Management of Increased Intracranial Pressure and Intracranial Shunts
Pathophysiology of ICP
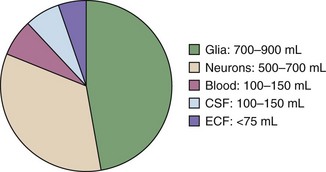
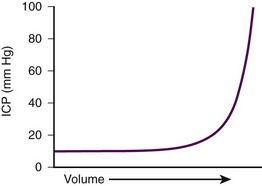
The components of the calvaria are the brain parenchyma, cerebrospinal fluid (CSF), the venous blood supply, and the arterial blood supply (Fig. 59-1). CSF and the venous blood supply have the greatest ability to change their volume to compensate for increases in pressure. These dynamic changes in the relative proportion of the cranial content may not affect the patient if ICP is not excessive. However, if a pathologic process overwhelms the compensatory mechanisms, the result will be a nearly exponentially increase in ICP (Fig. 59-2).
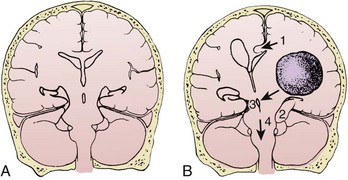
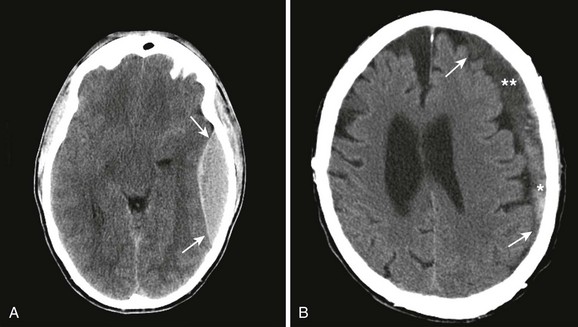
Normal supine ICP ranges from 5 to 15 mm Hg. Transient increases in ICP as high as 80 to 100 mm Hg occur with coughing or straining. Other factors that can transiently increase ICP are movement, pain, and fever. A space-occupying lesion such as a tumor, hematoma, abscess, or foreign body can also raise ICP. Figure 59-3 demonstrates that the area and cause of the increased ICP will determine where shifts occur to result in brain herniation.
Brain
IIH, formerly known as pseudotumor cerebri, is a chronic condition characterized by increased CSF pressure not caused by a tumor, edema, hydrocephalus, or change in CSF composition. It occurs most frequently in obese women. Symptoms may include headache, nausea, and blurry vision. The headache is typically worse on waking or with exertion. In general, patients with IIH have normal findings on neurologic examination except for the frequent presence of papilledema. Although the precise pathophysiology remains unclear, severe cases can lead to permanent loss of vision. Commonly associated conditions are listed in Box 59-1. IIH is a diagnosis of exclusion. Because of its often subtle symptoms and normal findings on computed tomography (CT), it is often not suspected or diagnosed on initial clinical evaluation.
Bleeding in the brain can occur spontaneously (as in the case of hemorrhagic stroke or spontaneous subarachnoid hemorrhage) or can be a result of trauma. In addition to the mass effect of the blood itself, the associated edema in both instances contributes to further increases in ICP. Diffuse axonal injury (DAI) may occur in isolation or in conjunction with intracerebral bleeding. With DAI it is believed that the axons are not actually torn, but instead suffer significant injury that may lead to edema (shearing effect).1
CSF
CSF is produced by the choroid plexus at a daily rate of 500 mL. It flows from the ventricles into the cisternae of the subarachnoid space and is drained by the arachnoid villi of the dural sinuses to maintain a constant volume of 100 to 150 mL. Obstructive hydrocephalus occurs when flow is blocked at any point in the ventricular system by clotted blood, tumor, colloid cyst, edema, or primary stenosis. Communicating hydrocephalus is due to impedance of flow beyond the ventricular system at the level of the basal cisternae or lack of absorption by the arachnoid villi. Communicating hydrocephalus can occur with both infection and subarachnoid hemorrhage (Fig. 59-4).
Blood
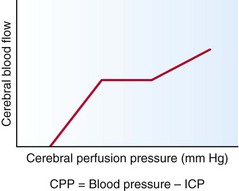
Up to a certain range, cerebral blood flow (CBF) is maintained by an autoregulatory mechanism despite fluctuations in cerebral perfusion pressure (CPP) (Fig. 59-5). Constant CBF can typically be maintained at any CPP between 60 and 160 mm Hg. Once CPP is out of the autoregulatory zone, CBF is linearly related to CPP. CPP lower than 60 mm Hg can lead to ischemia, whereas CPP higher 160 mm Hg can result in hypertensive encephalopathy.
