some general concepts should be applied when choosing the dose of intravenous anesthetic. Administering a larger-than-required dose virtually guarantees significant hypotension:
 Elderly patients require lower doses compared with nonelderly adults, and children require higher doses (on a milligram-per-kilogram scale) compared with adults.
Elderly patients require lower doses compared with nonelderly adults, and children require higher doses (on a milligram-per-kilogram scale) compared with adults. Ketamine:
Ketamine:
 In contrast to GABA-mimetic drugs, ketamine:
In contrast to GABA-mimetic drugs, ketamine:
 Causes sympathetic nervous system activation (resulting in increased heart rate and blood pressure) and therefore is the least likely to induce hypotension. However, it is still a direct myocardial depressant (reduces contractility), and in states in which the sympathetic nervous system is already highly activated (e.g., cardiogenic shock), ketamine retains potential for inducing hypotension.
Causes sympathetic nervous system activation (resulting in increased heart rate and blood pressure) and therefore is the least likely to induce hypotension. However, it is still a direct myocardial depressant (reduces contractility), and in states in which the sympathetic nervous system is already highly activated (e.g., cardiogenic shock), ketamine retains potential for inducing hypotension. Propofol:
Propofol:
 Is a strong myocardial depressant and should be given very cautiously, if at all, in patients with a weak heart (low systolic function).
Is a strong myocardial depressant and should be given very cautiously, if at all, in patients with a weak heart (low systolic function). Is lipophilic and does not dissolve in water; therefore a lipid-containing, milk-colored emulsion is the vehicle in which it is administered. The emulsion stings veins when it is injected. It is often mixed with lidocaine, a local anesthetic, to reduce the stinging. A newer formulation called fospropofol is water soluble.
Is lipophilic and does not dissolve in water; therefore a lipid-containing, milk-colored emulsion is the vehicle in which it is administered. The emulsion stings veins when it is injected. It is often mixed with lidocaine, a local anesthetic, to reduce the stinging. A newer formulation called fospropofol is water soluble.Advanced
 Etomidate and propofol preferentially act at GABAA receptors that contain β1 and β2 subunits (not to be confused with the autonomic receptors with similar names). The β2 subunit is probably the more important for mediating hypnotic and muscle-relaxing actions.
Etomidate and propofol preferentially act at GABAA receptors that contain β1 and β2 subunits (not to be confused with the autonomic receptors with similar names). The β2 subunit is probably the more important for mediating hypnotic and muscle-relaxing actions. Barbiturates also facilitate the actions of GABA at multiple sites in the CNS, but in contrast to benzodiazepines they appear to increase the duration of the GABA-gated chloride channel openings. At high concentrations the barbiturates may also be GABA-mimetic, directly activating chloride channels.
Barbiturates also facilitate the actions of GABA at multiple sites in the CNS, but in contrast to benzodiazepines they appear to increase the duration of the GABA-gated chloride channel openings. At high concentrations the barbiturates may also be GABA-mimetic, directly activating chloride channels.FYI
 Dextromethorphan, an NMDA antagonist, is a commonly used cough suppressant and is found in many over-the-counter cough remedies.
Dextromethorphan, an NMDA antagonist, is a commonly used cough suppressant and is found in many over-the-counter cough remedies.Local Anesthetics
MOA (Mechanism of Action)
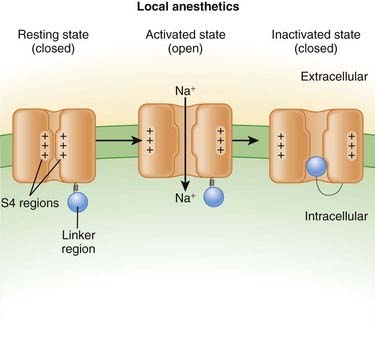
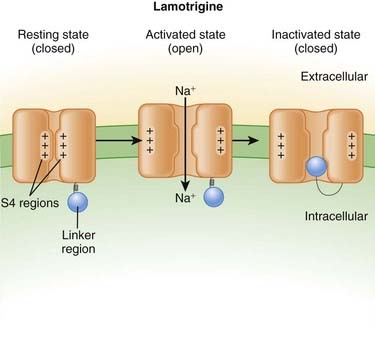
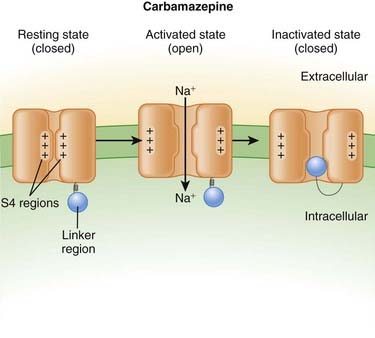
 Neuronal transmission requires that an action potential be propagated from one end of a neuron to the other. Voltage-gated sodium channels open up as the wave of depolarization travels from one end of the neuron to the other. The opening of these ion channels permits sodium to enter the cell, causing depolarization, and is the primary method by which the wave of depolarization occurs.
Neuronal transmission requires that an action potential be propagated from one end of a neuron to the other. Voltage-gated sodium channels open up as the wave of depolarization travels from one end of the neuron to the other. The opening of these ion channels permits sodium to enter the cell, causing depolarization, and is the primary method by which the wave of depolarization occurs. Local anesthetics bind these voltage-gated sodium channels. The ion channels can exist in three states: resting, activated (open), and inactivated. The local anesthetic binds them in the inactivated state and prevents them from transitioning to the open state; thus the ion channel remains closed and unresponsive to incoming depolarizing currents (Figure 21-4).
Local anesthetics bind these voltage-gated sodium channels. The ion channels can exist in three states: resting, activated (open), and inactivated. The local anesthetic binds them in the inactivated state and prevents them from transitioning to the open state; thus the ion channel remains closed and unresponsive to incoming depolarizing currents (Figure 21-4).
 The local anesthetic works on the intracellular side of the ion channel. Therefore the drug must first cross the lipid cell membrane before it can exert its action.
The local anesthetic works on the intracellular side of the ion channel. Therefore the drug must first cross the lipid cell membrane before it can exert its action. Neurons that are covered in myelin are more difficult to block because the myelin impedes entry of the drug into the cell.
Neurons that are covered in myelin are more difficult to block because the myelin impedes entry of the drug into the cell. Larger-diameter nerves are more difficult to block than are smaller-diameter nerves. They require higher doses and are blocked for shorter durations.
Larger-diameter nerves are more difficult to block than are smaller-diameter nerves. They require higher doses and are blocked for shorter durations. There are three major classes of nerve function: sensory, motor, and autonomic. All types of nerves are susceptible to blockade by local anesthetic, but to varying degrees because of myelination and diameter:
There are three major classes of nerve function: sensory, motor, and autonomic. All types of nerves are susceptible to blockade by local anesthetic, but to varying degrees because of myelination and diameter:
 Voltage-gated sodium channels are the primary ion channels in phase 0 of the cardiac action potential. Therefore, local anesthetics are also classified as antiarrhythmics, specifically type 1b. This mechanism of action is discussed in more detail in the discussion of Na+ channel blockers in Chapter 11 and is also responsible for cardiac toxicity of local anesthetics.
Voltage-gated sodium channels are the primary ion channels in phase 0 of the cardiac action potential. Therefore, local anesthetics are also classified as antiarrhythmics, specifically type 1b. This mechanism of action is discussed in more detail in the discussion of Na+ channel blockers in Chapter 11 and is also responsible for cardiac toxicity of local anesthetics.Pharmacokinetics
 Local anesthetics are most commonly injected, but other routes of administration include topical application: oral sprays, creams, and vaporized forms (for airways).
Local anesthetics are most commonly injected, but other routes of administration include topical application: oral sprays, creams, and vaporized forms (for airways). There are important factors that dictate the potency, speed of onset, and duration of a local anesthetic:
There are important factors that dictate the potency, speed of onset, and duration of a local anesthetic:
 Lipid solubility: Increased lipid solubility results in more drug being able to cross the cell membrane and bind the ion channel. The property of increased lipid solubility influences:
Lipid solubility: Increased lipid solubility results in more drug being able to cross the cell membrane and bind the ion channel. The property of increased lipid solubility influences:
 pH: Local anesthetics are weak bases. Therefore when the environment is acidic, the following reaction occurs: B + H+ → BH+ (where B = base and represents the local anesthetic). From this equation, it can be seen that local anesthetics will be ionized (BH+) and therefore hydrophilic (not lipid soluble) in an acid environment. Therefore, acidic tissues (such as an abscess or any infection) are very difficult to anesthetize with local anesthetic. An acid pH will decrease potency, speed of onset, and duration.
pH: Local anesthetics are weak bases. Therefore when the environment is acidic, the following reaction occurs: B + H+ → BH+ (where B = base and represents the local anesthetic). From this equation, it can be seen that local anesthetics will be ionized (BH+) and therefore hydrophilic (not lipid soluble) in an acid environment. Therefore, acidic tissues (such as an abscess or any infection) are very difficult to anesthetize with local anesthetic. An acid pH will decrease potency, speed of onset, and duration. Ester local anesthetics are predominantly metabolized via ester hydrolysis by pseudocholinesterase. Ester hydrolysis is a fast reaction, and therefore ester local anesthetics have a shorter duration of action.
Ester local anesthetics are predominantly metabolized via ester hydrolysis by pseudocholinesterase. Ester hydrolysis is a fast reaction, and therefore ester local anesthetics have a shorter duration of action.| Agent | Duration Plain (minutes) | Duration with Epinephrine (minutes) |
|---|---|---|
| 2-Chloroprocaine | 20-30 | 30-45 |
| Procaine | 15-30 | 30 |
| Lidocaine | 30-60 | 120 |
| Mepivacaine | 45-90 | 120 |
| Prilocaine | 30-90 | 120 |
| Bupivacaine | 120-240 | 180-240 |
| Ropivacaine | 120-240 | 180-240 |
Side Effects
 Side effects more commonly occur when the toxic dose is approached or exceeded or if any dose is accidently injected into a blood vessel.
Side effects more commonly occur when the toxic dose is approached or exceeded or if any dose is accidently injected into a blood vessel. CNS toxicity: The action of local anesthetic will also influence the neurons in the brain and gives rise to the following signs and symptoms:
CNS toxicity: The action of local anesthetic will also influence the neurons in the brain and gives rise to the following signs and symptoms:
 Cardiovascular toxicity occurs at usually about 3 times the dose that is required to produce CNS toxicity, although this ratio is variable for different anesthetics. Bupivacaine is the most cardiotoxic. Electrical disturbances of the heart, including heart block, ventricular tachycardia, and ventricular fibrillation can occur, and these complications are life-threatening. Bupivacaine-induced ventricular fibrillation can be very resistant to treatment (defibrillation and antiarrhythmic treatment).
Cardiovascular toxicity occurs at usually about 3 times the dose that is required to produce CNS toxicity, although this ratio is variable for different anesthetics. Bupivacaine is the most cardiotoxic. Electrical disturbances of the heart, including heart block, ventricular tachycardia, and ventricular fibrillation can occur, and these complications are life-threatening. Bupivacaine-induced ventricular fibrillation can be very resistant to treatment (defibrillation and antiarrhythmic treatment). Consequences of unintentional intravascular injection: Toxicity can occur very suddenly and with low doses of anesthetic if the anesthetic is injected directly into an artery or vein. If the anesthetic also contains epinephrine, the patient will experience symptoms related to tachycardia and hypertension as well.
Consequences of unintentional intravascular injection: Toxicity can occur very suddenly and with low doses of anesthetic if the anesthetic is injected directly into an artery or vein. If the anesthetic also contains epinephrine, the patient will experience symptoms related to tachycardia and hypertension as well.Important Notes
 Onset time and duration are both dose dependent. Higher doses and higher concentrations of solution of local anesthetic will result in faster onset times and longer durations of action. The addition of low-dose epinephrine to the local anesthetic also prolongs the duration of action because it causes vasoconstriction and reduces blood flow, which slows the washout of the drug from the site of action.
Onset time and duration are both dose dependent. Higher doses and higher concentrations of solution of local anesthetic will result in faster onset times and longer durations of action. The addition of low-dose epinephrine to the local anesthetic also prolongs the duration of action because it causes vasoconstriction and reduces blood flow, which slows the washout of the drug from the site of action. The location of injection strongly determines the effect of the local anesthetic. Only a segment of a neuron needs to be bound with local anesthetic to completely block its function (compared with blocking the entire length of the neuron). Therefore if local anesthetic is administered upstream to a large nerve, a very large downstream distribution of sensation can be blocked. This is the principle behind spinal anesthetics, in which a small dose (usually 1 to 3 mL) of a local anesthetic is administered to the cerebral spinal fluid in the lumbar spine and the result is often complete loss of all sensation and motor activity from the chest down to the toes!
The location of injection strongly determines the effect of the local anesthetic. Only a segment of a neuron needs to be bound with local anesthetic to completely block its function (compared with blocking the entire length of the neuron). Therefore if local anesthetic is administered upstream to a large nerve, a very large downstream distribution of sensation can be blocked. This is the principle behind spinal anesthetics, in which a small dose (usually 1 to 3 mL) of a local anesthetic is administered to the cerebral spinal fluid in the lumbar spine and the result is often complete loss of all sensation and motor activity from the chest down to the toes!
 Small doses of epinephrine are often added to local anesthetic to prolong the duration of anesthesia. The epinephrine acts as a vasoconstrictor and thus reduces blood flow to the site of injection and reduces metabolism of the local anesthetic.
Small doses of epinephrine are often added to local anesthetic to prolong the duration of anesthesia. The epinephrine acts as a vasoconstrictor and thus reduces blood flow to the site of injection and reduces metabolism of the local anesthetic.
 Injection of local anesthetic is painful (in addition to the pain of the needle). This is because the local anesthetic initially activates Na+ channels, triggering initial depolarization before rendering the neurons inactive.
Injection of local anesthetic is painful (in addition to the pain of the needle). This is because the local anesthetic initially activates Na+ channels, triggering initial depolarization before rendering the neurons inactive.Advanced
 Structure: Local anesthetics contain a benzene (lipophilic) section connected to a hydrophilic section (often an amide). The connection between them is via either an ester or an amide bond, which is how the two categories of local anesthetics are designated.
