Pancreatic Necrosectomy
Acute pancreatitis is a significant cause of morbidity and mortality in the United States, occurring in approximately 44 per 100,000 adults and accounting for more than 200,000 hospital admissions each year [1]. Of those patients, more than 80% have a benign course and recover without significant morbidity or recurrence [2]. However, in the minority of patients who suffer complications, the outcomes can be devastating. The most feared complication is the development of pancreatic necrosis, which is estimated to occur in 10% to 25% of all cases of acute pancreatitis [3,4]. The risk of mortality from necrotizing pancreatitis has been estimated between 10% and 20% [5–7] compared with an overall mortality of at most 5% to 10% for acute pancreatitis in general [8]. In those patients who develop necrosis, mortality is bimodal in its temporal distribution [9,10]. Early deaths are attributed mostly to severe multisystem organ failure within the first few days of onset [11], whereas late deaths tend to occur in the setting of infection and systemic sepsis [10,12].
Pancreatic necrosis is generally stratified by the presence or absence of infection. The presence of infection has been associated with as much as a 3-fold increase in mortality in severe necrotizing pancreatitis [1]. Infected necrosis has until relatively recently been considered an indication for urgent operative pancreatic necrosectomy [13]. This dogma has been challenged in recent years by the emergence of evidence suggesting that extended nonoperative management is feasible in a subset of stable patients even in the face of documented infection [14,15]. Open pancreatic necrosectomy itself carries a mortality of 7% to 43% [16], and complications such as postoperative abscess or pseudocyst formation, hemorrhage, bowel injury, pancreatic fistula, and late pancreatic exocrine and endocrine insufficiencies are common [17]. Minimally invasive techniques, including laparoscopic and endoscopic procedures, have begun to gain traction as alternatives to open debridement in selected situations in which operation cannot be otherwise avoided in patients [18].
Definition
At a 1992 international consensus conference in Atlanta, expert opinion was used to establish uniform terminology and a clinically based classification system for acute pancreatitis and its complications [19]. According to the Atlanta classification, the term pancreatic necrosis refers to diffuse or focal areas of nonviable pancreatic parenchyma typically associated with peripancreatic fat necrosis (Table 1). The diagnosis requires contrast-enhanced imaging for confirmation and, by definition, must involve approximately 30% or more of pancreatic parenchyma. The necrosis may be focal or diffuse but rarely involves the entire gland. In the Atlanta classification, pancreatic necrosis is specifically distinguished from other complications of severe acute necrotizing pancreatitis, such as pseudocyst, pancreatic abscess (which occurs in close proximity to the pancreas), and hemorrhagic pancreatitis. Consensus regarding the precise usage of these terms allows for clearer documentation of natural history and more accurate comparison of the results of therapy across different centers. However, in practice, these distinctions often fail to capture the unique context of an individual patient of which there is often more ambiguity in the clinical presentation than these discrete terminologies otherwise imply. Although the Atlanta classification currently remains the standard system for terminology in acute pancreatitis, its application in reported series has been less than uniform, and several groups have suggested modifications that consider the presence of local or systemic complications and the number and type of remote organ system failures [20–22].
Pathophysiology and prevention of sterile and infected necrosis
Acute pancreatitis has many causes, most commonly alcohol abuse and gallstones, each accounting for approximately 35% of cases [23]. Less-common risk factors include hypertriglyceridemia, hypercalcemia, post–endoscopic retrograde cholangiopancreatography pancreatitis, genetic factors, and anatomic anomalies such as pancreatic divisum [23]. Irrespective of the underlying cause, the development of pancreatitis is thought to derive from inappropriate activation of trypsin and other enzymes within the pancreatic acinar cells and the surrounding parenchyma [24]. Normally, the pancreatic acinar cell synthesizes and stores digestive enzymes in precursor forms within zymogen granules along with natural protease inhibitors, such as pancreatic secretory trypsin inhibitor. Excessive pancreatic stimulation or other insults may, in predisposed individuals, overwhelm or disrupt the normal mechanisms that prevent pancreatic autodigestion [24]. In particular, unregulated activation of enzymes, such as trypsin and elastase, may trigger parenchymal proteolytic damage, leading to influx of inflammatory cells and production of soluble proinflammatory mediators, such as interleukin (IL) 1 and tumor necrosis factor α [25–27].
