Chapter 220 Management of Symptomatic Osteoporotic Vertebral Compression
Vertebroplasty
Vertebral Augmentation
Kyphoplasty and vertebroplasty currently are used to treat neurologically intact patients suffering from vertebral compression fractures resulting from osteoporosis and certain neoplasms. Percutaneous vertebroplasty (PV) is an imaging-guided procedure that reinforces a compromised vertebra with polymethylmethacrylate (PMMA), alleviating pain and improving the patient’s mobility. Initially described in 1987 as a treatment for painful hemangiomas,1 the procedure now is used most widely to treat fractures and destructive lesions that cause vertebral collapse and pain. The goal of vertebral augmentation procedures is to reduce fracture pain by stabilizing the vertebral fracture, allowing patients to return to their daily living activities and exercise.
The primary indications for PV are persistently painful compression fractures, most commonly related to osteoporosis, that are unresponsive to correct medical treatment, and benign or malignant osteolytic neoplasms (e.g., hemangioma, metastasis, and myeloma). In the setting of osteoporotic fracture, PV is done primarily for pain management and secondarily to prevent further collapse.2 For neoplasm, the indications are somewhat broader, and the procedure may be done for pain management and/or stabilization. PV is not an ablative procedure, but reinforcement may reduce pain while facilitating other therapies such as radiation therapy or surgical resection and fixation and minimizing the risk of further collapse or fracture.
The specific indications for PV are as follows:
• Osteoporotic compression fractures causing pain refractory to nonsurgical therapy and interfering with normal activities of daily living
• Multiple compression deformities resulting in, or threatening to result in, respiratory or gastrointestinal compromise or loss of balance, increasing fall risk
• Unstable compression fractures showing movement or progressive collapse
• Osteolysis due to malignant or benign neoplasms with fracture or impending risk of fracture
Relative contraindications include the following:
• An asymptomatic and stable fracture
• A patient showing symptomatic improvement with time
• Prophylactic vertebral augmentation in the absence of any acute compression fracture
Other, less common, relative contraindications are (1) retropulsion of fracture fragment(s) causing substantial spinal canal compromise (e.g., a burst-type fracture); (2) neoplasm extending into the epidural space with substantial spinal canal compromise; (3) severe vertebral collapse such that it would be technically challenging to place a needle; (4) chronic stable fracture without pain; and (5) treatment of more than three levels at any one time. Acute traumatic fracture of a nonosteoporotic vertebra also is considered a contraindication.
Technical Performance of Vertebroplasty
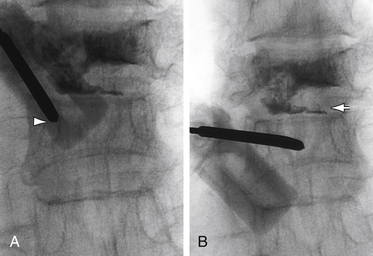
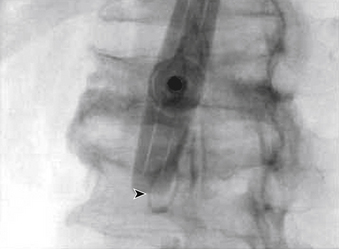
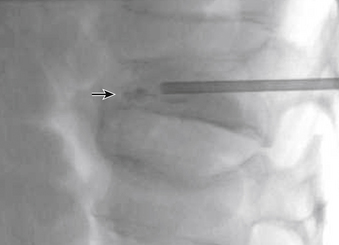
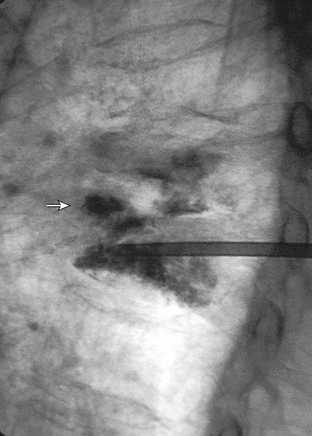
The traditional mode for PV has been to perform bilateral injections via a transpedicular or dorsolateral (parapedicular) approach using a two-needle technique (Figs. 220-1 to 220-5). Unipedicular injections also can provide substantial vertebral reinforcement when enough cement is injected to cross the midline of the vertebral body and provide a similar distribution to bipedicular injections.3
The modalities used for imaging are either fluoroscopy or CT. PV can be accomplished with either single-plane or biplane fluoroscopy. Biplane fluoroscopy is considered ideal because one can view orthogonal projections without changing tube position, greatly reducing procedure time. CT requires repositioning the gantry between needle manipulations, imaging, and injection, consuming useful injection. CT fluoroscopy allows the user to generate real-time CT sections while performing procedures, but concerns with CT fluoroscopy revolve around the increased radiation dose delivered to personnel and the operator in the room during the procedure. For most purposes, C-arm fluoroscopy provides adequate imaging, is readily available, and is less time-consuming.
