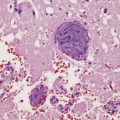
Multiple sclerosis
Demyelination is characterized by destruction of normal myelin with relative preservation of axons. By convention, the term demyelination excludes disorders in which there is a failure to form myelin normally (dysmyelination) or a loss of myelin as a result of axonal degeneration. The central nervous system (CNS), peripheral nervous system, or both, may be affected by demyelinating diseases. Disorders characterized by a loss of myelin due to an inherited defect of metabolism are considered in Chapter 22.
MULTIPLE SCLEROSIS (MS)
 Geographic and migration studies suggest an environmental etiologic agent.
Geographic and migration studies suggest an environmental etiologic agent.
 Family, twin, racial, human leukocyte antigen (HLA) and genome-wide association studies indicate that genetic factors play a role.
Family, twin, racial, human leukocyte antigen (HLA) and genome-wide association studies indicate that genetic factors play a role.
 Immunologic studies provide evidence of an autoimmune disorder, possibly precipitated by a viral infection.
Immunologic studies provide evidence of an autoimmune disorder, possibly precipitated by a viral infection.
CLASSIFICATION
Classical (also known as Charcot-type) MS is classified according to its clinical course as:
 Relapsing remitting (RRMS), in which the patient experiences multiple acute attacks, each followed by clinical improvement.
Relapsing remitting (RRMS), in which the patient experiences multiple acute attacks, each followed by clinical improvement.
 Secondary progressive (SPMS), in which after years of RRMS, the patient enters a stage in which there is no recovery between acute attacks.
Secondary progressive (SPMS), in which after years of RRMS, the patient enters a stage in which there is no recovery between acute attacks.
 Primary progressive (PPMS), in which the patient experiences progressive disease without episodes of recovery.
Primary progressive (PPMS), in which the patient experiences progressive disease without episodes of recovery.
 Relapsing progressive (RPMS), in which repeated acute attacks are superimposed on progressive disease without episodes of recovery.
Relapsing progressive (RPMS), in which repeated acute attacks are superimposed on progressive disease without episodes of recovery.
Until relatively recently, neuromyelitis optica (Dévic’s disease) was classified as a form of multiple sclerosis but in view of its distinct pathogenesis and pathology, it is now regarded as a separate disease (see Chapter 20).
CLASSIC (CHARCOT-TYPE) MS
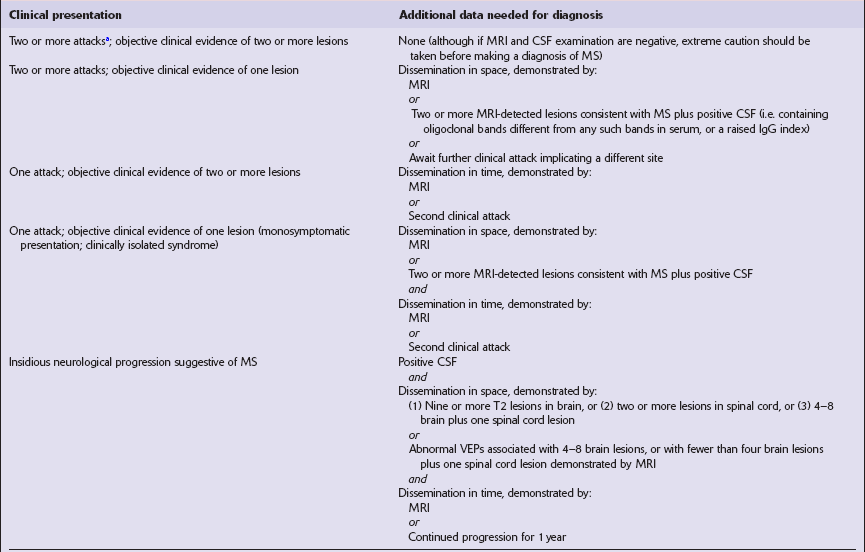
 Vary in size, shape, number, and distribution (Fig. 19.1).
Vary in size, shape, number, and distribution (Fig. 19.1).
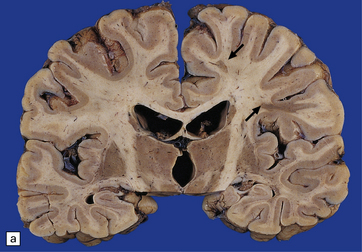
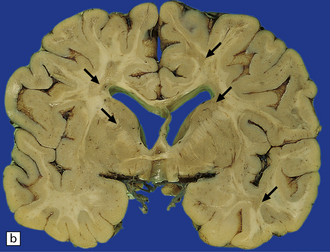
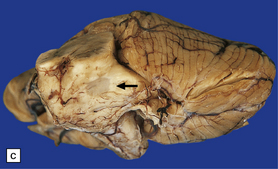

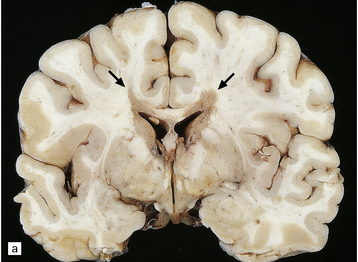
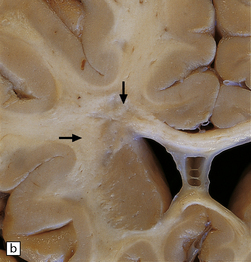
19.1 Plaques in MS: macroscopic appearance.
(a,b) Scattered plaques (arrows) of varying shapes, sizes, and locations in coronal brain slices from two patients with MS. (c) Plaques may be visible as slightly depressed areas of gray discoloration on the surface of the pons (arrow), or (d) on the surface of the spinal cord (arrow).
 May extend to the surface of the brain stem and spinal cord, forming gray depressions on external examination (Fig. 19.1).
May extend to the surface of the brain stem and spinal cord, forming gray depressions on external examination (Fig. 19.1).
 May be seen in the olfactory tracts and are frequently present in the optic nerves (Fig. 19.2).