Necrosis in acute pancreatitis is thought to be multifactorial. Microvascular damage may be induced by reactive oxygen species and other noxious agents [28], as well as compromised tissue oxygen delivery mediated by the vasoactive effects of endothelin and the inhibition of bradykinin [28]. Underresuscitation of patients during the early phases of acute pancreatitis is thought to lead to pancreatic hypoperfusion as the consequence of mesenteric vasoconstriction. It has been reported that the development of pancreatic necrosis is significantly more likely in patients who show early evidence of hemoconcentration, indicating inadequate replenishment of pancreatitis-associated fluid losses due to vomiting and third-space losses [29]. Phospholipase A2 has been implicated as a potential prognostic marker and an important mediator of injury [30]. Necrosis of the peripancreatic vasculature may lead to hemorrhage. Significant bleeding can occur if there is erosion into one of the larger vessels, most commonly involved vessels include the splenic and gastroduodenal arteries or the portal vein [31,32]. These patients often present with massive hematemesis or intraperitoneal hemorrhage and may require urgent embolization or surgical intervention with high-attendant mortality of approximately 60% [31,33].
Infection of pancreatic necrosis is thought to develop via several mechanisms. Systemic and intraperitoneal sites of inflammation have been shown experimentally to induce translocation of intestinal microbes that are then postulated to directly extend into or otherwise seed foci of necrosis [34]. Mesenteric hypoperfusion and retroperitoneal inflammation lead to impaired intestinal motility and consequent bacterial overgrowth, as well as mucosal barrier disruption and an overall repression of host immune responses [35]. Secondary seeding of pancreatic necrosis may derive from remote sites of bacterial colonization or infection, such as central intravascular lines or indwelling urinary catheters. The most common bacteria isolated from the pancreas are gram-negative enteric organisms, such as Escherichia coli and Proteus mirabilis [36]. However, gram-positive cocci are frequently isolated as well [37], suggesting nonenteric sources, including contamination during diagnostic needle aspiration, may also be contributory.
Whether antibiotic prophylaxis is effective in the prevention of pancreatic infection and reduction in mortality in the setting of severe acute pancreatitis remains somewhat controversial. Although the experimental and theoretical rationale for antibiotic prophylaxis is compelling [38,39], clinical trials have yielded conflicting results, depending on patient inclusion criteria, choice of antibiotic, and timing of administration. Concerns over the possible selection for resistant bacteria and the potential for the emergence of fungal infection have been raised [40]. The most recent systematic review of 7 studies comprising a total of 404 patients found no difference in either mortality or prevention of secondary pancreatic infection [41]. Meta-analysis has further shown no difference in the need for surgical intervention [42]. Given the potential concerns and lack of evidence to support their use, prophylactic antibiotics should not be used routinely in clinical practice. Probiotic (rather than antibiotic) prophylaxis has been suggested as an alternative approach to the prevention of infection in pancreatic necrosis by maintaining normal gut flora. However, a prospective double-blind multicenter trial failed to show a reduction in the risk of infectious complications and was also associated with an unexpected increase in mortality in the probiotic group [43].