Cement Handling
The two most popular preparations used for PV are Cranioplastic Type I Slow Set (Codman, Johnson & Johnson) and Simplex P (Stryker-Howmedica-Osteonics). Simplex P is approved by the FDA as a structural device for use in pathologic fractures in bones throughout the body, but the approval does not specify PV per se. Simplex P was the original PMMA used for the first PV by Deramond in 1984 and has remained popular for this application in Europe and the United States. In a comparison of three types of PMMA in cadavers (Cranioplastic, Osteobond, and Simplex P), vertebrae were significantly stronger after cement injection, regardless of cement type. However, Simplex P restored stiffness to initial values, whereas vertebrae injected with Cranioplastic were significantly less stiff than in their initial state.4
These preparations (mixed according to the package insert) produce cement that is difficult to inject and poorly visualized by fluoroscopy (although quite suitable for radiography). Thus the addition of an opaque agent (sterile barium, tantalum, or tungsten) is required. Sterile barium preparations are available from Parallax (Mountain View, CA) or Bryan Corporation (Woburn, MA). It has been determined that PMMA mixtures containing approximately 25% to 30% by weight of barium sulfate will provide opacification sufficient for the performance of fluoroscopically guided PV.4,5
With respect to how much cement mixture to inject, an in vitro study demonstrated that initial vertebral body strength is restored with as little as 2 mL of cement, but significantly greater stiffness requires 4 to 8 mL, depending on vertebral level and type of cement.6 These data provide guidance on the cement volumes needed to restore biomechanical integrity and parallel the clinical experience that many patients do well without having the cement fill an entire vertebral body. Hence, the trend has been to use less cement, minimizing the risk of complications from extravasation.
New biomaterials such as nanoparticles and Orthocomp (a glass-ceramic-reinforced BisGMA/BisEMA/TEGDMA matrix composite) are being actively pursued, and it is likely that specific formulations will be available for PV in the near future.7
Treating Tumors
Vertebroplasty may play an important role in palliation for the patient with vertebral metastases and in improving independence and function during more definitive systemic therapy. Tumors that are particularly radiosensitive are most appropriate to PV, because local control can be obtained as easily after stabilization as before. Tumors that require resection with a surgical margin, such as primary malignancies or locally aggressive benign tumors, are not appropriate for PV. Although there is some theoretical margin of tumor-kill associated with the thermal effect of the PMMA mass,8 the application of PV in the setting of neoplasm is not intended as an ablative procedure, and the goal is to provide structural support primarily, with the secondary goal of pain relief. The amount of PMMA used may be greater in an osteolytic spinal lesion than in a compression fracture, because the trend to minimize injectant volume in osteoporosis (i.e., filling the fracture line) does not apply in an erosive or destructive lesion.
Metastases are the lesions most commonly treated by PV, but large, symptomatic hemangiomas also may require ablation and stabilization with PMMA injection. Hemangiomas have a benign histology, but may grow aggressively and cause pain through either tissue distortion or pathologic fracture. PV stabilizes the vertebral body and obliterates the vascular sinusoids that make up the mass of the hemangioma. Subsequent surgery may then be focused on decompression, if needed.9 In this fashion, PV is another adjuvant treatment akin to intralesional sclerosis or embolotherapy preoperatively. PV also is effective in treating vertebral metastases that result in pain or instability, providing immediate and long-term pain relief.10 MRI is crucial for preoperative planning to evaluate the soft tissue extent of the tumor (e.g., spinal canal involvement, neuroforaminal encroachment, and status of the posterior longitudinal ligament).
Complications
Fortunately, major complications are uncommon. The most common complication is radicular pain caused by migration of cement into the epidural venous plexus. In most patients, intradiscal and paravertebral leaks of cement have no clinical importance.11 However, there have been case reports of severe neurologic complications, underscoring the need for appropriate safeguards as outlined previously.12 Permanent paralysis has been reported, but is exceptionally uncommon if the procedure is performed in a controlled, image-guided fashion (by using a biplane real-time fluoroscopy suite). Rib, pedicle, or transverse process fractures also have been noted. One case has been reported of a pulmonary embolism caused by acrylic cement. This rare complication was believed to have occurred because perivertebral venous migration was not recognized.13 The anticipated complication rate is higher when treating neoplasms (10%) than for osteoporotic compression fractures (1% to 3%).
Outcomes
Taylor et al. performed a systematic review and meta-regression to compare the efficacy and safety of balloon kyphoplasty and vertebroplasty for the treatment of vertebral compression fractures, and to examine the prognostic factors that predict outcome. They found level III evidence to support balloon kyphoplasty and vertebroplasty as effective therapies in the management of patients with symptomatic osteoporotic vertebral compression fractures refractory to conventional medical therapy. Although there was a good ratio of benefit to harm for both procedures, balloon kyphoplasty appeared to offer the better adverse event profile.14
In a follow-up study, the same authors concluded that in direct comparison to conventional medical management, patients undergoing kyphoplasty experienced superior improvements in pain, functionality, vertebral height, and kyphotic angle, at least up to 3 years postprocedure. Reductions in pain with kyphoplasty appeared to be greatest in patients with newer fractures. The authors concluded that there are prospective studies of low bias, with follow-up of 12 months or more, that demonstrate balloon kyphoplasty to be more effective than medical management of osteoporotic vertebral compression fractures and at least as effective as vertebroplasty.15
Kallmes et al. randomly assigned 131 patients who had one to three painful osteoporotic vertebral compression fractures to undergo either vertebroplasty or a simulated procedure without cement injection (served as control group).16 The primary outcomes were scores on the modified Roland-Morris Disability Questionnaire (RDQ) and patients’ ratings of average pain intensity during the preceding 24 hours at 1 month. Patients were allowed to cross over to the other study group after 1 month. Interestingly, both groups had immediate improvement in disability and pain scores after the intervention. At 1 month, there was no significant difference between the vertebroplasty group and the control group in either the RDQ score or the pain rating. The authors found a trend toward a higher rate of clinically meaningful improvement in pain (a 30% decrease from baseline) in the vertebroplasty group at 1 month. At 3 months, there was a higher crossover rate in the control group than in the vertebroplasty group. There was one serious adverse event in each group. The authors concluded that improvements in pain and pain-related disability associated with osteoporotic compression fractures in patients treated with vertebroplasty were similar to the improvements in a control group.