May be seen in the olfactory tracts and are frequently present in the optic nerves (Fig. 19.2).
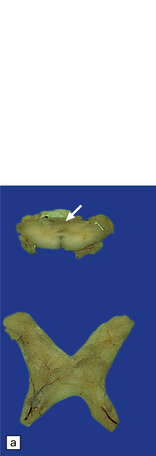
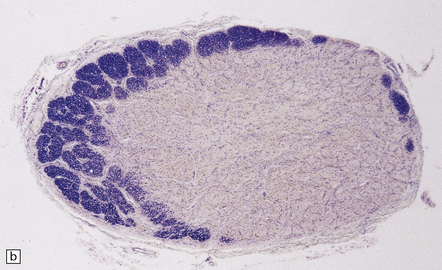
19.2 Optic chiasm and nerves in MS.
(a) Grayish brown discoloration and atrophy of demyelinated optic chiasm and spinal white matter, most marked in the posterior cervical columns (arrow). (b) Transverse section through optic nerve in which only a peripheral crescent of myelin can still be stained.
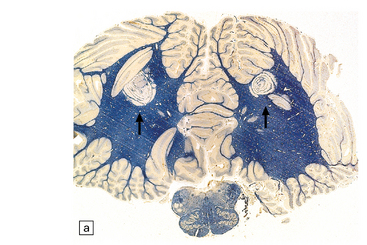
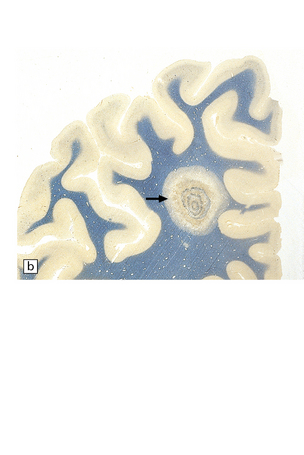
 Are often present adjacent to the lateral angles of the lateral ventricles on sectioning the cerebrum (Fig. 19.3).
Are often present adjacent to the lateral angles of the lateral ventricles on sectioning the cerebrum (Fig. 19.3).
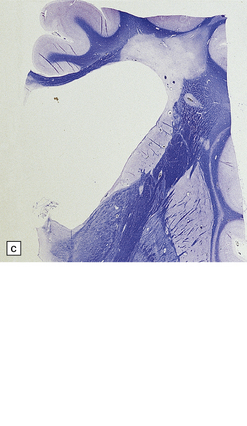
19.3 Plaques adjacent to the superolateral angle of the lateral ventricles.
(a,b) In MS, plaques are often found around the superolateral angles of the lateral ventricles (arrows). (c) Staining for myelin reveals a well-circumscribed zone of demyelination (arrows).
 Can occur anywhere in the white matter, at the junction between the cerebral gray and white matter (Fig. 19.4), and within the cortical gray matter and deep gray nuclei (Fig. 19.5), which include myelinated axons as well as neuronal somata and dendrites.
Can occur anywhere in the white matter, at the junction between the cerebral gray and white matter (Fig. 19.4), and within the cortical gray matter and deep gray nuclei (Fig. 19.5), which include myelinated axons as well as neuronal somata and dendrites.
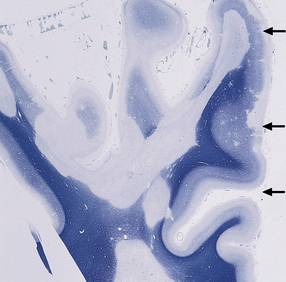
19.4 Subpial and junctional plaques.
Multiple plaques of demyelination. Here occurring in the subpial region (arrows) and at the junction between cerebral cortex and white matter.
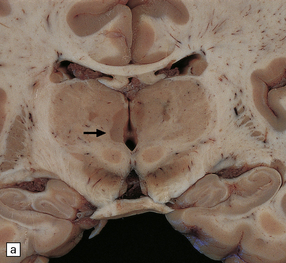
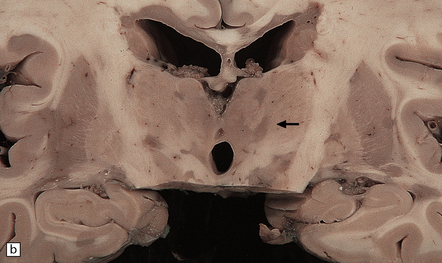
19.5 Multiple plaques in the deep cerebral gray matter.
(a,b) These appear as dark gray-brown patches within the basal ganglia and thalamus (arrows to some).
 May occur in the cerebellar white matter and peduncles, in the floor of the fourth ventricle, elsewhere in the brain stem (Fig. 19.6, see also Fig. 19.1c), and in the spinal cord (Fig. 19.6, see also Fig. 19.2a).
May occur in the cerebellar white matter and peduncles, in the floor of the fourth ventricle, elsewhere in the brain stem (Fig. 19.6, see also Fig. 19.1c), and in the spinal cord (Fig. 19.6, see also Fig. 19.2a).
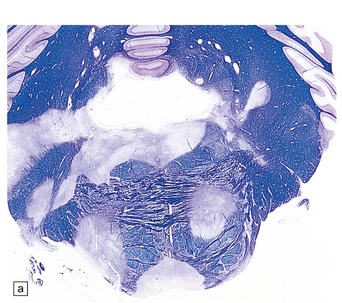
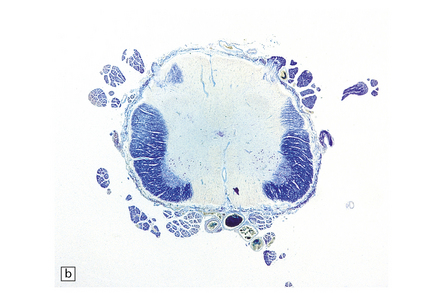
19.6 Plaques of demyelination in the brain stem and spinal cord.
(a) Plaques in the base and tegmentum of the pons. Histology often reveals involvement of the brain stem in MS although the plaques may be difficult to discern on gross examination of the fixed brain. In this case, the weak luxophilic staining in some of the plaques reflects partial remyelination. (b) Extensive demyelination in the lumbar spinal cord. Note the preservation of anterior horn cells.
