Microbiological Laboratory Techniques
After reading this chapter, the student will be able to:
• Describe the general concept of aseptic techniques used in laboratory preparation and analysis
• Explain and differentiate between sterilization, disinfection, and sanitization
• Describe the different types of culture media and their possible physical state
• Discuss inoculation, incubation, and isolation
• Describe the various fixation and staining techniques to identify microbes with the light microscope
• Describe the fixation and staining methods used in electron microscopy
• Classify the bacteria according to their shape for morphological identification
• Describe the different culture characteristics of microbes used for the purpose of identification
• Discuss the physiological, biochemical, and genetic characteristics used for microbial identification purposes
Aseptic Technique in Laboratory Preparation and Analysis
Medical and clinical laboratories test biological specimens to determine the health status of a patient and to identify the disease-causing pathogen for the purpose of appropriate treatment. Research laboratories include basic, clinical, and pharmaceutical laboratories, each dealing with a specific aspect of microbiological research.
Microorganisms are everywhere in the environment. In order to selectively identify specific microbes they must be grown in controlled laboratory environments. Beginning with pure sterile cultures, the key is to control the factors to which the cultures are subjected. In other words, when working with microbial cultures, it is necessary to ensure that organisms are selectively introduced into the culture and that other environmental organisms do not contaminate it. Aseptic technique is a procedure that is performed under sterile conditions, a method that prevents the introduction of unwanted organisms or contaminants into an environment. This process is characterized by strict adherence to details. The use of aseptic technique controls, limits, or prevents contamination by fomites. A fomite is any inanimate object or substance capable of transporting pathogens from one medium or individual to another. Aseptic technique is essential in the microbiology laboratory to prevent any contamination of laboratory personnel (see Chapter 5, Safety Issues), cultures, supplies, and equipment.
Air currents must be controlled by closing laboratory doors and windows to prevent microbes on surfaces from becoming airborne and entering the cultures. When handling cultures to prepare slides or to transfer organisms to another medium, the transfer loops and needles need to be sterilized by flame or incinerator before and after use (Figure 4.1). Furthermore, culture plates are held in a position that minimizes exposure of the medium to the environment. When removing lids/stoppers from test tubes, the lids should remain held in the hand and not placed on other surfaces such as countertops during the transfer of materials from one tube to another. Flaming is one of the physical methods of sterilization and must always be applied to the lips of test tubes and also of flasks whenever culture liquid is poured from one container to another, as in the case of pouring culture plates (Figure 4.2).

One of the primary inoculation tools in the microbiology laboratory is the loop. Metal loops must be sterilized in the flame of a Bunsen burner by heating the wire until it glows. Presterilized, disposable plastic loops are also available for inoculation.

Bacteria and fungi are everywhere in the environment, including the air. The mouth of a tube or bottle opening is a potential point of contamination and flaming of the opening in direct flame immediately after opening and before closing is an effective technique to prevent potential contamination on this exposed surface.
Sterilization and disinfection procedures are daily routines in microbiological laboratories. They are essential to ensure that cultures, containers, media, and equipment are handled in such a way that only the desired organism will grow and others will be eliminated or excluded.
Sterilization
Sterilization is the destruction or removal of all microorganisms, including bacteria and their endospores, viruses, fungi, and prions. This can be accomplished by physical methods such as heat, radiation, and filtration, or by chemical methods. The wide application of sterilization processes makes it necessary to impose strict control measures to validate the results. When using dry heat or moist heat sterilization, physical, chemical, or biological indicators can be used to validate the desired results (Table 4.1). The general resistance of microbes to methods of sterilization ranges from bacterial endospores, with the highest resistance to sterilization, to vegetative cells, with moderate to least resistance.
TABLE 4.1
Methods for Validating Dry or Moist Heat Sterilization
| Physical Methods | Chemical Methods | Biological Test Organism | |
| Dry heat | Temperature-recording charts | Color change indicator | Bacillus subtilis var. niger |
| Moist heat | Temperature-recording charts | Color change indicator | Bacillus stearothermophilus |

Physical and chemical methods of sterilization are applied in the microbiological laboratory to ensure that equipment and materials are free of microorganisms. Aseptic technique is the first and most important step in ensuring that manipulation of specimens during investigative procedures does not infect laboratory personnel or contaminate cultures or the laboratory environment (see Chapter 5, Safety Issues). Bacteria are found practically everywhere including fingertips and bench tops and therefore it is essential to minimize contact with these surfaces. Only sterile items are free of potentially contaminating microorganisms and once a sterile object comes in contact with a nonsterile surface, the object can no longer be considered sterile. The most commonly used instrument in the microbiology laboratory for the sterilization of media and glassware is the autoclave (Figure 4.3). A detailed discussion of the different types of physical and chemical methods of sterilization is provided in Chapter 19 (Physical and Chemical Methods of Control; for overviews see Tables 19.3 and 19.5).

The autoclave is one of the primary tools for sterilization of equipment, containers, media, and biohazardous wastes. It is essentially a large pressure cooker that raises the temperature of steam to about 121° C under 15–20 psi pressure. The size and configuration of autoclaves vary but the basic operation is same.
Disinfection
Disinfectants are applied to inanimate surfaces, medical equipment, and other man-made objects whereas antiseptics are used to disinfect skin. The term disinfection refers to the use of a physical process or the use of a chemical agent to destroy vegetative microbes and viruses. This does not include bacterial endospores. The ideal disinfectant would result in complete sterilization without harming other forms of life. Unfortunately, ideal disinfectants as such do not exist and most of them only partially sterilize. In addition to the most resistant pathogens, endospores, other bacteria, and viruses are also highly resistant to many disinfectants.
Substances that kill bacteria are bactericidal and those that interfere with cell growth and reproduction are bacteriostatic. Disinfectants and antiseptics are bactericidal and bacteriostatic depending on the concentration applied. All disinfectants are by their nature potentially harmful, even toxic, to humans and animals. They should be handled with appropriate care to avoid harm to the handler or recipient. The type of disinfectant to be used depends on the surface or material to be disinfected. Specifics on the different types of disinfectants and their particular effectiveness are described in Chapter 19, with an overview in Table 19.5 (Chemicals Used in the Control of Microbes).
Sanitization
Several applications in everyday life and medicine do not require sterilization, disinfection, or antisepsis but need to reduce microorganisms in order to control possible infections or spoilage of substances. Sanitization achieves this by using any cleansing technique that mechanically removes microorganisms and other debris to reduce contamination to safe levels. Often the sanitizer used is a compound such as soap or detergent. Restaurants, dairies, breweries, and other food industries handle soiled utensils on a daily basis and must take appropriate measures to sanitize them for prevention of infection, spoilage, and contamination. This includes controlling microbes to a minimal level during preparation and processing.
