Current Management of Small Bowel Obstruction
Although common, small bowel obstruction (SBO) remains one of the most challenging clinical problems treated by surgeons. Responsible for up to 300,000 hospital admissions every year in North America, SBO arises from multiple etiologies and manifests as a diverse panoply of clinical presentations [1]. Initial evaluation should center on differentiating those patients who need urgent exploration from those who may undergo a safe, nonoperative trial. The wide range of etiologies, however, combined with specific, and often unique, patient parameters, renders this decision difficult. Traditionally, the decision between urgent operative intervention and initial nonoperative management has hinged on the distinction between complete and partial obstruction. However, the clinical diagnosis of complete obstruction is imprecise, and the complete/partial dichotomy has not eliminated avoidable obstruction-associated ischemia and necrosis. Rather than trying to predict those patients at risk for ischemic complications, we may do better to define clinical parameters that predict failure of nonoperative management and offer prompt operation to patients demonstrating these parameters. Hopefully, such an approach, codified into practice management guidelines, will minimize both ischemia and hospital length of stay associated with SBO. After reviewing the pathogenesis and pathophysiology of SBO, this article outlines newly developed and refined management and surgical techniques to reach these goals.
Pathogenesis
SBO implies compromised luminal patency and as such is differentiated from the functional abnormalities of ileus and dysmotility disorders. Adhesive disease, neoplasia, and hernias are the 3 most common causes in the western world, collectively accounting for 80% of all obstructions [2,3]. In developing countries, hernias, intussusceptions, and volvulae predominate [4].
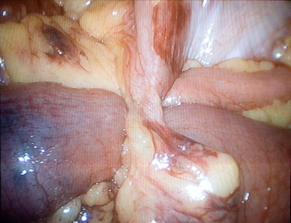
SBO most frequently arises from adhesive disease (49%), which in turn is almost always related to prior operation (Fig. 1) [2]. Only a minority of patients with adhesions will experience SBO. Although 93.0% of patients with more than one abdominal operation will have adhesions at autopsy, only 4.6% of patients with a prior abdominal procedure will have an adhesive SBO [5,6]. Adhesive SBO is frequently a relapsing disease: up to 30% of patients who undergo celiotomy for obstruction will require reexploration for recurrence [2,7].
Barmparas and colleagues [5] reviewed the English literature and collected more than 440,000 reported patients with postceliotomy adhesive SBO to examine risk factors. The likelihood of subsequent SBO varied among different index operative procedures, and was greatest after open adnexal operations (23.9%), followed by ileal pouch anal-anastomosis (19.3%), and open total abdominal hysterectomy (9.5%). Laparoscopic cholecystectomy (0.2% vs 7.1%), laparoscopic total abdominal hysterectomy (0.0% vs 15.6%), and laparoscopic adnexal operations (0.0% vs 23.9%), but not laparoscopic appendectomies (1.3% vs 1.4%), all resulted in fewer adhesive obstructions than their open counterparts. Overall, open procedures were associated with twice as many adhesive obstructions as was laparoscopy, but the investigators cautioned that interpretation of this collective comparison was hampered by limited follow-up, heterogeneity between the primary studies, and selection biases. Indeed, early review of the Conventional versus Laparoscopic-Assisted Surgery In Colorectal Cancer CLASICC (CLASICC) trial data demonstrated no statistical difference between adhesive obstructions after laparoscopic (2.5%) versus open resections (3.1%) for colorectal cancer at 3-year follow-up [8].
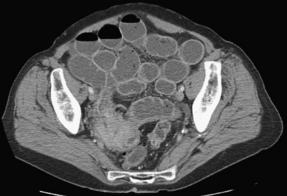
Neoplasia, the second leading cause of SBO, is responsible for 16% of SBO admissions (Fig. 2) [2]. Although primary small bowel tumors do cause obstruction, colorectal and ovarian metastases are the most common malignant etiologies (41% and 28%, respectively) [9]. Breast cancer and malignant melanoma are the 2 most common nonabdominal tumors that can cause obstruction [10]. Neoplastic masses causing obstruction may be a result of the primary tumor, peritoneal metastases, or bulky lymph node metastases. The mechanism of obstruction may be direct compression, malignant adhesion with consequent torsion, knuckling, or internal herniation. The median time from the diagnosis of cancer to the first episode of obstruction is about 1 year, but decades can pass from the time of initial diagnosis to obstruction. Median survival is on the order of 3 to 6 months after onset of initial obstructive symptoms and is almost universally fatal [9,10].
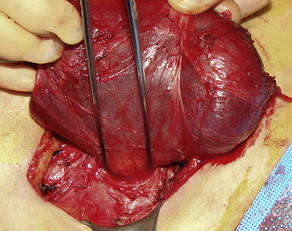
Hernias cause 15% of SBOs [2]. There are 2 main categories of hernia: external and internal. Among external hernias, incarceration is encountered most frequently from inguinal and incisional hernias, but is also seen with femoral, umbilical, traumatic, and peristomal hernias (Fig. 3). Whereas external hernias are diagnosed on physical examination, internal hernias are generally diagnosed in the operating room, and on occasion, by preoperative computed tomography (CT). Internal hernias can be further classified into congenital or acquired types. For example, an obturator hernia, resulting from protrusion of intra-abdominal contents through the obturator foramen created by the pubic bones and ischium, is considered a congenital internal hernia (Fig. 4) [11]. Obturator hernias, generally present in elderly women after significant weight loss, are associated with a 25% mortality owing to the difficulty in diagnosis and the comorbidities of the patient population [12]. Omental and paraduodenal defects, as well as the foramen of Winslow are additional, but uncommon, sites of congenital herniation [13].
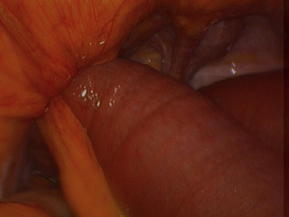
Acquired internal hernias arise after surgical manipulation. With the rapid increase in the rate of laparoscopic bariatric procedures, the rate of internal hernia is rising [14]. Surgeons must be knowledgeable in the diagnosis and treatment of the specific hernias that can result from Roux-En-Y Gastric Bypass including those through mesocolic, Petersen, and mesomesenteric defects (Fig. 5) [13,14]. The incidence has been reported to be as high as 3.1%, but this is likely declining with greater awareness of the need to close the defects at the primary operation [15]. The mesocolic defect is created for the retrocolic passage of the Roux limb. Petersen defect is bordered by the transverse mesocolon superiorly, the Roux limb and its mesentery anteriorly, and the proximal-most jejunum and its mesentery posteriorly [16]. Mesomesenteric defects are created by inadequate apposition of mesentery after bowel resection with anastomosis; after gastric bypass, a large such defect exists between the mesentery of the biliopancreatic limb anteriorly and the mesentery of the alimentary limb/common channel posteriorly. Symptomatic hernias can occur at any time after bypass, especially as the patients lose weight, allowing for widening of defects not closed properly during the initial procedure. As an additional consideration in patients with gastric bypass, a large remnant stomach on CT after gastric bypass must be recognized as an abnormal finding indicative of biliopancreatic limb obstruction.
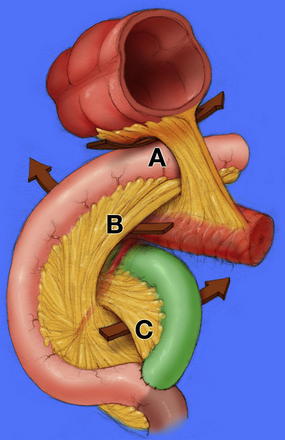
Fig. 5 Internal hernia defects: (A) mesocolic, (B) Petersen, (C) mesomesenteric.
(From Kendrick ML, Dakin GF. Surgical approaches to obesity. Mayo Clin Proc 2006;81(10 Suppl):S18–24; with permission.)
The risk of strangulation with any symptomatic hernia, internal or external, is significant and has been reported to be 28%, as they tend to be closed loop obstructions; consequently, symptomatic hernias should be repaired [7,17]. Asymptomatic and minimally symptomatic hernias, on the other hand, are safe to observe. In the largest randomized, clinical trial, Fitzgibbons and colleagues [18] studied symptomatic and minimally symptomatic hernias, defined as easily reducible hernias without pain during limited physical activity. Patients in the observation arm had similar symptoms at follow-up as those patients undergoing repair. Of those patients who underwent watchful waiting, the risk of incarceration was minimal (0.0018 hernia-related events per patient-year). Thirty-one percent of those initially observed underwent operative repair for symptom progression within 3.2 years of median follow-up. This study was performed in white men older than 40, so its applicability to other ethnic groups, younger patients, and women is unknown.
The remaining 20% of SBOs have multiple, but much less common, causes. The inflammatory changes of Crohn disease may result in acute, subacute, or chronic bowel obstructions. Although most patients will require surgical resection at some point in their disease process, the aim is to avoid intervention until absolutely necessary and, therefore, these patients are managed primarily with medical treatment [19]. It is imperative, however, to ensure there is no evidence for perforation. Gallstone ileus results from a fistulous connection from the gallbladder to the gastrointestinal tract with passage of a large gallstone that obstructs the bowel lumen [20]. Remaining causes include radiation enteritis, bezoar/foreign body, volvulus, hematomas, and abscesses (Fig. 6).