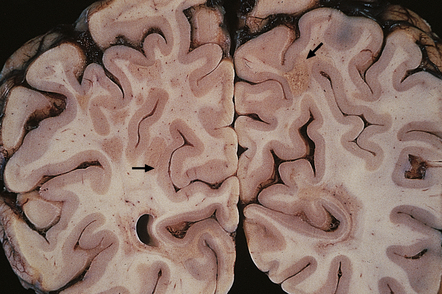
Plaques containing many lipid-laden macrophages tend to appear slightly yellow or chalky white rather than gray (Fig. 19.7), while old plaques and, rarely, fulminant acute plaques may contain foci of cavitation (Fig. 19.8).
MICROSCOPIC APPEARANCES
White matter demyelination
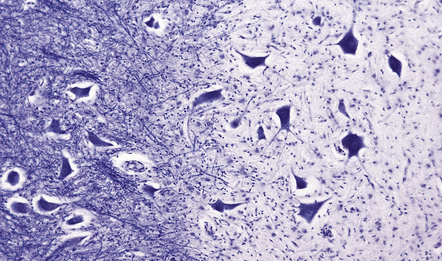
In the white matter (Fig. 19.9), it is probable that perivascular inflammation consisting of lymphocytes and macrophages occurs at a very early stage, as does disruption of the blood–brain barrier, resulting in a high signal on MRI enhanced with gadolinium–DTPA and an interstitial accumulation of serum proteins that can be demonstrated immunohistochemically (Fig. 19.10). It is believed that inflammation and disruption of the blood–brain barrier precede myelin destruction.
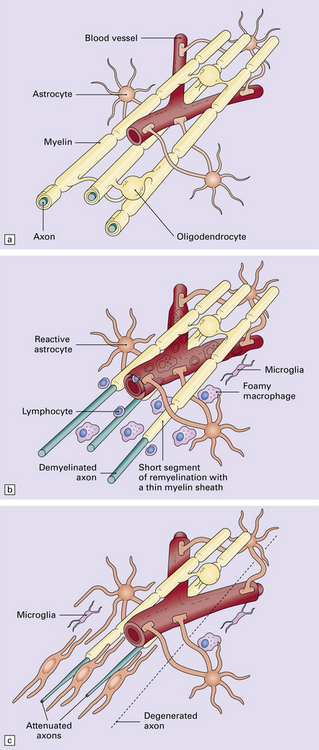
19.9 Different stages of disease activity in multiple sclerosis plaques in the white matter.
(a) Normal white matter. (b) During active demyelination, there is perivascular and parenchymal infiltration by lymphocytes and macrophages. Many of the macrophages are distended by lipid material and appear ‘foamy’. Remyelinating activity probably commences at an early stage. (c) Inactive plaques are hypocellular, most of the cells within them being astrocytes, there is some loss of axons and those that remain have a reduced caliber.
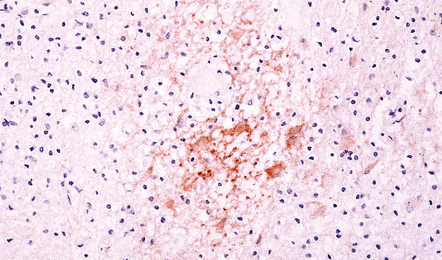
19.10 Disruption of the blood–brain barrier in MS.
Perivascular transthyretin (prealbumin) immunoreactivity in an active plaque. Enhancement of vascular permeability to serum proteins occurs at an early stage of demyelination, and immunoreactivity for albumin transthyretin, and other proteins can be demonstrated in the interstitium of active plaques.
Established lesions can be subdivided according to the stage of demyelination into active, inactive, and shadow plaques, although several more complicated staging schemes have been devised (Table 19.2). The demyelinating activity can vary considerably in different parts of the same plaque.
Table 19.2

aClusters of microglia and a few perivascular inflammatory cells but no demyelination.
bCategory included to encompass findings that might be expected in patients undergoing treatment.
Adapted from van der Valk P, De Groot CJA. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol 2000; 26:2–10.
Active plaques
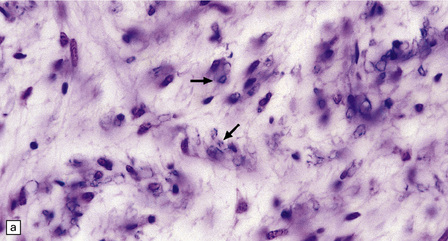
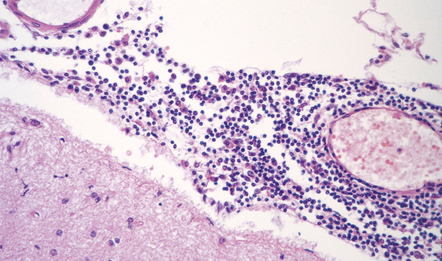
Active plaques are hypercellular lesions containing a relatively dense perivascular and parenchymal infiltrate of lymphocytes and macrophages (Fig. 19.11), and scattered reactive astrocytes, which may be quite pleomorphic (Fig. 19.12). The inflammation tends to be greatest towards the edge of the plaque and in the contiguous intact white matter (Fig. 19.13). The lymphocytes in these regions are mostly T cells (Fig. 19.14). CD4-positive (helper) cells predominate in earlier lesions and the actively demyelinating regions of older lesions, while CD8-positive (suppressor/cytotoxic) cells are more numerous in less active regions.
