CHAPTER 11 Monitoring
Monitoring
Most studies that have determined the rate of cardiac arrests resulting from anesthesia have found a threefold to fivefold greater risk among children than adults (Graff et al., 1964; Keenan and Boyan, 1985). In children younger than 1 year, the incidence increases to 9.2 to 17 per 10,000 anesthesias, or 10 times the adult incidence (Olsson and Hallen, 1988; Cohen et al., 1990). Factors contributing to cardiac arrests in anesthetized children are likely to be related to the cardiovascular or respiratory system (Salem et al., 1975). Flick and others (2007) have reviewed cardiac arrest data at the Mayo Clinic. The incidences of cardiac arrest and mortality during noncardiac procedures were 2.9:10,000 and 1.6:10,000, respectively. However, the incidence of cardiac arrest in children undergoing cardiac operations was 127:10,000. The incidence of other serious complications is also greater for infants than for adults in the operating room (Tiret et al., 1988) and in the postanesthesia care unit (PACU) (Cohen et al., 1990). These data indicate that children are a high-risk population and should be monitored with particular attention to cardiovascular and respiratory variables.
Guidelines for the intraoperative monitoring of patients under anesthesia have been published by the American Society of Anesthesiologists (ASA) (2005) (see Box 10-1). These standards mandate the continuous presence of an anesthesiologist or a nurse anesthetist throughout the conduct of anesthesia and require continuous monitoring of oxygenation, electrocardiographic status, and adequacy of ventilation and circulation. The minimum standard for monitoring oxygenation includes an oxygen analyzer in the anesthesia breathing circuit, sufficient illumination to evaluate the patient’s color, and a quantitative method such as pulse oximetry, except under extenuating circumstances. Tracheal intubation must be verified by physical examination and the qualitative detection of carbon dioxide in the exhaled gas. Regardless of whether endotracheal intubation has been performed, continuous capnography is required unless it is invalidated by the nature of the patient, procedure, or equipment. Furthermore, quantitative monitoring of the volume of expired gas is strongly encouraged. The ASA also recommends monitoring of ventilation using observation of chest excursion and the reservoir breathing bag, as well as auscultation of breath sounds. When ventilation is controlled by a mechanical ventilator, there should be in continuous use a device that is capable of detecting disconnection of components of the breathing system, and the device must give an audible signal when its alarm threshold is exceeded.
Physical Examination
Observation
The anesthesiologist can gain a tremendous amount of information from observation alone. Anesthetic depth can be inferred from the rate and pattern of respiration, and airway obstruction can be detected by chest wall retractions or “seesaw” paradoxical motion. The skin and mucous membranes should be continually assessed to confirm adequate oxygenation, because a pulse oximeter reading can significantly lag behind other indices of hypoxemia when placed on an extremity (Reynolds et al., 1993), or it may not detect a pulse at all during intense vasoconstriction. In rare circumstances, pulse oximetry falsely indicates normal saturations during hypoxic conditions (Costarino et al., 1987).
Auscultation
Esophageal stethoscopes are contraindicated in patients with esophageal atresia or in those who have a disease process involving the proximal portion of the esophagus. They confer a rigid feel to the esophagus, which might be mistaken for the trachea (Schwartz and Downes, 1977). As a result, the esophageal stethoscope is relatively contraindicated in neck dissections where the trachea is a critical landmark, such as a tracheostomy.
Electrocardiography
In children, the normal heart rate varies with age (Table 11-1). The normal heart rate of the newborn ranges from 120 to 160 beats per minute, although lower rates (e.g., 70) are frequently observed during sleep, and higher rates (>200) are common during anxiety or pain. Heart rates tend to decrease with age and in parallel with decreases in oxygen consumption. In addition, many children have a noticeable variation in heart rate with respiration (i.e., sinus arrhythmia).
TABLE 11-1 Normal Resting Heart Rates of Infants and Children
| Age | Heart Rate (beats/min) | |
|---|---|---|
| Mean | Range (±2 SD) | |
| 0 to 24 hr | 119 | 94 to 145 |
| 1 to 7 days | 133 | 100 to 175 |
| 8 to 30 days | 163 | 115 to 190 |
| 1 to 3 mo | 152 | 124 to 190 |
| 3 to 12 mo | 140 | 111 to 179 |
| 1 to 3 yr | 126 | 98 to 163 |
| 3 to 5 yr | 98 | 65 to 132 |
| 5 to 8 yr | 96 | 70 to 115 |
| 8 to 16 yr | 77 | 55 to 105 |
Modified from Liebman J, Plonsey R, Gillette PC, editors: Pediatric electrocardiography, Baltimore, Md, 1982, Williams & Wilkins.
Systemic Arterial Pressure
Noninvasive Measurement
Blood pressure is easily measured noninvasively in children and small infants using oscillotonometry. In children, oscillometric measurements of systolic arterial pressure (Bruner et al., 1981; Friesen and Lichtor, 1981) and mean arterial pressure (Kimble et al., 1981) usually correlate well with the Riva Rocci mercury column method, as well as with direct arterial pressure measurement, but oscillometric measurements tend to underestimate the diastolic component. During routine uncomplicated cases, measurement of blood pressure should be performed every 3 to 5 minutes while the child is anesthetized—determinations that are too frequent can result in limb ischemia. The blood pressure cuff is most commonly placed on the upper arm but can be placed on the forearm, thigh, or calf. There is inconsistent correlation of measurements obtained between the upper and lower limbs.
The width of the blood pressure cuff should cover approximately two thirds of the total length of the upper arm (or other extremity portion to which it is applied). A cuff that is too small or too narrow incompletely occludes the artery, resulting in the premature return of detectable flow and hence falsely increasing the pressure measurement (Park et al., 1976; Kimble et al., 1981). The error can be as great as 30 mm Hg. A cuff that is too wide can dampen the arterial wave and result in a falsely low pressure, but the magnitude of this error is small (Kimble et al., 1981). Blood pressure increases gradually throughout childhood (Figs. 11-1 and 11-2) and depends on the height of the child: taller children demonstrate a higher blood pressure (Table 11-2). Blood pressure ranges in premature infants have been defined (Table 11-3) and vary depending on the health status of the infant and mother.
Direct Measurement
There are no absolute contraindications to placing an arterial catheter, but a risk-benefit analysis should be performed in patients with a hypercoagulable state or bleeding disorder. The radial artery is a favored site for arterial cannulation because the vessel is superficial and easily accessible. Other anatomic sites frequently used are the ulnar, dorsalis pedis, posterior tibial, and femoral arteries. The axillary artery has gained favor because of increased collateral blood flow compared with the brachial or femoral artery (Lawless and Orr, 1989; Cantwell et al., 1990; Greenwald et al., 1990; Piotrowski and Kawczynski, 1995). In general, the brachial artery should be avoided because of the risk for median nerve damage and poor collateral flow around the elbow. Umbilical vessels are an alternative site for cannulating the aorta and inferior vena cava in neonates. In determining a site, one needs to consider the history of that vessel (i.e., whether it has been cannulated before), its collateral flow, the experience of the person inserting the catheter, and special physiologic issues (e.g., whether it arises on an aortic root proximal to the ductus arteriosus) or surgical issues (e.g., whether it arises from a vessel likely to be clamped or sacrificed during the procedure). Cannulation of vessels with good collateral flow, such as the arch vessels of the wrist or foot, may reduce the risk for ischemic tissue damage distal to the catheter.
As the largest superficial vessel, the femoral artery can be cannulated most predictably in situations when intense peripheral vasoconstriction may accompany low cardiac output and blood pressure. In less dire circumstances, the selection of a vessel may reflect a variety of anatomic and physiologic characteristics exhibited by certain vessels. The pedal vessels exhibit pressure wave amplification that results in pressure determinations exceeding aortic values by as much as 30% (Park et al., 1983).
A Doppler flow transducer is occasionally useful to locate an artery that is difficult to palpate. Surgical cutdown may be the preferred option when percutaneous placement is likely to be difficult or has failed. Indwelling arterial catheters are associated with several possible complications. Proximal emboli, distal ischemia, arterial thrombosis, and infection are common to all sites. Thrombosis of the radial artery is generally temporary, although it is more likely to persist after a cutdown (Miyasaka et al., 1976). Although small flush volumes (0.3 mL) in radial arterial catheters can be detected in the aortic arch vessels, cerebral infarcts have not been reported (Edmonds et al., 1980). The tip of an umbilical artery catheter should be placed in either a high (above the diaphragm) or a low (below L3) position to avoid direct flushing into the renal arteries. Despite these precautions, as many as 10% of neonates exhibit hypertension as a late complication attributed to umbilical artery catheterization (Bauer et al., 1975; Plumer et al., 1976; Horgan et al., 1987). Minor complications of umbilical artery monitoring include vasospasm of the lower extremity vessels, which are more common with low tip placement. Major complications (e.g., necrotizing enterocolitis, renal artery thrombosis) occur independent of location (Mokrohisky et al., 1978; Umbilical Artery Catheter Trial Study Group, 1992). The rarity of clinical complications is remarkable given that the incidence of aortic thrombosis on removal of umbilical artery catheters approaches 95% in some series (Neal et al., 1972), although most series define the incidence at 12% to 31% of neonates (Symansky and Fox, 1972; Horgan et al., 1987; Seibert et al., 1987).
Systolic Pressure Variation
Systolic pressure variation is a noninvasive way to determine volume status and fluid responsiveness. It is defined as the difference between the maximal and minimal values of systolic blood pressure during a positive pressure breath. Initially during a positive pressure breath, there is a transient increase in systolic blood pressure (delta up) followed within four or five beats by a decrease in systolic blood pressure (delta down). Increases in intrathoracic pressure during positive pressure ventilation cause a decrease in systolic blood pressure because of decreased preload to the right ventricle, increased afterload to the right ventricle, and decreased afterload to the left ventricle. This decrease is greater during hypovolemia. Systolic pressure changes in response to respiratory variation have been used to determine hypovolemia (Greilich and Johnston, 2007).
where ΔPP% is respiratory change in pulse pressure (mm Hg).
Michard and others (1999) demonstrated a strong relationship in adult ventilated patients between pulse pressure changes and cardiac output. Patients with pulse pressure changes (ΔPP) that are greater than 10% may be fluid responsive and benefit from the administration of intravenous fluids.
Changes in the pulse oximetry waveform (plethysmographic waveform amplitude) have been shown to predict fluid responsiveness (Pizov et al., 2010). Bedside use of this variable is challenging. The “pleth variability index” (Masimo Corp., Irvine, CA) automatically calculates the waveform amplitude variation and may predict fluid responsiveness noninvasively (Cannesson et al., 2008a, 2008b).
Central Venous Pressure
Catheters of various sizes (2.5 to 10 French), lengths, and composition are available for pediatric applications (Cook Critical Care, Bloomington, IN, and other companies). Selection is based on the size of the patient (Andropoulos et al., 2001) and the purpose of the catheter. The composition of the catheter depends on its intended use. Teflon is fairly resistant to thrombus formation, but concerns about perforation by catheters have prompted the development of softer catheter materials, especially for long-term use (e.g., Silastic and polyurethane). The catheters are generally inserted via the Seldinger technique, using landmarks that are similar to those used in adults.
There are no absolute contraindications to placing a central venous catheter, but each site has potential risks. All sites share the common complications of infection (site cellulitis, bacteremia), venous thrombosis with potential emboli, air embolism, catheter malfunction (occlusion, dislodgment, or fractures), dysrhythmias (when the catheter tip is in the heart), and bleeding. Universal precautions and sterile technique should be used when placing a central venous catheter. The risks involved in cannulating the internal jugular vein include carotid artery puncture, Horner’s syndrome, pneumothorax, and injury to the thoracic duct when the left internal jugular vein is cannulated. The high approach to the internal jugular vein, at the midpoint of the sternocleidomastoid muscle, results in comparable success with fewer complications than lower approaches (Coté et al., 1979). Two-dimensional ultrasound scanning improves localization of the internal jugular vein and increases the success rate of central venous cannulation in adults and children (Verghese et al., 2002; Hind et al., 2003). Using this device, Alderson and others (1993) reported an 18% prevalence of anatomic variations in children younger than 6 years that would preclude or significantly hinder the successful cannulation of the internal jugular vein using anatomic landmarks alone. In addition, Hong and colleagues (2010) reported that rotating the head away from the neutral position increases the degree of carotid artery and internal jugular vein overlap, and decreases the incidence of lateral positioning of the internal jugular vein to the carotid artery.
Mixed Venous Oxygenation and Monitoring
Dueck and coworkers (2005) found a significant variation between central venous saturations and mixed venous saturations in the same patient, so individual values of Scvo2 cannot be substituted for Svo2 values. However, although the absolute values do not correlate, there is a correlation between the trends in Scvo2 values and in Svo2 values. Perez and colleagues (2009), in a retrospective pediatric study, identified a correlation between right atrial and mixed venous oxygen saturations. Scvo2 is used clinically in pediatric patients. Continuous monitoring of Scvo2 can be performed in neonates, infants, and children.
