Chapter 72 Management of liver failure
Liver Failure
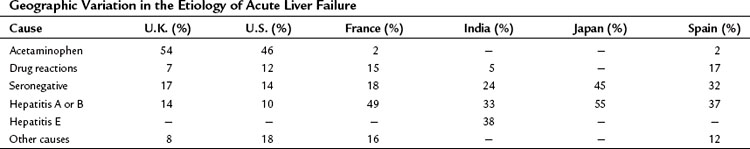
Acute liver failure is a heterogeneous condition that incorporates a range of clinical syndromes. The dominant factors that give rise to this heterogeneity include the underlying etiology, which varies significantly from country to country (Table 72.1); the age of the patient; and the duration of time over which the disease evolves. Natural history studies indicate that survival rates without liver transplantation range from 10% to 90%, with the best outcomes seen in patients with pregnancy-related syndromes, acetaminophen overdose, and hepatitis A. Survival rates are also poor in older patients and possibly in very young children.
Etiology of Acute Liver Failure
Viral
Acute liver failure is a very uncommon complication of hepatitis A infection, occurring in 0.14% to 0.35% of hospitalized patients and in 0.4% of all cases seen in the United States. The incidence of acute liver failure following hepatitis B infection is 1% to 4% of hospitalized patients. In early studies, hepatitis D coinfection or superinfection was thought to increase the risk, as it was found in 34% to 43% of patients with acute liver failure as a result of hepatitis B infection compared with 4% to 19% of less severe cases. However, the prevalence of hepatitis D appears to have decreased dramatically in recent years. Vaccination and antiviral therapy with drugs that have a rapid onset of action (e.g., lamivudine, tenofovir) should alter the observed pattern of hepatitis B–related acute liver failure (Sjögren, 2003). The risk of developing acute liver failure after exposure to hepatitis C appears to be low, although well-documented cases have been reported. Hepatitis E is common in parts of Asia and Africa but is being increasingly recognized in Western countries. The natural history of hepatitis E in India has recently been revised to show that, although pregnant females are more likely to develop acute liver failure, the outcome is independent of sex, pregnancy status, and trimester of pregnancy (Bhatia et al, 2008).
Seronegative hepatitis accounts for 14% to 32% of acute liver failure in the Western world. In the United States, a proportion of these events are considered to be related to acetaminophen (Davern et al, 2006). There is little evidence to suggest that an unrecognized viral infection is to blame because most cases are sporadic. Unidentified toxins or autoimmune processes may be the underlying mechanisms. Middle-aged females are most frequently affected, and the risk of developing acute liver failure has been calculated at 2.3% to 4.7% of hospitalized cases.
Drugs
Acetaminophen (paracetamol) overdose is the commonest cause of acute liver failure in the United Kingdom and the United States (Bernal, 2003; Lee, 2003). In the United Kingdom, excess amounts are usually taken with suicidal or parasuicidal intent; only 8% of such events were ascribed to inadvertent overdose with therapeutic use. However, in the United States, unintentional overdoses accounted for at least 48% of cases (Larson et al, 2005). Susceptibility to developing acetaminophen hepatotoxicity is increased in people with liver enzyme induction as a consequence of antiepileptic therapy, regular alcohol consumption, or malnutrition.
Drug-induced liver injury resulted in liver-related death in 3% to 4% of cases in a prospective study of 300 cases (Chalasani et al, 2008). More than 100 different agents were implicated, with 46% being associated with antimicrobials and 15% with central nervous system agents. Some of the more common offending drugs linked to acute liver failure are listed in Box 72.1. The risk of developing acute liver failure from an idiosyncratic reaction ranges from 0.001% for nonsteroidal antiinflammatory drugs (NSAIDs) to 1% for the isoniazid/rifampicin combination. Nontherapeutic drugs, such as MDMA (3,4-methylenedioxymethamphetamine; “ecstasy”) and some herbal remedies, can also cause acute liver failure. The diagnosis is made on the basis of a temporal relationship between exposure to the drug or hepatotoxin and the development of acute liver failure.