Diagnosis
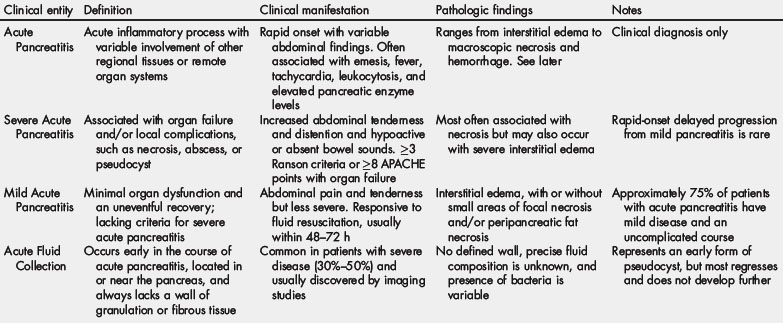
Computed tomography (CT) is particularly useful in establishing the presence of pancreatic necrosis, and CT-based grading systems have been proposed as predictors of clinical outcomes [44]. Early noncontrast CT can predict severity and mortality with reasonable accuracy [45], although intravenous contrast-infused protocols are necessary to establish definitively the presence of necrosis (Fig. 1) [46]. By definition, the CT criteria for pancreatic necrosis are diffuse or focal areas of nonenhancement (density less than approximately 50 Hounsfield units) involving more than 30% or 3 cm of the pancreas. Magnetic resonance imaging (MRI), and in particular the combination of T1- and T2-weighted sequences, can also be used to establish the diagnosis and extent of pancreatic necrosis [47]. The additional information provided by MRI cholangiopancreatography (MRCP) may be useful to evaluate ductal anatomy and the presence of bile duct stones, but otherwise there are few, if any, differences between CT and MRI with respect to diagnostic accuracy, prediction of severity, or other outcomes [48].
Various scoring systems have been devised to predict severity and estimate mortality in acute pancreatitis, beginning with the original Ranson score [49]. The Computed Tomography Severity Index (CTSI), also known as the Balthazar score, is the most widely used radiological system (Table 2) [50,51]. The presence of necrosis features prominently in the CTSI. Although several studies indicate that the CTSI is more accurate in assessing severity of pancreatitis than physiologic scoring systems, such as the APACHE-II (Acute Physiology and Chronic Health Evaluation II), the traditional Ranson criteria, or biomarkers such as C-reactive protein (CRP) levels [52–54], clinical outcomes may correlate more closely with the Ranson criteria or the APACHE-II scores [55].
Table 2 The CTSI (Balthazar score). Patients are assigned a score from 1 to 10 based on CT findings as well as the extent of pancreatic necrosis
| Balthazar grade | Radiological findings | Points |
|---|---|---|
| A | Normal CT | 0 |
| B | Focal or diffuse enlargement of the pancreas | 1 |
| C | Pancreatic gland abnormalities; peripancreatic inflammation | 2 |
| D | Single fluid collection | 3 |
| E | ≥2 fluid collections and/or gas in or adjacent to pancreas | 4 |
| Amount of necrosis | Points |
|---|---|
| None | 0 |
| 0%–30% | 2 |
| 30%–50% | 4 |
| ≥50% | 6 |
Data from Balthazar E, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174(2):331–6.
CT-guided (or, less commonly, ultrasound-guided) aspiration of necrotic pancreatic tissue was introduced in the 1980s as a means to establish the presence or absence of infection and, for many years, formed a standard part of the treatment algorithm in many centers [37,39,56–62]. This practice was based on the prevailing field bias at the time in favor of early operative debridement of infected necrosis and for continued nonoperative management of otherwise-stable patients with sterile necrosis [13]. Theoretical concerns about percutaneous aspiration include the potential for conversion of sterile necrosis to infected necrosis by instrumentation. One study showed a secondary infection rate of 22%, although with a very small sample size [63]. In general, rates of secondary infection are extremely low and virtually noncontributory to the overall infection rate, and thus, image-guided needle aspiration should still be considered a safe diagnostic tool [64]. However, with increased adoption of nonoperative and minimally invasive alternatives even in cases of infected necrosis, the application of this practice has become somewhat less routine. Various serum markers have been proposed as noninvasive alternatives to the diagnosis of necrosis and the presence of infection. Procalcitonin levels of 1.8 ng/mL or more on 2 consecutive days predicted infected necrosis and differentiated it from sterile necrosis with a sensitivity of 95%, specificity of 88%, and accuracy of 90% in one study [65]. A large multicenter trial showed superiority of procalcitonin to CRP in predicting multisystem organ failure in patients with acute pancreatitis [66]. CRP is readily available in most centers and carries a sensitivity and specificity of more than 75% in predicting necrosis at levels greater than 200 mg/L [67,68]. Various other markers, such as IL-6 and IL-8 [69] and serum amyloid A [70], have also been correlated with disease severity, although these tests are not widely available for routine use.
