Massive Hemoptysis
Hemoptysis varies in amount from intermittent blood-streaked sputum to massive arterial bleeding with asphyxiation or exsanguination. Massive hemoptysis is defined as the expectoration of blood from the respiratory tract in life-threatening quantities. Clinical definitions of massive hemoptysis focus on selected quantities of coughed blood between 200 and 1000 mL over 24 hours or less, with greater than 600 mL being the most common criterion.1 Quantification of the amount of coughed blood is unreliable, often subjective, and fails to account for blood remaining in the lungs. The adverse clinical effects of hemoptysis such as impaired gas exchange, airway obstruction, or hypotension may be more relevant for defining a life-threatening condition.2
Fortunately, massive hemoptysis is rare and accounts for 4% to 18.5% of all cases of hemoptysis in recent studies.3–5 Incidence studies are problematic because of the use of variable definitions. Although mortality rates as high as 71%6 were reported in the past, mortality rates in recent studies of massive hemoptysis range from 0% to 38%.2,3,7
Anatomic Considerations
Pulmonary Circulation
Prospective angiographic studies for hemoptysis in which both pulmonary and bronchial circulations have been imaged do not exist. Bleeding from the pulmonary arterial circulation accounts for less than 10% of massive hemoptysis cases and has been noted in a variety of destructive pulmonary lesions including tuberculosis, lung abscess, and aspergillosis.2,8 Aneurysms of the pulmonary artery, arteriovenous malformations (AVMs), and pulmonary artery rupture have also been reported.
Bronchial Circulation
Bleeding from the higher pressure bronchial circulation has been estimated to cause 88% of the cases of massive and submassive hemoptysis.9 The bronchial arteries arise from the descending aorta with considerable anatomic variation. The one or two bronchial arteries that supply each lung in the majority of individuals10 arise from the area near the first and second intercostal arteries. Particularly on the right side, the bronchial arteries may arise directly from the proximal first intercostal artery. The arteries course along the trachea, major bronchi, and bronchioles and have terminal communications with the pulmonary capillaries or pulmonary venules. The small-vessel bronchial supply to the trachea and major bronchi drains into the azygos vein with direct communication to the superior vena cava. Aneurysmal dilation of bronchial arteries (Dieulafoy’s vascular malformation) has been noted in some patients with hemoptysis, and it can occasionally be visualized endobronchially and noted on bronchial arteriography.11
A direct anastomotic communication between the bronchial and pulmonary arterioles has been sought to explain the preservation of lung parenchyma after injuries to the pulmonary vascular supply. Anatomic studies have found that intermeshing of pulmonary and bronchial capillary networks is the most common anastomotic arrangement that prevents pressurization of the pulmonary arterioles with systemic pressures.12 However, in chronic inflammatory diseases of the airways, anatomic anastomoses have been found that allow direct pressurization of the pulmonary artery with systemic pressures.13 The extent to which these vascular communications are related to hemoptysis remains unknown.
Bronchial arteries vasodilate in the presence of cholinergic, β2-adrenergic, and some nonadrenergic, noncholinergic agonists. Although the effect of β2-agonists on the course of hemoptysis remains unstudied, the balance between improved mucociliary clearance of blood affected by β2-agonists and detrimental bronchial artery dilation should be considered.14 Other physiochemical maneuvers can influence bronchial blood flow—cold air causes blanching of the human airway,15 humidified air decreases bronchial blood flow compared with dry gas,16 and increased alveolar pressure decreases bronchial blood flow by applied pressure at the capillaries.12
Pulmonary Venous Abnormalities
Bleeding from the pulmonary veins is most likely in cardiac disease such as mitral stenosis or mitral regurgitation. Focal varices of the pulmonary veins that are occasionally visualized on chest radiography, but are best identified on the venous phase of pulmonary arteriography, have been described.17,18
Causes of Hemoptysis
Bronchiectasis, tuberculosis or its sequelae, lung cancer, and aspergilloma (mycetoma) account for the largest proportion of massive hemoptysis cases. However, almost any of the many causes of hemoptysis (Box 46.1) can become massive on rare occasions. Clues to specific diagnoses are obtained by history, physical examination, chest radiography, and chest computed tomography (CT). Additionally, it is important to consider the demographics of the patient population when considering the cause of hemoptysis. For example, there is a predominance of causes related to infectious diseases (i.e., mycobacterial or parasitic) and their long-term complications in individuals from developing and impoverished countries. In contrast, malignancies and bronchiectasis are more common causes of massive hemoptysis in developed countries.
Bronchiectasis
Bronchiectasis is the usual cause of massive hemoptysis in patients with CF and occurs in approximately 4.1% of patients during their lifetime.19 Massive hemoptysis is more common in patients over the age of 18 years and has been associated with pancreatic insufficiency, Staphylococcus aureus colonization, reduced lung function, and diabetes.19 Although Pseudomonas aeruginosa remains the predominant pathogen associated with decline in lung function, its presence does not translate into an increased incidence of hemoptysis. Origin of the bleeding is most often upper lobe bronchial or systemic arteries. These patients are difficult to treat because of minimal pulmonary reserve in the majority of patients at the time of hemoptysis. Guidelines for treatment of massive hemoptysis in CF patients recommend empiric antibiotic coverage to include S. aureus, discontinuation of nonsteroidal anti-inflammatory drugs, discontinuation of airway clearance therapies, discontinuation of aerosolized hypertonic saline, and use of BAE to control bleeding.20 Although inhaled tobramycin and dornase alfa use were associated with a lower hemoptysis incidence,19 consensus was not reached on whether to discontinue these therapies in massive hemoptysis.20
Lung Malignancy
Lung cancer is associated with hemoptysis in 20% to 30% of cases and may be the presenting manifestation.21 Although the clinical course of hemoptysis is often that of chronic blood-streaked sputum, massive hemoptysis may occur as a terminal event. Massive hemoptysis is most commonly associated with squamous cell type22; cavitation within the carcinoma23; and central endobronchial position, occasionally with invasion into the pulmonary arteries. The blood supply to most lung carcinomas is derived from diffuse neovascularization from the bronchial circulation, making BAE effective in some cases.
Other less common cancers can bleed when found in the lung. Kaposi sarcoma has a high incidence of bloody pleural effusions and hemoptysis. Angiosarcomas are vascular tumors that may bleed continuously from small tumor sites.24 Choriocarcinomas may bleed profusely, particularly after initiation of chemotherapy. Metastatic disease including renal, ovarian, and breast cancer have rarely been associated with hemoptysis.
Massive hemoptysis may also result from interventions that treat lung malignancies. Endobronchial brachytherapy has resulted in massive, fatal hemoptysis and was found to be associated with direct contact of the brachytherapy applicator and tracheobronchial walls near great vessels.25 Radiofrequency ablation of lung neoplasms has been associated with massive bleeding due to intraparenchymal hemorrhage and pulmonary artery pseudoaneurysm.26,27
Lung Abscess
Lung abscesses are commonly found in parenchymal areas prone to aspiration. The indolent course of these infections allows time for hypertrophy of the bronchial circulation within the walls of the abscess cavity. Additionally, these cavities may enlarge and erode into major pulmonary arteries and other thoracic vessels, including the aorta.28 With either of these abnormalities, bleeding can be massive and recurrent. Although the abscesses are focal and amenable to surgery, the patient who chronically aspirates because of alcoholism or dementia may have other contraindications for surgery.
Chronic Bronchitis
Chronic bronchitis rarely causes massive hemoptysis, but it is a frequent cause of mild hemoptysis. The pathologic lesions responsible for bleeding are likely dilated bronchial arteries that are eroded during active inflammation of the airways.29 A comprehensive management approach is required to treat hemoptysis and ensure that other lesions such as lung cancer are not present.
Other Pulmonary Infections
Although hemoptysis can complicate any bacterial or fungal pneumonia, massive hemoptysis is rare unless tissue necrosis is present. Tissue necrosis is a hallmark of anaerobic, staphylococcal, and actinomycotic30 pneumonias but can occur with many different bacterial causes. Septic pulmonary emboli, particularly from staphylococcal species, have a high incidence of concomitant lung cavitation.31 Mycotic pulmonary artery aneurysms may also be hidden within pneumonias and can be diagnosed by pulmonary arteriography or multidetector CT angiography.32
Hemoptysis is particularly common in fungal pneumonias that invade the vasculature. Invasive Aspergillus can be found in nonimmunosuppressed patients with chronic obstructive pulmonary disease (COPD)33 but is more commonly found in the persistently neutropenic patient. A characteristic radiographic pattern of cavitation and hemoptysis follows the recovery of neutrophils and should be anticipated in at-risk patients.34,35 The use of prophylactic surgery to resect areas of infarcted lung tissue has been advocated36,37 because of the high mortality rate associated with medical management of the hemoptysis. However, such surgery remains high risk, and controlled trials have not been performed. Although more rare, invasive pulmonary mucormycosis38 may produce similar findings. Hemoptysis complicates primary coccidioidal infections in 15% of cases and may approach a 50% incidence in patients with chronic coccidioidal cavities. Histoplasmosis, cryptococcosis, and blastomycosis can also cause hemoptysis. Frequent and sometimes massive hemoptysis can occur with the parasitic diseases paragonimiasis,39 echinococcosis,40 strongyloidiasis, and ancylostomiasis.
