Management of Diabetes Mellitus in Children
Presentation of Diabetes Mellitus
Distinguishing Between Type 1 and Type 2 Diabetes in Children
Management of Diabetes Mellitus
Type 2 Diabetes Mellitus in Children and Adolescents
New Therapies for Type 2 Diabetes
Prevention of Type 2 Diabetes Mellitus
Hypoglycemia in Children With Diabetes Mellitus
Symptoms and Signs of Hypoglycemia
Impact of Hypoglycemia on the Child’s Brain
Screening for Other Autoimmune Diseases in Type 1 Diabetes
Presentation of Diabetes Mellitus
Most children with newly diagnosed type 1 diabetes mellitus (T1DM) present with classic symptoms (polyuria, polydipsia, weight loss) for a few days to several weeks. Other presentations include recent onset of enuresis in a previously toilet trained child, failure to gain weight appropriately in a growing child, perineal candidiasis, especially in a prepubertal child, recurrent skin infections, irritability, and deteriorating school performance.1 The frequency of diabetic ketoacidosis (DKA) at diabetes onset varies widely by geographic location, ranging from 15% to 67% in Europe and North America, and DKA is even more common in developing countries.2,3 There is an inverse correlation between the frequency of DKA and the background incidence of T1DM in different populations. DKA at initial presentation is more frequent in infants, toddlers, and preschool age children (up to two thirds of toddlers present in DKA), in children who do not have a first-degree relative with TIDM, and in children whose families are of lower socioeconomic status.2
Prospective follow-up of high-risk subjects shows that the diagnosis of type 1 diabetes can be made in most asymptomatic individuals when metabolic abnormalities are still relatively mild.4,5 The progression of T1DM tends to follow a characteristic clinical course that includes an abrupt onset of classical symptoms that rapidly disappear after insulin replacement therapy is begun. A temporary remission (“honeymoon phase”) often follows with partial recovery of endogenous insulin secretion, demonstrable by plasma C-peptide levels and characterized by stable near-to-normal blood glucose levels and decreasing insulin requirements.1 Severe DKA and young age at presentation reduce the likelihood of a remission phase. Recurrence or persistence of the autoimmune attack on β cells invariably leads to further β cell destruction and progressive decline in insulin production, leading eventually to complete cessation of insulin production in most cases of childhood onset T1DM.
Distinguishing Between Type 1 and Type 2 Diabetes in Children
Both T1DM and T2DM most often present during puberty, a period of life characterized by a physiologic reduction in insulin sensitivity of approximately 30%.6 The increasing incidence of T2DM in youth and the current high prevalence of overweight and obesity in children and adolescents have presented clinicians with a diagnostic challenge when evaluating a patient with new-onset diabetes mellitus. Distinguishing between T1DM and T2DM may be difficult because considerable overlap in presentation may occur. The overall frequency of obesity at diagnosis of T1DM, irrespective of race, gender, and age of onset, has tripled in the past decade, and a recent report indicates that one fourth of patients with T1DM are obese.7 In contrast to T2DM in adults, in which ketonuria is unusual, a substantial fraction of adolescents with T2DM have ketonuria (24% to 63%) or even DKA (5% to 46%) at presentation. Insulin requirements typically decrease after several weeks of treatment for TD2M, which may resemble the remission or “honeymoon” period of T1DM. Measuring pancreatic autoantibodies and markers of insulin secretion (fasting C-peptide levels) at the time of diagnosis helps to distinguish between T1DM and T2DM in obese patients. A fasting plasma C-peptide level >0.85 ng/mL suggests T2DM.8 Plasma C-peptide levels, however, may initially be temporarily low in T2DM owing to glucotoxicity and lipotoxicity, and rechecking the level after several weeks or even months of therapy will sometimes demonstrate hyperinsulinism, helping to establish a diagnosis of T2DM. A recent report suggests that a fasting insulin-like growth factor binding protein-1 (IGFBP-1) level, whose secretion is acutely inhibited by insulin and, therefore, is a marker of insulin action, is another useful biochemical parameter to assist the clinician in making the distinction. An IGFBP-1 concentration ≤3.6 ng/mL is highly suggestive of T2DM.8 Several recent reports have described autoantibody positivity in children with clinical features of T2DM. Latent autoimmune diabetes in youth (LADY) has been proposed to describe this subgroup. A binary classification is not always possible at the time of diagnosis; clearly, some patients have clinical and biochemical features of both types of diabetes. Irrespective of the type of diabetes, the choice of initial therapy must be made on the basis of the metabolic state, as determined by clinical assessment. Subsequent therapy is then modified, if necessary, guided by the individual patient’s response to treatment.
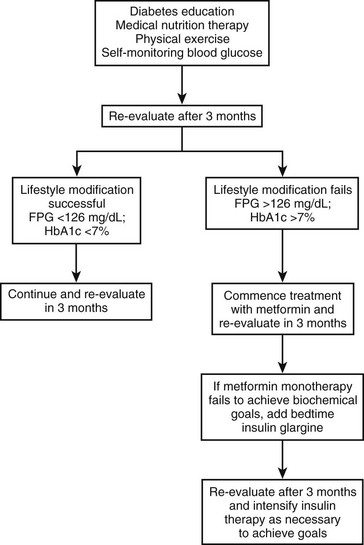
Management of Diabetes Mellitus
Initial Management of Newly Diagnosed Type 1 Diabetes Mellitus
Whenever possible, the child with DKA should be cared for in a health care facility that has nursing staff trained in DKA management and access to a clinical chemistry laboratory that can provide frequent and timely measurement of serum chemistries. Children with signs of severe DKA (long duration of symptoms, compromised circulation, depressed level of consciousness) and those at increased risk for cerebral edema (<5 years of age, new-onset diabetes) should be treated in a pediatric intensive care unit or in a children’s ward that specializes in diabetes and can provide equivalent resources and supervision of care.9
The diagnosis of diabetes in a child is a crisis for the family, who require considerable emotional support and time for adjustment and healing. Shocked, grieving, and overwhelmed parents typically require at least 2 to 3 days to acquire basic or “survival” skills while they are coping with the emotional upheaval that typically follows the diagnosis of diabetes in a child. Even if they are not acutely ill, children with newly diagnosed T1DM usually are admitted to hospital for metabolic stabilization, diabetes education, and self-management training. However, outpatient or home-based management is preferred at some centers that have the appropriate resources.10 Outpatient or home-based management may offer several advantages: the stress of a hospital stay can be avoided, the outpatient setting or the patient’s home is a more natural learning environment for the child and family, and ambulatory treatment possibly reduces the cost of care for the health care system and the family. The literature comparing initial hospitalization with home-based and/or outpatient management of children who are not acutely ill with newly diagnosed T1DM has recently been critically reviewed. The results are inconclusive owing to insufficient high-quality data. The data suggest that where adequate outpatient and/or home initial management of T1DM in children at diagnosis can be provided, there is no disadvantage in terms of metabolic control nor increase in acute complications, hospitalizations, psychosocial or behavioral problems, or total costs.10 The decision concerning whether a child with newly diagnosed diabetes should be admitted to hospital depends on several factors. Of these, the most important are the severity of the child’s metabolic derangements, the family’s psychosocial circumstances, and the resources available at the treatment center.
Outpatient Diabetes Care
Optimal care of children with T1DM is complex and time consuming. Children with diabetes should be managed by a multidisciplinary diabetes team that provides diabetes education and care in collaboration with the child’s primary care physician.11 The team should consist of a pediatric endocrinologist or pediatrician with training in diabetes, a pediatric diabetes nurse educator (DNE), a dietitian trained in pediatric nutrition, and a mental health professional, either a clinical psychologist or a social worker. A member of the diabetes team should always be available by telephone to provide guidance and support to parents and patients and to respond to metabolic crises that require immediate intervention.
Initial Diabetes Education
Education is the keystone of diabetes care, and structured self-management education is vital to a successful outcome.11 Diabetes education is the process of providing the person with the knowledge and skills needed to perform diabetes self-care, to manage crises, and to make lifestyle changes to successfully manage the disease. The diabetes education curriculum should be adapted to the individual child and family. Parents and children with newly diagnosed diabetes are anxious and frequently overwhelmed, and cannot assimilate a large amount of abstract information. Therefore, the education program should be staged. Initial educational goals should be limited to essential survival skills so that the child can be safely cared for at home and return to his or her daily routine. Initial diabetes education and self-management training should include information on what causes diabetes, how it is treated, how to administer insulin, basic meal planning, self-monitoring of blood glucose and ketones, recognition and treatment of hypoglycemia, and how and when to contact a member of the diabetes team for advice.
Psychosocial Issues
Diabetes presents family members with the task of being sensitive to the balance between the child’s need for a sense of autonomy and mastery of self-care activities and the need for ongoing family support and involvement. The struggle to balance independence and dependence in relationships between the child and family members presents a long-term challenge and raises different issues for families at different stages of child and adolescent development. Focusing on normal developmental tasks at each stage of the child’s growth and development provides the most effective structure with which to address this concern (see reference 12 for details).
Current rates of psychological ill health in diabetic youth are disturbingly high, and longitudinal data indicate that mental health issues in childhood are likely to persist into early adulthood and possibly beyond. It is important to note that such mental health issues appear to be prognostic of maladaptive lifestyle practices and long-term problems with diabetes control and earlier than expected onset of complications. Based on these considerations, mental health should be given equivalence to, and perhaps even precedence over, other complications screening undertaken in diabetes clinics. Routine screening for behavioral disturbance should begin in children at the time of diabetes diagnosis, with further assessment of parental mental health and family functioning for those children identified as “at risk.” Interventions can then be targeted to the specific needs of individual children and families.13
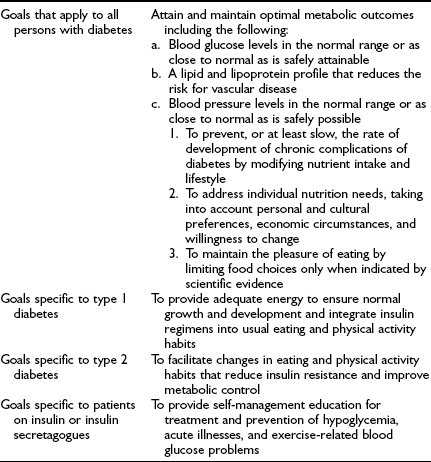
Goals of Therapy
The Diabetes Control and Complications Trial (DCCT)14,15 and a similar smaller study in Sweden, the Stockholm Diabetes Intervention Study,16 ended the debate about whether the microvascular complications of diabetes are caused by hyperglycemia and can be prevented or ameliorated. The U.K. Prospective Diabetes Study (UKPDS) in adults with type 2 diabetes17,18 provided additional scientific evidence for the importance of glycemic control. These clinical trials and long-term follow-up observations of the DCCT cohort unequivocally demonstrate the importance of lowering glycated hemoglobin (HbA1c) values to reduce the risk of development and progression of retinopathy, nephropathy, neuropathy, and macrovascular disease. Treatment regimens that reduce average HbA1c to ≈7% (about 1% above the upper limit of normal) are associated with fewer long-term microvascular and macrovascular complications.14,15,19 Moreover, improved glycemic control is associated with a sustained decreased rate of development of diabetic complications.20,21
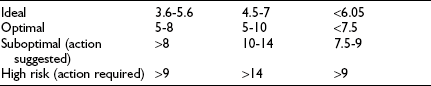
The aim of diabetes management is to achieve recommended glycemic targets known to reduce the risk for long-term complications; however, no international consensus has been attained on appropriate targets for children of different ages. Biochemical goals of treatment for children and adolescents have recently been published by the International Society for Pediatric and Adolescent Diabetes (ISPAD): ideal <6.05%, optimal <7.5%, and suboptimal 7.5% to 9.0%; action is required when the value exceeds 9.0%.22 The ISPAD guidelines are accompanied by the following statement: “… each child should have their targets individually determined with the goal of achieving a value as close to normal as possible while avoiding severe hypoglycemia as well as frequent mild to moderate hypoglycemia.” The recommendations of a sample of national diabetes organizations are shown in Table 23-1.
Table 23-1
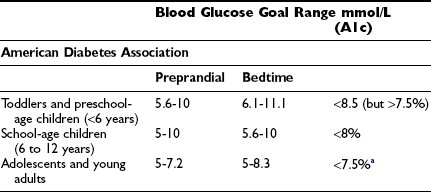



aA lower goal (<7%) is reasonable if it can be achieved without excessive hypoglycemia.
b“In very young children, … glycaemic targets need to be at the upper part of these ranges or a little higher.”
cExtreme caution is required to avoid severe hypoglycemia.
dTargets should be graduated to the child’s age.
eAppropriate for most patients.
fConsider for patients in whom these targets can be achieved safely.
g“… without frequent disabling hypoglycemia.”
hThese targets are intended as guidelines, and each child should have his or her targets individually determined with the goal of achieving a value as close to normal as possible while avoiding severe hypoglycemia, as well as frequent mild to moderate hypoglycemia.22
Management of young children with diabetes, especially those younger than 5 years old, must balance opposing risks of hypoglycemia (see section on Hypoglycemia below) and future vascular complications. The relative contribution of the prepubertal years to the development of microvascular complications has been uncertain; however, recent evidence indicates that longer prepubertal duration of diabetes increases the risk for retinopathy and, possibly, microalbuminuria in adolescence and young adulthood, but at a slower rate than in the postpubertal years.23
The risk for microalbuminuria increases steeply with HbA1c >8%.24,25 Based on these considerations, an HbA1c of ≤8.0% is a reasonable general goal for children with diabetes; however, biochemical goals should be individualized, taking into account both medical and psychosocial considerations. Less stringent treatment goals are appropriate for preschool-age children, those with developmental handicaps, psychosocial challenges, and lack of appropriate family support, for children who have experienced severe hypoglycemia or have hypoglycemia unawareness.
Insulin Therapy
Within days to months of diagnosis, most children with T1DM are severely insulin deficient and depend on insulin replacement for survival. The aim of insulin replacement therapy is to simulate as closely as possible patterns of plasma insulin levels that occur in nondiabetic individuals; however, truly physiologic replacement of insulin remains an elusive goal. Insulin pump therapy and multiple daily insulin injections are the two methods that most closely mimic insulin secretion. The first step in choosing an insulin regimen is to establish glycemic goals. For most patients, this means that more than one half of plasma glucose values should fall within the following ranges: preprandial 90 to 130 mg/dL (5 to 7.2 mmol/L), bedtime 100 to 140 mg/dL (5.6 to 7.8 mmol/L), and 1 to 2 hours postprandial <180 mg/dL (10 mmol/L) (see Table 23-1).
The initial route of insulin administration is determined by the severity of the child’s condition at presentation. Insulin is preferably given intravenously as treatment for DKA. Children who are metabolically stable without vomiting or significant ketosis may be started with subcutaneous (SC) insulin administration. SC insulin treatment in the newly diagnosed child should, ideally, be started with at least three injections per day or a basal-bolus regimen (Table 23-2). Some clinicians have recently started insulin pump therapy at the time of diagnosis, regardless of the severity of presentation or the age of the child.