Structure: Local anesthetics contain a benzene (lipophilic) section connected to a hydrophilic section (often an amide). The connection between them is via either an ester or an amide bond, which is how the two categories of local anesthetics are designated.FYI
 Amide local anesthetics have an –i– in the first part of the generic drug name (lidocaine, prilocaine, bupivacaine), whereas esters (tetracaine, procaine, cocaine) do not.
Amide local anesthetics have an –i– in the first part of the generic drug name (lidocaine, prilocaine, bupivacaine), whereas esters (tetracaine, procaine, cocaine) do not.Baclofen
MOA (Mechanism of Action)
γ-Aminobutyric Acid (GABA)
 GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
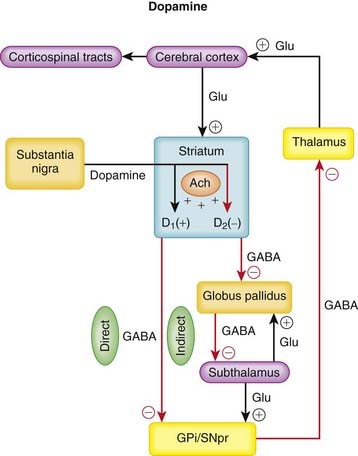
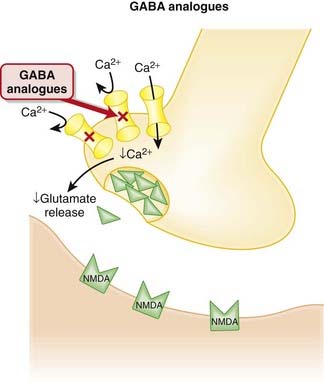
 Binding of GABA to GABAB receptors leads to the opening of the potassium (K+) channel, facilitating K+ efflux (leaving the cell) and thus cellular hyperpolarization (making the inside more negative).
Binding of GABA to GABAB receptors leads to the opening of the potassium (K+) channel, facilitating K+ efflux (leaving the cell) and thus cellular hyperpolarization (making the inside more negative). Note that most other GABA-mimetic drugs (benzodiazepines and intravenous anesthetics) are GABAA receptor modifiers, whereas baclofen increases GABA activity at GABAB receptors (Figure 21-5).
Note that most other GABA-mimetic drugs (benzodiazepines and intravenous anesthetics) are GABAA receptor modifiers, whereas baclofen increases GABA activity at GABAB receptors (Figure 21-5). Spasticity occurs because of increased activity of reflex circuits in the spinal cord. Normally there is descending inhibition of these circuits coming from the brain. The interruption or absence of this inhibition results in increased activity of the reflex circuit, resulting in increased muscle tone, clonus (repeated involuntary tremorlike contractions), and spasticity. Increased GABAB activity in the spinal cord decreases this reflex activity.
Spasticity occurs because of increased activity of reflex circuits in the spinal cord. Normally there is descending inhibition of these circuits coming from the brain. The interruption or absence of this inhibition results in increased activity of the reflex circuit, resulting in increased muscle tone, clonus (repeated involuntary tremorlike contractions), and spasticity. Increased GABAB activity in the spinal cord decreases this reflex activity.Side Effects
 The GABAB receptor is an important component of both spinal cord and brain neurotransmission; therefore the side effects are primarily related to brain and spinal cord dysfunction
The GABAB receptor is an important component of both spinal cord and brain neurotransmission; therefore the side effects are primarily related to brain and spinal cord dysfunction Urinary: dysuria (pain on urination), enuresis (incontinence), hematuria (blood in urine), nocturia (night urination), urinary retention (inability to empty bladder), impotence, and inability to ejaculate. Bladder function is abnormal in patients with spinal cord injuries; the urinary tract is under control of the autonomic nervous system (ANS), which is usually impaired in patients with spinal cord dysfunction.
Urinary: dysuria (pain on urination), enuresis (incontinence), hematuria (blood in urine), nocturia (night urination), urinary retention (inability to empty bladder), impotence, and inability to ejaculate. Bladder function is abnormal in patients with spinal cord injuries; the urinary tract is under control of the autonomic nervous system (ANS), which is usually impaired in patients with spinal cord dysfunction.Important Notes
 Avoid abrupt withdrawal of the drug; abrupt withdrawal of intrathecal baclofen has resulted in severe spasticity:
Avoid abrupt withdrawal of the drug; abrupt withdrawal of intrathecal baclofen has resulted in severe spasticity:
 Severe overdose of baclofen can mimic brain death: loss of brainstem reflexes (pupillary changes in response to light, corneal reflexes, gag reflex, cough reflex), apnea, loss of motor responses to painful stimuli.
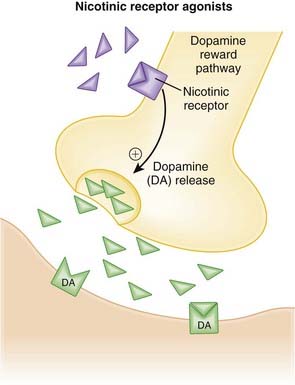
Severe overdose of baclofen can mimic brain death: loss of brainstem reflexes (pupillary changes in response to light, corneal reflexes, gag reflex, cough reflex), apnea, loss of motor responses to painful stimuli. Baclofen has been recently investigated for treatment of addiction, including cigarettes, alcohol, and cocaine. The basis for use in this indication is that GABAB can blunt the dopamine surge in the ventral striatum, which is an important mediator of the reward pathways, and can thus blunt the experienced reward that occurs with drug use.
Baclofen has been recently investigated for treatment of addiction, including cigarettes, alcohol, and cocaine. The basis for use in this indication is that GABAB can blunt the dopamine surge in the ventral striatum, which is an important mediator of the reward pathways, and can thus blunt the experienced reward that occurs with drug use.Nondepolarizing Neuromuscular Blockers
MOA (Mechanism of Action)
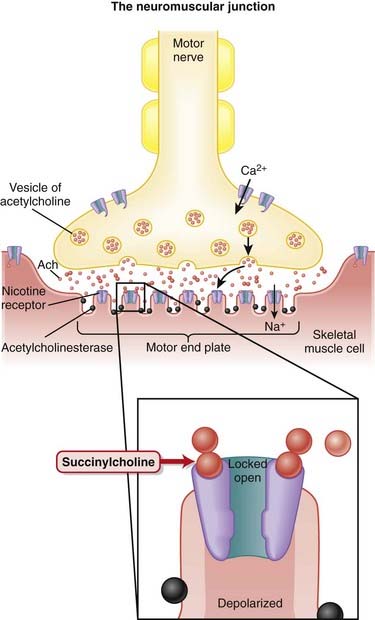
 Voluntary skeletal muscle contraction occurs when a motor neuron is depolarized. The distal end of the motor neuron is part of the NMJ, which is the anatomic connection between the neuron and muscle. The presynaptic membrane is the motor nerve, and the postsynaptic membrane is the motor end plate of the muscle cell.
Voluntary skeletal muscle contraction occurs when a motor neuron is depolarized. The distal end of the motor neuron is part of the NMJ, which is the anatomic connection between the neuron and muscle. The presynaptic membrane is the motor nerve, and the postsynaptic membrane is the motor end plate of the muscle cell. The depolarizing motor neuron releases acetylcholine, and the ACh crosses the synapse and binds to nicotinic ACh receptors on the muscle cell. The binding of ACh to the motor end plate induces small mini-depolarizations. When enough mini-depolarizations occur, a full action potential is created in the muscle cell, which results in an increase in intracellular calcium levels and subsequent actin-myosin interactions, resulting in contraction (Figure 21-6).
The depolarizing motor neuron releases acetylcholine, and the ACh crosses the synapse and binds to nicotinic ACh receptors on the muscle cell. The binding of ACh to the motor end plate induces small mini-depolarizations. When enough mini-depolarizations occur, a full action potential is created in the muscle cell, which results in an increase in intracellular calcium levels and subsequent actin-myosin interactions, resulting in contraction (Figure 21-6). NMJ blockers bind the nicotinic ACh receptor and competitively antagonize ACh, thereby preventing the signal from the neuron to be communicated to the muscle. These drugs do not cause any depolarization of the muscle when they bind; therefore they are referred to as nondepolarizing. This is in contrast to the other class of paralytics, which are depolarizing.
NMJ blockers bind the nicotinic ACh receptor and competitively antagonize ACh, thereby preventing the signal from the neuron to be communicated to the muscle. These drugs do not cause any depolarization of the muscle when they bind; therefore they are referred to as nondepolarizing. This is in contrast to the other class of paralytics, which are depolarizing. Neuromuscular blockade can be partial or complete. If partial, then the strength of muscle contraction is reduced.
Neuromuscular blockade can be partial or complete. If partial, then the strength of muscle contraction is reduced. All skeletal muscles become paralyzed, including muscles for breathing, speech, and eyelid movement and all other skeletal muscle. Smooth muscles and cardiac muscle are not paralyzed because contraction is not dependent on the same neuromuscular transmission. Therefore the heart does not stop beating and smooth muscle function such as pupillary reflexes to light and gastrointestinal motility are not affected.
All skeletal muscles become paralyzed, including muscles for breathing, speech, and eyelid movement and all other skeletal muscle. Smooth muscles and cardiac muscle are not paralyzed because contraction is not dependent on the same neuromuscular transmission. Therefore the heart does not stop beating and smooth muscle function such as pupillary reflexes to light and gastrointestinal motility are not affected. Paralytics do not affect level of consciousness; it is very difficult to know the exact level of consciousness of a person who is paralyzed, and therefore unconsciousness must be achieved before a patient is paralyzed.
Paralytics do not affect level of consciousness; it is very difficult to know the exact level of consciousness of a person who is paralyzed, and therefore unconsciousness must be achieved before a patient is paralyzed.Reversal of Blockade
 If the patient remains paralyzed for longer than desired, then reversal medication can be administered to facilitate return of muscle strength.
If the patient remains paralyzed for longer than desired, then reversal medication can be administered to facilitate return of muscle strength. Because these drugs are all competitive antagonists of ACh, increasing the concentration of ACh in the synaptic cleft will result in more ACh binding to ACh receptors on the motor end plate, and thus strength will be restored.
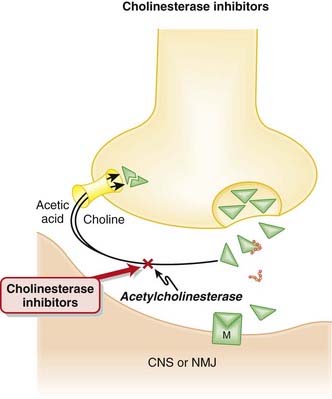
Because these drugs are all competitive antagonists of ACh, increasing the concentration of ACh in the synaptic cleft will result in more ACh binding to ACh receptors on the motor end plate, and thus strength will be restored. Cholinesterase is the enzyme that breaks down ACh. Anticholinesterases are the drugs that inhibit cholinesterase, resulting in increased levels of ACh everywhere in the body.
Cholinesterase is the enzyme that breaks down ACh. Anticholinesterases are the drugs that inhibit cholinesterase, resulting in increased levels of ACh everywhere in the body.
 Increased ACh is desirable only in the motor end plate. ACh, however, will stimulate all muscarinic and nicotinic receptors in the body and can lead to the following cholinergic side effects (mediated by muscarinic receptors):
Increased ACh is desirable only in the motor end plate. ACh, however, will stimulate all muscarinic and nicotinic receptors in the body and can lead to the following cholinergic side effects (mediated by muscarinic receptors):
 Blocking the muscarinic receptors with an anticholinergic drug such as atropine or glycopyrrolate will reduce the incidence of these muscarinic side effects without influencing the effects on nicotinic receptors. Anticholinergic drugs are therefore routinely administered with anticholinesterase drugs.
Blocking the muscarinic receptors with an anticholinergic drug such as atropine or glycopyrrolate will reduce the incidence of these muscarinic side effects without influencing the effects on nicotinic receptors. Anticholinergic drugs are therefore routinely administered with anticholinesterase drugs.Pharmacokinetics
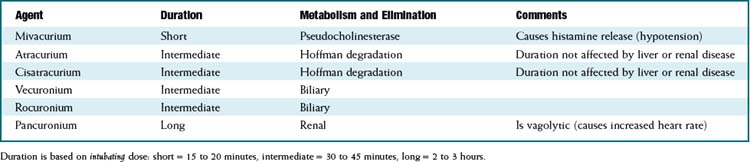
 There are important differences in duration of action and method of metabolism among the different drugs in this class. See Table 21-4.
There are important differences in duration of action and method of metabolism among the different drugs in this class. See Table 21-4.
 Hoffman degradation is simply spontaneous breakdown of the molecule at physiologic pH and temperature. This process occurs despite renal or hepatic failure, and therefore the duration of action of atracurium and cisatracurium is essentially unchanged in patients with renal or hepatic impairment.
Hoffman degradation is simply spontaneous breakdown of the molecule at physiologic pH and temperature. This process occurs despite renal or hepatic failure, and therefore the duration of action of atracurium and cisatracurium is essentially unchanged in patients with renal or hepatic impairment. Decreased metabolism or elimination results in prolonged duration of action for the drug. This is undesirable when the drug is being used for surgery and the surgery is finished but the patient is still paralyzed; the anesthesiologist must then wait before waking the patient. Reversal medications can be given, but if the degree of paralysis is too high, then full reversal cannot be achieved.
Decreased metabolism or elimination results in prolonged duration of action for the drug. This is undesirable when the drug is being used for surgery and the surgery is finished but the patient is still paralyzed; the anesthesiologist must then wait before waking the patient. Reversal medications can be given, but if the degree of paralysis is too high, then full reversal cannot be achieved.Important Notes
 Potency and time to onset: Onset time is determined by the dose administered. For a given drug, giving a larger dose will result in a faster onset of paralysis. For drugs that are more potent (e.g., pancuronium is the most potent), a smaller dose is required for paralysis, but because a smaller dose is required, more potent drugs have a slower onset of action.