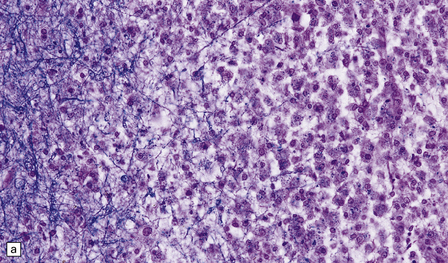
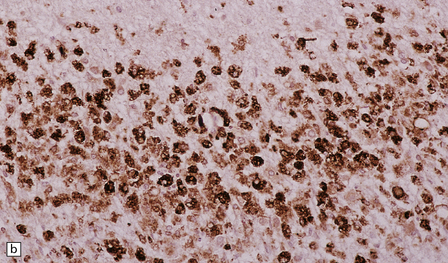
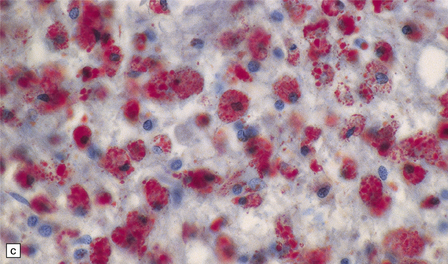
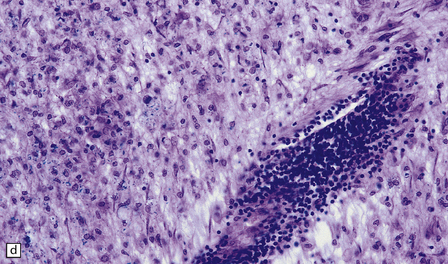
19.11 Active MS plaque.
(a) Sheets of foamy macrophages towards the margin of an active plaque. Some contain granules of dark blue (luxophilic) myelin debris. (b) CD68 immunoreactivity of macrophages in an active plaque. (c) The macrophages contain numerous lipid droplets, stained here with oil red-O. (d) Perivascular and scattered interstitial lymphocytes in an active plaque.
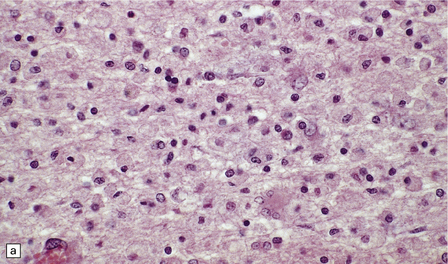
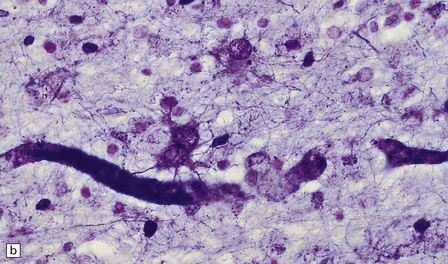
19.12 Reactive astrocytes in MS plaques.
(a) Reactive astrocytes with abundant homogeneous eosinophilic cytoplasm are scattered amongst macrophages and lymphocytes. Note that one of the astrocytes is multinucleated. (b) Large reactive astrocytes are clearly demonstrated in a Holzer-stained section of this MS plaque.
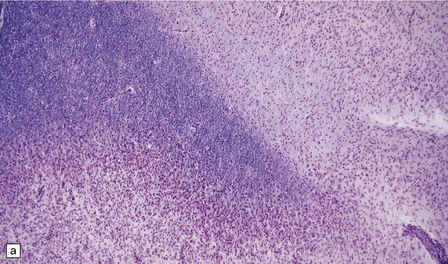

19.13 Variation in disease activity in an MS plaque.
(a) Marked variation in the density of the cellular infiltrate in two contiguous regions of demyelination. (b) Chronic plaque with a hypocellular center, but a densely cellular actively demyelinating periphery.
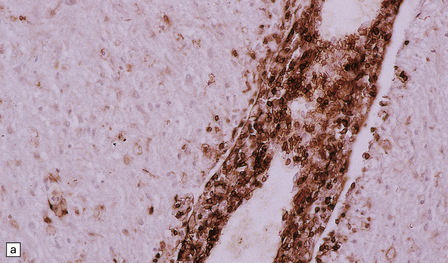
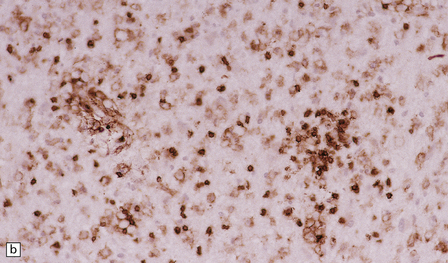
19.14 Lymphocytic inflammation in MS.
Most of the perivascular (a) and interstitial (b) lymphocytes are T cells, as shown by their CD45RO immunoreactivity.
The cytoplasm of the macrophages appears foamy towards the edge of the plaque owing to their accumulation of material that reacts strongly with oil red O (see Fig. 19.11) and with stains for myelin. The macrophages express major histocompatibility complex (MHC) class II antigens, as do some astrocytes in zones of active demyelination. MHC class II antigen expression is not a feature of normal CNS tissue, but occurs in several inflammatory disorders including MS.
Superimposed on this archetypal pattern of classic MS are several variations that differ in the composition of inflammatory cells, the relative preservation of certain myelin proteins, the appearance of the edge of the plaques, and the extent of remyelination (see Shadow plaques, below) (Table 19.3). These patterns vary between patients but are broadly consistent for all plaques within any one patient, suggesting that there is pathogenetic heterogeneity in MS.
Table 19.3
Structural and immunological features of different patterns of active MS lesions
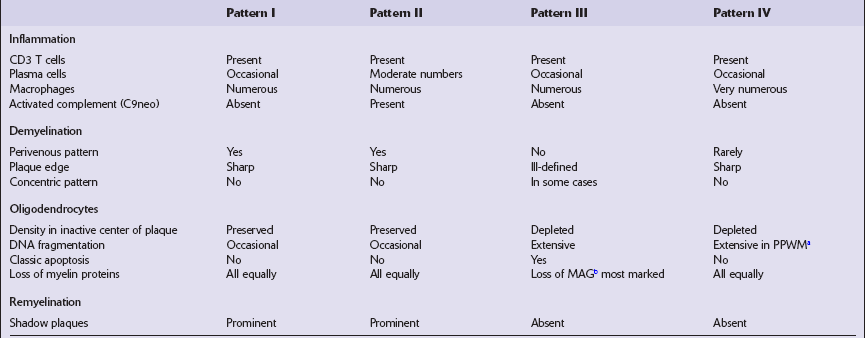
bMyelin-associated glycoprotein.