Aspergillus Fungus Balls
Hemoptysis occurs in more than half of patients with pulmonary Aspergillus fungus balls.41 The cavity walls are richly vascularized by branches of the bronchial circulation, and enlargement of these cavities may also extend into large branches of the pulmonary artery. The mechanism of hemoptysis is likely multifactorial from secondary bacterial invasion of the fungal cavity, microinvasion of the cavity wall by Aspergillus (semi-invasive aspergillosis), or less commonly by truly invasive disease. Therapy of hemoptysis depends on the underlying cause for the cavitary lung disease.
Systemic antifungal therapy remains controversial because there is not a well-defined means to diagnose semi-invasive disease.42,43 Intracavitary amphotericin B, however, instilled via a transthoracic catheter has proved successful at dissolution and sclerosis of the cavity with control of hemoptysis and is a viable option in patients who are poor surgical candidates.44,45 Alternatively, antifungal therapy with ketoconazole,46 miconazole,47 or amphotericin B48 has been given endobronchially for fungus ball dissolution and hemoptysis control, but these techniques are effective in less than half of patients.49 External beam radiotherapy of 3.5 Gy given once per week has been used as an adjunctive measure in nonoperable patients.50
Surgery remains the therapy of choice in patients with adequate pulmonary reserve due to the recurrent nature of hemoptysis. Simple aspergilloma (no abnormality in surrounding lung) has an excellent response to surgical resection, most commonly a lobectomy. Complex aspergilloma with surrounding pleural and parenchymal involvement usually requires pneumonectomy and is associated with variable success.51 Fungus balls complicating sarcoidosis usually occur in patients with bilateral upper lobe cavitary disease (stage IV), in which underlying lung function prohibits resection.
Cardiovascular Causes
Mitral stenosis is one of the most common cardiac abnormalities that can present with hemoptysis.52 The risk of hemoptysis is likely related to the elevation of pulmonary venous pressure and the rapidity with which the stenosis developed. Clinical examination may reveal an opening snap or diastolic murmur. An echocardiogram should be obtained for definitive diagnosis.
Other causes of increased pulmonary venous pressure, such as mitral regurgitation or severe congestive cardiomyopathy, may also produce hemoptysis that usually presents with radiographic pulmonary edema and a prodrome of pink frothy sputum. Fibrosing mediastinitis,53 pulmonary veno-occlusive disease,54 and congenital pulmonary venous stenosis55 are less common causes of pulmonary venous congestion.
Interstitial Lung Disease
Only a few interstitial lung diseases are prone to hemoptysis. Lymphangioleiomyomatosis (LAM) is a disease of smooth muscle proliferation around pulmonary lymphatics, airways, and vasculature. Any of the triad of hemoptysis, pneumothorax, and chylothorax should suggest the diagnosis in a woman of childbearing age.56 The airway granulomas of sarcoidosis have also been associated with hemoptysis, although the presence of traction bronchiectasis may be a more common cause. Additionally, the erosion of calcified hilar lymph nodes into the vasculature can produce hemoptysis. Other interstitial lung diseases that cause hemoptysis usually have diffuse alveolar hemorrhage.
Broncholithiasis
Therapy of hemoptysis involves broncholith removal. Broncholith removal by rigid or flexible bronchoscopy is usually successful when the broncholith is free. When broncholiths are partially embedded in the airway wall, removal is best facilitated by rigid bronchoscopy57 or by thoracic surgery. Surgical options include lymph node resection with or without bronchoplasty or lobectomy. One surgical series had a 34% complication rate and a 15% rate of recurrent or persistent disease.58
Diffuse Alveolar Hemorrhage
Diffuse alveolar hemorrhage (DAH) most commonly presents abruptly and may or may not be associated with frank hemoptysis. The early manifestations are often confused with other alveolar filling processes such as pulmonary edema, bacterial pneumonia, or the acute respiratory distress syndrome (ARDS). Consideration of DAH is prompted by the presence of diffuse infiltrates on chest radiograph, the presence of systemic manifestations of vasculitis, the association of hemoptysis, hypoxemic respiratory failure, and the common finding of anemia.59 Unfortunately, the many causes of DAH are often differentiated on the basis of laboratory tests that may not be routine in many hospitals. Furthermore, specific therapy is often not initiated until a definitive diagnosis has been established by biopsy.
The differential diagnosis of DAH is listed in Box 46.2. Although some of these diseases have no specific therapy, establishing a diagnosis and instituting supportive care help to avoid unnecessary diagnostic and therapeutic maneuvers. Many of the vasculitides are stabilized only with aggressive immunosuppressive therapy, which would not be appropriate for infectious diseases that can present with the same features in a chest radiograph.
Bronchoscopy with transbronchial biopsy and bronchoalveolar lavage (BAL) are usually performed in a patient with unknown pulmonary infiltrates in the intensive care unit (ICU) if respiratory compromise is not severe. Although often not sufficient to establish a specific diagnosis, finding a bloody lavage, hemosiderin-laden macrophages, and the lack of specific pathogens can presumptively yield a diagnosis of DAH. The diagnosis of DAH is best made by quantitation of hemosiderin in alveolar macrophages obtained by BAL. A Prussian blue stain is graded by the methods of Kahn and colleagues60; hemosiderin scores above 100 are virtually diagnostic of alveolar hemorrhage. Hemosiderin-laden macrophages may not appear in BAL fluid until 48 to 72 hours after acute hemorrhage, resulting in a low sensitivity for hemosiderin scores in this setting.61
Immunologic Lung Disease
The differential diagnosis of DAH is narrowed significantly if renal abnormalities are present. Although pulmonary-renal syndromes can often be stabilized with high-dose corticosteroids alone pending further evaluation, directed therapy depends on the measurement of specific autoantibodies and evaluation of a renal biopsy. Alveolar hemorrhage is a hallmark of anti–glomerular basement membrane (GBM) antibody disease (Goodpasture’s disease). This disease is 75% male-predominant and follows a flulike prodrome in 30% of patients. Pulmonary hemorrhage is the initial manifestation in 90%, and an abnormal urinalysis is found in 80%. An iron-deficiency anemia from sequestration of iron within pulmonary alveolar macrophages is commonly associated with the disease. The IgG antibodies reacting to a component of type IV collagen are found in linear deposits on the basement membrane of both alveoli and glomeruli and are circulating in 90% of cases.62 In the appropriate clinical setting, the presence of circulating anti-GBM antibodies is sufficient to make a diagnosis without biopsy and institute plasmapheresis with or without plasma exchange for severe pulmonary or renal disease. After initial stabilization, corticosteroids and immunosuppressive medications will usually prevent further antibody production. Treatment with rituximab is also an option for patients intolerant of or refractory to standard therapy.63
Alveolar hemorrhage is an unusual manifestation of Wegener’s granulomatosis.64 The classic triad of renal dysfunction, upper airway disease, and pulmonary infiltrates is present in less than 20% of patients at presentation; however, pulmonary infiltrates are present in 45%.65 The pulmonary findings are characterized by nodules that may cavitate, lobar infiltrates that are often transient, upper airway obstruction from the granulomatous inflammation that follows airway ulceration, prominent interstitial markings with or without hilar and mediastinal adenopathy, or alveolar hemorrhage. Antineutrophil antibodies against proteinase 3 in cytoplasmic granules (c-ANCA) are found in the serum of 85% to 90% of patients with active Wegener’s granulomatosis and are 97% specific for the diagnosis.66
Among the many causes of rapidly progressive crescentic glomerulonephritis (RPGN) are small-vessel vasculitides such as microscopic polyangiitis that are associated with pulmonary hemorrhage in a third of patients.67 Therapy of these vasculitides and Wegener’s granulomatosis usually includes corticosteroids and immunosuppressive therapy. In patients with life-threatening respiratory failure, extracorporeal membrane oxygenation (ECMO) has proved lifesaving in patients with ANCA-positive vasculitides and SLE awaiting onset of systemic therapy.68,69 Use of recombinant factor VIIa has also been reported in this setting.70,71
Immunocompromised Host
DAH complicates autologous and allogeneic bone marrow transplantation in up to 21% of cases.72,73 Risk factors include age younger than 40 years, the presence of underlying solid tumors, renal insufficiency, and severe mucositis. The typical presentation is characterized by onset near the time of leukocyte recovery and is heralded by high fever and diffuse pulmonary infiltrates that prompt BAL. Typically the lavage fluid appears progressively bloody over serial aliquots, and no pathogenic organisms are recovered on bacterial, fungal, or viral culture. Mortality rate has been reported from 80% to 100% despite aggressive supportive care72,74 but may be improved with corticosteroid therapy.75 The optimal dosage and duration of corticosteroid treatment remain controversial, but standard regimens include 1 g/day methylprednisolone administered for 3 days and thereafter tapered over 2 months.75 The use of recombinant factor VIIa in patients with life-threatening DAH refractory to steroids has been reported.71 More recent reports reveal a favorable prognosis in patients with early (first 30 days) versus late DAH and autologous versus allogeneic transplants with an overall mortality rate of 48%.73
A similar syndrome characterized by fever and pulmonary infiltrates has been noted in 5% of transplant patients, but hemoptysis is rare. Pulmonary capillaritis following lung transplant also can result in DAH. Hemoptysis is seen in up to 25% of cases, with fulminant respiratory failure seen in 18%.76 This form of acute allograft rejection appears less responsive to corticosteroid therapy than acute lung rejection but has a more favorable response to plasmapheresis. No long-term adverse effects on allograft function are apparent.