Degermation is the process by which the numbers of microbes on the human skin are reduced by scrubbing, immersion in chemicals, or both. Some examples of degermation include the process of presurgical scrubbing of the hands with sterile brushes and germicidal soap before putting on sterile surgical gloves, the application of alcohol wipes to the skin, and the cleansing of a wound with germicidal soap and water.
Culture Techniques
Microbiologists use five basic procedures to examine and characterize microbes: Inoculation, Incubation, Isolation, Inspection (observation), and Identification—the five “I’s.” To culture a microorganism a small sample, the inoculum, is introduced into a culture medium usually with a platinum wire probe streaked across its surface. This process is called inoculation and the growth that appears on or in the medium is the culture. A culture can be pure—containing one type of organism, or mixed—containing two or more species.
Types of Culture Media
Nutritional requirements of particular microorganisms range from a few simple inorganic compounds to a complex list of specific inorganic and organic chemicals (Table 4.2). Access to carbon, the essential component required for molecular life, is obtained in different ways by microorganisms. Autotrophs acquire carbon from carbon dioxide in the atmosphere and heterotrophs obtain their carbon from organic compounds. This diversity is seen in the different types of media needed to ensure the growth of the organism for investigation. Media vary in nutrient content and consistency and can be classified according to their physical state, chemical composition, and functional type.
TABLE 4.2
Nutritional Requirements for Microorganisms*
| Macronutrients | Growth Factors (Vitamins) | Micronutrients (Trace Elements) |
| Carbon | p-Aminobenzoic acid | Boron |
| Hydrogen | Folic acid | Chromium |
| Oxygen | Biotin | Cobalt |
| Nitrogen | Cobalamin (B12) | Copper |
| Phosphorus | Lipoic acid | Iron |
| Sulfur | Nicotinic acid (niacin) | Manganese |
| Potassium | Pantothenic acid | Molybdenum |
| Magnesium | Riboflavin | Nickel |
| Sodium | Thiamine | Selenium |
| Calcium | Vitamin B6 | Tungsten |
| Iron | Vitamin K group | Vanadium |
| Hydroxamates | Zinc |
*Not all microbes need all of the nutrients listed; therefore, for optimal growth environments media with specific nutrients are necessary for specific microorganisms.
Physical State of Media
Liquid media are water-based solutions that do not solidify at temperatures above freezing and flow freely in the containers when tilted. Most commonly, liquid media are supplied in tubes or bottles and are called broths, milks, or infusions. A common laboratory medium is nutrient broth, which contains beef extract and peptone (partially digested protein) dissolved in water. Methylene blue milk and litmus milk are opaque liquids prepared from skim milk powder and dyes. After inoculation, growth occurs throughout the container. Enriched broths are used to grow bacteria that are present in few numbers such as in small specimen samples obtained from patients.
Semisolid media contain a limited amount of a solidifying agent such as agar or gelatin, giving the medium a clotlike consistency. Semisolid media are often used to determine motility and growth patterns of bacteria.
Solid media are dispensed in Petri plates or slanted in tubes or bottles to provide firm and maximal surfaces for growing bacteria or fungi. By far the most widely used and effective of these media is agar, composed of a complex polysaccharide from the red alga Gelidium. Agar is solid at room temperature and liquefies at the boiling temperature of water. Once in liquid form it does not solidify until it cools to 42° C. It then can be inoculated and poured in liquid form at temperatures that will not harm the microbes or the handlers. Agar added to media simply gels them into a solid form. Any medium containing 1% to 5% agar usually has the agar in the name of the specific medium as, for example, nutrient agar, phenylethyl alcohol agar, blood agar, and others.
Chemical Classification of Media
Depending on their chemical content media can be classified as complex or nonsynthetic media and as chemically defined or synthetic media.
• Chemically defined media or synthetic media are media with a defined, exact chemical composition. They are prepared by means of an exact formula, adding precise amounts of inorganic and/or organic chemicals to distilled water. Some of these media contain minimal amounts of chemicals such as some salts and a source of carbon; others are special media containing a variety of precisely measured substances.
• Complex media or nonsynthetic media contain at least one component that cannot be chemically defined and thus the medium cannot be represented by an exact chemical formula. Complex media contain extracts from animals, plants, or yeast. They may include blood, serum, meat extracts, milk, yeast extracts, soybean digests, and peptone.
Functional Types of Media
General-purpose media are designed to grow a broad spectrum of microbes that do not have any special growth requirements. Other media are available for special growth conditions of selected organisms. These include enriched, selective, and differential media.
• Enriched media contain complex organic substances such as blood, serum, hemoglobin, or growth factors for the growth needs of specific species. An example is blood agar, made by adding sterile sheep, horse, or rabbit blood to a sterile agar base. It is widely used to grow certain streptococci and other pathogens. Another enriched medium is chocolate agar. Chocolate agar is enriched with heat-treated blood, which turns brown and gives the medium the color and thus its name.
• Selective media inhibit the growth of selected organisms while allowing the growth of others. These media are useful in isolating bacteria or fungi from specimens that contain several different organisms. For example, mannitol salt agar contains 7.5% NaCl, inhibitory to most human pathogens with the exception of the genus Staphylococcus, which thrives in mannitol salt agar and consequently its growth can be amplified in mixed samples.
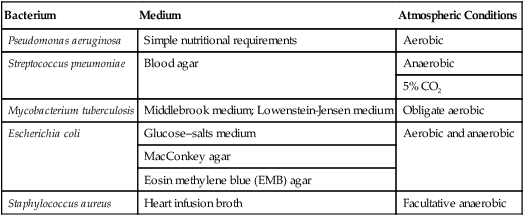
• Differential media can grow several different organisms that show visible differences. These differences can be variations in colony size or color, a change in medium color, or the formation of gas bubbles and precipitates. Dyes can be used as differential agents because many of them are pH indicators that change color in response to acid or base production by a specific microbe. For example, MacConkey agar contains neutral red, which is a dye that is yellow when neutral and pink or red when acidic. Escherichia coli, a bacterium common to the intestinal tract, produces acid when it metabolizes the lactose in the medium and develops red or pink colonies. In contrast, Salmonella does not give off acid and therefore remains in a natural off-white color. A comparison of general, selective, and differential media is shown in Figure 4.4.