Adapted from Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000; 47:707–717.
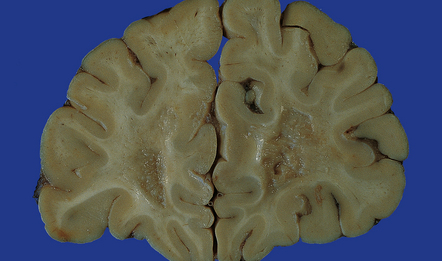
Silver impregnation or immunohistochemistry for neurofilament proteins usually reveals preservation of most axons (Fig. 19.15), although some axonal degeneration is usually demonstrable in plaques with active demyelination (Fig. 19.16). Even in the absence of frank degeneration, scattered axons may show other evidence of injury, in the form of abnormal accumulation of anterogradely transported proteins such as β-amyloid precursor protein (Fig. 19.16). Although axons in the normal-appearing white matter away from plaques usually look normal, quantitative studies have shown mild loss of axons from these regions also. On electron microscopy, macrophages containing myelin debris can be seen in direct contact with axons undergoing demyelination (Fig. 19.17). Most neurons are relatively preserved within plaques that involve gray matter (Fig. 19.18), although subtle abnormalities can be detected in some, and recent studies have shown evidence of occasional neuronal apoptosis.
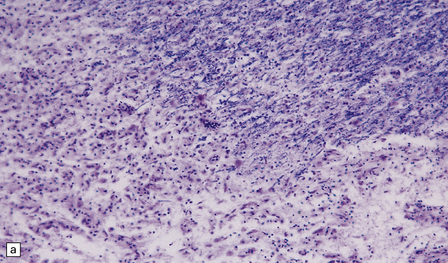
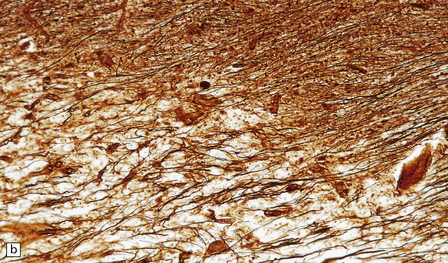
19.15 Relative preservation of axons.
In adjacent sections through a poorly defined edge of an area of active demyelination, a Luxol fast blue/cresyl violet stain shows loss of myelin (a), but silver impregnation of axons (b) shows that they are relatively preserved.
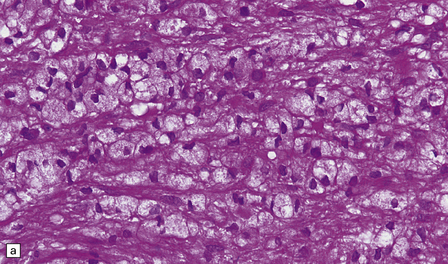
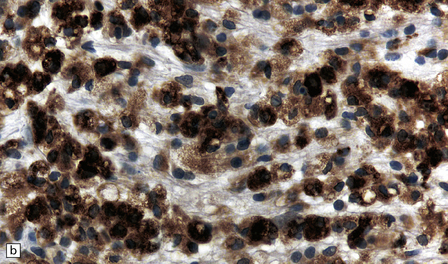
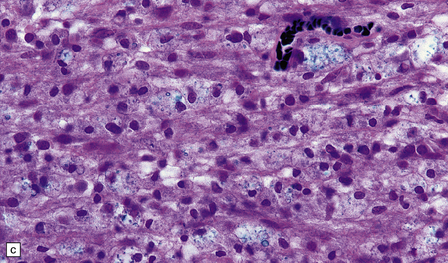
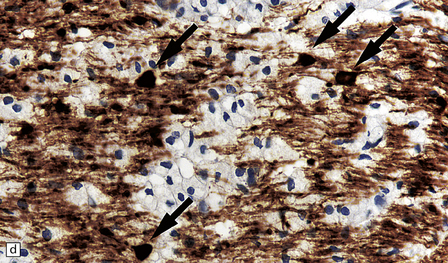
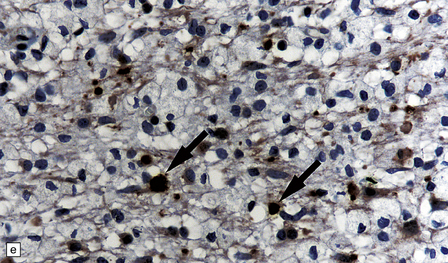
19.16 Axonal damage in MS.
(a) Biopsy of an active plaque, containing numerous foamy cells. (b) Immunohistochemistry for CD68 shows the foamy cells to be macrophages. (c) There is complete loss of myelin but the foamy cells contain fragments of myelin debris that is stained blue with solochrome cyanin. (d) Although most of the axons are preserved, immunohistochemistry for neurofilament protein reveals scattered axonal swellings (arrows). (e) Some of the axonal swellings contain accumulations of β-amyloid precursor protein (arrows).
