Chapter 68 Myopic Macular Degeneration
Pathologic myopia is a major cause of legal blindness and low vision worldwide,1–5 and its prevalence is increasing in modern society, presumably due to an increase of near activities. In patients with pathologic myopia, various kinds of myopic macular degeneration develop in the posterior fundus, and these are the cause of visual impairment.6,7 There are new ideas concerning the pathogenesis of pathologic myopia and novel treatments for myopic macular degeneration. Although myopic macular degeneration had been an incurable degenerative disorder, the recent application of photodynamic therapy (PDT) with verteporfin and anti-vascular endothelial growth factor (VEGF) therapy has enabled treatment of myopic choroidal neovascularization (CNV). With these advances, myopic macular degeneration is now a curable disorder in some patients to some extent. Importantly, a novel pathology called “myopic macular retinoschisis” has been described using optical coherence tomography (OCT).8 The OCT examinations showed that retinoschisis was present in some eyes with pathologic myopia before the development of myopic macular holes. With the description of myopic macular retinoschisis, vitrectomy has been actively performed to reduce the chance of progression to more serious conditions like macular holes or macular retinal detachment.
Epidemiology
Myopia is more common among Asian populations, especially in East Asian countries, than among white, black, or Hispanic people. The prevalence of myopia (spherical equivalent (SE) < –0.5 or –1.0 D) is reported to range from 17% to 43% among Asian populations.9–17 Among white populations in Europe, the USA, and Australia, it is reported to be between 13% and 27%.18–21 In Hispanic and black populations, it is reported to be 17% and 21%, respectively.19, 22 The prevalence of high myopia (SE < –5.0 or –6.0 D) is also greater in Asian populations, ranging from 1.7% to 9.1%, than that in white, Hispanic, and black populations (2.0%, 2.4%, and 1.0%, respectively) (Table 68.1).9–22
Pathologic myopia is one of the major causes of visual impairment and blindness worldwide. For example, myopic macular degeneration is the leading cause of blindness in Japan, the second most common cause in Denmark4 and in China,23 and the third cause of blindness in Latinos 40 years and older in USA.3, Myopic macular degeneration was the leading cause of impaired vision for persons aged between 55 and 75 years in the Netherlands.3,23,24 In Italy and Taiwan, it is the second most common cause of low vision.25,26 The impact of myopic retinopathy on visual impairment is of great concern because it is often bilateral and irreversible, and it frequently affects individuals during their most productive years.7,27
To date, three population-based studies have estimated the prevalence of myopic retinopathy. The Blue Mountains Eye Study in Australia, which focused on a white population, reported that the prevalence of myopic retinopathy was 1.2%.27 In the Beijing Eye Study in China and the Hisayama Study in Japan, the prevalence was 3.1% and 1.7%, respectively.28a Study participants’ characteristics, methodology, and study design (for example, the definition of myopic retinopathy) were different between those studies, therefore the precise racial difference in myopic retinopathy prevalence is unknown. But it may be higher in East Asian populations than in white populations and the prevalence of high myopia is probably higher as well (Table 68.2).
The prevalence of myopic retinopathy is increased with advancing age in those population-based studies. Histological studies have shown a decreased density of photoreceptors, ganglion cells, retinal pigment epithelium (RPE), and optic nerve fibers with age.29,30 Several studies using OCT have examined subjects with healthy eyes with neither high myopia nor hyperopia and reported a negative relationship between retinal thickness and age.31,32 In addition to the axial elongation of the eyeball in highly myopic eyes, increasing age may contribute to the pathogenesis of myopic retinopathy by causing retinal thinning.
Although it is reported that men have longer axial length than women,33,34 many hospital-based studies showed higher prevalence of myopic retinopathy in women than in men.35–37 For example, Hayashi et al. showed that, of 429 consecutive patients with pathologic myopia, 147 were men and 282 were women, which is about twice as many cases in women as in men.7 Comparable findings were also observed in population-based studies. In the Blue Mountains Eye Study, the prevalence of myopic retinopathy in men was 0.06% and that in women was 0.4%. In the Hisayama Study, the prevalence of myopic retinopathy in men and in women was 1.2% and 2.2%, respectively. The Beijing Eye Study did not report the prevalence of myopic retinopathy by sex, but the numbers of female/male subjects with and without myopic retinopathy were 75/57 and 570/489, respectively. It suggests that not only greater axial length but other risk factors such as genetic factors and lifestyle risk factors may contribute to the pathogenesis of myopic retinopathy.
