Neurologic Criteria for Death in Adults
The clinical examination in brain death is the most unequivocal in neurology. Most of the time, establishing the diagnosis of brain death on clinical grounds is not a difficult task, as long as providers follow established protocols and guidelines for the interpretation of findings during clinical examination.1,2 However, there may be confusion based on the variability of the interpretation of current guidelines,3,4 hurdles to clinical examination in specific patient populations such as trauma victims, laws from specific jurisdictions, and the changing face of outcomes after severe brain injury in an era of advancements in critical care and resuscitative medicine. In this chapter we will review current procedures and recommendations for the evaluation of comatose patients presumed brain dead, the concept of brain death substantiated by anatomic and physiologic bases, important concepts for the determination of brain death in the setting of novel interventions such as hypothermia and extracorporeal circulation, and how to avoid pitfalls during the evaluation of patients presumed brain dead.
Historical Perspective and Definitions
The concept of brain death is modern to medicine but the definition of death has historically been associated with physiologic cessation of cardiopulmonary function. With advancements in mechanical ventilation, life support, and resuscitation medicine, a new state in which a patient’s cardiopulmonary functions could be sustained in the absence of neurologic function emerged. In 1959, Mollaret and Goulon described 23 patients with irreversible coma, whose clinical syndrome was characterized by the absence of brainstem function, spontaneous respiratory function, and cardiovascular collapse that ensued without the use of vasopressors, in what became the birth of the concept of brain death.5 In 1963, Harvard neurologists proposed that a patient be certified as dead in the setting of coma, absence of brainstem reflexes, apnea for 30 minutes, and an isoelectric electroencephalogram (EEG) tracing in all leads for more than 30 minutes,6 despite the presence of cardiac function. The first guideline (the Harvard criteria) for deciding brain death was established in 1968.7 This concept has been accepted worldwide, although its fundamental meaning is not exactly globally uniform yet. Some countries, such as the United States, accept the concept of brain death as “whole brain death,” which is defined as irreversible loss of all cortical and brainstem function. The Medical Royal Colleges of the United Kingdom established the definition of brain death on a “lower brain” concept of brainstem death.8 In the opinion of the Royal Medical College, permanent unconsciousness secondary to neuronal death of brainstem structures such as the reticular activating system (RAS) is irreversible.8
In 1981, a Presidential Commission in the United States was formed to address the issue of death by neurologic criteria.9 The President’s Commission established the foundations of the criteria currently used in the United States for the diagnosis of brain death: the cause of brain death should be known and irreversible and no improvement in neurologic condition should occur during a period of observation; the period of observation was left at the discretion of the physician, but periods of 6 to 12 hours were recommended depending on the availability of confirmatory testing such as EEG or cerebral perfusion scans.9 Though the President’s Commission suggested periods of observation of 24 hours for cases of ischemia-anoxia,9 more recent clinical experience in the era of therapeutic hypothermia and published guidelines suggest a period of observation of up to 72 hours in patients with hypoxic-ischemic coma.10 The President’s Commission guidelines for the determination of brain death culminated in a proposal for a legal definition that led to the Uniform Determination of Death Act (UDDA) in 1981. Under the law, the determination of death must be made with accepted medical standards and can be established only if an individual “has sustained either: 1) irreversible cessation of circulatory and respiratory functions, or 2) irreversible cessation of all functions of the entire brain, including the brain stem.”
In 1995, the American Academy of Neurology (AAN) published the practice parameter to delineate the medical standards for the determination of brain death.11 The guideline emphasized the three clinical findings necessary to confirm irreversible cessation of all brain functions including the brainstem: (1) known cause and presence of coma, (2) absence of brainstem reflexes, and (3) apnea.11 Despite this publication, there is considerable practice variation in the adherence to the AAN guidelines for the determination of brain death, particularly in areas related to definitions of acceptable core temperature, number of required examinations, ancillary testing to be used,3 and deficiencies in documentation.3 In response to this phenomenon, the AAN published an evidence-based update of the 1995 Practice Parameter12 to answer the main questions related to practice variability in the determination of brain death.3,13
In the United States, most state laws have adopted the UDDA, and some have added their own amendments. All states and the District of Columbia have statutes for determining brain death based on the UDDA, but certain statutes differ in minor points such as the requirement that determination of brain death should be done by two different physicians, the use of confirmatory testing, and the notification of next of kin before the declaration of brain death. To this end, it is important for the practitioner to understand local hospital policies for the declaration of brain death that should abide by the laws of their respective jurisdictions. Additional information about specific status in jurisdictions of the United States can be downloaded from http://www.braindeath.org.