In infants, continuous monitoring of Scvo2 can be achieved with a fiberoptic probe. One type of fiberoptic probe is designed as a percutaneous catheter from CeVOX (Pulsion Medical Systems AG, Munich, Germany). This 2-F probe is 31 cm in length and measures central venous oxygen saturations using spectrophotometry. Muller and coworkers (2007) described placing the catheter percutaneously through a 16-gauge single-lumen catheter in the femoral or subclavian vein. There were only three patients in this study, so accurate correlation with central venous blood samples cannot be determined.
The Pediasat system (Edwards Life Sciences) has been described in infants and children having orthopedic, craniofacial, and cardiac surgery. It comes in four sizes (4.5 F, 5 cm; 4.5 F, 8 cm; 5.5 F, 8 cm; and 5.5 F, 15 cm) and provides continuous readings of central venous oxygen saturation. Liakapolous and colleagues (2007) and Ranucci and colleagues (2008) demonstrated good correlation between Scvo2 values from the Pediasat system when compared with co-oximetry values obtained from blood samples drawn from the distal port.
Pulmonary Artery Catheters
Since its introduction in 1970, indications for the use of the flow-directed balloon-tipped pulmonary artery (Swan-Ganz) catheter in pediatric patients have been slow to evolve. Although the validity and value of the data that these catheters generate remain controversial in pediatrics, the technical difficulties and complications associated with their use are significant. Pulmonary artery pressure measurement can help guide therapy in children with elevated or volatile pulmonary vascular resistance, but the interpretation of the flow data they generate is hindered by several factors. First, the desired cardiac output varies according to age, disease state, and other elements of management that alter metabolic demand in complex ways, thereby introducing significant uncertainty in assigning a target value. Second, the prevalence of intracardiac communications that permit shunting of blood causes discrepancies in pulmonary and systemic blood flow that may vary continuously and are difficult to quantify. Finally, despite several studies demonstrating reasonable accuracy when thermodilution is compared with other methods of flow determination, such as the Fick equation (Freed and Keane, 1978) and dye dilution (Colgan and Stewart, 1977), the precision of these determinations in small infants is low and has a 25% intersample variability. In patients with congenital heart malformations, for example, measurement errors are introduced by shunting and complex anatomy, and the risks of improper placement of the flow-directed pulmonary artery catheter are increased. Alternatively, directly placed pulmonary artery catheters can provide the necessary information regarding pulmonary vascular resistance and residual left-to-right shunts, and left atrial catheters reflect filling and diastolic function of the left ventricle after cardiac surgery.
Pulmonary artery catheters can be difficult to insert, especially in infants or in children with low cardiac output. They may be placed in any vein used for access to the central venous system, but the most reliable veins are the right internal jugular and the femoral. In infants and children smaller than 15 kg, it is technically difficult to place an introducer sheath in the neck vessels; the femoral veins are preferable. Multilumen catheters capable of thermodilution are available in two sizes, 5 and 7 F, with four options for the right atrium–to–pulmonary artery interluminal distance. Catheter recommendations are based on age (Table 11-4). The proper placement of these catheters can take a long time, and thus the assistance of fluoroscopy is recommended for infants and children less than 30 kg and for larger children who have a low cardiac output.
TABLE 11-4 Guidelines for Multilumen Pulmonary Artery Catheters for Infants and Children
| Age (yr) | Catheter Size (F) | CVP to Pulmonary Artery Port Distance (cm) |
| Newborn to 3 | 5 | 10 |
| 3 to 8 | 5 | 15 |
| 8 to 14 | 7 | 20 |
| >14 | 7 | 30 |
Cardiac output can be estimated in children through indicator dilution (e.g., thermodilution or dye dilution) and noninvasive techniques. Doppler determinations of aortic blood velocity can be used to quantify systemic flow if the angle of the incident ultrasound beam and the cross-sectional area of the aorta are reliably determined (Alverson et al., 1982). Transthoracic and transesophageal evaluations of Doppler cardiac output in children have proved to be less promising (Notterman et al., 1989; Muhiudeen et al., 1991). Thoracic bioimpedance, a method that estimates stroke volume on the basis of changes in thoracic impedance, has been applied to children as small as 3.6 kg. Although some correlation exists between bioimpedance and indicator dilution methods, reproducibility is poor (O’Connell et al., 1991). Further details and the complexities encountered in the measurement of cardiac output in children are beyond the scope of this chapter but have been reviewed previously (Tibby and Murdoch, 2002).
A noninvasive cardiac output monitor has been developed that determines cardiac output via the Fick principle for rebreathed CO2 (Respironics; Novametrix Medical Systems Inc., Wallingford, CT) (Capek and Roy, 1988). The noninvasive cardiac output monitor has been clinically validated in adults and is approved by the U.S. Food and Drug Administration (FDA) for use, but it requires tidal volumes of 200 mL or greater (Guzzi et al., 2003; Watt et al., 2004).
Transesophageal Echocardiography
The value of transesophageal echocardiography (TEE) for monitoring hemodynamics and to evaluate preoperative and postoperative cardiac anatomy has been appreciated from the time of its introduction to the operating room. In the infancy of TEE in the late 1970s, an M-mode transducer was passed into the esophagus, plotting the distance of structures from the transducer on the y-axis and time on the x-axis (Frazin et al., 1976). Although they provided valuable information, M-mode images were too limited alone. In 1982, Schlüter and colleagues (1982) described their experience using a transducer capable of two-dimensional images mounted on a gastroscope. The usefulness of the images obtained was readily apparent, and since that time technology has catapulted the field of TEE to the forefront of cardiovascular monitoring. Now, multiple companies (Phillips, Acuson/Siemens, General Electric) manufacture advanced TEE-specific probes capable of two- and three-dimensional imaging on top of the original M-mode. Doppler has also been incorporated, providing the examiner the ability to extrapolate vast amounts of information from their patients.
A modification of the Bernoulli equation allows an examiner to estimate pressure gradients using the measured velocities. Simplified, the change in pressure between two points is equal to four times the maximum velocity squared (Holen et al., 1977; Hatle et al., 1978). Using this principle, the stenosis of valves, severity of aortic coarctation, obstruction caused by muscle bundles or membranes, and a multitude of other clinical questions can be answered.
TEE has played a vital role in improving outcomes in pediatric patients with congenital heart disease. Typically, examinations are performed preoperatively to confirm anatomy, and postoperatively to assess repairs and evaluate function. One study looking at 865 consecutive examinations demonstrated alterations to surgical plans based on preoperative TEE examinations in 2% of patients. The same study found that 12% of patients had post-bypass examinations that led to surgical interventions. Many of these interventions saved patients from unnecessary revision operations and their associated morbidity. In addition to surgical interventions, medical management with drugs and fluids was affected in 20% of the examined patients (Bettex et al., 2003).
An earlier report found that pediatric patients leaving the operating room without residual defects seen on the echocardiographic examination had a risk for reoperation of 3%, versus 42% for those who were found to have residual defects (Ungerleider et al., 1989). This ability to detect problems early provides a significantly improved outcome and subsequently reduces costs, as the need for repeat operations is reduced (Randolph et al., 2002).
As evidence supporting the value of TEE in cardiac surgery has increased, recommendations for proper performance of the examination have matured. The American Society of Echocardiographers and the Society of Cardiovascular Anesthesiologists have developed guidelines for adult intraoperative TEE examinations to encourage complete examinations with standardized views and nomenclature so as to improve communication between various care providers (Shanewise et al., 1999). Discussion of the complete examinations is beyond the scope of this chapter, but some of the probe locations and angles are useful for obtaining basic information regarding a patient’s condition (Table 11-5). Similar guidelines have also been published to assist clinicians with the pediatric examination (Lai et al., 2006).
| Location | Angle (degrees) | Structures Visualized |
| Transgastric | 0-20 | Left and right ventricles, and both atrioventricular valves |
| 80-100 | Two-chamber view of left side | |
| 90-120 | Long axis of left side, including left ventricular outflow tract | |
| Midesophageal | 0-20 | Standard four-chamber view |
| 30-60 | Aortic valve on short axis, coronary arteries | |
| 60-90 | Right ventricular inflow and outflow tracts | |
| 80-110 | Bicaval view | |
| 120-160 | Long-axis view of aortic valve and left ventricular outflow tract | |
| Upper esophageal | 0 | Aortic arch on long axis |
| 90 | Aortic arch on short axis |
Modified from Shanewise JS, Cheung AT, Aronson S et al: ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography, J Am Soc Echocardiogr 12:884, 1999.
Although the benefits have been demonstrated, providers must also be aware of the complications associated with the TEE examination. Problems that may be encountered include damage to the oral cavity, esophagus, or stomach; compromised ventilation; inadvertent extubation; right main stem advancement; vascular compression; and arrhythmias. In light of these serious but rare events, caution should precede placement of the transesophageal probe (Stevenson, 1999). Contraindications to the TEE examination include an unrepaired tracheoesophageal fistula, esophageal web, and recent esophageal or gastric surgery. Failure to ascertain a history of such events may lead to significant damage or even perforation of the esophagus.
Temperature
Temperature monitoring is vital during pediatric anesthesia, as children may exhibit hypothermia or hyperthermia, both of which can have profound physiologic consequences (see Chapter 6, Thermoregulation).
Urine Output
Urine output often reflects intravascular volume status and cardiac output. Proper assessment of urine output requires recognition of the physiologic mechanisms that exert an affect on urine flow in children. During the first week of life, the glomerular filtration rate and renal plasma flow are only 25% of normal adult values (Arant, 1978). The neonatal kidney is limited in its ability to concentrate the urine (Simpson and Stephenson, 1993). By the end of the first week of life, the kidneys begin to reach absorption thresholds for sodium and glucose that approach adult levels.
Normal newborns produce between 0.5 and 4 mL urine/kg per hour in the first 3 hours of life (Strauss et al., 1981). Urine flow, which initially ranges from 15 to 60 mL/kg per day, reaches as much as 120 mL/kg per day by the end of the first week of life, with 90% of neonates producing 0.5 to 5 mL/kg per hour (Douglas, 1972; Guignard, 1982). In the neonate who is less than 1 week old, urine flow alone is not a sensitive index of changes in cardiac output or intravascular volume. The limited capacity of the neonatal kidneys to compensate for diminished or excessive intravascular volume demands more precise management of blood and fluid replacement in these infants. Beyond the neonatal period, a urine flow of 0.5 to 1 mL/kg per hour usually indicates adequate renal perfusion and function.
Noninvasive Respiratory Gas Monitoring
Carbon Dioxide
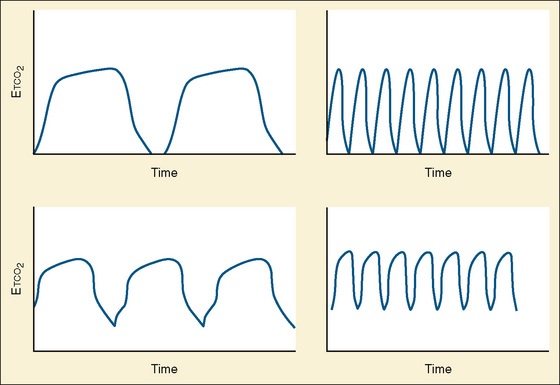
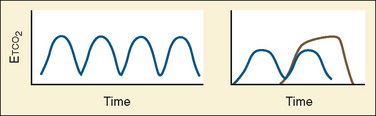
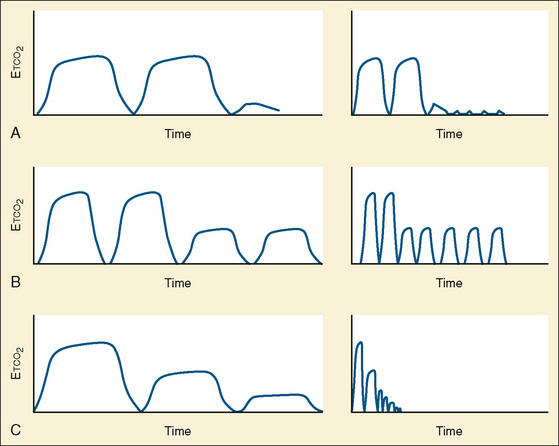
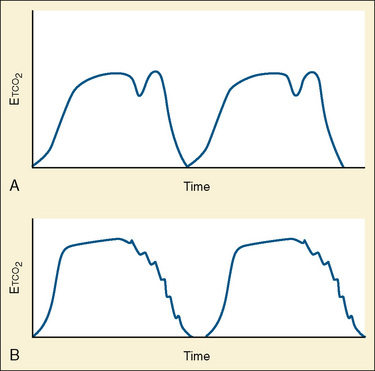
Capnometry is the instantaneous measurement of CO2 in the breathing circuit; it depicts this information in a continuous graphic display in which both the quality and the quantity of ventilation can be evaluated (Figs. 11-3 to 11-6).