Pathogenesis
Hereditary or genetic factors play important roles in the development of pathologic myopia. There is a large international linkage study on familial high myopia, in which linkage analyses were performed on 1201 samples from Asian, African American, and Caucasian families, finding that the MYP1, MYP3, MYP6, MYP11, MYP12, and MYP14 loci were replicated.38 Recent genomewide studies identified susceptibility loci at 15q14 and 15q25 for myopia.39,40 However, the genetic influence on the development of myopic macular degeneration is not clear. The genetic risk factors of age-related macular degeneration related to rs11200638 of HTRA1, and rs1061170 (Y402H) of complement factor H did not appear to contribute significantly to the development of CNV in a highly myopic elderly Japanese population.41 It was reported that the responsiveness of myopic CNV to PDT had a correlation with common coagulation balance gene polymorphisms.42
Various factors, such as aging, and biomechanical factors in addition to hereditary factors, have been considered to contribute to the development of myopic macular degeneration in highly myopic eyes.43–45 Excessive axial elongation and posterior staphyloma (defined below) formation are critical features of pathologic myopia and these are considered important for the development and progression of myopic macular degeneration.44 Large-scale studies show that a peripapillary crescent, chorioretinal atrophy, and posterior staphyloma are significantly related to increased axial length.28,44 Above all, the incidence, size, and type of peripapillary crescent have the strongest correlation with the axial length;6,44 more than 95% of eyes with an axial length of 26.5 mm or more have a peripapillary crescent, while 0% of eyes with an axial length of 21.4 mm or less have a peripapillary crescent.44 Chorioretinal atrophy has also been related directly to increased axial length.
In addition to the influence of biomechanical factors, aging is an important factor for the development of posterior staphyloma and subsequent myopic macular degeneration. A posterior staphyloma is rarely found in highly myopic patients younger than 40 years of age.46 The posterior staphyloma develops as patients age, and this accelerates the further mechanical extension of the posterior fundus and causes the development of myopic macular degeneration. The incidence of myopic chorioretinal atrophy increases with age, and these changes are rarely seen in persons less than 20 years old.6,44 Childhood high myopes do not develop myopic macular degeneration or posterior staphyloma.47
The development of myopic CNV and lacquer cracks is not directly correlated with axial length. The incidence of myopic CNV peaks in the fourth decade. There may be biological mechanisms of neovascular membrane formation other than axial elongation or aging.6 Steidl and Pruett48 reported that eyes with a shallow staphyloma had the high frequency of CNV. They suggested that it is possible that eyes with a shallow staphyloma may be healthier and more metabolically active with well-perfused chorioretinal tissue and good capacity to respond to injury by neovascular ingrowth. It appears that the influence of aging and mechanical factors is rather complicated and different lesions may have different pathogenetic influences.
Histopathology
The sclera
Scleral thinning and localized ectasia of the posterior sclera are characteristic changes of the eyes with pathologic myopia. Microscopically, the normal sclera consists of interwoven bands or bundles of collagen fibers.43 These are usually well integrated and in longitudinal section present a relatively homogeneous appearance throughout their extent. The architectural changes in the longitudinal fibers in pathologic myopia consist of thinning of the collagen bundles, a reduction in refringence of the bundle edges, and the loss of longitudinal fiber striations. The cross-sectional fibers demonstrate dissociation such that the individual fibers separate from one another. There is also a reduction in the size of the individual dissociated fibers. The more advanced examples of architectural disorganization are found in and about the regions of the posterior pole and peripapillary sclera. It has also been reported that the elastic fibers within the sclera showed a definite decrease in the number of fibers.
Electron microscopic analyses showed that a predominance of collagen fibrils of small diameter, usually averaging below 60–70 nm, was found.43 Fibrils of a very fine diameter were also observed. Also, the cross-sectioned fibrils showed a marked increase in the prevalence of fissured or “star-shaped” forms. Most of the ultramicroscopic alterations seen in the myopic sclera indicate a derangement of the growth and organization of the fibrils. These may be products of defective fibrillogenesis. These aspects of development are thought to be under the control of the acidic glycosaminoglycan composition of the interfibrillar substance. It is also conceivable that this picture corresponds to abnormal fibril growth in the presence of an accentuated breakdown or catabolism of the sclera.