It should be noted that there can be multiple causes of obstruction within the same patient. For instance, patients with colorectal cancer who undergo colectomy and have postoperative radiation therapy for recurrence may have any combination of adhesive disease, hernia, malignancy, and radiation enteritis causing their obstruction. In these patients, diagnosis of the specific etiology is difficult and will usually become apparent only upon operative exploration.
Pathophysiology
A fundamental understanding of the pathophysiology of SBO and strangulation obstruction is necessary for an understanding of the changes in the peritoneal cavity, bowel wall, and mesentery, which are closely associated with the patient’s clinical course and CT findings. Regardless of the cause of obstruction, the pathophysiologic effects of bowel obstruction are similar; the symptoms result from a blockage of normal intestinal transit. As luminal fluids pool proximal to the blockage, the patient may experience nausea and vomiting. Vague, diffuse abdominal pain may result from persistent peristalsis both above and below the obstruction. The patient may continue to have bowel function in the form of flatus and stool early in the process owing to distal peristalsis, even with complete obstructions. Abdominal distention results from increased intestinal volume. As the obstruction progresses, the bowel wall is stimulated to secrete fluid and electrolytes and may result in postobstructive diarrhea [21,22]. If not decompressed, a positive feedback loop of distension and secretion occurs. As the pressure approaches 30 mm Hg, the terminal lacteals become occluded, resulting in bowel wall lymphedema [23]. This causes the luminal pressure to increase further, which, if left untreated, will lead to venous outflow obstruction at the postcapillary venules at pressures greater than 50 mm Hg. The increased venous hydrostatic pressure leads to alterations in Starling forces, causing substantially increased filtration across the capillary bed into the bowel lumen, further exacerbating intravascular volume depletion. Progressive volume depletion will ultimately result in dehydration, hypotension, tachycardia, and metabolic acidoses. Further unchecked, venous hypertension will lead to microvascular or macrovascular arterial compression and, consequently, bowel wall necrosis (Fig. 7). Sepsis from either bacterial translocation through the compromised gut-mucosal barrier or free perforation may result, which, if left untreated, is fatal.
Diagnosis and workup
The approach to SBO should be systematic and should focus on (1) securing and confirming the diagnosis, (2) identifying the etiology, and (3) determining likelihood of strangulation. Each step is aided by a thorough history and physical examination, pertinent laboratory evaluation, and judicious radiographic imaging. The information gained will optimize eventual surgical decision making.
History and physical examination
The diagnosis of SBO should be made primarily on history and physical examination. Signs and symptoms include colicky abdominal pain, nausea and vomiting, obstipation, and abdominal distension. The patient with partial obstruction may describe borborygmi, often quite graphically and with the corroboration of a companion. The patient’s history will usually point to a specific cause. The vast majority of patients with adhesive obstructions will have had a prior abdominal operation. Suspicion of malignancy is heightened by an unoperated abdomen in the absence of radiation or inflammatory bowel disease, as well as unintentional weight loss and/or night sweats. Despite the rarity of gallstone ileus, intermittent chronic right upper quadrant pain in an elderly patient with obstructive symptoms should call this entity to mind.
The consulting surgeon must take heed of abnormal vital signs and perform a thorough physical examination. Orthostatic changes in heart rate and blood pressure indicate early volume depletion, whereas more severe hypovolemia will manifest as frank hypotension and tachycardia. Sepsis secondary to advanced bowel ischemia or perforation will amplify hypotension and tachycardia, and patients with neglected SBO may present with combined hypovolemic and septic shock.
Typical abdominal physical findings of SBO include diffuse abdominal tenderness, distension, and tympany. The abdominal tenderness of uncomplicated SBO is mild; more severe tenderness follows development of bowel ischemia culminating in the exquisite percussion tenderness seen with necrotic bowel. The degree of abdominal distension increases with more distal levels of obstruction and will be minimal for proximal jejunal obstructions. Incisional scars indicate previous operations, which may not have been reported. Palpation of incisional scars and the periumbilical, inguinal, and femoral regions may reveal the firm, tender bulge of an incarcerated hernia. The rare palpable mass suggests advanced malignancy or a long-standing inflammatory process. A metastasis in the pouch of Douglas may be manifest as a hard anterior mass on digital rectal examination, a “Blumer shelf.” Rushes and tinkles heard on auscultation of the abdomen add nothing to the signs and symptoms. Indeed, Dr Charles H. Mayo, speaking before the American Surgical Association in 1922, stated, “I should prefer to see a stomach tube rather than a stethoscope hanging around the neck of my surgical intern” [24].
Laboratory testing
Currently available laboratory tests do not contribute to the diagnosis of SBO, but they can confirm clinical suspicion of volume depletion, guide volume resuscitation, and assist recognition of bowel ischemia. Hypovolemia may manifest as azotemia with an elevated blood urea nitrogen to creatinine ratio. Patients with associated vomiting are at risk for hypochloremic, hypokalemic metabolic alkalosis. Leukocytosis has been implicated as a marker of strangulation, although a normal white blood cell count does not rule out ischemia [25]. Last, patients with severe volume depletion or sepsis from perforation or bacterial translocation will present with metabolic acidosis.
Imaging
Abdominal flat and upright radiographs, along with a chest radiograph, can confirm the diagnosis of SBO, but are accurate only in 50% to 60% of patients, and can be normal in 21% of patients with confirmed obstruction [2,26,27]. X-ray interpretation relies on the recognition of bowel gas patterns. As defined by plain radiograph, complete bowel obstruction comprises dilated small bowel (>3 cm), air-fluid levels, and the absence of colonic gas. Although this pattern is diagnostic of obstruction, other findings are possible. For example, the absence of bowel gas shadows is consistent with proximal and closed loop obstructions and/or obstructed bowel completely distended and exclusively distended with fluid.
CT findings of SBO include small bowel dilatation with air-fluid levels, collapsed distal bowel, and, at times, a distinct transition point. CT has replaced upper gastrointestinal series/small bowel follow-through and enteroclysis for confirmation of a clinical diagnosis of SBO [14,28,29]. Although CT has greater sensitivity, its main utility lies in its ability to diagnose the underlying cause of the obstruction [30–32]. Closed loop obstruction, internal hernia, tumor, intussusception, and volvulus all give distinctive CT images. General findings of SBO absent these specific findings suggest adhesive disease in the proper setting.
Beyond making a diagnosis, CT findings can guide the decision to pursue immediate exploration or nonoperative management. CT findings of free intraperitoneal fluid, mesenteric edema, pneumatosis, and portal venous gas correlate with small bowel ischemia [25,33]. As discussed later in this article, CT findings can predict need for operative intervention; further, the water-soluble oral contrast agents used have been shown to be therapeutic and decrease the need for operative intervention [25,33–39]. New techniques in multidetector-row CT with 3-dimensional reconstructing techniques may allow for even greater diagnostic ability. In a limited series, Hong and colleagues [29] were able to demonstrate an improved ability to locate the site of obstruction and diagnose its cause compared with conventional CT. As a final imaging consideration, magnetic resonance imaging (MRI) in the form of enterography and enteroclysis has been used successfully in small studies [40,41]. Issues of cost and accessibility severely limit the current usefulness of MRI as a front-line technique for imaging SBO, however.
Management
Resuscitation
The initial step in management of SBO consists of correcting the fluid and metabolic derangements that result from protracted vomiting and intraluminal fluid sequestration. Aggressive crystalloid resuscitation should be performed simultaneously with the diagnostic workup. The essential end points of resuscitation for noncomplicated SBO should be normal blood pressure and heart rate with a urinary output of 0.5 mL/kg/h monitored with an indwelling bladder catheter. Correction of electrolyte abnormalities should also be undertaken concurrently. In patients presenting with hypovolemic and/or septic shock, central venous pressure measurement may be a useful guide. All patients with SBO require nasogastric tube decompression regardless if operative or nonoperative management is undertaken. Nasogastric decompression improves mortality, ameliorates symptoms, minimizes aspiration, and provides access for radiographic contrast administration.
Deciding between operative or nonoperative management
The challenge of SBO management lies in deciding on the appropriate treatment plan. An optimal approach will (1) provide immediate operative exploration in patients with strangulation obstructions, (2) facilitate early recognition of patients without strangulation who will not resolve without intervention, and (3) minimize unnecessary operations.
Delay to operative exploration for strangulation obstruction will increase mortality and morbidity, including a greater frequency of bowel resection; hence the adage, “Never let the sun rise or set on a small bowel obstruction” [1,25,39,42,43]. Implicit in this quotation is an ability to promptly and precisely recognize the presence of strangulation. Unfortunately, this predictive ability has been elusive, as even the most experienced surgeons are correct only half of the time [17]. The clinical markers of strangulation are numerous, as demonstrated in Box 1 [25,42–44]. Patients who present with signs of intestinal ischemia comprise the minority of patients with SBO, but patients with signs of ischemia will have a strangulation obstruction up to 45% of the time [39]. Therefore, any patient with these features and a concurrent diagnosis of SBO has a strangulation obstruction until proven otherwise and should undergo emergent celiotomy after resuscitation.