In a prospective study of 30 consecutive patients, Muijs et al. analyzed clinical and radiologic outcome 36 months after percutaneous vertebroplasty for osteoporotic vertebral compression fractures unresponsive to conservative treatment for at least 8 weeks. The authors also examined the quality of life (QOL). The authors reported good pain relief and significant increase in QOL scores, despite finding asymptomatic leakage of cement in 47 of 58 (81%) of treated vertebrae. The authors concluded that percutaneous vertebroplasty in the treatment of chronic vertebral compression fractures results in an immediate, significant, and lasting reduction in back pain, as well as overall improvement in physical and mental health.17
A meta-analysis to study the amount of pain reduction using the visual analog scale (VAS) with kyphoplasty and vertebroplasty in the treatment of osteoporotic vertebral compression fractures revealed that both procedures reduce pain in symptomatic osteoporotic vertebral compression fractures that have failed conservative treatment.18
Pain relief and risk of complications associated with vertebroplasty versus kyphoplasty were evaluated by Eck et al.19 The authors identified a total of 1036 abstracts for potential inclusion. Of these, 168 studies met the inclusion criteria. Mean preoperative and postoperative VAS scores for vertebroplasty were 8.36 and 2.68, respectively, with a mean change of 5.68 (P < .001). The mean preoperative and postoperative VAS scores for kyphoplasty were 8.06 and 3.46, respectively, with a mean change of 4.60 (P < .001). Statistically greater improvement was found with vertebroplasty versus kyphoplasty (P < .001). The risk of new fracture was 17.9% with vertebroplasty versus 14.1% with kyphoplasty (P < .01). The risk of cement leak was 19.7% with vertebroplasty versus 7.0% with kyphoplasty (P < .001). The authors concluded that vertebroplasty had a significantly greater improvement in pain scores but also had statistically greater risk of cement leakage and new fracture.
Outcomes in Patients with Vertebral Compression Fractures
Buchbinder et al. performed a multicenter, randomized, double-blind, placebo-controlled trial. Participants had one or two painful osteoporotic vertebral fractures that were of less than 12 months’ duration and unhealed. Participants were randomly assigned to undergo either vertebroplasty or a sham procedure.20 The primary outcome was overall pain at 3 months. These authors found no beneficial effect of vertebroplasty as compared with a sham procedure in patients with painful osteoporotic vertebral fractures, at 1 week or at 1, 3, or 6 months after treatment.
Masala et al. reviewed 624 patients with 1253 compression fractures that were treated by percutaneous vertebroplasty and found a statistically significant improvement in the patients’ quality of life by 12 months.21 In a separate retrospective study, Masala et al. evaluated the effectiveness, costs, and cost-effectiveness of percutaneous vertebroplasty.22 After 2 weeks of analgesic therapy, 153 patients presented with refractory pain and were offered treatment by percutaneous vertebroplasty. A total of 58 patients accepted and underwent percutaneous vertebroplasty, while 95 refused and underwent conservative medical therapy. Significant reduction in VAS and improvement in ambulation and activities of daily living were observed in both groups at 1 week, 3 months, and 12 months. These results were significantly superior in the percutaneous vertebroplasty group at 1 week and 3 months. Percutaneous vertebroplasty was significantly more cost-effective than medical therapy with regard to VAS and activities of daily living at 1 week. By 3 months, percutaneous vertebroplasty was more cost-effective than medical therapy with regard to ambulation. No significant difference in cost-effectiveness was found between the two groups at 12 months. The authors concluded that percutaneous vertebroplasty should be considered the treatment of first choice in symptomatic acute osteoporotic vertebral fractures with refractory pain after a short period of analgesic therapy.
Effects of Vertebral Augmentation on Restoration of Vertebral Height and Sagittal Alignment
Hiwatashi et al.23 compared restoration of vertebral body height and wedge angle and cement leakage with kyphoplasty and vertebroplasty in 40 patients with osteoporotic compression fractures (57 vertebrae). Kyphoplasty and vertebroplasty both improved vertebral body height and the wedge angles (P < .05), but these differences were not statistically significant when the two techniques were compared (P > .05). Cement leakage into the paravertebral soft tissues or veins was found in 18% of the kyphoplasty patients and 49% of vertebroplasty patients (P < .01). Cement leakage into the disc space occurred in 12% of the kyphoplasty patients and in 25% of vertebroplasty patients (P < .01). There were no complications related to cement leakage. 23
In a cadaveric study, Luo et al. compared the ability of vertebroplasty and kyphoplasty to restore spine mechanical function, and vertebral body shape, following vertebral fracture.24 The authors reported that vertebroplasty and kyphoplasty were equally effective at restoring mechanical function to an injured spine, but only kyphoplasty was able to reverse minor vertebral wedging.
Erkan at al. evaluated the biomechanics of transpedicular and extrapedicular vertebroplasty in terms of height restoration, strength, and stiffness.25 Both extrapedicular and transpedicular PMMA techniques increased strength but reduced stiffness compared with the intact condition. The extrapedicular technique achieved greater height restoration, and the authors attributed this result to the technique’s easier access to the fracture site.