Before 1998, capnography was considered a standard monitor by the ASA for confirming the initial placement and continuous presence of an endotracheal tube. This section of the ASA monitoring standards was updated in 1998 and states that capnography should be used to confirm adequate ventilation during general anesthesia with or without an endotracheal tube (during laryngeal mask airway, face mask, or natural-airway anesthesia). Specifically, these guidelines state, “Continual monitoring for the presence of expired carbon dioxide shall be performed unless invalidated by the nature of the patient, procedure or equipment…. Continual end-tidal carbon dioxide analysis, in use from the time of endotracheal tube/laryngeal mask placement, until extubation/removal or initiating transfer to a postoperative care location, shall be performed using a quantitative method such as capnography, capnometry or mass spectroscopy” (ASA, 2003).
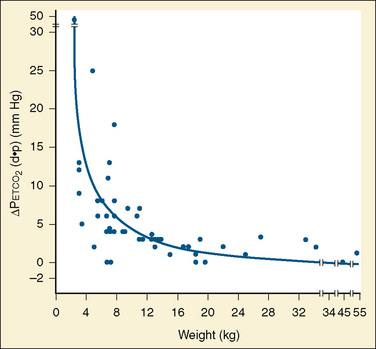
The degree to which Petco2 reflects Paco2 is subject to many variables, some technical and others physiologic. The technical issues of primary importance in the accurate measurement of mean alveolar CO2 tension include the volume and flow rate of exhaled gas, the aspirating flow rate (for sidestream analyzers), the fresh gas flow rate, the type of breathing circuit, and the circuit location of the sampling chamber (mainstream analyzers) or lumen of the aspirating tubing (sidestream analyzers). These variables are of particular importance in the small neonate whose small exhaled volumes at low flow rates are often diluted by high fresh gas flows or aspirating gas flows (Badgwell et al., 1987, 1993; Rich et al., 1990; Spahr-Schopfer et al., 1993). Badgwell and others (1987) demonstrated exponential increases in the discrepancy between Petco2 and Paco2 values with progressive reduction in patients who weighed less than 12 kg (Fig. 11-7). The coaxial distal sampling tube that they advocated dramatically improved the correlation.
The physiologic variable that introduces the most significant error in Petco2 is dead space ventilation (Swedlow, 1986). Apart from children with severe pulmonary pathology or acute events such as pulmonary embolus, the most prevalent pediatric population in whom substantial dead space ventilation occurs are those with cyanotic congenital heart disease, particularly right-to-left shunts (Burrows, 1989; Fletcher, 1991).
Monitoring Oxygen and Carbon Dioxide
Cutaneous Oxygen Tension
In 1972, a miniature Clark polarographic oxygen electrode, similar to those used in in vitro blood gas analysis, became available for application to the skin. When a probe heats the skin to 42° to 44° C, the cutaneous oxygen tension (Pso2) approaches arterial oxygen tension because the skin blood flow and permeability to oxygen are increased (Barker and Tremper, 1985). The correlation may be better in neonates because their epidermis is less keratinized and their cutaneous capillary bed is denser. In fact, skin heating alters oxygen dissociation and may even result in a Pso2 value that is higher than the Pao2 value (Lubbers, 1981). In older children and adults, as the keratinized layer thickens, the diffusion gradient for oxygen becomes more significant. In practice, transcutaneous gas monitors are subject to the effects of these and myriad other nonlinear variables that influence skin perfusion, such as hypotension, hypothermia, and pharmacologic agents. They are the only noninvasive monitors that can provide information regarding significant hyperoxemia (Monaco et al., 1982; Rafferty et al., 1982; Barker and Tremper, 1985). The correlation, especially outside the physiologic range of Pao2, is variable and depends on the individual conditions, and the data produced may differ from those provided by the Pso2 by a substantial yet unpredictable amount (American Academy of Pediatrics, 1989). Such results led Barker and Tremper (1985) to propose a more reasonable role for this monitor in determining peripheral tissue oxygen delivery (perfusion) rather than arterial oxygenation.
Cutaneous Carbon Dioxide Tension
Cutaneous carbon dioxide tension (Psco2) measurement using a variant of the Severinghaus electrode is also available (Nosovitch et al., 2002). Although Psco2 is always higher than Paco2 as a result of tissue carbon dioxide production and the increased metabolism caused by a heating sensor, these monitors accurately follow trends in arterial carbon dioxide tension. The predictable gradient from arterial to cutaneous CO2 tensions enables the monitors to calculate the gradient and display a “corrected” value. These devices are less altered by changes in skin temperature and perfusion than are cutaneous oxygen analyzers. The reasonably good correlation of end-tidal and arterial CO2 tensions in all but extremely small subjects has limited the interest in cutaneous CO2 tension monitoring in the operating room to very rare situations.
Pulse Oximetry
Pulse oximetry, invented by Takuo Aoyagi in 1974 and developed as a clinical monitor a decade later, has markedly improved patient safety, especially during the perioperative period (Yelderman and New, 1983; Aoyagi, 2003; Severinghaus, 2007). It provides an estimate of the oxyhemoglobin saturation in arterial blood with a probe on a fingertip (New, 1985). The pulse oximeter uses plethysmography to determine the systolic portion of the cardiac cycle. During systole, there is a greater volume of blood in a pulsatile arterial vascular bed. This vascular bed is positioned between a sensor that contains a two-wavelength (red and infrared, 660 and 940 nm) light-emitting diode and a photodiode receptor (the wavelength varies depending on the manufacturer). Less light is transmitted in systole than in diastole because of the increased volume of the arterial bed. Sophisticated algorithms based on the amount of light absorbed differentiate systole from diastole. Oxygenated and deoxygenated blood absorb different quantities of light, proportional to their concentrations or the percentage of saturation according to the Beer-Lambert law. Once systole is identified through plethysmography, the arterial saturation during this period is determined by using the ratio of light absorption at the two different wavelengths through this vascular bed. The ratio is matched to data acquired over a range of experimentally determined saturations and ratios of light absorption stored in the instrument’s memory to determine the arterial saturation. Using the ratio of light absorption at two wavelengths makes unnecessary the calibration and zeroing to adjust to the size of the patient or to skin pigment (Aoyagi, 2003). In the 80% to 100% saturation range, arterial saturation values determined by this method correlated well with in vitro measurements (New, 1985). Based on the algorithm used to determine systole and the time-averaging process used over several cardiac cycles, different pulse oximeters have slightly different responses to a variety of clinical situations. Because the device must identify the pulse-added absorption, it may confuse motion of the extremity to which the sensor is attached with pulsating motion and abort the display of saturation data or, worse, display inaccurate data (see later).
Pulse oximetry was introduced into pediatric practice in the United States in the late 1980s (Salyer, 2003). It serves as an early warning signal of impending or actual hypoxemia, often before the onset of cyanosis, frequently reminding anesthesiologists of the alarming rapidity with which infants develop hypoxemia as compared with toddlers and older children (Xue et al., 1996). Continuous-use pulse oximetry is included in the basic monitoring standards of the ASA (2003). Anesthesiologist-blinded studies have demonstrated that the use of pulse oximetry facilitates earlier recognition and fewer episodes of hypoxemia (Coté et al., 1988, 1991). Pulse oximetry has rapidly evolved into a standard monitor during pediatric anesthesia and, as a result, has never been subjected to rigorous outcome studies with a true control group (an anesthetic without a pulse oximeter) (Cohen et al., 1988). There are no outcome studies that demonstrate proven benefit from the use of pulse oximetry (Moller et al., 1993a, 1993b; Pedersen et al., 2001).
A number of well-described limitations of pulse oximetry affect the accuracy of commercially available pulse oximeters. Among other factors, ambient lighting conditions, motion, peripheral circulation to the extremity, body temperature, and abnormal hemoglobins can interfere with accuracy and consistency of pulse oximeter performance (Schramm et al., 1997; Trivedi et al., 1997). Of all these factors, motion artifact is clinically the most common problem, resulting in inaccurate readings, data loss, missed alarms, and false alarms. Patient motion (e.g., shivering and seizures in adults and children, as well as kicking, stretching, and crying in infants) causes movement of venous blood, as well as other normally nonpulsatile body fluid components (e.g., tissue fluid in edematous patients), along with the arterial blood. The pulsatile components of the body fluids other than arterial blood may lead to falsely low saturation readings. If motion is combined with low perfusion at the sensor site, then the venous blood makes a more significant contribution to the pulsatile component and drives the Sao2 as measured with a pulse oximeter (or SpO2) even lower. Various algorithm-based motion rejection methods used by manufacturers to minimize the impact of motion artifact and reduce false alarms include averaging saturation data over longer periods of time, freezing values until uncorrupted signals are present, and implementing alarm delays (Petterson et al., 2007). Other technologies use parallel processing of multiple sophisticated algorithms to isolate the biological signal in the presence of noise. When compared with conventional and other motion-tolerant pulse oximeters, Signal Extraction Technology (Masimo Corp., Irvine, Calif) has been reported to have a better performance in a variety of pediatric clinical settings, including newborn and pediatric intensive care units, the PACU, and the pediatric sleep lab (Ahlborn et al., 2000; Bohnhorst et al., 2000; Malviya et al., 2000; Barker, 2002; Brouillette et al., 2002; Trang et al., 2004; Workie et al., 2005).
Although the pulse oximeter is a continuous monitor, it does not instantaneously reflect the arterial saturation or the degree of desaturation. Most commercially available pulse oximeters display the average SpO2 numbers of the most recent 5 to 15 pulses, depending on models and manufacturers, causing a delay in detecting desaturation. When the patient is breathing high concentrations of oxygen and the blood is fully saturated, a substantial decrease in Pao2 can occur without a change in Sao2. Reynolds and associates (1993) detected desaturation in children 30 seconds earlier in probes placed centrally (forehead) than in those placed on a finger. By the time the value indicated by a peripheral sensor had decreased 5%, the value indicated on a central sensor was 30% to 40% lower (Agashe et al., 2006). The precise mechanism for this discrepancy remains unknown, although Severinghaus and Naifeh (1987) postulated that it reflects peripheral blood transit, capillary composition, and oxygen utilization. Centrally placed reflective forehead pulse oximeter sensors are reported to respond faster and to be less likely to be compromised by vasoconstriction than transmittance finger probes (Choi et al., 2010).
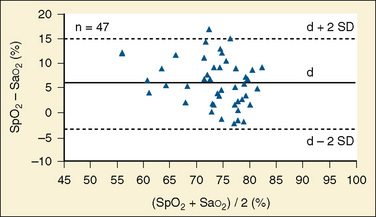
Pulse oximeters are designed to warn practitioners when the arterial saturation decreases to below normal, not to serve as quantitative devices in hypoxemic patients. Compared with measured arterial saturation in children with cyanotic congenital heart disease, most pulse oximeters exhibit a progressively positive bias, typically reaching 5% to 15% at an Sao2 of 60%, in addition to a significant reduction in precision (±8% to 10%) (Gidding, 1992; Schmitt et al., 1993) (Fig. 11-8). More recently, a pulse oximetry sensor designed specifically for patients who are chronically hypoxemic, such as children with congenital heart disease, has become available. This specialized sensor, called the Blue sensor, has reported precision of less than ± 4% between saturations of 60% and 80%. When compared with a standard pulse oximetry sensor on postoperative palliative cardiac surgery patients, the Blue sensor had a bias of 0.21 and an accuracy root-mean-square (RMS) error of 3.6% compared with Sao2 values, whereas the standard sensor had a bias of 0.86 and an accuracy RMS error of 6.4% compared with Sao2 values (Cannesson et al., 2008c). The Blue sensor demonstrates considerable improvement in the accuracy of pulse oximetry monitoring of cyanotic children.
Interference with the expected spectrophotometric absorption pattern also causes errors in measurement. Low hemoglobin (<5 g/dL) and abnormal hemoglobin species (e.g., methemoglobin, carboxyhemoglobin) cause inaccurate saturation estimates by pulse oximeters, which become progressively inaccurate as Sao2 decreases (New, 1985; Barker and Tremper, 1987; Barker et al., 1989; Watcha et al., 1989). In contrast, unusual or abnormal hemoglobin molecules, such as fetal hemoglobin and sickle cell hemoglobin, apparently have little effect on the saturation measurement (Jennis and Peabody, 1987; Ortiz et al., 1999). Intravenous dyes such as methylene blue and indocyanine green, affect the expected light absorption and produce spurious information (Ralston et al., 1991). Aberrant radiation (e.g., electromagnetic energy from the electrocautery, infrared heat lamps, operating room lights) and infrared light pulses from lasers and neuronavigation systems also cause incorrect saturation determinations (Brooks et al., 1984; Costarino et al., 1987; Hanowell et al., 1987; Mathes et al., 2008; Zhang et al., 2009).