Surgical indications and timing of intervention
Historically, the mortality of severe acute necrotizing pancreatitis was so high that surgical dogma mandated operative intervention by debridement, drainage, and open packing. By the 1980s, an association between the presence of infection and overall mortality was recognized, and a more selective approach began to be adopted. While in Europe, early surgical debridement was endorsed for all cases of sterile necrosis, in American centers, the practice of early CT-guided aspiration of areas of necrosis became the standard practice, with operative intervention reserved for cases of documented infection or rapid clinical deterioration [8,28,71]. The guidelines from the International Association of Pancreatology (IAP, 2002) [72], based mostly on level II-2 evidence, endorsed the use of fine-needle aspiration biopsy (FNAB) and recommended nonoperative management for FNAB-negative necrosis except in the presence of organ failure that does not improve with nonoperative therapy. However, current practice continues to evolve. Several groups began to report good results with initial nonoperative management of infected necrosis in selected clinically stable patients [14,15,73–75]. Most practitioners agree that most patients with sterile necrosis resolve without surgical intervention [6,76,77]. False-negative culture results may occur in as many as 20% of patients, and infection may be present with minimal signs. In most centers, FNAB is no longer routine. Even staunch advocates for early surgical intervention in patients with suspected infected necrosis are now more willing to accept a clinical picture consistent with infection as sufficient indication, such as persistent fevers, tachycardia, adynamic ileus, and continued or progressive multisystem organ failure. Surgical intervention continues to be indicated in patients with progressive multiorgan dysfunction, clinical deterioration, and failure to respond to supportive therapy [75].
A critical question is the timing of surgical intervention, and clinical practice has evolved considerably in this regard over the past few years. In the past, when the major option was open transperitoneal necrosectomy, surgical intervention was generally undertaken early in the course of the illness, typically within a few days of onset [78] because it was assumed that early operation, particularly in cases of infection, improves the otherwise-dismal prognosis. Several earlier studies suggested that early intervention within the first 48 to 72 hours is associated with particularly high mortality, and the IAP guidelines of 2002 recommended against surgical intervention within the first 14 days of onset of the illness unless there were specific overriding indications [72]. Subsequent studies have provided evidence that mortality remains high even within the first 14 to 21 days of presentation but can be reduced to less than about 8% when operation is delayed beyond 28 to 30 days [5,79]. One prospective randomized trial examining early versus late necrosectomy was stopped because of high mortality in the early operative group [80]. One explanation for the apparent benefit of delayed surgery is that, over time, the definition and demarcation between normal and necrotic tissues improve to the point to which dissection can be less extensive, the risk of hemorrhage and injury to surrounding organs can be reduced, and unnecessary removal of otherwise-viable pancreatic tissues can be limited [75]. Although the current practice is to delay surgical intervention wherever possible, it is acknowledged that the prolonged use of antibiotics, so typical of this approach, is associated with the emergence of resistant strains and fungal superinfection [75].
Surgical techniques
Open pancreatic necrosectomy
The traditional approach to patients with infected pancreatic necrosis has been open necrosectomy with drain placement and continual postoperative lavage. As described by Beger and colleagues [81], open necrosectomy is effective in removing the necrotic and infected tissues. However, mortality rates as high as 50% have been reported depending on the timing of intervention and patient selection [18], and the risks of bowel injury, postoperative fistula, recurrent abscess, and wound complications are considerable. Open necrosectomy is still generally considered to be the standard by which other alternatives should be judged and is less dependent on advanced technology or experience with emerging alternative modalities [82]. The details of the technique of pancreatic necrosectomy vary among surgeons and across institutions, but the general principles include complete debridement of necrotic tissue and wide drainage of infected compartments [66,77,79–83].