Other Etiologies
Wilson disease may present as acute liver failure, usually during the second decade of life. It is characterized clinically by a Coombs test negative for hemolytic anemia and demonstrable Kayser-Fleischer rings in the majority of cases. Poisoning with Amanita phalloides mushrooms is most commonly seen in central Europe, South Africa, and the West Coast in the United States. Severe diarrhea, often with vomiting, is a typical feature and commences 5 hours or more after ingestion of the mushrooms; liver failure develops 4 to 5 days later. Autoimmune chronic hepatitis may present as acute liver failure, but by then, it is usually beyond rescue with corticosteroid or other immunosuppressive therapy. Budd-Chiari syndrome (see Chapter 77) may present with acute liver failure, and the diagnosis is suggested by hepatomegaly and confirmed by the demonstration of hepatic vein thrombosis. Malignancy infiltration, especially with lymphoma, is another rare cause of acute liver failure that is typically associated with hepatomegaly. Ischemic hepatitis is being increasingly recognized as a cause of acute liver failure, especially in older patients. Other unusual causes of acute liver failure include heat stroke and sepsis.
Pediatric Causes
Children are at risk of developing some of the causes of acute liver failure discussed above, but they also are vulnerable to other unique underlying causes, especially in the category of metabolic disease. Neonatal hemochromatosis may occur within the first few weeks of life and has been treated by liver transplantation as early as 5 days of age. Acute liver failure as a result of mitochondrial disorders may be triggered by bacterial infections and are characterized by high lactate levels in blood. Other metabolic causes include tyrosinemia, galactosemia, and fructose intolerance. Young infants can develop acute liver failure with viral infections, such as adenovirus, coxsackie virus, and cytomegalovirus (see Chapter 71).
Diagnosis
The etiology of acute liver failure must be accurately identified; the appropriate investigations are outlined in Tables 72.2 and 72.3 and cover the key essential investigations and those that are relevant in certain circumstances, respectively. Imaging assesses the size and shape of the liver and screens for portal hypertension; however, the detection of portal hypertension does not always indicate chronic liver disease because ascites and/or splenomegaly may be seen in subacute liver failure and in the acute presentation of Wilson disease.
Table 72.2 Core Investigation of the Etilogy of Acute Liver Failure
| Etiology | Investigation |
|---|---|
| Hepatitis A virus (HAV) | IgM anti-HAV |
| Hepatitis B (HBV) + D (HDV) | IgM anticore, HBV DNA (NB HBsAg may be negative) |
| Hepatitis E virus (HEV) | Anti-HEV |
| Paracetamol | Drug levels in blood |
IgM, immunoglobulin M; NB HbsAg, hepatitis B surface antigen.
Table 72.3 Extended Investigation of Etilogy of Acute Liver Failure
| Etiology | Investigation |
|---|---|
| Idiosyncratic drug reactions | Eosinophil count, histology |
| Autoimmune | Autoantibodies, immunoglobulins |
| Pregnancy-related syndromes | |
| Fatty liver | Ultrasound, uric acid, histology |
| HELLP syndrome | Platelet count |
| Toxemia | Serum transaminases |
| Wilson disease | Urinary copper, ceruloplasmin, alkaline phosphatase/bilirubin ratio, AST/ALT ratio, slit-lamp examination |
| Budd-Chiari syndrome | Ultrasound or venography |
| Malignancy | Imaging, histology |
| Ischemic hepatitis | Transaminases |
HELLP, hemolysis, elevated liver enzymes and low platelets; AST/ALT, aspartate aminotransferase/alanine aminotransferase.