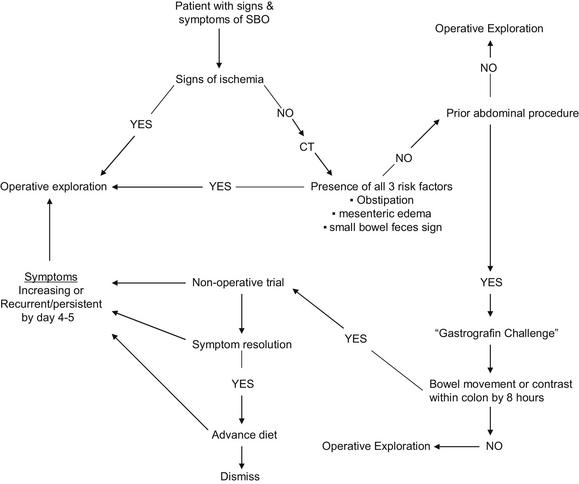
Especially vexing are those patients who harbor a strangulated obstruction and fail to display any of the features outlined in Box 1 [25,39]. To combat this discrepancy, the presence of “complete” SBO was traditionally used as a tool to screen for patients with either silent strangulation or at risk of developing strangulation. Used in this way, the diagnosis of complete SBO implies the need for emergent/urgent operation; however, complete SBO is actually defined as the presence of dilated small bowel with air fluid levels in the absence of colonic gas on abdominal radiograph [45]. Further, the differentiation of complete versus partial SBO is fraught with some subjective elements: time to last flatus and the patient’s memory thereof, and absence versus paucity of colonic gas as visualized by plain radiograph versus CT. An additional difficulty with the complete versus partial paradigm follows from the finding that 31% to 43% of patients with complete SBO or peritonitis will not require bowel resection [39,42,43]. Given these concerns, the authors believe the traditional focus of differentiating complete from partial SBO should change to one of predicting failure of nonoperative management with the goal of exploring patients with predicted failure as soon as possible. This approach promises not only early exploration for patients with silent strangulation, but also shortened hospital stay for all. Two potential decision-aids are emerging to assist with such an approach: (1) response to water-soluble oral contrast meals and (2) predictive models.
Water-soluble oral contrast agents such as methylglucamine diatrizoate (Gastrografin) and sodium diatrizoate/meglumine diatrizoate (Urografin) are high-osmolar liquids used in CT and other gastrointestinal radiographic studies. Oral or nasogastric administration of these agents with subsequent imaging has been shown to both predict and reduce the need for operative intervention in patients presenting with SBO without signs of strangulation [35–38,46–50]. The methodological variability between studies has been significant and impedes interpretation of the general technique; different agents, different doses, and different durations from administration to assessment have been used. In the trials studying methylglucamine diatrizoate, a dose of 50 to 100 mL was administered, whereas the single sodium diatrizoate/meglumine diatrizoate trial used 40 mL. Failure of contrast to pass into the colon as detected by plain radiograph predicted need for operation, but this assessment was made at times varying from 4 to 72 hours. Several studies demonstrated an apparent therapeutic effect of the water-soluble agent: patients in the contrast arms underwent fewer operative explorations than those in the control groups [35–38,47]. This therapeutic effect was not demonstrated in all studies, however [48–50]. Both the predictive and therapeutic utility of water-soluble contrast administration is supported by a meta-analysis performed by Branco and colleagues [50]. The investigators showed that if upper gastrointestinal contrast reached the colon within 8 hours of administration, then 99% of patients would not require operation. On the other hand, 90% of those without contrast in the colon at 8 hours required operation. Further, patients receiving contrast had a reduced need for operation compared with controls (20% vs 29%, P = .007). Although 508 patients were included in the meta-analysis, the investigators caution that the primary studies’ significant methodological differences limit the certainty of the conclusions: only 3 of the 9 studies analyzed were randomized, controlled trials. Another limitation was the lack of data regarding missed strangulation obstructions. First studied in 1994, water-soluble contrast administration in SBO management has many proponents, but widespread acceptance awaits accrual of better data demonstrating efficacy.
An alternative, but also complementary, approach involves the development of predictive models based on combinations of readily available clinical parameters. The goal of a predictive model for SBO management is to identify, soon after surgical consultation, those patients who will ultimately require operative exploration. Two early studies attempted to develop predictive systems based on clinical, laboratory, and plain radiographic data; both initiatives failed [3,51]. Newer models incorporate data from state-of-the-art CT imaging. This modality provides remarkable images of the small bowel mesentery and bowel wall, demonstrating such signs as mesenteric edema, mesenteric swirling, and reduced bowel wall enhancement [52–55]. This technology rekindled interest in predicting the need for exploration, leading to recent development of 2 models attempting to predict the need for operative exploration in SBO [25,33,56]. The goal of these models is to identify those patients who will undergo operative exploration for SBO during their hospital stay in an attempt to reduce missed strangulation obstructions and minimize time to operation for all who will require it. An initial attempt by Jones and colleagues [56] identified 7 CT features based on retrospective review that were associated with the need for exploration. The scoring system did not incorporate history and physical examination findings and was not validated in a separate population, limiting its applicability.
More recently, the authors retrospectively screened data from history, presenting physical examination, and initial imaging data to identify predictive factors useful for modeling [25]. Four features were found to predict the need for abdominal exploration: vomiting and CT findings of free intraperitoneal fluid, mesenteric edema, and the lack of the small bowel feces sign. These 4 features in combination predicted a 16-fold increase in the need for operation during the same hospital stay. Those patients with all 4 predictive features undergoing operation within 12 hours of admission experienced a lower mortality than those with the features undergoing later operation. The model was validated and improved in a separate, prospective cohort. Within the new cohort, vomiting was not predictive and free intraperitoneal fluid was only minimally so. The prospective data collection allowed analysis of the clinical sign of obstipation (lack of flatus for 12 hours) and this was found to be predictive. Based on these most recent data, we have eliminated free intraperitoneal fluid and vomiting as discriminatory features but have added obstipation. Our newest model identifies patients with mesenteric edema, lack of the small bowel feces sign, and obstipation as high risk. Of the 29 of 100 patients with these features (mesenteric edema, lack of the small bowel feces sign, and obstipation) on admission, 9 (31%) had strangulation obstructions and 22 (76%) required abdominal exploration before dismissal; these results reflect a concordance index for the need for exploration, a measure of the predictive ability of a model, of 0.77 which is comparable with that of other clinically used biomedical models [57]. Sixty-nine patients presented without signs of strangulation and 2 or fewer features; within this low-risk subgroup, only one patient suffered a missed strangulation obstruction (1.4%). On the basis of these findings, the authors recommend urgent exploration for any patient presenting with signs of strangulation or all 3 of the new model features present on admission (Fig. 8).
There are novel molecular markers that are being investigated as predictors of intestinal ischemia and, therefore, strangulation obstruction. Fatty acid binding protein is present in enterocytes lining the gastrointestinal tract. The enterocytes are the intestinal cells most sensitive to ischemia. Fatty acid binding protein is released into the systemic circulation after an onset of intestinal mucosal hypoxia; serum levels have a 50% positive predictive value for small bowel ischemia [58]. Procalcitonin has been used as an inflammatory marker in sepsis [59]. No human studies have been performed, but animal models have demonstrated an ability for procalcitonin to predict small intestinal strangulation, but interestingly not large bowel ischemia [60,61].
Both the oral contrast meal and the predictive models are appropriate methods of determining the need for exploration in patients lacking signs of strangulation, but each approach has limitations. The oral contrast meal cannot determine the presence of strangulation until hours after admission. The time required for the oral contrast meal challenge could allow initial noncritical ischemia to progress to a point of irreversible strangulation, or allow strangulation to progress to sepsis [39,62]. Conversely, predictive models are not as directly therapeutic as oral contrast can be. We emphasize that the 2 methods complement each other. If a patient does not demonstrate criteria predicting the need for urgent exploration, an oral contrast challenge may be therapeutic and further identify those patients who will fail nonoperative management.
Nonoperative management
If a nonoperative trial is initiated, then serial abdominal examinations and assessment of vital signs are mandatory to monitor for signs of developing small bowel ischemia. When bowel function returns, a diet is slowly reinitiated. If tolerated, then the patient may be dismissed. Exploration should be performed if bowel function does not return after 3 to 5 days or, generally, if symptoms return after hospital dismissal [6,25,44]. Patients whose bowel function returns without operation experience a shorter time to SBO recurrence (153 vs 411 days) compared with patients treated operatively [63]. There is increased bacterial gut translocation in humans in the setting of SBO, but to date, no data exist to recommend for or against gut decontamination [44,64]. Finally, the use of a long intestinal tube (ie, Baker tube) offers no benefit over nasogastric decompression [65].
No matter the method of treatment, there is a significant rate of SBO recurrence; however, patients who undergo operative exploration for SBO will have a lower recurrence rate (34% to 40% vs 26% to 32%) than patients treated nonoperatively [63,66,67]. Select patients will resolve with nonoperative management, but re-present with intermittent, recurrent episodes of SBO. These patients may choose exploration to avoid the discomfort of the repeated episodes. When the frequency of episodes begins to either compromise the patient’s participation in work and favored recreational activities or threaten the patient’s nutrition, the surgeon should recommend operation. For those with a known chronically agglutinated or “frozen” abdomen, repeated courses of nonoperative management will usually carry less risk than operation. However, the onset of weight loss is a sign that exploration by a surgeon experienced in the reoperative abdomen should be seriously considered; endoscopic dilatation of strictures or chronic home parenteral nutrition may be the only other options.