Determination of Brain Death
The most common causes of brain death in the adult population are traumatic brain injury (TBI), aneurysmal subarachnoid hemorrhage (SAH), hypoxic-ischemic injury, and fulminant hepatic failure.14 It is estimated that the diagnosis of brain death is made at least 25 to 30 times a year in large referral centers, but this number may be lower in nonacademic centers.14 The determination of brain death is based on the UDDA and supported by the AAN Practice Parameter.11,12 The diagnosis is based on the establishment of three main criteria: (1) known cause and presence of coma, (2) absence of brainstem reflexes, and (3) apnea. Therefore, the clinical diagnosis, when considering accepted guidelines, is the most unequivocal in neurology.
In general, most jurisdictions require that the determination of brain death be made by a licensed physician, which in most of the cases should be an attending physician who has experience in the assessment of comatose patients and the legal requirements applicable to the jurisdiction of practice. Some centers require the testing to be performed by two different physicians, and some centers require one of these physicians to be a neurologist, neurosurgeon, or critical care specialist. In the United States, 42% of top neuroscience centers required the brain death examination to be documented by a neurologist or neurosurgeon and only 35% required that an attending neurologist or neurosurgeon be involved; of the 42% centers, residents could document the examination in up to 65% of the centers.3 In those circumstances in which the patient is a potential organ donor, the clinical team may not be directly involved with the discussions of organ procurement, as a conflict of interest may be apparent.
Clinical Evaluation of Coma and Prerequisites
Practitioners should first determine the cause of coma by history, physical examination, neuroimaging, and laboratory testing. Exclusion of central nervous system (CNS) depressants should be attempted by careful history taking, drug screen testing, and calculation of drug clearance using the rule of five times the drugs half-life (assuming a normal hepatic and renal function) or drug plasma levels below therapeutic ranges.12 In the setting of abnormal hepatic or renal function, or after use of therapeutic hypothermia, caution should be taken before entertaining the diagnosis of brain death based on changes in drug metabolism inherent to these clinical settings, and to avoid catastrophic and embarrassing misdiagnoses.15 In these circumstances, more than recommended times for observation are suggested or the use of confirmatory testing may be sought. The legal limit for ethanol is a blood alcohol content of 0.08%, which is a practical threshold below which an examination to determine brain death could reasonably proceed.12 In those patients exposed to pentobarbital for the management of intracranial hypertension, an accepted level in which a clinical examination can proceed without chance of confounding is 10 µg/mL.12 There should be no temporal administration of neuromuscular blockers and this can be assessed definitely by the presence of train-of-four twitches with maximal ulnar stimulation. Acid-base status and electrolyte levels must not be severely deviated from the norm, but the presence of signs and symptoms of diabetes insipidus does not preclude the diagnosis of brain death; however, treatment with fluids and vasopressors such as vasopressin will target both sodium and blood pressure requirements to allow for adequate neurologic assessment. With more cardiac arrest survivors being exposed to therapeutic hypothermia, practitioners should now achieve a normal core temperature, defined as near-normal temperature or core temperature higher than 36° C, before attempting to determine brain death. Patients should have normal blood pressure, defined as systolic blood pressure (SBP) equal to or higher than 100 mm Hg, as the neurologic examination is usually reliable at this level of blood pressure.