Histologic assessment of liver tissue may aid the diagnosis of the cause of acute liver failure (see Chapter 70A). Confluent necrosis is the most common histologic finding; it may be zonal or may involve all of the parenchyma. Necrosis that is zonal within the acinus and coagulative or eosinophilic is more likely to be secondary to a toxic insult or ischemia. The features of necrosis and parenchymal collapse may be interspersed with evidence of regeneration, either occurring in a diffuse pattern of small areas throughout the liver or in randomly occurring larger nodules that give the maplike pattern that has often been described in this condition. The latter pattern is most commonly seen in patients with subacute liver failure.
Liver biopsy may also be of assistance in differentiating acute liver failure from established cirrhosis and acute alcoholic hepatitis, whether or not it is associated with cirrhosis. The former scenario can usually be distinguished on clinical grounds, but acute alcoholic hepatitis can present with clinical features very similar to subacute liver failure. A history of high alcohol consumption may not be available, but the diagnosis of acute alcoholic hepatitis is suggested by the investigational findings outlined in Box 72.2.
Prognosis
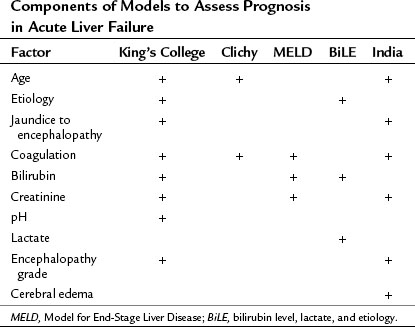
The use of transplantation has intensified the need for early indicators of prognosis, so that those in need of this intervention could be identified as quickly as possible (see Chapter 97C). The King’s College criteria, with some modifications, are still widely used to identify the patients most in need of liver transplantation (Boxes 72.3 and 72.4). The acetaminophen criteria have been found to be specific but lack adequate sensitivity, and serum lactate levels (>3.5 before resuscitation and 3.0 after) have been suggested as an alternative or supplementary assessment of prognosis in these patients (Bernal et al, 2002). Another problem with these criteria is the difficulty applying the coagulation parameters outside the United Kingdom. The scale on which prothrombin times are reported in the United States is dramatically shorter than in the United Kingdom, reflecting differences in technique; in addition, the reagents used and the implementation of the international normalized ratio (INR) have not entirely resolved this problem because of loss of accuracy at higher readings. The Model for End-Stage Liver Disease (MELD) score is being increasingly used to prognosticate in acute liver failure. In France, the factor V levels are used in preference to either the prothrombin time or INR; levels below 20% in patients younger than 30 years and below 30% in older patients are indicative of a poor prognosis once encephalopathy develops. In Germany, the BiLE score—a combination of serum bilirubin level, lactate, and etiology—is used (Hadem et al, 2008). A study from India used a combination of six demographic, clinical, and laboratory variables (Dhiman et al, 2007). In reality, these different prognostic tools use a total of 10 variables (Table 72.4), and considerable commonality is found among them (O’Grady, 2007).
Box 72.3 Indicators of a Poor Prognosis in Paracetamol-Induced Acute Liver Failure
Management
Overall Strategy
In acute liver failure, the overall strategy is a combination of intensive medical care (see Chapter 73), liver transplantation in selected patients (see Chapter 97C), and, to some degree, the use of a liver support device (Chapter 73). Management starts with identification of etiology and an initial assessment of prognosis that is then progressively updated as each individual’s clinical course evolves. Early referral to specialist centers that offer liver transplantation is recommended; monitoring is instituted, and a decision on the need for immediate liver transplantation is reviewed. Patients are then treated for the complications that may develop to the point of recovery, death, or transplantation. Although considerable variation is found among centers and countries, the outcomes in fully functional programs approximate 40% survival without transplantation, and 40% proceed to liver transplantation; a 20% mortality rate is reported without transplantation.