Signs and Symptoms
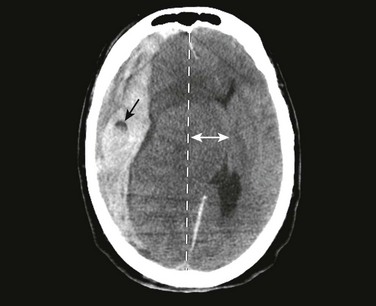
Signs and symptoms of severely increased ICP include a decreasing level of consciousness, papilledema, cranial nerve palsies, and lateralizing neurologic deficits. When any of these are noted, particularly when CT confirms the presence of a mass effect such as hydrocephalus or a midline shift, urgent intervention is necessary (Fig. 59-6). Neurosurgical consultation for possible invasive means of reducing ICP and monitoring is indicated. Medical management of increased ICP should also proceed without delay.
Medical Treatment of Increased ICP
Sedation and Paralytics
Rapid assessment of findings on the patient’s neurologic examination should be performed before sedation and paralysis (RSI). If time allows, premedicate the patient with lidocaine, 1 to 1.5 mg/kg, 3 minutes before intubation while preoxygenating the patient. Lidocaine has been reported to blunt the rise in ICP associated with laryngoscopy and may protect against some hypoxia-related dysrhythmias; however, its true value is unproven (see Chapters 4 and 5). Fentanyl is an excellent drug for control of pain, which if untreated, can lead to increased ICP. Fentanyl can also be given during the pretreatment phase of RSI (3 minutes before administration of the paralytic agent) if time allows. Caution should be exercised, however, to avoid precipitous drops in blood pressure, which can actually threaten CBF and exacerbate the brain injury. It is for this reason that we recommend titrating fentanyl up to the target dose rather than giving a full dose (typically 3 µg/kg) up front. We also recommend caution in brain-injured patients with concomitant trauma, who may have exaggerated episodes of hypotension with narcotics and sedatives. A defasciculating dose 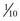 of the intubating dose of a nondepolarizing neuromuscular blocking agent may also be administered as pretreatment. If given 3 minutes before a paralyzing dose of succinylcholine, it counteracts the transient fasciculations and increase in ICP that occur with the administration of succinylcholine. It is important to note, however, that practice variations are common, and though theoretically attractive, none of these pretreatment options have been demonstrated to improve patient-oriented outcomes.2
of the intubating dose of a nondepolarizing neuromuscular blocking agent may also be administered as pretreatment. If given 3 minutes before a paralyzing dose of succinylcholine, it counteracts the transient fasciculations and increase in ICP that occur with the administration of succinylcholine. It is important to note, however, that practice variations are common, and though theoretically attractive, none of these pretreatment options have been demonstrated to improve patient-oriented outcomes.2
Induction (sedation) may be achieved with drugs that block the rise in ICP, such as etomidate (0.3 mg/kg intravenously [IV]), thiopental (50 to 100 mg IV or 3 to 5 mg/kg IV), or propofol (1 mg/kg IV).3–5 Etomidate is preferred over thiopental in patients with unstable hemodynamic status. Etomidate has two significant benefits—it has minimal effect on systemic blood pressure and does not appear to increase ICP.3,4 Propofol has gained acceptance as a sedative in patients with increased ICP because of its short duration of action and depression of cerebral metabolism and oxygen consumption. This may have a neuroprotective effect. However, propofol can cause profound decreases in systemic blood pressure and with higher doses and extended periods of use can be associated with significant morbidity.5
The paralytic agent of choice is succinylcholine (1.5 mg/kg in adults and up to 2.5 mg/kg in pediatric patients). Nondepolarizing agents are appropriate when contraindications to succinylcholine exist (see Chapter 5). Sedatives and paralytics should be short acting to facilitate close monitoring of the patient’s neurologic status.
Oxygenation and Hyperventilation
As the airway is secured, adequate supplemental oxygen should be provided, with titration down rapidly from the initial Fio2 of 1.0 used for RSI to ensure oxygen saturation greater than 90%.1
In general, avoid hyperventilation in patients with brain injury because low Pco2 levels cause cerebral vasoconstriction, which results in decreased CBF in the critical hours following injury.1,6 Hyperventilation is also associated with poor survival and neurologic outcomes.6,7 For the majority of brain-injured patients, the target is thus eucapnia with a Pco2 of 35 to 40 mm Hg.6 Nevertheless, if a patient displays evolving signs of brain herniation (e.g., anisocoria, hemiparesis, asymmetric posturing, Cushing’s reflex, or rapid deterioration in GCS score), hyperventilation may be necessary to arrest the process. Current recommendations target a Pco2 of 28 to 35 mm Hg in these scenarios as a temporizing measure until surgical intervention to lower ICP can occur.6
Head Position
If not in shock, elevate the patient’s head to decrease ICP. This position allows drainage of cerebral veins. Feldman,8 Ng,9 and their colleagues demonstrated that elevation of the head to 30 degrees significantly reduces ICP in most patients without impairing CBF, CPP, or cerebral metabolism. Raising the head of a patient with hypotension, however, exacerbates any decrease in the patient’s mean arterial pressure (MAP) and hence lowers CPP. If head elevation is to be used, MAP must be maintained above 90 mm Hg to facilitate a CPP of approximately 60 mm Hg.10 It is also important to avoid neck rotation and flexion or any other intervention that could result in compression of the jugular vein. If the jugular veins are compressed, venous outflow from the head can be further compromised.