Bleeding Diathesis
Leukemia patients may be particularly susceptible to DAH when chemotherapy-induced thrombocytopenia is combined with diffuse alveolar damage from other causes. Viral infections, sepsis, radiation, chemotherapy agents with pulmonary toxicity, and leukostasis from blast counts exceeding 80,000 cells/mm3 may all produce diffuse alveolar damage in leukemia.77 Therapy is directed toward correction of thrombocytopenia and supportive care of lung injury.
Drug-Induced Alveolar Hemorrhage
Hemoptysis due to drug-induced alveolar hemorrhage is rare and may result from therapeutic medications, illicit drugs, and other agents. Implicated agents include crack cocaine,78 amiodarone,79 nitrofurantoin,80 D-penicillamine,81 retinoic acid,82 propylthiouracil,83 infliximab,84 inhaled resins containing trimellitic anhydride,85 and various chemotherapeutic agents.86–89
Vascular Abnormalities
Almost all blood vessels that course through the thoracic cavity have been associated with fistula formation to an airway with resultant hemoptysis. Often this occurs in the setting of endovascular infection, inflammation, congenital or acquired stenoses, aneurysms of these vessels, or chest surgery. Some of the rare vascular-to-airway fistulas that have been described include (1) carotid artery to trachea in a patient with occult laryngeal cancer90; (2) various abdominal arterial supplies to pulmonary sequestrations91; (3) syphilitic aneurysms of the ascending aorta and other thoracic arteries to pulmonary parenchyma92; (4) coronary artery bypass grafts to pulmonary parenchyma or bronchial artery93,94; (5) splenopulmonary shunt in portal hypertension following splenectomy95; (6) left ventricular pseudoaneurysms96 to pulmonary parenchyma; and (7) vena caval–bronchial fistulas.97 Several of the more common bronchovascular communications deserve comment.
Pulmonary Artery Aneurysms
Aneurysms of the pulmonary artery remain rare causes of hemoptysis.98 Mycotic aneurysms are commonly caused by Mycobacterium tuberculosis, syphilis, S. aureus, and streptococcal species. Poststenotic dilation may occur in congenital pulmonary artery strictures. Structural vascular abnormalities such as those found in Marfan syndrome can also affect the pulmonary arteries.
Behçet’s disease, characterized by oral ulcers, uveitis, arthritis, and cutaneous vasculitis, is the only common large-vessel vasculitis that affects the pulmonary arteries.99 These multiple aneurysms may resolve with high-dose corticosteroid therapy or cyclophosphamide.100,101
An idiopathic syndrome characterized by fatal hemoptysis from pulmonary artery aneurysms, associated with fever and recurrent superficial and deep venous thromboembolism, was originally reported in 1959 by Hughes and Stovin.102 Although infection and angiodysplasia have been proposed as possible causes of the aneurysms, the current consensus is that vasculitis is the primary process.103
Arteriovenous Malformations
Pulmonary arteriovenous malformations (PAVMs) present with progressive hypoxemia, paradoxical emboli, or bleeding complications including hemoptysis or hemothorax.104 Although the majority of these lesions are likely congenital telangiectasias that enlarged over years,105 acquired arteriovenous malformations (AVMs) have been noted after chest surgery and trauma and have been associated with actinomycosis, schistosomiasis, cirrhosis, and metastatic carcinoma.106 The hereditary Osler-Weber-Rendu disease (OWR) is associated with hemorrhagic telangiectasias in many organ systems. Approximately 15% of OWR patients have pulmonary arteriovenous aneurysms,107 and up to 36% of patients with a single PAVM and 57% of patients with multiple PAVMs have OWR.108 Bronchial artery telangiectasias with bleeding109 have also been described, although the pathogenic relationship to pulmonary artery telangiectasias remains speculative. Treatment of PAVMs, particularly if hemoptysis has developed, is to obliterate the lesion with BAE.110
Trauma
Hemoptysis following major trauma requires emergent thoracic surgical consultation and management. Although some cases will be simple lung contusions that manifest as a focal radiographic abnormality on chest radiograph with blood-streaked sputum present, approximately 15% of thoracic trauma victims with hemoptysis need early exploration. The majority of cases with hemoptysis need bronchoscopy to localize bleeding and exclude a tracheobronchial rupture, which can be clinically silent for weeks. The most common reason for emergent thoracotomy remains pulmonary hemorrhage. Pneumonorrhaphy (suture repair of the lung) is preferred for minor injuries; lobectomy and pneumonectomy, performed for more severe injuries, carry mortality rates of 55% and 89%, respectively.111
Vascular Monitoring Catheters
Use of pulmonary artery catheters has decreased significantly and complications causing hemoptysis occur rarely. Pulmonary artery catheters should be inflated in the proximal pulmonary circulation for 20 seconds or less to obtain pulmonary artery occlusion pressures. More distal and prolonged inflation can cause fatal pulmonary artery dissection, pseudoaneurysm formation, or pulmonary artery rupture.112,113 Endovascular damage may predispose to thrombus formation and pulmonary infarction. Preventive measures include placement of the catheter at an insertion distance when full inflation is required to obtain an occlusion pressure, slowly inflating the balloon (never inflating against resistance), full inflation of the balloon to prevent the catheter tip from projecting beyond the balloon, and daily monitoring of catheter position with chest radiography.
If hemoptysis occurs with a pulmonary artery catheter in place, rapid diagnosis and treatment are required. Risk factors for pulmonary artery rupture include concomitant anticoagulation, cardiopulmonary bypass, balloon migration, hypothermia, advanced age, and pulmonary hypertension.113 Surgical resection of the involved lobe or angiographic ablation of the involved pulmonary artery has been successful in decreasing the incidence of recurrent and often fatal hemoptysis. Other successful interventions for acute conditions have included proximal reinflation of the pulmonary artery catheter to stop blood flow to the pulmonary artery segment that is bleeding; high levels (18 mm Hg) of positive end-expiratory pressure to decrease the pulmonary artery to bronchial pressure gradient; resumption of cardiopulmonary bypass for patients in cardiac surgery; and operative banding of the pulmonary artery, which can be unclamped 48 hours later.113,114
Management
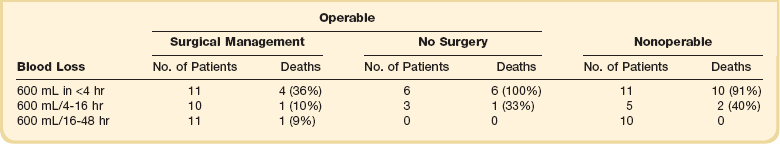
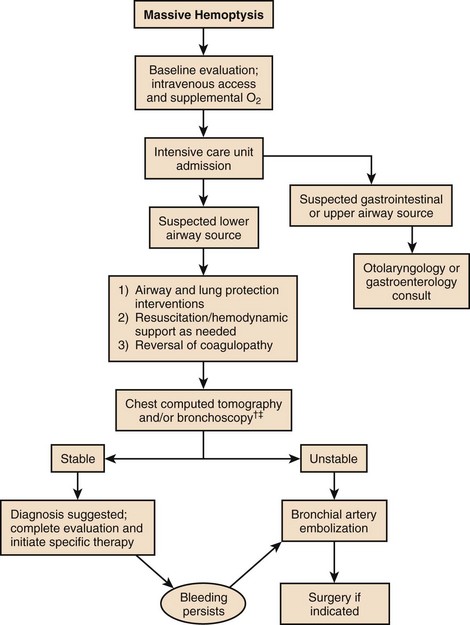
Massive hemoptysis should be managed within a framework of expeditious therapy to stabilize and resuscitate the patient. Figure 46.1 is a suggested algorithm for management. A multidisciplinary collaborative approach involving intensivists, pulmonologists, interventional radiologists, and thoracic surgeons is optimal to improve outcomes.7 The urgency and aggressiveness of management are influenced by the rate of bleeding. The study by Crocco and colleagues6 (Table 46.1) demonstrated that the incidence of death was 71% in patients with 600 mL of hemoptysis in less than 4 hours, compared with 22% and 5% mortality rate if 600 mL of hemoptysis occurred in 4 to 16 hours and 16 to 48 hours, respectively. Sputum containers should be placed at the bedside of patients who are not intubated to allow measurement of blood loss and an estimate of the bleeding rate. High bleeding rates, hemodynamic instability, and severe oxygenation failure signal the need for rapid evaluation and treatment. A critical care setting is optimal for patients with massive hemoptysis.