Patients with acute-on-chronic liver failure often require, and benefit from, intensive monitoring. This is especially appropriate when deterioration has been triggered by a specific, reversible complication, especially variceal bleeding (see Chapter 75A, Chapter 75B, Chapter 75C ). Patients with hepatorenal failure are also frequently admitted to intensive care, which is particularly appropriate in patients who are active candidates for liver transplantation. Liver support systems, particularly the Molecular Adsorbents Recirculation System (MARS; Gambro, Stockholm, Sweden), are being increasingly used in this situation.
General Measures in Liver Failure
A number of drugs have well-defined roles in specific etiologies of acute liver failure. N-acetylcysteine is established as an antedote to paracetamol hepatotoxicity when given within 16 to 24 hours of the overdose, but it has also been shown to be beneficial in reducing mortality rates and cerebral edema when begun as late as 36 hours after the overdose. In the United States, a trial of N-acetylcysteine in nonacetaminophen acute liver failure showed improved transplant-free survival of 52%, compared with 30% in patients receiving placebo, when patients were started on therapy prior to the onset of grade 3 to 4 encephalopathy (Lee et al, 2009). Penicillin, and possibly silymarin, should be added at the earliest opportunity to the standard supportive measures in patients with Amanita phalloides toxicity. Patients with Wilson disease complicated by encephalopathy do not respond to chelating therapy, but penicillamine should be considered for acute presentations not yet complicated by encephalopathy. Acute liver failure caused by autoimmune hepatitis rarely responds to immunosuppressive therapy. Patients with severe acute alcoholic hepatitis may benefit from corticosteroid therapy or treatment with pentoxifylline; however, neither of these medications is indicated in acute-on-chronic episodes of alcoholic cirrhosis in the absence of a component of alcoholic hepatitis.
Management of Specific Complications
Neurologic Complications
Cerebral edema is common in hyperacute and acute liver failure but unusual in subacute liver failure. Clinically apparent cerebral edema has only been reported on an anecdotal basis in patients with cirrhosis (excluding Wilson disease). Emerging evidence suggests that the incidence of cerebral edema is decreasing with modern management protocols, but the precise reasons for this change have yet to be determined (Stravitz & Kramer, 2009). Nevertheless, in individual patients, cerebral edema remains an important complication that may cause death or may disqualify patients from transplantation; on that basis, treatment of cerebral edema retains its prominent role in management protocols. The clinical features of cerebral edema include systemic hypertension, “decerebrate” posturing, hyperventilation, abnormal pupillary reflexes, and, ultimately, impairment of brain stem reflexes and functions. Papilledema is rarely seen. The risk to life with cerebral edema can be from classic brain stem herniation or hypoxic brain damage.
Encephalopathy with acute-on-chronic liver failure is managed with dietary protein restriction and lactulose and phosphate enemas, usually with good effect. Treatment of triggering events is also fundamental to the management of encephalopathy, and empiric antibiotics and intravenous fluids are standard components of care (Box 72.5). With the possible exception of subacute liver failure, specific management options for encephalopathy have limited impact in acute liver failure. L-ornithine L-asparate given in a dose of 30 g/day for 3 days failed to lower ammonia levels or improve survival in acute liver failure (Acharya et al, 2009). Liver support devices can reduce ammonia and lighten encephalopathy, but no single system has demonstrated improvement in patient survival (McKenzie et al, 2008; Stradlbauer et al, 2009).
Mannitol (0.25 to 0.5 mg/kg) is the first-line treatment for surges in intracranial pressure. This process is repeated as determined by the pattern of clinical relapses, but the serum osmolarity should not be allowed to exceed 320 mOsm. Sodium thiopentone (phenobarbitone) was shown to control cerebral edema that had become unresponsive to mannitol in an uncontrolled study, but it has not been subjected to a controlled trial, nor has it been shown to improve the survival rate. Acute, but not chronic, hyperventilation was reported to be of benefit in reducing critical surges in intracranial hypertension. Nevertheless, hyperventilation to pco2 levels below 25 mm Hg is routinely incorporated as a first-line treatment in protocols in the United States. Hypothermia, hypertonic saline infusions, and indomethacin have also been shown in small studies to be useful adjuncts in the management of intracranial hypertension (Auzinger & Wendon, 2008; Stravitz & Kramer, 2009).