Ruger et al. used a cadaver model to evaluate the ability of a novel high-viscosity PMMA cement and vertebroplasty kit to correct the kyphosis angle of wedge compression fractures.26 The authors reported that high-viscosity vertebroplasty effectively reduced and stabilized thoracolumbar wedge compression fractures and may represent a one-step solution for restoring vertebral body dimensions following thoracolumbar compression fractures, while minimizing the risk of cement leakage and associated complications in vivo.
Vertebral Augmentation and Adjacent Vertebral Compression Fractures
Kobayashi et al. found that prophylactic cement injection into nonfractured vertebrae adjacent to fractured vertebrae may prevent new compression fractures after vertebroplasty for osteoporotic patients.27 The authors performed vertebroplasty on 89 consecutive patients with osteoporotic compression fractures. They then treated a second subset of 155 consecutive patients with osteoporotic compression fractures with vertebroplasty at the fractured level. In addition, the authors performed prophylactic cement injection by injecting cement into the adjacent nonfractured vertebrae during the same procedure. They then evaluated the frequency of new vertebral fractures and the efficacy of prophylactic therapy. In the nonprophylactic group, 15 of 89 patients (16.8%) developed new fractures within 3 months, and 20 of 89 patients (22.4%) developed new, painful compression fractures within 1 year. These fractures occurred mostly in adjacent vertebrae, particularly in the vertebra immediately superior to the treated one, and occurred in the lower thoracic and upper lumbar spine. In the prophylactic group, 7 of 155 patients (4.5%) developed new compression fractures within 3 months (P = .0020), and 15 of 155 patients (9.7%) developed new compression fractures within 1 year (P = .0079).
Oakland et al. performed a biomechanical assessment of prophylactic vertebral reinforcement adjacent to vertebroplasty using a three-vertebrae cadaveric segment under dynamic loads that represented increasing activity demands. The effects of reducing the elastic modulus of the cement used in the intact vertebrae also were assessed. The authors concluded that under normal physiologic loads associated with moderate physical activity, prophylactic augmentation adjacent to vertebroplasty showed little evidence of inducing fractures, although loads representing more strenuous activities may generate adjacent and periaugmentation compromise. Reducing the elastic modulus of the cement in the adjacent intact vertebrae appeared to have no significant effect on the incidence or location of the induced fracture or the overall height loss of the vertebral segment.28
Nouda et al. used a cadaver model to compare the effects of treatment by vertebroplasty with PMMA cement and vertebroplasty with calcium phosphate cement on the creation of adjacent vertebral body fractures. The authors found that there was a difference in results related to fracture location, with specimens treated with PMMA cement developing fractures at the adjacent level and specimens treated with calcium phosphate cement developing fractures at the augmented level.29
Experience in Metastases and Multiple Myeloma
In a study undertaken to assess the safety and efficacy of percutaneous vertebroplasty in 64 patients with osteolytic lesions due to multiple myeloma, Masala et al. showed excellent postoperative pain reduction. However, there was no difference in pain levels at 1 and 6 months, and no procedure-related complications were observed.30
Lee et al. reviewed 19 patients with spinal metastasis from solid organ adenocarcinomas. The authors found that PV was an effective palliative procedure in patients with compression fractures secondary to metastatic malignancy and that its use can be successfully combined with radiation therapy and chemotherapy.31 Tseng treated 57 patients (78 vertebrae) with spinal metastatic tumor with PV. The authors found marked pain reduction and significant reduction in the amount of narcotic and non-narcotic analgesics.32 McDonald performed a retrospective review of clinical outcome data from 67 multiple myeloma patients treated with PV. Outcome measures including the Roland Morris Disability Questionnaire, VAS, and VAS with rest and activity improved by 11 (48%; P < .0001), 2.7 (25%; P < .001), and 5.3 (48%; P < .0001) points, respectively, with persistent improvement at 1 year (P < .01; P < .03; P < .001). Eighty-two percent and 89% of patients experienced a significant improvement in subjective rest pain and activity pain, respectively. Subjective scores achieved durable improvements, with 65% of patients requiring reduced narcotics after vertebroplasty and 70% having improved mobility.33
Future Considerations
In a randomized study, Yang et al. compared the outcomes in patients with metastatic spinal tumors treated with either vertebroplasty alone or vertebroplasty combined with interstitial implantation of iodine-125 seeds.34 A total of 80 patients with metastatic spinal tumors were randomized to receive vertebroplasty alone (40 cases) or vertebroplasty combined with iodine-125 seed implantation (40 cases). Visual analogue scores and Karnofsky performance scores were superior in the iodine-125 group. The authors concluded that clinical outcomes can be enhanced by the addition of interstitial implantation of iodine-125 seeds to the treatment protocol.
Belkoff S.M., Maroney M., Fenton D.C., Mathis J.M. An in vitro biomechanical evaluation of bone cements used in percutaneous vertebroplasty. Bone. 1999;25:23S-26S.
Erkan S., Wu C., Mehbod A.A., et al. Biomechanical comparison of transpedicular versus extrapedicular vertebroplasty using polymethylmethacrylate. J Spinal Disord Tech. 2010;23(3):180-185.