Choroid and retinal pigment epithelium
The degenerative changes found in pathologic myopia initially appear to involve the choriocapillaris – Bruch’s membrane – RPE complex. The changes subsequently affecting the choroid were essentially degenerative and atrophic. Thinning of the choroid and loss of the choriocapillaris were reported. Choroidal vascular occlusions are a prominent feature of the disease, and this process appears to affect the smaller-diameter vessels initially. The choroidal vessels appear to be fewer and to have thinner walls than seen normally. There is a generalized loss of the normal connective tissue framework of the choroid with some degree of compaction of the vessels. Although the large-sized choroidal vessels tend to be most resistant, these, too, may undergo occlusion in the late stages of the disease. Recent studies using enhanced-depth imaging of OCT or high-penetrance OCT also showed significant thinning of the choroid in highly myopic eyes.49
Animal models
Rhesus monkeys, chickens, fish, tree shrews, marmosets, and guinea pigs have long been used as animal models of experimental myopia by inducing form deprivation myopia by lid suture or by wearing plastic goggles (Figs 68.1 and 68.2).50–52 Chicks have long been the main animal model for experimental myopia because myopia accompanying a dramatic increase of the axial length can be induced easily by wearing plastic goggles in about 2 weeks.53 Therefore, many studies were performed using chick models of experimental myopia; these studies identified responsible factors which cause excessive eye growth. However the sclera of chickens differs from that of humans. The chicken has the classic vertebrate sclera, consisting of a layer of cartilage surrounded by layers of fibrous connective tissue, whereas in most mammals, including primates and rodents, the cartilage has been lost.
Based on the benefit of having similar scleral components, mice have commonly been used as examples of experimental myopia (Fig. 68.3),54,55 although there is some drawback that the induced myopia is not as severe as in chickens because mice are not “visual animals.” Mouse model advantages also include the availability of numerous knockout mutants, more advanced gene microarrays for screening the transcriptome, and a completely sequenced genome.
The transcriptome of neurosensory retina without RPE was analyzed with Affymetrix GeneChip mouse genome 430 2.0 arrays after unilateral retinal image degradation by use of frosted goggles in mice,56 and some genes were identified as being altered, including a downregulation of the early growth response 1 (Egr-1) gene. The refractions of homozygous Egr-1 knockout mice were some 4–5 D less hyperopic relative to the wild type.58 Zebrafish have also been considered as a model of experimental myopia. Downregulation of zebrafish lumican gene expression manifested ocular enlargement resembling axial myopia due to disruption of the collagen fibril arrangement in the sclera and resulted in scleral thinning.58 The lumican gene, which encodes one of the major keratan sulfate proteoglycans in the vertebrate cornea and sclera, has been linked to axial myopia in humans. Veth and colleagues59 have used zebrafish to identify a genetically complex, recessive mutant that shows risk factors for glaucoma, including adult-onset severe myopia, elevated intraocular pressure, and progressive retinal ganglion cell pathology. Positional cloning and analysis of a noncomplementing allele indicated that nonsense mutations in low-density lipoprotein receptor-related protein 2 (lrp2) underlie the mutant phenotype.
Features of the myopic fundus
Myopic conus
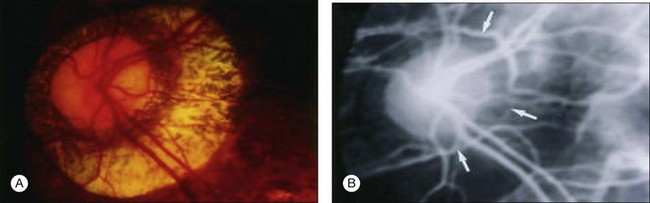
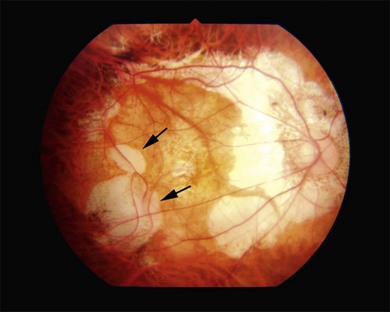
Myopic conus is one of the earliest features which develop in the posterior fundus of highly myopic eyes. Due to a mechanical expansion in the peripapillary sclera, the optic disc in eyes with pathologic myopia is surrounded by a concentric area of depigmentation, the so-called myopic conus. The myopic crescent usually appears as a white, sharply defined area where the inner surface of the sclera is seen distinctly (Fig. 68.4). According to its range, the myopic conus is divided into the temporal conus, the nasal conus, the inferior conus, and the annular conus (Fig. 68.4). Sometimes the myopic conus becomes remarkably large. The myopic conus and tesselated fundus are the earliest lesions which develop in eyes with pathologic myopia, and these lesions can be observed even in children and young individuals.47 When we perform indocyanine green angiography, we see the Zinn – Haller arterial ring in the middle of the myopic conus in eyes with a large annular conus (Fig. 68.5).60,61 The inner zone of myopic conus might develop as a result of mechanical stretching, and the outer zone might be the result of a secondary circulatory disturbance and mechanical stretching.