Operative management
Traditionally, the operative management of patients with SBO has been through open celiotomy, but there is increasing evidence for the efficacy of laparoscopic management [68–79]. Laparoscopy has been adopted more slowly for SBO management than for other abdominal procedures owing to the technical challenges imposed by bowel distension, compromised exposure, and fear of iatrogenic bowel injuries during bowel manipulation [70]. The main theoretical advantages beyond reduction in postoperative pain, decreased length of stay, and fewer wound complications is the promise of less operative trauma, minimization of adhesion formation, and the potential for fewer subsequent adhesive obstructions [5,6,80,81]. In addition, laparoscopy is a diagnostic tool and may be converted to an open celiotomy when necessary [82]. Laparoscopic exploration and enterolysis may be converted to an open celiotomy when necessary. No clinical trials exist comparing laparoscopic to open techniques. The largest review to date studied 93 patients with adhesive SBO. Of the 90 patients approached initially with laparoscopy, 26% were converted to celiotomy [83]. Conversion was necessitated by abdominal distension preventing adequate exposure (41%), iatrogenic perforation (41%), extensive adhesions (30%), and strangulation obstruction necessitating small bowel resection (8%). Preoperative features associated with success of the laparoscopic technique included lower American Society of Anesthesiologists score and prior celiotomy. Patients who underwent open celiotomy had a longer duration of stay, but there was no mortality difference between the groups (6%).
Advanced laparoscopic skills will minimize conversion rates, but there will always be an irreducible percentage of cases converted owing to the hostile nature of the peritoneal cavity from which some obstructions arise. Appropriate patient selection is of paramount importance for the success of laparoscopy, and the surgeon should be familiar with factors correlating with conversion so that his or her judgment is optimal [79,82,84]. In a multi-institutional review, Lee and colleagues [67] demonstrated that a focal, isolated transition point on CT was predictive of laparoscopic success 100% of the time versus 17% if this finding was not present. Comprehensive meta-analysis by Ghosheh and Salameh [79] showed that laparoscopy will be successful 66% of the time. Factors associated with conversion to celiotomy were dense adhesions (28%), need for bowel resection (23%), inability to determine the etiology of obstruction (13%), iatrogenic injury (10%), malignancy (7%), poor visualization (4%), and hernia (3%). Other reported factors consistent with a need to convert from laparoscopy to celiotomy are the number of previous abdominal procedures, bowel distension greater than 4 cm, documented dense adhesions, and a complete obstruction [80,82,83]. In patients with these features, especially if in combination, primary celiotomy should be strongly considered, and, if a laparoscopic approach is chosen, there should be a low threshold for conversion. With a 14% to 19% rate of iatrogenic bowel injuries, it must be emphasized that conversion is not a failure, but “sound surgical judgment” [72,76,79,84–86]. Clearly, appropriate patient selection is of paramount importance for success of the laparoscopic approach. When the cautions are heeded, however, successful laparoscopy will result in shorter hospital stay and decreased morbidity [86].
Small bowel ischemia may develop with SBO through incarceration in an internal or external hernia, closed loop obstruction, volvulus, or severe nondecompressed distension. Intraoperative identification of ischemic bowel demands a decision regarding resection. Necrotic, strangulated bowel, dark blue to black in color and generally with a foul odor, must be resected at the first exploration to prevent sepsis. However, less severe ischemic changes may be reversible and challenge the surgeon to determine the necessity of resection. After release of the obstruction, originally ischemic bowel may regain viability as indicated by such signs as a return toward normal color, palpable arterial pulsations, audible Doppler signals on the antimesenteric border, and visible peristalsis [87]. In the only clinical trial to date assessing the accuracy of detecting reversible versus nonreversible ischemia, Bulkley and colleagues [87] concluded that clinical judgment alone was accurate, as assessed by the patient’s clinical course, 89% of the time, but led to a 49% rate of unnecessary bowel resection. Doppler faired slightly worse with an accuracy of 84%. Fluorescein dye assessment (1000 mg intravenous sodium fluorescein) under a long-wave (3600 A) ultraviolet (“Woods”) lamp was able to accurately identify viable and nonviable bowel 100% of the time. Nonviable bowel has 3 distinct categories: (1) patchy (≥5 mm areas of nonfluorescence), (2) perivascular (nonfluorescence of the antimesenteric border), and (3) nonfluorescent. All other patterns are considered viable. Laparoscopic fluorescein dye techniques have been developed, but not studied in humans [88–90].
Second-look celiotomy remains an important tool to assess bowel viability over time [91]. Gangrenous bowel is resected at the initial operation, but ischemic bowel with potential for reversibility is left in situ, potentially eliminating unnecessary bowel resection. If resection has been performed leaving questionably viable margins, anastomosis should be deferred initially. Temporary abdominal closure is used, and a “second-look” celiotomy is performed at 18 to 48 hours. Bowel that remains ischemic or otherwise unsuitable for anastomosis can be resected at that time. Animal models have suggested fluorescein techniques are superior to second-look celiotomy but no human studies have directly compared the methods [92,93]. Adding to the armamentarium, a second-look laparoscopic technique for assessment of bowel viability has been described and can be useful after laparoscopic enterolysis of questionably viable bowel not requiring immediate resection [94]. When bowel necrosis leads to sepsis and intraoperative hemodynamic instability, abbreviating the primary operation in a “damage control” fashion after resection of the clearly necrotic bowel will minimize physiologic insults and allow prompt initiation of intensive care unit resuscitation. This is often the most rapid course to improve the systemic derangements of septic shock. The optimized mesenteric blood flow will theoretically minimize later bowel resection. In such cases, enterolysis should be limited to only that necessary for resection of necrotic bowel and anastomoses should be deferred.
Management of obstruction by specific etiology
Adhesive disease
Adhesions are formed through a complex process, but generally start from peritoneal mesothelial lining disruption caused by operative dissection or other trauma. This is followed by fibrin deposition on raw surfaces and formation of a spanning fibrin mass connecting adjacent tissues. The adhesion matures with the addition of fibroblasts and consequent collagen deposition. Over time, the adhesions will evolve as the collagen is remodeled. An adhesion can lead to obstruction through several mechanisms: severe knuckling and kinking of bowel in a gnarled mass, volvulus about the fixed point of the adhesion, and incarceration in an internal hernia. Several pharmacologic agents have been investigated for their potential to prevent adhesion formation; these include fibrinolytics, steroids, and nonsteroidal anti-inflammatory medications [95–97]. These agents have successfully diminished adhesion formation in animal models, but their use in humans is limited owing to hemostatic and wound-healing concerns; none have been shown to prevent subsequent obstruction. In contrast, hyaluronic acid–carboxymethylcellulose membrane (Seprafilm) has been shown to decrease adhesion formation and incidence of early postoperative obstruction (7% vs 14%) [98,99].
The potential ability to minimize late postoperative SBO is more important than lessening the adhesion burden. The only data on the effect of hyaluronic acid–carboxymethylcellulose on late postoperative SBO comes from a prospective, randomized trial performed by Fazio and colleagues [100]. Of 1701 patients undergoing bowel resection, 840 were randomized to receive hyaluronic acid–carboxymethylcellulose and 861 were randomized to a control group. Overall, the rate of postoperative obstruction at 3.5 years was the same: 12% in each arm. In the subgroup of patients undergoing resection for adhesive SBO, however, the patients receiving hyaluronic acid–carboxymethylcellulose had fewer subsequent obstructions than did controls: 1.8% required reoperation versus 3.4% (P <.05). In a separate subgroup of 90 patients who had prior adhesiolysis for SBO, the incidence of reexploration for SBO was not statistically different between the study (17%) and control (10%) groups. Hyaluronic acid–carboxymethylcellulose membranes should be used with caution, however, because the safety evaluation for the clinical trial performed by Fazio and colleagues [100] demonstrated significantly more anastomotic leaks: 2% in the study arm compared with fewer than 1% in the control arm (P <.05). This difference in leak rate, however, was attributed to wrapping of the anastomosis with the membrane [101]. Fazio and colleagues [100] recommend carefully weighing the risks and benefits of hyaluronic acid–carboxymethylcellulose membranes on a case-to-case basis.
Other materials have been studied to reduce the rate of adhesion formation, including statins, melatonin, collagen gel, phosphatidylcholine, and even honey, but none have been shown to reduce the rate of obstruction [102]. To minimize adhesion formation and postoperative obstruction, all operations should be performed with gentle handling of tissues, minimization of tissue plane disruption, and debridement of infectious and ischemic debris. Further, omentum should be used as a barrier to protect bowel from raw dissection or incisional surfaces and to fill postresection pelvic voids.