In the later stages of the neurologic complications, the emphasis of management changes to the preservation of cerebral perfusion pressure, increased oxygen delivery to the brain, and manipulation of the neuronal microcirculation to promote cerebral oxygen extraction (Auzinger & Wendon, 2008; Stravitz & Kramer, 2009). The patient should be positioned with the trunk at an angle of 20 to 30 degrees to horizontal. The options for increasing the mean arterial pressure, and consequently improving cerebral perfusion pressure, are outlined in the section in this chapter dealing with hemodynamics. These adjustments are made to maintain a cerebral perfusion pressure greater than 50 mm Hg if possible. At this stage of the complication, spontaneous recovery is unlikely without liver transplantation, and hepatectomy is useful to secure transient improvement. Occult seizure activity may contribute to neurologic instability in patients with grade 4 encephalopathy. Phenytoin and diazepam are effective therapies despite the theoretic consideration that the latter may aggravate the underlying encephalopathy.
The use of intracranial pressure monitoring is controversial and has not been subjected to clinical trials (Stravitz & Kramer, 2009). Early detection of cerebral edema and a facility with the appropriate equipment to constantly monitor this complication help to optimize therapeutic interventions. Intracranial pressure monitoring allows earlier and more accurate detection of pressure changes—especially in the ventilated patient, in whom most of the clinical signs are masked—and it facilitates careful monitoring of the intracranial pressure during high-risk therapeutic interventions, such as hemodialysis and tracheal suctioning. It is also considered valuable during orthotopic liver transplantation because increases in intracranial pressure often occur during the dissection and reperfusion phases of the transplant operation. The most commonly used system places transducers on or through a small nick in the dura. The risk of intracranial hemorrhage is a deterrent, although the studies that have systematically addressed safety have favored the use of intracranial pressure monitoring, especially using fiberoptic extradural or subdural devices. Proponents argue that this has been shown to be effective and relatively safe, despite the attendant coagulopathy. Epidural transducers were associated with a low complication rate of 3.8%, but subdural and parenchymal devices had higher complication rates of 20% and 22%, respectively. Noninvasive monitoring using transcranial Doppler ultrasonography may provide an alternative way of assessing intracranial pressure and cerebral perfusion pressure in this setting (Aggarwal et al, 2008).
Infection
Immunoparesis is a feature of acute liver failure (Antonides et al, 2008); it is therefore no surprise that bacterial and fungal infections are prominent complications in all varieties of this condition (see Chapter 9, Chapter 10, Chapter 11 ). Considerable overlap is seen between the clinical features of infection and the systemic immune response syndrome (SIRS), but microbial infection can also be seen in the absence of these typical markers of sepsis. Infection is one of the most common causes of death and plays an integral role in the evolving cycle of hemodynamic instability and multisystem failure (Rolando et al, 2000). It also frequently delays or disqualifies potential candidates from emergency liver transplantation. Bacterial infection occurs in up to 80% of patients, and fungal infection occurs in 30%. Systemic fungal infection is notoriously difficult to diagnose in the setting of acute liver failure, and a high index of suspicion is required, especially in high-risk patients and in those with a very high white cell count or an arrest in the normalization of the parameters of coagulation activity during an apparent recovery phase. Risk factors for both bacterial and fungal sepsis include coexisting renal failure, cholestasis, treatment with thiopentone, and liver transplantation. Surveillance cultures are required on a regular basis (Auzinger & Wendon, 2008; Stravitz & Kramer, 2009).