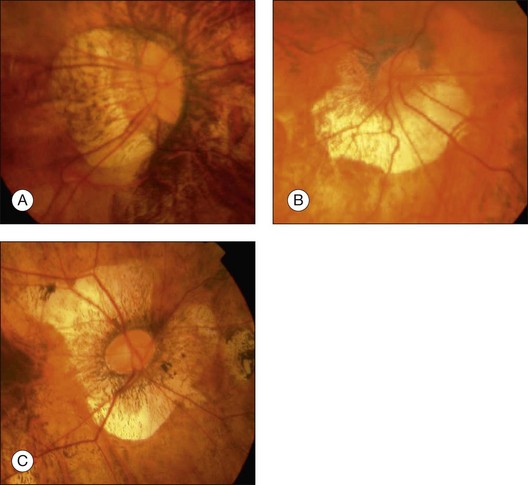
Fig. 68.4 Various types of myopic conus. (A) Temporal conus; (B) inferior conus; (C) annular or ring conus.
Posterior staphyloma
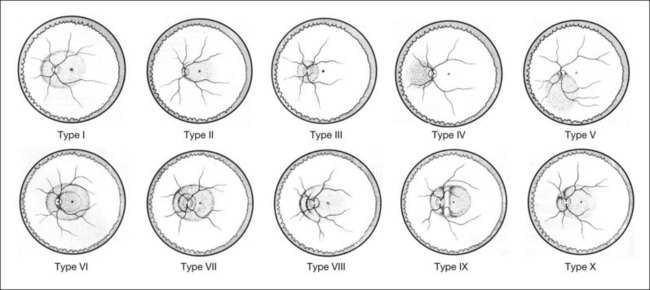
A posterior staphyloma is an outward protrusion of all layers of the posterior eye globe and is considered a hallmark lesion of pathological myopia. There are 10 different types of staphyloma according to Curtin (Fig. 68.6).62 Types I through V are basic staphylomas, and types VI through X are compound staphylomas. A posterior staphyloma is not common in children with pathologic myopia, and the incidence of staphyloma is significantly higher in older patients (96.7% in those ≥50 years of age) than in younger patients (80.7% in those <50 years).46 Earlier studies on human cadaver eyes suggested the possibility that eyes with pathologic myopia were not simply elongated but were deformed and did not have a spherical shape.
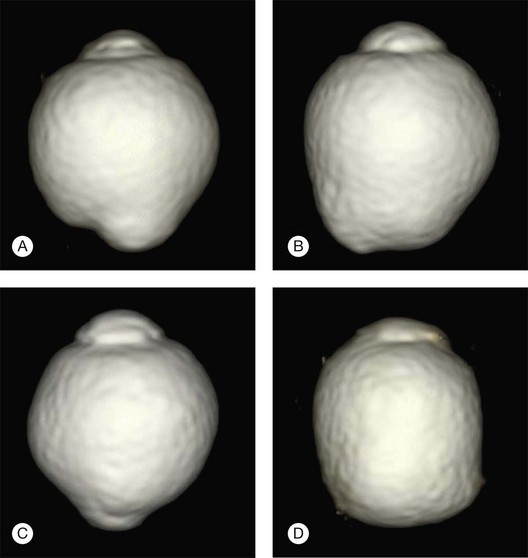
With the development of three-dimensional magnetic resonance imaging analysis of human globes in highly myopic patients, it is possible to demonstrate distinctly different shapes of eyes with pathologic myopia (Fig. 68.7).63 This clearly supports the idea that pathologic myopia is a disease accompanied by deformity of the globe. The eye is an exact optical device and the fact that its shape is different from normal spherical shape could cause serious visual problems. The correlation between specific eye shape in eyes with pathologic myopia and its effect on visual impairment are now being investigated.
Myopic chorioretinal atrophy
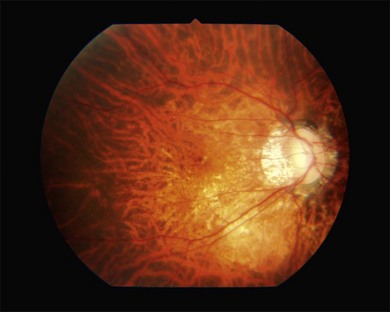
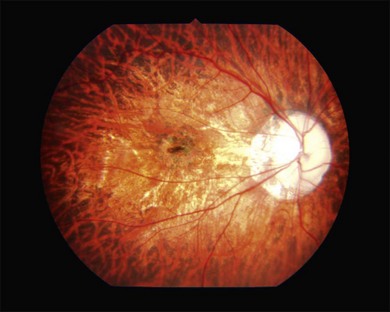
Two types of myopic chorioretinal atrophy develop in the posterior fundus. According to Tokoro, there is diffuse chorioretinal atrophy and patchy chorioretinal atrophy.6 Diffuse chorioretinal atrophy is observed as yellowish-white and ill-defined chorioretinal atrophy (Fig. 68.8), and patchy chorioretinal atrophy is observed as grayish-white and well-defined chorioretinal atrophy (Fig. 68.9). Different from diffuse chorioretinal atrophy, the patchy chorioretinal atrophy is caused by complete loss of choriocapillaris and there is a corresponding absolute scotoma.