An upper midline laparotomy or left-sided subcostal incision is used to gain access to the peritoneal cavity, depending on radiologic findings of the extent of the necrosis and extension into retroperitoneal spaces. A midline incision has the advantage of preservation of the rectus abdominis muscles, as well as avoidance of the tracts from previously placed percutaneous drains. Preservation of the transverse mesocolon is recommended because it can serve as a partial barrier to the remainder of the abdominal contents once debridement is completed. Blunt dissection is used to elevate the omentum, stomach, transverse colon, and mesocolon off of the pancreatic body and tail. Access to the pancreatic head and uncinate process is gained via the lesser sac, and extensive mobilization of the duodenum is done via the Kocher maneuver. The necrosum can usually be finger dissected away from the underlying vasculature and a frequently normal pancreas because often the necrotic tissue is more peripancreatic than pancreatic per se. The area posterior to the superior mesenteric vessels should be carefully debrided and drained. Normal-appearing pancreas should be left undisturbed if possible, even at the expense of leaving a small amount of necrotic tissue behind. Necrosis may extend into the perirenal spaces and paracolic gutters, and these regions should be entered and thoroughly explored depending on preoperative imaging [83].
Once all the necrotic tissue has been removed, the adequacy of drainage should be assessed. Percutaneously placed catheters may be repositioned within the necroma cavities or may be exchanged. Additional catheters should be large bore, at least 28F. Ideally, the choice of surgical drainage catheter should allow for later guidewire exchange if necessary. Removal of the gallbladder is also recommended before leaving the abdomen because frequently a chronic smoldering cholecystitis is present and contributes to sepsis. However, if inflammation and infection obscures exposure and safe visualization of hilar structures, cholecystectomy may be deferred and tube cholecystostomy should be considered. If choledocholithiasis is suspected, intraoperative cholangiogram may be advisable [83].
Second-look and repeated procedures are occasionally necessary after open necrosectomy, although it is usually the goal to achieve complete and thorough debridement at the time of initial operation. Some surgeons opt for open packing followed by planned reexploration every 48 to 72 hours [84,85], but this is probably not necessary unless there has been significant hemorrhage or a question of viability of intestinal segments that may have been compromised by operative injury or mesenteric inflammation (particularly the transverse colon). After granulation tissue begins to appear in the necroma cavity, the abdomen may be closed with or without the use of additional closed drainage catheters [84], provided that postoperative edema and bowel distention have resolved sufficiently. Because multiple forays into the abdomen in this setting are associated with an increased risk of gastrointestinal fistula and other complications [86], the alternative of closed packing and lavage technique is attractive. Multiple-lumen drainage catheters are used in this setting for postoperative irrigation with balanced salt solution or peritoneal dialysis fluid instilled via the smaller lumen to facilitate evacuation of larger necrotic debris through the larger lumen [66,86]. Closed packing involves the use of multiple gauze-filled Penrose drains and closed-suction catheters. This option is also useful to control minor bleeding from raw surfaces after debridement [87]. Drains and packing are removed slowly after a minimum of 7 days, allowing for the slow collapse of the necroma cavity [88]. In experienced hands, mortality rates after open necrosectomy are as low as 15% depending on patient selection and timing. Because of the morbidity associated with multiple laparotomies, closed techniques are generally preferred [86].
Videoscopic surgical techniques
Because of the high mortality and complication rates associated with open necrosectomy, there has been an increasing interest in applying techniques of advanced minimally invasive videoscopic surgical alternatives in the treatment of pancreatic necrosis [89]. Transperitoneal (laparoscopic) and retroperitoneal (intracavitary) approaches have both been used.