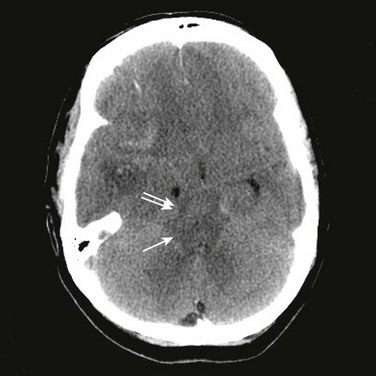
A temporal cause of severe brain injury must be established. Irreversibility from neurologic injury is recognized by the extent of the injury, the devastation in neurologic findings, and the lack of improvement. Neuroimaging is useful in establishing an acute neurologic catastrophe that is compatible with the clinical diagnosis. In most cases, a computed tomography (CT) scan will reveal specific findings such as diffuse cerebral edema, mass lesions with severe shift of midline structures, and herniation (Fig. 62.1).
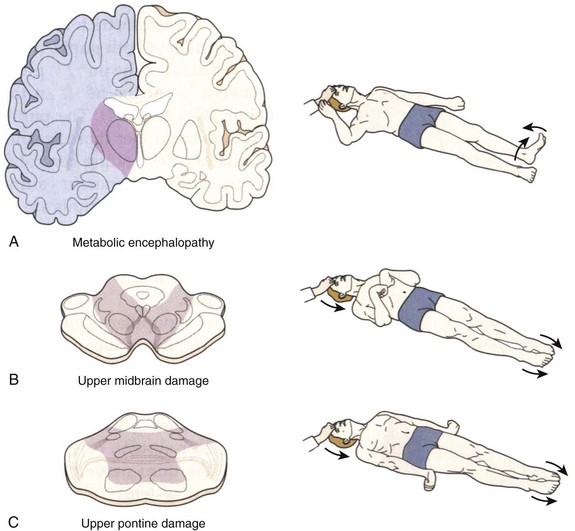
Coma is established by the lack of all evidence of responsiveness. That is, eye opening or eye movement to noxious stimuli must be absent. The motor responses to noxious stimuli should not be flexor or extensor but spinally mediated. The clinical differentiation of motor responses may require experience and specific training in neurology or neurosurgery. When flexor posturing of the upper extremities and extension of the lower extremities (decorticate) or extensor posturing of both the upper and lower extremities (decerebrate) is observed, the diagnosis of brain death cannot be entertained and an additional period of observation is recommended (Fig. 62.2). The basis of these responses is related to the patency of the rubrospinal and vestibulospinal tracts, and lower segments of the medulla that connect with the spinal cord.16 Brain death patients typically exhibit spinal cord–mediated motor responses characterized by “en bloc” flexor responses of the lower extremities with flexion of the hip, flexion of the knee, and dorsiflexion of the ankle and toes (the so-called triple flexion response or Babinski en bloc spinal cord sign) (see Fig. 62.2). More complex movements that have been referred to as brain death–associated reflexes (Lazarus sign, spinal man, spinal reflexes, or spinal automatisms) have been described in patients who otherwise meet all other brain death criteria.17 When in doubt, the neurologic examination of a comatose patient becomes equivocal, and in these circumstances, additional observation or a confirmatory test may be warranted.
Absence of Brainstem Reflexes
All segments of the brainstem should be tested in the clinical examination. The highest segments are examined by documentation of the pupillary response to bright light in both eyes (cranial nerves II and III). The usual presentation is dilated fixed pupils, which may be asymmetrical. Constricted pupils may mean drug intoxication or lesions at the level of the pons causing de-efferentation from sympathetic fibers and unopposed activation of parasympathetic centers located in the rostral area of the midbrain (Edinger-Westphal nucleus). Absence of ocular movements using oculocephalic and oculovestibular reflex testing can be achieved after assuring integrity of the cervical spine (cranial nerves III, IV, VI, and VIII [vestibular part]). The oculocephalic reflex is tested by briskly rotating the head side to side and vertically. Vertical oculocephalic movements are important for the determination of brain death as injuries in the lateral portions of the pons may manifest with bilateral palsies of horizontal eye movements but would spare the vertical eye movements. The oculovestibular or cold-caloric reflex is tested by irrigating each ear with 50 mL of iced water and observing the response for up to 1 minute. The absence of corneal reflexes (cranial nerves VII, V, and others) is demonstrated by touching the cornea with a cotton swab or gauze. Painful noxious stimulation in the cranium, trunk, or limbs should not produce any facial movement or grimacing (cranial nerve VII). Absence of pharyngeal (cranial nerve IX) or gag reflex (cranial nerve IX) is demonstrated by stimulating the back of the mouth or oropharynx with a suction device or tongue blade. The tracheal reflex (cranial nerve X) is tested by examining the cough response to tracheal stimulation provided by suctioning. Additional cranial nerve reflexes such as the “jaw jerk” (cranial nerve V) may be assessed for completeness and to follow established guidelines.11 Any unanticipated movements of any segment of the face, trunk, or limbs during brainstem examination implies intact brainstem efferent connections and precludes the diagnosis of brain death. In trauma victims with facial trauma in whom adequate examination of brainstem patency cannot be substantiated, the use of confirmatory testing may be warranted.