Fluid Management
The goal in fluid management is to maintain euvolemia; head-injured patients with hypotension have double the mortality of normotensive patients. Patients with increased ICP may be hypovolemic from profuse vomiting, decreased fluid intake, or hemorrhage. Distributive shock may also be present and occurs as a result of loss of vasomotor sympathetic tone, especially in patients with concomitant cervical spine injury. Only isotonic or hypertonic fluids should be used (normal or hypertonic saline) to avoid worsening the brain edema. Vasopressors may also be necessary. A target CPP of 60 mm Hg and MAP of greater than 90 mm Hg are recommended.11
Diuresis
Mannitol has two properties—it initially acts as a volume expander and then serves as an osmotic agent. On administration of mannitol, intravascular volume expands and blood viscosity decreases, which results in augmentation of CBF. Once volume expansion has occurred, osmotic movement of fluid from the cellular compartment to the intravascular compartment begins and results in a decrease in ICP. The osmotic effect usually occurs within 15 minutes. The half-life of mannitol ranges from 90 minutes to 6 hours.11 Its effect is most pronounced in patients with CPP lower than 70 mm Hg.11
Hypertonic saline is an alternative to mannitol in patients who are hypotensive. Theoretically, hypertonic saline is an ideal resuscitation fluid for patients with concomitant head injury and hemorrhagic shock because it can effectively expand intravascular volume while causing osmotic diuresis of the brain. However, its efficacy in human studies remains uncertain, so its use should be reserved for patients with contraindications to mannitol. Although hypertonic saline is generally administered in boluses of 100 to 250 mL of 3% to 7% saline, there is no consensus on the preferred concentration and volume of administration.11,12
Seizure Prophylaxis
Controlling seizure activity is important in the early stages of traumatic brain injury to avoid hypoxia and aspiration, as well as elevation of ICP and possible herniation. In the absence of a seizure, it is currently controversial whether or not prophylactic anticonvulsant medication should be routinely administered after spontaneous intracranial hemorrhage. If a seizure occurs, anticonvulsant therapy is warranted. However, current literature supports the use of prophylactic anticonvulsant therapy for the first week following traumatic brain injury, with the recognition that this has no effect on the occurrence of late seizures.13 Rapidly acting benzodiazepines such as lorazepam and diazepam are first-line therapy, followed by anticonvulsant agents such as pentobarbital or phenytoin. Fosphenytoin can be given more rapidly (up to 150 mg/min IV by infusion) than phenytoin, which can be administered no faster than 50 mg/min IV. Phenytoin can induce hypotension and lower CPP, even at rates below 50 mg/min. Levetiracetam has also been used in cases of severe head injury and stroke. Although beneficial effects of prophylactic anticonvulsants on outcomes have not been established, in the ED setting the risks associated with the administration of anticonvulsants are probably small in comparison to the potentially devastating secondary injury that results from uncontrolled seizure activity.
Patients who are paralyzed, either chemically or by their neurologic disease, are difficult to monitor for seizure activity without the use of electroencephalography (EEG). These patients may manifest seizures by subtle rhythmic movements of the extremities, tonic gaze deviation, an elevation in heart rate or blood pressure, or spikes on an ICP monitor (if one is placed by the neurosurgeon). Whenever possible, avoid giving paralytic agents so that seizure activity can be monitored. If seizures are not controlled despite upward titration of benzodiazepines, phenytoin, or other agents, endotracheal intubation and induction of a barbiturate or propofol coma may be necessary. Pentobarbital has been used to treat uncontrolled elevations in ICP when other medical and surgical treatments have failed. It decreases CBF, metabolism, oxygen consumption, and cerebral edema. It also scavenges free radicals.14 Pentobarbital is given at a loading dose of 10 mg/kg over a 30-minute period followed by infusion at 1 to 3 mg/kg/hr with an EEG monitor available for burst suppression. Intensive monitoring of the patient’s hemodynamic status is necessary because of pentobarbital’s hypotensive effect.
