Nephrogenic systemic fibrosis
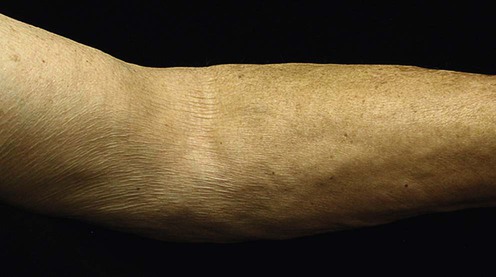
(From Nazarian, R.M., et al., 2011. Quantitative assessment of dermal cellularity in nephrogenic systemic fibrosis: a diagnostic aid. J Am Acad Dermatol 64(4), 741–7.)
Second-line therapies

(From Nazarian, R.M., et al., 2011. Quantitative assessment of dermal cellularity in nephrogenic systemic fibrosis: a diagnostic aid. J Am Acad Dermatol 64(4), 741–7.)