Potency and time to onset: Onset time is determined by the dose administered. For a given drug, giving a larger dose will result in a faster onset of paralysis. For drugs that are more potent (e.g., pancuronium is the most potent), a smaller dose is required for paralysis, but because a smaller dose is required, more potent drugs have a slower onset of action. Potency of muscle relaxants is measured using the effective dose in 95% of the population (ED95). For each drug, the ED95 dose is different. As a general rule, an intubating dose (probably the most common indication for paralyzing drugs) is twice the ED95.
Potency of muscle relaxants is measured using the effective dose in 95% of the population (ED95). For each drug, the ED95 dose is different. As a general rule, an intubating dose (probably the most common indication for paralyzing drugs) is twice the ED95. The degree of paralysis can be measured with a small battery-powered device called a nerve stimulator. It essentially delivers a small electric shock. Electrodes (usually just electrocardiographic patches) are applied on top of the motor nerve of interest (usually the ulnar nerve at the wrist or the facial nerve at the temple). When the electrical shock is applied, a muscle that is not paralyzed will vigorously contract; a partially paralyzed muscle will demonstrate a small twitch, and a fully paralyzed muscle will not contract at all.
The degree of paralysis can be measured with a small battery-powered device called a nerve stimulator. It essentially delivers a small electric shock. Electrodes (usually just electrocardiographic patches) are applied on top of the motor nerve of interest (usually the ulnar nerve at the wrist or the facial nerve at the temple). When the electrical shock is applied, a muscle that is not paralyzed will vigorously contract; a partially paralyzed muscle will demonstrate a small twitch, and a fully paralyzed muscle will not contract at all.
 If four electrical shocks are applied in succession, the test is called a train of four. This is important because each successive shock will release a slightly smaller amount of ACh from the presynaptic nerve; because the drugs are competitive antagonists, the partially blocked muscle will demonstrate progressively smaller contractions, a phenomenon called fade. A patient can have zero fade when as many as 50% of the nicotinic receptors are still occupied; therefore four full contractions with the nerve stimulator TOF test does not guarantee that the patient will have 100% strength.
If four electrical shocks are applied in succession, the test is called a train of four. This is important because each successive shock will release a slightly smaller amount of ACh from the presynaptic nerve; because the drugs are competitive antagonists, the partially blocked muscle will demonstrate progressively smaller contractions, a phenomenon called fade. A patient can have zero fade when as many as 50% of the nicotinic receptors are still occupied; therefore four full contractions with the nerve stimulator TOF test does not guarantee that the patient will have 100% strength.Advanced
 Nondepolarizing muscle relaxant blockade is prolonged by inhaled anesthetics. This is relevant for anesthesiologists because inhaled anesthetics are very commonly coadministered with muscle relaxants.
Nondepolarizing muscle relaxant blockade is prolonged by inhaled anesthetics. This is relevant for anesthesiologists because inhaled anesthetics are very commonly coadministered with muscle relaxants. During a long surgery a long-acting muscle relaxant might be used. However, near the end of surgery the anesthesiologist might want a shorter-acting, more titratable drug. However, mixing drugs has an unexpected synergistic effect: one would expect that converting from a long-duration to a short-duration drug would result in a shorter duration of action, but, in fact, in combination the two drugs frequently potentiate each other and the result can be a much longer duration of block than would have been expected from either drug. Mixing nondepolarizing drugs is not recommended.
During a long surgery a long-acting muscle relaxant might be used. However, near the end of surgery the anesthesiologist might want a shorter-acting, more titratable drug. However, mixing drugs has an unexpected synergistic effect: one would expect that converting from a long-duration to a short-duration drug would result in a shorter duration of action, but, in fact, in combination the two drugs frequently potentiate each other and the result can be a much longer duration of block than would have been expected from either drug. Mixing nondepolarizing drugs is not recommended.FYI
 Curare is the historical prototype of nondepolarization neuromuscular blockers, but it is no longer used clinically. Curare (also called D-tubocurare) was the first paralytic used in anesthesia, but it has been replaced by newer agents. It was introduced to anesthesia around 1940. It was discovered in South America and was first used in poison arrows for hunting. It is harvested from the plant Strychnos toxifera. The toxin strychnine is also from this genus of plant (but from a different species).
Curare is the historical prototype of nondepolarization neuromuscular blockers, but it is no longer used clinically. Curare (also called D-tubocurare) was the first paralytic used in anesthesia, but it has been replaced by newer agents. It was introduced to anesthesia around 1940. It was discovered in South America and was first used in poison arrows for hunting. It is harvested from the plant Strychnos toxifera. The toxin strychnine is also from this genus of plant (but from a different species). Take care not to confuse the following terms, which are polar opposites of each other: anticholinergic and anticholinesterase. The former blocks muscarinic activity, whereas the latter increases both muscarinic and nicotinic receptor activity.
Take care not to confuse the following terms, which are polar opposites of each other: anticholinergic and anticholinesterase. The former blocks muscarinic activity, whereas the latter increases both muscarinic and nicotinic receptor activity. Drugs that are GABA-mimetic (they increase the action of the inhibitory neurotransmitter GABA) are also muscle relaxants. However, these drugs are not paralytic drugs. They act in the brain or spinal cord and reduce afferent transmission to the muscle. Examples of these drugs include benzodiazepines (GABAA enhancers), baclofen (GABAB enhancers), cyclobenzaprine, and methocarbamol.
Drugs that are GABA-mimetic (they increase the action of the inhibitory neurotransmitter GABA) are also muscle relaxants. However, these drugs are not paralytic drugs. They act in the brain or spinal cord and reduce afferent transmission to the muscle. Examples of these drugs include benzodiazepines (GABAA enhancers), baclofen (GABAB enhancers), cyclobenzaprine, and methocarbamol.Depolarizing Neuromuscular Blockers
Description
Depolarizing neuromuscular blockers are paralyzing drugs also known as muscle relaxants.
MOA (Mechanism of Action)
 Voluntary skeletal muscle contraction occurs when a motor neuron is depolarized. The distal end of the motor neuron is part of the neuromuscular junction (NMJ), which is the anatomic connection between the neuron and muscle. The presynaptic membrane is the motor nerve, and the postsynaptic membrane is the motor end plate of the muscle cell.
Voluntary skeletal muscle contraction occurs when a motor neuron is depolarized. The distal end of the motor neuron is part of the neuromuscular junction (NMJ), which is the anatomic connection between the neuron and muscle. The presynaptic membrane is the motor nerve, and the postsynaptic membrane is the motor end plate of the muscle cell. The depolarizing motor neuron releases acetylcholine (ACh), which crosses the synapse and binds to nicotinic ACh receptors on the muscle cell. The binding of ACh to the motor end plate induces small mini-depolarizations via opening sodium channels. When enough mini-depolarizations occur, a full action potential is created in the muscle cell, which results in an increase in intracellular calcium levels and subsequent actin-myosin interactions, resulting in contraction (Figure 21-7).
The depolarizing motor neuron releases acetylcholine (ACh), which crosses the synapse and binds to nicotinic ACh receptors on the muscle cell. The binding of ACh to the motor end plate induces small mini-depolarizations via opening sodium channels. When enough mini-depolarizations occur, a full action potential is created in the muscle cell, which results in an increase in intracellular calcium levels and subsequent actin-myosin interactions, resulting in contraction (Figure 21-7). Succinylcholine irreversibly binds the nicotinic ACh receptor. Succinylcholine causes opening of the sodium channel controlled by the ACh receptor and a subsequent short-lived myofibril contraction (fasciculation); therefore it is referred to as depolarizing. However, because succinylcholine is not metabolized by cholinesterase, it remains bound to the nicotinic receptor for a longer duration. The sodium channel is locked in the open position, and the muscle stays in a depolarized state. However, the intracellular calcium is taken back up by the sarcoplasmic reticulum after its initial release, lowering the intracellular calcium level, and the actin-myosin coupling dissociates. For as long as the myocyte remains depolarized by the open sodium channel, it cannot accept a new action potential, and the intracellular calcium levels remain low.
Succinylcholine irreversibly binds the nicotinic ACh receptor. Succinylcholine causes opening of the sodium channel controlled by the ACh receptor and a subsequent short-lived myofibril contraction (fasciculation); therefore it is referred to as depolarizing. However, because succinylcholine is not metabolized by cholinesterase, it remains bound to the nicotinic receptor for a longer duration. The sodium channel is locked in the open position, and the muscle stays in a depolarized state. However, the intracellular calcium is taken back up by the sarcoplasmic reticulum after its initial release, lowering the intracellular calcium level, and the actin-myosin coupling dissociates. For as long as the myocyte remains depolarized by the open sodium channel, it cannot accept a new action potential, and the intracellular calcium levels remain low.
 Neuromuscular blockade can be partial or complete. If partial, then the strength of muscle contraction is reduced.
Neuromuscular blockade can be partial or complete. If partial, then the strength of muscle contraction is reduced. All skeletal muscles become paralyzed; this includes muscles for breathing, speech, and eyelid movement and all other skeletal muscles. Smooth muscles and cardiac muscle are not paralyzed. Therefore the heart does not stop beating and smooth muscle function such as pupillary reflexes to light and gastrointestinal motility are not affected.
All skeletal muscles become paralyzed; this includes muscles for breathing, speech, and eyelid movement and all other skeletal muscles. Smooth muscles and cardiac muscle are not paralyzed. Therefore the heart does not stop beating and smooth muscle function such as pupillary reflexes to light and gastrointestinal motility are not affected.Pharmacokinetics
Indications
 Electroconvulsive therapy (ECT): With this treatment, an electric shock is administered to the brain, which induces a seizure. The paralytic is of benefit so that the limbs do not demonstrate vigorous tonic-clonic activity, which could cause injury. The patient is given a short-acting anesthetic before both the paralysis and the electric shock.
Electroconvulsive therapy (ECT): With this treatment, an electric shock is administered to the brain, which induces a seizure. The paralytic is of benefit so that the limbs do not demonstrate vigorous tonic-clonic activity, which could cause injury. The patient is given a short-acting anesthetic before both the paralysis and the electric shock.Contraindications
 Risk of hyperkalemia:
Risk of hyperkalemia:
 Hyperresponders: In some patients there are extrajunctional (outside the NMJ) nicotinic receptors on the muscle that ACh can bind to; this binding can cause a greater-than-normal release of potassium.
Hyperresponders: In some patients there are extrajunctional (outside the NMJ) nicotinic receptors on the muscle that ACh can bind to; this binding can cause a greater-than-normal release of potassium.
 Preexisting hyperkalemia: In patients with renal failure, potassium levels are often elevated (and would be further elevated 0.5 to 1.0 mEq/L with succinylcholine). However, if the potassium level is normal, patients with renal failure are normal responders and therefore it is safe to administer succinylcholine.
Preexisting hyperkalemia: In patients with renal failure, potassium levels are often elevated (and would be further elevated 0.5 to 1.0 mEq/L with succinylcholine). However, if the potassium level is normal, patients with renal failure are normal responders and therefore it is safe to administer succinylcholine. Myotonic dystrophy: In these patients the depolarizing effect of succinylcholine is dramatically prolonged and results in widespread increased tone in all muscles. Patients who have this reaction have muscles of respiration also contracted, and therefore it is very difficult to ventilate these patients. Succinylcholine is absolutely contraindicated in these patients.
Myotonic dystrophy: In these patients the depolarizing effect of succinylcholine is dramatically prolonged and results in widespread increased tone in all muscles. Patients who have this reaction have muscles of respiration also contracted, and therefore it is very difficult to ventilate these patients. Succinylcholine is absolutely contraindicated in these patients.Side Effects
 Hyperkalemia: Intracellular potassium is released from muscle cells on depolarization, leading to a small rise in serum potassium.
Hyperkalemia: Intracellular potassium is released from muscle cells on depolarization, leading to a small rise in serum potassium. Bradycardia: Because the chemical structure of succinylcholine is essentially two ACh molecules bound together end to end, it is not surprising that it can mimic ACh at muscarinic receptors. Although it does not produce a widespread muscarinic response, bradycardia and asystole have occurred. This effect is more pronounced in children, and atropine is sometimes coadministered before succinylcholine to prevent bradycardia.
Bradycardia: Because the chemical structure of succinylcholine is essentially two ACh molecules bound together end to end, it is not surprising that it can mimic ACh at muscarinic receptors. Although it does not produce a widespread muscarinic response, bradycardia and asystole have occurred. This effect is more pronounced in children, and atropine is sometimes coadministered before succinylcholine to prevent bradycardia.Important Notes
 Because succinylcholine binds irreversibly, there are important differences between it and the nondepolarizing drugs, which bind competitively:
Because succinylcholine binds irreversibly, there are important differences between it and the nondepolarizing drugs, which bind competitively:
 Increasing the ACh concentration in the synaptic cleft via administration of anticholinesterase drugs is not effective in reversing the block.
Increasing the ACh concentration in the synaptic cleft via administration of anticholinesterase drugs is not effective in reversing the block. The train-of-four (TOF) test (see the discussion of nondepolarizing NMJ blockers) exhibits different results with succinylcholine versus nondepolarizers:
The train-of-four (TOF) test (see the discussion of nondepolarizing NMJ blockers) exhibits different results with succinylcholine versus nondepolarizers:
• With each stimulation, the amount of ACh released is slightly less than with the previous stimulation; because succinylcholine is not competitively bound, the concentration of ACh in the synaptic cleft does not influence the strength of contraction. The strength of contraction is solely dependent on nicotinic receptor occupancy. Therefore there is no fade when there is partial blockade by succinylcholine, which is in contrast to nondepolarizers.
 Anticholinesterase drugs also inhibit pseudocholinesterase, the enzyme that breaks down succinylcholine, so in fact both ACh and succinylcholine levels would be increased in the synaptic cleft. This is a second reason why paralytic reversal drugs are not effective with succinylcholine.
Anticholinesterase drugs also inhibit pseudocholinesterase, the enzyme that breaks down succinylcholine, so in fact both ACh and succinylcholine levels would be increased in the synaptic cleft. This is a second reason why paralytic reversal drugs are not effective with succinylcholine.