Steroids
Steroids have been shown to be beneficial in patients with vasogenic edema—edema associated with brain tumors. Steroids decrease CSF production, stabilize membranes, and restore normal membrane permeability.15–17 Dexamethasone is usually administered at 10 to 20 mg IV as a loading dose, followed by 4 to 10 mg every 6 hours. No studies support the use of steroids in head-injured patients; in fact, steroid use in this population was found to increase mortality in a recent randomized trial.18,19
Hypothermia
Lower body temperatures have been associated with a decrease in blood flow, ICP, and metabolism. The previous method of keeping core temperatures lower than 30°C has been abandoned. It fell out of favor because of the complications of cardiac arrhythmias and difficulty maintaining such profound hypothermia. More recently, mild therapeutic hypothermia (32°C to 34°C) has become popular in the management of post–cardiac arrest victims who do not regain consciousness. Although one study has shown a benefit in survival and decreased ICP with mild hypothermia (32°C to 34°C),20 in the setting of traumatic brain injury and stroke it is not currently recommended.21 Hyperthermia increases ICP and must be avoided.
Skull Trephination
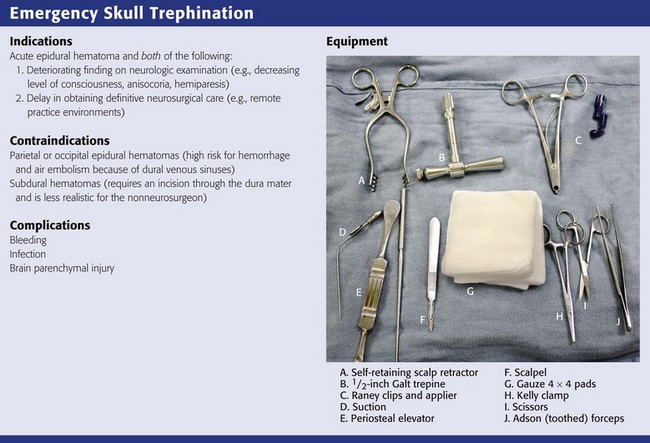
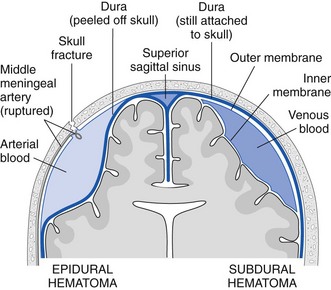
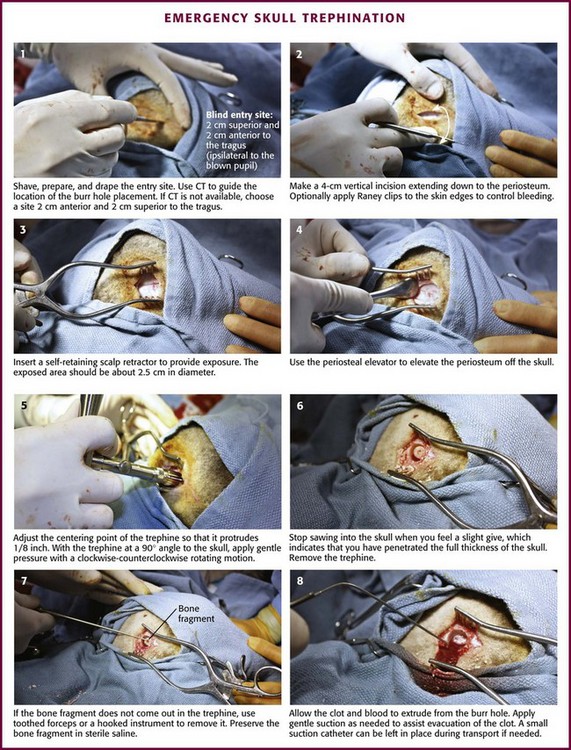
Skull trephination or burr hole placement is a technique that has been traced back to the Neolithic age.22 Though rarely performed by nonsurgeons, it can be a lifesaving procedure for severely head-injured patients. In a patient with an acute epidural hematoma and deteriorating findings on neurologic examination (decreasing level of consciousness, anisocoria, or hemiparesis), skull trephination has been shown to improve both short- and long-term neurologic outcomes.23,24 Trephination is an appropriate treatment of acute subdural hematoma as well, but this requires incision through the dura mater and is less realistic for non-neurosurgeons (Figs. 59-7 and 59-8). Trephination may be lifesaving if performed before transport in selected patients from remote practice environments without neurosurgical capability. This heroic procedure is not standard nor is it an expected skill for most emergency clinicians; nonetheless, it may be attempted when the situation is dire.
Trephination in the ED should be performed only in the temporal regions. Trephination in the parietal and occipital regions is associated with a much higher risk for hemorrhage and air embolism because of their proximity to the dural venous sinuses. These approaches should generally be performed only after the results of CT are available for guidance.23
In practice environments without access to CT, it is not unreasonable to attempt this procedure before transport of the patient when all other measures to lower ICP have failed and signs of evolving brain herniation are present. The blind approach for temporal trephination is depicted in Figure 59-9 and involves locating an area 2 cm anterior and 2 cm superior to the tragus on the side of the suspected hematoma.23 In the majority of cases the appropriate side for trephination is ipsilateral to pupillary dilation and contralateral to motor paresis.