Masala S., Anselmetti G.C., Marcia S., et al. Percutaneous vertebroplasty in multiple myeloma vertebral involvement. J Spinal Disord Tech. 2008;21(5):344-348.
Muijs S.P., Nieuwenhuijse M.J., Van Erkel A.R., Dijkstra P.D. Percutaneous vertebroplasty for the treatment of osteoporotic vertebral compression fractures: evaluation after 36 months. J Bone Joint Surg [Br]. 2009;91(3):379-384.
Nouda S., Tomita S., Kim A., et al. Adjacent vertebral body fracture following vertebroplasty with polymethylmethacrylate or calcium phosphate cement: biomechanical evaluation of the cadaveric spine. Spine (Phila Pa 1976). 2009;34(24):2613-2618.
Padovani B., Kasriel O., Brunner P., Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20(3):375-377.
Taylor R.S., Taylor R.J., Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (Phila Pa 1976). 2006;31(23):2747-2755.
Weill A., Chiras J., Simon J.M., et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199(1):241-247.
1. Galibert P., Deramond H., Rosat P., Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166-168.
2. Bostrom M.P., Lane J.M. Future directions. Augmentation of osteoporotic vertebral bodies. Spine (Phila Pa 1976). 1997;22(Suppl 24):39S-42S.
3. Tohmeh A.G., Mathis J.M., Fenton D.C., et al. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine (Phila Pa 1976). 1999;24:1772-1776.
4. Belkoff S.M., Maroney M., Fenton D.C., Mathis J.M. An in vitro biomechanical evaluation of bone cements used in percutaneous vertebroplasty. Bone. 1999;25:23S-26S.
5. Jasper L.E., Deramond H., Mathis J.M., Belkoff S.M. The effect of monomer-to-powder ratio on the material properties of cranioplastic. Bone. 1999;25:27S-29S.
6. Belkoff S.M., Mathis J.M., Jasper L.E., Deramond H. The biomechanics of vertebroplasty: the effect of cement volume on mechanical behavior. Spine (Phila Pa 1976). 2001;26(14):1537-1541.
7. Belkoff S.M., Mathis J.M., Erbe E.M., Fenton D.C. Biomechanical evaluation of a new bone cement for use in vertebroplasty. Spine (Phila Pa 1976). 2000;25(9):1061-1064.
8. Deramond H., Wright N.T., Belkoff S.M. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999;25(Suppl 2):17S-21S.
9. Ide C., Gangi A., Rimmelin A., et al. Vertebral haemangiomas with spinal cord compression: the place of preoperative percutaneous vertebroplasty with methyl methacrylate. Neuroradiology. 1996;38(6):585-589.
10. Weill A., Chiras J., Simon J.M., et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199(1):241-247.
11. Cotten A., Dewatre F., Cortet B., et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200(2):525-530.
12. Harrington K.D. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg. 2001;83:1070-1073.
13. Padovani B., Kasriel O., Brunner P., Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20(3):375-377.
14. Taylor R.S., Taylor R.J., Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (Phila Pa 1976). 2006;31(23):2747-2755.
15. Taylor R.S., Fritzell P., Taylor R.J. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085-1100.
16. Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579.
17. Muijs S.P., Nieuwenhuijse M.J., Van Erkel A.R., Dijkstra P.D. Percutaneous vertebroplasty for the treatment of osteoporotic vertebral compression fractures: evaluation after 36 months. J Bone Joint Surg [Br]. 2009;91(3):379-384.
18. Gill J.B., Kuper M., Chin P.C., et al. Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007;10(4):583-590.
19. Eck J.C., Nachtigall D., Humphreys S.C., Hodges S.D. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488-497.
20. Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557-568.
21. Masala S., Petrella M.C., Massari F., et al. Percutaneous vertebroplasty in 1,253 levels: results and long-term effectiveness in a single centre. Eur Radiol. 2009;19(1):165-171.
22. Masala S., Konda D., Vinicola V., et al. Cost-effectiveness of percutaneous vertebroplasty in osteoporotic vertebral fractures. Eur Spine J. 2008;17(9):1242-1250.
23. Hiwatashi A., Yoshiura T., Noguchi T., et al. Kyphoplasty and vertebroplasty produce the same degree of height restoration. AJNR Am J Neuroradiol. 2009;30(7):1388-1393.
24. Luo J., Bertram W., Sangar D., et al. Is kyphoplasty better than vertebroplasty in restoring normal mechanical function to an injured spine? Bone. 2009;46(4):1050-1057.
25. Erkan S., Wu C., Mehbod A.A., et al. Biomechanical comparison of transpedicular versus extrapedicular vertebroplasty using polymethylmethacrylate. J Spinal Disord Tech. 2010;23(3):180-185.
26. Rüger M., Schmoelz W. Vertebroplasty with high-viscosity polymethylmethacrylate cement facilitates vertebral body restoration in vitro. Spine (Phila Pa 1976). 2009;34(24):2619-2625.
27. Kobayashi N., Numaguchi Y., Fuwa S., et al. Prophylactic vertebroplasty: cement injection into non-fractured vertebral bodies during percutaneous vertebroplasty. Acad Radiol. 2009;16(2):136-143.
28. Oakland R.J., Furtado N.R., Wilcox R.K., et al. Preliminary biomechanical evaluation of prophylactic vertebral reinforcement adjacent to vertebroplasty under cyclic loading. Spine J. 2009;9(2):174-181.