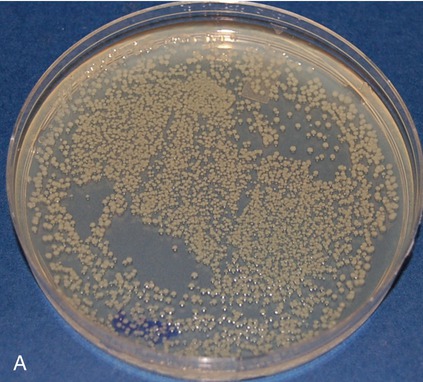
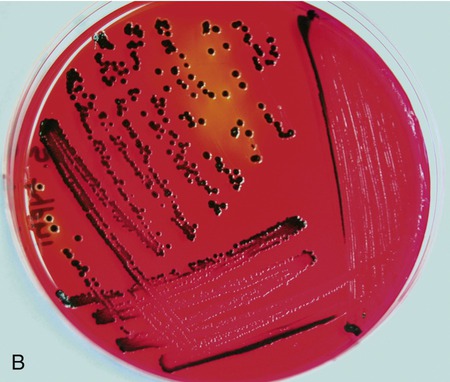
Pictured are (A) tryptic soy agar—a complex medium used as an all-purpose growth medium, and (B) xylose lysine deoxycholate agar—a chemically defined agar that is both selective and differential and used primarily in selecting for and differentiating gram-negative enteric bacilli, especially Shigella, Salmonella, and Providencia. Yellow indicates the organism is utilizing the carbohydrate xylose and the black colonies indicate the production of hydrogen sulfide.
HEALTHCARE APPLICATION
Growth Requirements of Selected Bacteria
| Bacterium | Medium | Atmospheric Conditions |
| Pseudomonas aeruginosa | Simple nutritional requirements | Aerobic |
| Streptococcus pneumoniae | Blood agar | Anaerobic |
| 5% CO2 | ||
| Mycobacterium tuberculosis | Middlebrook medium; Lowenstein-Jensen medium | Obligate aerobic |
| Escherichia coli | Glucose–salts medium | Aerobic and anaerobic |
| MacConkey agar | ||
| Eosin methylene blue (EMB) agar | ||
| Staphylococcus aureus | Heart infusion broth | Facultative anaerobic |
Live Media
Viruses and certain bacteria that cannot grow on artificial media require cell cultures or host animals to grow. In the early times of microbiology animals were used to confirm the pathogenicity of bacteria isolated from cases of human infections (see Koch’s postulates in Chapter 1, Scope of Microbiology). Today, animal inoculations are rarely used in diagnostic laboratories. Fluid or tissue suspensions from patients suspected to have viral, fungal, or protozoal infections are occasionally injected intraperitoneally into mice or hamsters; however, there are few pathogens that will not grow on some artificial medium. Of note is Mycobacterium leprae, the causative agent for leprosy, which can be cultured only in the footpads of mice or a species of armadillo.
Incubation and Isolation
After inoculation, the media are incubated by placing their containers in a temperature-controlled chamber or incubator, to facilitate the growth process. The incubator temperature in the laboratory is generally between 20° C and 40° C. The concentration of atmospheric gases may also be controlled in some incubators if required for the growth of a certain microbe. The incubation period varies from a day to several weeks, during which time the microbe multiplies and produces growth that can be visualized without a microscope. Microbial growth in a liquid medium manifests itself as cloudiness, sediment, scum, or color. On solid media the growth appears as colonies of various sizes, shapes, color, and texture.
Identification of bacterial cultures depends on isolating colonies that contain an uncontaminated single species of an organism—a pure culture. A pure culture can be obtained by three different techniques: streak plate, pour plate, or spread plate.
• Streak plate: The procedure of streaking a plate with an inoculating loop spreads millions of cells over the surface of a solid medium, and then separates individual cells at a distance from the others. Usually samples, such as lake water, contain a mixture of bacteria and isolation of individual bacterial strains needs to be performed in the laboratory for purposes of identification. A classic technique used for this purpose is the streak plate procedure by which bacteria from a sample are spread over the surface of an agar plate, using a series of strikes, basically “thinning” the inoculum. After spreading, bacteria are separated at a distance from one another and can multiply to form isolated colonies. These colonies can then be used to produce pure cultures by streaking a new sterile plate or inoculating a broth with inoculum from the isolated colony. The isolated cultures are usually subjected to the streak plate procedure a few times until a single bacterial strain exists on the Petri plate. There are a number of different methods for mechanically diluting microorganisms on a streak plate, as shown in Figure 4.5.
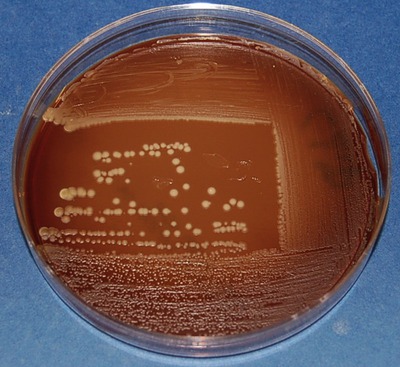
The streak plate is used primarily to produce isolated colonies from the inoculum. These isolated colonies represent pure cultures of the organism and can be used to produce additional pure cultures, to perform biochemical testing, or both. The plate shows the quadrant streaking pattern applied with a sterile loop.
• Pour plate: The pour plate technique can be used to determine the number of microbes per milliliter or the number of microbes per gram in a specimen, as well as to suspend isolated cells for culture. The advantage of this technique is that it does not require previously prepared plates and is often used to assay foods for bacterial contamination. With this technique, bacteria are inoculated into a tube of melted agar that has been cooled to 45° C to 47° C, mixed, and then poured into a sterile Petri plate (Figure 4.6).
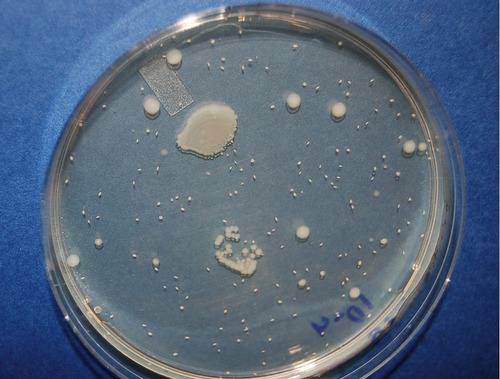
The pour plate can be used to produce isolated colonies as well as to perform plate counts. The technique involves adding the inoculum to warm liquid agar in a Petri plate and allowing the agar to cool and solidify. The colonies will grow on the surface as well as throughout the thickness of the agar. The smaller, oval-shaped colonies on the pour plate in this photo are actually embedded in the agar.
• Spread plate: The number of bacteria in a solution can be quantified by using the spread plate technique. With this technique a small amount of a liquid inoculum is placed in the center of an agar medium plate and spread evenly over the surface with a bent sterile glass, metal, or plastic (disposable) rod (Figure 4.7). Plates are then allowed to absorb the inoculum before being inverted for incubation. After the colonies are grown, they are counted and the number of bacteria in the original sample is calculated.