Once the proper location is identified, either with a blind approach or by using the results of CT, make a 4-cm vertical incision through the skin and temporalis fascia. Divide the temporalis muscle. Once the periosteum is identified, incise it. Expose the skull by elevating the periosteum (with a periosteal elevator if available). Insert a trephine or hand drill into the area underneath the periosteum.23 While holding the drill perpendicular to the skull, apply continuous gentle downward pressure and rotate the drill in an alternating clockwise-counterclockwise motion. As progress is made with the hand drill, gradually reduce pressure to avoid inadvertent “plunging” into the brain parenchyma. The operator will know when penetration through both the outer and inner tables of the skull has been accomplished once resistance against the drill is no longer felt. After skull penetration has been accomplished, remove the round piece of bone that has been cored out (with the diameter of the drill) and place it in saline.
In many cases, epidural blood and clots under pressure will extrude from the site on full penetration of the skull. Insertion of a suction catheter into the trephinated space may be necessary, however, for full evacuation of the clotted material. If easily identified, the bleeding artery (usually the middle meningeal artery) may be clamped.23 After successful trephination, the patient’s status should immediately be reassessed for signs of improvement. In a significant minority of patients, false localizing signs may lead the clinician to suspect a hematoma on the wrong side. Thus, if no improvement is noted with trephination on the side of the suspected hematoma, the procedure may be repeated on the opposite side. However, in all cases the delay in definitive neurosurgical care caused by attempts at trephination must be weighed against the possible benefits of the procedure. Moreover, trephination should ideally be performed after consultation with the accepting neurosurgeon.24
Complications of the procedure include bleeding, infection, and injury to the brain parenchyma.
Intracranial Shunts
Intracranial shunt malfunctions are often diagnosed in the ED. In many cases the patient’s significant other or caretaker provides the subtle clues that lead to the diagnosis. Depending on the age of the patient, recurrent symptoms of increased ICP may include changes in behavior, headache, nausea, vomiting, and visual disturbances. In children, lethargy, poor feeding, vomiting, ataxia, decreased or increased activity level, fever, diaphoresis, and fussiness can all be indications of shunt malfunction. Accordingly, in children, lethargy and shunt site swelling are the most predictive signs of shunt malfunction.25 Failure of upward gaze is another sensitive sign of nonacute shunt malfunction (sunset eyes). Cranial nerve and visual field examination should be routine in the assessment of older, cooperative patients with intracranial shunts.
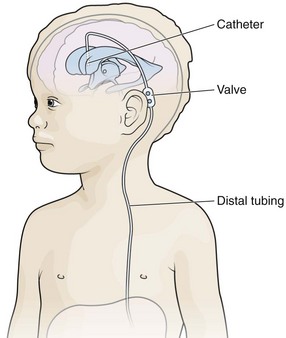
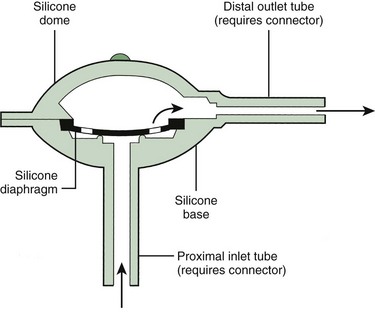
The essential elements of any shunt system include the proximal and distal catheters, a valve, and a reservoir. A wide array of valves, catheters, and other devices are available for use in shunt systems. The valve allows unidirectional flow, incorporates a pumping chamber, and regulates the pressure at which flow will occur across it. The reservoir is usually located between two valves. The proximal valve allows flow from the ventricles to the reservoir, whereas the distal valve allows flow from the reservoir to the distal catheter (Fig. 59-10). Many different types of shunt systems incorporating a variety of designs are available (Fig. 59-11). Some have unique characteristics, such as a double dome, whereas in others, valves are absent altogether.
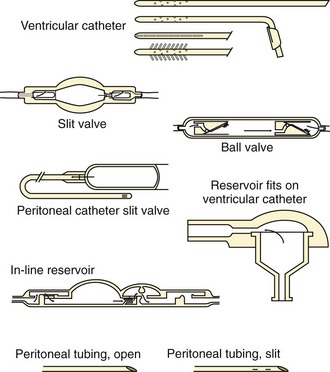
Figure 59-11 Shunt components. (From Rengachary SS, Wilkins RH, eds. Principles of Neurosurgery. Philadelphia: Mosby; 1994.)