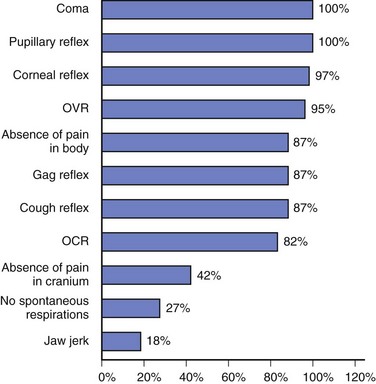
Deviations from accepted guidelines during clinical examination of comatose patients presumed brain dead may be associated with false-positive results, so a thorough examination of all segments of the brainstem is required. Areas of particular deviation from the AAN Practice Parameter for the determination of brain death include failure of testing for pain above the foramen magnum, the oculocephalic reflex, the jaw-jerk reflex, and establishing the absence of spontaneous respirations3 (Fig. 62.3).
Apnea
Complete absence of breathing drive must exist to confirm the diagnosis of brain death. The absence of respiratory drive is tested with a CO2 challenge, which requires all of the following: (1) normotension, (2) normothermia, (3) euvolemia, (4) eucapnia (PaCO2 35-45 mm Hg), (5) absence of hypoxia, and (6) no prior evidence of CO2 retention (chronic obstructive pulmonary disease [COPD], severe obesity, or sleep apnea syndrome).12 The test is started by preoxygenating the patient with 1.0 FIO2 for 10 minutes to achieve a PaO2 of more than 200 mm Hg using a respiratory rate of 10 per minute to achieve eucapnia. If the patient remains hemodynamically stable and with oxygen saturation more than 95%, the patient is disconnected from the ventilator and oxygenation is preserved by placing a catheter through the endotracheal tube and close to the level of the carina delivering O2 at 1.0 FIO2 with a flow of 6 L/minute. During the following 8 to 10 minutes, the practitioner should look carefully for respiratory movements (abdominal or chest excursions and may include a brief gasp).12 The test should be aborted if oxygen saturation drifts lower than 85% for more than 30 seconds. If this is the case, the test can be repeated later using a T-piece, continuous positive airway pressure (CPAP) with 10 cm H2O, and O2 with 1.0 FIO2 with a flow of 12 L/minute.12,18 The test is considered positive if respiratory movements are absent and the PaCO2 is higher than 60 mm Hg or 20 mm Hg change from baseline supporting the diagnosis of brain death.12
Confirmatory Testing
The role of confirmatory testing in brain death differs among jurisdictions.19 In the United States and the United Kingdom, they are discretionary.11,12,20 According to the AAN guidelines, confirmatory tests are required only when specific components of the clinical examination cannot be reliably assessed.11,12 In some European countries such as Italy, France, and the Netherlands, among others, these tests are mandatory,19,20 however, there is still significant variability in the approach to determining brain death in the world.19
A specialist in the neurosciences and any other skilled physician should be able to determine brain death using clinical criteria alone, so the main purpose of confirmatory testing besides being a diagnostic safeguard is to support the diagnosis of brain death in the setting of failure to complete a thorough neurologic examination or an apnea test. The important thing about confirmatory testing is that these tests should never replace the clinical examination and should never be ordered before attempts to complete a thorough neurologic examination.21
Origins of Confirmatory Testing in Brain Death
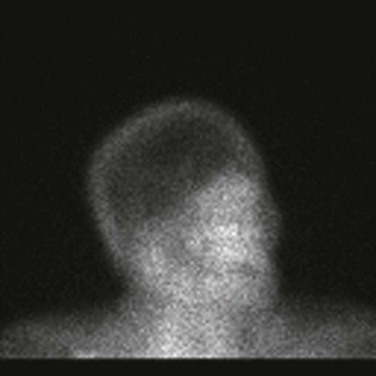