Table 23-2
Insulin Regimens Used to Treat Children and Adolescents
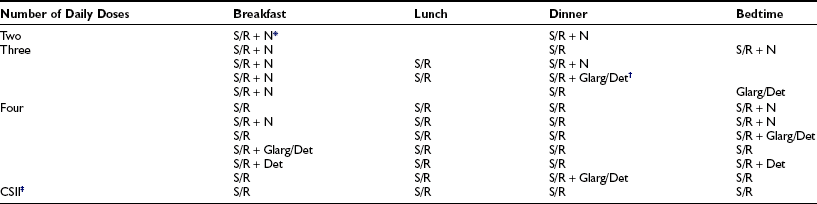
Intensified insulin therapy is defined as the use of at least three daily doses of insulin or CSII.
*Premixed combinations such as either 70% NPH and 30% regular, or 70% protamine-crystallized aspart (PA) and 30% soluble insulin aspart, or 75% neutral protamine lispro (NPL) and 25% insulin lispro are usually used in twice-daily fixed-dose insulin regimens.
†Insulin glargine is almost always given once daily, with breakfast or in the evening with dinner or at bedtime. According to the manufacturers, both glargine and detemir should be given as a separate injection and cannot be mixed with another insulin in the same syringe. However, recent studies suggest that rapid-acting insulin analogues can be mixed with glargine in the same syringe with no detrimental effect on insulin action, provided they are injected immediately.238,239
‡CSII, continuous subcutaneous insulin infusion (pump), boluses are given with meals and snacks together with basal insulin throughout the day and night.
Three major categories of insulin preparations, classified according to their time course of action, are available (Table 23-3). Various insulin replacement regimens consisting of a mixture of short- or rapid-acting insulin and an intermediate- or long-acting insulin are used in children and adolescents (see Table 23-2) and typically are given two to four (or more) times daily. Clear superiority of any one regimen in children and adolescents, in terms of metabolic outcomes, has not been demonstrated.26,27 All insulin regimens have the same general goal: to provide basal insulin throughout the day and night and additional (prandial) insulin to cover meals and snacks.
Table 23-3
Insulin Preparations Classified According to Their Pharmacodynamic Profiles
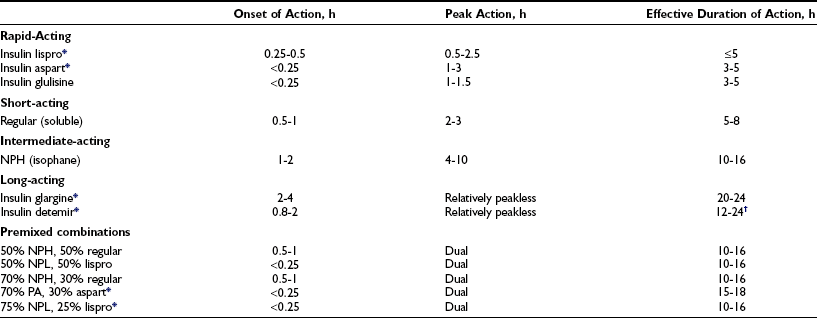
Pharmacodynamic effects of lispro insulin and insulin aspart appear to be equivalent.240
These data are for human insulins and are approximations from studies in adult test subjects. Time action profiles are estimates only. The kinetics of NPH insulin may be more rapid in children.241 The times of onset, peak, and effective duration of action vary within and between patients and are affected by numerous factors, including size of dose, site and depth of injection, dilution, exercise, temperature, regional blood flow, and local tissue reactions.
*Insulin analogue developed by modifying the amino acid sequence and/or chemical adducts of the human insulin molecule.
†Dose dependent; 12 hours for 0.2 U/kg; 20-24 hours for ≥0.4 U/kg.
When a two-dose regimen is used, the total daily dose is typically divided so that about two thirds is given before breakfast and one third is given in the evening. With a three-dose regimen, short- or rapid-acting insulin is administered before the evening meal, and the second dose of intermediate- or a long-acting insulin is given at bedtime rather than before the evening meal. The initial ratio of rapid- to intermediate-acting insulin at both times is approximately 1:2. Toddlers and young children typically require a smaller fraction of short- or rapid-acting insulin (10% to 20% of the total dose) and proportionately more intermediate- or long-acting insulin. Regular insulin is given at least 30 minutes before eating; rapid-acting insulin (lispro, aspart, glulisine) is given 5 to 15 minutes before eating (depending on the pre-meal blood glucose value). In toddlers and young children with unpredictable eating habits, rapid-acting insulin may be given immediately after the meal (dose based on estimated actual carbohydrate consumed) to prevent hypoglycemia from incorrect insulin dosing owing to the child’s not eating the entire meal.28,29
Insulin Therapy in Young Children: Technical Considerations
Caring for young children with diabetes is challenging for many reasons, one of which is the need to accurately and reproducibly measure and inject tiny doses of insulin that is supplied in a concentration of 100 units/mL (U 100 insulin). To administer a dose of 1 unit requires the ability to accurately measure 10 µL (1/100 mL) of insulin. When the dose is less than 2 U of U 100 insulin, neither parents of diabetic children nor skilled pediatric nurses are able to measure the dose accurately.30 Furthermore, a dose change of 0.25 U translates into a volume difference of 2.5 µL in a 300 µL (3/10 cc or 30 unit) syringe. When parents attempt to measure insulin doses in increments of 0.25 U of insulin (e.g., 3.0, 3.25, 3.5 U) using a standard commercial 30 unit (300 µL) syringe, they consistently measure more than the prescribed amount.31 Therefore, to enhance the accuracy and reproducibility of small doses, insulin should be diluted to U 10 (10 units/mL) with the specific diluent available from the insulin manufacturers. When U 10 insulin is used, each line (“unit”) on a syringe is actually 0.1 U of insulin.
Intensified Insulin Therapy in Children: Little evidence is available to guide clinical decisions concerning the risk-benefit ratio of strict control in the preadolescent patient. Clinical trials comparable to the DCCT have not been conducted in prepubertal children; nevertheless, it is reasonable to extrapolate that prepubertal children will also benefit from strict control of their diabetes.
A three-dose insulin regimen with mixed short- or rapid- and intermediate-acting insulins before breakfast, only short- or rapid-acting insulin before dinner, and intermediate- or long-acting insulin at bedtime may significantly ameliorate these problems.32,33 Intensive insulin regimens that employ intermediate-acting insulin demand consistency in the daily meal schedule, amounts of food consumed at each meal, and the timing of insulin injections.
Basal-Bolus Regimens and Continuous Subcutaneous Insulin Infusion
Insulin therapy with at least three injections each day or with continuous subcutaneous insulin infusion (CSII) using an insulin pump can more closely simulate normal diurnal insulin profiles, overcome many of the limitations inherent in a two-dose regimen, and permit greater flexibility with respect to timing and content of meals. Doses of rapid-acting insulin are adjusted meal-to-meal based on preprandial glucose values, anticipated carbohydrate intake, and physical activity. A peakless long-acting insulin, such as insulin glargine or detemir, can be used to provide basal insulin (typically 40% to 60% of the total daily dose) and is used together with short- or rapid-acting insulin injected before each meal (basal-bolus regimen). Insulin glargine is an insulin analogue, produced by recombinant DNA technology, whose duration of action is approximately 24 hours. It has little peak activity and is administered once daily, either before breakfast or in the evening with dinner or at bedtime. It should be injected at about the same time each day, whereas short- or rapid-acting insulin is injected separately before each meal, whenever it is eaten. Insulin glargine has been used safely in children and adolescents,34 and because it does not have the peak of activity characteristic of NPH, Lente, and Ultralente insulins,35 it can reduce nocturnal hypoglycemic episodes without jeopardizing glycemic control.33,36 More recently, insulin detemir has become available as an alternative long-acting, peakless basal insulin.37 Detemir has effects similar to those of glargine during the first 12 hours after administration; thereafter its effects wane; accordingly, it usually has to be administered twice daily in patients with severe insulin deficiency.38
In 1996, less than 5% of patients starting pump therapy were <20 years of age. Over the past several years, a worldwide marked increase has occurred in the number of children and adolescents using CSII (pump) therapy39; a current estimate is that more than 80,000 children and adolescents worldwide are using a pump to deliver insulin. An insulin pump has one unique advantage over insulin injections—the ability to program changes in basal dosage to meet an anticipated increase or decrease in need (Fig. 23-1C). This feature can be advantageous in combating the dawn phenomenon (especially in adolescents) or preventing hypoglycemia during or after strenuous exercise. In addition to programming various basal rates, the use of dual-wave and square-wave bolus delivery significantly lowers 4-hour postprandial blood glucose levels.40 Also, the infusion set typically has to be replaced only every 2 to 3 days, sparing the child the discomfort of repeated injections. A meta-analysis of randomized controlled clinical trials concluded that CSII resulted in a small (≈0.5%) improvement in HbA1c.41
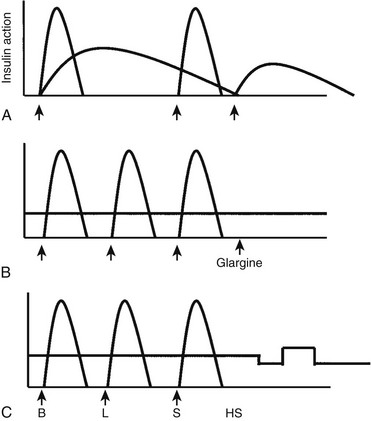
FIGURE 23-1 Insulin regimens. A, Schematic representation of idealized insulin action provided by a regimen consisting of a mixture of rapid-acting insulin (lispro or aspart) and intermediate-acting insulin (NPH or Lente) before breakfast, rapid-acting insulin (lispro or aspart) before supper, and intermediate-acting insulin (NPH or Lente) at bedtime. B, Schematic representation of idealized insulin action provided by an insulin regimen consisting of four daily injections: rapid-acting insulin (lispro or aspart) before each meal (B, L, S) and a separate injection of insulin glargine, at bedtime (as shown here) or at dinner or breakfast. C, Schematic representation of idealized insulin effect provided by continuous subcutaneous insulin infusion via an insulin pump with insulin aspart or lispro. In this figure, alternative basal rates are illustrated; insulin delivery is shown to decrease from midnight to 3 am and to increase before breakfast. B, Breakfast; HS, bedtime; L, lunch; S, supper. Arrows indicate times of insulin injection or bolus before meals.
Although an insulin pump is a complex and sophisticated medical device that requires extensive training in its proper use, with appropriate education and training and with support from parents and a school nurse, many children can manage the added responsibility of using an insulin pump and can benefit from its advantages.39,42 Only short- or rapid-acting insulin is used with CSII; therefore, any interruption in the delivery of insulin rapidly leads to metabolic decompensation. To reduce this risk, meticulous care must be devoted to the infusion system, and blood glucose levels must be measured at least four times daily. Increased lifestyle flexibility, reduced blood glucose variability, improved glycemic control, and reduced frequency of severe hypoglycemia are all documented advantages of CSII.39 Success requires motivation to achieve normal blood glucose levels, frequent blood glucose monitoring, record-keeping, carbohydrate counting, and frequent contact with the diabetes team. Patients must understand that to be successful, CSII therapy requires more time, effort, and active involvement in diabetes care by patients and parents, as well as considerable education and support from the diabetes team. The individual who is unable to master a multiple-dose injection regimen is not likely to be successful with CSII. Despite concerns that it might have adverse psychosocial consequences owing to the added burden of treatment, especially in adolescents, the opposite effect has been observed. Short-term studies have shown that more aggressive and successful management of their diabetes by teenagers can be accompanied by enhanced psychosocial well-being.43 In teenagers, CSII offers a treatment option that can lead to improved control and can lower the risk for severe hypoglycemia.44
Owing to physiologic peripheral insulin resistance of puberty,45 adolescents require large doses of rapid- or short-acting insulin to control postprandial blood glucose excursions. However, a large increase in the dose of regular insulin delays its peak effect (to 3 to 4 hours) and prolongs its total duration of action to 6 to 8 hours. Puberty does not cause hepatic insulin resistance; therefore, hyperinsulinemia suppresses hepatic glucose production for several hours and increases the risk for postprandial hypoglycemia, especially at night between 10 pm and 2 am.46 This is an important reason to recommend use of rapid-acting insulin analogs (lispro, aspart, or glulisine) in preference to regular (soluble) insulin in treating adolescents, especially before the evening meal, to reduce the risk for nocturnal hypoglycemia.
Medical Nutrition Therapy
Nutritional management is one of the cornerstones of the management of all types of diabetes mellitus, and nutrition education is an essential component of a comprehensive program of diabetes education for patients and their families.47 There is no “diabetic diet” per se. Nutrition therapy should be individualized, with consideration given to the patient’s usual eating habits and other lifestyle factors. Monitoring clinical and metabolic parameters, including height and weight, blood pressure, blood glucose, HbA1c, and lipids, as well as quality of life, is crucial to ensure successful outcomes. Diabetes management that combines frequent self-monitoring of blood glucose with intensive insulin therapy and mastery of carbohydrate counting enables children and adolescents to enjoy dietary flexibility while maintaining glycemic control in the target range.
Patients with both T1DM and T2DM have the same goals: namely, to achieve and maintain target blood glucose and HbA1c levels (Table 23-4). The initial focus of medical nutrition therapy (MNT), however, differs between the two major types of diabetes. Children with T2DM typically are obese at presentation, and great emphasis is placed on weight loss, limiting caloric intake, and distributing meals evenly throughout the day. In T2DM, even modest weight reduction alone increases sensitivity to insulin and improves fasting and postprandial plasma glucose levels. Similarly, moderate caloric reduction decreases plasma glucose levels. In adults, structured, intensive lifestyle programs involving participant education, individualized counseling, reduced energy and fat intake (30% of total energy), regular physical activity, and frequent participant contact are necessary to produce long-term weight loss of 5% to 7% of starting weight.48 Accordingly, lifestyle changes that lead to weight loss are the cornerstone of therapy in patients with T2DM. In contrast, in the child with T1DM, the primary goal is to match insulin delivery with carbohydrate consumption to achieve blood glucose levels in the age-specific target range (see Table 23-1).
Carbohydrate
Approximately 60% to 70% of total energy should be obtained from carbohydrate and monounsaturated fat.49 Dietary dogma had been to avoid simple sugars and replace them with complex carbohydrates. This belief was based on the assumption that simple sugars are more rapidly digested and absorbed than starches and would aggravate hyperglycemia to a greater degree. The glycemic index (GI), proposed in 1981 as an alternative system for classifying carbohydrate-containing foods, measures the glycemic response after ingestion of carbohydrate. GI is defined as the incremental area under the plasma glucose response curve after consumption of a standard amount of carbohydrate from a test food relative to that of a control food, either white bread or glucose. Glycemic and hormonal responses to a large number of carbohydrates have been systematically examined and their GIs defined. There is a wide spectrum of biological responses to different complex and simple carbohydrates with so much overlap that they cannot be simply classified into two distinct groups. Even a single food produces a substantially different glycemic response when prepared in different ways. The physical structure and form of a carbohydrate-containing food, in addition to its chemical composition, influence postprandial glycemia by altering its rate of digestion and absorption. Fruits and milk cause a lower glycemic response than most starches, and sucrose causes a glycemic response similar to that of bread, rice, and potatoes. In general, most refined starchy foods have a high GI, whereas nonstarchy vegetables, fruits, and legumes tend to have a low GI.