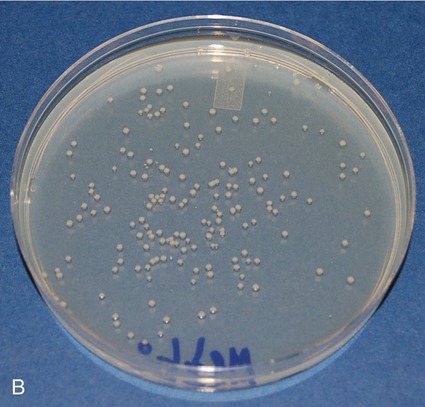
The spread plate is used primarily to perform cell counts on a sample. The original sample is serially diluted, a measured amount of inoculum is then spread over the surface of the agar, and the colonies are counted after incubation. A, Sterilization of the spreader stick and observance of aseptic technique are critical as any contamination will be spread over the plate and prevent an accurate count of the colonies of interest. The stick is sterilized by immersing it in alcohol, igniting it, and allowing it to burn off before and after use. B, This photo shows a spread plate after 24 hours of incubation. The colonies are evenly distributed over the agar surface and can easily be counted in order to calculate the original cell density of the sample.
In the laboratory environment a mixed culture can contain two or more easily identifiable and differentiated microorganisms, using differential media. On the other hand, a contaminated culture is a culture that once was pure or mixed but was contaminated unintentionally with unwanted organisms.
Fixation and Staining
In order to observe microorganisms under the microscope the specimens must be specially prepared. Some organisms can be observed in the living state, but most often the specimens are dead or killed by chemical fixatives such as glutaraldehyde. Materials that have been dried on glass slides are called smears and are fixed to the slide with chemicals or heat (heat-fixed). Preparation of samples for electron microscopy requires different techniques, which are discussed separately. In order for fixed organisms to become visible under the light microscope, staining of the specimens is required.
Negative and Simple Stains
Most biological materials provide little contrast from their surroundings and must be stained for visualization. Dyes that are used to enhance the contrast of a cell can be positively or negatively charged, which allows the dye to attach itself to different components of the cell or to be repelled by the cell.
Negative Stain
Negative staining is an indirect staining process by which negatively charged dyes stain the background of the slide. Acidic stains such as India ink or nigrosin, when applied to bacteria, produce a dark background against the organisms. The organisms appear colorless because they do not pick up the stain, the result of the presence in their cell wall of negatively charged proteins that repel the stain (Figure 4.8). Negative stain techniques do not require fixation of the smear. This staining procedure is extremely valuable for identifying spirochetes, yeast capsules, and some other bacterial capsules.
Simple Stain
In simple staining, a single stain is applied to the specimen after fixation onto the slide. Simple stains are positively charged and are attracted to the negatively charged bacteria.
The most commonly used simple stains in microbiology include the methylene blue, crystal violet, and safranin stains. A drop of stain is put on the slide containing the specimen and allowed to react with the smear (specimen) for 30 to 60 seconds. The stain is then rinsed off with water, blotted or air dried, and the slide is ready for microscopic examination. Simple staining allows observation of the shape, size, and groupings of bacteria (Figure 4.9). Sometimes endospores can be identified because they do not take up stain and therefore appear colorless. However, endospores can be stained by special procedures (see Special Stains, later in this chapter).
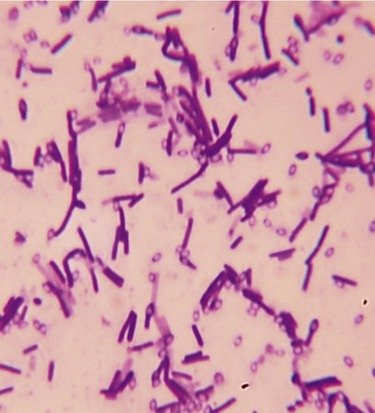
This micrograph illustrates a simple stain of Bacillus cereus, using crystal violet as the positively charged stain. The positively charged stain is attracted to the negatively charged cell wall. The clear egg-shaped voids inside some cells and scattered outside the cells are endospores that resist taking up much of the stain.
Differential Stains
Differential stains are more complex than simple stains and use more than one stain to differentiate cellular components. This application of more than one stain to a fixed smear is often used to demonstrate different staining characteristics of bacterial cells and allows the differentiation between bacterial species.
Gram Stain
The Gram stain is the most widely used differential stain in microbiology. It is a four-step staining process that differentiates bacteria into gram-negative and gram-positive groups. In the first step, crystal violet is applied to the smear on the slide, which is then rinsed with water. Next, Gram’s iodine, a mordant (a chemical that fixes the dye to intensify a staining reaction) is applied and the slide is again rinsed with water. At this point all bacteria appear purple. Now a decolorizing agent, a mixture of acetone and ethyl alcohol or just 95% ethyl alcohol, is applied to the slide. Gram-positive bacteria with their thick peptidoglycan layer will retain some of the crystal violet, but gram-negative bacteria with their thin peptidoglycan layer will not retain crystal violet and will be colorless after this step. The slide is then rinsed again with water. In the final step safranin, a counterstain, is applied followed by a last rinse with water (Box 4.1). Bacteria that retain crystal violet, the primary stain, along with the safranin, are called gram-positive bacteria and appear purple/blue. Gram-negative bacteria appear red due to the safranin counterstain because the crystal violet was lost during the decolorizing step (Figure 4.10).
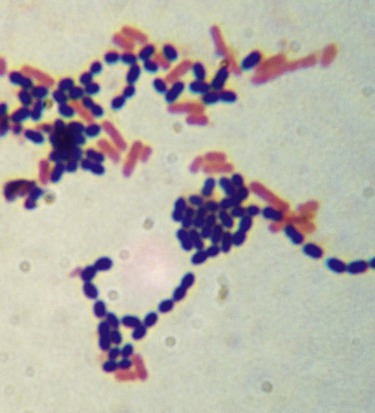
The Gram stain involves a four-step process in which the results will indicate whether the cells have a thick peptidoglycan layer in the cell wall (gram positive) or a thin layer (gram negative). This photo shows a mix of gram-positive cocci (dark purple/blue spheres), and gram-negative bacilli (red/pink rods).
The Gram staining procedure is most reliable with young bacterial cultures. However, as cultures age, some gram-positive bacteria may appear gram negative because they lose their ability to retain crystal violet. The Gram staining properties of the bacteria are due to the composition and structure of their cell walls. Gram-positive bacteria contain a much larger amount of peptidoglycan (see Chapter 3, Cell Structure and Function) in their cell wall than do gram-negative bacteria. Because of the different composition of their cell walls, the bacteria can also react differently to antimicrobial drugs.
Acid-fast Stain
Acid-fast staining is used primarily for the diagnosis of tuberculosis, nocardiosis, and cryptosporidiosis, a disease commonly found in patients with AIDS. Acid-fast organisms contain mycolic acid in their cell wall, which resists simple stains. The primary stain in this procedure is basic carbolfuchsin followed by counterstaining with acid alcohol (Box 4.2), a more powerful decolorizer than the ethyl alcohol used in Gram staining. Acid-fast organisms retain the primary stain whereas others are decolorized and become counterstained (Figure 4.11).