29. Nouda S., Tomita S., Kim A., et al. Adjacent vertebral body fracture following vertebroplasty with polymethylmethacrylate or calcium phosphate cement: biomechanical evaluation of the cadaveric spine. Spine (Phila Pa 1976). 2009;34(24):2613-2618.
30. Masala S., Anselmetti G.C., Marcia S., et al. Percutaneous vertebroplasty in multiple myeloma vertebral involvement. J Spinal Disord Tech. 2008;21(5):344-348.
31. Lee B., Franklin I., Lewis J.S., et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009;45(9):1597-1602.
32. Tseng Y.Y., Lo Y.L., Chen L.H., et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of pain induced by metastatic spine tumor. Surg Neurol. 2008;70(Suppl 1):78-83.
33. McDonald R.J., Trout A.T., Gray L.A., et al. Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol. 2008;29(4):642-648.
34. Yang Z., Yang D., Xie L., et al. Treatment of metastatic spinal tumors by percutaneous vertebroplasty versus percutaneous vertebroplasty combined with interstitial implantation of 125I seeds. Acta Radiol. 2009;50(10):1142-1148.
Kyphoplasty
Osteoporosis is a metabolic disorder that may result in devastating medical problems if not treated appropriately. However, even in the best-treated patients, fractures may occur. Two different minimally invasive percutaneous vertebral augmentation methods for cement application into the vertebral body for the management of symptomatic compression fractures without neurologic impairment have been developed: percutaneous vertebroplasty (PV) and kyphoplasty. In PV, polymethylmethacrylate (PMMA) cement is injected percutaneously into a vertebral body. Kyphoplasty is an advanced surgical technique that evolved from a marriage of PV with balloon angioplasty. It has a number of potential advantages, including lower risk of cement extravasation and better restoration of vertebral body height. A cannula is introduced into the vertebral body, via a transpedicular or extrapedicular route, followed by insertion of an inflatable bone tamp, which, when deployed, reduces the compression fracture and restores the vertebral body toward its original height. This then creates a cavity to be filled with bone cement. The cement augmentation can now be completed with more control into the low-pressure environment of the preformed cavity with viscous, partially cured cement. Using a cannula for bone filler with a steel stylet as a plunger enables the operator to apply cement at considerably higher viscosity than is possible with injection through a 5-mL syringe and 11-gauge needle. Both the higher cement viscosity and controlled fill reduce the risk of cement extravasation. Filling is performed under continuous lateral fluoroscopic guidance similar to vertebroplasty. The procedure can be performed under general anesthesia or local anesthesia with IV sedation; most patients are able to return home the same day.
Surgical Indications
Kyphoplasty currently is indicated for progressive, painful osteoporotic vertebral compression fractures (VCFs) in the absence of neurologic signs. Even though the natural history of vertebral compression fractures is for two thirds of patients to eventually become pain free, one must appreciate that (1) not one of those vertebral bodies ever regains its normal height, (2) the presence of a vertebral collapse predicts increased mortality, and (3) there is a five-fold increase in further fractures at adjacent or remote levels within 1 year.1–3 Therefore, the indications are pain relief and protection of sagittal spinal alignment.
Clinical Results
Our published data from the Cleveland Clinic show that kyphoplasty provides a safe and effective treatment for pain and disability in patients with vertebral compression fractures secondary to osteoporosis and multiple myeloma.4–8 Outcome data were obtained by administering the Short Form-36 health survey (SF-36) and visual analogue scale (VAS) for pain rating. In addition, the patients underwent detailed neurologic and radiographic examinations preoperatively and postoperatively. In our experience there were no clinically significant cement leaks and no perioperative complications attributable to the inflatable bone tamp or tools. Preoperative and postoperative SF-36 data are available on over 210 patients with average follow-up periods of 10.8 months. SF-36 scores improved in every category, statistically significant in all but the General Health modality. Physical function improved from 15.0 to 30.0 (P < .001). Role-physical improved from 9.3 to 27.3 (P = 0.004). Bodily pain improved from 22.0 to 32.0 (P ≤ .001). Vitality improved from 30.0 to 40.0 (P ≤ .001). Social function improved from 37.5 to 50.0 (P ≤.001). Role-emotional improved from 33.3 to 66.7 (P = .39). Mental health improved from 64.0 to 72.0 (P < .001). General health was unchanged from 52.0 to 55.0 (P = .051). The VAS scores improved from a preoperative level of 7.0 to an initial postoperative level of 3.2 (P < .0001). At last follow-up examination, the value remained unchanged at 3.4 (P < .0001).
Ledlie et al. reported functional and radiographic outcomes in the first 117 consecutive patients with 151 fractures.9 Their report focused on the results for the subset of 77 patients completing follow-up. With regard to pain as rated by the patient using a 10-point VAS, the mean pain score was decreased from 8.9 ± 1.5 before surgery to 2.8 ± 2.9 at 1 week postoperatively, and stayed low at 1.5 ± 1.8 at 2 years postoperatively. The proportion of patients who were fully ambulatory increased from 45% before surgery to 85% at 1 week postoperatively, and 88% at the 2-year follow-up.
Garfin et al. reported clinical results from a total of 155 subjects with 214 VCFs who underwent balloon kyphoplasty at 19 study centers from August 2000 to June 2001.10 Mean pain ratings decreased from 15.0 (full mark: 20) before surgery to 6.0 within 7 days after kyphoplasty (P < .001), a 60% reduction, and remained decreased throughout follow-up to 24 months. The mean number of days of the previous 28 days spent in bed decreased from 8.8 at baseline to 1.9, 2.2, 0.7, and 1.4 at 1, 3, 12, and 24 months after kyphoplasty.