The usefulness of low-GI diets in individuals with T1DM continues to be controversial, and data are sparse in children. A meta-analysis of randomized controlled clinical trials, some of which have included children, shows that low-GI diets have modest long-term beneficial effects on blood glucose and lipid concentrations.50
The glycemic load of meals and snacks is more important than the source or type of carbohydrate. The glycemic load, defined as the weighted average of the GI of individual foods multiplied by the percentage of dietary energy as carbohydrate, has been proposed as a method to characterize the impact of foods and dietary patterns with different macronutrient composition on glycemic responses. For example, a carrot has a high GI but a low glycemic load, whereas a potato has both a high GI and a high glycemic load. Although the use of low-GI foods may reduce postprandial glycemic excursions and may have long-term benefit on HbA1c levels, emphasis should be on the total amount of carbohydrate consumed, and its source should be a secondary consideration.51
Carbohydrate Counting and Exchange Lists
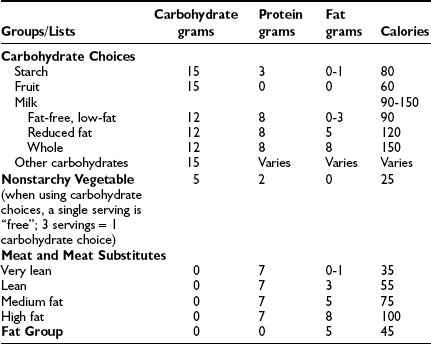
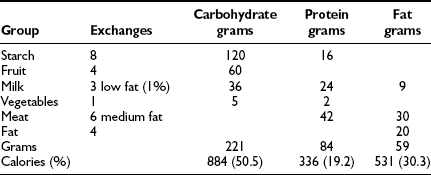
Carbohydrate counting is a meal planning method that entails counting the amount of carbohydrate or the number of carbohydrate servings eaten at each meal and snack. Carbohydrate is the main nutrient in starches, fruits, milk, and sugar-containing foods and has the greatest effect on blood glucose levels. Therefore, it is the most important macronutrient to control in order to maintain optimal glycemic control. With the use of exchange lists, one starch choice is considered to be equivalent to one fruit or milk choice; each contains approximately 15 grams of carbohydrate and is equal to one “carbohydrate choice” (Table 23-5). The “nutrition facts” on food labels list the portion size and total amount of carbohydrate measured in grams per serving. Carbohydrate counting allows flexibility in food choices and minimizes “cheating,” as all foods can be included in the meal plan. Table 23-6 shows an example of a patient’s daily meal plan, incorporating both exchange servings and grams of carbohydrate.
Fiber, which refers to the indigestible portion of a plant, influences the digestion, absorption, and metabolism of many nutrients. Inclusion of plant fiber in the diet may benefit patients with diabetes by diminishing postprandial glycemia. Certain soluble plant fibers significantly reduce serum cholesterol concentrations and decrease fasting serum triglyceride levels in patients with diabetes who have hypertriglyceridemia. Dietary fiber guidelines for children with diabetes are the same as for nondiabetic children and can be readily achieved by increasing the consumption of minimally processed foods, such as grains, legumes, fruits, and vegetables. Among diabetic adolescents using intensive insulin treatment methods, optimal blood glucose control is more common in those who have a higher intake of fiber, fruits, and vegetables.52
Fat
A carbohydrate-containing meal that also has a high content of saturated fat significantly increases and prolongs the glycemic effect of the meal and requires anticipatory adjustment of the dose of insulin to combat the effect. Excessive saturated fat, cholesterol, and total energy lead to increased blood levels of cholesterol and triglycerides. Because hyperlipidemia is a major determinant of atherosclerosis, and patients with T1DM eventually develop atherosclerosis and its sequelae, the meal plan should attempt to mitigate this risk factor. The consumption of saturated fat can be reduced by eating less red meat, whole milk, and high-fat dairy foods and by eating more poultry, fish, and vegetable proteins, and by drinking more low-fat milk. Children and adolescents with well-controlled T1DM are not at high risk for dyslipidemia, but they should be screened and monitored according to recommended guidelines (see Chronic Complications section below). If the child or adolescent is growing and developing normally and has normal plasma lipid levels, less than 10% of energy should come from saturated fat, the daily intake of cholesterol should be less than 300 mg/day, and consumption of transunsaturated fatty acids should be minimized. Total dietary fat should be reduced in the obese child to reduce total energy consumption. The National Cholesterol Education Program (NCEP) Step II diet guidelines should be implemented in the patient with elevated LDL cholesterol (>2.6 mmol/L [100 mg/dL]). Total fat should constitute ≤30% of total calories, with <7% of calories from saturated fat, and dietary cholesterol should be limited to 200 mg/day.53
MNT Education and Formulation of the Meal Plan
MNT begins with an assessment by a registered dietitian, heeding the ethnic, religious, and economic factors pertaining to the individual patient and family. The meal plan must take account of the child’s school schedule, early or late lunches, physical education classes, after-school physical activity, and differences in a child’s activities on weekdays compared with weekends and holidays. Young children typically have three meals and two or three snacks daily, depending on the interval between meals, the age of the child, and the level of physical activity. Although their daily energy intake is relatively constant over time, young children adjust their energy intake at successive meals.54 The highly variable food consumption from meal to meal typical of normal young children is especially challenging when the child has T1DM. Rapid-acting insulin may be administered after the meal, based on estimation of the actual amount of carbohydrate consumed, and this diminishes parental anxiety.28,29 The purpose of snacks is to prevent hypoglycemia and hunger between meals. If the basal insulin component is adjusted appropriately, patients who use a basal-bolus insulin regimen or insulin pump therapy may not require snacks. Data from preprandial and postprandial blood glucose monitoring and individualized insulin-to-carbohydrate ratios are used to select insulin doses to match anticipated carbohydrate intake.
The dietitian’s role is to evaluate the patient’s and family’s knowledge and understanding of nutrition and to formulate an individualized meal plan. Even intensive insulin replacement regimens are not successful without careful attention to meal planning.55 Nutrition education, like all aspects of diabetes education, has to be an ongoing process with periodic review and revision of the meal plan and assessment of the child’s and parents’ levels of comprehension, ability to analyze and solve problems, and adherence to the nutrition goals. The patient with newly diagnosed diabetes and his or her parents should consult with a dietitian several times during the first few days after diagnosis. Within a few weeks of the child resuming his or her usual schedule and activities, the patient and family should review the meal plan with a dietitian, who also should be available to patients for telephone consultation. If the patient’s glycemic control is poor, if growth is failing, if weight gain is excessive, or if other problems related to MNT should arise, the dietitian should be re-consulted.
The Meal Plan: The individualized meal plan must be simple, practical, and easy to modify, and should offer foods that are interesting, tasty, and affordable. Dietary strategies principally are determined by the patient’s insulin replacement regimen (Table 23-7). We advocate meal planning adapted to the ethnic, religious, and economic circumstances of each family and based on a combination of carbohydrate counting and the exchange system. Each list in the exchange system for meal planning indicates the appropriate size or volume of each food exchange. Each portion of food within a group is exchangeable because it contains approximately the same nutritional value in terms of calories, carbohydrates, protein, and fat. By prescribing the meal plan in terms of a number of exchanges for each meal, the consistency of total calories and the proportions of nutrients can be maintained, while allowing the patient to choose among numerous foods. Accurate measurement of portion sizes has to be learned, and weighing and measuring of foods helps to achieve familiarity with the sizes of food portions specified in the exchange list. Weighing and measuring food should be viewed as an educational exercise to train the eye and need not be continued indefinitely; however, if blood glucose control appears inexplicably to deteriorate, it is useful to resume weighing and measuring of food portions to ensure that amounts are accurate. The exchange system should not be used in isolation; rather, it should be one component of a nutritional program directed by a trained dietitian. An example of how this system is applied to a hypothetical patient is illustrated below. An 11-year-old girl’s height is 144 cm (50th percentile on the Centers for Disease Control and Prevention growth chart) and her weight is 37.4 kg (50th percentile). Her daily energy requirement to support growth in the 50th percentile is 1756 calories. An appropriate distribution of macronutrients consists of 50% of total calories from carbohydrate, 20% as protein, and 30% as fat (see Table 23-6).
Exercise: Children with diabetes are encouraged to participate in sports and to include regular exercise in their lives. Participation in physical exercise normalizes the child’s life, enhances self-esteem, improves physical fitness, helps to control weight, and may improve glycemic control. Regular exercise increases insulin sensitivity, cardiovascular fitness, and lean body mass, improves blood lipid profiles, and lowers blood pressure.
Hypoglycemia usually can be prevented by a combination of anticipatory reduction in pre-exercise insulin dose or temporary interruption or reduction of basal insulin infusion (with CSII) and/or supplemental snacks before, during, and after activity, depending on the intensity and duration of the physical activity and its timing. Nearly all forms of activity lasting longer than 30 minutes require some adjustment to food and/or insulin. Continuous moderate-intensity exercise tends to cause a lesser decline in blood glucose levels than is produced by intermittent high-intensity exercise of short duration.56 The optimal strategy depends on the timing of the exercise relative to the child’s meal plan and on the insulin regimen. When the content and size of the snack are selected, consideration is given to several factors, including the current blood glucose level, the action of insulin most active during and after the period of anticipated exercise, the interval since the last meal, and the duration and intensity of physical activity. The appropriate amount is learned by trial and error; however, a useful initial guide is to provide up to 1 gram of carbohydrate per kg of body mass per hour of strenuous exercise. Prolonged and strenuous exercise in the afternoon or evening should be followed by a 10% to 30% reduction in pre-supper or bedtime dose of intermediate-acting insulin or long-acting insulin or an equivalent reduction in overnight basal insulin delivery in patients using CSII. In addition, to reduce the risk for nocturnal or early-morning hypoglycemia caused by the lag effect of exercise, the bedtime snack should be larger than usual and should contain carbohydrate, protein, and fat. Parents should be encouraged to monitor the blood glucose concentration in the middle of the night until they are experienced in modifying the evening dose of insulin after exercise.
Blood glucose monitoring is essential for the active child with diabetes because it allows identification of trends in glycemic responses. Records should include blood glucose levels and information on the timing, duration, and intensity of exercise, as well strategies used to maintain glucose concentrations in the target range. Blood glucose levels should be measured before, during, and after exercise and, to prevent nocturnal hypoglycemia, before bedtime (Table 23-8).
Table 23-8
Practical Guidelines for Exercise
• Consider the timing, mode, duration, and intensity of exercise.
• Eat a carbohydrate-containing meal 1 to 3 hours before exercise.
• Measure blood glucose level:
• If BG <90 mg/dL (5 mmole/L) and levels are decreasing, extra calories are needed.
• If BG 90-270 mg/dL (5-15 mmole/L), extra calories may not be needed, depending on duration of exercise and individual’s response to exercise.
• If BG >270 mg/dL (>15 mmole/L) and urine or blood ketones are increased, delay exercise until levels are restored to normal with supplemental insulin.
• If the exercise is aerobic, determine whether insulin or additional carbohydrate will be needed based on the peak insulin activity.
• If insulin dose is to be changed for long-duration moderate- to high-intensity activity, reduce pre-meal insulin dose by 50% 1 hour before exercise. On subsequent days, adjust dose based on measured individual response.
• Inject insulin into a site that will not be affected by exercising muscles.
• If additional carbohydrate is required, start with 1 g/kg/h of moderate- to high-intensity exercise performed during peak insulin activity; less carbohydrate is required as time elapsed since last injection increases.
• Alter the amount of carbohydrate on subsequent days based on measured individual response.
Adapted from Riddell and Iscoe.244
In the child with poorly controlled diabetes, vigorous exercise can aggravate hyperglycemia and ketoacid production; accordingly, a child with ketonuria should not exercise until satisfactory biochemical control has been restored (see Table 23-8).
Type 2 Diabetes Mellitus in Children and Adolescents
Until recently, most children with diabetes had T1DM; however, as early as 1916, a phenotypically distinct form of diabetes, now classified as type 2 diabetes mellitus (T2DM), was recognized in childhood.57 Over the past 10 to 20 years, an alarming increase in the prevalence of pediatric T2DM has been reported from pediatric diabetes centers in North America58 and elsewhere in the world,59,60 and T2DM now accounts for up to 33% of new cases of diabetes in adolescents at centers that serve large numbers of minority youth.61,62 At least 90% of patients with newly diagnosed T2DM are obese,58 and the increased prevalence of pediatric T2DM temporally coincides with the increase in obesity noted in children in the United States; it has more than doubled in the past 20 years. In 2003-2004, 17.1% of U.S. children aged 2 to 19 years were overweight, defined as body mass index ≥95th percentile.63 As in adults, obesity in childhood is associated with insulin resistance, hyperinsulinism, and decreased insulin-stimulated glucose metabolism compared with nonobese children64,65 (Table 23-9). Factors that explain the increased prevalence of pediatric T2DM and strategies for primary prevention have been reviewed recently.66 The pathophysiology of T2DM is discussed in Chapter 15.
Table 23-9
Risk Factors for Type 2 Diabetes in Youth
• Insulin resistance: usually associated with obesity
• Family history of type 2 diabetes in first- or second-degree relative
• Ethnicity: African American, Hispanic, Pacific Islander, Native American, Canadian First Nation
• Maternal gestational diabetes
• Small size for gestational age (intrauterine growth restriction)
Treatment
The general goals of treatment for T2DM are the same as those outlined above for T1DM: to normalize fasting and postprandial blood glucose concentrations. However, in addition to blood glucose control, from the outset treatment must include management of comorbidities such as obesity, dyslipidemia, hypertension, and microalbuminuria. The goals of treatment and the recommended standards of care for pediatric patients with T2DM are described in Tables 23-10 and 23-11. The UKPDS showed that intensive glycemic control in T2DM decreased the risk for microvascular complications by up to 25% for each 1% reduction in HbA1c.18 A multifactorial approach that addresses associated risk factors has been shown in adults to be essential to prevent or reduce complications, including cardiovascular disease (CVD).67 Evidence suggests that T2DM in children and adolescents may have a more rapid clinical course; therefore, optimal management is required to prevent diabetes-associated complications.68
Table 23-10
Goals of Treatment for Type 2 Diabetes Mellitus75
• Achieve and maintain near-normoglycemia
• Maintain a reasonable weight (BMI <95th percentile) and normal growth
• Prevent or treat diabetes-associated comorbidities, including dyslipidemia, hypertension, and microalbuminuria
• Achieve overall improvement in physical and emotional well-being
*Blood pressure goals must be adjusted for age, height, and gender.