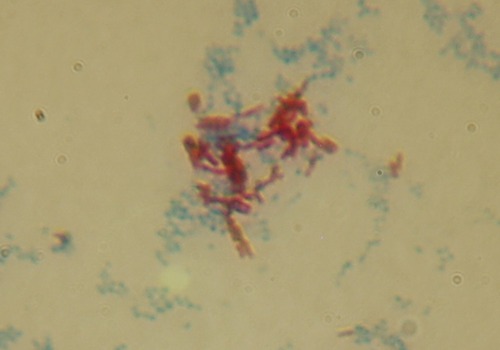
Certain bacteria have mycolic acid in the cell wall, which resists conventional chemical staining. By means of either heat or chemicals, the acid-fast stain can be “driven” into the cell where it will resist decolorization. This micrograph is of Mycobacterium smegmatis (an acid-fast bacillus shown in red) and Staphylococcus aureus (a non–acid-fast coccus shown in blue). S. aureus was used as a negative control.
The two main acid-fast staining procedures are the Ziehl-Neelson procedure and the Kinyoun procedure. The Ziehl-Neelson technique requires heating the slide during the basic carbolfuchsin step, but the other steps are performed on cooled slides. The Kinyoun technique is an alternative procedure that does not require heat. The lipid-rich wall of acid-fast bacteria makes them resistant to some dyes, antimicrobial drugs, and disinfectants.
Other Differential Stains
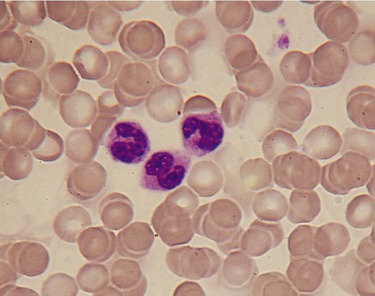
Wright’s stain is the most widely used differential stain in clinical hematology laboratories. It is used to stain and differentiate the various types of blood cells (Figure 4.12), and it can also be used to examine spirochetes, intracellular bacteria, and some infectious protozoans and can identify malaria-causing protozoans. Protozoans and fungi are stained by a variety of other procedures as well.
Special Stains
Special staining procedures are necessary for the demonstration of structures such as bacterial flagella, capsules, and endospores. The endospore stain is similar to the acid-fast stain in that it requires heat or chemicals to enter the endospores. The stain distinguishes the spores from the cells in their vegetative state. Capsule stains allow visualization of an unstructured protective layer surrounding some bacteria and fungi—the microbial capsules. Capsules do not react with most stains, but the background of the slide can be stained with a stain such as India ink, and the cell itself with the capsule can be stained with a simple stain. The capsule will appear as a clear halo, surrounding the cell with a stained background. Flagellar staining reveals flagella too small to be seen with the light microscope unless they are coated on the outside and then stained. Flagellar staining works best with fresh, young cultures because these structures can be damaged or lost in older cells.
Fixation and Staining for Electron Microscopy
Transmission Electron Microscopy
Transmission electron microscopy (TEM) in biological science yields higher resolution than light microscopy for studying cells/ultrastructure, macromolecules, bacteria, and viruses. Transmission electron microscopy in microbiology generally is used for the observation and identification of microorganisms within cells and tissues, for observation of organisms attached to cells, and for details of cellular structures. The operating environment of a TEM dictates the use of special preparation techniques. Whereas light microscopy uses stains that color the microorganisms to differentiate them, EM staining uses metals to block the transmission of some of the electron beam through the sample and increase contrast.
Tissue samples or culture specimens are prepared by immersion in a special chemical fixative such as glutaraldehyde for a specific amount of time, usually between 12 and 24 hours. After the initial fixation period, the specimens are postfixed in 1% osmium tetroxide (OsO4) for approximately 1 hour. This procedure is followed by staining of the entire specimen with a solution of uranyl acetate, a specific stain for transmission electron microscopy. The samples or specimens are then dehydrated through a series of increasing concentrations of ethanol. After dehydration, the samples are embedded in liquid epoxy resin, which infiltrates the specimens. After embedding at room temperature, the samples are poured into special dishes and placed in an incubator held at a specific temperature for a specified period of time, during which the epoxy resin polymerizes into hard plastic blocks. These hard plastic specimen blocks are ready for the preparation of ultrathin sections with an ultramicrotome equipped with glass or diamond knives. The ultrathin sections are between 260 angströms (Å) and 640 Å thick and float on top of the water in the boat of the knife (an angström is equal to 0.1 nanometer [nm] or 1 × 10–10 meter [m]). The sections are then picked up with a small metallic grid and stained before examination with the electron microscope.
Another fixation technique for TEM is cryofixation, which provides structural and biochemical preservation of cells that, as a result, more closely resemble their living state than is obtained by preparation via chemical fixation. This technique uses rapid cooling rates during which the intracellular ice crystals formed within the cells on freezing are smaller than 5 nm, which is the minimal resolution required for ultrastructural studies. Before freezing, the specimens are coated with cryoprotective materials. After freezing, the specimens are sectioned with a cryoultramicrotome before observation with the TEM. Cryofixation is a useful tool in immunofluorescence. Immunofluorescence and ultrathin cryosections are used to reveal the presence and localization of antigens in cells and tissues.
Scanning Electron Microscopy
Specimen preparation for scanning electron microscopy (SEM) uses both chemical fixation and cryofixation techniques. In addition, hydrated materials such as biological samples must be dehydrated after fixation. This is typically done by passing the specimens through a graded series of ethanol treatments (see the previous description of sample preparation for TEM). The samples for SEM observation must then be completely dried by the critical-point drying method. This complicated process involves the replacement of liquid in the cells with gas to create a completely dry specimen with minimal or no cellular distortion. Freeze-fixed samples may also be freeze-dried. The completely dried sample is then mounted on an aluminum stub with special silver conductive glue. In the next step, if it is a nonmetallic sample (hair, insect, or biological tissue) the mounted specimen is sputter coated with gold to make it electrically conductive, after which it can be examined by SEM. If it is a metallic specimen, then it can be directly mounted and scanned.
Identification Techniques
To identify unknown microorganisms, it is essential to use their morphological, physiological, and chemical characteristics. This is readily done in the laboratory with differentially stained slides prepared from organisms grown on or in various media, recording observations of growth characteristics, and observing biochemical reactions. Microscopy in the microbiological laboratory is generally used for the detection and preliminary identification of microorganisms and, together with other laboratory techniques, more positive accurate identification of microorganisms can be achieved.
Morphology
The morphological characteristics of cells and organisms are the major data components used for their identification and classification. On the basis of their morphological characteristics, cellular organisms are divided into prokaryotes and eukaryotes (see Chapter 3, Cell Structure and Function). Morphological characteristics are also the basis for determining whether eukaryotes are plant or animal cells. In other words, morphological characteristics are used for the preliminary identification of many bacteria and the definite identification of most fungi and parasites.