Lacquer cracks
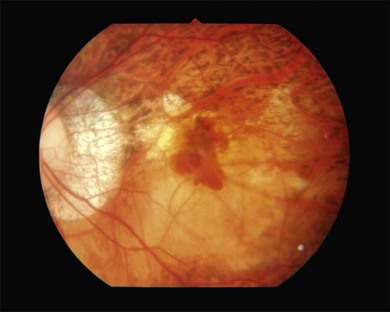
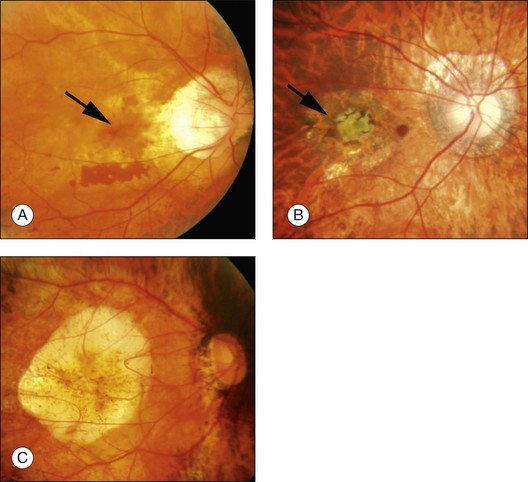
Lacquer cracks are linear ruptures of Bruch’s membrane in the macular area of highly myopic eyes and are observed as yellowish linear lesions in the macula (Fig. 68.10). Lacquer cracks are more easily detected by fluorescein angiography, fundus autofluorescence imaging, and indocyanine green angiography.64,65 With time, lacquer cracks increase in number and also increase their width.66 When new lacquer cracks develop, the choriocapillaris is also damaged and subretinal bleeding may occur.67 Subretinal bleeding without CNV is a visible sign of new lacquer crack formation, and after absorption of bleeding, lacquer cracks appear as yellowish linear lesions. Lacquer cracks are known as precursor lesions of myopic CNV.65,68 Often, CNV tends to develop along the foveal edge of chorioretinal atrophy which was formed by increased width of lacquer cracks.
Myopic chorodial neovascularization
Macular CNV is a one of the most frequent complications that reduce the central vision in patients with pathologic myopia (Fig. 68.11). Myopic CNV develops in 10% of highly myopic patients,68 and 30% of the patients who have a CNV in one eye eventually develop CNV in the other eye. Due to a thin, stretched fundus, the bleeding does not usually overlie the CNV and thus, CNV is easily observed ophthalmoscopically. Myopic CNV is almost always so-called classic CNV and CNV shows distinct hyperfluorescence throughout the entire angiographic phase. Especially for small CNV, fluorescein angiography is a powerful tool to detect CNV.
Myopic macular retinoschisis or myopic foveoschisis
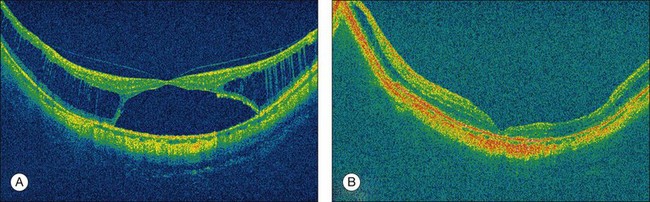
Myopic macular retinoschisis (also known as myopic foveoschisis69 or myopic traction maculopathy70) was first identified using OCT by Takano and Kishi in 1999.8 Myopic macular retinoschisis is found in 9% of highly myopic eyes with posterior staphyloma,71 and 50% of patients progress to more serious complications like full-thickness macular hole or macular retinal detachment within 2 years.72 Myopic macular retinoschisis is considered to be caused by various factors. The rigidity of the internal limiting membrane (ILM) can induce significant traction on the retina.4 OCT examinations of serial sections along the entire posterior vascular arcade showed that the paravascular abnormalities, such as paravascular lamellar holes,73 vascular microfolds,73–75 and paravascular retinal cysts,73 are frequently found in eyes with myopic macular retinoschisis. Although the mechanism of the development of this condition is not fully clear, the glial cells like astrocytes which exist abundantly around the retinal vessels can migrate and proliferate through the paravascular lamellar holes. These cells can produce collagen and facilitate the proliferative and contractile response of ILM. Studies have shown that vitrectomy is useful to treat myopic macular retinoschisis in some patients (Fig. 68.12).69,76 The need for ILM peeling remains controversial; however, it is appropriate to consider when apparent ILM traction is recognized on preoperative OCT images.