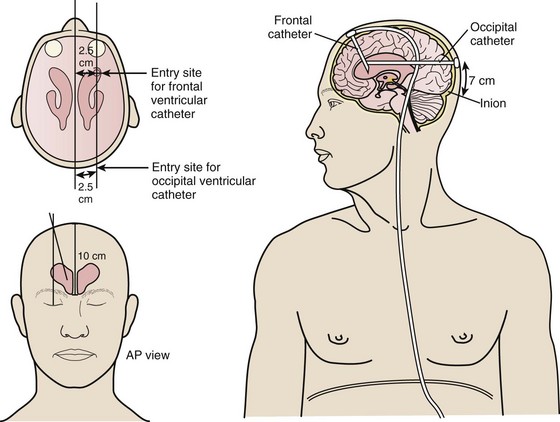
In the ventriculoperitoneal (VP) shunt system, a ventricular catheter is placed in the right frontal horn of the lateral ventricle (nondominant hemisphere) and connected to a subcutaneous valve traversing the temporal aspect (Fig. 59-12). This valve is then connected to a distal catheter threaded subcutaneously into the neck and finally into the peritoneum or another body compartment (Fig. 59-13). Ventricular catheters can be either straight or angled, with the latter having the option of a reservoir component attachment. Valves come in four different types (ball, diaphragm, miter, slit), each with unique flow characteristics. The distal catheter has either an open or closed end. Identifying the type of shunt in place is often difficult unless the patient or caretaker has the information available. Moreover, the skin overlying the subcutaneous component of the shunt in the temporal regions can scar, thus rendering palpation of the shunt type impossible.
Shunt Assessment
The characteristics of valve pumping may be useful in diagnosing shunt malfunction in some cases.26 Partial shunt valve compression should be done because full depression and release may lead to suction of the choroid plexus. If the CT scan shows narrow (slit) ventricles, indicative of overdrainage, further valve compression may cause blockage of the shunt by suction of the choroid plexus. When palpating the shunt, a normal refill time of 15 to 30 seconds should be observed. If the valve fills more slowly than this but can be compressed easily, the obstruction is proximal to the valve. If the valve is not compressible, the blockage is either at the valve or distal to it. Proximal obstruction is more common than distal obstruction. Other sources of proximal blockage include blood clots or debris related to the surgical procedure. Sources of distal occlusion include malposition, infection, shunt disconnection, and pseudocyst formation. The entire shunt tract and surgical incisions should be examined for signs of wound infection, disruption of the tubing, or CSF leakage around the tract. Definitive treatment usually requires shunt revision.
Infection is usually due to skin organisms overlying the valve that may ulcerate the skin and colonize the shunt.27 Typical organisms include Staphylococcus epidermidis and Staphylococcus aureus, as well as gram-negative bacilli, which are thought to be introduced into the system during manipulation of the site.28 Approximately 70% of these infections are seen within 2 months of shunt placement (see later under “Special Considerations—Postoperative Shunt Complications”). Other risk factors associated with shunt infection include perioperative infection and dental or urologic instrumentation. Two studies have shown that approximately 8% of neurosurgical patients with implanted shunts acquire infections.29,30
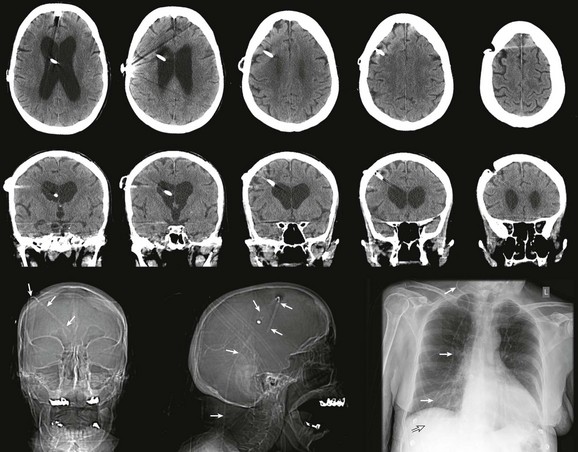
Radiographic evaluation of shunt function in the ED begins with a non–contrast-enhanced head CT scan and shunt series radiographs. Shunt series radiographs consist of anteroposterior (AP) and lateral skull, AP chest, and AP abdominal radiographs (Figs. 59-14 and 59-15). These films may reveal kinking, breakage, or disconnection of the catheter (Fig. 59-16). Head CT has a sensitivity of 83% in detecting shunt obstruction and a negative predictive value of 93%. Shunt series radiographs have a sensitivity of 20% and a negative predictive value of 22%. When combined, the two tests have a sensitivity of 88% and a negative predictive value of 95%. Because they are complementary, it is important to obtain both studies.31–33 It should be noted, however, that the finding of enlarged ventricles on CT reveals little about shunt function unless comparison with previous images or serial scans show progressive ventricular expansion.