Table 23-11
Recommended Standards of Care for Patients With Type 2 Diabetes Mellitus47
Currently, no evidence-based guidelines are available for the management of T2DM in children and adolescents; however, as for T1DM, a multidisciplinary diabetes team that consists of a physician, a diabetes nurse educator, a registered dietitian, an exercise physiologist, and a behavioral specialist or social worker is essential. Results of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study on the treatment of T2DM in children and adolescents are eagerly anticipated. This study is expected to provide much needed information on the natural history of T2DM and the efficacy of various treatments for youth T2DM (Table 23-11).69
Nonpharmacologic Therapy
Weight Control and Physical Activity: Although weight loss and increased physical activity are the first-line therapies for prevention and management of T2DM and its comorbidities, the optimal strategy is still controversial. A recent review by the Cochrane Collaboration found few to no high-quality long-term data available on the optimal dietary treatment of T2DM in adults.70
Nutrition and lifestyle approaches to diabetes prevention and treatment should be given at least as much importance as drug therapy. A family-centered rather than patient-oriented approach usually is more successful. Patients and their families must acknowledge that lifestyle modifications such as eating a balanced diet, maintaining a healthy weight, and exercising regularly are essential.71 Nutrition recommendations should be culturally appropriate and sensitive to family resources. As a general rule, patients should be advised to restrict starches and refined carbohydrates, including sugary drinks and “fast foods.” Intake of salad, nonstarchy vegetables, fruits, and whole grains should be encouraged. Less than 30% of the daily caloric requirement should come from fat.
Increasing evidence suggests that a low-GI diet may have beneficial effects on metabolic control.50 A meta-analysis of randomized controlled trials comparing low-GI versus conventional or high-GI diets found a mean reduction in HbA1c in favor of low-GI diets. A low-GI diet may reduce insulin secretion and improve insulin sensitivity, and by reducing insulin secretion, may downregulate malonyl-CoA carboxylase activity, thereby decreasing formation of fatty acids and triglycerides. The amount of dietary fiber should also be increased as it reduces insulin levels, promotes weight loss, improves lipid profiles, and lowers cardiovascular risk. A recent study in adults comparing the effects of three different diets—low-GI, high-GI, and low-carbohydrate diets—showed no differences in HbA1c levels; however, a reduction in C-reactive protein (CRP) and a decrease in postprandial glucose concentrations was seen with the low-GI diet.72
Regular physical activity facilitates weight loss, increases high-density lipoprotein (HDL) cholesterol levels, lowers blood pressure, and improves metabolic control. Fasting serum insulin concentrations decrease and insulin sensitivity improves in obese children who exercise regularly.73,74 Youth with T2DM should participate in regular aerobic exercise with a gradual increase in the frequency, intensity, and duration, aiming for at least 30 minutes daily of moderate/intense physical activity. Exercise tolerance is reduced in obese children; therefore, advice to increase physical activity should be realistic and individualized. To increase children’s physical activity, the amount of time devoted to sedentary activities (screen time) must be strictly limited.
Pharmacologic Therapy
Oral Agents: Symptomatic patients with severe hyperglycemia, weight loss, and ketosis or ketoacidosis require a period of insulin therapy (similar to the treatment of T1DM) until fasting and postprandial glycemia have been restored to normal or near normal. Similarly, when the type of diabetes has not been defined, the patient initially should be treated with insulin (see Fig. 23-1).75
Less than 10% of children and adolescents with T2DM achieve adequate glycemic control with lifestyle changes alone. Most require pharmacologic therapy76; however, data on the efficacy and safety of oral antihyperglycemic agents in the pediatric population are sparse. Metformin monotherapy is recommended as the first choice for asymptomatic or mildly symptomatic patients. Some clinicians initiate pharmacologic therapy upon diagnosis, whereas others prescribe medication only after a 2 to 3 month trial of behavior modification and lifestyle intervention has failed, as evidenced by persistent or worsening hyperglycemia.
Metformin.: Metformin is currently the only oral hypoglycemic agent specifically approved for pediatric use by the U.S. Food and Drug Administration (FDA) in children over 10 years of age, when given alone or in combination with insulin. Metformin is safe and efficacious in pediatric patients with T2DM.77,78 It suppresses basal hepatic glucose production and increases insulin-mediated glucose uptake in skeletal muscle, but it does not affect insulin secretion or cause hypoglycemia. Metformin causes a mild reduction in triglyceride and LDL concentrations. Its anorectic effect may contribute to modest weight loss.
Its most common side effects are nausea, vomiting, abdominal pain, and diarrhea. Lactic acidosis is a rare, potentially fatal side effect. Provided that it is not administered to patients with renal insufficiency (metformin is excreted unchanged in the urine) or poor tissue perfusion, the risk of lactic acidosis is not greater than that of other antihyperglycemic agents.79 Metformin must be discontinued before radiographic studies with contrast agents or surgery under general anesthesia is performed; in patients with renal, liver, or heart disease; and whenever tissue perfusion is poor. Because the absorption of vitamin B12 and/or folic acid may be compromised, patients are advised to take a daily multivitamin.
Insulin Secretagogues (Sulfonylureas and Meglitinides).: Although sulfonylureas have been used in adults for longer than half a century, only limited evidence of their efficacy in children has been found. A recent 24-week, randomized, single-blind comparative study in T2DM pediatric patients, showed that glimepiride was as safe and effective as metformin in terms of reduction of HbA1c and incidence of hypoglycemia. The glimepiride-treated group, however, showed greater weight gain compared with patients treated with metformin.80
Thiazolidinediones (TZDs).: TZDs are insulin sensitizers that act on the nuclear receptor peroxisome proliferator–activated receptor-gamma (PPAR-γ) and increase insulin sensitivity in muscle and adipose tissue. TZDs have favorable effects on lipid metabolism. Side effects include weight gain and fluid retention, which contraindicates their use in patients with heart failure. At the present time, TZDs are not approved for use in children, but clinical trials in pediatric T2DM are currently in progress.
Alpha-Glucosidase Inhibitors.: Inhibition of alpha-glucosidase works in the intestinal lumen, where these agents competitively inhibit enzymes that hydrolyze polysaccharide into simple sugars. Their main effect is on starches, but cleavage of sucrose to glucose and fructose is also reduced. The result is a delay in absorption of dietary carbohydrates until they have passed to the mid or distal small intestine, resulting in reduced postprandial glucose concentrations. Alpha-glucosidase inhibitors have had limited acceptance because of their gastrointestinal side effects, which include diarrhea, flatulence, and bloating. No clinical trials in children have been reported.
Insulin Therapy: Although many insulin regimens have been studied and successfully used in adults with T2DM, no comparable data exist in pediatric T2DM. As described earlier, symptomatic patients are treated with insulin to relieve symptoms of hyperglycemia (e.g., blurred vision, polydipsia). Metformin is added after normalization of blood glucose and correction of dehydration.
Insulin therapy may be necessary in asymptomatic or mildly symptomatic patients who fail to achieve adequate glycemic control (HbA1c <7%) after 3 to 6 months of lifestyle intervention and treatment with maximum doses of metformin. Long-acting insulin analogues (glargine or detemir) may be added to metformin. A suitable starting dose is 0.2 unit/kg/day at bedtime. Twice-daily premixed insulin regimens (see Table 23-3) have been efficacious in adults with T2DM, with a 2.8% reduction in HbA1c reported after 28 weeks of therapy.81 A short trial with premixed insulin analogues was also beneficial in children.82 Other strategies include the use of a long-acting insulin combined with a meglitinide before meals. Basal-bolus therapy (once-a-day long-acting insulin and short-acting insulin before meals) may be a suitable option in the motivated patient who is willing to perform carbohydrate counting. Side effects of insulin therapy include hypoglycemia, increased appetite, and weight gain.
New Therapies for Type 2 Diabetes
The discovery of amylin and glucagon-like peptide 1 (GLP-1) and the development of synthetic analogues of these hormones have led to the widespread use of these agents for the treatment of diabetes in adults. Published experience of their use in children is minimal.83
Comorbidities
Hypertension
Strict control of blood pressure in adults results in significant reduction in cardiovascular morbidity and mortality.84,85 Similar effects with reduction in the risk of premature CVD would be expected to occur in children. Management of hypertension, including weight control, regular exercise, a low-fat and low-sodium diet, smoking cessation, and abstinence from the use of alcohol, is recommended for all hypertensive patients. In the absence of end-organ damage or comorbid conditions, the goal is to reduce blood pressure to <95th percentile for age, height, and gender. If lifestyle intervention is unsuccessful, pharmacologic treatment should be initiated.86 Angiotensin-converting enzyme (ACE) inhibitors (e.g., captopril, enalapril, lisinopril, fosinopril) are the drugs of choice in children with diabetes and/or proteinuria. Delay in the progression of diabetic nephropathy in adult patients with diabetes mellitus treated with ACE inhibitors has been proved. Beneficial effects have also been reported in children with T1DM.87 If the highest recommended dose is ineffective, or if the child experiences side effects, a second drug from a different class, such as angiotensin receptor blockers (ARBs), calcium channel blockers, cardioselective β-blockers, and/or diuretics, may be used.88
Hyperlipidemia
Dyslipidemia in childhood tracks into adulthood; therefore, it is not unreasonable to assume that not treating lipid disorders in children with diabetes increases the risk for CVD later in life. In youth with dyslipidemia, initial therapy consists of weight control, exercise, optimization of glycemic control, discontinuation of tobacco use (if applicable), and a reduced-fat diet, consistent with Step 1 American Heart Association (AHA) guidelines. Total and saturated fat intake should account for <30% and <10%, respectively, of the total calories consumed.89
Despite compliance with lifestyle recommendations, some children with hyperlipidemia will require lipid-lowering drug therapy. Currently, the AHA recommends 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) as first-line pharmacologic treatment in children with hyperlipidemia.90 Statins are approved for use in children as young as 10 years old. Randomized clinical trials in the pediatric age group have shown safety and efficacy similar to those observed in adult studies.91,92
The addition of lipid-lowering drugs is recommended when LDL-C levels are >190 mg/dL and in patients with LDL-C >160 mg/dL and a family history of early CVD or other risk factors. Similarly, if after 6 to 12 months of medical nutrition therapy and lifestyle changes LDL-C levels remain >130 mg/dL, drug therapy is indicated. Lipid-lowering medications are not recommended in premenarcheal girls and boys younger than 8 to 10 years, unless the risk for atherosclerosis is particularly high, in which case aggressive therapy is appropriate.53
Prevention of Type 2 Diabetes Mellitus
In youth at increased risk for developing T2DM, the child’s primary health care provider should emphasize primary prevention by focusing on preventing obesity. Lifestyle modification should be implemented in overweight and obese children and in those with prediabetes, that is, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and/or the metabolic syndrome.49 Weight reduction by means of dietary changes, increased aerobic physical activity, general community health promotion, and health education are the most important preventive strategies. Diabetes prevention trials in adults show that nutrition and lifestyle interventions delay the onset of the disease.48,93 In middle-aged, overweight Finnish subjects with IGT, reducing weight and increasing physical activity decreased the cumulative incidence of diabetes after 4 years to 11% in the intervention group compared with 23% in the control group. The reduction in the incidence of diabetes (58%) was directly associated with changes in lifestyle.93 In obese adults with IGT, the Diabetes Prevention Program (DPP) in the United States, an intensive program of lifestyle modification with the goals of 7% weight loss and 150 minutes of physical activity per week, decreased the risk for progression to diabetes by 58%.48 These studies have demonstrated that T2DM can be delayed or prevented by changes in lifestyle and/or pharmacologic intervention in high-risk adult subjects. Although still unproven, a similar approach would be expected to be equally efficacious in children and adolescents. In established T2DM, secondary prevention should focus on the prevention of microvascular and macrovascular complications.
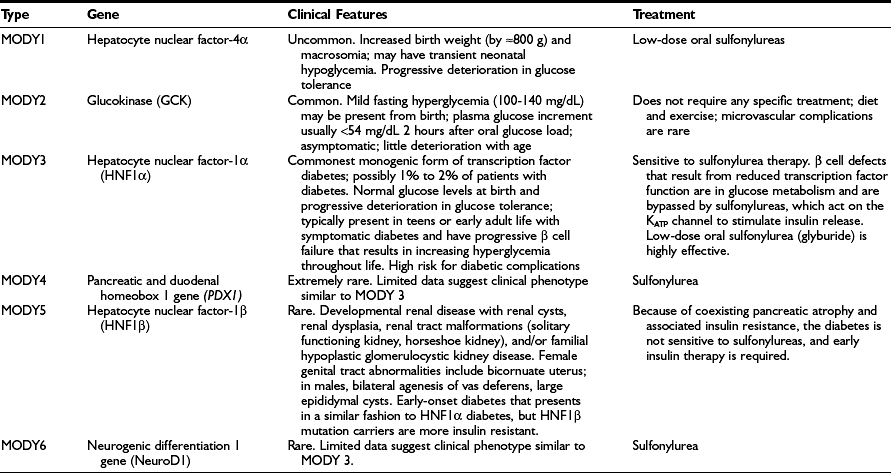
Monogenic Diabetes
Diabetes resulting from mutations that primarily reduce β cell function accounts for 1% to 2% of diabetes cases, and numerous genetic subtypes have been described.94 Patients who were previously categorized on the basis of their clinical characteristics as having maturity-onset diabetes of the young (MODY) now can usually be classified by specific genetic subgroup. Definition of the genetic subgroup can result in appropriate treatment, genetic counseling, and prognosis.94 The term MODY was used to describe children and young adults with autosomal dominantly inherited diabetes that, despite having a young age of onset (at least one family member diagnosed before 25 years of age), was not insulin dependent, as patients had moderate but insufficient circulating C-peptide levels 5 years after diagnosis.95,96 “Maturity-onset” implies a resemblance to T2DM, but all subtypes are not only different from each other but differ from T2DM. Patients with a clinical diagnosis of T1DM who have a two- or three-generation family history of diabetes with evidence of non–insulin dependence should be suspected of having monogenic diabetes. Absence of pancreatic autoantibodies and detection of C-peptide in the presence of hyperglycemia beyond the honeymoon increase the probability that the patient has monogenic diabetes. Genetic testing for HNF1A mutations (the most common transcription factor mutation that causes monogenic diabetes) is recommended in any young person with apparent T1DM who is antibody negative and has a parent with diabetes, especially if there is preservation of C-peptide in both the child and the parent.94 A monogenic form of diabetes should also be suspected in cases of young-onset apparent T2DM when obesity and features of insulin resistance are absent.
The different genetic subtypes are shown in Table 23-12. They differ in terms of age of onset, pattern of hyperglycemia, response to treatment, and associated extrapancreatic manifestations.94
Maternally Inherited Diabetes and Deafness
Maternally inherited diabetes associated with young-onset, bilateral sensorineural deafness (MIDD) should raise suspicion for the mitochondrial point mutation, m.3242A>G, which accounts for 1.5% of Japanese patients with diabetes, but only 0.4% in Europeans and other ethnic groups.97 Abnormal mitochondrial metabolism results in abnormal adenosine triphosphate (ATP) generation and defective glucose-induced insulin secretion, reduction in β cell mass, and insulin deficiency. The mutation causes mitochondrial dysfunction resulting in myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. The mean age of diagnosis is 37 years (range, 11 to 68 years). Diabetes in MIDD usually presents insidiously, similar to type 2 diabetes; however, approximately 20% of patients have an acute presentation that resembles that of T1DM, and a minority of patients present with DKA.