Multiple-Wavelength Pulse Oximetry
Pulse oximeters that use two wavelengths of light are subject to interference from other hemoglobin species that absorb light across similar spectra. Dyshemoglobins such as carboxyhemoglobin and methemoglobin cannot be detected by pulse oximetry, but their presence in arterial blood can bias the oximeter’s estimate of Sao2. Multiple-wavelength pulse oximetry (Pulse CO-Oximetry; Masimo Corp., Irvine, CA) has been developed to detect and measure both carboxyhemoglobin and methemoglobin levels noninvasively and continuously, although the accuracy of Sao2 estimations from these devices is still compromised (Barker et al., 2006). Pulse CO-Oximetry devices that measure carboxyhemoglobin have been shown to be useful in the emergency room for detection of occult carbon monoxide poisoning, and for monitoring and guiding emergency treatment of CO exposure (Suner et al., 2008; Bledsoe et al., 2009; Piatkowski et al., 2009). Methemoglobinemia can be caused by a wide variety of oxidizing drugs and their metabolites and by exposure to industrial chemicals such as aniline dyes, chlorates, and bromates. The clinical usefulness of noninvasive methemoglobin detection and monitoring by Pulse CO-Oximetry has been demonstrated for the diagnosis and treatment of severe methemoglobinemia in the hospital setting (Annabi and Barker, 2009).
Plethysmographic Waveform Analysis
For many years, clinicians have used the shape and amplitude of the plethysmographic waveform from pulse oximetry as a crude and imprecise method to assess blood volume and cardiac output (Partridge, 1987). Respiratory variations in pulse oximetry plethysmographic waveform amplitude can predict fluid responsiveness noninvasively in mechanically ventilated patients, but this method of assessment is technically challenging—neither automated nor continuous, and not available in clinical practice (Cannesson et al., 2007). An index that provides an automatic and continuous calculation of the respiratory variations in the waveform amplitude is now available with some pulse oximeters. The pleth variability index has been shown to predict fluid responsiveness during cardiac surgery (Cannesson et al., 2008b) and to predict changes in cardiac index with changes in positive end-expiratory pressure after cardiac surgery in mechanically ventilated, sedated patients (Desebbe et al. 2010). This index may allow the optimization of fluid loading noninvasively during and after surgery, but further validation is required before these results can be extrapolated to the general population.
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is another tool available to the anesthesiologist that provides information regarding tissue oxygenation. Its applications range from cerebral oximetry during circulatory arrest, to monitoring for compartment syndrome in the setting of trauma (Tobias and Hoernschemeyer, 2007; Bernal et al., 2010). Although NIRS continues to be studied for its impact on patient outcomes, it is increasing popular as a noninvasive monitor of vital data.
NIRS relies on principles similar to those of pulse oximetry, exploiting the variable absorption of light by color-containing compounds in the body. Infrared light emitted by the device enters the skin and scatters through the tissues beneath, some energy being absorbed and some reflected. The portion of the light that returns to the skin is analyzed and conclusions are drawn about the content of the tissues it passed through (Cohn, 2007).
The basis for analysis of the reflected light is found in the Beer-Lambert law. According to this law, reduction in the intensity of light passing through a substance depends on the absorbance of the substance and its thickness. These principles are used to quantify concentrations of oxygenated hemoglobin, which absorbs light at 805 nm, and deoxygenated hemoglobin, which absorbs at 730 nm, and to determine the regional oxygen saturation (rSo2) of the tissue examined (Tobias, 2006).
NIRS technology is commercially available from only a few companies. The INVOS Cerebral/Somatic Oximeter (Somanetics Corp., Troy, MI), which has been on the market for a long time, provides real-time monitoring capability for the rSo2 of blood in tissues beneath the probe. To target specific tissues such as the brain or kidneys and not the skin and soft tissues between the probe and these organs, the device has been designed to eliminate data from the superficial tissues. The probe incorporates a light-emitting diode and two sensors located at fixed distances from the light. Infrared light passing through the superficial tissue is picked up by the closest sensor, and infrared light passing through the deeper tissues is picked up by the further sensor. Information from the superficial tissues is subtracted from the data returning from deeper tissues to provide accurate estimates of the deep tissue oxygenation (Tobias, 2006).
The Fore-Sight (Cass Medical Systems, Branford, CT) uses laser light at four wavelengths. Based on the same principles as the Sonametics device, it also accounts for the spurious information collected from the superficial tissues to focus on the deeper areas of interest (Chakravarti et al., 2008). Both devices have approved uses in pediatrics, and probes of different sizes fit this population.
Clinicians presented with data on tissue oxygenation must know how to respond to it, and much has been written about the clinical usefulness of NIRS. Cerebral oximetry has been the most widely studied use of NIRS, and here NIRS has proved to be both a sensitive and specific indicator of cerebral tissue oxygenation (Al-Rawi et al., 2001). Kurth and coworkers (2002) noted thresholds for normal and abnormal values in a piglet model. They found baseline cerebral rSo2 values of 68%, and they found that lactate began rising when the rSo2 dropped to 44%, electroencephalographic changes started at around 42%, and ATP loss occurred at 33%. This information, along with corroborating findings in adult studies, has led to a weak consensus that an rSo2 reduction of 20% from baseline, or an absolute value of 50%, serves as an indication of potential hypoxic injury and warrants intervention.
To establish the value of cerebral oximetry in the pediatric population, Hoffman and colleagues (2008) studied patients treated for hypoplastic left heart syndrome, and they demonstrated improved neurologic scores at age 4 to 5 years in patients who were monitored with cerebral oximetry at the time of their surgery and treated for abnormal values (rSo2 < 55%). This is encouraging, but most agree that there is still insufficient evidence to support clinical decisions based on NIRS values alone (Hirsch et al., 2009).
NIRS has generated interest in additional applications, including shock, compartment syndrome, plastics, and regional perfusion. Adult literature demonstrates NIRS ability to detect compartment syndrome as well as improvement in tissue oxygenation after fasciotomy (Giannotti et al., 2000). Studies of free flaps found that NIRS identified early evidence of flap failure prior to routine clinical evidence (Holzle et al., 2006).
Bispectral Index
In 1996, the FDA approved the use of the bispectral index (BIS) monitor (Aspect Medical Systems, Nattick, MA), a device based on electroencephalography (EEG) and used to predict the relative level of hypnosis, or unconsciousness, in anesthetized patients (Rosow and Manberg, 1998). Using a patch affixed to the patient’s forehead, the BIS monitor integrates various EEG descriptors into a single, dimensionless, empirically calibrated number ranging from 0 to 100, where 0 represents electrical silence and 100 represents full wakefulness. A state of unconsciousness consistent with BIS values less than 60 usually ensures a lack of intraoperative recall (Glass et al., 1997). In adults, titration of anesthetics to a targeted BIS value between 40 and 60 results in the administration of relatively lower doses of anesthetics and earlier awakening (Gan et al., 1997). Studies in adults suggest that routine BIS monitoring is associated with reduced intraoperative awareness during high-risk surgical procedures (e.g., microlaryngeal surgery, cesarean section, cardiac bypass) (Myles et al., 2003).
In anesthetized children on mechanical ventilation, as well as in those spontaneously breathing through a face mask, BIS values are inversely proportional to the end-tidal concentration of halothane, sevoflurane, and desflurane (Denman et al., 2000; Davidson et al., 2001; Degoute et al., 2001; Tirel et al., 2006; Kern et al., 2007). Similar relationships between the depth of anesthesia (minimum alveolar concentration [MAC], plasma concentration) and BIS values have been studied during total intravenous anesthesia (TIVA), with or without additional opioids, in children with target-controlled infusion of propofol (Jeleazcov et al., 2007; Tirel et al., 2008; Rigouzzo et al., 2008; Malherbe et al., 2010). BIS values during sevoflurane anesthesia appear to be proportionately less in children with quadriplegic cerebral palsy and those who are mentally compromised (Choudhry and Brenn, 2002; Valkenburg et al., 2009).
In children, at a given depth of anesthesia (i.e., MAC), there are significant nonlinear inverse correlations between BIS values and age: BIS values are higher in toddlers than in older children in all studies, including TIVA with propofol. Furthermore, in addition to age-related variations in BIS values, interindividual variabilities in BIS index are much higher in infants and children than in adults (Tirel et al.; 2006, Kern et al., 2007; Rigouzzo et al., 2008). These characteristics of BIS in children may be associated with age-related differences in brain maturation and synapse formation throughout childhood (Watcha, 2001). The reliability of the BIS index is further diminished in infants younger than 1 year (Tirel et al., 2006; Jeleazcov et al., 2007; Kern et al., 2007; Rigouzzo et al., 2008).
The clinical benefits of measuring BIS and maintaining appropriate levels of anesthesia, such as reduced risk for intraoperative awareness and improved recovery time, may still be valid in pediatric patients, with the realization of certain characteristics of and differences in children. In adolescents undergoing scoliosis surgery, BIS can predict voluntary patient movement in response to commands during the intraoperative wake-up test (McCann et al., 2002). BIS monitoring was also used successfully for the intraoperative wake-up test in a neonate undergoing neurosurgery for the repair of myelomeningocele (Govindarajan et al., 2006). BIS monitoring in children aged 3 to 18 years who are undergoing tonsillectomy and adenoidectomy is associated with reduced recovery times (Bannister et al., 2001). However, in the same study, BIS monitoring did not affect recovery times in children younger than 3 years who were undergoing hernia repair.
More recently, interest in the efficacy of BIS in monitoring the depth of sedation has increased in other specialties, including critical care, emergency medicine, dentistry, and general pediatrics (Kerssens and Sebel, 2006; Malviya et al., 2006, 2007; Sadhasivam et al., 2006; Froom et al., 2008; Baygin et al., 2010). Also, BIS monitoring has been used in documenting positive sedative effects of regional and spinal blockade with local anesthetics in children, with or without general anesthesia (Davidson et al., 2006; Hermanns et al., 2006). Future studies are expected to further delineate the use of BIS in the pediatric population.
Neurophysiologic Monitoring
Neurophysiologic techniques provide important and reliable alternative tools for assessment of function of the pediatric CNS (Sclabassi and Krieger, 1995). These techniques provide objective measures of the functioning of the CNS and can serve to localize, warn of, and document deterioration in neuronal function. Intraoperatively, continuous monitoring of the area of the CNS that is at risk from surgical and anesthetic manipulation provides immediate insight into the effects of these manipulations. Rather than waiting to evaluate the neurologic examination of a child in the postoperative period, continuous IOM provides an immediate view of the integrity of the CNS, permitting changes in operative and anesthetic technique to minimize or correct the deleterious effects of intraoperative manipulations. Advantages of these methods are that the results are objective and quantifiable, the site of the lesion can be identified, and clinically latent and evolving lesions can frequently be demonstrated. These techniques have roles to play both in the diagnostic investigation of pediatric CNS function and in the field of intraoperative assessment of CNS function.
Technical Methodology
The bioelectrical activity at the scalp and the surface of the body is sensed using metal electrodes, and it is transferred through conducting leads to recording amplifiers. Subdermal needle electrodes are used in the operating room and may be used as stimulating electrodes as well as recording electrodes. The positions of the recording electrodes should be chosen in relation to the expected distribution of the responses to be recorded. Many laboratories place scalp electrodes at sites determined by the international 10-20 system (Jasper, 1958). This system, originally devised for EEG recordings, specifies the position of 21 evenly spaced locations on the scalp. Recording electrodes are placed symmetrically to provide control recordings from the side contralateral to the surgery, even when electrodes may not be positioned in the standard recording sites. Electrodes that are not in the operative field but are on the scalp and not accessible during surgery are either sutured or stapled in place. Electrodes on the face, which are placed to record electromyographic activity, are taped in place. Electrodes in the operative field are placed by the surgeons using sterile technique, usually early in the procedure.
Neurophysiologic signals are amplified using differential amplifiers (Goff, 1974), in which two input channels to the amplifier are differenced. This differencing has the effect of eliminating identical (in-phase) signal components that might be present at each recording electrode (presumably noise), and retaining the signals that are different (out of phase) and presumably produced by physiologic generators. The effectiveness with which a differential amplifier rejects in-phase signals compared with its ability to amplify out-of-phase signals is called the common mode rejection ratio (CMRR). Differential amplifiers typically have CMRRs of greater than 10,000:1 (80 dB). For efficient rejection of in-phase signals, it is extremely important that the impedances of each electrode in a pair be not only as low as possible but as similar as possible, because any inequality in electrode impedance will produce amplitude differences in the in-phase activity that will be amplified along with the desired signal.
After signal amplification, the most effective method for extracting a signal of interest from background noise is signal averaging. Signal averaging is in effect a cross-correlation between a point-process defined by the occurrence of the stimuli and the recorded evoked activity (i.e., an optimal filter) (Lee, 1960). In averaging, the signal component at each point is coherent and adds directly, whereas the background and noise components tend to be statistically independent and summate in a more or less root-mean-square fashion.