In the earlier years of refining the clinical picture of brain death, there was a desire to demonstrate absence of brain function with information different from clinical data. In the 1950s Jouvet22 in France and C. Miller Fisher23 in the United States began to use electrocerebral science to study a group of patients who would have poor neurologic outcomes. Although these studies provided the basis for defining brain death by the Ad Hoc Committee of the Harvard Medical School7 a year later, Beecher, who served as the chairman of the committee, mentioned in an editorial that EEG was “not essential to a diagnosis of reversible coma, but provides valuable supporting data”24 and this statement was later endorsed by the American Neurological Association.25 Additional confirmatory tests, which can be divided into those that test for electrical function and those that test for blood flow, have been introduced and are aimed at confirming cerebral death (Table 62.1). Electrophysiologic tests include EEG, brainstem auditory evoked potentials (BAERs), and somatosensory evoked potentials (SSEPs); blood flow tests include four-vessel cerebral angiography, transcranial Doppler (TCD), CT angiogram, magnetic resonance (MR) angiogram, and nuclear brain scan (Fig. 62.4). More recent ancillary tests used for the determination of brain death include the bispectral index scale monitor26 (BIS, mathematical algorithm of EEG), jugular bulb venous oxygen saturation (SjvO2),27 and brain tissue oxygenation (PbtO2).28
Table 62.1
Historical Time Frame for Confirmatory Tests Used in Determination of Brain Death
| Year | Reported Study* | Test |
| 1959 | Löfstedt and von Reis39 | Cerebral angiogram |
| 1959 | Fischgold and Mathis40 | EEG |
| 1969 | Goodman et al41 | Nuclear brain scan |
| 1974 | Yoneda et al42 | TCD |
| 1976 | Starr43 | BAEP |
| 1978 | Rappaport et al45 | CT angiogram |
| 1978 | Rangel46 | CT angiogram |
| 1981 | Goldie et al44 | SSEP |
| 1992 | Jones and Barnes47 | MRI |
*Studies cited in complete list of references for this chapter provided online.
Adapted from Wijdicks EF: The case against confirmatory tests for determining brain death in adults. Neurology 2010;75:77-83; used with permission from Lippincott Williams & Wilkins.
Accuracy of Confirmatory Tests
Practitioners must be careful at the time of interpreting the results of confirmatory tests and be knowledgeable of the technology being used, as there are disparities in the accuracy of confirmatory tests that could lead to potential pitfalls in the determination of brain death (Box 62.1). Based on the absence of gold standards from a physiologic and pathologic perspective,29 the results of confirmatory testing may confound the practitioner in two ways: tests can be labeled as false positives or false negatives when they are compared against each other. False positives occur when the test suggests brain death and the patient does not meet clinical criteria. False negatives are more common (Table 62.2), and they occur when the patient is clinically brain dead but the test shows otherwise,21 which may be more common with EEG.30
Table 62.2
False-Negative Rates with Confirmatory Tests Used in Determination of Brain Death
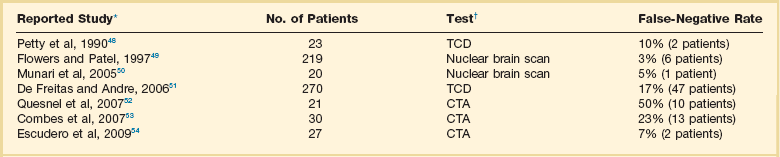
CTA, computed tomographic angiogram; TCD, transcranial Doppler.