Malignant SBO
Malignant SBO is defined as obstruction from known incurable, intra-abdominal neoplasm; obstruction is attributable to extrinsic compression or luminal obturation by tumor mass, bulky lymphadenopathy, or diffuse peritoneal carcinomatosis [103]. Any metastatic or primary tumor may be responsible for a malignant SBO, but colorectal and ovarian neoplasms are the most common causes; 10% to 28% and 20% to 50% of patients with these neoplasms, respectively, will be complicated by SBO [9,104]. Obstruction from primary tumors of the jejunum or ileum are rare, as they represent only 1% to 2% of all gastrointestinal neoplasms; these unusual tumors are most often resectable.
The goals of SBO management for patients with incurable, malignant SBO should be to improve quality of life and maximize days out of hospital. The patient and surgeon should collaboratively define additional goals that incorporate the patient’s specific desires. Recognizing that life expectancy averages 3 to 6 months, reasonable goals include regaining the ability to eat, relieving abdominal distension, reducing nausea and vomiting, and dismissal to home [103–105]. Despite multiple options among surgical, medical, and endoscopic/interventional techniques, little outcome data exist to guide decision making. This forces clinicians to rely on anecdotal and personal experience to guide therapy and places great responsibility on the surgeon to exercise thoughtful judgment when making recommendations [10,106].
Surgical procedures and plans must focus on maximizing palliation and minimizing both complications and time in hospital. The simplest procedure that provides symptom relief should be chosen. If intraperitoneal conditions are safe for anastomosis, bypass of the obstructed segment will often be best [107]. If the creation of a safe anastomosis is not feasible, a proximal diverting loop stoma remains an option. The palliative benefit of both bypass and enterostomy diminishes when functional bowel length is shortened to a degree that may require home intravenous hydration to treat volume depletion and electrolyte abnormalities. Anastomosis should be performed only when conditions are clearly favorable; one should always be suspicious of a lurking distal obstruction and remember that anastomotic leak will likely deprive the patient of any time outside the hospital. Surgeons must be flexible and creative when faced with unexpected intraoperative findings, but always make decisions that maximize the patient’s likelihood of participating in meaningful relationships with family and friends before the patient’s death. Up to 80% of a patient’s obstructive symptoms will resolve with this approach, but complications are substantial with morbidity and mortality rates of up to 90% and 41%, respectively [104,107–110]. Also, re-obstruction rates, although imprecisely defined with current data, can be substantial [111]. Palpable intra-abdominal masses, ascites, carcinomatosis, multiple transition points, and poor clinical status have all been implicated in the failure of surgical management [112].
Medical management should center on the use of analgesics, antiemetics, and antisecretory agents and should be used in conjunction with the other modalities. Palliation via endoscopic and interventional techniques often provides helpful and potentially lower-risk options [113]. Self-expanding metal stents (SEMS) are small-diameter bare metal frames that are passed endoscopically or fluoroscopically via guide wire through the obstruction and expanded, allowing for an increase in lumen size. Although success with gastric outlet obstructions has been demonstrated, suitable focal obstructing lesions beyond the ligament of Treitz are rare. Patients with carcinomatosis and multiple obstructing points will fail SEMS management [113,114]. Decompressive gastrostomy tubes will provide some palliation of intractable nausea and vomiting. Percutaneous endoscopic gastrostomy (PEG) may be performed as a sole intervention when celiotomy is contraindicated by extent of disease or medical condition; preprocedure CT can be used to ensure malignant masses are not interposed between the stomach and abdominal wall. Malignant ascites, which can lead to postprocedure local wound complications and difficulty with transillumination, is a relative contraindication to PEG. Large-volume paracentesis has been reported to facilitate successful PEG placement in the face of ascites [115]. Patients with prior gastric or esophageal resection can undergo tube pharyngostomy or percutaneous jejunostomy placement [116,117].
The surgeon must remember that malignant SBO can present situations wherein no intervention can be performed without harming the patient. These situations must be recognized and explained to the patient and family gently and truthfully. End-of-life planning and care is often facilitated by consultation with colleagues with expertise in palliative care.
Early postoperative SBO
Up to 2% of patients after intraperitoneal operations will develop early postoperative SBO. Unfortunately, a standard definition does not exist; some authors have defined early postoperative SBO as that occurring within the first 10 days and some as that occurring up to 6 weeks after celiotomy or laparoscopy [118,119]. Of patients who prove to have early postoperative SBO, 10% to 20% will develop strangulation. This is a dangerous situation with reported mortality ranging between 2% and 18% [52,119–123]. Differentiating postoperative ileus from early postoperative SBO by clinical criteria is challenging. CT imaging greatly aids the distinction, however. In one study of 36 patients, CT not only differentiated early postoperative SBO from ileus with 100% sensitivity and specificity, but this modality also identified the nature of the obstruction [124]. The reason for reoperation included adhesion, abscess, hematoma, intussusceptions, and stricture.
Initial management of both prolonged postoperative ileus and early postoperative SBO consists of nasogastric decompression and parenteral nutritional support. Presumed ileus associated with fever, leukocytosis, and/or frank sepsis should prompt abdominal CT imaging. In the absence of nonabdominal sources of infection, CT will likely reveal a postoperative septic process such as abscess and/or leak. Rarely in this setting, CT will reveal signs of obstruction and secondary small bowel ischemia. CT imaging should also be performed in the absence of infectious concerns for presumed ileus persisting beyond 10 days to rule out SBO.
The decision to reexplore or continue nonoperative management for early postoperative SBO should take 2 factors into consideration: (1) 90% of early postoperative SBO will resolve spontaneously, and (2) the window for safe reoperation is short [122]. Indeed, the decision is intimately dependent upon the time elapsed since operation. By 10 to 14 days, dense, hypervascular adhesions develop, creating an agglutinated peritoneal environment hostile to enterolysis and making surgical reexploration dangerous because of the risk of iatrogenic bowel injury and fistula formation [24]. This hostile peritoneal environment generally persists for 6 weeks. To maximize the contribution of CT to early postoperative SBO management, it should be performed before peritoneal agglutination to provide the opportunity for early intervention. In the event that a definitive diagnosis of SBO can be made before 10 to 14 days, reoperation is reasonable if a surgically correctable cause, such as abscesses or fascial dehiscence/hernia, can be identified. Management of early postoperative SBO after the 10-day to 14-day window becomes a very long-term endeavor requiring patience from both the surgeon and the patient. Diagnosis after the window of opportunity has elapsed is best managed with long-term gastric decompression either by percutaneous endoscopic gastrostomy if technically feasible or a small-bore, flexible nasogastric tube. Home parenteral nutrition support will be necessary until reexploration is feasible. If strangulation or other obstruction-related ischemia is present within the 10-day to 6-week window, then the risks of exploration are outweighed by the consequences of bowel ischemia, and exploration is required. Based on our clinical experience, reoperations beyond postoperative day 10 to 14 for postlaparoscopy SBO appear safe and feasible in selected patients. This is in clear contrast to our clinical experience of reoperation after celiotomy and may be the result of a more focal etiology (isolated band, internal hernia) and the lower likelihood of diffuse agglutination; however, this has not been substantiated in clinical trials (Michael L. Kendrick, MD, Rochester, MN, personal communication, November 2010).
Hernia
Hernias can be broadly categorized into external and internal subtypes. Incarcerated external hernias in inguinal, femoral, umbilical, parastomal, and incisional locations are diagnosed on physical examination. Manual reduction with gentle sedation is often successful and can eliminate the need for emergent herniorrhaphy. After reduction of an incarcerated hernia, patients require hospital admission for close observation to exclude the possibilities of a reduced ischemic segment and reduction en masse [125]. The hernia defect should be repaired before hospital dismissal to prevent recurrence of the incarceration. Unsuccessful reduction requires emergent repair because of the high risk of strangulation. A patient with a Richter hernia will not present with typical signs of SBO, such as nausea and vomiting, as only the antimesenteric wall of the bowel is incarcerated. This type of hernia may not be palpable on physical examination, but should be considered in any patient with abdominal pain. Dehiscence of small laparoscopic cannula wounds provides opportunity for hernias of the Richter type. Internal hernias can be congenital or acquired postoperatively. Operations that include bowel resections or Roux-en-Y reconstruction create multiple foramina that, if not closed, can lead to herniation (see Fig. 1). Congenital internal hernias are rare, but there are multiple types including obturator, paraduodenal, transmesenteric, and transomental. These hernias all require urgent repair with reduction of the bowel contents and closure of the defect. It has been suggested that obturator hernias, which are relatively difficult to repair primarily because the tissues do not reapproximate easily, do not require closure because of the low recurrence rate of between 0% and 10%. The possibility, however, that this low recurrence rate is related to poor long-term follow-up in the elderly population affected must be considered [126].
Crohn disease
The pathophysiology of Crohn disease leads to several types of obstructive processes. In the acute inflammatory phase, the diameter of the small bowel lumen decreases owing to the transmural inflammation and smooth muscle spasm. These patients do not develop strangulation and can be managed medically with hydration, nasogastric decompression, and parenteral nutrition. Anti-inflammatories such as steroids and infliximab are generally reserved for patients who fail to resolve with these measures. Strictures, another major cause of SBO in patients with Crohn disease, result from chronic inflammation, collagen deposition, and scar retraction. Strictures will generally not respond to anti-inflammatory medications [127].