Airway and Lung Protection
Double-Lumen Endotracheal Tubes
Devices utilized for single-lung ventilation have also been applied to the management of hemoptysis.115 A double-lumen endotracheal tube is an alternative to a single-lumen endotracheal tube for airway management in massive hemoptysis. Successful placement of this type of endotracheal tube requires training and experience.116 The independent isolation of each mainstem bronchus allows for single-lung ventilation and isolation of the unaffected lung from blood contamination when bleeding is localized to one lung. The smaller suction ports of each independent lumen can cause difficulty in suctioning blood. Endobronchial evaluation requires a pediatric bronchoscope or double-lumen tube removal once bleeding has been controlled. Proximal airway masses may preclude placement of a double-lumen endotracheal tube.
Localization of Bleeding
Localization of bleeding facilitates diagnostic and therapeutic efforts regardless of the amount of hemoptysis. Because blood in the mouth can originate from the gastrointestinal tract or from diverse sites in the sinuses, nasal airway, or upper airway proximal to the larynx, an initial evaluation is needed to confirm that bleeding is from the lung. One series of hemoptysis patients found an upper airway source of bleeding in 10%.117 The characteristics of the expectorated blood and clinical presentation of the patient often help in differentiating hemoptysis from upper airway or gastrointestinal sources (Table 46.2). A pH assessment of the blood (low pH expected in gastric hemorrhage) and observation of expectorated sputum can be performed at the bedside.
Table 46.2
Features of Hemoptysis and Patient Presentation
| Hemoptysis Feature | Clinical Presentation |
| Blood usually bright red | Often dyspneic |
| Portion of blood usually frothy Alkaline pH |
Hypoxemia |
| Blood usually mixed with sputum | Preceding cough common |
| Alveolar macrophages may be present in sputum smear | Anemia and melena uncommon |
Chest Computed Tomography
The importance of chest CT in the diagnosis of acute hemoptysis and localization of bleeding has increased with advances in technology. In a series of 80 patients with massive hemoptysis, emergency high-resolution computed tomography (HRCT) was not only equivalent to bronchoscopy in localizing bleeding (70% vs. 73%), but it was more efficient than bronchoscopy for identifying the cause of bleeding (77% vs. 8%). Findings on HRCT also directly affected treatment in more than 30% of patients in this study.118 HRCT also allows the adequate prediction of nonbronchial systemic arterial supply, which can be the cause of bleeding in massive hemoptysis and a significant cause of recurrent bleeding after successful BAE.119 Contrast enhancement increases the yield by identifying vascular abnormalities (i.e., thoracic aneurysm or AVMs) that would allow for more timely surgical referral.
Use of multidetector CT angiography to visualize the bronchial and nonbronchial systemic vasculature has been found to increase the number of pulmonary artery vaso-occlusions and reduce the number of urgent surgical resections compared to single-detector helical CT.120 In CF patients with massive hemoptysis, electrocardiographically synchronized, prospectively triggered multidetector CT angiography of the aorta accurately predicted the location of ectopic bronchial arteries. The use of this technique was felt to decrease the BAE radiation dose and contrast volume and likely reduced table time compared to a conventional complete aortogram.121
Bronchoscopy
Although the diagnostic yield of bronchoscopy remains low in hemoptysis due to causes other than endobronchial carcinoma, it remains a vital tool in the management of acute massive hemoptysis in unstable patients. Localization of bleeding can facilitate immediate interventions for hemorrhage control including appropriate patient positioning, selective intubation, endobronchial tamponade, endobronchial infusions, laser photocoagulation, and guidance for BAE or surgical resection. Recent studies, however, have suggested that patients with lateralizing radiography and known causation who are candidates for BAE do not need prior bronchoscopy unless bronchoscopic airway management is necessary.20,122,123 This strategy can avoid delays in definitive therapy, reduce cost, and avoid the risk of airway compromise from sedation associated with bronchoscopy.
Hemorrhage Control
Expectant Therapy
The additional factor that must be considered in cough suppression is the necessary removal of blood clots that can cause endobronchial obstruction. Particularly when a central airway lesion is responsible for bleeding, the suctioning necessary to remove blood clots may be associated with rebleeding and perpetuation of a vicious cycle. Blood clots left unsuctioned, however, may cause atelectasis, which is detrimental to patient weaning from the ventilator. Although endobronchial streptokinase (1000 IU/mL; total dose 30,000 to 80,000 IU) has been used for dissolution of central blood clots,124 airway stabilization can usually be obtained with serial bronchoscopies and suctioning alone.
Endobronchial Tamponade
Acute lung bleeding may be amenable to control by endobronchial tamponade with balloon-tipped devices known as bronchial blockers.125–128 Several types of devices are available: Fogarty vascular embolectomy catheter, a single-lumen endotracheal tube with an enclosed moveable bronchial blocker, and a wire-guided bronchial blocker.129 Regardless of the selected device, experience is required for successful placement and safe utilization.116 The blocker is directed to the bleeding bronchus under guidance of fiberoptic bronchoscopy. By advancing the blocker to the smallest subsegment to which bleeding can be visualized, bleeding can be contained and diagnostic workup can continue. Other balloon catheters can also be used for this purpose,130 although they must be carried to the bleeding site on the outside of the bronchoscope by the bronchoscopic shuttle technique.131 Multiple catheters can be placed if hemoptysis is multifocal and catheters can be left in place for 24 to 48 hours until bleeding is controlled.128
Endobronchial Infusions
Although a variety of agents have been infused into the airway through the bronchoscope to control bleeding, no studies directly compare the agents with each other or with other modalities of therapy. Nevertheless, the potential advantage of these agents is their administration during bronchoscopy for localization of bleeding. Thrombin and fibrinogen-thrombin mixtures have been used in some sites to provide a hemostatic clot in the area of bleeding with good success.132,133 Commercial fibrinogen is not available in the United States for patient use. Oxidized regenerated cellulose mesh, a biodegradable cellulose fabric, is an alternative procoagulant used in patients with massive hemoptysis and showed a 98% success rate in a series of 57 patients.134 Once deployed in the area of hemorrhage, it absorbs blood, swelling into a gelatinous mass that promotes tamponade and coagulation. Endobronchial sealing with n-butyl cyanoacrylate, a biocompatible glue with prothrombotic properties, has also been used with success in small case series.135
Topical vasoconstrictors including iced saline,136 epinephrine,137 and vasopressin or vasopressin derivatives138,139 have also been used effectively for airway bleeding in anecdotal reports. The likely mechanism is vasoconstriction of bronchial arteries. These may be a safe, effective alternative in patients without access to BAE or surgery, or in unstable patients in need of a temporizing intervention until definitive therapy is available.
Laser Photocoagulation
The neodymium : yttrium aluminum garnet (Nd : YAG) or argon plasma laser has been used successfully for airway carcinoma with persistent hemoptysis. Recognizing that carcinomatous bleeding is usually progressive and can be life threatening, aggressive photocoagulation of the endobronchial site may provide the only possibility for palliation after chemotherapy and radiation have been exhausted. Success has been reported in approximately 60% of cancer patients with hemoptysis.140 Appropriate training, however, is imperative to ensure appropriate patient selection and to avoid catastrophic complications such as tracheal fire or vessel perforation.141
Bronchial Artery Embolization
Bronchial artery embolization (BAE) was first described in 1974 to control massive hemoptysis in the nonsurgical patient.142 Subsequent studies on safety and efficacy have confirmed immediate, safe control of hemoptysis in 79% to 100% of a variety of patients, resulting in early use of BAE in the management strategy.143–151 The technique for BAE localizes the bronchial arteries supplying the lobe that is bleeding. A formal bronchial arteriogram is performed to ensure that there is no communication to the anterior spinal artery, to determine whether a vascular pathologic condition is present, and to ensure that the bleeding area of lung parenchyma is served by the vessel. Only rarely will vascular extravasation indicative of bleeding be observed, usually in massively bleeding patients. Angiographic signs that suggest a source of bleeding include hypertrophied/enlarged/tortuous bronchial arteries with parenchymal hypervascularity, bronchial artery aneurysms, and bronchial artery to pulmonary vein or pulmonary artery shunting.152 Angiographic technique usually begins with injection of contrast agent in the descending aorta just below the left subclavian artery to identify bronchial arteries supplying the majority of the lung and phrenic arteries supplying the lung bases. Identification of anomalous origins of the bronchial arteries may require a full arch aortogram in some patients.153 For pathologic conditions of the lower lung, a selective phrenic artery injection is used if the entire lung is not visualized by bronchial injections. Rarely, these lower injections may demonstrate a pulmonary sequestration. For pathologic conditions of the upper lung zone, a unilateral subclavian artery injection is done to exclude nonbronchial systemic collateral arteries.