*Studies cited in complete list of references for this chapter provided online.
†All tests evaluated by comparison with clinical confirmation of brain death.
Adapted from Wijdicks EF: The case against confirmatory tests for determining brain death in adults. Neurology 2010;75:77-83; used with permission from Lippincott Williams & Wilkins.
Recommendations for Confirmatory Testing
Current evidence-based guidelines from the AAN recommend the use of only one confirmatory test for the determination of brain death in those cases in which an additional period of observation, or an apnea test, or clinical examination is not feasible to fully establish the clinical criteria of brain death.12 In a large study of brain death determination at the Mayo Clinic, the apnea test was aborted in 3% of patients and in 7% of patients the apnea test was not performed as it was not deemed to be safe, a situation that occurred more frequently in patients with polytrauma or with chest trauma.31 In practice, practitioners should anticipate that 1 in 10 patients with devastating neurologic injury will be unable to have an apnea test, and declaration of brain death may not be possible on clinical grounds. Families should be informed about the unlikely event of meaningful recovery and in these circumstances, if the patient is a candidate for organ donation, donation after circulatory determination of death (DCDD) may be considered; most hospitals have protocols for donation under DCDD,21 and this approach could potentially increase the supply of deceased donor organs.32
Special Circumstances
Determination of Brain Death After Cardiac Arrest in the Era of Therapeutic Hypothermia
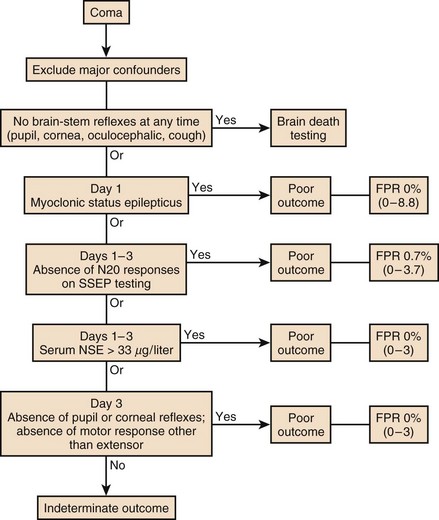
About 450,000 Americans have cardiac arrest annually.33 The face of prognostication after cardiac arrest has changed in recent years based on clinical trials suggesting a robust effect of therapeutic hypothermia on clinical outcomes.34 In 2006, the AAN published an algorithm to facilitate prognostic determination in patients who are resuscitated within 24 hours after cardiac arrest10 (Fig. 62.5). The algorithm may require modification as more information accrues on the effects of hypothermia and with validation of other tests for poor and favorable outcomes35 but the most important feature of the new clinical practice parameter was the recommendation to practitioners to wait at least 24 hours before entertaining the diagnosis of brain death. Despite the practice parameter a recent study shows that prognostication after cardiac arrest occurs on the basis of insufficient clinical data, which may lead to early withdrawals on the basis of self-fulfilling prophecies of doom.36
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) is increasingly used as a means of extracirculatory support to patients in severe, reversible cardiac or respiratory failure. Patients presumed to have brain death may have earlier withdrawal of ECMO support,37 which would limit the possibility of organ donation in patients who would otherwise be declared brain dead. In these circumstances, confirmatory testing may be warranted. A protocol of apnea testing involving the addition of CO2 to the oxygenator to a target of 60 mm Hg or more than 20 mm Hg from the baseline normal CO2 value may be used in lieu of the conventional protocol for apnea testing, but this protocol requires future validation.38
References
1. American Medical Association. An appraisal of the criteria of cerebral death. A summary statement. A collaborative study. JAMA. 1977; 237:982–986.
2. Flowers, WM, Jr., Patel, BR. Accuracy of clinical evaluation in the determination of brain death. South Med J. 2000; 93:203–206.
3. Greer, DM, Varelas, PN, Haque, S, Wijdicks, EF. Variability of brain death determination guidelines in leading US neurologic institutions. Neurology. 2008; 70:284–289.