Wardlaw et al. reported the results of their randomized controlled trial at 21 sites in 8 countries11 in which 300 patients were randomly assigned by a computer-generated sequence to review kyphoplasty treatment (n = 149) or nonsurgical care (n = 151). Among them, 138 participants in the kyphoplasty group and 128 controls completed follow-up at 1 month. By use of repeated measures mixed effects modeling, all 300 randomized participants were included in the analysis. The mean SF-36 physical component summary (PCS) score improved by 7.2 points (95% CI, 5.7–8.8), from 26.0 at baseline to 33.4 at 1 month, in the kyphoplasty group, and by 2.0 points (95% CI, 0.4–3.6), from 25.5 to 27.4, in the nonsurgical group (difference between groups 5.2 points, 2.9–7.4, P < .0001). The frequency of adverse events did not differ between groups. These results suggest that balloon kyphoplasty is an effective and safe procedure for patients with acute vertebral fractures and can be considered as an early treatment option.
In addition to good clinical results, height restoration by kyphoplasty has been reported in several studies. Our initial results showed height restoration in 70% of 70 fractured vertebrae treated with kyphoplasty.4 In patients in whom the vertebral fractures were reduced by kyphoplasty, vertebral height was increased by a mean of 46.8%.
Ledlie et al. reported their radiographic measurement results for ventral and midline points of the fractured vertebrae using the two nearest normal vertebrae as reference points.12 Pairs of preoperative and 2-year follow-up radiographs for 85 treated fractures were evaluated for vertebral height measurement. Mean preoperative ventral height (Ha), mid-height (Hm), and posterior height (Hp) were 61.3% ± 23.8%, 61.0% ± 20.1%, and 86.5% ± 18.4%, respectively. Compared to preoperative heights, mean Ha, Hm, and Hp were significantly higher (P < .001) at all postoperative intervals. Following kyphoplasty (1–6 weeks), mean Ha, Hm, and Hp were 81.2% ± 20.9%, 86.9% ± 17.9%, and 95.1% ± 10.1%, respectively. At 2-year follow-up, mean Ha, Hm, and Hp were 80.9% ± 19.8%, 88.1% ± 16.6%, and 91.4% ± 12.8%, respectively. There was no correlation between height increase and patient age, gender, fracture level, or fracture age.9
Garfin et al. reported the results of midline height restoration rates. The mean preoperative mid-vertebral height was 65% of the predicted height. Eighty-two percent had at least 10% lost height restored between pretreatment and posttreatment. The mean percent of mid-vertebral lost height restored was 32% overall and 44% among those with measurable height restoration.10
Complications
Many kyphoplasty studies have provided details on the number of cases that involved cement leakage. Tayler’s meta-analysis showed that a total of 189 (9%) cement leakages were reported in 2239 vertebrae that underwent kyphoplasty.13 This corresponds to 81 cement leaks per 1000 fractures undergoing kyphoplasty per year. One leak (0.001%) was reported by Majd et al.14 to be symptomatic L1 radiculopathy. A few cases also were reported as inappropriate cement injection and needle placement in the early phase.15 In our series of patients,4,6 cement extravasation was seen in less than 10% of cases. No problems were identified clinically as a result of these extravasations, either immediately after surgery or at final follow-up. In one patient, a myocardial infarction occurred as a result of fluid overload during the procedure.
Ledlie et al. reported three complications in their series.9 Two weeks after kyphoplasty, a pulmonary embolism was diagnosed in a patient with a history of deep vein thrombosis (no evidence of PMMA leakage to the lungs by CT). One patient with preexisting metastatic, cardiac, vascular, severe chronic obstructive pulmonary disease required mechanical ventilation and subsequently died 5 days postoperatively. A third patient had perioperative confusion and generalized weakness that gradually resolved (negative brain CT and neurologic workup). No complications were related to the kyphoplasty technique. Minor cement extravasations were observed in 17 of 151 (11.3%) treated fractures, but none of the leaks were associated with any clinical consequence.
Garfin et al. also reported that extravasation of PMMA outside the vertebral body occurred in 21 of 214 (10%) treated levels.10 All PMMA exravasations were asymptomatic: the cement remained in the immediate area of the treated vertebrae, and no medical or surgical intervention was required to remove the extravasated PMMA.
Kyphoplasty versus Vertebroplasty
Although both vertebroplasty and kyphoplasty provide excellent pain relief, kyphoplasty has the potential to improve spine biomechanics and decrease the risk of cement extravasation. PV usually will not expand the vertebral body or regain normal spine alignment. Hiwatashi et al. reported the increase in vertebral body height after vertebroplasty in preoperative MRI and postoperative CT scans to measure the vertebral heights.16 They measured the heights of 85 vertebral bodies in 37 patients before and after vertebroplasty. The results showed that the average increase in vertebral body height was 2.5 mm ventrally, 2.7 mm centrally, and 1.4 mm dorsally. However, they did not distinguish height corrections from positioning, and it is still unclear how much was corrected by the procedure itself. The significance of this methodology to measure the height between MRI and CT also is uncertain. Preliminary data indicate that kyphoplasty may restore near-normal height, preventing kyphosis that leads to respiratory and digestive problems. Restoration of height and sagittal alignment also may work to protect vulnerable vertebral levels above or below the site(s) treated by minimizing force transfer.