 Phase 1 versus phase 2 blockade: Phase 1 blockade is the TOF stimulation test result whereby no fade (all contractions are equal in size) is demonstrated. Phase 2 blockade occurs when repeated doses or infusions of succinylcholine are administered. Phase 2 blockade does demonstrate fade (the 4 contractions get smaller and smaller) just like nondepolarizing paralytics. However, it is no longer clinically common to administer repeated doses or infusions of succinylcholine, so phase 2 blockade with succinylcholine is not commonly seen.
Phase 1 versus phase 2 blockade: Phase 1 blockade is the TOF stimulation test result whereby no fade (all contractions are equal in size) is demonstrated. Phase 2 blockade occurs when repeated doses or infusions of succinylcholine are administered. Phase 2 blockade does demonstrate fade (the 4 contractions get smaller and smaller) just like nondepolarizing paralytics. However, it is no longer clinically common to administer repeated doses or infusions of succinylcholine, so phase 2 blockade with succinylcholine is not commonly seen. Potency of muscle relaxants is measured using the effective dose in 95% of the population (ED95). For each drug, the ED95 dose is different. As a general rule, an intubating dose (probably the most common indication for paralyzing drugs) is twice the ED95.
Potency of muscle relaxants is measured using the effective dose in 95% of the population (ED95). For each drug, the ED95 dose is different. As a general rule, an intubating dose (probably the most common indication for paralyzing drugs) is twice the ED95.Advanced
 In attempts to reduce muscle pain after succinylcholine-induced fasciculations, a defasciculating dose of a nondepolarizing paralytic (such as rocuronium or vecuronium) is given 5 minutes beforehand. This is only a very small dose so as not to produce paralysis in the awake patient; usually about 5% to 10% of the ED95 is used (
In attempts to reduce muscle pain after succinylcholine-induced fasciculations, a defasciculating dose of a nondepolarizing paralytic (such as rocuronium or vecuronium) is given 5 minutes beforehand. This is only a very small dose so as not to produce paralysis in the awake patient; usually about 5% to 10% of the ED95 is used ( to
to  of the intubating dose). Lidocaine has also been used to reduce postsuccinylcholine muscle pain.
of the intubating dose). Lidocaine has also been used to reduce postsuccinylcholine muscle pain. The paradoxic paralysis seen with an overdose of reversal (anticholinesterase) drugs results from the myocytes being continuously saturated with too much ACh, resulting in the sodium channels remaining in the open state, mimicking the action of succinylcholine. Organophosphates (insecticides and chemical warfare agents such as sarin) are anticholinesterases, and organophosphate poisoning results in a cholinergic syndrome (muscarinic side effects) and, in addition, paralysis.
The paradoxic paralysis seen with an overdose of reversal (anticholinesterase) drugs results from the myocytes being continuously saturated with too much ACh, resulting in the sodium channels remaining in the open state, mimicking the action of succinylcholine. Organophosphates (insecticides and chemical warfare agents such as sarin) are anticholinesterases, and organophosphate poisoning results in a cholinergic syndrome (muscarinic side effects) and, in addition, paralysis. Probably the only reasons that succinylcholine remains in clinical practice despite the prominent side effects that are not present in nondepolarizing paralytics are its short onset of action and short duration of action. Rapacuronium, a fast-acting and ultra-short–duration nondepolarizing paralytic threatened to put an end to succinylcholine use, but it was withdrawn from the market in 2001 because of risk of bronchospasm.
Probably the only reasons that succinylcholine remains in clinical practice despite the prominent side effects that are not present in nondepolarizing paralytics are its short onset of action and short duration of action. Rapacuronium, a fast-acting and ultra-short–duration nondepolarizing paralytic threatened to put an end to succinylcholine use, but it was withdrawn from the market in 2001 because of risk of bronchospasm.Nicotine
MOA (Mechanism of Action)
 There are several nicotine receptors, composed of alpha (α) and beta (β) subunits. The various α subunits and β subunits all appear in different combinations throughout the body. The most common subunits are α4β2, α3β4, and α7.
There are several nicotine receptors, composed of alpha (α) and beta (β) subunits. The various α subunits and β subunits all appear in different combinations throughout the body. The most common subunits are α4β2, α3β4, and α7. The α4β2 subunit is the most common nicotinic receptor in the brain and is believed to be the subunit most responsible for nicotine addiction.
The α4β2 subunit is the most common nicotinic receptor in the brain and is believed to be the subunit most responsible for nicotine addiction. Nicotine binds to presynaptic nicotinic α4β2 receptors, which in turn leads to the release of dopamine into the nucleus accumbens, the “reward” pathway of the brain (Figure 21-8).
Nicotine binds to presynaptic nicotinic α4β2 receptors, which in turn leads to the release of dopamine into the nucleus accumbens, the “reward” pathway of the brain (Figure 21-8). In addition to dopamine, binding of nicotine to α4β2 receptors leads to release of a variety of other neurotransmitters such as acetylcholine, norepinephrine, serotonin, GABA, endorphins, and glutamate. Many of these neurotransmitters are associated with mood, whereas others (GABA, endorphins) tend to have a calming or euphoric effect. Chronic cigarette smoking also appears to reduce levels of enzymes such as monoamine oxidase (MAO) that break down many of these neurotransmitters, including dopamine.
In addition to dopamine, binding of nicotine to α4β2 receptors leads to release of a variety of other neurotransmitters such as acetylcholine, norepinephrine, serotonin, GABA, endorphins, and glutamate. Many of these neurotransmitters are associated with mood, whereas others (GABA, endorphins) tend to have a calming or euphoric effect. Chronic cigarette smoking also appears to reduce levels of enzymes such as monoamine oxidase (MAO) that break down many of these neurotransmitters, including dopamine. Chronic nicotine use leads to up-regulation of α4β2 receptors. Although this may seem counterintuitive, the up-regulation appears to be in response to receptor desensitization.
Chronic nicotine use leads to up-regulation of α4β2 receptors. Although this may seem counterintuitive, the up-regulation appears to be in response to receptor desensitization. Aside from stimulation of the reward pathway, nicotine acts on other nicotinic receptors in other areas of the body. For example the α3β4 subunit likely mediates the cardiovascular effects of nicotine. Nicotine acts as a vasoconstrictor, increases heart rate and contractility, and may cause endothelial dysfunction.
Aside from stimulation of the reward pathway, nicotine acts on other nicotinic receptors in other areas of the body. For example the α3β4 subunit likely mediates the cardiovascular effects of nicotine. Nicotine acts as a vasoconstrictor, increases heart rate and contractility, and may cause endothelial dysfunction.Pharmacokinetics
 Nicotine is rapidly absorbed, has a rapid onset of action when delivered by the inhalational route (cigarette smoking), and has a short elimination half-life (1 to 2 hours). The quick onset of action and the short half-life both contribute to the addictive properties of smoking.
Nicotine is rapidly absorbed, has a rapid onset of action when delivered by the inhalational route (cigarette smoking), and has a short elimination half-life (1 to 2 hours). The quick onset of action and the short half-life both contribute to the addictive properties of smoking. Oral absorption of nicotine is poor, and nicotine acts as a gastric irritant, so nicotine is not currently available for oral administration.
Oral absorption of nicotine is poor, and nicotine acts as a gastric irritant, so nicotine is not currently available for oral administration.Side Effects
Full Agonists
Local Effects
Partial Agonists
 Nausea: The mechanism has not been described; however, other agents that elevate dopamine levels in the CNS also promote nausea.
Nausea: The mechanism has not been described; however, other agents that elevate dopamine levels in the CNS also promote nausea.Serious
 Neuropsychiatric: Events such as depression, agitation, hostility, and suicidality have been observed in the postmarketing period (see Important Notes for further details). The mechanism is unknown; however, agents that elevate dopamine levels in the CNS have been known to produce some of the same side effects.
Neuropsychiatric: Events such as depression, agitation, hostility, and suicidality have been observed in the postmarketing period (see Important Notes for further details). The mechanism is unknown; however, agents that elevate dopamine levels in the CNS have been known to produce some of the same side effects.Important Notes
 Despite the acute effects of nicotine on the cardiovascular system (see Mechanism of Action), there is no clear evidence that nicotine itself increases risk of cardiovascular events. Recent concerns have arisen about an increased risk of mortality after coronary artery bypass surgery; however, these findings need to be confirmed by well-designed prospective studies. Currently the risk-to-benefit ratio of nicotine replacement in patients with cardiovascular disease is unknown.
Despite the acute effects of nicotine on the cardiovascular system (see Mechanism of Action), there is no clear evidence that nicotine itself increases risk of cardiovascular events. Recent concerns have arisen about an increased risk of mortality after coronary artery bypass surgery; however, these findings need to be confirmed by well-designed prospective studies. Currently the risk-to-benefit ratio of nicotine replacement in patients with cardiovascular disease is unknown. Nicotine also inhibits apoptosis and promotes angiogenesis, both of which would be expected to promote tumor growth. However, there is also no clear evidence that chronic use of nicotine replacement medication has a carcinogenic effect.
Nicotine also inhibits apoptosis and promotes angiogenesis, both of which would be expected to promote tumor growth. However, there is also no clear evidence that chronic use of nicotine replacement medication has a carcinogenic effect. A common dilemma is what to do with pregnant patients who wish to use nicotine replacement therapies to quit smoking and maintain abstinence. This is once again a risk-to-benefit assessment. Ideally a patient would abstain from smoking without any medical interventions during pregnancy, but if this is not possible the potential effects of multiple toxins in cigarette smoke must be weighed against the risk posed by nicotine itself.
A common dilemma is what to do with pregnant patients who wish to use nicotine replacement therapies to quit smoking and maintain abstinence. This is once again a risk-to-benefit assessment. Ideally a patient would abstain from smoking without any medical interventions during pregnancy, but if this is not possible the potential effects of multiple toxins in cigarette smoke must be weighed against the risk posed by nicotine itself. High doses of nicotine are toxic, and this is particularly evident in children. Used transdermal patches typically contain significant amounts of nicotine, and patients are advised to dispose of them carefully so they are not accidently accessible to children and pets.
High doses of nicotine are toxic, and this is particularly evident in children. Used transdermal patches typically contain significant amounts of nicotine, and patients are advised to dispose of them carefully so they are not accidently accessible to children and pets. Reports of serious neuropsychiatric events with varenicline have emerged in the postmarketing period. These include depression, agitation, hostility, and suicidal thoughts or attempts. The interpretation of these findings is complicated by their similarity to the classic side effects of nicotine withdrawal.
Reports of serious neuropsychiatric events with varenicline have emerged in the postmarketing period. These include depression, agitation, hostility, and suicidal thoughts or attempts. The interpretation of these findings is complicated by their similarity to the classic side effects of nicotine withdrawal. Given their respective mechanisms, it is not expected that the combination of varenicline along with nicotine replacement therapies would provide any additional advantages over using either agent alone. Providing additional nicotine would simply increase competition for nicotinic receptors, reducing the effects of varenicline.
Given their respective mechanisms, it is not expected that the combination of varenicline along with nicotine replacement therapies would provide any additional advantages over using either agent alone. Providing additional nicotine would simply increase competition for nicotinic receptors, reducing the effects of varenicline.Advanced
Pharmacogenetics
 The rate of nicotine metabolism is determined in part by polymorphisms in the genes for CYP2A6 as well as uridine 5′-diphosphate (UDP)–glucuronosyltransferases (UGTs), a secondary pathway of elimination. These differences are believed to explain why women metabolize nicotine more rapidly than men and why patients of Caucasian and Hispanic descent metabolize nicotine more rapidly than patients of Asian and Black descent.
The rate of nicotine metabolism is determined in part by polymorphisms in the genes for CYP2A6 as well as uridine 5′-diphosphate (UDP)–glucuronosyltransferases (UGTs), a secondary pathway of elimination. These differences are believed to explain why women metabolize nicotine more rapidly than men and why patients of Caucasian and Hispanic descent metabolize nicotine more rapidly than patients of Asian and Black descent.Evidence
Nicotine Replacement Therapies for Smoking Cessation
 A 2008 Cochrane review (111 trials, N > 40,000 participants) compared nicotine replacement therapies to other interventions and placebo as an aid to smoking cessation. Overall, nicotine replacement therapies performed better than control for improving abstinence rates (relative risk [RR] 1.58). Abstinence rates for individual interventions varied slightly: gum, RR 1.43; patch, RR 1.66; inhaler, RR 1.90; nasal spray, RR 2.02; and tablets or lozenges, RR 2.00. Only one study compared quit rates between nicotine replacement and other active comparators (bupropion), and quit rates were lower for the nicotine patch in this study, compared with bupropion.
A 2008 Cochrane review (111 trials, N > 40,000 participants) compared nicotine replacement therapies to other interventions and placebo as an aid to smoking cessation. Overall, nicotine replacement therapies performed better than control for improving abstinence rates (relative risk [RR] 1.58). Abstinence rates for individual interventions varied slightly: gum, RR 1.43; patch, RR 1.66; inhaler, RR 1.90; nasal spray, RR 2.02; and tablets or lozenges, RR 2.00. Only one study compared quit rates between nicotine replacement and other active comparators (bupropion), and quit rates were lower for the nicotine patch in this study, compared with bupropion.Partial Agonists versus Placebo or Active Comparators for Smoking Cessation and Relapse Prevention
 A 2008 Cochrane review (nine trials, 7267 participants) compared varenicline with either placebo or bupropion for smoking cessation and relapse prevention. The pooled RR for continuous abstinence after 6 months versus placebo was 2.3. Varenicline also performed better than bupropion (RR 1.5) and nicotine replacement therapy (RR 1.3) for continuous abstinence at 12 months.
A 2008 Cochrane review (nine trials, 7267 participants) compared varenicline with either placebo or bupropion for smoking cessation and relapse prevention. The pooled RR for continuous abstinence after 6 months versus placebo was 2.3. Varenicline also performed better than bupropion (RR 1.5) and nicotine replacement therapy (RR 1.3) for continuous abstinence at 12 months.
FYI
 Nicotine vaccines are currently in development. The vaccines would stimulate antibodies to nicotine, which would then form complexes with nicotine and prevent absorption of nicotine across the blood-brain barrier. Early results are promising, although positive responses are typically seen in those with sufficient antibody production.