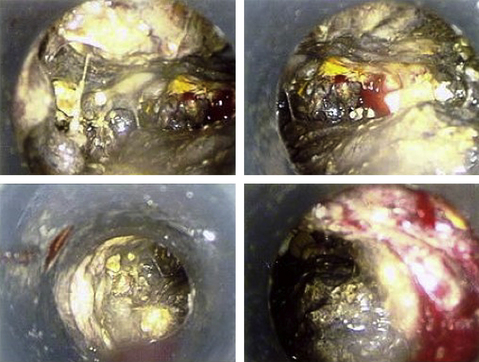
Laparoscopic transperitoneal pancreatic necrosectomy has been confirmed as a safe and feasible option in patients requiring surgery for pancreatic necrosis [87,90–92]. This technique in essence accomplishes the same goals of open surgery via several small anterior abdominal wall incisions for positioning of 5- to 15-mm trocars and traditional laparoscopic instrumentation. Pneumoperitoneum is created through insufflation of carbon dioxide. Similar to open operation, an infracolic approach is used to gain access to the lesser sac, and according to surgeon preference, a larger incision can be used for a hand port to aid dissection, gain access to deeper loculations and compartments, and control bleeding. Laparoscopic and hand-assisted port placement is outlined in Fig. 2, and a typical videoscopic view of necrosis is illustrated in Fig. 3. Drainage catheters are left in the unroofed necroma cavities, allowing for postoperative closed lavage as with open operation [92]. Advocates of the transperitoneal approach argue that it accomplishes the same extent of debridement and drainage of pancreatic necrosis as the tried-and-true open procedure. A key advantage to this technique is the avoidance of full laparotomy, a factor that may be important in critically ill patients with compromised respiratory status, provided that they can tolerate pneumoperitoneum. Repeat laparoscopic intervention is simpler and safer than repeat laparotomy, and wound complications, such as infection and hernia formation, are less common [93]. Review of results of transperitoneal laparoscopic necrosectomy [94] indicates likely selection and publication bias and limited documentation of the presence of infection. About 11% of patients required laparotomy, and overall mortality was 7% [94].
Several groups have reported a retroperitoneal approach in which the necroma cavity is directly accessed without entry into the peritoneal cavity [95,96]. This procedure has been described as laparoscopic or nephroscopic, but, by definition, neither term is strictly appropriate. In the literature, this procedure has variably been called intracavitary or percutaneous necrosectomy or, increasingly commonly, video-assisted retroperitoneal debridement (VARD). Although there are no direct comparisons to laparoscopic transperitoneal techniques, VARD is touted to achieve lower operative mortality than open necrosectomy (19% vs 38%), as well as lower overall morbidity, postoperative organ failure, and need for intensive care unit management [94,97]. This approach is conceptually appealing in that it preserves compartmentalization of the infection and avoids contamination of virgin spaces, including the peritoneal cavity. As a result, the intracavitary procedure is less likely to incite an overwhelming systemic inflammatory or septic response that may otherwise be the consequence of release of infected necrosis at open operation. Avoidance of major laparotomy incision and repeat transabdominal procedures is thought to reduce the risk of bowel injury, fistula formation, hemorrhage, and wound complications, although these benefits are to date imperfectly documented.