Although an initial retrospective report implicated infliximab in the development of acute obstructions, 2 prospective studies have shown that infliximab is efficacious in the acute setting and is not associated with obstruction [128,129]. The Crohn’s Therapy, Resource, Evaluation, and Assessment Tool (TREAT) was a prospective observational study of patients with Crohn disease that, on univariate analysis, showed a high incidence of strictures and obstructions in patients treated with infliximab versus placebo (1.95 events/100 patient-years vs 0.99 events/100 patient-years; P<.001). On multivariate analysis, however, only the severity and duration of Crohn disease, steroid use, and the presence of ileal disease, not infliximab, were independently associated with obstruction [128]. A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-Term Treatment Regimen (ACCENT 1) was a multicenter, prospective, randomized, placebo-controlled clinical trial comparing the effects of increasing doses of infliximab on 545 patients with moderate to severe Crohn disease who failed conventional therapy [129]. The patients receiving infliximab had the same incidence of obstruction as those patients who received placebo (3% vs 2% vs 3%).
Perforation with abscess can create similar symptoms to strangulation obstructions with abdominal pain, nausea, vomiting, and peritonitis. CT imaging will help to differentiate these processes. In addition, it should be remembered that a high proportion of patients with Crohn disease will have had prior operative explorations and are at risk for internal hernia and adhesive disease. In addition, clinicians need to be aware of the risk for malignant transformation with subsequent malignant SBO.
Radiation enteritis
Radiation enteritis is a distinct entity that differs from SBO, but causes similar symptoms such as diarrhea, nausea, weight loss, and abdominal pain as a result of chronic ischemia from obliterative arteritis. There are acute and chronic forms of the disease. The acute phase occurs during or shortly after radiotherapy and is self-limited with medical management. The chronic form is a result of the ischemic changes that lead to stricture, fibrosis, ulceration, and fistula. These pathologic findings, found in isolation or in combination, result in poor absorption, diminished intestinal transit, and bacterial overgrowth. In the presence of stricture, the patient’s symptoms will arise both from the stricture and from functional dysmotility. Considering this dual etiology, the hostile nature of the radiated abdomen, and the potential for tumor recurrence, operation for stricture should be undertaken only after the surgeon has become thoughtfully convinced that the stricture dominates the clinical picture and that the abdomen is indeed operable. Given that most of these patients will have had prior resections and known intra-abdominal tumor burden, the etiology can be difficult to elucidate. Patients with intra-abdominal tumor resection and adjuvant radiotherapy will have a 4 times greater risk of SBO than patients who underwent resection only [130]. As the dose increases and the radiation field widens, the rate of SBO will increase. The use of small bowel exclusion techniques during radiotherapy will decrease the incidence of SBO [131]. The care and goals of therapy in these patients mirrors that of malignant SBO, as the abdominal milieu can be hostile and there is significant risk for injury. For patients having a persistent or recurrent malignancy, the goal of prima non nocere must be foremost in the surgeon’s mind.
Summary
SBO is a common disease with multiple causes. The most significant advances over the past several years have involved, first, decision-making techniques to promptly and accurately identify patients who will require exploration, and, second, the increasing use of laparoscopic techniques. “Complete” bowel obstruction is becoming an outdated term, as treatment algorithms use predictive models and oral contrast challenges to select patients for operation without recourse to the notion of “complete obstruction.” Laparoscopic techniques are gaining acceptance as a primary modality in the treatment of SBO. Appropriate patient selection is necessary for success, but successful laparoscopic SBO management can reduce postoperative pain, minimize hospital stay, and may lead to fewer adhesions, possibly preventing further adhesive SBO.
Strangulation obstruction is the major cause of morbidity and mortality in SBO. Although unrecognized strangulation obstructions remain, their incidence is decreasing with the new protocols in development. Future efforts should focus on incorporating predictive models into management with the goal of eliminating unrecognized strangulation obstructions. Further refinement of the predictive models incorporating outcomes of oral contrast challenges and molecular biomarker data may allow surgeons to reach this goal. In addition, the benefit of the elimination of interpractitioner variability conferred by standardized protocols will in itself improve patient outcomes.
References
[1] N.F. Ray, W.G. Denton, M. Thamer, et al. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1-9.
[2] P. Mucha. Small intestinal obstruction. Surg Clin North Am. 1987;67(3):597-620.
[3] L.S. Bizer, R.W. Liebling, H.M. Delany, et al. Small bowel obstruction. Surgery. 1981;89(4):407-413.
[4] C. Holcombe. Surgical management in tropical gastroenterology. Gut. 1995;36:9-11.
[5] G. Barmparas, B.C. Branco, B. Schnüriger, et al. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14:1619-1628.
[6] M.R. Cox, I.F. Gunn, M.C. Eastman, et al. The operative aetiology and types of adhesions causing small bowel obstruction. Aust N Z J Surg. 1993;63:848-852.
[7] M.L. Kendrick. Partial small bowel obstruction: clinical issues and recent technical advances. Abdom Imaging. 2009;34:329-334.
[8] G.W. Taylor, D.G. Jayne, S.R. Brown, et al. Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. Br J Surg. 2010;97:70-78.
[9] G. Miller, J. Boman, I. Shrier, et al. Small-bowel obstruction secondary to malignant disease: an 11-year audit. Can J Surg. 2000;43(5):353-358.
[10] R.S. Krouse. The international conference on malignant bowel obstruction: a meeting of the minds to advance palliative care research. J Pain Symptom Manage. 2007;34:S1-S6.
[11] H.K. Lee, S.J. Park, B.H. Yi. Diagnostic imaging. Multidetector CT reveals diverse variety of abdominal hernias. Diagn Imaging. 2010;32(5):27-31.
[12] J.M. Bergstein, R.E. Condon. Obturator hernia: current diagnosis and treatment. Surgery. 1996;119(2):133-136.
[13] B.D. Newsom, J.S. Kukora. Congenital and acquired internal hernias: unusual causes of small bowel obstruction. Am J Surg. 1986;152(3):279-285.
[14] M.L. Kendrick, G.F. Dakin. Surgical approaches to obesity. Mayo Clin Proc. 2006;81(10):S18-S24.
[15] M.E. Lockhart, F.N. Tessler, C.L. Canon, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roentgenol. 2007;188:745-750.
[16] W. Petersen. Ueber darmyerschlingung nach der gastro-enterostomie [Concerning twisting of the intestines following a gastroenterostomy]. Arch Klin Chir. 1900;62:94.
[17] M.G. Sarr, G.B. Bulkley, G.D. Zuidema. Preoperative recognition of intestinal strangulation. Am J Surg. 1983;145(1):176-182.
[18] R.J. Fitzgibbons Jr., A. Giobbie-Hurder, J.O. Gibbs, et al. Watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA. 2006;295(3):285-292.
[19] S.C. Nga, G.A. Lieda, N. Arebia, et al. Clinical and surgical recurrence of Crohn’s disease after ileocolonic resection in a specialist unit. Eur J Gastroenterol Hepatol. 2009;21(5):551-557.
[20] M.D. Zielinski, L.E. Ferreira, T.H. Baron. Successful endoscopic treatment of colonic gallstone ileus using electrohydraulic lithotripsy. World J Gastroenterol. 2010;16(12):1533-1536.
[21] R. Shields. The absorption and secretion of fluid and electrolytes by the obstructed bowel. Br J Surg. 1965;52:774-779.
[22] H.K. Wright, J.J. O’Brien, M.D. Tilson. Water absorption in experimental closed segment obstruction of the ileum in man. Am J Surg. 1971;121:96-99.
[23] L.F. Nadrowski. Pathophysiology and current treatment of intestinal obstruction. Rev Surg. 1974;31:381-407.
[24] C.H. Mayo. The cause and relief of acute intestinal obstruction. JAMA. 1922;79:194-197.
[25] M.D. Zielinski, P.W. Eiken, M.P. Bannon, et al. Small bowel obstruction—who needs an operation? A multivariate prediction model. World J Surg. 2010;34(5):910-919.
[26] D.D. Maglinte, S.N. Gage, B.H. Harmon, et al. Obstruction of the small intestine: accuracy and role of CT in diagnosis. Radiology. 1993;188:61-64.
[27] D.D.T. Maglinte, B.L. Reyes, B.H. Harmon, et al. Reliability and the role of plain film radiography and CT in the diagnosis of small-bowel obstruction. AJR Am J Roentgenol. 1996;167:1451-1455.
[28] D.D. Maglinte, G.N. Bender, D.E. Heitkamp, et al. Multidetector-row helical CT enteroclysis. Radiol Clin North Am. 2003;41:249-262.
[29] S.S. Hong, A.Y. Kim, S.B. Kwon, et al. Three-dimensional CT enterography using oral Gastrografin in patients with small bowel obstruction: comparison with axial CT images or fluoroscopic findings. Abdom Imaging. 2010;35:556-562.
[30] A.J. Megibow, E.J. Balthazar, K.C. Cho, et al. Bowel obstruction: evaluation with CT. Radiology. 1991;180:313-318.
[31] T. Fukuya, D.R. Hawes, C.C. Lu, et al. CT diagnosis of small bowel obstruction: efficacy in 60 patients. AJR Am J Roentgenol. 1992;158:765-769.