Inactive plaques
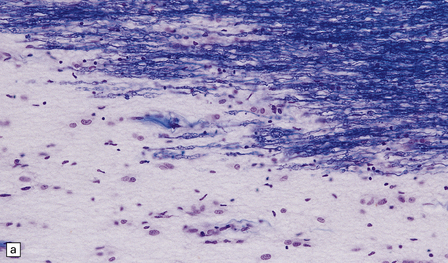
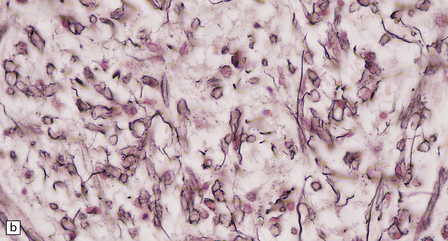
Inactive plaques are hypocellular, densely gliotic lesions, often showing a marked loss of oligodendrocytes (Fig. 19.19). Groups of axons may be in direct apposition, a relationship that may facilitate ephaptic ‘cross-talk’ (i.e. non-synaptic spread of excitation from one axon to another adjacent axon). Stains for myelin usually show that the plaque margins are sharply defined. There is a reduction in caliber and variable depletion of axons, the extent of which is less marked at the periphery of the plaque (Fig. 19.20). MRI and ultrastructural studies have shown that the loss of axons is associated with a concomitant increase in the amount of extracellular space. Severe axonal loss may be associated with cavitation.
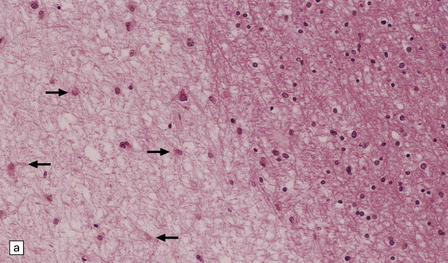
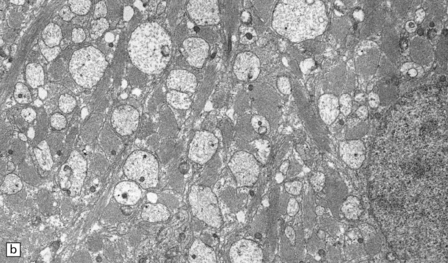
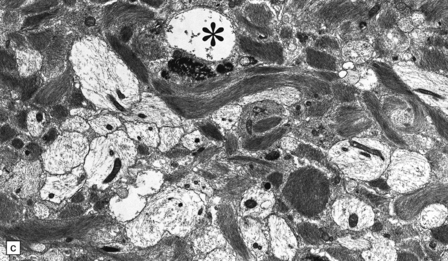
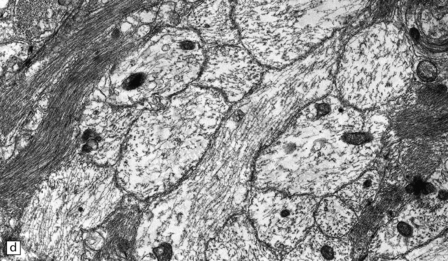
19.19 Inactive plaques.
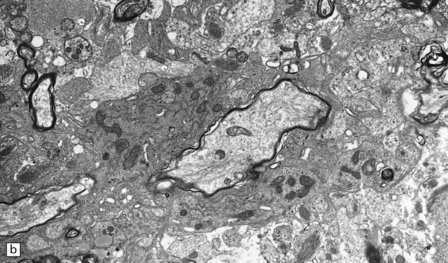
(a) A markedly hypocellular inactive plaque with obvious loss of oligodendrocytes. Most of the cells within the plaque are astrocytes (arrows). (b) Electron micrograph showing chronic inactive plaque of demyelination. Note the paucicellularity, as evidenced by the presence of only a single nucleus in this field. (c) Higher magnification shows that the remaining (relatively electron-lucent) axons are surrounded by a meshwork of astrocyte processes containing bundles of electron-dense glial intermediate filaments. The asterisk indicates an electron-lucent profile that is probably a degenerating axon. (d) Groups of demyelinated axons may be in direct apposition. (d, Reproduced with permission from Love S, Gradidge T, Coakham HB. Trigeminal neuralgia due to multiple sclerosis: ultrastructural findings in trigeminal rhizotomy specimens. Neuropathol Appl Neurobiol 2001; 27: 238-44.)
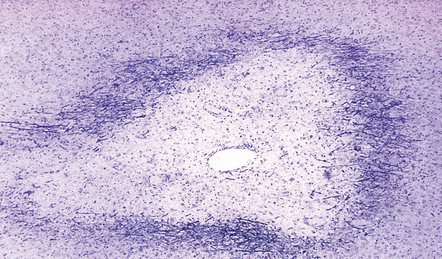
Shadow plaques
Shadow plaques are recognized by light microscopy as plaques with reduced but not absent myelin staining (Fig. 19.21). Examination of well-preserved resin-embedded material has shown that shadow plaques contain remyelinated axons, with relatively thin myelin sheaths. The zone of remyelination that constitutes a shadow plaque often appears to be confined to part of a larger zone of demyelination. Remyelination probably commences within weeks of demyelination. Demyelination and remyelination can occur repeatedly and concurrently in the same plaque (Fig. 19.22). Foci of remyelination in the brain stem and spinal cord may occasionally be mediated by invading Schwann cells rather than oligodendrocytes (Fig. 19.23).
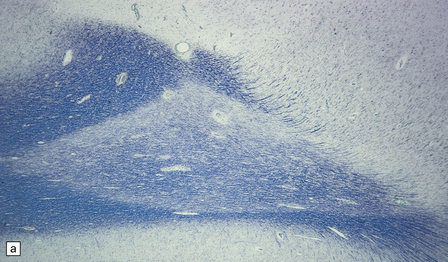
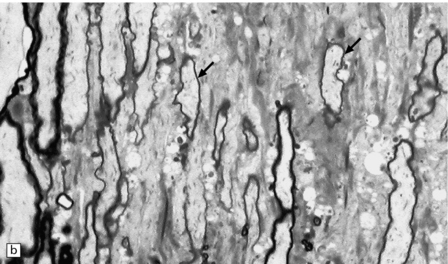
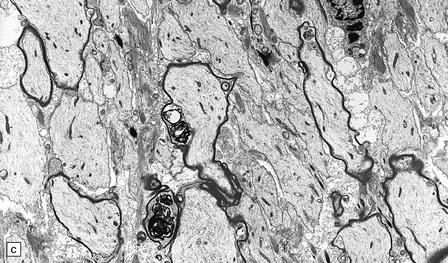
19.21 Remyelination in MS.
(a) Well-defined shadow plaque. The fibers stain for myelin albeit less intensely than in the adjacent subcortical white matter. (b) Semithin resin-embedded section through the edge of a plaque shows that it contains many nerve fibers with relatively thin myelin sheaths (arrows). (c) Electron microscopy shows an admixture of demyelinated and thinly remyelinated fibers.