Natural course and treatment of myopic CNV
Myopic CNV is not intensely active, and thus has a tendency to regress spontaneously, and progresses from an active phase (Fig. 68.13A) to a scar phase (Fig. 68.13B). In the scar phase, the CNV is covered by proliferated RPE cells and is observed as a dark pigmented spot (Fuchs spot). After CNV regression, well-defined chorioretinal atrophy gradually develops and enlarges around the Fuchs spot and this causes a progressive visual decrease in the long term. This phase is called atrophic CNV (Fig. 68.13C).
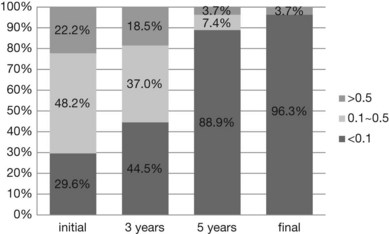
The prognosis of myopic CNV is poor. A natural history study with 10-year follow-up showed that, at the onset of CNV, 70% had a visual acuity better than 20/200, and 22% had a visual acuity better than 20/40. Three years after the onset of CNV, 56% retained a visual acuity of better than 20/200. At 5 and 10 years after onset, however, visual acuity dropped to 20/200 or less in 89% and 96%, respectively (Fig. 68.14).37,77 The mechanism by which chorioretinal atrophy develops and enlarges around the regressed CNV in eyes with pathologic myopia is not clear. Because chorioretinal atrophy around the CNV affects the final visual outcome of eyes with myopic CNV, we need to determine the effect of treatment against myopic CNV on chorioretinal atrophy development.
Anti-VEGF therapy and PDT with verteporfin (Visudyne) have recently been used to treat myopic CNV. The Verteporfin in the Photodynamic Therapy Study Group study, a randomized, prospective, clinical trial performed to confirm the safety and efficacy of the treatment of myopic CNV with PDT, reported a significantly better visual outcome at 12 months;78 however, the visual increase was not significant at 24 months,79 indicating that the long-term efficacy of PDT is not confirmed. PDT acts by producing selective choriocapillaris endothelial damage, which stabilizes the myopic CNV. Recently, a long-term study evaluating the effectiveness of PDT on myopic CNV showed that better visual outcome was associated with better initial best-corrected visual acuity (BCVA) and larger lesions in younger patients treated by PDT.80 Also, 71% of the highly myopic eyes with juxtafoveal CNV had BCVA of 0.5 or better at 4 years after PDT.35
Currently, bevacizumab (Avastin) and ranibizumab (Lucentis) are the most commonly used intravitreal anti-VEGF agents. It has been reported that 40% of patients have a significant (three lines or more) visual improvement at 1-year follow-up.36,81 The duration of symptoms, baseline visual acuity, and location of the CNV are factors that are predictive of the final visual acuity.81,82 CNV sometimes recurs, typically within 6 months of the initial injection.83 The number of treatments during the first year may depend on the loading dose regimen; however, the number of required treatments has been reported to range from 2.4 to 4.9 times annually.81–83 Several prospective and retrospective investigations have shown that anti-VEGF therapies offer more beneficial effects for visual outcomes compared with PDT.84–86 The influence of anti-VEGF therapies on the development of chorioretinal atrophy around myopic CNV needs to be evaluated to prove the long-term effectiveness of this treatment, since the outcome of 2-year results is not consistent.85,87–90
1 Krumpaszky HG, Ludtke R, Mickler A, et al. Blindness incidence in Germany. A population-based study from Wurttemberg-Hohenzollern. Ophthalmologica. 1999;213:176–182.
2 Munier A, Gunning T, Kenny D, et al. Causes of blindness in the adult population of the Republic of Ireland. Br J Ophthalmol. 1998;82:630–633.
3 Cotter SA, Varma R, Ying-Lai M, et al. Causes of low vision and blindness in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2006;113:1574–1582.
4 Buch H, Vinding T, La Cour M, et al. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111:53–61.
5 Iwase A, Araie M, Tomidokoro A, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi study. Ophthalmology. 2006;113:1354–1362.
6 Tokoro T. Types of fundus changes in the posterior pole. In: Tokoro T, ed. Atlas of posterior fundus changes in pathologic myopia. Tokyo: Springer-Verlag; 1998:5–22.
7 Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–1611. 1611 e1–4
8 Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–476.
9 Dandona R, Dandona L, Naduvilath TJ, et al. Refractive errors in an urban population in Southern India: the Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci. 1999;40:2810–2818.