Hemodynamic Instability and Oxygen Debt
The hemodynamic changes in liver failure are very similar to those observed in SIRS. The early hemodynamic profile reflects a hyperdynamic circulation with increased cardiac output and reduced systemic peripheral vascular resistance. Profound vasodilation may cause relative hypovolemia, and invasive monitoring is used to determine appropriate fluid regimens and adequate intravascular volumes. Progressive disease leads to circulatory failure, either as a result of a falling cardiac output or an inability to maintain an adequate mean arterial pressure. Patients with acute liver failure and sepsis exhibit a different hemodynamic profile than patients in septic shock in the absence of liver disease (Tsai et al, 2008). The patients with acute liver failure had higher cardiac indexes and oxygen delivery but lower systemic vascular indexes and oxygen extraction/consumption. Circulatory failure is another important contraindication to liver transplantation and is a common mode of death in liver failure.
Hemodynamic monitoring using volumetric indices of preload is preferred over pressure-derived variables (Auzinger & Wendon, 2008). Circulatory failure is initially managed with appropriate fluid replacement in combinations of colloids, crystalline fluids, and blood products as clinically appropriate. Persistent hypotension after volume repletion indicates vasopressor agents, with a clear preference for norepinephrine as first-line therapy (Auzinger & Wendon, 2008; Stravitz & Kramer, 2009). Dopamine and vasopressin may also be used, although vasopressors may cause or aggravate an oxygen debt, but prostacyclin and N-acetylcysteine have been shown to improve these parameters in patients receiving vasopressors. Some patients with acute liver failure who develop resistance to inotropes may have a hypoadrenal profile that responds to hydrocortisone (Harry et al, 2002).
Renal Failure
Urea synthesis is impaired in acute liver failure. Consequently, serum creatinine levels are preferred for the purposes of monitoring renal function. Renal failure developed in 75% of patients with acetaminophen-related acute liver failure and in 30% of acute liver failure from other causes. Renal failure after an acetaminophen overdose is a consequence of direct renal toxicity and develops early in the course of the illness. Early renal dysfunction is also seen in Wilson disease, Amanita poisoning, and pregnancy-related syndromes. In the other etiologies, renal impairment develops relatively late and progresses from a stage of “functional” or prerenal failure (urinary sodium <10 mmol/L; urine/plasma osmolarity ratio >1.1) to acute tubular necrosis. The presence of SIRS correlated with the development of renal dysfunction in nonacetaminophen etiologies of acute liver failure but not in acetaminophen-induced disease (Leithead et al, 2009).
Optimization of intravascular filling is essential in patients with deteriorating renal function. The prophylactic use of dopamine has been common practice, but its benefits have been challenged, especially in the setting of profound vasodilation that is typical of acute liver failure. The metabolic complexity of combined liver and renal failure suggests that early intervention with hemodialysis, preempting standard indications, is prudent in the setting of acute liver failure. Continuous filtration systems are associated with less hemodynamic instability and run a lower risk of aggravating latent or established cerebral edema than does intermittent hemodialysis. Some evidence suggests that high-volume, continuous venovenous hemofiltration may reduce vasopressor requirements through modulation of proinflammatory mediators (Auzinger & Wendon, 2008). The role for renal support therapy is less well defined in acute-on-chronic liver failure, but MARS therapy may be especially useful in deeply jaundiced patients with renal dysfunction.
Coagulopathy
Poor correlation is reported between the laboratory and clinical manifestations of the coagulopathy of acute liver failure. This has been attributed to the fact that the decrease in the procoagulant and anticoagulant proteins is proportional (Stravitz & Kramer, 2009). The use of prophylactic repletion of coagulation has never been practiced in the United Kingdom except before significant invasive procedures. Recent data from the United States show that 92% of patients were given fresh frozen plasma at a mean of 14 units during the first week of hospitalization, even though it was acknowledged that only a minority of patients had gastrointestinal bleeding or intracranial pressure monitoring (Munoz et al, 2008). The practice of prophylactic repletion of clotting factors is also controversial because it may interfere with assessment of prognosis, especially in patients being considered for liver transplantation. The highest risk of bleeding is seen in those with an associated thrombocytopenia or a frank DIC syndrome rather than in the patients with isolated severe prolongations of prothrombin time. It has been proposed that the lack of coagulation factors does not result in a bleeding diathesis unless accompanied by an inadequate platelet scaffold on which to propagate a clot (Stravitz & Kramer, 2009). Clinical bleeding is managed by infusions of fresh frozen plasma, cryoprecipitate, and platelets as required. Limited data are available on the utility of recombinant activated Factor VII in this setting (Caldwell et al, 2004).