Several variants of the VARD procedure have been described. Typically, the patient is placed in a right lateral decubitus position to gain access to the left side of the retroperitoneum (into which pancreatic necrosis most commonly extends). Intraoperative fluoroscopy or ultrasonography is used to aid access by modified Seldinger technique into the retroperitoneal compartment and the necroma cavity. Direct percutaneous needle puncture or, alternatively, the track of a percutaneous drainage catheter placed under CT guidance may be used (see later). Under fluoroscopic guidance, a long (40–60 cm) floppy guidewire is placed into the cavity over which a 5-mm trocar is placed. After initial suction drainage of liquid debris, gentle insufflation is performed and the cavity is inspected with a 0° 5-mm scope. The track is then dilated to accommodate a 15- or 18-mm trocar using a Kittner dissector sponge as a guide. Next, without insufflation, the cap of the trocar is removed, and the 5-mm scope and a 5-mm grasper are inserted coaxially into the cavity for debridement of solid material. The assistant steadies the trocar and directs it systematically around in a circular direction to all regions of the space. At intervals, the cap is replaced, gentle insufflation is restored, and the cavity is revisualized to direct the trocar to deeper spaces and sites of heavy contamination. With the cap removed, jet lavage irrigation followed by gravity drainage into a receptacle is performed. An important difference between the retroperitoneal approach and open necrosectomy is that complete debridement of all necrotic materials is not necessarily the goal of the initial procedure. Materials that cannot be fully visualized or easily debrided are left behind, and a large drainage tube is left in place for postoperative irrigation. Because the peritoneal cavity is not breached, postoperative intra-abdominal adhesions are avoided, and thus repeated access to the necroma cavity is both simple and safe. Subsequent examinations may be performed every few days until the cavity is deemed to have been adequately cleared of debris and purulent loculations [98]. Multiple debridement procedures are usually necessary, with the median number of operations reported as 3 in one study [98]. In addition to this procedure, a significant number of patients (14%) may require conversion to, or subsequent, open procedure because of inability to entirely evacuate the necrosum [98] or because of complications such as hemorrhage.
Endoluminal therapy
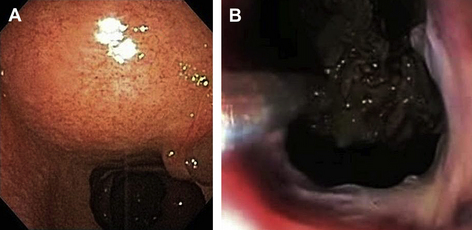
Endoscopic techniques have been applied to the treatment of complications of acute pancreatitis, such as pancreatic pseudocyst and, to a lesser extent, pancreatic necrosis, for more than 2 decades [99]. For the most part, this technique has consisted of simple drainage and pigtail or nasocystic catheter placement via transgastric, transduodenal, or transpapillary routes. More recently, endoscopic intervention has been extended into the necroma space to accomplish debridement and irrigation similar to other videoscopic procedures. The natural orifice transluminal endoscopic surgery (NOTES) has been increasingly applied in the treatment of pancreatic necrosis, either as definitive therapy or for temporization [100]. In most cases, the endoscope is passed via the mouth into the stomach, and the indentation of the lumen by the extrinsic necroma is identified (Fig. 4A). Most often, endoscopic ultrasonography is used to more precisely visualize the target area and avoid vascular structures. An enterotomy is made through the posterior wall of the stomach or, in some cases, the medial wall of the duodenum, to reach the lesser sac and necroma cavity [101]. A multiple-channel endoscope is inserted into the cavity (Fig. 4B), and biopsy forceps, baskets, and other standard instruments are used in conjunction with generous irrigation and suction. Tissue debris is brought back into the gastrointestinal lumen to pass by peristalsis. Extensive debridement through what is essentially a controlled internal fistula can generally be achieved safely, although there is an ever-present risk of iatrogenic hemorrhage [102]. Most reports of this technique have been favorable, although usually retrospective and small, with mortality rates of 0% to 15% [103–106]. One larger multicenter study (the GEPARD [Geriatric Patients Right to Adequate Destination] study) cited a success rate of 80%, with a morbidity and mortality of 26% and 7.5%, respectively. In this study, 4% required subsequent intervention by conventional surgical techniques [107]. Most surgeons now consider endoscopy and the NOTES a viable alternative to open or laparoscopic surgery in the drainage of pancreatic necrosis when used in patients with appropriately located and accessible collections.
Image-guided drainage procedures
Interventional radiology plays a key role in the management of complications of severe acute pancreatitis. In limited instances, percutaneous radiologic-guided drainage is used as definitive therapy [108], but more frequently it serves as an adjunct during the course of other interventional therapy (eg, up-sizing of surgical drains) [83]. Percutaneous drainage may be appropriate as primary intervention in patients who are considered otherwise unfit for surgical or endoscopic intervention, particularly to drain fluid collections, such as pseudocysts [109]. Because the relatively smaller caliber of even the largest pigtails catheters cannot effectively evacuate thick debris and organized areas of necrosis, percutaneous methods are usually avoided in the setting of pancreatic necrosis. Recently, however, percutaneous drainage has been used more aggressively to temporarily control systemic sepsis and allow definitive operation to be deferred to a safer delayed time frame. Contrary to established surgical dogma, it has been found that complete resolution of illness can be achieved in as many as one-third of appropriately selected patients with infected necrosis by percutaneous drainage and extended antibiotic therapy alone, despite failing to address the solid components of the necroma cavity [110].