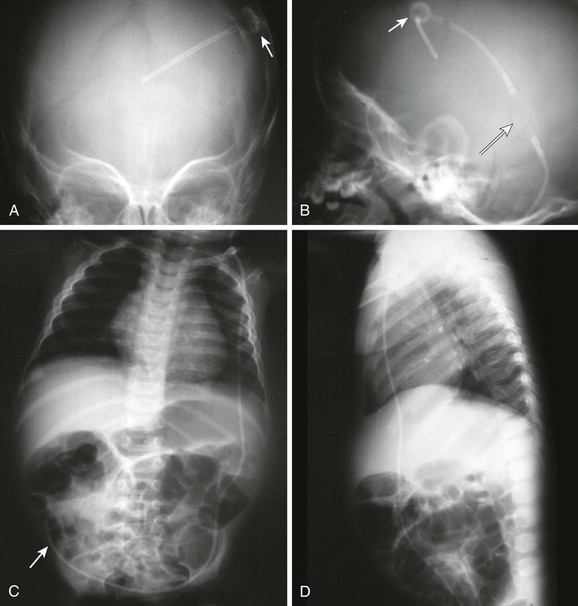
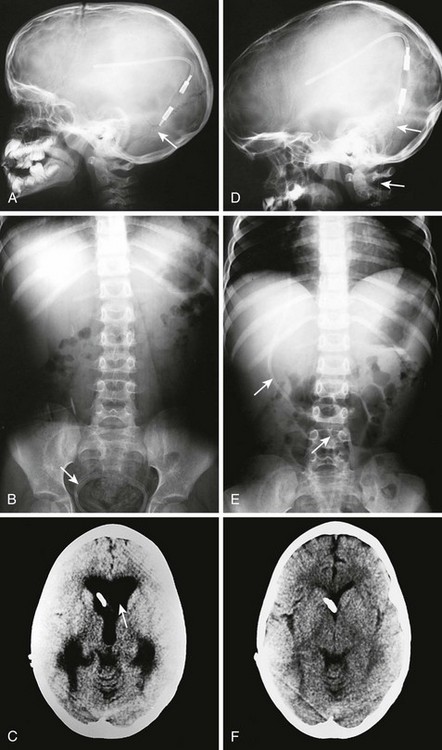
Figure 59-15 Conventional radiography standard shunt series. A standard shunt series includes skull, chest, and abdominal radiographs. A, This anteroposterior (AP) skull radiograph reveals the proximal intracranial portion of the shunt and a Rickham reservoir (arrow) over the burr hole. A companion non–contrast-enhanced head CT scan (see Fig. 59-14) is usually performed to further evaluate shunt function. B, The lateral skull radiograph better reveals a cylindrical Holter valve (long arrow) several centimeters distal to the Rickham reservoir (short arrow). C, This AP chest and abdominal radiograph reveals the distal portion of the shunt (arrow). Note the length of the catheter to allow patient growth. D, The lateral chest and abdominal view redemonstrates the course of the shunt.
Shunt Tapping
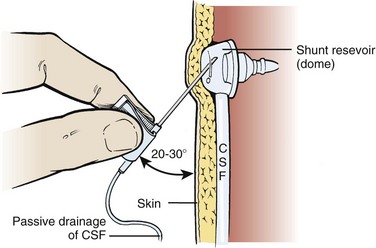
Clip the scalp hair over and around the reservoir, prepare the skin with a surgical scrub brush for 10 minutes, followed by the application of a povidone-iodine solution, and then allow it to fully dry. After appropriate draping, infiltrate the skin with 1% plain lidocaine to a level of adequate local anesthesia. Use a 25-gauge butterfly needle with tubing and enter the reservoir percutaneously at a 20- to 30-degree angle (Fig. 59-17). Lack of CSF flow from the reservoir indicates a proximal obstruction unless the ventricles are completely deflated (slit ventricles syndrome). Even so, a small amount of CSF should be obtained within the tubing, thus ensuring that entry into the lumen of the reservoir has been accomplished. If the ventricles are deflated and only a small amount of CSF is aspirated, hold the end of the tubing 5 to 10 cm below the level of the reservoir to see whether CSF will fill the tubing and eventually begin to drip at the rate of 2 to 3 drops/min. If CSF is readily aspirated from the reservoir, hold the tubing vertically to obtain an indication of intraventricular pressure. This pressure reading, as well as the ease with which CSF is aspirated, will give some indication of proximal obstruction. To assess runoff or patency of the distal end, apply pressure to the tubing proximal to the reservoir and then deflate the reservoir without any resistance. If any resistance is encountered when deflating the reservoir, suspect a distal obstruction.