Nicotine vaccines are currently in development. The vaccines would stimulate antibodies to nicotine, which would then form complexes with nicotine and prevent absorption of nicotine across the blood-brain barrier. Early results are promising, although positive responses are typically seen in those with sufficient antibody production.Dopamine and Dopamine Agonists
MOA (Mechanism of Action)
 Parkinson’s disease is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Dopamine is a catecholamine that is synthesized in these dopaminergic neurons and is released onto two pathways in the striatum that regulate coordinated movement.
Parkinson’s disease is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Dopamine is a catecholamine that is synthesized in these dopaminergic neurons and is released onto two pathways in the striatum that regulate coordinated movement.
 The level of activity of the cells of the globus pallidus interna (GPi) and substantia nigra pars reticulata (SNpr) depends on the balance of input between excitatory glutamate (Glu) (+) and inhibitory GABA (−) (Figure 21-9).
The level of activity of the cells of the globus pallidus interna (GPi) and substantia nigra pars reticulata (SNpr) depends on the balance of input between excitatory glutamate (Glu) (+) and inhibitory GABA (−) (Figure 21-9). When the cells of the GPi and SNpr become more active, this increases release of inhibitory GABA (−) onto the thalamus, which in turn inhibits the release of excitatory Glu (+) onto the cerebral cortex.
When the cells of the GPi and SNpr become more active, this increases release of inhibitory GABA (−) onto the thalamus, which in turn inhibits the release of excitatory Glu (+) onto the cerebral cortex. This reduction in release of Glu (+) onto the cerebral cortex is what leads to inhibition of movement.
This reduction in release of Glu (+) onto the cerebral cortex is what leads to inhibition of movement. Because the level of activity of the GPi and SNpr depends on the input of GABA and Glu from the direct and indirect pathways, these pathways must be in balance for normal movement to be maintained.
Because the level of activity of the GPi and SNpr depends on the input of GABA and Glu from the direct and indirect pathways, these pathways must be in balance for normal movement to be maintained. The balance between these direct and indirect pathways is disrupted in Parkinson’s disease. Low levels of dopamine released onto these receptors leads to greater activation of D2 than D1 receptors, which results in a net inhibitory effect on movement.
The balance between these direct and indirect pathways is disrupted in Parkinson’s disease. Low levels of dopamine released onto these receptors leads to greater activation of D2 than D1 receptors, which results in a net inhibitory effect on movement. A balance also exists between dopamine and acetylcholine in the striatum. When dopamine levels are reduced, this leads to a relative excess of acetylcholine, an imbalance that also interferes with proper movement. That is why some patients are treated with anticholinergics in the early stages of Parkinson’s disease.
A balance also exists between dopamine and acetylcholine in the striatum. When dopamine levels are reduced, this leads to a relative excess of acetylcholine, an imbalance that also interferes with proper movement. That is why some patients are treated with anticholinergics in the early stages of Parkinson’s disease.Pharmacokinetics
 Levodopa is the precursor of dopamine and is readily and completely converted to dopamine by the enzyme dopa decarboxylase (DDC). Levodopa is rapidly absorbed when administered orally, with a short plasma half-life of 1 to 3 hours. Dietary amino acids may compete for absorption sites in the small intestine; therefore administration with meals may delay absorption and reduce peak plasma concentrations.
Levodopa is the precursor of dopamine and is readily and completely converted to dopamine by the enzyme dopa decarboxylase (DDC). Levodopa is rapidly absorbed when administered orally, with a short plasma half-life of 1 to 3 hours. Dietary amino acids may compete for absorption sites in the small intestine; therefore administration with meals may delay absorption and reduce peak plasma concentrations. Once converted, dopamine is then metabolized and inactivated by catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). Another approach to enhancing dopaminergic transmission is to inhibit these enzymes, thereby increasing dopamine levels. These agents, COMT inhibitors and MAO inhibitors, are discussed in Chapters 21 and 23.
Once converted, dopamine is then metabolized and inactivated by catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). Another approach to enhancing dopaminergic transmission is to inhibit these enzymes, thereby increasing dopamine levels. These agents, COMT inhibitors and MAO inhibitors, are discussed in Chapters 21 and 23.Important Notes
 l-Dopa is almost always administered with a peripheral decarboxylase inhibitor. The reason for this is that l-dopa is readily converted to dopamine by decarboxylases before it even reaches the CNS. This dopamine is then converted to inactive metabolites and eliminated, or it acts on peripheral dopamine receptors, resulting in a number of side effects.
l-Dopa is almost always administered with a peripheral decarboxylase inhibitor. The reason for this is that l-dopa is readily converted to dopamine by decarboxylases before it even reaches the CNS. This dopamine is then converted to inactive metabolites and eliminated, or it acts on peripheral dopamine receptors, resulting in a number of side effects. Decarboxylase inhibitors such as carbidopa are not able to cross into the CNS; they therefore allow l-dopa to be converted to dopamine in the CNS but prevent this from occurring before l-dopa reaches the CNS.
Decarboxylase inhibitors such as carbidopa are not able to cross into the CNS; they therefore allow l-dopa to be converted to dopamine in the CNS but prevent this from occurring before l-dopa reaches the CNS. Initially, the therapeutic effects of dopamine appear to extend beyond its relatively short half-life. This is likely a result of the remaining ability of dopaminergic neurons in the substantia nigra to store and release dopamine. However, after this ability is inevitably lost through progression of Parkinson’s disease, doses of l-dopa begin to wear off quickly, in keeping with its short plasma half-life.
Initially, the therapeutic effects of dopamine appear to extend beyond its relatively short half-life. This is likely a result of the remaining ability of dopaminergic neurons in the substantia nigra to store and release dopamine. However, after this ability is inevitably lost through progression of Parkinson’s disease, doses of l-dopa begin to wear off quickly, in keeping with its short plasma half-life. A longstanding debate with l-dopa therapy is over the potential for dopamine itself to accelerate the cell death in the substantia nigra. The metabolism of dopamine produces free radicals, and free radical damage is considered to play a key role in the pathogenesis of Parkinson’s disease. However, a definitive link has yet to be established, and until it is, l-dopa will likely remain the standard of care for management of Parkinson’s disease symptoms.
A longstanding debate with l-dopa therapy is over the potential for dopamine itself to accelerate the cell death in the substantia nigra. The metabolism of dopamine produces free radicals, and free radical damage is considered to play a key role in the pathogenesis of Parkinson’s disease. However, a definitive link has yet to be established, and until it is, l-dopa will likely remain the standard of care for management of Parkinson’s disease symptoms.Evidence
Parkinson’s Disease
Ropinirole versus Bromocriptine
 A 2001 Cochrane review (three trials, N = 482 patients) found that ropinirole and bromocriptine had similar effects in improving off-time and reducing l-dopa dose, without increasing adverse events such as dyskinesia. The authors noted that the three included studies might not have enough power to distinguish between agents. Off-time refers to wearing off of the effects of the drug, severely limiting the mobility of the patient.
A 2001 Cochrane review (three trials, N = 482 patients) found that ropinirole and bromocriptine had similar effects in improving off-time and reducing l-dopa dose, without increasing adverse events such as dyskinesia. The authors noted that the three included studies might not have enough power to distinguish between agents. Off-time refers to wearing off of the effects of the drug, severely limiting the mobility of the patient.Pramipexole versus Placebo
 A 2000 Cochrane review (four trials, N = 669 patients) compared pramipexole with placebo in patients with Parkinson’s disease and long-term complications from l-dopa. Pramipexole reduced off-time, improved motor impairments and disability, and reduced l-dopa dose requirements, but it also increased dyskinetic adverse events.
A 2000 Cochrane review (four trials, N = 669 patients) compared pramipexole with placebo in patients with Parkinson’s disease and long-term complications from l-dopa. Pramipexole reduced off-time, improved motor impairments and disability, and reduced l-dopa dose requirements, but it also increased dyskinetic adverse events.Early Parkinson’s Disease: L-Dopa versus Bromocriptine
 A 2007 Cochrane review examined six trials (N = 850 patients), but the studies were too heterogeneous for a meta-analysis to be conducted. The authors arrived at a qualitative conclusion that bromocriptine may be beneficial in delaying motor complications and dyskinesias, with comparable effects on impairment and disability.
A 2007 Cochrane review examined six trials (N = 850 patients), but the studies were too heterogeneous for a meta-analysis to be conducted. The authors arrived at a qualitative conclusion that bromocriptine may be beneficial in delaying motor complications and dyskinesias, with comparable effects on impairment and disability.FYI
 In the future we will likely discover that the pathophysiology of Parkinson’s disease is much more complicated than described in the Mechanism of Action section and that several other neurotransmitters may be involved, including substance P and dynorphin. The significance of these transmitters in the pathophysiology of Parkinson’s disease is an active area of research.
In the future we will likely discover that the pathophysiology of Parkinson’s disease is much more complicated than described in the Mechanism of Action section and that several other neurotransmitters may be involved, including substance P and dynorphin. The significance of these transmitters in the pathophysiology of Parkinson’s disease is an active area of research. For example, the previously described model would suggest that ideally, dopamine agonists should nonselectively stimulate both D1 and D2 receptors. However, most of the clinically useful dopamine agonists stimulate D2 receptors more than D1 receptors, and some are even considered to be D2 selective agonists.
For example, the previously described model would suggest that ideally, dopamine agonists should nonselectively stimulate both D1 and D2 receptors. However, most of the clinically useful dopamine agonists stimulate D2 receptors more than D1 receptors, and some are even considered to be D2 selective agonists.Catechol-O-Methyl Transferase (COMT) Inhibitors
MOA (Mechanism of Action)
 Parkinson’s disease is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Dopamine is a catecholamine that is synthesized in these dopaminergic neurons and is released onto two pathways in the striatum that regulate coordinated movement.
Parkinson’s disease is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Dopamine is a catecholamine that is synthesized in these dopaminergic neurons and is released onto two pathways in the striatum that regulate coordinated movement.
 The balance between these direct and indirect pathways is disrupted in Parkinson’s disease. Low levels of dopamine released onto these receptors lead to greater activation of D2 than D1 receptors, which results in a net inhibitory effect on movement.
The balance between these direct and indirect pathways is disrupted in Parkinson’s disease. Low levels of dopamine released onto these receptors lead to greater activation of D2 than D1 receptors, which results in a net inhibitory effect on movement. COMT inhibitors increase the amount of dopamine available to the CNS. COMT is one of the two major enzymes involved in the metabolism of catecholamines (epinephrine, norepinephrine, and dopamine). Thus one of the ways COMT inhibitors increase dopamine is by inhibiting its breakdown (Figure 21-10).
COMT inhibitors increase the amount of dopamine available to the CNS. COMT is one of the two major enzymes involved in the metabolism of catecholamines (epinephrine, norepinephrine, and dopamine). Thus one of the ways COMT inhibitors increase dopamine is by inhibiting its breakdown (Figure 21-10). The COMT inhibitors also free up transporters for levodopa:
The COMT inhibitors also free up transporters for levodopa:
Pharmacokinetics
Contraindications
 Concomitant use with nonselective MAOIs: This includes the use of an MAO-A and an MAO-B inhibitor in combination. The MAO pathway becomes the key metabolic route for epinephrine and norepinephrine in the presence of COMT blockade. There should be at least a 2-week washout before initiation of treatment with a COMT inhibitor. Caution should also be exercised in patients on MAO-B selective inhibitors, as these become nonselective at higher doses.
Concomitant use with nonselective MAOIs: This includes the use of an MAO-A and an MAO-B inhibitor in combination. The MAO pathway becomes the key metabolic route for epinephrine and norepinephrine in the presence of COMT blockade. There should be at least a 2-week washout before initiation of treatment with a COMT inhibitor. Caution should also be exercised in patients on MAO-B selective inhibitors, as these become nonselective at higher doses.Side Effects
 Dopamine-related effects include dyskinesia (peak dose), vivid dreams, hallucinations, nausea, hypotension.
Dopamine-related effects include dyskinesia (peak dose), vivid dreams, hallucinations, nausea, hypotension.Evidence
COMT Inhibitors versus Dopamine Therapy in Parkinson’s Disease Patients with Motor Complications on L-Dopa
 A 2004 Cochrane review compared COMT inhibitors (tolcapone) with pergolide (one trial, N = 203 over 12 weeks) and bromocriptine (one trial, N = 146 over 8 weeks). Tolcapone allowed for a greater reduction in l-dopa dose than bromocriptine and was similar to pergolide. Tolcapone produced similar benefits in motor impairment and disability ratings versus both bromocriptine and pergolide. The studies were underpowered to detect statistical differences for these efficacy outcomes, and there were no studies involving entacapone.
A 2004 Cochrane review compared COMT inhibitors (tolcapone) with pergolide (one trial, N = 203 over 12 weeks) and bromocriptine (one trial, N = 146 over 8 weeks). Tolcapone allowed for a greater reduction in l-dopa dose than bromocriptine and was similar to pergolide. Tolcapone produced similar benefits in motor impairment and disability ratings versus both bromocriptine and pergolide. The studies were underpowered to detect statistical differences for these efficacy outcomes, and there were no studies involving entacapone.Cholinesterase Inhibitors
Description
Cholinesterase inhibitors are a collection of agents that increase acetylcholine levels.
MOA (Mechanism of Action)
 Acetylcholine is the main neurotransmitter in the parasympathetic arm of the ANS and a major neurotransmitter in the CNS.
Acetylcholine is the main neurotransmitter in the parasympathetic arm of the ANS and a major neurotransmitter in the CNS. Acetylcholinesterase inhibitors bind to this enzyme, inhibiting its activity and leading to an increase in acetylcholine in the synapse, thus enhancing cholinergic transmission (Figure 21-11).
Acetylcholinesterase inhibitors bind to this enzyme, inhibiting its activity and leading to an increase in acetylcholine in the synapse, thus enhancing cholinergic transmission (Figure 21-11). Alzheimer’s disease is characterized by profound cell death in the brain, with a progressive decline in cognitive function. The death of neurons leads to a reduction in the amount of acetylcholine released into the synapse as well as the amount of postsynaptic receptors on which acetylcholine acts.