Anesthetic techniques
The type of anesthesia as well as the patient’s blood pressure, cerebral blood flow, body temperature, hematocrit, and blood gas tensions all affect the functioning of the patient’s CNS and thus intraoperatively observed neurophysiologic measures (Grundy et al., 1981; Grundy, 1983; McPherson, 1994; Sloan, 1998). The neurophysiologist must discuss the anesthetic plan with the anesthesiologist before the start of the procedure to ensure that no conflicts exist over the required anesthetic and neurophysiologic monitoring. Both the neurophysiologist and anesthesiologist must understand one another’s needs and develop a plan for monitoring and anesthesia that allows both individuals to provide appropriate care for each patient. The halogenated hydrocarbon inhalation agents tend to reduce the amplitude of somatosensory evoked responses (Salzman et al., 1986), and to suppress alpha motor neuron activity that interferes with obtaining MEPs. The best SEPs are often recorded when a narcotic relaxant technique (consisting of an opioid, nitrous oxide (<65%), and a muscle relaxant) is used, whereas the best MEPs are often recorded with TIVA. Thus, when both types of responses are needed, a balancing act with regard to anesthesia is required. Boluses of medications produce more disruption of signals than constant infusions. Regardless of how medications are delivered, the anesthesiologist must inform the neurophysiologist of medication administration, changes in patient temperature or blood pressure, and any change in the patient’s condition.
In many situations, the use of halogenated hydrocarbon inhalation agents is desired to help control blood pressure. Once baseline responses have been obtained and compared with preoperative responses, many children can maintain their responses to an isoflurane level of approximately 0.3 MAC, whereas many adults can maintain their responses to 0.5 MAC or higher. This is highly variable, and it strongly depends on the individual patient’s reaction to the inhalation agent. A slow increase in isoflurane until either the blood pressure is controlled or the responses significantly deteriorate usually leads to satisfactory results; however, a small number of patients cannot maintain their SEPs with any inhalation agent on board. Propofol, etomidate, and ketamine also appear to maintain SEPs at anesthetic concentrations and may be particularly useful when signals are expected to be difficult to obtain (Schubert et al., 1990; Kalkman et al., 1991; Taniguchi et al., 1992; McPherson, 1994). Close cooperation between the anesthesiologist and neurophysiologist is important, because some patients, particularly those with immature nervous systems, exhibit sensitivity to anesthetic agents (Sloan, 1998).
Neurophysiologic Measures
Maturational Effects
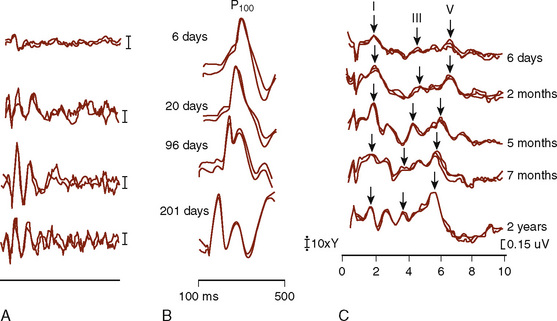
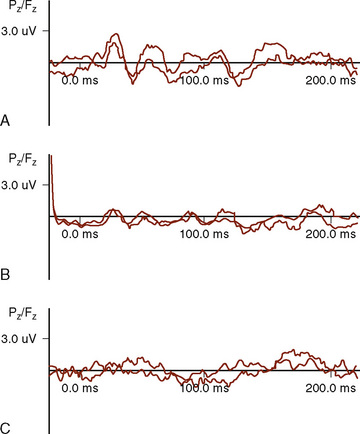
The functional assessment of the pediatric CNS presents difficult and unique problems. The pediatric CNS differs from the adult CNS in that it is maturing over the first several years of life; that is, the neural tissue, the myelin coating of the axonal processes, and the vascular supply to the CNS all show significant changes. These developmental anatomic changes are reflected in maturational functional changes as measured by ascending SEP and by descending MEP activity, VEPs, and BAEPs (Starr et al., 1977; Cracco et al., 1979; Guthkelch et al., 1982). A number of factors contribute to the maturational changes of evoked potentials, and the use of age- and size-matched normal controls is essential. For intraoperative monitoring purposes, infants act as their own controls. Central and peripheral myelination is believed to be completed by 5 years of age, and from then until maturity, the dominant factor affecting SEP latency is height (Yakovlev and Lecours, 1967; Gilmore et al., 1985) (Fig. 11-9).
General monitoring procedures
Electroencephalography
The functioning of the cerebral cortex is extremely sensitive to changes in arterial oxygenation and insufficient cerebral blood flow or an inadequate partial pressure of oxygen; this sensitivity is rapidly reflected in the EEG (Meyer and Marx, 1972). Oxidative metabolism supplies the energy for maintenance of the membrane potential of nerve cells, and the EEG depends directly on the transmembrane potentials of neurons, reflecting disturbances of cerebral metabolism such as hypoxia. Some factors that may contribute to ischemic events in surgical patients are decreased oxygen-carrying capacity resulting from hypovolemia, and decreased cerebral perfusion pressure resulting from factors associated with decreased systemic arterial pressure, increased intracranial pressure, and mechanical obstruction of cerebral vessels (Freye, 1990). It is thought that having two channels of continuous EEG monitoring is adequate, because the problems are related more to global or hemispheric effects than to precise focality. The EEG can be observed both as the ongoing unprocessed signal and in a Fourier-transformed representation. The electroencephalographic appearances of any ischemic or hypoxic events are similar, and differentiation between the various putative causative factors is made by being particularly attentive to the clinical situation. For example, blood pressure, ECG, oxygen saturation, administered drugs, and surgical manipulations may all have an observable effect. Other concurrent factors that may alter the EEG are changes in the depth of anesthesia, temperature changes, and changes in CO2 content. These factors can be recognized by their relatively slow onset, lasting for several minutes, in contrast to the changes of ischemia, which generally occur within seconds. In some situations, the EEG may be acutely depressed on injection of an anesthetic that rapidly passes the blood-brain barrier. Such situations may be found with the use of high-dosage opioid anesthesia, in which fentanyl induces an immediate and marked reduction in fast-frequency activity in the EEG, with an increase in low-frequency, high-amplitude activity in the delta range (Freye, 1990).
A simplified but useful summary of possible changes includes the following:
Somatosensory and Motor Evoked Potentials
Somatosensory Evoked Potentials (Ascending Activity)
SEPs are a sequence of potentials generated in the peripheral nerves, dorsal horn nuclei, dorsal column pathways, and dorsal column nuclei of the spinal cord; the medial lemniscal pathways of the brainstem; and the thalamus and thalamocortical and parietal regions of the brain after the application of a transient electrical stimulus to a peripheral nerve (Sclabassi et al., 1993b). When recorded from electrodes on the surface of the body, the potentials of interest are very small, ranging in size from 2 to 5 mcV, and occur in approximately the first 100 msec after the application of a stimulus (often referred to as early and middle latency potentials). Evoked potentials are described in terms of latency and amplitude. Latency is the time measured from the application of a stimulus to the point of maximum amplitude of the evoked potential. Some types of SEP have more than one peak, and the time between peaks is the interpeak latency. The amplitude is the voltage difference between two peaks of opposite polarity, or a reference potential. Measurements of latencies, amplitudes, and interpeak latencies characterize SEP recordings. Changes in these measurements during a surgical procedure may represent injury to the neural tissue between the stimulus generator and the recording electrode.
Median and Ulnar Nerve Evoked Potentials
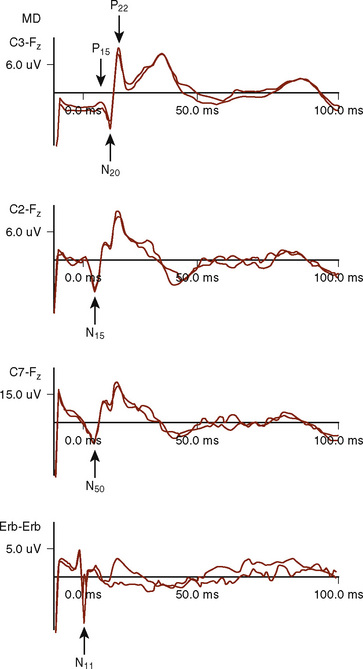
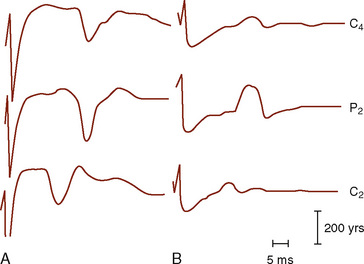
The median (MSPs) and ulnar (USPs) nerve evoked potentials are all useful in assessing the brachial plexus, upper spinal cord, brainstem, and telencephalon. One important distinction is that the USPs provide information rostral to T1, whereas the MSPs provide information rostral to C6 (Fig. 11-10). At Erb’s point, the response consists of an apparently triphasic (positive-negative-positive) nerve action potential, reflecting the passage of the mixed nerve volley through the brachial plexus. This component is usually labeled N11 for the large negative-going component. At the cervical C7 recording site, the main component is a negative peak occurring at 14-msec latency, N14, with an associated complex structure. It has been postulated that these waves are generated in the dorsal roots, dorsal horn, posterior columns, and structures of the lower brainstem. During spinal fusions, monitoring of the brachial plexus may also alert the surgeon and anesthesiologist to compressive positioning of the arms.
Common Peroneal and Posterior Tibial Nerve Evoked Potentials
In our experience, SEPs are extremely sensitive and specific to spinal cord injury, whether it occurs in the dorsal or the ventral pathway. This is confirmed in the literature (Nuwer et al., 1995), where a false-negative rate of 0.063% was found for 51,263 spinal cases in which SEPs were the only potentials monitored. Furthermore, the negative predictive value (i.e., the likelihood of normal spinal cord function in the presence of stable SEPs) was 99.93%. This is a significant improvement over the 0.72% to 1.4% incidence of spinal cord injury reported for unmonitored cases (MacEwen et al., 1975).
Dermatomal Evoked Potentials
Pudendal nerve responses, a special type of dermatomal response, are particularly useful, especially in patients with spina bifida. The pudendal nerve carries sensory fibers from the penis, urethra, anus, and pelvic floor muscles and supplies motor innervation to the bulbocavernosus and pelvic floor muscles, the external urethral sphincter, and the external anal sphincter. Cortical responses to electrical stimulation of the dorsal nerve of the penis, the urethra, and the urinary bladder have all been described (Badr et al., 1982; Haldeman et al., 1982). Pudendal nerve responses are of similar morphology to the tibial nerve SEP (TSP) and are best recorded from the same area of the scalp (Fig. 11-11).
Motor Evoked Potentials (Descending Activity)
Because SEPs reflect function in the dorsal columns of the spinal cord, they do not directly assess the integrity of descending spinal motor tracts. It is possible to have focal damage to the motor areas in the spinal cord in which the SEPs remain normal. Accordingly, misleading results have occasionally been obtained when using SEPs alone for intraoperative monitoring and diagnosis (Lesser et al., 1986), but this is rare (Nuwer et al., 1995). MEPs, which may be either evoked EMGs or compound action potentials, can be used to test the integrity of the motor pathways through either electrical or magnetic stimulation (Merton and Morton, 1980; Barker et al., 1985). MEPs can be obtained via stimulation of the motor areas of the brain or spinal cord through the intact skin, direct stimulation of exposed neuronal tissue, or direct root stimulation (e.g., during the release of a tethered cord), and recording of a stimulus-related response either as a compound muscle action potential (CMAP) or as an efferent compound nerve action potential (CNAP) (Fig. 11-12).
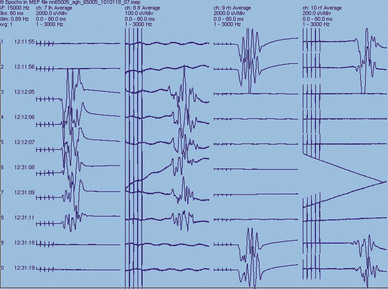
Transcranial electrical stimulation may be used to elicit motor responses. There is no general consensus about the location of recording electrodes, outside of specific muscle groups for evoked CMAPs or over the obvious peripheral nerves for evoked CNAPs, nor is there a general consensus concerning which class of these activities is more advantageous to record. CNAPs allow the patient to receive neuromuscular blockade agents. One of the most important stimulation parameters for eliciting reliable MEPs is the interstimulus interval (ISI) of a burst of stimuli (Taylor et al., 1993). It has been found that a burst of stimuli with ISIs between 2 and 5 msec (500 to 200 Hz) produce a maximal response by overcoming the depressed effect of general anesthesia (mentioned earlier) (Kalkman et al., 1995). The significant parameters and morphologic features of MEPs are response threshold, onset latency, central conduction time, and response size (Fig. 11-13).
Brainstem Auditory Evoked Potentials
Visual System
VEPs are used to aid in determining the functional integrity of the visual system, primarily in the region of the optic nerves, chiasm, and optic radiations (Albright and Sclabassi, 1985). The recorded activity is generated either at the retina (electroretinogram) or at the cortex.
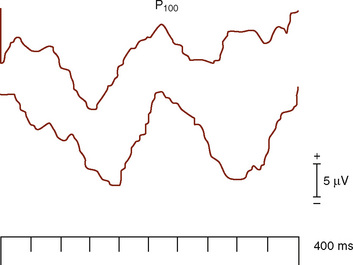
Stimulation of the visual system using a bright flash is not recommended for diagnostic purposes because of intersubject variability (Ciganek, 1961), except in select situations. In the operating room, this is a very helpful and effective technique (Fig. 11-14).