No studies have evaluated the optimal embolization material for control of hemoptysis. Gelatin sponge particles, polyvinyl alcohol particles, or liquid polymers (e.g., n-butyl cyanoacrylate) with a predetermined polymerization time have all been used with success.154 Velour, polyurethane particles of varying size, metal coils, protein macroaggregates, and fibrinogen-thrombin mixtures155 have also been instilled. Combinations of materials are used in a significant number of patients.150,156 Liquid sclerosants, such as absolute alcohol or Gelfoam powder, should be used with caution because they may pass into the smallest vessels at the bronchial surface, producing bronchial necrosis.157
Significant complications of BAE are rare when appropriate technique is employed. Chest pain, dysphagia, and fever are the most common complications. The most devastating complication is spinal cord infarction resulting from embolization of the anterior spinal artery, which arises from the bronchial artery circulation in approximately 5% of normal patients. However, with proliferation of the bronchial artery circulation, as occurs in CF, communication to the anterior spinal artery may be found in up to 55% of cases.158 By performing high-quality bronchial arteriograms to define vascular anatomy and avoiding the anterior spinal artery by wedging the angiography catheter distal to its takeoff (or by avoiding the vessel altogether), a safe procedure can almost always be ensured. The recent introduction of microcatheter technology (i.e., “superselective” catheterization) allows achievement of a more distal, safe catheter position and has greatly reduced the number of aborted procedures and complications arising from anterior spinal artery embolization.
Recurrent hemoptysis is common after BAE and can occur immediately, in the first several weeks to months (early), or after several years (late). Immediate recurrence of hemoptysis in the first few days after BAE may occur in approximately 10% of patients144 and may be due to several causes. For the patient who continues to bleed during the procedure, the appropriate blood vessel usually has not been embolized. Other common causes include bleeding from nonbronchial systemic or pulmonary vessels, particularly if not evaluated initially, and lysis of the hemostatic plug in the embolized bronchial artery. Recurrence of hemoptysis in the first several months after BAE may be related to incomplete embolization, although later recurrence at 1 to 2 years may be related to neovascularization or recanalization related to inflammation or disease progression.159 Reporting of outcomes varies considerably in BAE studies but early recurrence of bleeding may occur in 3.5% to 26% of patients.145,147,149,150 Late recurrence of bleeding may occur in 10% to 40% of patients.143,145,146,149,150 The incidence of recurrence is not uniform in all lung diseases, and higher recurrence rates are noted in Aspergillus fungus balls, bronchiectasis, tuberculosis, and lung cancers.* Use of superselective embolization, which provides less of a stimulus for neovascularization, may improve recurrence rates.161 BAE can be considered a palliative intervention for the acute cessation of massive hemoptysis that allows for a more controlled evaluation of the patient for potentially curative interventions.
Surgery
A significant question in control of massive hemoptysis is whether conservative management techniques including endobronchial tamponade and BAE are better than emergent surgery in improving outcomes. Given the high success rates of BAE and low associated morbidity and mortality rates, more conservative techniques are now considered first-line therapy over emergent surgery. The proportion of patients with massive hemoptysis who undergo emergent or urgent surgery often is not reported, but recent studies found 12% to 14% of patients required surgical intervention.151,162 Although perioperative and hospital mortality rates were high in past experience, more recent studies report a mortality rate of less than 15%.162–165 The outcome of surgery is likely influenced by the cause and severity of hemoptysis, patient comorbid conditions, extent of lung resection, and experience of the surgical team. Absolute indications for surgery do not exist, but patients with vascular disruptions (e.g., leaking aortic aneurysm, AVMs, pulmonary artery rupture, chest trauma); focal fungal disease, tuberculosis, or bronchiectasis; or failed BAE or early recurrence of bleeding after BAE should be considered for urgent surgery in the setting of massive hemoptysis.148,160 Mycetomas, active tuberculosis, and bronchiectasis represent a large majority of the causes of hemoptysis in surgical reports.
Patients with fibrosis and adhesions between the lung and chest wall, commonly seen in tuberculosis, fungal disease, and bronchiectasis, have significant surgical risks because they often require pneumonectomy. Physiologic lung exclusion, in which the bronchus and pulmonary artery of the involved lobe or lung are surgically interrupted, leaving the pulmonary veins intact, appears to be a viable alternative in such patients. In a series of 20 patients, Dhaliwal and colleagues166 reported control of bleeding in all patients with no fatality and no significant morbidity. Video-assisted thoracoscopic procedures may be successful in some patients.167
Other Therapies
As new devices and interventions are developed for the management of other pulmonary and nonpulmonary conditions, some may be adapted to treat massive hemoptysis. Covered self-expanding stents have been used to occlude a bleeding site in a patient not treatable by BAE or surgery.168,169 Recombinant activated factor VII has been used to control hemoptysis in DAH70,170 and focal bleeding associated with community-acquired pneumonia,171 thoracic trauma,172 and CF.173 Recombinant activated factor VII is not approved for use in hemoptysis, and clinicians must weigh adverse effects, thrombotic risk, and availability of other interventions when considering this treatment option.
References
1. Jean-Baptiste, E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000; 28:1642.
2. Sakr, L, Dutau, H. Massive hemoptysis: An update on the role of bronchoscopy in diagnosis and management. Respiration. 2010; 80:38.
3. Hirshberg, B, Biran, I, Glazer, M, et al. Hemoptysis: Etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997; 112:440.
4. Fidan, A, Ozdogan, S, Oruc, O, et al. Hemoptysis: A retrospective analysis of 108 cases. Respir Med. 2002; 96:677–680.
5. Uzun, O, Atasoy, Y, Findik, S, et al. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Resp J. 2010; 4:131.
6. Crocco, JA, Rooney, JJ, Fankushen, DS, et al. Massive hemoptysis. Arch Intern Med. 1968; 121:495.
7. Shigemura, N, Wan, IY, Yu, SCH, et al. Multidisciplinary management of life-threatening massive hemoptysis: A 10-year experience. Ann Thorac Surg. 2009; 87:849.
8. Khalil, A, Parrot, A, Nedelcu, C, et al. Severe hemoptysis of pulmonary arterial origin: Signs and role of multidetector row CT angiography. Chest. 2008; 133:212.
9. Remy, J, Remy-Jardin, M, Voisin, C. Endovascular management of bronchial bleeding. In: Butler J, ed. The Bronchial Circulation. New York: Marcel Dekker, 1992.
10. Liebow, AA. Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat. 1965; 117:19.
11. Katoh, O, Yamada, H, Hiura, K, et al. Bronchoscopic and angiographic comparison of bronchial arterial lesions in patients with hemoptysis. Chest. 1987; 91:486.
12. Charan, NB, Albert, RK, Lakshminarayan, S, et al. Factors affecting bronchial blood flow through bronchopulmonary anastomoses in dogs. Am Rev Respir Dis. 1986; 134:85.
13. Liebow, AA, Hales, MR, Lindskog, GF. Enlargement of the bronchial arteries and their anastomoses with the pulmonary arteries in bronchiectasis. Am J Pathol. 1949; 25:211.
14. Ullah, MI, Fegan, O. Potential hazard of nebulised salbutamol in patients with haemoptysis. Br Med J. 1983; 286:844.
15. McFadden, ER. Respiratory heat and water exchange; physiological and clinical implications. J Appl Physiol. 1983; 54:331.
16. Agostoni, P, Arena, V, Doria, E, et al. Inspired gas relative humidity affects systemic to pulmonary bronchial blood flow in humans. Chest. 1990; 97:1377.
17. Ferretti, GR, Arbib, F, Bertrand, B, et al. Haemoptysis associated with pulmonary varices: Demonstration using computed tomographic angiography. Eur Respir J. 1998; 12:989.
18. Umaya, T, Monden, Y, Harada, K, et al. Pulmonary varices: A case report and review of the literature. Jpn J Surg. 1988; 18:359.
19. Flume, PA, Yankaskas, JR, Ebeling, M, et al. Massive hemoptysis in cystic fibrosis. Chest. 2005; 128:729–738.
20. Flume, PA, Mogayzel, PJ, Robinson, KA, et al. Cystic fibrosis pulmonary guidelines, pulmonary complications: Hemoptysis and pneumothorax. Am J Resp Crit Care Med. 2010; 182:298.
21. Hamilton, W, Peters, TJ, Round, A, et al. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax. 2005; 60:1059–1065.
22. Miller, RR, McGregor, DH. Hemorrhage from carcinoma of the lung. Cancer. 1980; 46:200.
23. Panos, RJ, Barr, LF, Walsh, TJ, Silberman, HJ. Factors associated with fatal hemoptysis in cancer patients. Chest. 1988; 94:1008.