10 Saw SM, Chan YH, Wong WL, et al. Prevalence and risk factors for refractive errors in the Singapore Malay Eye Survey. Ophthalmology. 2008;115:1713–1719.
11 Wong TY, Foster PJ, Hee J, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci. 2000;41:2486–2494.
12 Saw SM, Gazzard G, Koh D, et al. Prevalence rates of refractive errors in Sumatra, Indonesia. Invest Ophthalmol Vis Sci. 2002;43:3174–3180.
13 Bourne RR, Dineen BP, Ali SM, et al. Prevalence of refractive error in Bangladeshi adults: results of the National Blindness and Low Vision Survey of Bangladesh. Ophthalmology. 2004;111:1150–1160.
14 Gupta A, Casson RJ, Newland HS, et al. Prevalence of refractive error in rural Myanmar: the Meiktila Eye Study. Ophthalmology. 2008;115:26–32.
15 Wickremasinghe S, Foster PJ, Uranchimeg D, et al. Ocular biometry and refraction in Mongolian adults. Invest Ophthalmol Vis Sci. 2004;45:776–783.
16 Xu L, Li J, Cui T, et al. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–1683.
17 Sawada A, Tomidokoro A, Araie M, et al. Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115:363–370. e3
18 Wang Q, Klein BE, Klein R, et al. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–4347.
19 Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. 1997;38:334–340.
20 Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106:1066–1072.
21 Wensor M, McCarty CA, Taylor HR. Prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol. 1999;117:658–663.
22 Tarczy-Hornoch K, Ying-Lai M, Varma R. Myopic refractive error in adult Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2006;47:1845–1852.
23 Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113:1134 e1–11.
24 Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–658.
25 Cedrone C, Culasso F, Cesareo M, et al. Incidence of blindness and low vision in a sample population: the Priverno Eye Study, Italy. Ophthalmology. 2003;110:584–588.
26 Hsu WM, Cheng CY, Liu JH, et al. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111:62–69.
27 Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109:704–711.
28 Liu HH, Xu L, Wang YX, et al. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology. 2010;117:1763–1768.
28a Asakuma T, Yasuda M, Ninomiya T, et al. Prevalence and risk factors for myopic retinopathy in a Japanese population: The Hisayama Study. Ophthalmology. 2012. May 10 [Epub ahead of print]. PMID: 22578442
29 Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995;102:1853–1859.
30 Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17.
31 Eriksson U, Alm A. Macular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol. 2009;93:1448–1452.
32 Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87:899–901.
33 Lim LS, Saw SM, Jeganathan VS, et al. Distribution and determinants of ocular biometric parameters in an Asian population: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2010;51:103–109.
34 Fotedar R, Wang JJ, Burlutsky G, et al. Distribution of axial length and ocular biometry measured using partial coherence laser interferometry (IOL Master) in an older white population. Ophthalmology. 2010;117:417–423.
35 Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term results of photodynamic therapy for choroidal neovascularization in Japanese patients with pathologic myopia. Am J Ophthalmol. 2010.
36 Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab – a randomized controlled trial. Am J Ophthalmol. 2010;149:458–464. e1
37 Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003;110:1297–1305.
38 Li YJ, Guggenheim JA, Bulusu A, et al. An international collaborative family-based whole-genome linkage scan for high-grade myopia. Invest Ophthalmol Vis Sci. 2009;50:3116–3127.
39 Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42:897–901.
40 Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–905.
41 Nakanishi H, Gotoh N, Yamada R, et al. ARMS2/HTRA1 and CFH polymorphisms are not associated with choroidal neovascularization in highly myopic eyes of the elderly Japanese population. Eye (Lond). 2010;24:1078–1084.
42 Parmeggiani F, Gemmati D, Costagliola C, et al. Impact of coagulation-balance gene predictors on efficacy of photodynamic therapy for choroidal neovascularization in pathologic myopia. Ophthalmology. 2010;117:517–523.
43 Curtin BJ. Basic science and clinical management. In: Curtin BJ, ed. The myopias. New York: Harper and Row; 1985:177.
44 Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. I. The posterior fundus. Trans Am Ophthalmol Soc. 1970;68:312–334.
45 Tokoro T. Lacquer crack lesions and simple bleeding. In: Tokoro T, ed. Atlas of posterior fundus changes in pathologic myopia. Tokyo: Springer; 1998:101–123.
46 Hsiang HW, Ohno-Matsui K, Shimada N, et al. Clinical characteristics of posterior staphyloma in eyes with pathologic myopia. Am J Ophthalmol. 2008;146:102–110.
47 Kobayashi K, Ohno-Matsui K, Kojima A, et al. Fundus characteristics of high myopia in children. Jpn J Ophthalmol. 2005;49:306–311.
48 Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997;123:181–187.
49 Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–450.
50 Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68.
51 Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–1615.