Neonatal Diabetes Mellitus
These patients have little or no endogenous insulin secretion, and C-peptide is usually undetectable.98 Sulfonylureas bind to the SUR1 subunits of the KATP channel and close the channel in an ATP-dependent manner. Most patients with Kir6.2 and SUR1 neonatal diabetes can transfer from insulin to sulfonylurea and achieve good glycemic control.99–101 Most patients with KATP channel mutations are treated with glibenclamide (glyburide) at considerably higher doses (0.4 to 0.8 mg/kg/day) than are customarily used for the treatment of type 2 diabetes; this may cause transient diarrhea.99,102,103 Glibenclamide binds nonspecifically to SUR subunits in KATP channels in nerve, muscle, and brain, in addition to β cells, which ameliorates the neurologic symptoms.104 Patients with the severe form of DEND may not respond to sulfonylurea therapy.
Heterozygous mutations in the insulin gene (INS) could account for 15% to 20% of cases of PNDM.105 Affected infants have a low birth weight, which is typical of all subtypes of neonatal diabetes, but do not have extrapancreatic features. Diabetes is permanent and insulin dependent.
Transient neonatal diabetes mellitus (TNDM) is usually diagnosed in the first week of life (range, 1 to 81 days); birth weights (average, 2000 g) of affected infants are typically lower than those of PNDM. In 70% of cases, an abnormality of a region of chromosome 6q24 results in overexpression of the paternally expressed genes PLAGL1 (pleiomorphic adenoma gene–like 1, also termed tumor repressor ZAC) and HYMAI (hydatidiform mole–associated and imprinted gene).106,107 One third of patients with TNDM have macroglossia. Three types of abnormality have been described: 50% of cases of sporadic TNDM are due to paternal uniparental disomy; most familial cases are due to paternal duplication of 6q24; abnormal methylation of the maternal copy of chromosome 6 is found in sporadic cases. Most of the other cases of TNDM have KATP channel mutations distinct from those observed in PNDM.107,108 Therapy is with insulin; however, by a median of 12 weeks, insulin is no longer required.106 The rate of relapse is 50% to 60% at an average age of 14 years; this results from moderate β cell dysfunction. At the time of relapse, treatment may include dietary modification, oral hypoglycemic agents, and/or insulin.109
Cystic Fibrosis–Related Diabetes
At centers that routinely evaluate glucose tolerance in patients with cystic fibrosis, the prevalence of cystic fibrosis–related diabetes (CFRD) increases with age: 9% at age 5 to 9 years, 26% at 10 to 20 years, and approximately 50% by 30 years.110,111 Virtually all patients with exocrine insufficiency have β cell dysfunction. Whereas fasting insulin and C-peptide concentrations may be normal, an oral glucose tolerance test (OGTT) shows delayed and blunted peak insulin secretion. With worsening glycemic status, the impairment of first-phase insulin secretion becomes more pronounced. Secretion of other islet hormones, especially glucagon, is also impaired. The primary defect in CFRD is severe but not absolute insulin deficiency; ketoacidosis is rare. Most patients with CF are sensitive to insulin when they are healthy; however, infection and inflammation increase both peripheral and hepatic insulin resistance.112 Insulin resistance can rapidly become severe during infectious exacerbations.
An insidious decline in clinical status can occur 2 to 6 years before the diagnosis of CFRD, and pulmonary deterioration correlates with the baseline degree of insulin deficiency.113 Patients with CFRD have worse lung function, poorer nutritional status, and decreased survival compared with nondiabetic CF patients. Among a large cohort followed for 10 years, 25% of patients with CFRD survived at 30 years as compared with 60% of those without CFRD.114 It is important to identify patients with glucose intolerance before the onset of symptoms. Because normal fasting or random plasma glucose levels do not exclude CFRD, annual testing with a 2 hour OGTT should begin at age 10 years, at a time when the patient is clinically well.
The aims of treatment are to eliminate symptoms of hyperglycemia and to maintain adequate nutrition, growth, and lung function. Insulin is the only recommended therapy for CFRD.115 Insulin prevents protein catabolism and improves weight gain and pulmonary function. Because patients with CFRD typically have unusual dietary patterns with wide daily variation in carbohydrate timing and quantity, the ideal insulin replacement regimen is either flexible basal-bolus therapy with long-acting basal insulin (insulin glargine or detemir) combined with rapid-acting insulin with meals and to correct hyperglycemia (Fig. 23-1B), or an insulin pump. Many patients are unwilling, at least initially, to employ an intensive insulin regimen; in these circumstances, an insulin regimen involving fewer injections but providing adequate insulin coverage for the patient’s main carbohydrate-containing meals may be an acceptable compromise. Total daily insulin requirements frequently change and must be adapted to the patient’s individual needs, for example, during acute illness, with glucocorticoid therapy, and during periods of intensive enteral or parenteral nutrition.
Monitoring Diabetes Control
Self-Monitoring of Blood Glucose
Self-monitoring of blood glucose (SMBG) is the cornerstone of modern diabetes care. Most glucose meters now display plasma values, which are about 10% to 15% higher than those for whole blood. Patients/parents must be taught how to use these data to assess the efficacy of therapy and to adjust the components of their treatment regimen to achieve individual blood glucose goals. Most glucose meters have an electronic memory; however, it is valuable for patients/parents to keep written records of their results and to analyze the data for patterns and trends and to make adjustments when necessary. For most patients with T1DM, SMBG should be performed at least four times daily: before each meal and at bedtime. To minimize the risk for nocturnal hypoglycemia, blood glucose (BG) should be measured between midnight and 4 am once each week or every other week, and whenever the evening dose of insulin is adjusted. If HbA1c targets are not being met, patients should be encouraged to measure BG levels more frequently, including 90 to 120 minute after meals. Frequency of BG monitoring is an important predictor of glycemic control in children with T1DM.116 The optimal frequency of SMBG for patients with T2DM is not known but should be sufficient to facilitate attainment of the individual patient’s glycemic goals. Children who are able to perform SMBG independently must be properly supervised because it is not unusual for children to fabricate data with disastrous consequences.
Continuous Glucose Monitoring
The technology for continuous glucose monitoring (CGM) has evolved rapidly over the past several years. Current CGM devices measure glucose in the interstitial fluid by means of a short, thin subcutaneous probe that can be used for 3 to 7 days. The accuracy of CGM devices is improving but is not yet considered sufficient to substitute for SMBG performed with portable glucose meters. Furthermore, each newly placed CGM probe must be calibrated during a period of stable glycemia over several hours by performing simultaneous capillary blood glucose measurements. It is important to note that there is a several minute lag between actual plasma glucose and interstitial glucose concentrations. Thus, current CGM devices cannot substitute for SMBG; they are used as an adjunct to provide blood glucose information between SMBG measurements.117
The latest generation of continuous glucose monitoring devices reports the estimated plasma glucose values in real time (RT-CGM) every 1 to 5 minutes via a user interface. Several such RT-CGM devices are commercially available and have been approved for use in the United States and Europe. Information from RT-CGM allows the user to detect the early phases of a hyperglycemic or hypoglycemic episode, thereby enabling corrective action to be taken after confirmatory SMBG. Short-term (3 month) uncontrolled trials of current-generation RT-CGM have demonstrated improved hemoglobin A1c concentrations and a high level of patient satisfaction.118 Whether use of RT-CGM will lead to durable improvements in glycemia and/or reduction in risk of acute diabetic complications is unknown and is the subject of ongoing investigation.
Blood Ketone Testing
Meters that measure blood β-hydroxybutyric acid (βOHB) levels are now available for home use, but the reagent strips are expensive and the meters are not widely used. Quantification of blood βOHB, the predominant ketone body, is preferred over urine ketone testing for diagnosing and monitoring metabolic decompensation, as may occur with intercurrent illness, with pump failure, and in ketoacidosis.119 Blood ketone determination is helpful in avoiding emergency room visits120 and offers the advantage of accurately assessing improvement after the start of treatment.119
Glycated Hemoglobin or Hemoglobin A1c
More than 30 different methods are used to measure hemoglobin A1c, which has led to different nondiabetic reference ranges, because different glycated hemoglobin fractions are measured.121 The International Federation of Clinical Chemistry has developed a new reference method that precisely measures the concentration of glycated hemoglobin (betaN1-deoxyfructosyl-hemoglobin).122 A recent international study accurately determined the relationship between mean blood glucose (approximately 2700 glucose values per subject) over the preceding 3 months and the glycated hemoglobin concentration. Linear regression analysis between the A1C and average glucose values showed a tight correlation described by the following equation: Average glucose (mg/dL) = 28.7 × A1C − 46.7.123 It is anticipated that the new assay will be reported as “estimated average blood glucose” or “A1C-derived average glucose,” and the units will be mmol/L or mg/dL.124
Hypoglycemia in Children With Diabetes Mellitus
Hypoglycemia is the most common acute complication of the treatment of diabetes mellitus, and concern about hypoglycemia is a central issue in treating children with T1DM. It is the most important barrier to the pursuit and maintenance of near-normal glycemic control.125 Effectively managing the risk for hypoglycemia is especially important in the treatment of children and adolescents. Patients, parents, and the diabetes team have to continuously balance the risks of hypoglycemia against those of long-term hyperglycemia. The confidence of the patient and parents is often shaken after an episode of severe hypoglycemia, and fear of a recurrence may induce the patient or parents to change their diabetes management to prevent a recurrence. Altered behaviors may include overeating and/or deliberate selection of inadequate doses of insulin to maintain higher blood glucose levels that are perceived as being safe, resulting in deterioration of glycemic control.126–128 Concern about nocturnal hypoglycemia causes more anxiety for some parents than any other aspect of diabetes, including the fear of long-term complications. Some parents believe that an episode of severe hypoglycemia during the nighttime may go undetected or may not be treated in a timely fashion, leading to permanent brain damage or death.129
The normal glucagon response to hypoglycemia is lost early in the course of the disease,130,131 and patients with T1DM depend on sympathoadrenal responses to prevent or correct hypoglycemia.132 Mild hypoglycemia itself reduces epinephrine responses and symptomatic awareness of subsequent episodes of hypoglycemia.133–135 Little is known about counterregulatory responses in preschool-age children.
Symptoms and Signs of Hypoglycemia
Symptoms of hypoglycemia are caused by neuronal deprivation of glucose and may be autonomic (sweating, shaking, pallor, palpitations) or neuroglycopenic (difficulty concentrating, blurred vision, confusion, drowsiness, odd behavior, slurred speech, loss of coordination), or a combination of both autonomic and neuroglycopenic symptoms. The most common signs and symptoms of hypoglycemia in diabetic children are pallor, weakness, tremor, hunger, fatigue, drowsiness, sweating, and headache.136,137 In contrast to adolescents, autonomic symptoms are less common in children younger than 6 years old, whose symptoms of hypoglycemia are more often neuroglycopenic or nonspecific in nature.137 Behavioral changes (irritability, erratic behavior, inconsolable crying) are often the primary manifestation of hypoglycemia in young children, and this difference has important implications for parent education on hypoglycemia. Also, in contrast to adult patients, who usually are able to distinguish between autonomic and neuroglycopenic symptoms, children and their parents report that symptoms tend to cluster.138 The coalescence of autonomic and neuroglycopenic symptoms in children may indicate that both types of symptoms are generated at similar glycemic thresholds.
Biochemical hypoglycemia (with or without symptoms) is defined by the American Diabetes Association as any plasma glucose level ≤70 mg/dL.139 This is the plasma glucose level at which counterregulatory hormone responses are activated and awareness of symptoms occurs. It should be noted, however, that these responses may be triggered at higher glucose levels in healthy 8- to 16-year-olds and in children and adolescents with T1DM who have poor glycemic control.140 Hypoglycemia is classified in terms of its severity as mild, moderate, or severe. Most episodes are mild.137 Cognitive impairment usually does not accompany mild hypoglycemia, and older children are able to recognize the symptoms and treat themselves. Mild symptoms abate within about 15 minutes after treatment with a rapidly absorbed form of carbohydrate. Moderate hypoglycemia has both neuroglycopenic and adrenergic symptoms (e.g., mood changes, irritability, decreased attentiveness, drowsiness, behavior change). Preschool-age children invariably require assistance with treatment because they often are confused and their judgment may be impaired; also, weakness and lack of coordination may make self-treatment difficult. Moderate hypoglycemia causes more protracted symptoms and may require a second treatment with oral carbohydrate. Severe hypoglycemia is characterized by sufficient cognitive impairment that the assistance of another person is needed for treatment. Such events include episodes of unresponsiveness, unconsciousness, or convulsions requiring emergency treatment with glucagon or intravenous glucose. This definition is difficult to apply to very young children, who, by definition, require assistance for treatment of all episodes of hypoglycemia.
Children who have had diabetes for several years may describe a change in their symptoms over time. Autonomic symptoms tend to occur less frequently and to become more muted, and neuroglycopenic symptoms (e.g., drowsiness, difficulty concentrating, lack of coordination) are more common. Patients must learn to recognize the change in symptoms to prevent severe episodes.141 The blood glucose concentration at which symptoms occur varies among patients, and the threshold may vary in the same individual in parallel with antecedent glycemic control. Children with poorly controlled diabetes experience symptoms of hypoglycemia at higher blood glucose concentrations than those with good glycemic control, similar to adults with diabetes.140,142
Impact of Hypoglycemia on the Child’s Brain
Numerous studies have documented cognitive impairments and academic difficulties in children and adolescents diagnosed with T1DM in early childhood. Global intellectual deficits have been described, as well as specific neurocognitive impairments in memory, visuospatial skills, and attention (see reference 143 for review). Neuropsychological complications have been detected within 2 years of onset of diabetes.144,145 Children with long-term diabetes, especially those who developed the disease before the age of 6 years appear to be at greatest risk. However, it is difficult to dissect out the contributions of metabolic disturbances (hyperglycemia and hypoglycemia) and the psychosocial effects of chronic disease in a young child.146 Evidence linking hypoglycemia to neuropsychological defects has been found. For example, Rovet et al. observed specific defects associated with a history of severe hypoglycemic events,147,148 whereas Golden et al. found no evidence of an association with severe episodes and thought that asymptomatic hypoglycemia may be more important.149 Impaired intellectual development without a clear relationship to experienced hypoglycemia has also been reported.150 Thus, cognitive impairments in children with early-onset diabetes mellitus may result from a number of factors whose relative importance is still unclear, including severe hypoglycemia, recurrent asymptomatic hypoglycemia, psychosocial effects of chronic illness, and chronic hyperglycemia.146,151 The neurocognitive sequelae of intensive diabetes management in children whose brains are still developing are still largely unknown. Preliminary findings suggest poorer memory skills, presumably as the consequence of recurrent and severe hypoglycemia.152
Even in the absence of typical symptoms, cognitive function deteriorates at low blood glucose levels.153 Moderate and severe hypoglycemia is disabling, affects school performance, and makes driving a car or operating dangerous machinery hazardous153–156; the utmost effort should be made to avoid such events. Repeated or prolonged severe hyperinsulinemic hypoglycemia can cause permanent central nervous system damage, especially in very young children. Fortunately, hypoglycemia is a rare cause of death in children with T1DM.157
Frequency of Hypoglycemia
The true frequency of mild (self-treated) symptomatic hypoglycemia is almost impossible to ascertain because mild episodes are quickly forgotten and/or are not recorded. In a 12-month population-based study, Aman et al.136 found that mild episodes (managed by the child without assistance) occurred in 97% of children and occurred at least once a week in 53%. More recently, Tupola et al.158 prospectively examined the frequency of hypoglycemia (blood glucose <54 mg/dL) in 161 children and adolescents predominantly treated with multiple doses of insulin, who were asked to document hypoglycemia episodes in a 3 month diary. Fifty-two percent of the clinic population experienced episodes of hypoglycemia (0.6 hypoglycemia events per patient per month), of which 77% were mild.