4. Powner, DJ, Hernandez, M, Rives, TE. Variability among hospital policies for determining brain death in adults. Crit Care Med. 2004; 32:1284–1288.
5. Mollaret, P, Goulon, M. Le coma dépassé. Rev Neurol (Paris). 1959; 3–15.
6. Schwab, R, Potts, F, Bonazzi, A. EEG as an aid in determining death in the presence of cardiac activity (ethical, legal, and medical aspects). Electroencephalogr Clin Neurophysiol. 1963; 15:147.
7. Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. A definition of irreversible coma. JAMA. 1968; 205:337–340.
8. Diagnosis of brain death. Statement issued by the honorary secretary of the Conference of Medical Royal Colleges and their Faculties in the United Kingdom on 11 October 1976. BMJ. 1976; 2:1187–1188.
9. Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA. 1981; 246:2184–2186.
10. Wijdicks, EF, Hijdra, A, Young, GB, et al. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006; 67:203–210.
11. The Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters for determining brain death in adults (summary statement). Neurology. 1995; 45:1012–1014.
12. Wijdicks, EF, Varelas, PN, Gronseth, GS, Greer, DM. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010; 74:1911–1918.
13. Wang, MY, Wallace, P, Gruen, JP. Brain death documentation: Analysis and issues. Neurosurgery. 2002; 51:731–735.
14. Wijdicks, EF. The diagnosis of brain death. N Engl J Med. 2001; 344:1215–1221.
15. Webb, AC, Samuels, OB. Reversible brain death after cardiopulmonary arrest and induced hypothermia. Crit Care Med. 2011; 39:1538–1542.
16. Saper, CB. Brain stem modulation of sensation, movement, and consciousness. In: Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. New York: McGraw-Hill; 2000:889–909.
17. Jain, S, DeGeorgia, M. Brain death-associated reflexes and automatisms. Neurocrit Care. 2005; 3:122–126.
18. Levesque, S, Lessard, MR, Nicole, PC, et al. Efficacy of a T-piece system and a continuous positive airway pressure system for apnea testing in the diagnosis of brain death. Crit Care Med. 2006; 34:2213–2216.
19. Wijdicks, EF. Brain death worldwide: Accepted fact but no global consensus in diagnostic criteria. Neurology. 2002; 58:20–25.
20. Haupt, WF, Rudolf, J. European brain death codes: A comparison of national guidelines. J Neurol. 1999; 246:432–437.
21. Wijdicks, EF. The case against confirmatory tests for determining brain death in adults. Neurology. 2010; 75:77–83.
22. Jouvet, M. Diagnostique electro-sous-cortico-graphique de la mort du systeme nerveux central au cours de certain comas. Electroencephalogr Clin Neurophysiol. 1959; 11:805–808.
23. Fisher, CM. The neurological examination of the comatose patient. Acta Neurol Scand. 1969; 45(Suppl 36):1–56.
24. Beecher, HK. After the “definition of irreversible coma. ”. N Engl J Med. 1969; 281:1070–1071.
25. American Neurological Association. Revised statement regarding method for determining that the brain is dead. Trans Am Neurol Assoc. 1977; 192–193.
26. Escudero, D, Otero, J, Muniz, G, et al. The Bispectral Index Scale: Its use in the detection of brain death. Transplant Proc. 2005; 37:3661–3663.
27. Diaz-Reganon, G, Minambres, E, Holanda, M, et al. Usefulness of venous oxygen saturation in the jugular bulb for the diagnosis of brain death: Report of 118 patients. Intensive Care Med. 2002; 28:1724–1728.
28. Palmer, S, Bader, MK. Brain tissue oxygenation in brain death. Neurocrit Care. 2005; 2:17–22.
29. Wijdicks, EF, Pfeifer, EA. Neuropathology of brain death in the modern transplant era. Neurology. 2008; 70:1234–1237.
30. Young, GB, Shemie, SD, Doig, CJ, Teitelbaum, J. Brief review: The role of ancillary tests in the neurological determination of death. Can J Anaesth. 2006; 53:620–627.