Bacteria grown in culture media vary according to their shape, size, and arrangements. According to their shape, bacteria are classified as bacilli, cocci, spirilla, vibrios, spirochetes, or coccobacilli (see Chapter 6, Bacteria and Archaea). Some bacteria show variation in size and shape as a result of environmental conditions and age of the organisms; these are called pleomorphic bacteria. During cell division, the different types of bacteria produce cultures of varied appearance. These characteristics can be used for purposes of identification. Whereas size, shape, and arrangement of bacteria are the first step in identification, many bacteria may look similar and require additional laboratory methods other than microscopy for more specific identification.
Cultural Characteristics
Growth behavioral patterns exhibited by unknown bacteria in various types of culture media are important characteristics that can be observed on solid medium plates or slants, or in broths. For example, selective isolation of individual species enables the examiner to observe the shape and texture of the colony, its pigmentation and odor. As stated earlier, some bacterial species exhibit a pleomorphic appearance under different environmental conditions (such as a temperature change) and at different ages. It is therefore important to incubate replicate plates at more than one temperature. For purposes of continuity, descriptive terms, illustrated in Bergey’s Manual of Systematic Bacteriology, should be used in describing and recording cultural characteristics.
Growth requirements for fungi vary among species and also among strains. In general, fungi grow best on media that are formulated from the natural material from which they were isolated. Laboratory media include but are not limited to Sabouraud agar, hay infusion agar, and potato dextrose agar. More specific information about the growth requirements of fungi cultures is provided in Chapter 8 (Eukaryotic Microorganisms).
Growing algae in the laboratory also depends on the requirements of the specific algae and on the research interests of the scientists working with the selected species. Requirements for growth of algae cultures are also discussed in Chapter 8.
Culture Plates
Solid, agar-based media in sterile Petri dishes are routinely used to grow and subsequently identify colony characteristics such as shape, size, elevation, and margin type in particular. Inoculants from media on culture plates are used to select particular bacterial groups and to differentiate between two or more different species.
• Nutrient agar plates are the most commonly used general medium plates in the laboratory. This medium is safe for use in high school and college teaching laboratories because it does not selectively grow pathogenic bacteria.
• Tryptic (Trypticase) soy agar (TSA) is a general-purpose medium frequently used as the base medium of other agar plate types, for example, blood agar plates, which are made by enriching TSA plates with blood.
• Phenylethyl alcohol agar (PEA) is selective for gram-positive bacteria because it inhibits the growth of gram-negative bacteria. It is often used to isolate Staphylococcus and Streptococcus from specimens also containing gram-negative organisms.
• Blood agar plates (BAPs) are used both as enriched media and to differentiate between individual species on the basis of their ability to produce hemolysins (enzymes that lyse red blood cells).
• Chocolate agar (CHOC), a type of blood agar in which the blood cells have been lysed by heating the cells to 56° C, is used to grow fastidious respiratory bacteria. It should be noted that no chocolate is contained in the medium; it is named for the coloration only.
• Thayer-Martin agar (TM) is a chocolate agar designed to isolate Neisseria gonorrhoeae and N. meningitidis.
• MacConkey agar (MAC) is a selective and differential medium that differentiates between gram-negative bacteria, while inhibiting the growth of gram-positive bacteria. The addition of bile salts and crystal violet to the agar inhibits the growth of most gram-positive bacteria, making MAC selective. Lactose and neutral red are added to differentiate the lactose fermenters (pink colonies) from those not fermenting lactose (clear colonies).
• Xylose lysine deoxycholate (XLD) agar is used for the culture of stool samples, and is formulated to inhibit gram-positive bacteria, whereas the growth of gram-negative bacilli is facilitated. The colonies of lactose fermenters appear yellow.
• Mannitol salt agar (MSA) is a selective and differential medium that differentiates organisms that ferment mannitol. When mannitol fermentation occurs, lactic acid is produced and the pH will drop, causing the MSA plate to turn yellow. The salt component of the medium is selective for halophiles such as staphylococci. Organisms that cannot withstand a high salt content fail to grow.
Slants
An agar slant consists of agar-based medium in a culture tube. It is called a slant because the tube is placed at an angle during cooling to provide a large slanted surface for inoculation. After incubation the amount of growth is determined as:
Any pigments produced by the bacteria are associated with the colony or, if soluble, they diffuse into the growth medium. However, most bacteria do not produce pigments and their colonies are white or buff colored. The degrees of opacity of the growth can be classified as opaque, transparent, and partially transparent. The gross appearances of the different types of growth are illustrated in Figure 4.13 and are described as follows:
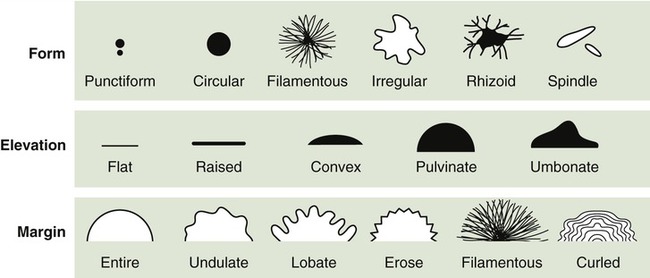
This drawing shows the different types of colony morphology that organisms can display as they grow on the surface or agar, whether in a slant or on a Petri plate.
• Filiform: This type of growth is characterized by uniform growth along the line of inoculation.
• Echinulate: The margins of growth have a jagged, toothed appearance.
• Beaded: Separate or semiconfluent colonies appear along the line of inoculation.
• Effuse: The growth appears thin, veil-like, and usually spreading.
Gelatin Stabs
The gelatin stab method uses deep tubes containing 12% nutrient gelatin. Gelatin causes liquids to solidify at temperatures below 28° C, but at temperatures above 28° C it remains liquid. Some bacteria produce gelatinase, an enzyme that hydrolyzes gelatin. In the presence of gelatinase, gelatin is hydrolyzed and can no longer gel; it remains liquid even below 28° C. A heavy inoculum from a pure culture of the test organism is stabbed into the medium (Figure 4.14) and incubated for at least 48 hours followed by refrigeration for about 30 minutes. If the organism has produced enough gelatinase, the contents of the tube will remain liquid or partially liquid and not solidify in the refrigerator. If liquefaction has occurred the appearance can be:
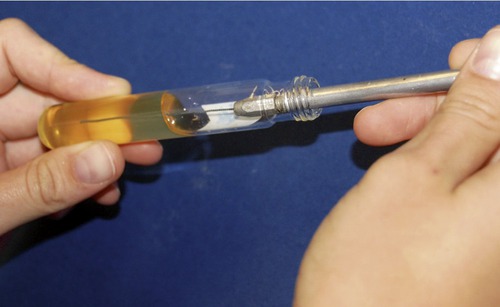
This photo shows a gelatin stab being made with a sterile needle. The needle is stuck down through the center of the agar to the bottom of the tube. This technique is often used in performing the gelatinase and oxidation–fermentation tests.