The literature is replete with reports of the frequency of severe hypoglycemia in children and adolescents with diabetes.158–183 However, various methods of collecting data, variability among clinic populations, ages of patients, therapeutic methods, intensity of treatment, and definitions of severe hypoglycemia make interpretation of the data and comparisons among the reports difficult.146,184 For example, in some studies, severe hypoglycemia is defined as loss of consciousness or seizure, whereas others included children who required assistance with treatment. In young children, all episodes of hypoglycemia require the assistance of a third party for treatment, regardless of the severity of the symptoms. It is not surprising, therefore, that the reported incidence of moderate or severe hypoglycemia varies widely in the pediatric diabetes population. The highest incidence of hypoglycemia in the DCCT was seen in intensively treated adolescents, with the rate of hypoglycemia requiring assistance reaching 85.7 events per 100 patient-years and 26.7 episodes of seizure or coma per 100 patient-years. Recent prospective studies with strict definitions of hypoglycemic events and well-described populations continue to show disturbingly high rates of severe hypoglycemia; younger children and patients with tight glycemic control are at greatest risk.171,176,178,185–187 However, the rate of severe hypoglycemia appears to have decreased in recent years.178–181,183,188 Studies of severe hypoglycemia published since 2000 that included both children and adolescents report an incidence rate of 8 to 36 episodes of severe hypoglycemia per 100 patient-years.116,178–180,183,188 A recent study from Asia and the Western Pacific Region, which defined severe hypoglycemia as any episode requiring assistance in the previous 3 months, reported an incidence of 73 per 100 patient-years, with significant variation among the participating countries.182 The widespread use of CSII and the availability of insulin analogues that can more closely mimic physiologic insulin replacement have contributed to the reduction in risk for severe hypoglycemia (Table 23-13).
Table 23-13
Incidence of Severe Hypoglycemia in Children and Adolescents
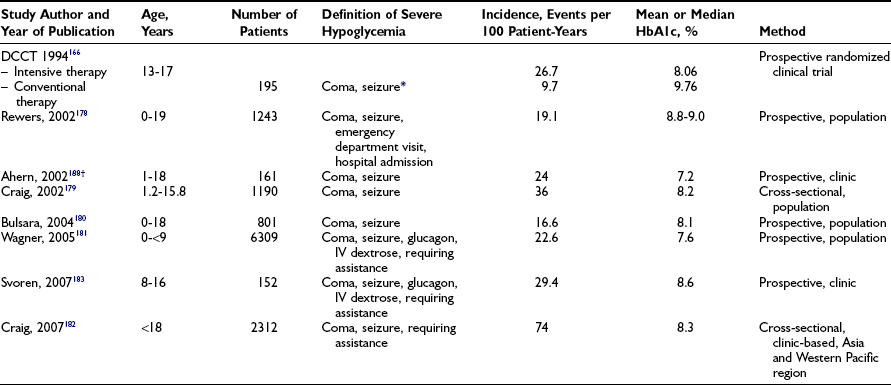
*In the DCCT, severe hypoglycemia (SH) was defined as an episode of hypoglycemia in which the patient required assistance with treatment from another person to recover; in addition, the blood glucose level had to be documented as <50 mg/dL and/or the clinical manifestations had to be reversed by oral carbohydrate, subcutaneous glucagon, or intravenous glucose. In the intensively treated adolescent cohort, 85.7 hypoglycemic episodes occurred per 100-patient years as compared with 27.8 in the conventionally treated group.
Many, but not all, studies have found an increased frequency of severe hypoglycemia in younger children* and in association with lower hemoglobin A1c concentrations.† Other factors associated with a higher risk for moderate and severe hypoglycemia are a prior history of severe hypoglycemia,163,168,187,190 relatively higher doses of insulin and low C-peptide secretion,‡ longer duration of diabetes,165,171,174,181 male gender,167,190 psychiatric disorders,178 and underinsurance178,186 (Table 23-14).
Causes of Hypoglycemia in Diabetes Mellitus
Hypoglycemia is the result of a mismatch between insulin dose, food consumed, and recent exercise. The numerous reasons it may occur in patients with T1DM are shown in Table 23-15.
Table 23-15
Causes of Hypoglycemia in Children and Adolescents With Diabetes Mellitus
• Insulin errors (inadvertent or deliberate)
• Reversal of morning and evening dose
• Reversal of short- or rapid-acting insulin and intermediate-acting insulin
• Improper timing of insulin in relation to food consumption
• Surreptitious insulin administration, suicide gesture or attempt
• Erratic or altered absorption
• Inadvertent intramuscular injection
• More rapid absorption from exercising limbs
• Unpredictable absorption from lipohypertrophy at injection sites
• Prolonged duration and/or increased intensity of physical activity
• Failure to reduce the dose of basal insulin to combat the “lag effect” of exercise
• Impaired gluconeogenesis and glycogenolysis from excessive consumption of ethanol
• Impaired cognition from use of ethanol (may cause hypoglycemia unawareness), marijuana, cocaine, other recreational drugs
Patient errors related to insulin dosage, erratic meal or snack times, decreased food intake, or unplanned exercise account for 50% to 85% of episodes of hypoglycemia in children and adolescents.136,161,163–165,169 After years of living with diabetes, some patients and/or their parents conduct their routine diabetes self-care practices without carefully thinking about the intricate interplay among insulin, food, and exercise.191
New and improved methods of replacing insulin (CSII and MDI regimens using rapid- and long-acting insulin analogues), education that specifically focuses on hypoglycemia,192 behavioral education approaches such as blood glucose awareness training, and intermittent continuous glucose monitoring may enable patients to maintain improved glycemic control with less risk for severe hypoglycemia than was previously possible.180,183,188 These claims have yet to be confirmed in large prospective studies. Several reports have shown that insulin pump therapy is associated with fewer hypoglycemic events despite improved glycemic control.188,193–195 This may be so because CSII permits lower (and adjustable) rates of basal insulin delivery compared with injection therapy, especially after exercise and at night when hypoglycemia is most common. Rapid-acting insulin analogues decrease the frequency of hypoglycemia,173 and basal-bolus therapy combining long-acting insulin analogues (glargine, detemir) with pre-meal rapid-acting insulin analogues (lispro, aspart) decreases the incidence of nocturnal hypoglycemia when compared with regimens using NPH combined with Regular insulin36 or insulin aspart.37
Nocturnal Hypoglycemia
Hypoglycemia, often asymptomatic, frequently occurs during sleep.196 Moderate and severe hypoglycemia are more common during the night and early morning (before breakfast) than during the daytime.171,197 In the DCCT, 55% of severe hypoglycemia events occurred during sleep and 43% occurred between midnight and 8 am.190,197 In children, up to 75% of severe hypoglycemia occurs during the nighttime hours.171
Both children and adults studied either in the hospital or at home with frequent intermittent or continuous blood glucose measurements during the night show a high incidence (14% to 47%) of asymptomatic hypoglycemia (see references 146 and 196 for review). Episodes during sleep may exceed 4 hours in duration, and up to half of these episodes may go undetected because the subject does not awake from sleep. The incidence of hypoglycemia on any given night is affected by numerous factors, including the insulin regimen, the timing and content of meals and snacks, and antecedent physical activity.198 Long after strenuous physical exercise has ended, a sustained increase in insulin action on muscle and liver is seen, along with blunting of the counterregulatory response to hypoglycemia.199,200 The highest frequency of asymptomatic nocturnal hypoglycemia occurs in children younger than 10 years of age.201–205 Low blood glucose concentrations in the early morning (before breakfast) are associated with a higher frequency of preceding nocturnal hypoglycemia. Knowledge of this fact is useful in counseling patients to modify the evening insulin regimen and bedtime snack to prevent more severe nocturnal hypoglycemia.
Sleep impairs counterregulatory hormone responses to hypoglycemia in normal subjects and in patients with diabetes mellitus.206,207 Because a rise in plasma epinephrine is normally the main hormonal defense against hypoglycemia, impaired counterregulatory hormone responses to hypoglycemia explain the increased susceptibility to hypoglycemia during sleep. Furthermore, asymptomatic nocturnal hypoglycemia may impair counterregulatory hormone responses.208 Thus, impaired defenses against hypoglycemia during sleep may contribute to the vicious cycle of hypoglycemia, impaired counterregulatory responses, and unawareness of hypoglycemia while either awake or asleep. Recurrent asymptomatic nocturnal hypoglycemia is therefore an important cause of hypoglycemia unawareness, which, in turn, leads to more frequent and severe hypoglycemia due to failure to experience autonomic warning symptoms before the onset of neuroglycopenia.209
Treatment
The goal is to restore the blood glucose level to normal as quickly as possible; aim to raise the blood glucose to 100 mg/dL. Except in preschool-age children, most episodes of symptomatic hypoglycemia are self-treated with rapidly absorbed carbohydrate such as glucose tablets, juices, soft drinks, candy, crackers, or milk. Glucose tablets raise blood glucose levels more rapidly than orange juice or milk, and the dosage is easily calibrated.210 Glucose tablets are the treatment of choice for children old enough to chew and safely swallow large tablets. The recommended dose is 0.3 grams glucose per kg body weight (5 to 20 grams, depending on the child’s body weight). The blood glucose concentration should be re-measured 15 minutes after treatment, and if the value does not exceed 70 to 80 mg/dL, treatment should be repeated. The glycemic response to oral glucose usually lasts less than 2 hours. Therefore, unless a scheduled meal or snack is due within an hour, the patient should be given a snack or a meal containing carbohydrate and protein.
Hypoglycemia frequently occurs when a child with diabetes is unable to consume or absorb oral carbohydrate because of nausea and vomiting caused by an intercurrent illness (e.g., gastroenteritis) or when oppositional behavior and food refusal occur in very young children. To maintain blood glucose concentrations in a safe range, parents seek emergency medical attention or attempt to force-feed oral carbohydrate to an ill child, which often leads to more vomiting. Mini-dose glucagon raises blood glucose by 60 to 90 mg/dL within 30 minutes, and its effect lasts approximately 1 hour. This method is effective in managing most situations of impending hypoglycemia at home. Using a U-100 insulin syringe and after dissolving 1 mg glucagon in 1 mL of diluent, children ≤2 years should receive 2 “units” (20 µg) of glucagon SC, and children older than 2 years should receive 1 unit (10 µg) per year of age up to 15 units (150 µg). If the blood glucose concentration does not increase within 30 minutes, twice the initial dosage should be administered.211,212
Severe reactions (unresponsiveness, unconsciousness, or convulsions) require emergency treatment with parenteral glucagon (IM or SC). The usual recommended dose is 0.5 mg if the child is <12 years and 1 mg if >12 years, or 10 to 30 mcg/kg.213,214 Glucagon raises blood glucose levels within 5 to 15 minutes and usually relieves symptoms of hypoglycemia. Symptoms of experimentally induced hypoglycemia in diabetic children are relieved within 10 minutes of giving glucagon by SC or IM injection. Mean blood glucose and plasma glucagon levels are slightly but not significantly higher after IM than SC injection. In children with diabetes and in healthy adults,215 no important differences have been noted between the effects of glucagon injected either SC or IM. The plasma glucagon levels attained are higher than those in the peripheral venous or portal blood of healthy adults during insulin-induced hypoglycemia, and they are probably higher than is necessary for maximal effect. The increase in blood glucose concentration after glucagon administration is sustained for at least 30 minutes. Therefore, it is unnecessary to repeat the dose or force the child to eat or drink for at least 30 minutes. Intranasal glucagon has a similar effect, but it is not available in the United States.216 In an emergency department or hospital, the preferred treatment is intravenous glucose (0.3 g per kg). After bolus administration of glucose, the glycemic response is transient; therefore, intravenous glucose infusion should continue until the patient is able to swallow safely.
If severe hypoglycemia was prolonged and the patient has had a seizure, complete recovery of cognitive and neurologic function may take many hours despite restoration of normal blood glucose levels.217 Permanent hemiparesis or other neurologic sequelae are rare218,219; however, the postictal period may be complicated by headache, lethargy, nausea, vomiting, and muscle ache.
Diabetic Ketoacidosis
DKA is comprehensively reviewed in Chapter 20. Aspects of DKA specifically related to children are briefly discussed here.
In Canada, the United States, and Europe, rates of hospitalization for DKA in established and new patients with T1DM age 0-19 years have remained stable at about 20 per 100,000 children.220 The risk for DKA in established T1DM is 1% to 10% per patient per year.174,178,221,222 This risk is increased in children with poor metabolic control or previous episodes of DKA, in peripubertal and adolescent girls, in children with psychiatric disorders, including those with eating disorders, and in those with difficult family circumstances, including lower socioeconomic status and lack of health insurance. In patients using CSII, interruption of insulin delivery, irrespective of the reason, is an important cause of DKA. Children rarely have DKA when insulin administration is closely supervised or performed by a responsible adult.223 In established patients, most instances of DKA probably are associated with insulin omission or treatment error, whereas the remainder are due to inadequate insulin therapy during intercurrent illness.224
Morbidity and Mortality of DKA in Children
DKA is the leading cause of acute morbidity and mortality in children with type 1 diabetes.9 Reported mortality rates from DKA in national population-based studies are reasonably constant in the range of 0.15% to 0.31%. In areas with sparse medical facilities, the risk of dying from DKA is greater, and children may die before receiving treatment.157 Cerebral edema accounts for 57% to 87% of all deaths from DKA.225,226 The incidence of cerebral edema has been fairly consistent between national population-based studies, at 0.46% in Canada to 0.87% in the United States. Mortality rates from cerebral edema in population-based studies are 21% to 25%. Significant morbidity occurs in 10% to 26% of survivors. Other causes of DKA-related morbidity and mortality include hypokalemia, hyperkalemia, hypoglycemia, sepsis, and other central nervous system (CNS) complications such as thrombosis.9
Cerebral edema typically occurs 4 to 12 hours after the start of treatment for DKA but can occur before treatment has begun or at any time during treatment. Symptoms and signs are variable and include onset of headache, change in neurologic status (restlessness, irritability, drowsiness, deterioration in level of consciousness), inappropriate slowing of the heart rate, and an increase in blood pressure.227 Cerebral edema is more common in children with severe DKA, new-onset T1DM, younger age, and longer duration of symptoms. The cause of cerebral edema remains poorly understood228 (Table 23-16).