Electromyography
Cranial Nerve Function
Cranial nerve function is monitored continuously during surgery for two reasons: first, to establish the location and orientation of the cranial nerves in the operative field, and second, to preserve functioning in the cranial nerves and their related brainstem nuclei (Sclabassi et al., 1993a). The major observed variables are the EMGs from the appropriate muscle group innervated by the cranial nerves of interest. In general, the cranial nerves ipsilateral to the operative side are monitored; when appropriate, bilateral activity is monitored.
In addition to monitoring the ongoing EMG activity related to the various cranial nerves, these cranial nerves may also be electrically stimulated. This is usually done to determine the location of the nerve in the operative field (because often the nerve is enveloped by tumor and may not be directly observable), or to determine the functional integrity of the nerve (Daube and Harper, 1989). The most common example of this procedure is the direct stimulation of nerve VII. The return path for the stimulating current is provided by a metal electrode inserted into the adjacent muscle mass. In some situations, when very precise localization of the nerve is required, bipolar stimulating electrodes are used. Most of the time, the question being asked is, Is the nerve there? These techniques are very useful when monitoring during surgery for posterior fossa tumors that extend to the floor of the fourth ventricle.
Agashe G.S., Coakley J., et al. Forehead pulse oximetry: headband use helps alleviate false low readings likely related to venous pulsation artifact. Anesthesiology. 2006;105(6):1111-1116.
Ahlborn V., Bohnhorst B., et al. False alarms in very low birthweight infants: comparison between three intensive care monitoring systems. Acta Paediatr. 2000;89(5):571-576.
Albright A.L., Sclabassi R.J. Cavitron ultrasonic surgical aspirator and visual evoked potential monitoring for chiasmal gliomas in children. J Neurosurg. 1985;63:138-140.
Alderson P.J., Burrows F.A., Stemp L.I., et al. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. Br J Anaesth. 1993;70:145.
Al-Rawi P.G., Smielewski P., Kirkpatrick P.J. Evaluation of a nearinfrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke. 2001;32(11):2492-2500.
Alverson D.C., Eldridge M., Dillon T., et al. Noninvasive pulsed Doppler determination of cardiac output in neonates and children. J Pediatr. 1982;101:46.
American Academy of Pediatrics Task Force on Transcutaneous Oxygen Monitors. report of consensus meeting, December 5 to 6, 1986. Pediatrics. 1989;83:122.
American Society for Testing and Materials (ASTM). Standard Specification for Particular Requirements for Anesthesia Workstations and their Components. West Conshohocken, PA: ASTM, 2005. (F-1195-99, and F-1850-00)
American Society of Anesthesiologists (ASA). Standards for basic anesthesia monitoring. 2005. Park Ridge, IL
Andropoulos D.B., Bent S.T., Skjonsby B., et al. The optimal length of insertion of central venous catheters for pediatric patients. Anesth Analg. 2001;93:883.
Annabi E.H., Barker S.J. Severe methemoglobinemia detected by pulse oximetry. Anesth Analg. 2009;108(3):898-899.
Aoyagi T. Pulse oximetry: its invention, theory and future. J Anesth. 2003;17:259-266.
Aoyagi T., Kishi M., Yamaguchi K,. Improvement of an earpicce oxi-meter. In Abstracts of the 13th Annual meeting of the Japan Society of Medical Electronics and Biological Engineering. Osaka, Japan. 1974:90-91. 26–27
Arant B.S.Jr. Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978;92:705.
Badgwell J.M., McLeod M.E., Lerman J., et al. End-tidal PCO2 measurements sampled at the distal and proximal ends of the endotracheal tube in infants and children. Anesth Analg. 1987;66:959.
Badgwell J.M., Kleinman S.E., Heavner J.E. Respiratory frequency and artifact affect the capnographic baseline in infants. Anesth Analg. 1993;77:708.
Badr G., Carlsson C.A., Fall M., et al. Cortical evoked potentials following stimulation of the urinary bladder in man. Electroencephalogr Clin Neurophysiol. 1982;54:494-498.
Bannister C.F., Brosius K.K., Sigl J.C., et al. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877.
Barker A.T., Jalinous R., Freeston I.L. Noninvasive magnetic stimulation of the human motor cortex. Lancet. 1985;2:1106.
Barker S.J. Motion-resistant pulse oximetry: a comparison of new and old models. Anesth Analg. 2002;95(4):967-972.
Barker S.J., Curry J., et al. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry. Anesthesiology. 2006;105:892.
Barker S.J., Tremper K.K. Transcutaneous oxygen tension: a physiological variable for monitoring oxygenation. J Clin Monit. 1985;1:130.
Barker S.J., Tremper K.K. The effect of carbon monoxide inhalation on pulse oximetry and transcutaneous PO2. Anesthesiology. 1987;66:677.
Barker S.J., Tremper K.K., Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology. 1989;70:112.
Bauer S.B., Feldman S.M., Gellis S.S., et al. Neonatal hypertension: a complication of umbilical-artery catheterization. N Engl J Med. 1975;293:1032.
Baygin O., Bodur H., Isik B. Effectiveness of premedication agents administered prior to nitrous oxide/oxygen. European J Anesthesiology. 2010;27:341-346.
Bernal N.P., Hoffman G.M., Ghanayem N.S., et al. Cerebral and somatic near-infrared spectroscopy in normal newborns. J Pediatr Surg. 2010;45(6):1306-1310.
Bettex D.A., Schmidlin D., Bernath M.A., et al. Intraoperative transesophageal echocardiography in pediatric congenital cardiac surgery: a two-center observational study. Anesth Analg. 2003;97(5):1275-1282.
Bissonnette B., Sessler D.I. Passive or active inspired gas humidification increases thermal steady-state temperatures in anesthetized infants. Anesth Analg. 1989;69:783.
Bledsoe B.E., Nowicki K., et al. Use of pulse co-oximetry as a screening and monitoring tool in mass carbon monoxide poisoning. Prehosp Emerg Care. 2009;14(1):131-133.
Bohnhorst B., Peter C.S., et al. Pulse oximeters’ reliability in detecting hypoxemia and bradycardia: comparison between a conventional and two new generation oximeters. Crit Care Med. 2000;28(5):1565-1568.
Brooks T.D., Paulus D.A., Winkle W.E. Infrared heat lamps interfere with pulse oximeters. Anesthesiology. 1984;61:630.
Brouillette R.T., Lavergne J., et al. Differences in pulse oximetry technology can affect detection of sleep-disorderd breathing in children. Anesth Analg. 2002;94(Suppl 1):47-53.
Bruner J.M., Krenis L.J., Kunsman J.M., et al. Comparison of direct and indirect measuring arterial blood pressure. Med Instrum. 1981;15:11.
Burrows F.A. Physiologic dead space, venous admixture, and the arterial to end-tidal carbon dioxide difference in infants and children undergoing cardiac surgery. Anesthesiology. 1989;70:219.
Cannesson M., Attof Y., et al. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology. 2007;106(6):1105-1111.
Cannesson M., Delannoy B., Morand A., et al. Does the pleth variability index indicate the respiratory-induced variation in the plethysmogram and arterial pressure waveforms. Anesth Analg. 2008;106(4):1031-1033.
Cannesson M., Desebbe O., et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008.
Cannesson M., Henaine R., et al. Clinical usefulness of new-generation pulse oximetry in the paediatric cardiac surgery setting. Ann Fr Anesth Reanim. 2008;27(10):808-812.
Cantwell G.P., Holzman B.H., Caceres M.J.. Percutaneous catheterization of the axillary artery in the pediatric patient. [published erratum appears in Crit Care Med. 1991;19:746. Crit Care Med. 1990;18:880.
Capek J.M., Roy R.J. Noninvasive measurement of cardiac output using partial CO2 rebreathing. IEEE Trans Biomed Eng. 1988;35:653.
Chakravarti S., Srivastava S., Mittnacht A.J. Near infrared spectroscopy (NIRS) in children. Semin Cardiothorac Vasc Anesth. 2008;12(1):70.
Choi S.J., Ahn H.J., et al. Comparison of desaturation and resaturation response times between transmission and reflectance pulse oximeters. Acta Anaesthesiol Scand. 2010;54(2):212.
Choudhry D.K., Brenn B.R. Bispectral index monitoring: a comparison between normal children and children with quadriplegic cerebral palsy. Anesth Analg. 2002;95:1582.
Ciganek L. The EEG response to light stimulus in man. Electroenceph Clin Neurophysiol. 1961;13:165-172.
Cohen D.E., Downes J.J., Raphaely R.C. What difference does pulse oximetry make? Anesthesiology. 1988;68:181.
Cohen M.M., Cameron C.B., Duncan P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70:160.
Cohn S.M. Near-infrared spectroscopy: potential clinical benefits in surgery. J Am Coll Surg. 2007;205:322-332.
Colgan F.J., Stewart S. An assessment of cardiac output by thermodilution in infants and children following cardiac surgery. Crit Care Med. 1977;5:220.
Costarino A.T., Davis D.A., Keon T.P. falsely normal saturation reading with the pulse oximeter. Anesthesiology. 1987;67:830.
Coté C.J., Jobes D.R., Schwartz A.J., et al. Two approaches to cannulation of a child’s internal jugular vein. Anesthesiology. 1979;50:371.
Coté C.J., Goldstein E.A., Coté M.A., et al. A single-blind study of pulse oximetry in children. Anesthesiology. 1988;68:184.
Coté C.J., Rolf N., Liu L.M.P., et al. Single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74:980.
Cracco J.B., Cracco R.Q., Stolove R. Spinal evoked potentials in man: a maturational study. Electroencephalogr Clin Neurophysiol. 1979;46:58.
Daube J.R., Harper C.M. Surgical monitoring of cranial and peripheral nerves. In: Desmedt J.E., editor. Neuromonitoring in surgery. Amsterdam: Elsevier; 1989:115.
Davidson A.J., McCann M., Devavaram P., et al. The differences in the bispectral index between infants and children during emergence from anesthesia after circumcision surgery. Anesth Analg. 2001;93:326.
Davidson A.J., Ironfield C.M., Skinner A.V., et al. The effect of caudal local anesthesia blockade on the Bispectral Index during general anesthesia. Pediatr Anesth. 2006;16:828-833.
Degoute C.S., Macabeo C., Dubreuil C., et al. EEG bispectral index and hypnotic component of anaesthesia induced by sevoflurane: comparison between children and adults. Br J Anaesth. 2001;86:209.
Denman W.T., Swanson E.L., Rosow D., et al. Pediatric evaluation of the bispectral index (BIS) monitor and correlation of BIS with end-tidal sevoflurane concentration in infants and children. Anesth Analg. 2000;90:872.
Desebbe O., Boucau C., et al. The ability of pleth variability index to predict the hemodynamic effects of positive end-expiratory pressure in mechanically ventilated patients under general anesthesia. Anesth Analg. 2010;110(3):792-798.
Douglas M. Urinary flow rates and urea excretion in newborn infants. Biol Neonate. 1972;21:321.
Dueck M.H., Klimek M., Appenrodt S., et al. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103:249-257.
Edmonds J.F., Barker G.A., Conn A.W. Current concepts in cardiovascular monitoring in children. Crit Care Med. 1980;8:548.
Fletcher R. The relationship between the arterial to end-tidal PCO2 difference and hemoglobin saturation in patients with congenital heart disease. Anesthesiology. 1991;75:210.
Flick R.P., Sprung J., Harrison T.E., et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center. Anesthesiology. 2007;106:226-237.
Frazin L., Talano J.V., Stephanides L., et al. Esophageal echocardiography. Circulation. 1976;54:102-108.
Freed M.D., Keane J.F. Cardiac output measured by thermodilution in infants and children. J Pediatr. 1978;92:39.
Freye E. Cerebral monitoring in the operating room and the intensive care unit. Boston: Kluwer Academic, 1990.
Friesen R.H., Lichtor J.L. Indirect measurement of blood pressure in neonates and infants utilizing an automatic noninvasive oscillometric monitor. Anesth Analg. 1981;60:742.
Froom S.R., Malan C.A., Mecklenburgh J.S., et al. Bispectral Index asymmetry and COMFORT score in paediatric intensive care patients. Br J Ananesth. 2008;100:690.
Gan T.J., Glass P.S., Windsor A., et al. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 1997;87:808.
Giannotti G., Cohn S.M., Brown M., et al. Utility of near-infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma. 2000;48:396-399.
Gidding S.S. Pulse oximetry in cyanotic congenital heart disease. Am J Cardiol. 1992;70:391.
Gilmore R.L., Bass N.H., Wright E.A., et al. Developmental assessment of spinal cord and cortical evoked potentials after tibial nerve stimulation: effects of age and stature on normative data during childhood. Electroencephalogr Clin Neurophysiol. 1985;62:241.
Glass P.S., Bloom M., Kearse L., et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836.
Goff W.R. Human average evoked potentials: procedures for stimulating and recording. In: Thompson R.F., Patterson M.M., editors. Bioelectric recording techniques. Part B: electroencephalography and human brain potentials. New York: Academic; 1974:101.