24. Palvio, DH, Paulsen, SM. Primary angiosarcoma of the lung presenting as intractable hemoptysis. Thorac Cardiovasc Surg. 1987; 35:105.
25. Hara, R, Itami, J, Aruga, T, et al. Risk factors for massive hemoptysis after endobronchial brachytherapy in patients with tracheobronchial malignancies. Cancer. 2001; 92:2623.
26. Nour-Eldin, NA, Naguib, NNN, Mack, M, et al. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011; 21:197.
27. Yamakado, K, Takaki, H, Takao, M, et al. Massive hemoptysis from pulmonary artery pseudoaneurysm caused by lung radiofrequency ablation: Successful treatment by coil embolization. Cardiovasc Intervent Radiol. 2010; 33:410.
28. Rogol, PR. Fatal hemoptysis due to lung abscess and pulmoaortic fistula. Chest. 1988; 94:441.
29. Spark, RP, Sobonya, RE, Armbruster, RJ, et al. Pathologic bronchial vasculature in a case of massive hemoptysis due to chronic bronchitis. Chest. 1991; 99:504.
30. Hamer, DH, Schwab, LE, Gray, R. Massive hemoptysis from thoracic actinomycosis successfully treated by embolization. Chest. 1992; 101:1442.
31. Zimmerman, JL, Dellinger, RP. Septic pulmonary emboli in the intravenous substance abuser. Probl Critical Care. 1987; 1:1.
32. Renie, WA, Rodeheffer, RJ, Mitchell, S, et al. Balloon embolization of a mycotic pulmonary artery aneurysm. Am Rev Respir Dis. 1982; 126:1107.
33. Pittokopitis, K, Herriott, DT, Shirey, JK. Massive fatal hemoptysis secondary to invasive aspergillosis in a patient with COPD. Chest. 1983; 83:583.
34. Albeda, SM, Talbot, GH, Gerson, SC, et al. Pulmonary cavitation and massive hemoptysis in invasive pulmonary aspergillosis. Am Rev Respir Dis. 1985; 131:115.
35. Kibbler, CC, Milkins, SR, Bhamra, A, et al. Apparent pulmonary mycetoma following invasive aspergillosis in neutropenic patients. Thorax. 1988; 43:108.
36. Wong, K, Waters, CM, Walesby, RK. Surgical management of invasive pulmonary aspergillosis in immunocompromised patients. Eur J Cardiothorac Surg. 1992; 6:18.
37. Pedhorecky, I, Urschel, J, Anderson, T. Resection of invasive pulmonary aspergillosis in immunocompromised patients. Ann Surg Oncol. 2000; 7:312.
38. Watts, WJ. Bronchopleural fistula followed by massive fatal hemoptysis in a patient with pulmonary mucormycosis. A case report. Arch Intern Med. 1983; 143:1029.
39. Razaque, MA, Mutum, SS, Singh, TS. Recurrent haemoptysis? Think of paragonimiasis. Trop Doct. 1991; 21:153.
40. Tekinbas, C, Turedi, S, Gunduz, A, Erol, MM. Hydatid cyst disease of the lung as an unusual cause of massive hemoptysis: A case report. J Med Case Rep. 2009; 3:21.
41. Rafferty, P, Biggs, BA, Crompton, GK, et al. What happens to patients with pulmonary aspergilloma? Analysis of 23 cases. Thorax. 1983; 38:579.
42. Shale, J, Faux, JA, Lane, DJ. Trial of ketoconazole in non-invasive aspergillosis. Thorax. 1987; 42:26.
43. Jennings, TS, Hardin, TC. Treatment of aspergillosis with itraconazole. Ann Pharmacother. 1993; 27:1206–1211.
44. Cochrane, LJ, Morano, JU, Norman, JR, et al. Use of intracavitary amphotericin B in a patient with aspergilloma and recurrent hemoptysis. Am J Med. 1991; 90:654.
45. Rumbak, M, Kohler, G, Eastrige, C, et al. Topical treatment of life threatening haemoptysis from aspergillomas. Thorax. 1996; 51:253–255.
46. Guleria, R, Gupta, D, Jindal, SK. Treatment of pulmonary aspergilloma by endoscopic intracavitary instillation of ketoconazole. Chest. 1993; 103:1301.
47. Hamamoto, T, Watanabe, K, Ikemoto, H. Endobronchial miconazole for pulmonary aspergilloma. Ann Intern Med. 1983; 98:1030.
48. Hargis, JL, Bone, RC, Stewart, J, et al. Intracavitary amphotericin B in the symptomatic pulmonary aspergilloma. Am J Med. 1980; 68:389.
49. Yamada, H, Kohno, S, Koga, H, et al. Topical treatment of pulmonary aspergilloma by antifungals. Chest. 1993; 103:1421.
50. Falkson, C, Sur, R, Pacella, J. External beam radiotherapy: A treatment option for massive haemoptysis caused by mycetoma. Clin Oncol. 2002; 14:233–235.
51. Shiraishi, Y, Katsuragi, N, Nakajima, Y, et al. Pneumonectomy for complex aspergilloma: Is it still dangerous? Eur J Cardiothorac Surg. 2006; 29:9–13.
52. Scarlat, A, Bodner, G, Liron, M. Massive haemoptysis as the presenting symptom in mitral stenosis. Thorax. 1986; 41:413.
53. Hicks, GL, Jr. Fibrosing mediastinitis causing pulmonary artery and vein obstruction with hemoptysis. N Y State J Med. 1983; 83:242.
54. Cohn, RC, Wong, R, Spohn, WA, et al. Death due to diffuse alveolar hemorrhage in a child with pulmonary veno-occlusive disease. Chest. 1991; 100:1456.
55. Reid, JM, Jamieson, MP, Cowan, MD. Unilateral pulmonary vein stenosis. Br Heart J. 1986; 55:599.
56. Taylor, JR, Ryu, J, Colby, TV, et al. Lymphangioleiomyomatosis: Clinical course in 32 patients. N Engl J Med. 1990; 323:1254.
57. Olson, EJ, Utz, JP, Prakash, UB. Therapeutic bronchoscopy in broncholithiasis. Am J Respir Crit Care Med. 1999; 160:766.
58. Potaris, K, Miller, DL, Trastek, VF, et al. Role of surgical resection in broncholithiasis. Ann Thorac Surg. 2000; 70:248.
59. Lara, AR, Schwarz, MI. Diffuse alveolar hemorrhage. Chest. 2010; 137:1164.
60. Kahn, FW, Jones, JM, England, DM. Diagnosis of pulmonary hemorrhage in the immunocompromised host. Am Rev Respir Dis. 1987; 136:155.
61. Sherman, JM, Winnie, G, Thomassen, MJ, et al. Time course of hemosiderin production and clearance by human pulmonary macrophages. Chest. 1984; 86:409–411.
62. Wilson, CB, Dixon, FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973; 3:74.
63. Arzoo, K, Sadeghi, S, Liebman, HA. Treatment of refractory antibody mediated autoimmune disorders with an anti-CD20 monoclonal antibody (rituximab). Ann Rheum Dis. 2002; 61:922–924.
64. Myers, JL, Katzenstein, AL. Wegener’s granulomatosis presenting with massive pulmonary hemorrhage and capillaritis. Am J Surg Pathol. 1987; 11:895.
65. Hoffman, GS, Kerr, GS, Leavitt, RY, et al. Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med. 1992; 116:488.
66. Kallenberg, CGM, Mulder, AHL, Tervaert, JWC. Antineutrophil cytoplasmic antibodies: A still-growing class of autoantibodies in inflammatory disorders. Am J Med. 1992; 93:675.
67. Savage, COS, Winearls, CG, Evans, DJ, et al. Microscopic polyarteritis: Presentation, pathology and prognosis. Q J Med. 1985; 56:467.
68. Ahmed, SH, Aziz, T, Cochran, J, et al. Use of extracorporeal membrane oxygenation in a patient with diffuse alveolar hemorrhage. Chest. 2004; 126:305–309.
69. Tandon, M, Reynolds, HN, Borg, U, et al. Life-threatening acute systemic lupus erythematosus: Survival after multiple extracorporeal modalities: A place for the multipotential extracorporeal service. ASAIO J. 2000; 46:146–149.
70. Henke, D, Falk, RJ, Gabriel, DA. Successful treatment of diffuse alveolar hemorrhage with activated factor VII. Ann Intern Med. 2004; 140:493–494.
71. Pastores, SM, Papadopoulos, E, Voigt, L, et al. Diffuse alveolar hemorrhage after allogeneic hemopoietic stem cell transplantation: Treatment with recombinant factor VIIa. Chest. 2003; 124:2400.
72. Robbins, RA, Linder, J, Stahl, MG, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. 1989; 87:511.
73. Afessa, B, Tefferi, A, Litzow, MR, et al. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002; 166:1364–1368.
74. Jules-Elysee, K, Gulati, S, Stover, DE, et al. Pulmonary complications in autologous bone marrow transplantation. Am Rev Respir Dis. 1990; 141:A604.