Table 23-16
Factors Associated With Increased Risk for Cerebral Edema
• An attenuated rise in measured serum sodium concentration during treatment
• Administration of bicarbonate to correct acidosis
• More profound hypocapnia at presentation (after adjustment for the degree of acidosis)
• Increased serum urea nitrogen at presentation, which may reflect more severe dehydration
Treatment for Cerebral Edema
Treatment should be initiated as soon as the condition is suspected. The rate of fluid administration should be reduced by one third and the head of the bed elevated. Give intravenous mannitol (0.5 to 1 g/kg) over 20 minutes, and repeat if necessary if there is no response within 30 minutes. Hypertonic saline (3%), 5 to 10 mL/kg over 30 minutes, has been used as an alternative to mannitol229 and is recommended if there is no response to mannitol. Intubation may be necessary for the patient with impending respiratory failure, but aggressive hyperventilation (to a PCO2 <22 mm Hg) has been associated with poor outcome and is not recommended.230 After treatment for cerebral edema has been started, a cranial CT scan should be obtained to rule out other possible intracerebral causes of neurologic deterioration (10% of cases), especially thrombosis or hemorrhage, which may benefit from specific therapy.
Screening for Other Autoimmune Diseases in Type 1 Diabetes
Autoimmune thyroid disorders are common in patients with T1DM.231 Approximately 22% of patients have thyroid autoantibodies; however, the reported prevalence of thyroid dysfunction varies widely. Asymptomatic individuals should be screened annually for thyroid dysfunction with a sensitive thyroid stimulating hormone (TSH) assay. Alternatively, some endocrinologists determine thyroid autoantibodies and measure TSH only in those with autoantibodies.232
In Western Europe, North America, and Australia, the mean prevalence of celiac disease among children and adults with T1DM is 4.1% (0% to 10.4%). Screening studies with endomysial or tissue transglutaminase antibodies show that 3.7% to 9.9% (mean, 7.4%) of children with T1DM screen positive, and of these, 80% have a positive biopsy. It has been suggested that all children with T1DM should be screened for celiac disease; however, the potential benefits and risks of screening diabetic children for celiac disease have not been systematically assessed.233 If screening is not routine, clinicians should consider the possibility of celiac disease and should screen by measuring tissue transglutaminase antibody in patients with suboptimal glycemic control, diarrhea, abdominal pain, poor growth, or recurrent hypoglycemia.
Anti-21-hydroxylase antibodies occur in 1.6% to 2.3% of individuals with T1DM; only 1 in 200 to 300, however, progress to develop clinical adrenocortical insufficiency.232 The risk increases to 1 in 30 in patients with two autoimmune processes (i.e., diabetes and thyroiditis). The development of adrenocortical insufficiency in T1DM is characterized by recurrent unexplained hypoglycemia and decreasing insulin requirements.
Screening for Long-Term Complications
Intensive glycemic control decreases the risk for microvascular disease, retinopathy, nephropathy and neuropathy, and macrovascular disease.234 In addition to hyperglycemia, several other modifiable risk factors contribute to and influence the risk for vascular complications. Use of tobacco considerably increases the risk for onset and progression of nephropathy and macrovascular disease. Hypertension, likewise, is associated with increased risk for and rate of progression of retinopathy, nephropathy, and macrovascular disease. Dyslipidemia contributes to the risk for macrovascular disease, nephropathy, and retinopathy. A family history of hypertension or nephropathy increases the risk for nephropathy.
Development of diabetic complications is insidious but usually can be detected years before the patient has symptoms or organ function is impaired. Systematic screening can detect abnormality at an early stage, when intervention to arrest, reverse, or retard the disease process will have the greatest impact. Diabetic retinopathy is rare before the onset of puberty or in patients who have had T1DM for less than 5 years. Therefore, annual dilated retinal examinations should begin 3 to 5 years after diagnosis once the child is 10 years of age or older.235 Temporary rapid progression of retinopathy may occur when metabolic control improves drastically, and in these circumstances, retinal examination should be performed more frequently.
Renal disease is first detected by persistent albuminuria. After 5 years of diabetes, an annual screening measurement of urine albumin and creatinine concentrations should be performed to detect microalbuminuria. Several methods can be used to screen for microalbuminuria. The most convenient and, therefore, preferred method is to measure the albumin-to-creatinine ratio in a random spot urine specimen. First-void collections upon arising in the morning avoid the confounding effects of increased albumin excretion induced by upright posture. Timed collections, over 24 hours or timed overnight, are more accurate but less convenient than spot samples. Albumin excretion is transiently elevated by hyperglycemia, exercise, and febrile illness. Because of marked day-to-day variability in albumin excretion, microalbuminuria should be confirmed in at least two of three collections over a 3- to 6-month period to establish the diagnosis of diabetic nephropathy before treatment is instituted. In contrast to the above recommendations for T1DM in children, monitoring of lipids, urinary albumin excretion, and screening eye examinations should begin at diagnosis in T2DM.236
Conclusion
In 1993, the DCCT recommended that most youth with diabetes should receive intensive therapy. Technological innovations since that time, including better pumps and insulin analogues that facilitate more physiologic insulin replacement, have made it possible to achieve tighter blood glucose control with reduced risk for severe hypoglycemia in children and adolescents with diabetes. Increased use of more physiologic insulin regimens, together with frequent blood glucose monitoring and patient empowerment, has made it possible to ensure normal growth and development and to safely achieve levels of blood glucose control that were previously unattainable. It is reasonable to expect that the benefits of sustained improvement in glycemic control will prevent, or at least delay, the appearance of the chronic complications of diabetes. Epidemiologic data provide evidence that this is already the case.237 The arduous and incessant task of controlling blood glucose in a child is difficult and frustrating, and the risk for hypoglycemia is always present. Members of the diabetes team must set realistic and attainable goals for each patient while constantly providing encouragement and support. The resources of a multidisciplinary health care team working in collaboration with the child’s primary care physician are essential for the successful management of childhood diabetes. Unfortunately, over the past decade, T2DM has emerged as a major new challenge for those who provide care for children with diabetes.
References
1. Couper, J, Donaghue, K. Phases of diabetes. Pediatr Diabetes. 2007;8(1):44–47.
2. Wolfsdorf, J, Glaser, N, Sperling, MA. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159.
3. Majaliwa, ES, Munubhi, E, Ramaiya, K, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30(9):2187–2192.
4. Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691.
5. Barker, JM, Goehrig, SH, Barriga, K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404.
6. Goran, MI, Gower, BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450.
7. Libman, IM, Pietropaolo, M, Arslanian, SA, et al. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875.
8. Katz, LE, Jawad, AF, Ganesh, J, et al. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007;8(2):53–59.
9. Dunger, DB, Sperling, MA, Acerini, CL, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child. 2004;89(2):188–194.
10. Clar C, Waugh N, Thomas S: Routine hospital admission versus out-patient or home care in children at diagnosis of type 1 diabetes mellitus, Cochrane Database Syst Rev (2):CD004099, 2007.
11. Swift, PG. Diabetes education. ISPAD clinical practice consensus guidelines 2006–2007. Pediatr Diabetes. 2007;8(2):103–109.
12. Anderson, BJ, Wolfsdorf, JI, Jacobson, AM. Psychosocial adjustment in children with type 1 diabetes. In: Lebovitz HE, ed. Therapy for diabetes mellitus and related disorders. ed 5. Alexandria, Virginia: American Diabetes Association; 2009:97–104.
13. Cameron, FJ, Northam, EA, Ambler, GR, et al. Routine psychological screening in youth with type 1 diabetes and their parents: a notion whose time has come? Diabetes Care. 2007;30(10):2716–2724.
14. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. [see comments]. New England Journal of Medicine. 1993;329(14):977–986.
15. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177–188.
16. Reichard, P, Nilsson, BY, Rosenqvist, U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus [see comments]. N Engl J Med. 1993;329(5):304–309.
17. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
18. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34): UK Prospective Diabetes Study (UKPDS) Group [see comments] [published erratum appears in Lancet 352(9139):1557, 1998 Nov 7]. Lancet. 1998;352(9131):854–865.
19. Nathan, DM, Cleary, PA, Backlund, JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653.
20. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381–389.
21. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287(19):2563–2569.
22. Rewers, M, Pihoker, C, Donaghue, K, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2007;8(6):408–418.
23. Donaghue, KC, Fairchild, JM, Craig, ME, et al. Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care. 2003;26(4):1224–1229.
24. Krolewski, AS, Laffel, LM, Krolewski, M, et al. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus [see comments]. N Engl J Med. 1995;332(19):1251–1255.
25. Warram, JH, Scott, LJ, Hanna, LS, et al. Progression of microalbuminuria to proteinuria in type 1 diabetes: nonlinear relationship with hyperglycemia. Diabetes. 2000;49(1):94–100.
26. Holl, RW, Swift, PG, Mortensen, HB, et al. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur J Pediatr. 2003;162(1):22–29.
27. de Beaufort, CE, Swift, PG, Skinner, CT, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30(9):2245–2250.
28. Rutledge, KS, Chase, HP, Klingensmith, GJ, et al. Effectiveness of postprandial Humalog in toddlers with diabetes. Pediatrics. 1997;100(6):968–972.
29. Tupola, S, Komulainen, J, Jaaskelainen, J, et al. Post-prandial insulin lispro vs. human regular insulin in prepubertal children with Type 1 diabetes mellitus. Diabet Med. 2001;18(8):654–658.
30. Casella, S, Mongilio, M, Plotnick, L, et al. Accuracy and precision of low-dose insulin administration. Pediatrics. 1993;91:977–986.
31. Silva, S, Clark, L, Goodman, S, et al. Can caretakers of children with IDDM accurately measure small insulin doses and dose changes? Diabetes Care. 1996;19:56–59.
32. Fanelli, CG, Pampanelli, S, Porcellati, F, et al. Administration of neutral protamine Hagedorn insulin at bedtime versus with dinner in type 1 diabetes mellitus to avoid nocturnal hypoglycemia and improve control. A randomized, controlled trial. Ann Intern Med. 2002;136(7):504–514.
33. Chase, HP, Dixon, B, Pearson, J, et al. Reduced hypoglycemic episodes and improved glycemic control in children with type 1 diabetes using insulin glargine and neutral protamine hagedorn insulin. J Pediatr. 2003;143(6):737–740.
34. Schober, E, Schoenle, E, Van Dyk, J, et al. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15(4):369–376.
35. Lepore, M, Pampanelli, S, Fanelli, C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–2148.
36. Murphy, NP, Keane, SM, Ong, KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26(3):799–804.
37. Robertson, KJ, Schoenle, E, Gucev, Z, et al. Insulin detemir compared with NPH insulin in children and adolescents with Type 1 diabetes. Diabet Med. 2007;24(1):27–34.
38. Porcellati, F, Rossetti, P, Ricci, NB, et al. Pharmacokinetics and pharmacodynamics of the long-acting insulin analog glargine after 1 week of use compared with its first administration in subjects with type 1 diabetes. Diabetes Care. 2007;30(5):1261–1263.
39. Phillip, M, Battelino, T, Rodriguez, H, et al. Consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Use of Insulin Pump Therapy in the Pediatric Age-Group. Diabetes Care. 2007;30(6):1653–1662.
40. Chase, HP, Saib, SZ, MacKenzie, T, et al. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19(4):317–321.
41. Pickup, J, Mattock, M, Kerry, S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324(7339):705.
42. Weissberg-Benchell, J, Antisdel-Lomaglio, J, Seshadri, R. Insulin Pump Therapy: A meta-analysis. Diabetes Care. 2003;26(4):1079–1087.
43. Grey, M, Boland, EA, Davidson, M, et al. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents [see comments]. Diabetes Care. 1998;21(6):902–908.
44. Boland, EA, Grey, M, Oesterle, A, et al. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes [see comments]. Diabetes Care. 1999;22(11):1779–1784.
45. Amiel, SA, Caprio, S, Sherwin, RS, et al. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab. 1991;72(2):277–282.
46. Mohn, A, Matyka, KA, Harris, DA, et al. Lispro or regular insulin for multiple injection therapy in adolescence. Differences in free insulin and glucose levels overnight. Diabetes Care. 1999;22(1):27–32.
47. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–S54.
48. Knowler, WC, Barrett-Connor, E, Fowler, SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
49. Franz, MJ, Bantle, JP, Beebe, CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25(1):148–198.
50. Brand-Miller, J, Hayne, S, Petocz, P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267.
51. Wolfsdorf, J, Quinn, M, Warman, K. Diabetes Mellitus. In: Duggan C, Watkins J, Walker W, eds. Nutrition in Pediatrics: Basic Science and Clinical Applications. ed 4. Hamilton, Ontario: BC Decker, Inc.; 2008:617–630.
52. Overby, NC, Margeirsdottir, HD, Brunborg, C, et al. The influence of dietary intake and meal pattern on blood glucose control in children and adolescents using intensive insulin treatment. Diabetologia. 2007;50(10):2044–2051.
53. Daniels, SR, Greer, F, Nutrition, tCo. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208.
54. Birch, LL, Johnson, SL, Andresen, G, et al. The variability of young children’s energy intake. N Engl J Med. 1991;324(4):232–235.
55. Delahanty, LM, Halford, BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care. 1993;16(11):1453–1458.
56. Guelfi, KJ, Jones, TW, Fournier, PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care. 2005;28(6):1289–1294.
57. Reisman, D. Mild diabetes in children. Am J Med Sci. 1916;151:40–45.
58. Fagot-Campagna, A, Pettitt, DJ, Engelgau, MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–672.
59. Kitagawa, T, Owada, M, Urakami, T, et al. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila). 1998;37(2):111–115.
60. Kadiki, OA, Reddy, MR, Marzouk, AA. Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0–34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract. 1996;32(3):165–173.
61. Pinhas-Hamiel, O, Dolan, LM, Daniels, SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents [see comments]. J Pediatr. 1996;128(5 Pt 1):608–615.
62. Neufeld, ND, Raffel, LJ, Landon, C, et al. Early presentation of type 2 diabetes in Mexican-American youth. Diabetes Care. 1998;21(1):80–86.
63. Ogden, CL, Carroll, MD, Curtin, LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555.
64. Caprio, S, Tamborlane, WV. Metabolic impact of obesity in childhood. Endocrinol Metab Clin North Am. 1999;28(4):731–747.
65. Steinberger, J, Moran, A, Hong, CP, et al. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr. 2001;138(4):469–473.
66. Ritchie, L, Ganapathy, S, Woodward-Lopez, G, et al. Prevention of type 2 diabetes in youth: etiology, promising interventions and recommendations. Pediatric Diabetes. 2003;4:174–209.
67. Gaede, P, Vedel, P, Larsen, N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393.
68. Gungor, N, Thompson, T, Sutton-Tyrrell, K, et al. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28(5):1219–1221.