The vertebroplasty technique is much more prone to cement leaks, because the PMMA is injected in a liquid state and will take the path of least resistance through any cracks in surrounding bone. In administering vertebroplasty, the operator injects the liquid cement, typically pausing or stopping once a leak becomes evident. On the other hand, in kyphoplasty, the expanded balloon creates a cavity and pushes bone to the edges of the cavity, thus sealing off potential fissures and cracks. Greater placement control is possible in a kyphoplasty, in which the operator can fill the cavity with a more viscous cement to the point at which the cement bolus reaches and interdigitates with the bony margins. The initial kyphoplasty findings show lower rates of cement extravasation compared with published vertebroplasty series, supporting the hypothesis that filling with high-viscosity cement into a previously formed cavity may be an improvement over the injection of low-viscosity liquid cement into the unreduced vertebral body.
Recent Articles in the New England Journal of Medicine
In the August 9, 2009, issue of the New England Journal of Medicine, two new studies were reported.17,18 Both were multicenter, randomized controlled trials that used a sham procedure rather than conventional conservative treatment as a control. In the study by Buchbinder et al.,17 a total of 78 patients were enrolled. Of those, 35 of 38 underwent PV, and 36 of 40 underwent a sham procedure in four Australian centers and completed the 6-month follow-up (91%). At 3 months, the mean reductions in the score for pain in the vertebroplasty and control groups were 2.6 ± 2.9 and 1.9 ± 3.3, respectively. Similar improvements were seen in both groups with respect to pain at night and at rest, physical functioning, quality of life, and perceived improvement. They concluded that there was no beneficial effect of vertebroplasty as compared with a sham procedure. In another randomized, controlled trial, the Investigational Vertebroplasty Safety and Efficacy Trial (INVEST), performed by Kallmes et al.,18 68 patients underwent PV and 63 underwent a sham procedure. Both groups had immediate improvement in disability and pain scores after the intervention. Although there was a trend toward a higher rate of clinically meaningful improvement in pain (a 30% decrease from baseline) in the vertebroplasty group (64% vs. 48%, P = .06), the two groups did not differ significantly on any secondary outcome measure at 1 month. They also concluded that improvements in pain and pain-related disability associated with osteoporotic compression fractures in patients treated with vertebroplasty were similar to the improvements in a control group.
Moreover, the quoted “sham” procedure of analgesic injection of the periosteum is a distinct treatment that to date has not been validated. It should not be considered a “sham.” Another questionable issue is that both studies were performed by an interventional radiologist, which introduces selection and technique bias into the studies. Based on reviews of other published randomized controlled trials comparing vertebral augmentation with conservative therapy,11,19 these surgical procedures contribute significantly to pain relief. Regardless of the shortcomings of the two New England Journal of Medicine articles, the question of the efficacy of vertebral augmentation needs further study. Ongoing randomized controlled trials will provide further information in the future.
Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568.
Garfin S.R., Buckley R.A., Ledlie J. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila Pa 1976). 2006;31:2213-2220.
Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579.
Khanna A.J., Reinhardt M.K., Togawa D., Lieberman I.H. Functional outcomes of kyphoplasty for the treatment of osteoporotic and osteolytic vertebral compression fractures. Osteoporos Int. 2006;17:817-826.
Wardlaw D., Cummings S.R., Van Meirhaeghe J., et al. Efficacy and safety of balloon kyphoplasty compared with nonsurgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016-1024.
1. Hasserius R., Karlsson M.K., Nilsson B.E., et al. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int. 2003;14:61-68.
2. Lindsay R., Silverman S.L., Cooper C., et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
3. Silverman S.L. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27-S31.
4. Lieberman I.H., Dudeney S., Reinhardt M.K., Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2001;26:1631-1638.
5. Dudeney S., Lieberman I.H., Reinhardt M.K., Hussein M. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20:2382-2387.
6. Coumans J.V., Reinhardt M.K., Lieberman I.H. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg. 2003;99:44-50.
7. Khanna A.J., Reinhardt M.K., Togawa D., Lieberman I.H. Functional outcomes of kyphoplasty for the treatment of osteoporotic and osteolytic vertebral compression fractures. Osteoporos Int. 2006;17:817-826.
8. Lieberman I., Reinhardt M.K. Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res. 2003;415:S176-S186.
9. Ledlie J.T., Renfro M.B. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila Pa 1976). 2006;31:57-64.
10. Garfin S.R., Buckley R.A., Ledlie J. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila Pa 1976). 2006;31:2213-2220.
11. Wardlaw D., Cummings S.R., Van Meirhaeghe J., et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016-1024.
12. Ledlie J.T., Renfro M. Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain, and activity levels. J Neurosurg. 2003;98:36-42.
13. Taylor R.S., Fritzell P., Taylor R.J. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085-1100.
14. Majd M.E., Farley S., Holt R.T. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5:244-255.
15. Garfin S.R., Yuan H.A., Reiley M.A. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976). 2001;26:1511-1515.
16. Hiwatashi A., Moritani T., Numaguchi Y., Westesson P.L. Increase in vertebral body height after vertebroplasty. AJNR Am J Neuroradiol. 2003;24:185-189.
17. Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568.
18. Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579.
19. Voormolen M.H., Mali W.P., Lohle P.N., et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28:555-560.