Gray matter demyelination
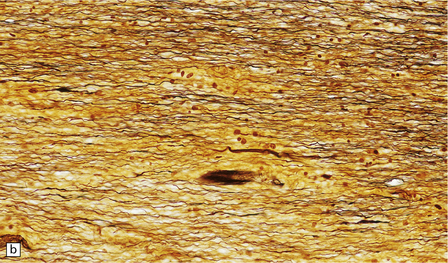
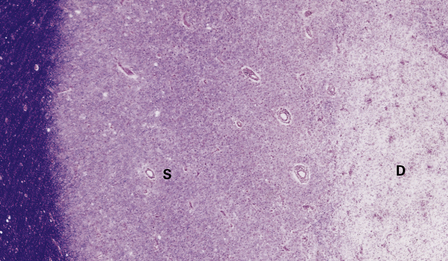
Gray matter demyelination is best demonstrated immunohistochemically, with antibody to myelin basic protein or proteolipid protein. The microscopic appearance of gray matter lesions varies somewhat according to their location. Within the cerebral cortex, several patterns of demyelination have been described: leukocortical, intracortical, subpial and transcortical (Fig. 19.24); the last two patterns are usually considered together. The numerical classification of Peterson and colleagues (2001) is often used: type I (leukocortical), type II (intracortical) and type III (subpial). Subpial plaques are the most common and intracortical plaques are relatively rare. Demyelination is also common in deep gray matter structures such as the basal ganglia, thalamus and brain stem (Fig. 19.24).
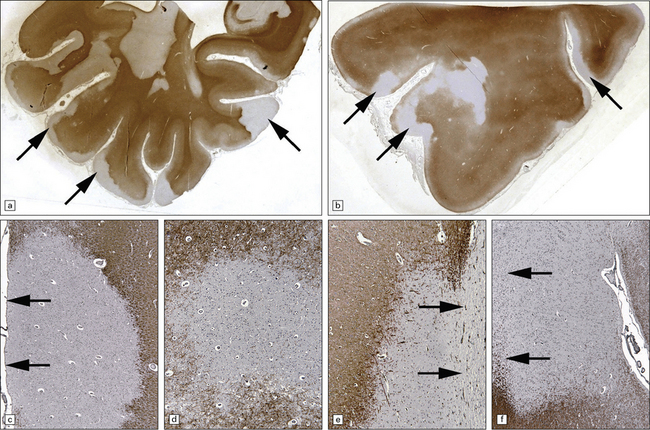
19.24 Cortical demyelination in multiple sclerosis, demonstrated by labeling of paraffin sections for myelin basic protein.
(a,b) Multiple well-defined areas of subpial and transcortical demyelination (arrows) in sections of temporal (a) and frontal (b) cortex. (c) Subpial plaque. The arrows indicate the pial surface. (d) Intracortical plaque. (e) Leukocortical plaque. The arrows indicate the junction between the cerebral cortex and under-lying white matter. (f) Transcortical plaque. The arrows indicate the junction between cortex and white matter. (Reproduced with permission from Gray E, Thomas TL, Betmouni S, et al. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol 2008; 18:86–95.)
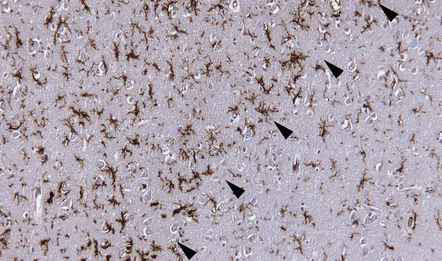
Ramified microglia are the predominant inflammatory cells in gray matter plaques (Fig. 19.25). Typical macrophages are usually absent but are occasionally seen at the edge of active cortical plaques, particularly leukocortical plaques. Within the gray matter, lymphocytes are sparse or absent but they may be present in the overlying leptomeninges (Fig. 19.26), sometimes forming discrete lymphoid aggregates that consist predominantly of B cells.
ACUTE (MARBURG-TYPE) MS
MACROSCOPIC AND MICROSCOPIC APPEARANCES
Sections contain multiple active plaques, all of which are hypercellular, with prominent perivascular lymphocytic cuffing, numerous foamy macrophages, and scattered pleomorphic reactive astrocytes (Fig. 19.27). The edges of the plaques tend to be poorly defined and some plaques are difficult to see macroscopically (Fig. 19.27). Occasionally, edema in the surrounding white matter produces a significant mass effect, simulating a neoplasm.