Alzheimer’s disease is characterized by profound cell death in the brain, with a progressive decline in cognitive function. The death of neurons leads to a reduction in the amount of acetylcholine released into the synapse as well as the amount of postsynaptic receptors on which acetylcholine acts. The efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease is believed to be a result of the enhanced cholinergic neurotransmission. Essentially, cholinesterase inhibitors maximize the remaining pool of acetylcholine by inhibiting its breakdown, thus reducing the decline in cognition.
The efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease is believed to be a result of the enhanced cholinergic neurotransmission. Essentially, cholinesterase inhibitors maximize the remaining pool of acetylcholine by inhibiting its breakdown, thus reducing the decline in cognition. Cholinesterase inhibitors are not believed to be disease-modifying agents, and once cell death has reached a certain threshold, their effects begin to wane as there are too few remaining synapses for cognition to be significantly affected.
Cholinesterase inhibitors are not believed to be disease-modifying agents, and once cell death has reached a certain threshold, their effects begin to wane as there are too few remaining synapses for cognition to be significantly affected. Cholinesterase inhibitors are also used to enhance acetylcholine levels at the neuromuscular junction (NMJ), facilitating transmission and movement. They can therefore be used in patients with disorders of the NMJ, such as myasthenia gravis, a condition characterized by a reduction in the number of acetylcholine receptors at the NMJ. Cholinesterase inhibitors increase the amount of acetylcholine available to bind to these receptors, thus increasing the probability of binding.
Cholinesterase inhibitors are also used to enhance acetylcholine levels at the neuromuscular junction (NMJ), facilitating transmission and movement. They can therefore be used in patients with disorders of the NMJ, such as myasthenia gravis, a condition characterized by a reduction in the number of acetylcholine receptors at the NMJ. Cholinesterase inhibitors increase the amount of acetylcholine available to bind to these receptors, thus increasing the probability of binding. Cholinesterase inhibitors can also be used to reverse the effects of competitive NMJ blockers used in anesthesia (see also Nondepolarizing Neuromuscular Blockers).
Cholinesterase inhibitors can also be used to reverse the effects of competitive NMJ blockers used in anesthesia (see also Nondepolarizing Neuromuscular Blockers).Pharmacokinetics
Contraindications
Important Notes
 The cholinesterase inhibitors used to treat Alzheimer’s disease are reversible inhibitors. Irreversible cholinesterase inhibitors lead to a profound increase in cholinergic neurotransmission, which in turn leads to convulsions, thick secretions that obstruct the airways, cardiac arrhythmias, and death. Irreversible cholinesterase inhibitors have been used as nerve gases in warfare (sarin gas) for decades and are also used in many pesticides.
The cholinesterase inhibitors used to treat Alzheimer’s disease are reversible inhibitors. Irreversible cholinesterase inhibitors lead to a profound increase in cholinergic neurotransmission, which in turn leads to convulsions, thick secretions that obstruct the airways, cardiac arrhythmias, and death. Irreversible cholinesterase inhibitors have been used as nerve gases in warfare (sarin gas) for decades and are also used in many pesticides.Evidence
Donepezil for Treatment of Alzheimer’s Disease
 A 2006 Cochrane review (24 trials, N = 5796 participants) assessed whether donepezil improves the well-being of patients with dementia from Alzheimer’s disease. Most of the participants had mild to moderate disease, and most studies were less than 6 months in duration. Donepezil improved cognition versus placebo. Some improvement was seen in global clinical scale, and improvements were also seen in activities of daily living and behavior but not in quality of life. Withdrawals were more frequent with donepezil 10 mg versus placebo. Results were similar for all severities of disease. Health resource use was reported in only two studies, and there were no differences versus placebo.
A 2006 Cochrane review (24 trials, N = 5796 participants) assessed whether donepezil improves the well-being of patients with dementia from Alzheimer’s disease. Most of the participants had mild to moderate disease, and most studies were less than 6 months in duration. Donepezil improved cognition versus placebo. Some improvement was seen in global clinical scale, and improvements were also seen in activities of daily living and behavior but not in quality of life. Withdrawals were more frequent with donepezil 10 mg versus placebo. Results were similar for all severities of disease. Health resource use was reported in only two studies, and there were no differences versus placebo.
Galantamine for Mild Cognitive Impairment or Alzheimer’s Disease
 A 2006 Cochrane review (10 trials, N = 6805 participants) assessed the clinical effects of galantamine in patients with mild cognitive impairment or probable or possible Alzheimer’s disease. In Alzheimer’s disease the authors found that galantamine was more effective than placebo in improving cognitive function, as well as some evidence of improvement on measures of activity of daily living and behavioral symptoms. In mild cognitive impairment, data from two trials suggest marginal clinical benefit but an increased incidence of death with galantamine.
A 2006 Cochrane review (10 trials, N = 6805 participants) assessed the clinical effects of galantamine in patients with mild cognitive impairment or probable or possible Alzheimer’s disease. In Alzheimer’s disease the authors found that galantamine was more effective than placebo in improving cognitive function, as well as some evidence of improvement on measures of activity of daily living and behavioral symptoms. In mild cognitive impairment, data from two trials suggest marginal clinical benefit but an increased incidence of death with galantamine.Rivastigmine for Treatment of Alzheimer’s Disease
 A 2009 Cochrane review (9 trials, N = 4775 participants) determined the efficacy and safety of rivastigmine for patients with dementia of the Alzheimer’s type. High-dose rivastigmine (6 to 12 mg daily) improved measures of cognition and activities of daily living versus placebo. These differences were statistically significant for cognition only when lower doses of rivastigmine were used. The incidence of side effects such as nausea, vomiting, diarrhea, anorexia, headache, syncope, abdominal pain, and dizziness was higher than with placebo. One trial compared patches with capsules, finding no differences in efficacy but a lower incidence of side effects with the lower dose patch versus capsules.
A 2009 Cochrane review (9 trials, N = 4775 participants) determined the efficacy and safety of rivastigmine for patients with dementia of the Alzheimer’s type. High-dose rivastigmine (6 to 12 mg daily) improved measures of cognition and activities of daily living versus placebo. These differences were statistically significant for cognition only when lower doses of rivastigmine were used. The incidence of side effects such as nausea, vomiting, diarrhea, anorexia, headache, syncope, abdominal pain, and dizziness was higher than with placebo. One trial compared patches with capsules, finding no differences in efficacy but a lower incidence of side effects with the lower dose patch versus capsules.Ergot Alkaloids
MOA (Mechanism of Action)
 Migraines are believed to be caused by cerebral vasodilation and subsequent activation of pain fibers.
Migraines are believed to be caused by cerebral vasodilation and subsequent activation of pain fibers. The activation of pain fibers may be mediated by the release of transmitters such as vasoactive intestinal peptide (VIP), substance P, and calcitonin gene-related peptide (CGRP).
The activation of pain fibers may be mediated by the release of transmitters such as vasoactive intestinal peptide (VIP), substance P, and calcitonin gene-related peptide (CGRP). Many ergots are agonists at serotonin 1B and 1D (5-HT1B and 5-HT1D) receptors and thus possess similar properties to those of the triptans, serotonin agonists used in the treatment of acute migraine. Stimulation of these receptors leads to vasoconstriction of cerebral blood vessels (Figure 21-12).
Many ergots are agonists at serotonin 1B and 1D (5-HT1B and 5-HT1D) receptors and thus possess similar properties to those of the triptans, serotonin agonists used in the treatment of acute migraine. Stimulation of these receptors leads to vasoconstriction of cerebral blood vessels (Figure 21-12). The 5-HT1B and 5-HT1D receptors are also presynaptic autoreceptors. Autoreceptors are receptors that when stimulated inhibit release of transmitters into the synaptic cleft. Thus the ergots inhibit the release of VIP, substance P, and CGRP, although a definitive link between these actions and the drugs’ efficacy in migraine has not been confirmed.
The 5-HT1B and 5-HT1D receptors are also presynaptic autoreceptors. Autoreceptors are receptors that when stimulated inhibit release of transmitters into the synaptic cleft. Thus the ergots inhibit the release of VIP, substance P, and CGRP, although a definitive link between these actions and the drugs’ efficacy in migraine has not been confirmed. In addition, the ergots are partial agonists at α1-adrenergic receptors. Stimulation of these receptors leads to vasoconstriction of cerebral vessels. It is believed that this vasoconstriction contributes to the efficacy of these agents in acute migraine, although a definitive link has not been established.
In addition, the ergots are partial agonists at α1-adrenergic receptors. Stimulation of these receptors leads to vasoconstriction of cerebral vessels. It is believed that this vasoconstriction contributes to the efficacy of these agents in acute migraine, although a definitive link has not been established.Pharmacokinetics
 Ergotamine undergoes extensive first-pass metabolism and has a very low oral bioavailability. In contrast, ergonovine and methylergonovine are rapidly absorbed and reach peak plasma levels in under 90 minutes, with plasma levels 10 times those of ergotamine.
Ergotamine undergoes extensive first-pass metabolism and has a very low oral bioavailability. In contrast, ergonovine and methylergonovine are rapidly absorbed and reach peak plasma levels in under 90 minutes, with plasma levels 10 times those of ergotamine. The biologic activity (vasoconstriction) of ergotamine (24 hours or more) extends well beyond its elimination half-life of 2 hours. The elimination half-life of ergonovine is even shorter than that of ergotamine.
The biologic activity (vasoconstriction) of ergotamine (24 hours or more) extends well beyond its elimination half-life of 2 hours. The elimination half-life of ergonovine is even shorter than that of ergotamine. Ergotamine (oral, sublingual, rectal) and dihydroergotamine (injectable, nasal spray) are available in several dosage forms. The availability of nonoral dosage forms is important because patients with migraine also often have nausea and vomiting.
Ergotamine (oral, sublingual, rectal) and dihydroergotamine (injectable, nasal spray) are available in several dosage forms. The availability of nonoral dosage forms is important because patients with migraine also often have nausea and vomiting. For postpartum hemorrhage, ergotamine should never be administered intravenously, because of concerns over excessive vasoconstriction leading to hypertension.
For postpartum hemorrhage, ergotamine should never be administered intravenously, because of concerns over excessive vasoconstriction leading to hypertension. Ergotamine derivatives are metabolized via CYP3A4. Given the dangers associated with excessive ergotamine levels (see Side Effects), the concomitant use of strong CYP3A4 inhibitors should be avoided.
Ergotamine derivatives are metabolized via CYP3A4. Given the dangers associated with excessive ergotamine levels (see Side Effects), the concomitant use of strong CYP3A4 inhibitors should be avoided.Contraindications
 Coronary artery disease: Vasoconstriction or spasm of coronary arteries may exacerbate symptoms and lead to myocardial ischemia.
Coronary artery disease: Vasoconstriction or spasm of coronary arteries may exacerbate symptoms and lead to myocardial ischemia. Hypertension: The pressor effects of the ergots may raise blood pressure, exacerbating hypertension.
Hypertension: The pressor effects of the ergots may raise blood pressure, exacerbating hypertension. Peripheral vascular disease may lead to limb ischemia because of vasoconstriction of stenotic arteries.
Peripheral vascular disease may lead to limb ischemia because of vasoconstriction of stenotic arteries.Side Effects
Important Notes
 The first use of ergots was to promote uterine contractions (an oxytocic) in childbirth in the 1500s. However, widespread use for this indication in the early 1800s was accompanied by an increase in the number of stillbirths. Excessive uterine contraction reduces blood flow to the fetus, and this is the likely reason for the stillbirths. In an early version of adverse drug reaction surveillance, ergot was no longer recommended for induction of labor but instead for postpartum hemorrhage, an indication that exists to this day.
The first use of ergots was to promote uterine contractions (an oxytocic) in childbirth in the 1500s. However, widespread use for this indication in the early 1800s was accompanied by an increase in the number of stillbirths. Excessive uterine contraction reduces blood flow to the fetus, and this is the likely reason for the stillbirths. In an early version of adverse drug reaction surveillance, ergot was no longer recommended for induction of labor but instead for postpartum hemorrhage, an indication that exists to this day. Caffeine is added to some ergot preparations (ergotamine oral and rectal) to both facilitate absorption and potentiate the analgesic effects. However, this also means that patients who are sensitive to caffeine may want to avoid these preparations. Caffeine (or caffeine withdrawal) can also be a precipitating factor with some migraine sufferers.
Caffeine is added to some ergot preparations (ergotamine oral and rectal) to both facilitate absorption and potentiate the analgesic effects. However, this also means that patients who are sensitive to caffeine may want to avoid these preparations. Caffeine (or caffeine withdrawal) can also be a precipitating factor with some migraine sufferers.FYI
 Ergot has been written about for centuries, although much of its history has been associated with its poisonous effects. Ergot is derived from a fungus found in grain; therefore there have been many instances throughout history in which mass ergot poisonings have occurred because of contaminated grain. Signs of ergot poisoning (ergotism or St. Anthony’s fire) include gangrene of the limbs, with some limbs falling off without loss of blood, and spontaneous abortion. These situations occur because of extreme vasoconstriction of vessels.
Ergot has been written about for centuries, although much of its history has been associated with its poisonous effects. Ergot is derived from a fungus found in grain; therefore there have been many instances throughout history in which mass ergot poisonings have occurred because of contaminated grain. Signs of ergot poisoning (ergotism or St. Anthony’s fire) include gangrene of the limbs, with some limbs falling off without loss of blood, and spontaneous abortion. These situations occur because of extreme vasoconstriction of vessels. The most notorious ergot alkaloid is lysergic acid diethylamide (LSD). The unique properties of LSD were discovered accidentally in 1943 by Dr. Albert Hoffman of Sandoz Pharmaceuticals. After accidentally ingesting LSD during an experiment, Dr. Hoffman experienced the full hallucinogenic effects of this compound. The onset of action of LSD occurs in within 30 to 60 minutes, with effects peaking in 1 to 6 hours and dissipating in 8 to 12 hours.