Gold M., Swartz J.S., Braude B.M., et al. Intraoperative anaphylaxis: an association with latex sensitivity. J Allergy Clin Immunol. 1991;87:662.
Goldman J.M., Petterson M.T., Kopotic R.J., et al. Masimo signal extraction pulse oximetry. J Clin Monit Comput. 2000;16:475.
Govindarajan R., Babalola O., Gad-el-Kareem M., et al. Intraoperative wake-up test in neonatal neurosurgery. Pediatr Anesth. 2006;16:451-453.
Graff T.D., Phillips O.C., Benson D.W., et al. Baltimore Anesthesia Study Committee: Factors in pediatric anesthesia mortality. Anesth Analg. 1964;43:407.
Greenwald B.M., Notterman D.A., DeBruin W.J., et al. Percutaneous axillary artery catheterization in critically ill infants and children. J Pediatr. 1990;117:442.
Greilich P.E., Johnston W.E. Invasive hemodynamic monitoring. In: Hahn R.G., Prough D.S., Svenson C.H., editors. Perioperative fluid therapy. New York: Informa Healthcare; 2007:29.
Grundy B.L. Intraoperative monitoring of sensory-evoked potentials. Anesthesiology. 1983;58:72-87.
Grundy B.L., Nash C.L., Brown R.H. Arterial pressure manipulation alters spinal cord function during the correction of scoliosis. Anesthesiology. 1981;54:249.
Guignard J.P. Renal function in the newborn infant. Pediatr Clin North Am. 1982;29:777.
Guthkelch A.N., Sclabassi R.J., Vries J.K. Changes in the visual evoked potentials of hydrocephalic children. Neurosurgery. 1982;11:599.
Guzzi L., Jaffe M., Orr J. Clinical evaluation of a new noninvasive method of cardiac output measurement: preliminary results in CABG patients [abstract]. Anesthesiology. 2003;89:A543.
Haldeman S., Bradley W.E., Bhatia N. Evoked responses from the pudendal nerve. J Urol. 1982;128:974-980.
Hanowell L., Eisele J.H.Jr, Downs D. Ambient light affects pulse oximeters. Anesthesiology. 1987;67:864.
Hatle L., Brubakk A., Tromsdal A., et al. Noninvasive assessment of pressure drop in mitral stenosis by Doppler ultrasound. Br Heart J. 1978;40:131.
Hermanns H., Stevens M.F., Werdehausen R., et al. Sedation during anaesthesia in infants. 2006;97:380-384.
Hind D., Calvert N., McWilliams R., et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327:361.
Hirsch J.C., Charpie J.R., Ohye R.G., et al. Near-infrared spectroscopy: what we know and what we need to know: a systematic review of the congenital heart disease literature. J Thorac Cardiovasc Surg. 2009;137:154. 112
Hoffman G.M., Mussatto K.M., Brosig C.L., et al. Cerebral oxygenation and neurodevelopmental outcome in hypoplastic left heart syndrome. Anesthesiology. 2008;109:A7.
Holen J., Aaslid R., Landmark K., et al. Determination of effective orifice area in mitral stenosis from non-invasive ultrasound Doppler data and mitral flow rate. Acta Med Scand. 1977;201:83.
Holzle F., Loeffelbein D.J., Nolte D., et al. Free flap monitoring using simultaneous non-invasive laser Doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34(1):25-33.
Hong J.Y., Koo B.N., Kim W.O., et al. Effect of head rotation on overlap and relative position of internal jugular vein to carotid artery in infants and children: a study of the anatomy using ultrasonography. J Crit Care. 2010;25(2):360.e9.
Horgan M.J., Bartoletti A., Polansky S., et al. Effect of heparin infusates in umbilical arterial catheters on frequency of thrombotic complications. J Pediatr. 1987;111:774.
Jasper H.H. Report of Committee on Methods of Clinical Examination in EEG: The Ten-Twenty System of the International Federation. Electroenceph Clin Neurophysiol. 1958;10:371-375.
Jeleazcov C., Schmidt J., Schmitz B., et al. EEG variables as measure of arousal during propofol anaesthesia for general surgery in children: rational selection and age dependence. Br J Anaesth. 2007;99:845-854.
Jennis M.S., Peabody J.L. Pulse oximetry: an alternative method for the assessment of oxygenation in newborn infants. Pediatrics. 1987;79:524.
Kalkman C.J., ten Brink S.A., Been H.D., et al. Variability of somatosensory cortical evoked potentials during spinal surgery: effects of anesthetic technique and high-pass filtering. Spine. 1991;16:924.
Kalkman C.J., Ubags L.H., Been J.D., et al. Improved amplitude of intraoperative myogenic evoked response after paired transcranial electrical stimulation during sufentanil/nitrous oxide. Anesthesiology. 1995;83:270-276.
Kamura J. Electrodiagnosis in diseases of nerve and muscle. Philadelphia: FA Davis, 1983.
Keenan R.L., Boyan P. Cardiac arrest due to anesthesia: a study of incidence and causes. JAMA. 1985;253:2373.
Kern D., Foucarde O., Mazoit J.X., et al. The relationship between bispectral index and endtidal concentration of sevoflurane during anesthesia and recovery in spontaneously ventilating children. Pediatr Anesth. 2007;17:249-254.
Kerssens C., Sebel P.S. To BIS or not BIS? That is the question. Anesth Analg. 2006;102:380-382.
Kimble K.J., Darnall R.A.Jr, Yelderman M., et al. An automated oscillometric technique for estimating mean arterial pressure in critically ill newborns. Anesthesiology. 1981;54:423.
Kurth C.D., Levy W.J., McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab. 2002;22:335-341.
Lai W.W., Geva T., Shirali G.S., et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413-1430.
Lawless S., Orr R. Axillary arterial monitoring of pediatric patients. Pediatrics. 1989;84:273.
Lee Y.W. Statistical theory of communication. New York: Wiley, 1960.
Lesser R.P., Raudzens P., Leuders H., et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
Liakopoulos O.J., Ho J.K., Yezbick A., et al. An experimental and clinical evaluation of a novel central venous catheter with integrated oximetry for pediatric patients undergoing cardiac surgery. Anesth Analg Dec. 2007;105(6):1598-1604.
Lubbers D.W. Theoretical basis of the transcutaneous blood gas measurements. Crit Care Med. 1981;9:721.
MacEwen G.D., Bunnell W.P., Sriram K. Acute neurological complications in the treatment of scoliosis. J Bone Joint Surg Am. 1975;57:404-408.
Malherbe S., Whyte S., Singh P. Total intravenous anesthesia and spontaneous respiration for airway endoscopy in children: a prospective evaluation. Pediatr Anesth. 2010;20:434-438.
Malviya S., Reynolds P.I., Voepel-Lewis T., et al. False alarms and sensitivity of conventional pulse oximetry versus the Masimo SET technology in the pediatric postanesthesia care unit. Anesth Analg. 2000;90:1336.
Malviya S., Voepel-Lewis T., Tait A.R., et al. A comparison of observational and objective measures to differentiate depth of sedation in children from birth to 18 years of age. Anesth Analg. 2006;102:389-394.
Malviya S., Voepel-Lewis T., Tait A.R., et al. Effect of age and sedative agent in the accuracy of Bispectral Index in detecting depth of sedation in children. Pediatrics. 2007;120:e461-e470.
Mathes A.M., Kreuer S., et al. The performance of six pulse oximeters in the environment of neuronavigation. Anesth Analg. 2008;107(2):541-544.
McCann M.E., Brustowicz R.M., Bacsik J., et al. The bispectral index and explicit recall during the intraoperative wake-up test for scoliosis surgery. Anesth Analg. 2002;94:1474.
McPherson R.W. General anesthetic considerations in intraoperative monitoring: effects of anesthetic agents and neuromuscular blockade on evoked potentials, EEG and cerebral blood flow. In: Loftus C.M., Traynelis V.C., editors. Intraoperative monitoring techniques in neurosurgery. New York: McGraw-Hill; 1994:97.
Merton P.A., Morton H.B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227.
Meyer J.S., Marx P.W.. The pathogenesis of EEG changes during cerebral anoxia. Van der Drift J.H.A., editor. Cardiac and vascular diseases: handbook of electroencephalography and clinical neurophysiology. vol. 14A. Amsterdam: Elsevier; 1972:5.
Michard F., Chemla D., Richard C., et al. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Resp Crit Care. 1999;159:935-939.
Miyasaka K., Edmonds J.F., Conn A.W. Complications of radial artery lines in the paediatric patient. Can Anaesth Soc J. 1976;23:9.
Mokrohisky S.T., Levine R.L., Blumhagen J.D., et al. Low positioning of umbilical-artery catheters increases associated complications in newborn infants. N Engl J Med. 1978;299:561.
Moller J.T., Johannessen N.W., Espersen K., et al. Randomized evaluation of pulse oximetry in 20,802 patients: II. Perioperative events and postoperative complications. Anesthesiology. 1993;78:445.
Moller J.T., Pedersen T., Rasmussen L.S., et al. Randomized evaluation of pulse oximetry in 20,802 patients: I. Design, demography, pulse oximetry failure rate, and overall complication rate. Anesthesiology. 1993;78:436.
Monaco F., Nickerson B.G., McQuitty J.C. Continuous transcutaneous oxygen and carbon dioxide monitoring in the pediatric ICU. Crit Care Med. 1982;10:765.
Muhiudeen I.A., Kuecherer H.F., Lee E., et al. Intraoperative estimation of cardiac output by transesophageal pulsed Doppler echocardiography. Anesthesiology. 1991;74:9.
Muhiudeen I.A., Roberson D.A., Silverman N.H., et al. Intraoperative echocardiography for evaluation of congenital heart defects in infants and children. Anesthesiology. 1992;76:165.
Muller M., Lohr T., Scholz S., et al. Continuous Svo2 Measurement in infants undergoing congenital heart surgery-first clinical experiences with a new fiberoptic probe. Pediatr Anesth. 2007;17:51-55.
Murthy L.C.T., Goyal R.M., et al. Masimo: a new reliable noninvasive method of detecting oxygen saturation in critically ill. Indian J Anaesthesiol. 2005;49:133-136.
Myles P.S., Symons J.A., Leslie K. Anaesthetists’ attitudes towards awareness and depth-of-anaesthesia monitoring. Anaesthesia. 2003;58:11.
Neal W.A., Reynolds J.W., Jarvis C.W., et al. Umbilical artery catheterization: demonstration of arterial thrombosis by aortography. Pediatrics. 1972;50:6.
New W.Jr. Pulse oximetry. J Clin Monit. 1985;1:126.
Niedermeyer E., Lopes da Silva F. Electroencephalography, ed 3. Baltimore: Williams & Wilkins, 1993.
Nosovitch M.A., Johnson J.O., Tobias J.D. Noninvasive intraoperative monitoring of carbon dioxide in children: end tidal versus transcutaneous techniques. Paediatr Anaesth. 2002;12:48.
Notterman D.A., Castello F.V., Steinberg C., et al. A comparison of thermodilution and pulsed Doppler cardiac output measurement in critically ill children. J Pediatr. 1989;115:554.
Nuwer M.R., Dawson E.G., Carlson L.G., et al. Somatosensory evoked potential spinal cord monitoring reduces neurological deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
O’Connell A.J., Tibballs J., Coulthard M. Improving agreement between thoracic bioimpedance and dye dilution cardiac output estimation in children. Anaesth Intensive Care. 1991;19:434.
Olsson G.L., Hallen B. Cardiac arrest during anaesthesia: a computer-aided study in 250,543 anaesthetics. Acta Anaesthesiol Scand. 1988;32:653.
Ortiz F.O., Aldrich T.K., et al. Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med. 1999;159:447-451.
Park M.K., Kawabori I., Guntheroth W.G. Need for an improved standard for blood pressure cuff size: the size should be related to the diameter of the arm. Clin Pediatr. 1976;15:784.
Park M.K., Robotham J.L., German V.F. Systolic pressure amplification in pedal arteries in children. Crit Care Med. 1983;11:286.
Partridge B.L. Use of pulse oximetry as a noninvasive indicator of intravascular volume status. J Clin Monit. 1987;3:263-268.
Pedersen T., Pedersen P., Moller A.M. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev. 2001. CD002013
Perez A., Eulmesekian P.G., Minces P.G., et al. Adequate agreement between venous oxygen saturation and the right atrium and pulmonary artery in critically ill children. Pediatr Crit Care Med. 2009;10(1):76-79.
Petterson M.T., Begnoche V.L., et al. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105(Suppl 6):S78-S84.
Piatkowski A., Ulrich D., et al. A new tool for the early diagnosis of carbon monoxide intoxication. Inhal Toxicol. 2009;21(13):1144-1147.
Piotrowski A., Kawczynski P. Cannulation of the axillary artery in critically ill newborn infants. Eur J Pediatr. 1995;154:57.
Pizov R., Eden A., Bystritski D., et al. Arterial and plethysmographic waveform analysis in anesthetized patients with hypovolemia. Anesthesiology. 2010;113:83-91.
Plumer L.B., Kaplan G.W., Mendoza S.A. Hypertension in infants: a complication of umbilical arterial catheterization. J Pediatr. 1976;89:802.