75. Chao, NJ, Duncan, SR, Long, GD, et al. Corticosteroid therapy for diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Ann Intern Med. 1991; 114:145.
76. Astor, TL, Weill, D, Cool, C, et al. Pulmonary capillaritis in lung transplant recipients: Treatment and effect on allograft function. J Heart Lung Transplant. 2005; 24:2091–2097.
77. Smith, LJ, Katzenstein, AA. Pathogenesis of massive pulmonary hemorrhage in acute leukemia. Arch Intern Med. 1982; 142:2149.
78. Forrester, JW, Steel, AW, Waldron, JA, et al. Crack lung: An acute pulmonary syndrome with a spectrum of clinical and histopathologic findings. Am Rev Respir Dis. 1990; 142:462.
79. Ravishankar, R, Samuels, LE, Kaufman, MS, et al. Amiodarone-associated hemoptysis. Am J Med Sci. 1998; 316:390.
80. Bucknall, CE, Adamson, MR, Banham, SW. Non fatal pulmonary haemorrhage associated with nitrofurantoin. Thorax. 1987; 42:475.
81. Sternlieb, I, Bennet, B, Scheinberg, IW. D-Penicillamine-induced Goodpasture’s syndrome in Wilson’s disease. Ann Intern Med. 1975; 83:673.
82. Nicolls, MR, Terada, LS, Tuder, RM, et al. Diffuse alveolar hemorrhage with underlying pulmonary capillaritis in the retinoic acid syndrome. Am J Respir Crit Care Med. 1998; 158:1302.
83. Nakamori, Y, Tominaga, T, Inoue, Y, et al. Propylthiouracil (PTU)-induced vasculitis associated with antineutrophil antibody against myeloperoxidase (MPO-ANCA). Intern Med. 2003; 42:529–533.
84. Panagi, S, Palka, W, Korelitz, BI, et al. Diffuse alveolar hemorrhage after infliximab treatment of Crohn’s disease. Inflamm Bowel Dis. 2004; 10:274–277.
85. Herbert, FA, Orford, R. Pulmonary hemorrhage and edema due to inhalation of resins containing trimellitic anhydride. Chest. 1979; 76:546.
86. Carron, PL, Cousin, L, Caps, T, et al. Gemcitabine-associated diffuse alveolar hemorrhage. Intensive Care Med. 2001; 27:1554.
87. de Gramont, A, Van Cutsem, E. Investigating the potential of bevacizumab in other indications: Metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology. 2005; 69(Suppl 3):46–56.
88. Tammaro, KA, Baldwin, PD, Lundberg, AS. Interstitial lung disease following erlotinib (Tarceva) in a patient who previously tolerated gefitinib (Iressa). J Oncol Pharm Pract. 2005; 11:127–130.
89. Vlahakis, NE, Rickman, OB, Morgenthaler, T. Sirolimus-associated diffuse alveolar hemorrhage. Mayo Clin Proc. 2004; 79:541–545.
90. Dellinger, RP, Savage, PJ, Carruth, C, et al. Tracheocarotid fistula secondary to laryngeal carcinoma presenting as massive hemoptysis. Chest. 1983; 84:222.
91. Hayakawa, K, Soga, T, Hamamoto, K, et al. Massive hemoptysis from a pulmonary sequestration controlled by embolization of aberrant pulmonary arteries: Case report. Cardiovasc Intervent Radiol. 1991; 14:345.
92. Boundy, K, Bignold, LP. Syphilitic aneurysm of the right subclavian artery presenting with hemoptysis. Aust N Z J Med. 1987; 17:533.
93. Belcher, E, Townsend, E, De Robertis, F. Saphenous vein graft bronchopulmonary fistula after coronary artery bypass grafting presenting as cough and subsequent massive hemoptysis. J Thorac Cardiovasc Surg. 2012; 143:e14.
94. Yoon, JY, Jeon, EY, Lee, IJ, Koh, SH. Coronary to bronchial artery fistula causing massive hemoptysis in patients with longstanding pulmonary tuberculosis. Korean J Radiol. 2012; 13:102.
95. Escoffier, JM, Le Treut, YP, Antoni, M, et al. Severe hemoptysis due to portal hypertension. Responsibility of acquired splenopulmonary shunt and treatment by proximal splenorenal anastomosis. Gastroenterol Clin Biol. 1991; 15:974.
96. Adkins, MS, Laub, GW, Pollock, SB, et al. Left ventricular pseudoaneurysm with hemoptysis. Ann Thorac Surg. 1991; 51:476.
97. Winkler, TR, Hanlin, RJ, Hinke, TD, et al. Unusual cause of hemoptysis: Hickman-induced cava-bronchial fistula. Chest. 1992; 102:1285.
98. Bartter, T, Irwin, RS, Nash, G. Aneurysms of the pulmonary arteries. Chest. 1988; 94:1065.
99. Cadman, EC, Lundberg, WB, Mitchell, MS. Pulmonary manifestations in Behçet’s syndrome. Arch Intern Med. 1976; 136:944.
100. Stricker, H, Malinverni, R. Multiple, large aneurysms of pulmonary arteries in Behçet’s disease. Arch Intern Med. 1989; 149:925.
101. Aktogu, S, Erer, OF, Urpek, G, et al. Multiple pulmonary arterial aneurysms in Behçet’s disease: Clinical and radiologic remission after cyclophosphamide and corticosteroid therapy. Respiration. 2002; 69:178–181.
102. Hughes, JP, Stovin, PGI. Segmental pulmonary artery aneurysms with peripheral venous thrombosis. Br J Dis Chest. 1959; 53:19.
103. Khalid, U, Saleem, T. Hughes-Stovin syndrome. Orphanet J Rare Dis. 2011; 6:15.
104. Swanson, KL, Prakash, UB, Stanson, AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience 1982-1997. Mayo Clin Proc. 1999; 74:671.
105. Teragaki, M, Akioka, K, Mitsutaka, Y, et al. Case report: Hereditary hemorrhagic telangiectasia with growing pulmonary arteriovenous fistulas followed for 24 years. Am J Med Sci. 1988; 195:545.
106. Prager, RL, Laws, KH, Bender, HW, Jr. Arteriovenous fistula of the lung. Ann Thorac Surg. 1983; 26:231.
107. Hodgson, CH, Burchell, HB, Good, CA, et al. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous fistula: Study of a large family. N Engl J Med. 1959; 26:625.
108. Bosher, LH, Jr., Blake, DA, Byrd, BR. An analysis of the pathologic anatomy of pulmonary arteriovenous aneurysms with particular reference to the applicability of local excision. Surgery. 1959; 45:91.
109. Lincoln, MJ, Shigeoka, JW. Pulmonary telangiectasia without hypoxemia. Chest. 1988; 93:1097.
110. Terry, PB, Barth, KH, Kaufman, SL, et al. Balloon embolization for treatment of pulmonary arteriovenous fistulas. N Engl J Med. 1980; 302:1189.
111. Thompson, DA, Rowlands, BJ, Walker, WD, et al. Urgent thoracotomy for pulmonary or tracheobronchial injury. J Trauma. 1988; 28:276.
112. Abreu, AR, Campos, MA, Krieger, BP. Pulmonary artery rupture induced by a pulmonary artery catheter: A case report and review of the literature. J Intensive Care Med. 2004; 19:91.
113. Coulter, TD, Weidemann, HP. Complications of hemodynamic monitoring. Clin Chest Med. 1999; 20:249.
114. Rice, PL, Pifarre, R, El-Etr, A, et al. Management of endobronchial hemorrhage during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1981; 81:800.
115. Campos, JH. Which device should be considered the best for lung isolation: Double-lumen endotracheal tube versus bronchial blockers. Curr Opin Anaesthesiol. 2007; 20:27.
116. Campos, JH, Hallam, EA, Van Natta, T, Kernstein, KH. Devices for lung isolation used by anesthesiologists with limited thoracic experience. Anesthesiology. 2006; 104:261.
117. DiLeo, MD, Amedee, RG, Butcher, RB. Hemoptysis and pseudohemoptysis: The patient expectorating blood. Ear Nose Throat J. 1995; 74:822.
118. Revel, MP, Fournier, LS, Hennebicque, AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? Am J Roentgenol. 2002; 179:1217–1224.
119. Yoon, W, Kim, YH, Kim, JK, et al. Massive hemoptysis: Prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003; 227:232–238.
120. Khalil, A, Fartoukh, M, Parrot, A, et al. Impact of MDCT angiography on the management of patients with hemoptysis. Am J Roentgenol. 2010; 195:772.
121. Hayes, D, Winkler, MA, Kirkby, S, et al. Preprocedural planning with prospectively triggered multidetector row CT angiography prior to bronchial artery embolization in cystic fibrosis patients with massive hemoptysis. Lung. 2012; 190:221.
122. Hsiao, EI, Kirsch, CM, Kagawa, FT, et al. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. Am J Roentgenol. 2001; 177:861–867.