The most notorious ergot alkaloid is lysergic acid diethylamide (LSD). The unique properties of LSD were discovered accidentally in 1943 by Dr. Albert Hoffman of Sandoz Pharmaceuticals. After accidentally ingesting LSD during an experiment, Dr. Hoffman experienced the full hallucinogenic effects of this compound. The onset of action of LSD occurs in within 30 to 60 minutes, with effects peaking in 1 to 6 hours and dissipating in 8 to 12 hours. Ergonovine goes by a number of different names (ergometrine, ergotocine, ergosterine, ergobasine), owing to the fact that it was discovered in four different laboratories almost simultaneously. In Europe, the names ergometrine and ergobasine have persisted, whereas ergonovine was adopted in the United States.
Ergonovine goes by a number of different names (ergometrine, ergotocine, ergosterine, ergobasine), owing to the fact that it was discovered in four different laboratories almost simultaneously. In Europe, the names ergometrine and ergobasine have persisted, whereas ergonovine was adopted in the United States.Triptans
MOA (Mechanism of Action)
 Migraines are believed to be caused by cerebral vasodilation and subsequent activation of pain fibers. The activation of pain fibers may be mediated by the release of transmitters such as VIP, substance P, and CGRP (Figure 21-13).
Migraines are believed to be caused by cerebral vasodilation and subsequent activation of pain fibers. The activation of pain fibers may be mediated by the release of transmitters such as VIP, substance P, and CGRP (Figure 21-13). Triptans are agonists at 5-HT1B and 5-HT1D receptors, leading to vasoconstriction of cerebral vessels. It is believed that this vasoconstriction contributes to the efficacy of these agents in acute migraine, although a definitive link has not been established.
Triptans are agonists at 5-HT1B and 5-HT1D receptors, leading to vasoconstriction of cerebral vessels. It is believed that this vasoconstriction contributes to the efficacy of these agents in acute migraine, although a definitive link has not been established. The 5H-T1B and 5H-T1D receptors are also presynaptic autoreceptors. Autoreceptors are receptors that when stimulated inhibit release of transmitters into the synaptic cleft. Thus the triptans inhibit the release of VIP, substance P, and CGRP, although a definitive link between these actions and their efficacy in migraine has not been confirmed.
The 5H-T1B and 5H-T1D receptors are also presynaptic autoreceptors. Autoreceptors are receptors that when stimulated inhibit release of transmitters into the synaptic cleft. Thus the triptans inhibit the release of VIP, substance P, and CGRP, although a definitive link between these actions and their efficacy in migraine has not been confirmed.Pharmacokinetics
 The triptans are intended to be used acutely and are characterized by a rapid onset and short half-life. Their time to peak plasma concentration and elimination half-lives are summarized in Table 21-5.
The triptans are intended to be used acutely and are characterized by a rapid onset and short half-life. Their time to peak plasma concentration and elimination half-lives are summarized in Table 21-5. Sumatriptan is available in several different dosage forms: tablet, nasal spray, and subcutaneous injection. Zolmitriptan is also available as a nasal spray. Zolmitriptan and rizatriptan are available in quick-dissolve formulations.
Sumatriptan is available in several different dosage forms: tablet, nasal spray, and subcutaneous injection. Zolmitriptan is also available as a nasal spray. Zolmitriptan and rizatriptan are available in quick-dissolve formulations.| Drug | Peak Plasma Concentration | Elimination Half-Life |
|---|---|---|
| Sumatriptan |
PO, Orally; SC, subcutaneously.
Contraindications
 Ischemic or vasospastic coronary artery disease: The triptans work by inducing vasoconstriction, and this may worsen existing ischemia or vasospasm.
Ischemic or vasospastic coronary artery disease: The triptans work by inducing vasoconstriction, and this may worsen existing ischemia or vasospasm.Important Notes
 Repeat doses of triptans can be administered if the headache returns. The interval between doses is based on the half-lives, with the shorter half-life oral agents allowing for repeat doses after 2 hours, and the longer-acting naratriptan allowing a repeat after 4 hours. Subcutaneous sumatriptan can be repeated only once in 24 hours (i.e., maximum 2 doses in 24 hours). All agents have a maximum dose allowed per 24 hours.
Repeat doses of triptans can be administered if the headache returns. The interval between doses is based on the half-lives, with the shorter half-life oral agents allowing for repeat doses after 2 hours, and the longer-acting naratriptan allowing a repeat after 4 hours. Subcutaneous sumatriptan can be repeated only once in 24 hours (i.e., maximum 2 doses in 24 hours). All agents have a maximum dose allowed per 24 hours.Evidence
Sumatriptan versus Other Therapies or Placebo
 A 2003 Cochrane review (25 trials, N = 16,200 patients) found that sumatriptan elicited significantly more pain-free responses at 2 hours at the 25-mg (NNT 5) and 100-mg (NNT 7.5) doses but not the 50-mg dose, compared with placebo. All doses were statistically different from placebo for pain relief. Adverse events were more common with sumatriptan 100 mg versus placebo (number needed to harm [NNH] 7).
A 2003 Cochrane review (25 trials, N = 16,200 patients) found that sumatriptan elicited significantly more pain-free responses at 2 hours at the 25-mg (NNT 5) and 100-mg (NNT 7.5) doses but not the 50-mg dose, compared with placebo. All doses were statistically different from placebo for pain relief. Adverse events were more common with sumatriptan 100 mg versus placebo (number needed to harm [NNH] 7).Topiramate
MOA (Mechanism of Action)
 Topiramate is thought to work via multiple mechanisms:
Topiramate is thought to work via multiple mechanisms:
 Topiramate also inhibits several carbonic anhydrase isozymes. At present it does not appear that this contributes to its antiseizure effect.
Topiramate also inhibits several carbonic anhydrase isozymes. At present it does not appear that this contributes to its antiseizure effect.Pharmacokinetics
Side Effects
Serious
Important Notes
 Topiramate is being tried in a variety of off-label indications, with varying degrees of success. The diversity of this agent is likely a reflection of its multiple mechanisms of action, although concerns have also been raised about its overuse and misuse. One of the major concerns is the significant cognitive effects associated with its use.
Topiramate is being tried in a variety of off-label indications, with varying degrees of success. The diversity of this agent is likely a reflection of its multiple mechanisms of action, although concerns have also been raised about its overuse and misuse. One of the major concerns is the significant cognitive effects associated with its use.Phenytoin
MOA (Mechanism of Action)
 Phenytoin slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing.
Phenytoin slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing. In secondarily generalized seizures, phenytoin prevents the rapid spread of seizure activity to other neurons.
In secondarily generalized seizures, phenytoin prevents the rapid spread of seizure activity to other neurons. A key feature of the efficacy of phenytoin is that it selectively targets channels that are opening and closing at high frequency. This is referred to as targeting in a use-dependent manner. This allows phenytoin to target rapidly firing neurons, typical of those seen in partial seizures, while minimizing its effects on spontaneous neuronal activity.
A key feature of the efficacy of phenytoin is that it selectively targets channels that are opening and closing at high frequency. This is referred to as targeting in a use-dependent manner. This allows phenytoin to target rapidly firing neurons, typical of those seen in partial seizures, while minimizing its effects on spontaneous neuronal activity. Phenytoin may also enhance the release of GABA and inhibit the release of glutamate from presynaptic neurons. GABA is a key inhibitory and glutamate a key excitatory neurotransmitter; hence the net effect would be inhibitory and would also contribute to reducing neuronal activity. It is not clear whether these effects on GABA and glutamate are related to sodium channel blockade.
Phenytoin may also enhance the release of GABA and inhibit the release of glutamate from presynaptic neurons. GABA is a key inhibitory and glutamate a key excitatory neurotransmitter; hence the net effect would be inhibitory and would also contribute to reducing neuronal activity. It is not clear whether these effects on GABA and glutamate are related to sodium channel blockade.Pharmacokinetics
 It exhibits saturation kinetics, meaning that at lower doses, metabolism is first order, then at higher doses the enzymes responsible for metabolizing phenytoin become saturated and metabolism switches to zero order. Toxicity can be reached quickly at higher doses.
It exhibits saturation kinetics, meaning that at lower doses, metabolism is first order, then at higher doses the enzymes responsible for metabolizing phenytoin become saturated and metabolism switches to zero order. Toxicity can be reached quickly at higher doses. Phenytoin is a CYP450 enzyme inducer and is therefore capable of interacting with other agents. Because its metabolism is saturable, phenytoin may also inhibit the metabolism of coadministered drugs that use the same CYP enzymes for elimination.
Phenytoin is a CYP450 enzyme inducer and is therefore capable of interacting with other agents. Because its metabolism is saturable, phenytoin may also inhibit the metabolism of coadministered drugs that use the same CYP enzymes for elimination.Contraindications
 Cardiac conduction abnormalities (intravenous phenytoin): Intravenous phenytoin, being a Na+ channel blocker, affects ventricular automaticity and is therefore contraindicated in patients at risk for being dependant on a ventricular escape rhythm, including those with sinus bradycardia, sinoatrial block, and second- and third-degree block.
Cardiac conduction abnormalities (intravenous phenytoin): Intravenous phenytoin, being a Na+ channel blocker, affects ventricular automaticity and is therefore contraindicated in patients at risk for being dependant on a ventricular escape rhythm, including those with sinus bradycardia, sinoatrial block, and second- and third-degree block.Side Effects
 CNS: Diplopia, ataxia, and nystagmus may occur. These types of sensory side effects may occur with agents that inhibit sodium channels, although a mechanism has not been established.
CNS: Diplopia, ataxia, and nystagmus may occur. These types of sensory side effects may occur with agents that inhibit sodium channels, although a mechanism has not been established. Gingival hyperplasia: The mechanism is multifactorial, but phenytoin stimulates fibroblasts, enhances formation of granulation tissue, and promotes collagen deposition.
Gingival hyperplasia: The mechanism is multifactorial, but phenytoin stimulates fibroblasts, enhances formation of granulation tissue, and promotes collagen deposition. Hirsutism: Excessive hair growth, typically facial hair, may occur in females. This can be a troubling and disfiguring side effect. The mechanism has not been established.
Hirsutism: Excessive hair growth, typically facial hair, may occur in females. This can be a troubling and disfiguring side effect. The mechanism has not been established.Evidence
Status Epilepticus (Refractory Seizures)
 A 2005 Cochrane review compared various agents used in status epilepticus. In the only study (198 patients) that had a phenytoin arm, intravenous lorazepam was more effective at terminating seizures than intravenous phenytoin (RR of 0.62 for seizures continuing). There was no difference in adverse events between the two agents.
A 2005 Cochrane review compared various agents used in status epilepticus. In the only study (198 patients) that had a phenytoin arm, intravenous lorazepam was more effective at terminating seizures than intravenous phenytoin (RR of 0.62 for seizures continuing). There was no difference in adverse events between the two agents.FYI
 Phenytoin was first synthesized in 1908, although it was not discovered to have anticonvulsant properties until 1938. Researchers at the time were looking for structural relatives of phenobarbital that lacked sedative properties and that were capable of suppressing electroshock convulsions in laboratory animals.
Phenytoin was first synthesized in 1908, although it was not discovered to have anticonvulsant properties until 1938. Researchers at the time were looking for structural relatives of phenobarbital that lacked sedative properties and that were capable of suppressing electroshock convulsions in laboratory animals.Lamotrigine
MOA (Mechanism of Action)
 Lamotrigine slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing.
Lamotrigine slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing. The broad spectrum of action of lamotrigine, including its varied uses in psychiatry, suggest that it may also work through other mechanisms distinct from Na+ channels. For example, lamotrigine may also inhibit Ca2+ channels and indirectly may also inhibit glutamate release from presynaptic nerve terminals, through its inhibition of either Na+ or Ca2+ channels or both.
The broad spectrum of action of lamotrigine, including its varied uses in psychiatry, suggest that it may also work through other mechanisms distinct from Na+ channels. For example, lamotrigine may also inhibit Ca2+ channels and indirectly may also inhibit glutamate release from presynaptic nerve terminals, through its inhibition of either Na+ or Ca2+ channels or both.Side Effects
 CNS effects include dizziness, ataxia, blurred vision, and double vision. These types of sensory side effects occur with agents that inhibit sodium channels, although a mechanism has not been established.
CNS effects include dizziness, ataxia, blurred vision, and double vision. These types of sensory side effects occur with agents that inhibit sodium channels, although a mechanism has not been established.Serious
 Rash: Rashes can range from mild to severe, including Stevens-Johnson syndrome and toxic epidermal necrolysis. These rashes can be fatal in patients who are otherwise compromised, or if treatment is not discontinued and/or if the rash is not treated. The exact mechanism behind these severe reactions is not known.
Rash: Rashes can range from mild to severe, including Stevens-Johnson syndrome and toxic epidermal necrolysis. These rashes can be fatal in patients who are otherwise compromised, or if treatment is not discontinued and/or if the rash is not treated. The exact mechanism behind these severe reactions is not known.Important Notes
 Lamotrigine is increasingly being used for psychiatric indications, including bipolar disorder. The idea to use lamotrigine in bipolar disorder first originated with the observation of its antiglutamatergic effects and the belief that this excitatory neurotransmitter may have a role in the pathogenesis of this illness.
Lamotrigine is increasingly being used for psychiatric indications, including bipolar disorder. The idea to use lamotrigine in bipolar disorder first originated with the observation of its antiglutamatergic effects and the belief that this excitatory neurotransmitter may have a role in the pathogenesis of this illness.Evidence
Lamotrigine versus Carbamazepine Monotherapy for Seizure Disorders
 A 2006 Cochrane review (five trials, N = 1384 participants) compared lamotrigine to carbamazepine as monotherapy in partial-onset or generalized-onset tonic-clonic seizures. The authors found that time to treatment withdrawal was improved with lamotrigine over carbamazepine (hazard ratio 0.55), whereas time to first seizure and freedom from seizure were not statistically different between lamotrigine and carbamazepine.
A 2006 Cochrane review (five trials, N = 1384 participants) compared lamotrigine to carbamazepine as monotherapy in partial-onset or generalized-onset tonic-clonic seizures. The authors found that time to treatment withdrawal was improved with lamotrigine over carbamazepine (hazard ratio 0.55), whereas time to first seizure and freedom from seizure were not statistically different between lamotrigine and carbamazepine.FYI
 Lamotrigine was initially developed as an antifolate agent, at a time when folate was believed to play a role in seizure development. Although it proved to be only a weak inhibitor of dihydrofolate reductase, its other pharmacologic properties as an Na+ channel blocker led to its success as an antiseizure medication.