Rafferty T.D., Marrero O., Nardi D., et al. Transcutaneous PO2 as a trend indicator of arterial PO2 in normal anesthetized adults. Anesth Analg. 1982;61:252.
Rajadurai V.S., Walker A.M., et al. Effect of fetal haemoglobin on the accuracy of pulse oximetry in preterm infants. J Pediatr Child Health. 1992;28(1):43-46.
Ralston A.C., Webb R.K., et al. Potential errors in pulse oximetry III: effects of interference, dyes, dyshaemoglobins and other pigments. Anaesthesia. 1991;46(4):291-295.
Randolph G.R., Hagler D.J., Connolly H.M., et al. Intraoperative transesophageal echocardiography during surgery for congenital heart defects. J Thorac Cardiovasc Surg. 2002;124:1176-1182.
Ranucci M., Isgrò G., De La Torre T., et al. Continuous monitoring of central venous oxygen saturation (Pediasat) in pediatric patients undergoing cardiac surgery:a validation study of a new technology. J Cardiothorac Vasc Anesth. 2008;22:847-852.
Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. East Norwalk, CT: Appleton & Lange, 1989.
Reynolds L.M., Nicolson S.C., Steven J.M., et al. Influence of sensor site location on pulse oximetry kinetics in children. Anesth Analg. 1993;76:751.
Rich G.F., Sullivan M.P., Adams M. Is distal sampling of end-tidal CO2 necessary in small subjects? Anesthesiology. 1990;73:265.
Rigouzzo A., Girault L., Louvet N., et al. The relationship between bispectral index and propofol during target-controlled infusion anesthesia: a comparative study between children and young adults. Pediatr Anesth. 2008;106:1109-1116.
Roger J., Roger A., Gastaut H. Electro-clinical correlation in 36 cases of vascular syndromes of brainstem. Electroencephalogr Clin Neurophysiol. 1954;6:164.
Rosow C., Manberg P. Bispectral index monitoring. Anesthesiol Clin North Am. 1998;2:89.
Sadhasivam S., Ganesh A., Robinson A., et al. Validation of the Bispectral Index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383-388.
Salem M.R., Bennett E.J., Schweiss J.F., et al. Cardiac arrest related to anesthesia. JAMA. 1975;233:238.
Salyer J.W. Neonatal and pediatric pulse oximetry. Respir Care. 2003;48:386.
Salzman S.K., Beckman A.L., Marks H.G., et al. Effects of halothane on intraoperative scalp recorded somatosensory-evoked potentials to posterior tibial nerve stimulation in man. Electroencephalogr Clin Neurophysiol. 1986;65:36-45.
Schlüter M., Langenstein B.A., Polster J., et al. Transoesophageal cross-sectional echocardiography with a phased array transducer system: technique and initial clinical results. Br Heart J. 1982;48:67.
Schmitt H.J., Schuetz W.H., Proeschel P.A., et al. Accuracy of pulse oximetry in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 1993;7:61.
Schramm W., Bartunek A., et al. Effect of local limb temperature on pulse oximetry and the plethysmographic pulse wave. Int J Clin Monit Comput. 1997;14:17-22.
Schubert A., Licina M., Lineberry P.J. The effect of ketamine on human somatosensory evoked potentials and its modification by nitrous oxide. Anesthesiology. 1990;72:33.
Schwartz A.J., Downes J.J. Hazards of a simple monitoring device, the esophageal stethoscope. Anesthesiology. 1977;47:64.
Sclabassi R.J., Krieger D.N. Neurophysiological diagnostic evaluation and intraoperative monitoring of the pediatric spinal cord. In: Pang D., editor. Disorders of the pediatric spine. New York: Raven Press; 1995:635.
Sclabassi R.J., Kalia K., Sekhar L., et al. Assessing brainstem function. Haines S.J., Heros R.C., editors. Surgery of the brainstem. Neurosurg Clin N Am. 1993;4:3,. 415
Sclabassi R.J., Krieger D.N., Weisz D., et al. Electrophysiological monitoring. In: Sekhar L.N., Janecka I., editors. Surgery of cranial base tumors: a color atlas. New York: Raven Press; 1993:83.
Seibert J.J., Taylor B.J., Williamson S.L., et al. Sonographic detection of neonatal umbilical-artery thrombosis: clinical correlation. AJR Am J Roentgenol. 1987;148:965.
Sessler D.I., McGuire J., Sessler A.M. Perioperative thermal insulation. Anesthesiology. 1991;74:875.
Severinghaus J.W., Naifeh K.H. Accuracy of response of six pulse oximeters to profound hypoxia. Anesthesiology. 1987;67:551.
Severinghaus J.W. Takuo Aoyogi: discovery of pulse oximetry. Anesth Analg. 2007;105:S1.
Shanewise J.S., Cheung A.T., Aronson S., et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. J Am Soc Echocardiogr. 1999;12:884-900.
Simpson J., Stephenson T. Regulation of extracellular fluid volume in neonates. Early Hum Dev. 1993;34:179.
Sloan T.B. Anesthetic effects on electrophysiological recordings. J Clin Neurophysiol. 1998;15:217-226.
Spahr-Schopfer I.A., Bissonnette B., et al. Capnometry and the paediatric laryngeal mask airway. Can J Anaesth. 1993;40:1038.
Standard Specification for Particular Requirements for Anesthesia Workstations and their Components West Conshohocken, PA, ASTM.
Starr A., Amlie R.N., Martin W.H., et al. Development of auditory function in newborn infants revealed by auditory brainstem potentials. Pediatrics. 1977;60:831.
Stevenson J.G. Incidence of complications in pediatric transesophageal echocardiography: experience in 1650 cases. J Am Soc Echocardiogr. 1999;12:527-532.
Strauss J., Daniel S.S., James L.S. Postnatal adjustment in renal function. Pediatrics. 1981;68:802.
Suner S., Partridge R., et al. Non-invasive Pulse CO-oximetry screening in the emergency department identifies occult carbon monoxide toxicity. J Emerg Med. 2008;34:441.
Swedlow D.B. Capnometry and capnography, the anesthesia disaster early warning system. Semin Anesth. 1986;5:194.
Symansky M.R., Fox H.A. Umbilical vessel catheterization: indications, management, and evaluation of the technique. J Pediatr. 1972;80:820.
Taniguchi M., Nadstawek J., Pechstein U., et al. Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery. 1992;31:89.
Taylor B.A., Fennelly M.E., Taylor A., et al. Temporal summation: the key to motor evoked potential spinal cord monitoring in the humans. J Neurol Neurosurg Psychiatry. 1993;56:104-106.
Taylor M.J. Evoked potentials in paediatrics. In: Halliday A.M., editor. Evoked potentials in clinical testing. Edinburgh: Churchill Livingstone; 1993:489-521.
Tibby S.M., Murdoch I.A. Measurement of cardiac output and tissue perfusion. Curr Opin Pediatr. 2002;14:303.
Tiret L., Nivoche Y., Hatton F., et al. Complications related to anaesthesia in infants and children: a prospective survey of 40,240 anaesthetics. Br J Anaesth. 1988;61:263.
Tirel O., Wodey E., Harris R., et al. The impact of age on bispectral index values and EEG bispectrum during anaesthesia with desflurane and halothane in children. Br J Anaesth. 2006;96:480-485.
Tirel O., Wodey E., Harris R., et al. Variation of bispectral index under TIVA with propofol in a paediatric population. Br J Anaesth. 2008;100:82-87.
Tobias J.D. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3(2):235-243.
Tobias J.D., Hoernschemeyer D.G. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27:311-313.
Trang H., Boureghda S., et al. Sleep desaturation: comparison of two oximeters. Pediatr Pulmonol. 2004;37(1):76-80.
Trivedi N.S., Ghouri A.F., et al. Effects of motion, ambient light, and hypoperfusion on pulse oximeter function. J Clin Anesth. 1997;9(3):179-183.
Umbilical Artery Catheter Trial Study Group. Relationship of intraventricular hemorrhage or death with the level of umbilical artery catheter placement: a multicenter randomized clinical trial. Pediatrics. 1992;90:881.
Ungerleider R.M., Greeley W.J., Sheikh K.H., et al. The use of intraoperative echo with color Doppler flow imaging to predict outcome after repair of congenital cardiac defects. Ann Surg. 1989;210:526-534.
U.S. Food and Drug Administration. Allergic reactions to latex-containing medical devices. FDA Med Bull. 2, 1991.
Valkenburg A.J., de Leeuw T.G., Tibboel D. Lower Bispectral Index values in children who are intellectually disabled. Anesth Analg. 2009;109:1428-1433.
Van der Drift J.H.A. The EEG in cerebrovascular disease. In: Vinken P.J., Bruyn G.W., editors. Handbook of clinical neurology. Amsterdam: Elsevier; 1972:267.
Verghese S.T., Nath A., Zenger D., et al. The effects of the simulated Valsalva maneuver, liver compression, and/or Trendelenburg position on the cross-sectional area of the internal jugular vein in infants and young children. Anesth Analg. 2002;94:250.
Watcha M.F. Investigations of the bispectral index monitor in pediatric anesthesia: first things first. Anesth Analg. 2001;92:805.
Watcha M.F., Connor M.T., Hing A.V. Pulse oximetry in methemoglobinemia. Am J Dis Child. 1989;143:845.
Watt R.C., Loeb R.G., Orr J. Comparison of a new noninvasive cardiac output technique with invasive bolus and continuous thermodilution. Anesthesiology. 2004;89:A536.
Workie F.A., Rais-Bahrami K., et al. Clinical use of new-generation pulse oximeters in the neonatal intensive care unit. Am J Perinatol. 2005;22(7):357-360.
Xue F.S., Lou J.K., Tong S.Y., et al. Study of the safe threshold of apneic period in children during anestehsia induction. J Clin Anesth. 1996;8:568-574.
Yakovlev P.I., Lecours A.R. The myelogenetic cycles of regional maturation in the brain. In: Minkowski A., editor. Regional development of the brain in early life. Oxford: Blackwell, pp 3–70, 1967.
Yelderman M., New W. Evaluation of pulse oximetry. Anesthesiology. 1983;59(4):349-352.
Zhang L., Bhattacharya S., et al. Alexandrite 755 nm laser-induced pulse oximetry dysfunction. Anesth Analg. 2009;108(1):386-387.

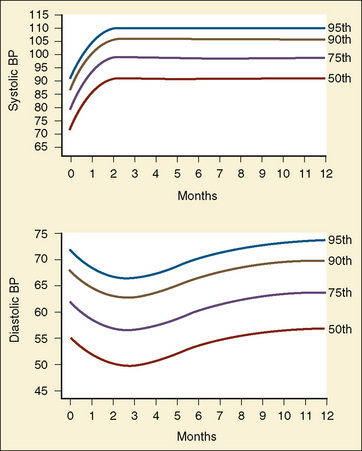
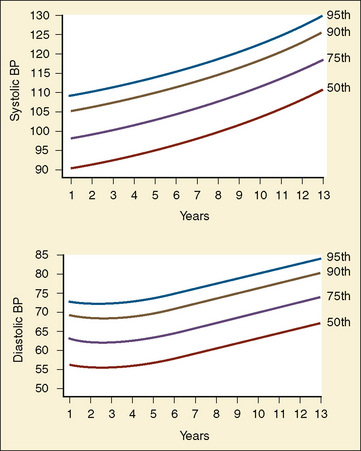
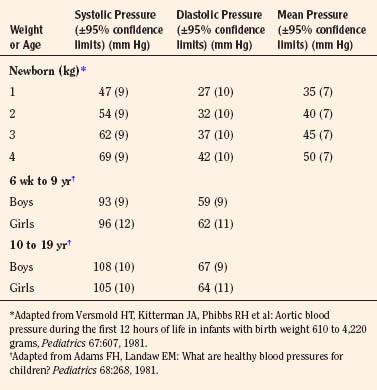
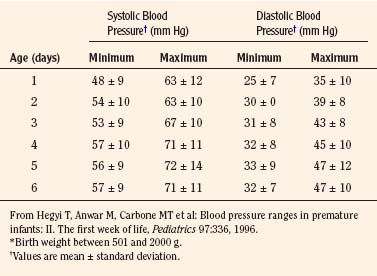
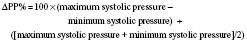
 ), cardiac output, hemoglobin, and arterial oxygen saturation.
), cardiac output, hemoglobin, and arterial oxygen saturation. (hypothermia, anesthesia) or an increase in oxygen delivery (increased hemoglobin, increased cardiac output, increased Pao2, increased Sao2). Mixed venous oxygenation is used to assess the balance between oxygen delivery and oxygen consumption for patients in the operating room and intensive care unit. It is a global index of tissue oxygenation.
(hypothermia, anesthesia) or an increase in oxygen delivery (increased hemoglobin, increased cardiac output, increased Pao2, increased Sao2). Mixed venous oxygenation is used to assess the balance between oxygen delivery and oxygen consumption for patients in the operating room and intensive care unit. It is a global index of tissue oxygenation.