52 Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251.
53 Troilo D. Experimental studies of emmetropization in the chick. Ciba Found Symp. 1990;155:89–102. discussion 102–14
54 Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010;51:1297–1303.
55 Barathi VA, Boopathi VG, Yap EP, et al. Two models of experimental myopia in the mouse. Vision Res. 2008;48:904–916.
56 Brand C, Schaeffel F, Feldkaemper MP. A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Mol Vis. 2007;13:920–932.
57 Schippert R, Burkhardt E, Feldkaemper M, et al. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007;48:11–17.
58 Yeh LK, Liu CY, Kao WW, et al. Knockdown of zebrafish lumican gene (zlum) causes scleral thinning and increased size of scleral coats. J Biol Chem. 2010;285:28141–28155.
59 Veth KN, Willer JR, Collery RF, et al. Mutations in zebrafish lrp2 Result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310.
60 Yasuzumi K, Ohno-Matsui K, Yoshida T, et al. Peripapillary crescent enlargement in highly myopic eyes evaluated by fluorescein and indocyanine green angiography. Br J Ophthalmol. 2003;87:1088–1090.
61 Ohno-Matsui K, Morishima N, Ito M, et al. Indocyanine green angiography of retrobulbar vascular structures in severe myopia. Am J Ophthalmol. 1997;123:494–505.
62 Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1977;75:67–86.
63 Moriyama M, Ohno-Matsui K, Hayashi K, et al. Topographical analyses of shape of eyes with pathologic myopia by high-resolution three dimensional magnetic resonance imaging. Ophthalmology. 2011;118:1626–1647.
64 Ohno-Matsui K, Morishima N, Ito M, et al. Indocyanine green angiographic findings of lacquer cracks in pathologic myopia. Jpn J Ophthalmol. 1998;42:293–299.
65 Ikuno Y, Sayanagi K, Soga K, et al. Lacquer crack formation and choroidal neovascularization in pathologic myopia. Retina. 2008;28:1124–1131.
66 Ohno-Matsui K, Tokoro T. The progression of lacquer cracks in pathologic myopia. Retina. 1996;16:29–37.
67 Ohno-Matsui K, Ito M, Tokoro T. Subretinal bleeding without choroidal neovascularization in pathologic myopia. A sign of new lacquer crack formation. Retina. 1996;16:196–202.
68 Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003;87:570–573.
69 Ikuno Y, Sayanagi K, Ohji M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137:719–724.
70 Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122:1455–1460.
71 Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135:338–342.
72 Shimada N, Ohno-Matsui K, Baba T, et al. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142:497–500.
73 Shimada N, Ohno-Matsui K, Nishimuta A, et al. Detection of paravascular lamellar holes and other paravascular abnormalities by optical coherence tomography in eyes with high myopia. Ophthalmology. 2008;115:708–717.
74 Shimada N, Ohno-Matsui K, Nishimuta A, et al. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology. 2007;114:2070–2076.
75 Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005;139:462–467.
76 Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003;110:1702–1707.
77 Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology. 2002;109:712–719.
78 Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial – VIP report no. 1. Ophthalmology. 2001;108:841–852.
79 Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial – VIP report no. 3. Ophthalmology. 2003;110:667–673.
80 Ruiz-Moreno JM, Amat P, Montero JA, et al. Photodynamic therapy to treat choroidal neovascularisation in highly myopic patients: 4 years’ outcome. Br J Ophthalmol. 2008;92:792–794.
81 Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol. 2009;147:94–100. e1
82 Calvo-Gonzalez C, Reche-Frutos J, Donate J, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol. 2011;151:529–534.
83 Chan WM, Lai TY, Liu DT, et al. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol. 2009;93:150–154.
84 Hayashi K, Ohno-Matsui K, Teramukai S, et al. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol. 2009;148:396–408.
85 Ikuno Y, Nagai Y, Matsuda S, et al. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2010;149:140–146.
86 Parodi MB, Iacono P, Papayannis A, et al. Laser photocoagulation, photodynamic therapy, and intravitreal bevacizumab for the treatment of juxtafoveal choroidal neovascularization secondary to pathologic myopia. Arch Ophthalmol. 2010.
87 Baba T, Kubota-Taniai M, Kitahashi M, et al. Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. Br J Ophthalmol. 2010;94:864–870.
88 Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010;248:937–941.
89 Hayashi K, Shimada N, Moriyama M, et al. Two year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathological myopia. Retina (in press).
90 Voykov B, Gelisken F, Inhoffen W, et al. Bevacizumab for choroidal neovascularization secondary to pathologic myopia: Is there a decline of the treatment efficacy after 2 years? Graefes Arch Clin Exp Ophthalmol. 2010;248:543–550.