[32] E.J. Balthazar. George W. Holmes lecture. CT of the small bowel obstruction. AJR Am J Roentgenol. 1994;162:255-261.
[33] M.D. Zielinski, P.W. Eiken, S.F. Heller, et al. Prospective observational validation of a multivariate small bowel obstruction model to predict the need for operative intervention. J Am Coll Surg. 2010;211(3):S22-S23.
[34] S. Argov, D. Itzkovitz, F. Wiener. A new method for differentiating simple intra-abdominal from strangulated small-intestinal obstruction. Curr Surg. 1989;46(6):456-460.
[35] Y. Zhang, Y. Gao, Q. Ma, et al. Randomised clinical trial investigating the effects of combined administration of octreotide and methylglucamine diatrizoate in the older persons with adhesive small bowel obstruction. Dig Liver Dis. 2006;38(3):188-194.
[36] S.C. Chen, K.J. Chang, P.H. Lee, et al. Oral urograffin in postoperative small bowel obstruction. World J Surg. 1999;23:1051-1054.
[37] S.C. Chen, F.Y. Lin, P.H. Lee, et al. Water-soluble contrast study predicts the need for early surgery in adhesive small bowel obstruction. Br J Surg. 1998;85:1692-1694.
[38] E. Feigin, D. Seror, A. Szold, et al. Water-soluble contrast material has no therapeutic effect on postoperative small bowel obstruction: results of a prospective randomized clinical trial. Am J Surg. 1996;171:227-229.
[39] B.T. Fevang, D. Jensen, K. Svanes, et al. Early operation or conservative management of patients with small bowel obstruction? Eur J Surg. 2002;168:475-481.
[40] C.G. Cronin, D.G. Lohan, A.M. Browne, et al. MR enterography in the evaluation of small bowel dilation. Clin Radiol. 2009;64:1026-1034.
[41] J.L. Fidler, L. Guimaraes, D.M. Einstein. MR imaging of the small bowel. RadioGraphics. 2009;29(6):1811-1825.
[42] K. Takeuchi, Y. Tsuzuki, T. Ando, et al. Clinical studies of strangulating small bowel obstruction. Am Surg. 2004;70:40-44.
[43] H. Tsumura, T. Ichikawa, E. Hiyama, et al. Systemic inflammatory response syndrome (SIRS) as a predictor of strangulated small bowel obstruction. Hepatogastroenterology. 2004;51:1393-1396.
[44] J.J. Diaz, F. Bokhari, N.T. Mowery, et al. Guidelines for management of small bowel obstruction. J Trauma. 2008;64:1651-1664.
[45] M.G. Sarr. How useful is methylglucamine diatrizoate solution in patients with small-bowel obstruction? Nat Clin Pract Gastroenterol Hepatol. 2006;3(8):432-433.
[46] A. Assalia, M. Schein, D. Kopelman, et al. Therapeutic effect of oral Gastrografin in adhesive, partial small-bowel obstruction: a prospective randomized trial. Surgery. 1994;115(4):433-437.
[47] S. Biondo, D. Pares, L. Mora, et al. Randomized clinical study of Gastrografin administration in patients with adhesive small bowel obstruction. Br J Surg. 2003;90:542-546.
[48] P. Kumar, L. Kaman, G. Singh, et al. Therapeutic role of oral water soluble iodinated contrast agent in postoperative small bowel obstruction. Singapore Med J. 2009;50(4):360-364.
[49] B.T. Fevang, D. Jensen, J. Fevang, et al. Upper gastrointestinal contrast study in the management of small bowel obstruction—a prospective randomised study. Eur J Surg. 2000;166(1):39-43.
[50] B.C. Branco, G. Barmparas, B. Schnuriger, et al. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg. 2010;97:470-478.
[51] W. Silen, M.F. Hein, L. Goldman. Strangulation obstruction of the small intestine. Arch Surg. 1962;85:137-145.
[52] B.J. O’Daly, P.F. Ridgway, N. Keenan, et al. Detected peritoneal fluid in small bowel obstruction is associated with the need for surgical intervention. Can J Surg. 2009;52(3):201-206.
[53] H.K. Ha, J.S. Kim, M.S. Lee, et al. Differentiation of simple and strangulated small-bowel obstructions: usefulness of known CT criteria. Radiology. 1997;204(2):507-512.
[54] R.D. Mallo, L. Salem, T. Lalani, et al. Computed tomography diagnosis of ischemia and complete obstruction in small bowel obstruction: a systematic review. J Gastrointest Surg. 2005;9:690-694.
[55] O. Makita, I. Ikushima, N. Matsumoto, et al. CT differentiation between necrotic and nonnecrotic small bowel in closed loop and strangulating obstruction. Abdom Imaging. 1999;24:120-124.
[56] K. Jones, A.J. Mangram, R.A. Lebron, et al. Can a computed tomography scoring system predict the need for surgery in small-bowel obstruction? Am J Surg. 2007;194:780-784.
[57] P.S. Kamath, R.H. Wiesner, M. Malinchoc, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
[58] D.R. Cronk, T.P. Houseworth, D.G. Cuadrado, et al. Intestinal fatty acid binding protein (I-FABP) for the detection of strangulated mechanical small bowel obstruction. Curr Surg. 2006;63(5):322-325.
[59] V. Pettilä, M. Hynninen, O. Takkunen, et al. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220-1225.
[60] B. Papaziogas, G. Anthimidis, I. Koutelidakis, et al. Predictive value of procalcitonin for the diagnosis of bowel strangulation. World J Surg. 2008;32:1566-1567.
[61] R. Ayten, O. Dogru, C. Camci, et al. Predictive value of procalcitonin for the diagnosis of bowel strangulation. World J Surg. 2005;29:187-189.
[62] N.A. Bickell, A.D. Federman, A.H. Aufses. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201:847-854.
[63] S.B. Williams, J. Greenspon, H.A. Young, et al. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005;48:1140-1146.
[64] P.M. Sagar, J. MacFie, P. Sedman, et al. Intestinal obstruction promotes gut translocation of bacteria. Dis Colon Rectum. 1995;38:640-644.
[65] P.R. Fleshner, M.G. Siegman, G.I. Slater, et al. A prospective, randomized trial of short versus long tubes in adhesive small bowel obstruction. Am J Surg. 1995;170:366-370.
[66] G. Miller, J. Boman, I. Shrier, et al. Natural history of patients with adhesive small bowel obstruction. Br J Surg. 2000;87:1240-1247.
[67] I.K. Lee, D.H. Kim, D.L. Gorden, et al. Selective laparoscopic management of adhesive small bowel obstruction using CT guidance. Am Surg. 2009;75:227-231.
[68] C.P. Fischer, D. Doherty. Laparoscopic approach to small bowel obstruction. Semin Laparosc Surg. 2002;9:40-45.
[69] G. Borzellino, S. Tasselli, G. Zerman, et al. Laparoscopic approach to postoperative adhesive obstruction. Surg Endosc. 2004;18:686-690.
[70] R. Chopra, C. Mcvay, E. Phillips, et al. Laparoscopic lysis of adhesions. Am Surg. 2003;69:966-968.
[71] E.L. Leon, A. Metzger, G.G. Tsiotos, et al. Laparoscopic management of small bowel obstruction: indications and outcome. J Gastrointest Surg. 1998;2:132-140.
[72] H. Levard, M.J. Boudet, S. Msika, et al. Laparoscopic treatment of acute small bowel obstruction: a multicentre retrospective study. ANZ J Surg. 2001;71:641-646.
[73] C. Wullstein, E. Gross. Laparoscopic compared with conventional treatment of acute adhesive small bowel obstruction. Br J Surg. 2003;90:1147-1151.
[74] J.J. Liauw, W.K. Cheah. Laparoscopic management of acute small bowel obstruction. Asian J Surg. 2005;28:185-188.
[75] K. Suzuki, Y. Umehara, T. Kimura. Elective laparoscopy for small bowel obstruction. Surg Laparosc Endosc Percutan Tech. 2003;13:254-256.
[76] M. Suter, P. Zermatten, N. Halkic, et al. Laparoscopic management of mechanical small bowel obstruction: are there predictors of success or failure? Surg Endosc. 2000;14:478-483.
[77] P. Strickland, D.J. Lourie, E.A. Suddleson, et al. Is laparoscopy safe and effective for treatment of acute small-bowel obstruction? Surg Endosc. 1999;13:695-698.
[78] H.J. Duepree, A.J. Senagore, C.P. Delaney, et al. Does means of access affect the incidence of small bowel obstruction and ventral hernia after bowel resection? Laparoscopy versus laparotomy. J Am Coll Surg. 2003;197:177-181.
[79] B. Ghosheh, J. Salameh. Laparoscopic approach to acute small bowel obstruction: review of 1061 cases. Surg Endosc. 2007;21:1945-1949.
[80] I. Qureshi, Z.T. Awad. Predictors of failure of the laparoscopic approach for the management of small bowel obstruction. Am Surg. 2010;76:947-950.
[81] R.I.S. Zbar, W.B. Crede, C.F. McKhann, et al. The post-operative incidence of small bowel obstruction following standard, open appendectomy and cholecystectomy: a six year retrospective cohort study at Yale-New Haven Hospital. Conn Med. 1997;57:123-127.