Lamotrigine was initially developed as an antifolate agent, at a time when folate was believed to play a role in seizure development. Although it proved to be only a weak inhibitor of dihydrofolate reductase, its other pharmacologic properties as an Na+ channel blocker led to its success as an antiseizure medication.γ-Aminobutyric acid (GABA) Analogues
Description
GABA analogues are primarily used in the treatment of seizures and management of neuropathic pain.
MOA (Mechanism of Action)
 Seizures are characterized by an abnormal increase in neural activity in the brain. Two approaches to treating seizures are to reduce activity of excitatory neurotransmitters and to enhance activity of inhibitory neurotransmitters.
Seizures are characterized by an abnormal increase in neural activity in the brain. Two approaches to treating seizures are to reduce activity of excitatory neurotransmitters and to enhance activity of inhibitory neurotransmitters. Gabapentin’s major mechanism in the treatment of seizure is via binding to the α2δ subunit of presynaptic voltage-gated Ca2+ channels in the brain. It is believed that by reducing the influx of Ca2+ at nerve terminals, the release of excitatory neurotransmitters such as glutamate is reduced, although the connection between the reduction of Ca2+ influx and inhibition of glutamate release has not been established (Figure 21-17).
Gabapentin’s major mechanism in the treatment of seizure is via binding to the α2δ subunit of presynaptic voltage-gated Ca2+ channels in the brain. It is believed that by reducing the influx of Ca2+ at nerve terminals, the release of excitatory neurotransmitters such as glutamate is reduced, although the connection between the reduction of Ca2+ influx and inhibition of glutamate release has not been established (Figure 21-17). The structure of gabapentin is derived from GABA; therefore it has long been thought of as simply a GABA agonist. However, gabapentin does not appear to bind to GABA receptors. Instead, it may promote the release of GABA. As with other antiseizure medications that work through GABA, enhancement of the actions of this inhibitory neurotransmitter appears to counteract the excess excitatory neurotransmission that leads to seizures.
The structure of gabapentin is derived from GABA; therefore it has long been thought of as simply a GABA agonist. However, gabapentin does not appear to bind to GABA receptors. Instead, it may promote the release of GABA. As with other antiseizure medications that work through GABA, enhancement of the actions of this inhibitory neurotransmitter appears to counteract the excess excitatory neurotransmission that leads to seizures.Pharmacokinetics
Side Effects
Evidence
Pregabalin as Adjunctive Therapy in Drug-Resistant Partial Seizures
 A 2008 Cochrane review (four trials, N = 1397 participants) compared a range of doses of pregabalin with placebo. The authors found that patients treated with pregabalin were more likely to experience a 50% reduction in seizures. However, pregabalin was not associated with freedom from seizures, and participants were significantly more likely to withdraw from treatment for any reason and for adverse events. Pregabalin elicited ataxia, dizziness, somnolence, and weight gain compared with placebo.
A 2008 Cochrane review (four trials, N = 1397 participants) compared a range of doses of pregabalin with placebo. The authors found that patients treated with pregabalin were more likely to experience a 50% reduction in seizures. However, pregabalin was not associated with freedom from seizures, and participants were significantly more likely to withdraw from treatment for any reason and for adverse events. Pregabalin elicited ataxia, dizziness, somnolence, and weight gain compared with placebo.Gabapentin for Acute and Chronic Pain
 A 2005 Cochrane review (15 studies, N = 1468 participants) examined RCTs assessing the analgesic effectiveness and adverse effects of gabapentin for pain management. Only one small study assessed acute pain, and it found no differences between gabapentin and placebo. In chronic pain, 42% of patients improved versus 19% on placebo.
A 2005 Cochrane review (15 studies, N = 1468 participants) examined RCTs assessing the analgesic effectiveness and adverse effects of gabapentin for pain management. Only one small study assessed acute pain, and it found no differences between gabapentin and placebo. In chronic pain, 42% of patients improved versus 19% on placebo.Carbamazepine
MOA (Mechanism of Action)
 Seizures are caused by abnormal electrical discharges in the brain. These abnormal discharges propagate, spreading to other parts of the brain and producing abnormal movements, thoughts, or sensations. Antiseizures drugs work by inhibiting either the initiation or the propagation of these discharges.
Seizures are caused by abnormal electrical discharges in the brain. These abnormal discharges propagate, spreading to other parts of the brain and producing abnormal movements, thoughts, or sensations. Antiseizures drugs work by inhibiting either the initiation or the propagation of these discharges. Sodium channels play an important role in the generation and propagation of action potentials. They exist in three conformations:
Sodium channels play an important role in the generation and propagation of action potentials. They exist in three conformations:
 Carbamazepine stabilizes the inactive form of the Na+ channel, which slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing.
Carbamazepine stabilizes the inactive form of the Na+ channel, which slows the rate of channel recovery from the inactivated state. This increases the threshold for action potentials and prevents repetitive firing. Carbamazepine and other Na+ channel blockers bind to the Na+ channel when it is open. Because rapidly firing neurons, such as those found in seizure disorders are open a greater percentage of the time, these drugs tend to be selective for abnormal electrical activity found in a seizure focus and do not suppress normal neuronal activity. This is referred to as use-dependent blockade and is essential for limiting the toxicity of these agents.
Carbamazepine and other Na+ channel blockers bind to the Na+ channel when it is open. Because rapidly firing neurons, such as those found in seizure disorders are open a greater percentage of the time, these drugs tend to be selective for abnormal electrical activity found in a seizure focus and do not suppress normal neuronal activity. This is referred to as use-dependent blockade and is essential for limiting the toxicity of these agents. In addition to its effects on sodium channels, carbamazepine has several other actions, interacting with adenosine receptors and inhibiting the uptake and release of norepinephrine from brain synaptosomes; it may also potentiate the postsynaptic actions of GABA. Carbamazepine may also potentiate glutamatergic transmission. Whether any of these actions contribute to its clinical efficacy is unclear at present.
In addition to its effects on sodium channels, carbamazepine has several other actions, interacting with adenosine receptors and inhibiting the uptake and release of norepinephrine from brain synaptosomes; it may also potentiate the postsynaptic actions of GABA. Carbamazepine may also potentiate glutamatergic transmission. Whether any of these actions contribute to its clinical efficacy is unclear at present.Pharmacokinetics
 Carbamazepine is slowly and erratically absorbed, with peak plasma concentrations occurring anywhere from 4 to 24 hours after an oral dose.
Carbamazepine is slowly and erratically absorbed, with peak plasma concentrations occurring anywhere from 4 to 24 hours after an oral dose. Carbamazepine (10,11-epoxide) and oxcarbazepine (10-monohydroxy; MHD) each have an active metabolite that contributes significantly to the activity of the drug. Oxcarbazepine is rapidly converted to its active metabolite and therefore acts more like a prodrug.
Carbamazepine (10,11-epoxide) and oxcarbazepine (10-monohydroxy; MHD) each have an active metabolite that contributes significantly to the activity of the drug. Oxcarbazepine is rapidly converted to its active metabolite and therefore acts more like a prodrug.Contraindications
Side Effects
Serious
 Dermatologic reactions may occur, including Stevens-Johnson syndrome and toxic epidermal necrolysis, which are both very severe reactions with possible fatal outcomes.
Dermatologic reactions may occur, including Stevens-Johnson syndrome and toxic epidermal necrolysis, which are both very severe reactions with possible fatal outcomes.Important Notes
Evidence
Seizure Disorder
Carbamazepine versus Phenytoin
 A 2002 Cochrane review (three trials, N = 551 participants) compared these two agents in patients with partial-onset or generalized tonic-clonic seizures, using individual patient data from three trials (551 patients). The authors found no differences between carbamazepine and phenytoin for time to 6- or 12-month remission, time to first seizure, or time to withdrawal.
A 2002 Cochrane review (three trials, N = 551 participants) compared these two agents in patients with partial-onset or generalized tonic-clonic seizures, using individual patient data from three trials (551 patients). The authors found no differences between carbamazepine and phenytoin for time to 6- or 12-month remission, time to first seizure, or time to withdrawal.Carbamazepine versus Lamotrigine
 A 2006 Cochrane review (five trials, N = 1384 participants) compared these two agents in patients with partial-onset or generalized-onset tonic-clonic seizures, using individual patient data from five trials (1384 patients). The time to treatment withdrawal was significantly improved with lamotrigine compared with carbamazepine, whereas the time to first seizure and freedom from seizure at 6 months favored carbamazepine, although not by a statistically significant amount.
A 2006 Cochrane review (five trials, N = 1384 participants) compared these two agents in patients with partial-onset or generalized-onset tonic-clonic seizures, using individual patient data from five trials (1384 patients). The time to treatment withdrawal was significantly improved with lamotrigine compared with carbamazepine, whereas the time to first seizure and freedom from seizure at 6 months favored carbamazepine, although not by a statistically significant amount.Barbiturates
MOA (Mechanism of Action)
 GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
GABA is the major inhibitory neurotransmitter in the CNS. GABA binds to three different types of receptors: GABAA, GABAB, and GABAC.
 Barbiturates increase the binding of GABA to GABAA receptors and increase the influx of Cl− into the neuron, resulting in hyperpolarization and decreased neuronal activity.
Barbiturates increase the binding of GABA to GABAA receptors and increase the influx of Cl− into the neuron, resulting in hyperpolarization and decreased neuronal activity. The overall net effect of this binding is a global reduction in CNS activity; barbiturates are CNS depressants (Figure 21-19).
The overall net effect of this binding is a global reduction in CNS activity; barbiturates are CNS depressants (Figure 21-19). Seizure activity is unwanted, increased neurologic activity. By suppressing all neural activity, the hope is to primarily suppress the neurons contributing to the seizure before depressing the neurons involved with normal function.
Seizure activity is unwanted, increased neurologic activity. By suppressing all neural activity, the hope is to primarily suppress the neurons contributing to the seizure before depressing the neurons involved with normal function.Pharmacokinetics
 Barbiturates can be administered orally, intravenously, intramuscularly, and, less commonly, rectally.
Barbiturates can be administered orally, intravenously, intramuscularly, and, less commonly, rectally. When administered via the intravenous route, a pronounced redistribution effect occurs: the drug levels in the blood are initially very high and equilibrate with the brain to have a strong effect. However, the drug also equilibrates with other tissues, and the concentration in the blood rapidly falls; the levels in the brain also rapidly fall in response to this, as the drug becomes redistributed to other tissues in the body.
When administered via the intravenous route, a pronounced redistribution effect occurs: the drug levels in the blood are initially very high and equilibrate with the brain to have a strong effect. However, the drug also equilibrates with other tissues, and the concentration in the blood rapidly falls; the levels in the brain also rapidly fall in response to this, as the drug becomes redistributed to other tissues in the body.Side Effects
Important Notes
 Phenobarbital has more selective antiseizure properties relative to its sedative properties. As a result, it is the most commonly used barbiturate for treatment of seizures.
Phenobarbital has more selective antiseizure properties relative to its sedative properties. As a result, it is the most commonly used barbiturate for treatment of seizures. Because of the narrow therapeutic index between antiseizure activity and sedation, most antiseizure medications are commonly measured in the blood to ensure that the target concentrations are appropriate. However, dosage should also be established on clinical grounds: the ultimate goal would be suppression of seizure activity and the absence of sedation. If both these goals are met, then the clinical outcome should out-prioritize the goal of meeting the blood levels. Some patients will require higher or lower blood levels (compared with the recommend range) to achieve these clinical endpoints.
Because of the narrow therapeutic index between antiseizure activity and sedation, most antiseizure medications are commonly measured in the blood to ensure that the target concentrations are appropriate. However, dosage should also be established on clinical grounds: the ultimate goal would be suppression of seizure activity and the absence of sedation. If both these goals are met, then the clinical outcome should out-prioritize the goal of meeting the blood levels. Some patients will require higher or lower blood levels (compared with the recommend range) to achieve these clinical endpoints. Tolerance occurs. Because of CNS receptor down-regulation and decreased sensitivity or responsiveness to continued agonism, progressively increasing doses are commonly required over time. Furthermore, the enzyme induction results in increased hepatic metabolism, a second factor that results in increased dose requirements over time.
Tolerance occurs. Because of CNS receptor down-regulation and decreased sensitivity or responsiveness to continued agonism, progressively increasing doses are commonly required over time. Furthermore, the enzyme induction results in increased hepatic metabolism, a second factor that results in increased dose requirements over time. Chronically administered barbiturates over time will virtually guarantee addiction and tolerance. This type of administration should be avoided if used for sedation.
Chronically administered barbiturates over time will virtually guarantee addiction and tolerance. This type of administration should be avoided if used for sedation. Some medications include a combination of a barbiturate and an opioid. Because both medications are addictive, these drugs have enormous addiction potential.
Some medications include a combination of a barbiturate and an opioid. Because both medications are addictive, these drugs have enormous addiction potential.FYI
 The structure of barbiturates replaces the two hydrogen atoms at the C5 position with seven and nine carbon atoms. This side chain determines potency and degree of seizure activity.
The structure of barbiturates replaces the two hydrogen atoms at the C5 position with seven and nine carbon atoms. This side chain determines potency and degree of seizure activity. Replacing the =O at C2 with a sulfur creates a thiobarbiturate, which has greater lipid solubility and thus faster onset of action and shorter duration of activity. Thiobarbiturates are therefore used as general anesthetics (Figure 21-20).
Replacing the =O at C2 with a sulfur creates a thiobarbiturate, which has greater lipid solubility and thus faster onset of action and shorter duration of activity. Thiobarbiturates are therefore used as general anesthetics (Figure 21-20). Ataxia (wide-stanced dizzy gait) is one hallmark of cerebellar dysfunction. Dysdiadokokinesis (difficulty placing your finger on an object when trying to point to an object in space) is another sign of cerebellar dysfunction.
Ataxia (wide-stanced dizzy gait) is one hallmark of cerebellar dysfunction. Dysdiadokokinesis (difficulty placing your finger on an object when trying to point to an object in space) is another sign of cerebellar dysfunction.