31. Wijdicks, EF, Rabinstein, AA, Manno, EM, Atkinson, JD. Pronouncing brain death: Contemporary practice and safety of the apnea test. Neurology. 2008; 71:1240–1244.
32. Halpern, SD, Barnes, B, Hasz, RD, Abt, PL. Estimated supply of organ donors after circulatory determination of death: A population-based cohort study. JAMA. 2010; 304:2592–2594.
33. Callans, DJ. Out-of-hospital cardiac arrest—The solution is shocking. N Engl J Med. 2004; 351:632–634.
34. Holzer, M, Bernard, SA, Hachimi-Idrissi, S, et al. Hypothermia for neuroprotection after cardiac arrest: Systematic review and individual patient data meta-analysis. Crit Care Med. 2005; 33:414–418.
35. Young, GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009; 361:605–611.
36. Perman, SM, Kirkpatrick, JN, Reitsma, AM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit Care Med. 2012; 40:719–724.
37. Thiagarajan, RR, Brogan, TV, Scheurer, MA, et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009; 87:778–785.
38. Muralidharan, R, Mateen, FJ, Shinohara, RT, et al. The challenges with brain death determination in adult patients on extracorporeal membrane oxygenation. Neurocrit Care. 2011; 14:423–426.
39. Löfstedt, S, von Reis, D. Diminution or obstruction of blood flow in the internal carotid artery. Opuse Med. 1959; 345–360.
40. Fischgold, H, Mathis, P, Obnubilations, coma et stupeurs: Etudes electroencephalographiques. Electroencephalogr Clin Neurophysiol. Suppl, 1959:27–68.
41. Goodman, JM, Mishkin, FS, Dyken, M. Determination of brain death by isotope angiography. JAMA. 1969; 209:1869–1872.
42. Yoneda, S, Nishimoto, A, Nukada, T, et al. To-and-fro movement and external escape of carotid arterial blood in brain death cases. A Doppler ultrasonic study. Stroke. 1974; 5:707–713.
43. Starr, A. Auditory brain-stem responses in brain death. Brain. 1976; 99:543–554.
44. Goldie, WD, Chiappa, KH, Young, RR, Brooks, EB. Brainstem auditory and short-latency somatosensory evoked responses in brain death. Neurology. 1981; 31:248–256.
45. Rappaport, ZH, Brinker, RA, Rovit, RL. Evaluation of brain death by contrast-enhanced computerized cranial tomography. Neurosurgery. 1978; 2:230–232.
46. Rangel, RA. Computerized axial tomography in brain death. Stroke. 1978; 9:597–598.
47. Jones, KM, Barnes, PD. MR diagnosis of brain death. Am J Neuroradiol. 1992; 13:65–66.
48. Petty, GW, Mohr, JP, Pedley, TA, et al. The role of transcranial Doppler in confirming brain death: Sensitivity, specificity, and suggestions for performance and interpretation. Neurology. 1990; 40:300–303.
49. Flowers, WM, Jr., Patel, BR. Radionuclide angiography as a confirmatory test for brain death: A review of 229 studies in 219 patients. South Med J. 1997; 90:1091–1096.
50. Munari, M, Zucchetta, P, Carollo, C, et al. Confirmatory tests in the diagnosis of brain death: Comparison between SPECT and contrast angiography. Crit Care Med. 2005; 33:2068–2073.
51. de Freitas, GR, Andre, C. Sensitivity of transcranial Doppler for confirming brain death: A prospective study of 270 cases. Acta Neurol Scand. 2006; 113:426–432.
52. Quesnel, C, Fulgencio, JP, Adrie, C, et al. Limitations of computed tomographic angiography in the diagnosis of brain death. Intensive Care Med. 2007; 33:2129–2135.
53. Combes, JC, Chomel, A, Ricolfi, F, et al. Reliability of computed tomographic angiography in the diagnosis of brain death. Transplant Proc. 2007; 39:16–20.
54. Escudero, D, Otero, J, Marques, L, et al. Diagnosing brain death by CT perfusion and multislice CT angiography. Neurocrit Care. 2009; 11:261–271.