[82] E. Farinella, R. Cirocchi, F. La Mura, et al. Feasibility of laparoscopy for small bowel obstruction. World J Emerg Surg. 2009;4:3.
[83] F.C. Grafen, V. Neuhaus, O. Schöb, et al. Management of acute small bowel obstruction from intestinal adhesions: indications for laparoscopic surgery in a community teaching hospital. Langenbecks Arch Surg. 2010;395:57-63.
[84] A. Nagle, M. Ujiki, W. Denham, et al. Laparoscopic adhesiolysis for small bowel obstruction. Am J Surg. 2004;187:464-470.
[85] B. Navez, J.M. Arimont, P. Guiot. Laparoscopic approach in acute small bowel obstruction. A review of 68 patients. Hepatogastroenterology. 1998;45:2146-2150.
[86] M. Khaikin, N. Schneidereit, S. Cera, et al. Laparoscopic vs. open surgery for acute adhesive small-bowel obstruction: patients’ outcome and cost-effectiveness. Surg Endosc. 2007;21:742-746.
[87] G.B. Bulkley, G.D. Zuidema, S.R. Hamilton, et al. Intraoperative determination of small intestinal viability following ischemic injury: a prospective, controlled trial of two adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment. Ann Surg. 1981;193(5):628-637.
[88] R. Horstmann, D. Palmes, D. Rupp, et al. Laparoscopic fluorometry: a new minimally invasive tool for investigation of the intestinal microcirculation. J Invest Surg. 2002;15:343-350.
[89] J.J. Mcginty, N. Hogle, D.L. Fowler. Laparoscopic evaluation of intestinal ischemia using fluorescein and ultraviolet light in a porcine model. Surg Endosc. 2003;17:1140-1143.
[90] P. Jiri, F. Alexander, P. Michal, et al. Laparoscopic diagnostics of acute bowel ischemia using ultraviolet light and fluorescein dye: an experimental study. Surg Laparosc Endosc Percutan Tech. 2007;17(4):291-295.
[91] T.A. Schneider, W.E. Longo, T. Ure, et al. Mesenteric ischemia: acute arterial syndromes. Dis Colon Rectum. 1994;37:1163-1174.
[92] G.B. Bulkley, L.G. Wheaton, J.D. Strandberg, et al. Assessment of small intestinal recovery from ischemic injury after segmental arterial, venous, and arterovenous occlusion. Surg Forum. 1979;30:210-213.
[93] T.F. Gorey. The recovery of intestine after ischaemic injury. Br J Surg. 1980;67(10):699-702.
[94] H. Yanar, K. Taviloglu, C. Ertekin, et al. Planned second-look laparoscopy in the management of acute mesenteric ischemia. World J Gastroenterol. 2007;13(24):3350-3353.
[95] A.S. Gervin, C.L. Puckett, D. Silver, et al. Serosal hypofibrinolysis: a cause of post-operative adhesions. Am J Surg. 1973;125:80-88.
[96] D.C. James, H. Ellis, T.B. Hugh. The effect of streptokinase on experimental intra-peritoneal adhesion formation. J Pathol Bacteriol. 1965;90:279-287.
[97] F.J. Montz, B.J. Monk, S.M. Lacy. Ketorlac tromethamine, a non-steroidal anti-inflammatory drug: ability to inhibit post-radical pelvic surgery adhesions in a porcine model. Gynecol Oncol. 1993;48:76-77.
[98] Y. Mohri, K. Uchida, T. Araki, et al. Hyaluronic acid-carboxycellulose membrane (Seprafilm) reduces early postoperative small bowel obstruction in gastrointestinal surgery. Am Surg. 2005;71:861-863.
[99] W.W. Vrijland, L.N. Tseng, H.J. Eijkman, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid–carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193-199.
[100] V.W. Fazio, Z. Cohen, J.W. Fleshman, et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1-11.
[101] D.E. Beck, Z. Cohen, J.W. Fleshman, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46:1310-1319.
[102] A. Imai, N. Suzuki. Topical non-barrier agents for postoperative adhesion prevention in animal models. Eur J Obstet Gynecol Reprod Biol. 2010;149:131-135.
[103] C.I. Ripamonti, A.M. Easson, H. Gerdes. Management of malignant bowel obstruction. Eur J Cancer. 2008;44:1105-1115.
[104] C. Ripamonti, E. Bruera. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer. 2002;12:135-143.
[105] D.S. Chi, R. Phaeton, T.J. Miner, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. Oncologist. 2009;14:835-839.
[106] T. Anthony, T. Baron, S. Mercadante, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34:S49-S59.
[107] H. Higashi, H. Shida, K. Ban, et al. Factors affecting successful palliative surgery for malignant bowel obstruction due to peritoneal dissemination from colorectal cancer. Jpn J Clin Oncol. 2003;33(7):357-359.
[108] G.P. Yazdi, B.W. Miedema, L.J. Humphrey. High mortality after abdominal operation in patients with large-volume malignant ascites. J Surg Oncol. 1996;62(2):93-96.
[109] A.D. Turnbull, J. Guerra, H.F. Starnes. Results of surgery for obstructing carcinomatosis of gastrointestinal, pancreatic, or biliary origin. J Clin Oncol. 1989;7(3):381-386.
[110] B. Lund, M. Hansen, F. Lundvall, et al. Intestinal obstruction in patients with advanced carcinoma of the ovaries treated with combination chemotherapy. Surg Gynecol Obstet. 1989;169(3):213-218.
[111] H.J. Solomon, K.H. Atkinson, J.V. Coppleson, et al. Bowel complications in the management of ovarian cancer. Aust N Z J Obstet Gynaecol. 1983;23(2):65-68.
[112] R.S. Krouse. Surgical management of malignant bowel obstruction. Surg Oncol Clin N Am. 2004;13:479-490.
[113] T.H. Baron. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep. 2009;11:293-297.
[114] A.S. Ross, C. Semrad, I. Waxman, et al. Enteral stent placement by double balloon enteroscopy for palliation of malignant small bowel obstruction. Gastrointest Endosc. 2006;64:835-837.
[115] L. Meyer, B. Pothuri. Decompressive percutaneous gastrostomy tube use in gynecologic malignancies. Curr Treat Options Oncol. 2006;7:111-120.
[116] M.L. Kendrick, M.G. Sarr. Prolonged gastrointestinal decompression of the inoperable abdomen: the forgotten tube pharyngostomy. J Am Coll Surg. 2000;191(2):221-223.
[117] P. Sparrow, E. David, R. Pugash. Direct percutaneous jejunostomy—an underutilized interventional technique? Cardiovasc Intervent Radiol. 2008;31:336-341.
[118] R.M. Stewart, C.P. Page, J. Brender, et al. The incidence and risk of early post-operative small bowel obstruction: a cohort study. Am J Surg. 1987;154:643-647.
[119] M.G. Sarr, D.M. Nagorney, D.C. McIlrath. Post-operative intussusception in the adult: a previously unrecognized entity? Arch Surg. 1981;116:144-148.
[120] J.C. Quatromoni, L. Rosoff, J.M. Halls, et al. Early post-operative small bowel obstruction. Ann Surg. 1980;191:72-74.
[121] E.R. Frykberg, J.W. Phillips. Obstruction of the small bowel in the early postoperative period. South Med J. 1989;82:169-173.
[122] J. Pickleman, R.M. Lee. The management of patients with suspected early post-operative small bowel obstruction. Ann Surg. 1989;210:216-219.
[123] P.A. Sykes, P.F. Schofield. Early post-operative small bowel obstruction. Br J Surg. 1974;61:594-600.
[124] D.H. Frager, J.W. Baer, A. Rothpearl, et al. Distinction between post-operative ileus and mechanical small bowel obstruction: value of CT compared with clinical and other radiographic findings. AJR Am J Roentgenol. 1995;164:891-894.
[125] H. Ravikumar, S. Babu, M.J. Govindrajan, et al. Reduction en-masse of inguinal hernia with strangulated obstruction. Biomed Imaging Interv J. 2009;5(4):e14.
[126] C.Y. Lo, T.G. Lorentz, P.W. Lau. Obturator hernia presenting as small bowel obstruction. Am J Surg. 1994 Apr;167(4):396-398.
[127] L.S. Toy, C. Abittan, A. Kornbluth, et al. Complete bowel obstruction following initial response to Infliximab therapy for Crohn’s disease: a series of a newly described complication [abstract]. Gastroenterology. 2000;118:A569.
[128] G.R. Lichtenstein, A. Olson, S. Travers, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101(5):1030-1038.
[129] S.B. Hanauer, B.G. Feagan, G.R. Lichtenstein, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549.
[130] F.J. Montz, C.H. Holschneider, S. Solh, et al. Small bowel obstruction following radical hysterectomy: risk factors, incidence, and operative findings. Gynecol Oncol. 1994;53:114-120.
[131] A.C. Mak, Rich Ta, T.E. Schultheiss, et al. Late complications of post-operative radiation therapy for cancer of the rectum and rectosigmoid. Int J Radiat Oncol Biol Phys. 1994;28:597-603.