Hybrid procedures
The combination of various minimally invasive interventional approaches has been proposed as an alternative to traditional open necrosectomy. Based on retrospectively reviewed results of hybrid therapy, members of the Dutch Pancreatitis Study Group proposed a registered, prospective, randomized, multicenter study (the PANTER [Pancreatitis, Necrosectomy Versus Step Up Approach] trial) to compare open necrosectomy to a step-up protocol [110]. Under the step-up protocol, patients initially undergo percutaneous or endoscopic drainage to control sepsis, which is followed by VARD after an appropriately delayed interval, if necessary, in patients who failed to improve or were otherwise persistently unwell [110]. A total of 88 patients were randomized. A significant reduction (69% vs 40%, comparing open vs step-up) in the primary end point of major complications or death was observed. Mortality was identical, and the difference between the groups was entirely accounted for by major complications, including new-onset or persistent multiple organ failure, fistulae, and major bleeding events (40% vs 69%). About 35% of patients who underwent initial percutaneous drainage as part of planned step-up did not require surgical intervention [110]. The step-up approach was associated with fewer incisional hernias and lower risk of new-onset diabetes [110].
Long-term outcomes
Little information regarding long-term results is available for any of the interventional options, including the need for hospitalization, reoperation, recurrent pancreatitis, length of stay, use of resources, cost-effectiveness, and the like. In some patients, fistula output through surgical or radiologic drains is persistent. In most instances, slow withdrawal of the catheter eventuates in fistula closure. In about 10% of cases, persistent fistula is usually because of pancreatic stricture or a disconnected distal (left) pancreatic segment. Definitive resolution may require resection (typically distal pancreatectomy and splenectomy) or internalization of the fistula track via Roux-en-y fistulojejunostomy [111].
Summary and recommendations
Several consensus guidelines for the management of pancreatic necrosis have been developed, including from the United Kingdom (1998, updated in 2005) [112,113], the IAP (2002) [72], and, most recently, the Japanese Society of Hepato-Pancreatic Surgery (2010) [75]. The authors’ recommendations, based on these guidelines and other published data, are summarized in Box 1. Patients with severe acute necrotizing pancreatitis should undergo radiologic imaging, and supportive care without prophylactic antibiotics should be initiated. In cases of suspected infected necrosis, confirmatory aspiration and culture may be performed according to institutional and practitioner preference. Sterile necrosis should generally respond to nonoperative management, and operation should be reserved for clinical deterioration and persistent unwellness past the 4-week mark. Infected necrosis may be an indication for surgical debridement and drainage by an approach (or combination of approaches) that is tailored to the specific anatomic circumstances and local experience. If possible, operative intervention should be delayed for a minimum of 2 weeks and preferably 4 weeks after onset. Antibiotics should target specific bacterial or fungal isolates whenever possible. Temporizing catheter drainage may be useful in controlling systemic sepsis, allowing for sequestration and organization of necrosis, infection, and inflammation in the retroperitoneum. Open necrosectomy remains the standard against which other interventions are judged. Videoscopic and endoluminal drainage and debridement are emerging as effective alternatives in experienced hands in selected patients. A step-up approach combining percutaneous and/or endoscopic drainage followed by VARD is particularly promising but requires further evaluation. Choice and timing of interventional therapy must be individualized for the unique clinical circumstances of each patient, ideally involving an experienced multidisciplinary team including surgeons, endoscopists, and radiologists.
Initial care and diagnosis
Indications and operative timing
Surgical procedure