• Crateriform: A saucer-shaped liquefaction
• Napiform: A turnip-like appearance
• Infundibular: A funnel-like or inverted cone
• Saccate: A tubular or cylindrical elongate sac
• Stratiform: Liquefaction to the walls of the tube in the upper region of the tube
The configuration of the liquefaction is not as significant as the fact that liquefaction takes place. It should be noted that some organisms produce the gelatinase at a very slow rate and need to be incubated for longer periods of time to determine whether or not liquefaction takes place. Pathogens that produce gelatinase often can cause extensive tissue damage in an infected patient.
Broths
When bacteria are grown in broths they may exhibit patterns of growth ranging from sediment at the bottom of the tube, turbid growth throughout the tube, or a pellicle—a thick growth at the top of the tube (Figure 4.15). Pellicle formation is sometimes due to an affinity for oxygen. It also may be the result of hydrophobic compounds present in the cell walls or the general formation of dry, light colonies. If an organism produces and releases soluble pigments, they will spread into the broth and change its color.
Physiological/Biochemical Characteristics
Specific biochemical patterns are blueprints for microbiologists to use in identifying microorganisms. Several biochemical tests can be used to identify their physiological characteristics. These tests include but are not limited to tests for hydrogen sulfide production, citrate use, phenylalanine deamination, litmus milk reactions, and the triple sugar iron test. These are just a few representative tests out of the many that can be used.
Hydrogen Sulfide Production
Certain bacteria such as Proteus vulgaris and Salmonella species produce hydrogen sulfide from the amino acid cysteine. The hydrogen sulfide production test is useful for the identification and differentiation of Salmonella species from other species of Enterobacteriaceae. Kligler’s iron agar and SIM (sulfur reduction, indole production, and motility) medium are used to detect the presence of sulfide. These media contain iron, which forms a dark precipitate when it reacts with hydrogen sulfide.
Citrate Use
The purpose of the citrate use test is to determine whether an organism, such as Enterobacter aerogenes and Salmonella typhimurium, is capable of using citrate as its sole carbon source for metabolism, with resulting alkalinity. This is a useful characteristic when differentiating species among enteric bacteria. The synthetic Koser’s citrate medium and Simmons citrate agar are used to detect citrate use by bacteria.
IMViC Tests
Enterobacteriaceae (enterics) are gram-negative bacteria that are common in the intestinal florae of humans and other animals. The IMViC tests are commonly used for the identification and differentiation of these bacteria including Klebsiella, Enterobacter, Proteus, and Escherichia coli. IMViC is an acronym that stands for indole, methyl red, Voges-Proskauer, and citrate. To obtain results of these tests, three test tubes are inoculated: tryptone broth or SIM agar (indole test), methyl red–Voges-Proskauer broth (MR-VP broth), and citrate slants.
Litmus Milk Reactions
Litmus milk reactions are used to determine the action of a microorganism on milk, which contains lactose, glucose, proteins, fats, minerals, and vitamins. Litmus milk containing 10% powdered skim milk and a small amount of litmus as a pH indicator is used. After incubation, different reactions can be observed depending on the biochemical reactions of a species within litmus milk. The various possible reactions are shown in Table 4.3.
TABLE 4.3
Changes That Can Occur in Litmus Milk
| Reaction | Condition of Medium | Explanation |
| Slight acid production | Pale pink indicator | Glucose but not lactose utilization |
| Acid reaction | Pink indicator | Glucose and lactose fermentation |
| Alkaline reaction | Blue or purple indicator | Amines or ammonia released from proteolytic bacteria |
| Litmus reaction | Culture becomes white | Result of rapidly reproducing bacteria, lowering the oxygen content at the bottom of the tube |
| Coagulation | Medium clotted, curd formation | Protein coagulation |
| Peptonization | Medium translucent | Caused by proteolytic bacteria |
Rapid Identification Tests
The identification of bacteria and fungi can be facilitated by the use of packaged kit systems, manufactured for the rapid identification of medically important bacteria and fungi. Rapid identification and reporting are becoming increasingly important because of newly emerging pathogens (see Chapter 18, Emerging Infectious Diseases), the recognition of old pathogens in different settings, increasing nosocomial infections, world travel, and the increase in multidrug-resistant organisms.
The term rapid method includes a wide variety of procedures and applies to all procedures that provide faster results than conventional methods. Rapid identification methods are available for microscopy, biochemical identification, as well as antigen and antibody detection. Commercially available kits for rapid identification include both manual and automated systems. Kits designed to perform several biochemical tests at the same time can identify bacteria within 4 to 24 hours. Hospital microbiology laboratories typically use fully automated computerized systems for the identification and classification of enteric bacterial species, with the final identification report available in as little as 6 hours. Miniaturized multimedia systems for bacterial identification also have been developed and are used when speed and cost-effective identification are needed. Some rapid identification methods for enteric bacteria are the Enterotube II, a tube with 12 compartments for different biochemical tests, the API 20 series with 20 tests, the BBL Crystal system with 30 tests, and the Biolog and VITEK systems with more than 90 tests.
HEALTHCARE APPLICATION
Examples of Manual and Automated Systems for Microbial Identification
| Name | Manufacturer | Organism(s) Identified |
| API/bioMérieux | bioMérieux Clinical Diagnostics | Enterobacteriaceae, other gram-negative bacilli, Staphylococcus, Streptococcus, Enterococcus, Neisseria, gram-positive bacilli, yeast, anaerobes |
| Vitek | ||
| BactiCard | Remel | Neisseria/Moraxella, Escherichia coli, Streptococcus, yeast |
| Biolog MicroPlate | Biolog | Gram-positive and gram-negative organisms, yeast, anaerobes |
| BBL Crystal E/NF* | Becton Dickinson Microbiology Systems | Enterobacteriaceae, other gram-negative bacilli, Neisseria, Haemophilus, gram-positive cocci and bacilli |
| Enterotube II | Becton Dickinson Diagnostic Systems | Enterobacteriaceae, other gram-negative bacilli |
| Gonochek | EY Laboratories | Neisseria/Moraxella |
| Micro-ID (Rapid ID) | Remel | Enterobacteriaceae, other gram-negative bacilli, Neisseria, Haemophilus, Streptococcus, Enterococcus, yeast, anaerobes, gram-positive bacilli |
| MicroScan (TouchScan) | Siemens Healthcare Diagnostics | Gram-positive and gram-negative organisms, Haemophilus, Neisseria |
| Oxi/Ferm | Becton Dickinson Microbiology Systems | Nonfermenter gram-negative bacteria |
| Uni-Yeast Tek | Remel | Nonfermenters, yeast |
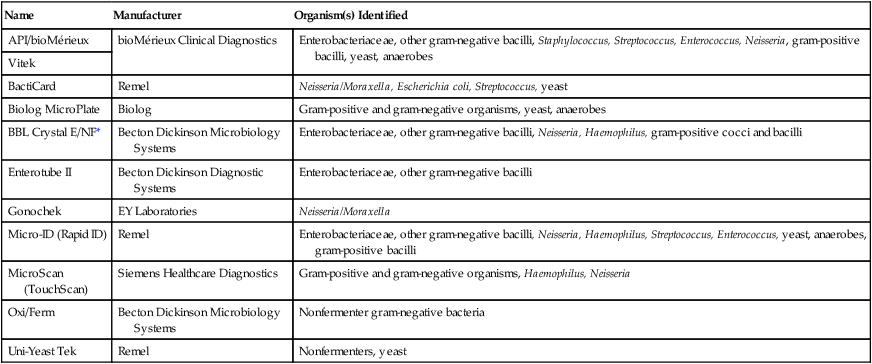
*E/NF, Enteric/nonfermenter.