Special Considerations—Postoperative Shunt Complications
Shunt Infection—Treatment and Prevention
Of all the potential complications associated with shunting procedures, infection is the most notorious and occurs in 2% to 10% of cases.26 Most shunt infections appear within the first 2 months after surgery. The diagnosis may be obvious in patients with variable systematic signs, wound infection, meningitis, peritonitis, or septicemia. It is also possible that the shunt may harbor an indolent infection without symptoms or signs. Culture of CSF obtained from a shunt tap may be negative even when the shunt is ultimately shown to be infected.34,35
References
1. Marik, PE, Varon, J, Trask, T. Management of head trauma. Chest. 2002;122:699.
2. Robinson, N, Clancy, M. In patients with head injury undergoing rapid sequence intubation, does pretreatment with intravenous lignocaine/lidocaine lead to an improved neurological outcome? A review of the literature. Emerg Med J. 2001;18:457.
3. Smith, DC, Bergen, JM, Smithline, H, et al. A trial of etomidate for rapid sequence intubation in the emergency department. Emerg Med Clin North Am. 2000;18:13.
4. Prior, JG, Hinds, CJ, Williams, J, et al. The use of etomidate in the management of severe head injury. Intensive Care Med. 1983;9:313.
5. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. XI. Anesthetics, analgesics, and sedatives. J Neurotrauma. 2007;24(suppl 1):S71–S76.
6. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. J Neurotrauma. 2007;24(suppl 1):S87–S90.
7. Muizelaar, JP, Marmarou, A, Ward, JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731.
8. Feldman, Z, Kanter, MJ, Robertson, CS, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76:207.
9. Ng, I, Lim, J, Wong, HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:597.
10. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(suppl 1):S59–S64.
11. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma. 2007;24(suppl 1):S15–S20.
12. Khanna, S, Davis, D, Peterson, B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144.
13. Schierhout, G, Roberts, I. Antiepileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. (6):2012. [CD000173].
14. Mihm, FG. Barbiturates for intracranial hypertension and local and global ischemia. In: Newfield P, Cottrell JE, eds. Handbook of Nueroanesthesia: Clinical and Physiologic Essentials. Boston: Little, Brown; 1983:60.
15. Weiss, MH, Nulsen, FE. The effect of glucocorticoids on CSF flow in dogs. J Neurosurg. 1970;32:452.
16. Braughler, JM, Hall, ED. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+, K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J Neurosurg. 1982;56:838.
17. Maxwell, RE, Long, DM, French, LA. The effects of glucosteroids on experimental cold-induced brain edema: gross morphological alterations and vascular permeability changes. J Neurosurg. 1971;34:477.
18. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. XV. Steroids. J Neurotrauma. 2007;24(suppl 1):S91–S95.
19. Roberts, I, Sandercock, P, Edwards, P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinical significant head injury (MRC CRASH trial): randomized placebo-controlled trial. Lancet. 2004;364:1324.
20. Shiozaki, T, Sugimoto, H, Taneda, M, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363.
21. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton, SL, Chestnut, RM, Ghajar, J, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermia. J Neurotrauma. 2007;24:S21–S25.
22. Zabihyan, S, Etemadrezaie, H, Baharvahdat, H. The origin of cranial surgery. World Neurosurg. 2010;74:7.
23. Smith, SW, Clark, M, Ruiz, E, et al. Emergency department skull trephination for epidural hematoma in patients who are awake but deteriorate rapidly. J Emerg Med. 2009;39:377.
24. Nelson, JA. Local skull trephination before transfer is associated with favorable outcomes in cerebral herniation from epidural hematoma. Acad Emerg Med. 2011;18:84.
25. Kim, TY, Stewart, G, Voth, M, et al. Signs and symptoms of cerebrospinal fluid shunt malfunction in the pediatric emergency department. Pediatr Emerg Care. 2006;22:28.
26. Youmans JR, ed. Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurosurgical Problems, 4th ed, Philadelphia: Saunders, 1996.
27. Bayston, R, Lari, J. A study of the sources of infection in colonized shunts. Dev Med Child Neurol Suppl. 1974;32:16.
28. Tuan, TJ, Thorell, EA, Simon, TD, et al. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 2011;30:734.
29. George, R, Leibrock, L, Epstein, M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51:804.
30. Renier, D, Lacombe, J, Pierre-Kahn, A. Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg. 1984;61:1072.
31. Pitetti, R. Emergency department evaluation of ventricular shunt malfunction: is the shunt series really necessary? Pediatr Emerg Care. 2007;23:137.
32. Griffey, RT, Ledbetter, S, Khorasani, R. Yield and utility of radiographic “shunt series” in the evaluation of ventriculo-peritoneal shunt malfunction in adult emergency patients. Emerg Radiol. 2007;13:307.
33. Zorc, JJ, Krugman, SD, Ogborn, J, et al. Radiographic evaluation for suspected cerebrospinal fluid shunt obstruction. Pediatr Emerg Care. 2002;18:337.
34. Rekate, HL. Parenchymal cerebrospinal fluid extravasation as a complication of computerized tomography. Case report. J Neurosurg. 1980;52:553.
35. Walters, BC, Hoffman, HJ, Hendrick, EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014.