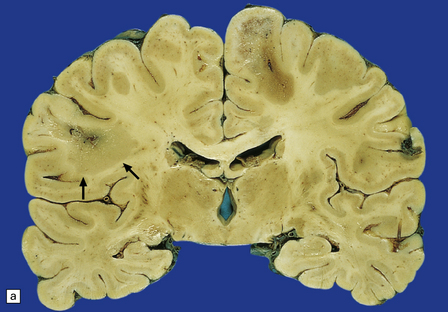
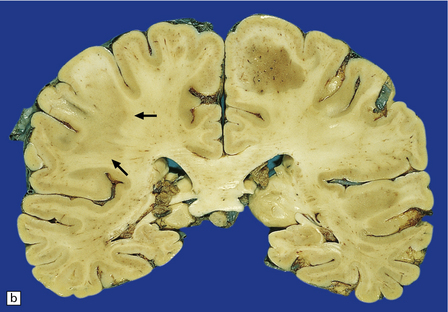
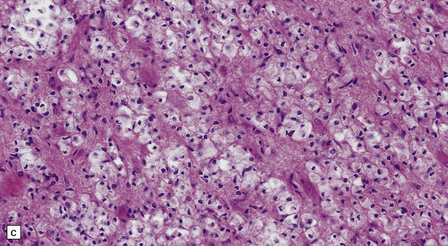
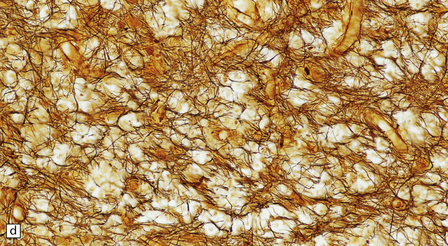
19.27 Acute MS.
(a,b) Adjacent coronal slices through the brain of a patient with acute MS. Note the multiple large plaques, some of which (arrows) are poorly defined. (c) Histology shows the features of active demyelination (i.e. sheets of foamy macrophages and scattered reactive astrocytes). (d) In an adjacent section, axons appear to be relatively preserved, although axonal swelling or fragmentation is common.
CONCENTRIC SCLEROSIS (BALÓ’S DISEASE)
MACROSCOPIC AND MICROSCOPIC APPEARANCES
The white matter usually contains multiple large plaques, some with central necrosis. The characteristic histologic feature is the presence of plaques composed of alternating, more or less concentric rings of demyelinated and myelinated white matter (Fig. 19.28). The plaques are usually hypercellular, with perivascular lymphocytic cuffing, foamy macrophages, and reactive astrocytes. Cases with typical Baló-type plaques are rare, but plaques containing bands or islands of preserved myelin are sometimes seen in classic MS (Fig. 19.29), predominantly in patients with a type III pattern of demyelinating activity (see Table 19.3).
REFERENCES
Pathology and pathogenesis of multiple sclerosis
Baranzini, S.E., Galwey, N.W., Wang, J., et al. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet.. 2009;18:2078–2090.
Barnett, M.H., Parratt, J.D., Pollard, J.D., et al. MS: is it one disease? Int MS J.. 2009;16:57–65.
Breij, E.C., Brink, B.P., Veerhuis, R., et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol.. 2008;63:16–25.
Gray, E., Thomas, T.L., Betmouni, S., et al. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol.. 2008;18:86–95.
Hu, W., Lucchinetti, C.F. The pathological spectrum of CNS inflammatory demyelinating diseases. Semin Immunopathol.. 2009;31:439–453.
Lassmann, H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci.. 2003;206:187–191.
Lill CM, Roehr JT, McQueen MB, et al. The MSGene Database. Alzheimer Research Forum. Available at http://www.msgene.org/.
Lovato, L., Willis, S.N., Rodig, S.J., et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain.. 2011;134:534–541.
Lucchinetti, C., Bruck, W., Parisi, J., et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol.. 2000;47:707–717.
Sawcer, S., Hellenthal, G., Pirinen, M., et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature.. 2011;476:214–219.
Stadelmann, C., Ludwin, S., Tabira, T., et al. Tissue preconditioning may explain concentric lesions in Balo’s type of multiple sclerosis. Brain.. 2005;128:979–987.
Storch, M.K., Piddlesden, S., Haltia, M., et al. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol.. 1998;43:465–471.
Disease of gray matter and normal-appearing white matter
Albert, M., Antel, J., Bruck, W., et al. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol.. 2007;17:129–138.
Bo, L. The histopathology of gray matter demyelination in multiple sclerosis. Acta Neurol Scand Suppl.. 2009:51–57.
Bo, L., Vedeler, C.A., Nyland, H.I., et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol.. 2003;62:723–732.
Howell, O.W., Reeves, C.A., Nicholas, R., et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain.. 2011;134:2755–2771.
Kooi, E.J., Geurts, J.J., van Horssen, J., et al. Meningeal inflammation is not associated with cortical demyelination in chronic multiple sclerosis. J Neuropathol Exp Neurol.. 2009;68:1021–1028.
Kutzelnigg, A., Faber-Rod, J.C., Bauer, J., et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol.. 2007;17:38–44.
Kutzelnigg, A., Lucchinetti, C.F., Stadelmann, C., et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain.. 2005;128:2705–2712.
DeLuca, G.C., Ebers, G.C., Esiri, M.M. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain.. 2004;127:1009–1018.
DeLuca, G.C., Williams, K., Evangelou, N., et al. The contribution of demyelination to axonal loss in multiple sclerosis. Brain.. 2006;129:1507–1516.
Dutta, R., Trapp, B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol.. 2011;93:1–12.
Ferguson, B., Matyszak, M.K., Esiri, M.M., et al. Axonal damage in acute multiple sclerosis lesions. Brain.. 1997;120:393–399.
Ghosh, N., DeLuca, G.C., Esiri, M.M. Evidence of axonal damage in human acute demyelinating diseases. J Neurol Sci.. 2004;222:29–34.
Gilmore, C.P., DeLuca, G.C., Bo, L., et al. Spinal cord atrophy in multiple sclerosis caused by white matter volume loss. Arch Neurol.. 2005;62:1859–1862.
Gilmore, C.P., DeLuca, G.C., Bo, L., et al. Spinal cord neuronal pathology in multiple sclerosis. Brain Pathol.. 2009;19:642–649.
Peterson, J.W., Bo, L., Mork, S., et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol.. 2001;50:389–400.
Schirmer, L., Antel, J.P., Bruck, W., et al. Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis. Brain Pathol.. 2011;21:428–440.
Stadelmann, C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol.. 2011;24:224–229.
Trapp, B.D., Peterson, J., Ransohoff, R.M., et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med.. 1998;338:278–285.
Trapp, B.D., Stys, P.K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol.. 2009;8:280–291.