123. Ramakantan, R, Bandekar, VG, Gandhi, MS, et al. Massive hemoptysis due to pulmonary tuberculosis: Control with bronchial artery embolization. Radiology. 1996; 200:691–694.
124. Maxwell, SL, Stauffer, JL. Endobronchial streptokinase for relief of tracheobronchial obstruction by blood clots. Chest. 1992; 101:1738.
125. Campos, JH. Which device should be considered the best for lung isolation: Double-lumen endotracheal tube versus bronchial blockers. Curr Opin Anaesthesiol. 2007; 20:27.
126. Murakawa, T, Ito, N, Fukami, T, et al. Application of lobe-selective bronchial blockade against airway bleeding. Asian Cardiovasc Thorac Ann. 2010; 18:485.
127. Spicek-Macan, J, Hodoba, N, Nikolic, I, et al. Exsanguinating tuberculosis-related hemoptysis: Bronchial blocker introduced through percutaneous tracheostomy. Minerva Anestesiol. 2009; 75:405.
128. Nishiumi, N, Nakagawa, T, Masuda, R, et al. Endobronchial bleeding associated with blunt chest trauma treated by bronchial occlusion with a univent. Ann Thorac Surg. 2008; 85:245.
129. Campos, JH. An update on bronchial blockers during lung separation techniques in adults. Anesth Analg. 2003; 97:1266.
130. Jolliet, P, Soccal, P, Chevrolet, J. Control of massive hemoptysis by endobronchial tamponade with a pulmonary artery balloon catheter. Crit Care Med. 1992; 20:1730.
131. Haruno, MM, Williams, JH. The flexible fiberoptic bronchoscopic shuttle. Chest. 1992; 102:944.
132. Tsukamoto, T, Sasaki, H, Nakamura, H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest. 1989; 96:473.
133. De Gracia, J, de la Rosa, D, Catalan, E, et al. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir Med. 2003; 97:790–795.
134. Valipour, A, Kreuzer, A, Koller, H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005; 127:2113–2118.
135. Bhattacharyya, P, Dutta A Samanta, AN, et al. New procedure: Bronchoscopic endobronchial sealing: A new mode of managing hemoptysis. Chest. 2002; 121:2066–2069.
136. Conlan, AA, Hurwitz, SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax. 1980; 35:901.
137. Dupree, H, Lewejohann, J, Gleiss, J, et al. Fiberoptic bronchoscopy of intubated patients with life threatening hemoptysis. World J Surg. 2001; 25:104–107.
138. Tuller, C, Tuller D Tamm, M, et al. Hemodynamic effects of endobronchial application of orinpressin versus terlipressin. Respiration. 2004; 71:397–401.
139. Breuer, H, Charchut, S, Worth, C. Endobronchial versus intravenous application of vasopressin derivative glypressin during diagnostic bronchoscopy. Eur Respir J. 1989; 2:225–228.
140. Hetzel, MR, Nixon, C, Edmondstone, WM, et al. Laser therapy in 100 tracheobronchial tumours. Thorax. 1985; 40:341.
141. Turner, JF, Jr., Wang, KP. Endobronchial laser therapy. Clin Chest Med. 1999; 20:107–122.
142. Remy, J, Viosin, C, Dupois, C, et al. Traitement des hemoptysies par embolisation de circulation systemique. Ann Radiol. 1974; 17:5.
143. Vidal, V, Therasse, E, Berthiaume, Y, et al. Bronchial artery embolization in adults with cystic fibrosis: Impact on the clinical course and survival. J Vasc Intervent Radiol. 2006; 17:953.
144. Ben Sarwar Zubairi, A, Tanveer-ul-Haq, Fatima, K, et al. Bronchial artery embolization in the treatment of massive hemoptysis. Saudi Med J. 2007; 28:1076.
145. Serasli, E, Kalpakidis, V, Iatrou, K, et al. Percutaneous bronchial artery embolization in the management of massive hemoptysis in chronic lung diseases. Immediate and long-term outcomes. Int Angiol. 2008; 27:319.
146. Slattery, MM, Keeling, AN, Lee, MJ. Outcome and complications of bronchial artery embolisation for life-threatening haemoptysis. Ir J Med Sci. 2009; 178:155.
147. Wang, GR, Ensor, JE, Gupta, S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: Utility and prognostic factors. J Vasc Intervent Radiol. 2009; 20:722.
148. Chun, J-Y, Belli, A-M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010; 20:558.
149. Daliri, A, Probst, NH, Jobst, B, et al. Bronchial artery embolization in patients with hemoptysis including follow-up. Acta Radiologica. 2011; 52:143.
150. Anuradha, C, Shyamkumar, NK, Vinu, M, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Intervent Radiol. 2012; 18:96.
151. Fartoukh, M, Khalil, A, Louis, L, et al. An integrated approach to diagnosis and management of severe haemoptysis in patients admitted to the intensive care unit: A case series from a referral center. Respir Res. 2007; 8:11.
152. Kalva, SP. Bronchial artery embolization. Tech Vasc Intervent Radiol. 2009; 12:130.
153. McPherson, S, Routh, WD, Nath, H, et al. Anomalous origin of bronchial arteries: Potential pitfall of embolotherapy for hemoptysis. J Vasc Intervent Radiol. 1990; 1:86.
154. Razavi, MK, Murphy, K. Embolization of bronchial arteries with n-butyl cyanoacrylate for management of massive hemoptysis: A technical review. Tech Vasc Intervent Radiol. 2007; 10:276.
155. Bense, L. Intrabronchial selective coagulative treatment of hemoptysis. Chest. 1990; 97:990.
156. Wang, GR, Ensor, JE, Gupta, S, et al. Bronchial artery embolization for management of hemoptysis in oncology patients: Utility and prognostic factors. J Vasc Intervent Radiol. 2009; 20:722.
157. Naar, CA, Soong, J, Clore, F, et al. Control of massive hemoptysis by bronchial artery embolization with absolute alcohol. Am J Roentgenol. 1983; 140:271.
158. Cohen, AM, Doershuk, CF, Stern, RC. Bronchial artery embolization to control hemoptysis in cystic fibrosis. Radiology. 1990; 175:401.
159. Hayakawa, K, Tanaka, F, Torizuka, T, et al. Bronchial artery embolization for hemoptysis: Immediate and long-term results. Cardiovasc Intervent Radiol. 1992; 15:154.
160. Kim, YG, Yoon, H-K, Ko, GY, et al. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Respirology. 2006; 11:776.
161. Tanaka, N, Yamakado, K, Murashima, S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Intervent Radiol. 1997; 8:65.
162. Andréjak, C, Parrott, A, Bazelly, B, et al. Surgical lung resection for severe hemoptysis. Ann Thorac Surg. 2009; 88:1556.
163. Metin, M, Sayar, A, Solak, O, et al. Emergency surgery for massive haemoptysis. Acta Chir Belg. 2005; 105:639.
164. Erdogan, A, Yegin, A, Gurses, G, Demircan, A. Surgical management of tuberculosis-related hemoptysis. Ann Thorac Surg. 2005; 79:299.
165. Brik, A, Salem, A-M, Shoukry, A, Shouman, W. Surgery for hemoptysis in various pulmonary tuberculous lesions: A prospective study. Interact Cardiovasc Thorac Surg. 2011; 13:276.
166. Dhaliwal, RS, Saxena, P, Puri, D, et al. Role of physiological lung exclusion in difficult lung resections for massive hemoptysis and other problems. Eur J Cardiothorac Surg. 2001; 20:25–29.
167. Parker, KL, Zervos, MD, Darvishian, F, Bizekis, CS. Video-assisted thoracoscopic lobectomy for pulmonary aspergilloma after life-threatening hemoptysis in a patient with lupus. Ann Thorac Surg. 2010; 89:291.
168. Brandes, JC, Schmidt, E, Yung, R. Occlusive endobronchial stent placement as a novel management approach to massive hemoptysis from lung cancer. J Thorac Oncol. 2008; 3:1071.
169. Chung, IH, Park, MH, Kim, DH, Jeon, GS. Endobronchial stent insertion to manage hemoptysis caused by lung cancer. J Korean Med Sci. 2010; 25:1253.
170. Meijer, K, de Graaff, W, Daenen, S, et al. Successful treatment of massive hemoptysis in acute leukemia with recombinant factor VIIa. Arch Intern Med. 2000; 160:2216.
171. Macdonald, JA, Fraser, JF, Foot, CL, Tran, K. Successful use of recombinant factor VII in massive hemoptysis due to community-acquired pneumonia. Chest. 2006; 130:577.
172. Tien, H, Gough, M, Farrel, R, et al. Successful use of recombinant activated factor VII in a patient with massive hemoptysis from a penetrating thoracic injury. Ann Thorac Surg. 2007; 84:1373.
173. Lau, EMT, Yozghatlian, V, Kosky, C, et al. Recombinant factor VII for massive hemoptysis in patients with cystic fibrosis. Chest. 2009; 136:277.