Nutrition
Although the standard patient with acute liver failure is well nourished at the onset of the illness, it is important to institute nutrition support as soon as possible (see Chapter 24). The catabolic rate increases in patients with acute liver failure, and this is most apparent in those with complicating sepsis and those undergoing liver transplantation. The theoretical problems that limit nutrition options are legion: gastrointestinal ileus, desire to minimize gastrointestinal protein, difficulty maintaining isoglycemia, fluid restrictions secondary to renal failure, amino acid ratios possibly mediating encephalopathy, difficulty handling lipids, aggravation of sepsis by intravenous feeding, and so on. Despite all these considerations, adequate nutrition support can be obtained in the majority of patients. An element of enteral nutrition is desirable to help maintain the integrity of the small intestinal mucosa, which is titrated against the volume of gastric aspirates and the development of diarrhea.
Acharya SK, et al. Efficacy of L-ornithine L-asparate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology. 2009;136:2159-2168.
Aggarwal S, et al. Non-invasive monitoring of cerebral perfusion pressure in patients with acute liver failure using transcranial Doppler ultrasonography. Liver Transpl. 2008;14:1048-1057.
Antonides CG, et al. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845-861.
Auzinger G, Wendon J. Intensive care management of acute liver failure. Curr Opin Crit Care. 2008;14:179-188.
Bernal B. Changing patterns of causation and the use of transplantation in the United Kingdom. Semin Liver Dis. 2003;23:227-237.
Bernal W, et al. Blood lactate as an early indicator of outcome in paracetamol-induced acute liver failure. Lancet. 2002;359:558-563.
Bhatia V, et al. A 20-year single experience with acute liver failure during pregnancy: is the prognosis really worse? Hepatology. 2008;48:1577-1585.
Caldwell SH, Chang C, Macik BG. Recombinant activated factor VII (rFVII) as a hemostatic agent in liver disease: a break from convention in need of controlled trials. Hepatology. 2004;39:592-598.
Chalasani N, et al. Causes, clinical features, and outcome from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
Davern TJ, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687-694.
Dhiman RK, et al. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-stage Liver Didease (MELD) and King’s College Hospital Criteria. Liver Transpl. 2007;13:814-821.
Hadem J, et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol. 2008;6:339-345.
Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395-402.
Larson AM, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372.
Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217-226.
Lee WM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
Leithead JA, et al. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non–paracetamol-induced acute liver failure. Gut. 2009;58:443-449.
McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis. 2008;28:210-217.
Munoz SJ, Rajender RK, Lee W. The coagulopathy of acute liver failure and implications for intracranial pressure monitoring. Neurocritical Care. 2008;9:100-107.
O’Grady JG. Prognostication in acute liver failure: a tool or an anchor? Liver Transpl. 2007;13:786-787.
Rolando N, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739.
Sjögren M. Immunization and the decline of viral hepatitis as a cause of acute liver failure. Hepatology. 2003;38:554-556.
Stradlbauer V, Wright GA, Jalan R. Role of artificial liver support in hepatic encephalopathy. Metabol Brain Dis. 2009;24:15-26.
Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev. Gastroenterol Hepatol. 2009;6:542-553.
Tsai MH, et al. Hemodynamic and metabolic studies on septic shock in patients with acute liver failure. J Crit Care. 2008;23:473-474.