Molecular Analysis
Serologic Analysis/Diagnosis
Immunologic techniques are used to detect, identify, and quantify antigens in clinical samples. Antibodies are sensitive and specific tools used to accomplish the detection of antigens from a virus, bacterium, fungus, or parasite. In addition, these techniques can evaluate the antibody response to infections (see Chapter 20, The Immune System). The specificity of the antibody–antigen interaction represents a powerful tool in the microbiology laboratory. In general, serological assays are designed to give negative or positive results; however, the antibody strength can be determined by a titer. A titer is a test that measures the presence and amount of antibodies in the blood in response to invasion by a particular antigen (see Chapter 20). In other words, the antibody level in the blood is a reflection of the body’s past exposure to an antigen, a foreign substance.
Genetic Analysis
Given the rapid changes in technologies, laboratory procedures in general, and genetic analysis in particular, the abbreviated presentation on genetic analysis that follows will soon require updating as a result of advancements made in the future.
Identification of a specific microbe from within a microbial community is difficult because of their enormous diversity in environmental samples, and growing cultures of microbes to identify species is slow and often error prone. The most precise method of classifying and identifying microorganisms is genetic analysis. Today’s technology allows the sequencing of entire genomes, which yields highly specific and informative data. This process is costly and labor intensive. As a result, scientists have been searching for ways to identify key segments of the genetic code that are short enough to be sequenced rapidly and can readily distinguish between species.
Whereas initially organisms were classified by their ratio of guanine to cytosine nucleotides, this method has been largely replaced by more discriminating methods. Genome structure and genetic sequence are major distinguishing characteristics of any organism. Specific strains of a microorganism can be differentiated on the basis of their DNA or RNA, or on the basis of DNA fragments produced by restriction enzymes—molecular scissors. Different restriction enzymes recognize specific DNA sites and cut the DNA at these sites. Depending on the restriction enzyme, the DNA is cleaved at different places, resulting in smaller fragments of different lengths. DNA or RNA fragments can be produced by different restriction enzymes, resulting in fragments of different lengths. The fragments of different sizes or structure can be distinguished by their rate of movement through an electrophoresis gel, which separates them, creating a specific profile (see Chapter 25, Biotechnology). Comparison of the patterns of DNA or RNA fragments generated by the restriction enzymes allows the identification of different strains of a species.
DNA probes can be chemically synthesized or generated by the cloning of specific genomic fragments. DNA probes are sensitive tools with which to detect, locate, and quantitate specific nucleic acid sequences of species that are difficult to grow. Rapid identification techniques have been developed by the use of DNA probes. If a probe binds to an organism’s DNA the organism can be identified. This technique is a valuable tool for the rapid identification of slow-growing microorganisms such as mycobacteria, fungi, and organisms that currently cannot be cultured in the laboratory environment.
Another genotypic method is nucleic acid sequence analysis. With this method, probes are used to localize specific nucleic acid sequences unique to a genus, species, or subspecies. The sequences are then amplified by the polymerase chain reaction (see the section on PCR in Chapter 25) and the amplified material is sequenced to define the identity of the isolates.
Most commonly used is the analysis of ribosomal DNA sequences because of their high species and subspecies specificity. This technique has also been used to determine the evolutionary relationship between organisms. Other molecular techniques used for identification are plasmid analysis, ribotyping, and analysis of chromosomal DNA fragments. Ribotyping is the use of universal probes that target specific ribosomal RNA coding sequences to detect band patterns. These methods have now been sufficiently modified so that they can be used for microbial identification in most clinical laboratories.
Summary
• Aseptic technique is essential in the microbiology laboratory to prevent contamination of cultures, laboratory personnel, specimens, and the environment.
• A variety of physical and chemical methods are applied to achieve sterilization, disinfection, and sanitation:
1. Sterilization destroys or removes all microorganisms and their endospores.
2. Disinfection destroys vegetative microbes and viruses but not endospores.
3. Sanitation is the mechanical removal of microorganisms and debris to reduce contamination to safe levels.
• In order to gather data about a particular microbe, a variety of tools, techniques, and media are used to grow microbes in the laboratory environment.
• Various types of media are available to meet the different nutrient requirements for the growth of different microorganisms.
• Culture media are inoculated, followed by incubation and isolation, in order to identify and study particular microbes.
• In order to observe microorganisms with the help of a light or electron microscope, the specimens require special preparation and the use of stains to identify the bacteria.
• Morphological characteristics of microorganisms are a major component for their initial identification and classification. Bacteria are classified according to their variability in shape and size.
• The cultural characteristics organisms exhibit when growing in a variety of media are also used for identification of unknown bacteria.
• Bacteria can also be identified by their physical and biochemical characteristics when growing in or on differential and selective media.
• The ultimate indicator for classification is via analysis at the DNA test level.
Review Questions
1. The destruction of all microorganisms and their endospores is referred to as:
2. The process by which the number of microbes on the human skin is reduced by scrubbing is called:
4. Media that contain complex organic substances such as blood for the growth of specific bacteria are referred to as:
5. Which of the following media are used to determine motility and growth patterns of bacteria?
6. Nigrosin is a stain used in:
7. The stain used to identify bacteria with a large amount of peptidoglycan in their cell walls is the:
8. The medium selective for the growth of Staphylococcus species is:
9. A growth that appears separate or semiconfluent is referred to as:
10. Which of the following media is not a differential medium based on fermentation?
11. A saucer-shaped liquefaction of gelatin stabs is said to have a(n) __________ appearance.
12. A culture that contains a single species of an organism is a(n) __________ culture.
13. A disinfectant is designed to be used only on __________ surfaces.
14. Bacteria that show variation in size and shape as a result of environmental conditions or age of the organisms are referred to as __________ bacteria.
15. Bacteria that appear red after Gram staining are said to be gram __________.
16. In correct order, describe the steps necessary in the Gram-staining procedure.
17. Compare and contrast differential and selective media.
18. Name and describe the action of three chemicals used for sterilization.
19. Name and describe the action of five commonly used disinfectants.
20. Describe the three different techniques that can be used to obtain organisms to produce pure cultures.