69. Zeitler, P, Epstein, L, Grey, M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8(2):74–87.
70. Nield L, Moore HJ, Hooper L, et al: Dietary advice for treatment of type 2 diabetes mellitus in adults, Cochrane Database Syst Rev (3):CD004097, 2007.
71. Wrotniak, BH, Epstein, LH, Paluch, RA, et al. The relationship between parent and child self-reported adherence and weight loss. Obes Res. 2005;13(6):1089–1096.
72. Wolever, TM, Gibbs, AL, Mehling, C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87(1):114–125.
73. Reinehr, T, Kiess, W, Kapellen, T, et al. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114(6):1569–1573.
74. Schmitz, KH, Jacobs, DR, Jr., Hong, CP, et al. Association of physical activity with insulin sensitivity in children. Int J Obes Relat Metab Disord. 2002;26(10):1310–1316.
75. Botero, D, Wolfsdorf, JI. Diabetes mellitus in children and adolescents. Arch Med Res. 2005;36(3):281–290.
76. Bobo, N, Evert, A, Gallivan, J, et al. An update on type 2 diabetes in youth from the National Diabetes Education Program. Pediatrics. 2004;114(1):259–263.
77. Jones, KL, Arslanian, S, Peterokova, VA, et al. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25(1):89–94.
78. Dunn, CJ, Peters, DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49(5):721–749.
79. Salpeter, SR, Greyber, E, Pasternak, GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163(21):2594–2602.
80. Gottschalk, M, Danne, T, Vlajnic, A, et al. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30(4):790–794.
81. Raskin, P, Allen, E, Hollander, P, et al. Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260–265.
82. Sellers, EA, Dean, HJ. Short-term insulin therapy in adolescents with type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17(11):1561–1564.
83. Jeha, GS, Heptulla, RA. Newer therapeutic options for children with diabetes mellitus: theoretical and practical considerations. Pediatr Diabetes. 2006;7(2):122–138.
84. Adler, AI, Stratton, IM, Neil, HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study [see comments]. Bmj. 2000;321(7258):412–419.
85. Estacio, RO, Jeffers, BW, Gifford, N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54–B64.
86. Miller, K. Pharmacological management of hypertension in paediatric patients. A comprehensive review of the efficacy, safety and dosage guidelines of the available agents. Drugs. 1994;48(6):868–887.
87. Hilgers, KF, Dotsch, J, Rascher, W, et al. Treatment strategies in patients with chronic renal disease: ACE inhibitors, angiotensin receptor antagonists, or both? Pediatr Nephrol. 2004;19(9):956–961.
88. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576.
89. Gidding, SS, Dennison, BA, Birch, LL, et al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–559.
90. McCrindle, BW, Urbina, EM, Dennison, BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115(14):1948–1967.
91. Clauss, SB, Holmes, KW, Hopkins, P, et al. Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatrics. 2005;116(3):682–688.
92. de Jongh, S, Ose, L, Szamosi, T, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation. 2002;106(17):2231–2237.
93. Tuomilehto, J, Lindstrom, J, Eriksson, JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350.
94. Murphy, R, Ellard, S, Hattersley, AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab. 2008;4(4):200–213.
95. Tattersall, RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974;43(170):339–357.
96. Fajans, SS. Scope and heterogeneous nature of MODY. Diabetes Care. 1990;13:49–64.
97. Murphy, R, Turnbull, DM, Walker, M, et al. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med. 2008;25(4):383–399.
98. Gloyn, AL, Pearson, ER, Antcliff, JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–1849.
99. Pearson, ER, Flechtner, I, Njolstad, PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–477.
100. Babenko, AP, Polak, M, Cave, H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355(5):456–466.
101. Rafiq, M, Flanagan, SE, Patch, AM, et al. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31(2):204–209.
102. Sagen, JV, Raeder, H, Hathout, E, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53(10):2713–2718.
103. Codner, E, Flanagan, S, Ellard, S, et al. High-dose glibenclamide can replace insulin therapy despite transitory diarrhea in early-onset diabetes caused by a novel R201L Kir6.2 mutation. Diabetes Care. 2005;28(3):758–759.
104. Slingerland, AS, Nuboer, R, Hadders-Algra, M, et al. Improved motor development and good long-term glycaemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia. 2006;49(11):2559–2563.
105. Stoy, J, Edghill, EL, Flanagan, SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104(38):15040–15044.
106. Temple, IK, Gardner, RJ, Mackay, DJ, et al. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49(8):1359–1366.
107. Flanagan, SE, Patch, AM, Mackay, DJ, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56(7):1930–1937.
108. Vaxillaire, M, Dechaume, A, Busiah, K, et al. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56(6):1737–1741.
109. Temple, IK, Shield, JP. Transient neonatal diabetes, a disorder of imprinting. J Med Genet. 2002;39(12):872–875.
110. Moran, A, Doherty, L, Wang, X, et al. Abnormal glucose metabolism in cystic fibrosis. J Pediatr. 1998;133(1):10–17.
111. Lanng, S, Thorsteinsson, B, Lund-Andersen, C, et al. Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late diabetic complications. Acta Paediatr. 1994;83(1):72–77.
112. Hardin, DS, Leblanc, A, Marshall, G, et al. Mechanisms of insulin resistance in cystic fibrosis. Am J Physiol Endocrinol Metab. 2001;281(5):E1022–1028.
113. Milla, CE, Warwick, WJ, Moran, A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891–895.
114. Finkelstein, SM, Wielinski, CL, Elliott, GR, et al. Diabetes mellitus associated with cystic fibrosis. J Pediatr. 1988;112(3):373–377.
115. Moran, A, Hardin, D, Rodman, D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract. 1999;45(1):61–73.
116. Levine, B, Anderson, B, Butler, D, et al. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139(2):197–203.
117. Wolpert, HA. The nuts and bolts of achieving end points with real-time continuous glucose monitoring. Diabetes Care. 2008;31(Suppl 2):S146–S149.
118. Weinzimer, S, Xing, D, Tansey, M, et al. FreeStyle navigator continuous glucose monitoring system use in children with type 1 diabetes using glargine-based multiple daily dose regimens: results of a pilot trial Diabetes Research in Children Network (DirecNet) Study Group. Diabetes Care. 2008;31(3):525–527.
119. Rewers, A, McFann, K, Chase, HP. Bedside monitoring of blood beta-hydroxybutyrate levels in the management of diabetic ketoacidosis in children. Diabetes Technol Ther. 2006;8(6):671–676.
120. Laffel, LM, Wentzell, K, Loughlin, C, et al. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278–284.
121. Sacks, DB, Bruns, DE, Goldstein, DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472.
122. Mosca, A, Goodall, I, Hoshino, T, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med. 2007;45(8):1077–1080.
123. Nathan, DM, Kuenen, J, Borg, R, et al. Translating the A1C Assay Into Estimated Average Glucose Values. Diabetes Care. 2008;31(8):1–6.
124. Sacks, DB. Global harmonization of hemoglobin A1c. Clin Chem. 2005;51(4):681–683.
125. Cryer, PE. Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43(11):1378–1389.
126. Cox, DJ, Irvine, A, Gonder-Frederick, L, et al. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–621.
127. Gonder-Frederick, LA, Clarke, WL, Cox, DJ. The emotional, social, and behavioral implications of insulin-induced hypoglycemia. Semin Clin Neuropsychiatry. 1997;2(1):57–65.
128. Clarke, WL, Gonder-Frederick, A, Snyder, AL, et al. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11(Suppl 1):189–194.
129. Santiago, JV. Nocturnal hypoglycemia in children with diabetes: an important problem revisited [editorial; comment]. J Pediatr. 1997;131(1 Pt 1):2–4.
130. Gerich, JE, Langlois, M, Noacco, C, et al. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(108):171–173.
131. Bolli, G, Calabrese, G, De Feo, P, et al. Lack of glucagon response in glucose counter-regulation in type 1 (insulin-dependent) diabetics: absence of recovery after prolonged optimal insulin therapy. Diabetologia. 1982;22(2):100–105.
132. Cryer, PE, Davis, SN, Shamoon, H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912.
133. Heller, SR, Cryer, PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223–226.
134. Cryer, PE. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes. 1992;41(3):255–260.
135. Dagogo-Jack, SE, Craft, S, Cryer, PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91(3):819–828.
136. Aman, J, Karlsson, I, Wranne, L. Symptomatic hypoglycaemia in childhood diabetes: a population-based questionnaire study. Diabet Med. 1989;6(3):257–261.
137. Tupola, S, Rajantie, J. Documented symptomatic hypoglycaemia in children and adolescents using multiple daily insulin injection therapy. Diabet Med. 1998;15(6):492–496.
138. McCrimmon, RJ, Gold, AE, Deary, IJ, et al. Symptoms of hypoglycemia in children with IDDM. Diabetes Care. 1995;18(6):858–861.
139. American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245–1249.
140. Jones, TW, Boulware, SD, Kraemer, DT, et al. Independent effects of youth and poor diabetes control on responses to hypoglycemia in children. Diabetes. 1991;40(3):358–363.
141. Dammacco, F, Torelli, C, Frezza, E, et al. Problems of hypoglycemia arising in children and adolescents with insulin-dependent diabetes mellitus. The Diabetes Study Group of The Italian Society of Pediatric Endocrinology & Diabetes. J Pediatr Endocrinol Metab. 1998;11(Suppl 1):167–176.
142. Boyle, PJ, Schwartz, NS, Shah, SD, et al. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988;318(23):1487–1492.
143. Northam, EA, Rankins, D, Cameron, FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nature clinical practice. 2006;2(2):78–86.
144. Northam, EA, Anderson, PJ, Jacobs, R, et al. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24(9):1541–1546.
145. Northam, EA, Anderson, PJ, Werther, GA, et al. Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care. 1998;21(3):379–384.
146. Jones, TW, Davis, EA. Hypoglycemia in children with type 1 diabetes: current issues and controversies. Pediatr Diabetes. 2003;4(3):143–150.
147. Rovet, JF, Ehrlich, RM, Hoppe, M. Specific intellectual deficits in children with early onset diabetes mellitus. Child Dev. 1988;59(1):226–234.
148. Rovet, JF, Ehrlich, RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study [see comments]. J Pediatr. 1999;134(4):503–506.
149. Golden, MP, Ingersoll, GM, Brack, CJ, et al. Longitudinal relationship of asymptomatic hypoglycemia to cognitive function in IDDM. Diabetes Care. 1989;12(2):89–93.
150. Schoenle, EJ, Schoenle, D, Molinari, L, et al. Impaired intellectual development in children with Type I diabetes: association with HbA(1c), age at diagnosis and sex. Diabetologia. 2002;45(1):108–114.
151. Ryan, CM. Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatr Diabetes. 2006;7(5):289–297.
152. Hershey, T, Bhargava, N, Sadler, M, et al. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed [see comments]. Diabetes Care. 1999;22(8):1318–1324.
153. Ryan, CM, Becker, DJ. Hypoglycemia in children with type 1 diabetes mellitus. Risk factors, cognitive function, and management. Endocrinol Metab Clin North Am. 1999;28(4):883–900.
154. Frier, BM, Matthews, DM, Steel, JM, et al. Driving and insulin-dependent diabetes. Lancet. 1980;1(8180):1232–1234.
155. Songer, TJ, LaPorte, RE, Dorman, JS, et al. Motor vehicle accidents and IDDM. Diabetes Care. 1988;11(9):701–707.
156. Ratner, RE, Whitehouse, FW. Motor vehicles, hypoglycemia, and diabetic drivers. Diabetes Care. 1989;12(3):217–222.
157. Edge, JA, Ford-Adams, ME, Dunger, DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81(4):318–323.
158. Tupola, S, Rajantie, J, Maenpaa, J. Severe hypoglycaemia in children and adolescents during multiple-dose insulin therapy. Diabet Med. 1998;15(8):695–699.
159. Goldstein, DE, England, JD, Hess, R, et al. A prospective study of symptomatic hypoglycemia in young diabetic patients. Diabetes Care. 1981;4(6):601–605.
160. Daneman, D, Frank, M, Perlman, K, et al. Severe hypoglycemia in children with insulin-dependent diabetes mellitus: frequency and predisposing factors [see comments]. J Pediatr. 1989;115(5 Pt 1):681–685.
161. Bergada, I, Suissa, S, Dufresne, J, et al. Severe hypoglycemia in IDDM children. Diabetes Care. 1989;12(4):239–244.
162. Soltesz, G, Acsadi, G. Association between diabetes, severe hypoglycaemia, and electroencephalographic abnormalities. Arch Dis Child. 1989;64(7):992–996.
163. Bhatia, V, Wolfsdorf, JI. Severe hypoglycemia in youth with insulin-dependent diabetes mellitus: frequency and causative factors. Pediatrics. 1991;88(6):1187–1193.
164. Egger, M, Gschwend, S, Smith, GD, et al. Increasing incidence of hypoglycemic coma in children with IDDM. Diabetes Care. 1991;14(11):1001–1005.
165. Limbert, C, Schwingshandl, J, Haas, J, et al. Severe hypoglycemia in children and adolescents with IDDM: frequency and associated factors. J Diabetes Complications. 1993;7(4):216–220.
166. The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. [see comments]. Journal of Pediatrics. 1994;125(2):177–188.
167. Dumont, RH, Jacobson, AM, Cole, C, et al. Psychosocial predictors of acute complications of diabetes in youth. Diabet Med. 1995;12(7):612–618.
168. Verrotti, A, Chiarelli, F, Blasetti, A, et al. Severe hypoglycemia in insulin-dependent diabetic children treated by multiple injection insulin regimen. Acta Diabetol. 1996;33(1):53–57.
169. Bognetti, F, Brunelli, A, Meschi, F, et al. Frequency and correlates of severe hypoglycaemia in children and adolescents with diabetes mellitus [see comments]. Eur J Pediatr. 1997;156(8):589–591.
170. Mortensen, HB, Hougaard, P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes [published erratum appears in Diabetes Care 20(7):1216, 1997 Jul]. Diabetes Care. 1997;20(5):714–720.
171. Davis, EA, Keating, B, Byrne, GC, et al. Hypoglycemia: incidence and clinical predictors in a large population- based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22–25.
172. Nordfeldt, S, Ludvigsson, J. Severe hypoglycemia in children with IDDM. A prospective population study, 1992–1994. Diabetes Care. 1997;20(4):497–503.
173. Chase, HP, Lockspeiser, T, Peery, B, et al. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care. 2001;24(3):430–434.
174. Rosilio, M, Cotton, JB, Wieliczko, MC, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. The French Pediatric Diabetes Group [see comments]. Diabetes Care. 1998;21(7):1146–1153.
175. Lteif, AN, Schwenk, WF, 2nd. Type 1 diabetes mellitus in early childhood: glycemic control and associated risk of hypoglycemic reactions [see comments]. Mayo Clin Proc. 1999;74(3):211–216.
176. Nordfeldt, S, Ludvigsson, J. Adverse events in intensively treated children and adolescents with type 1 diabetes. Acta Paediatr. 1999;88(11):1184–1193.
177. Danne, T, Mortensen, HB, Hougaard, P, et al. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidore Study Group. Diabetes Care. 2001;24(8):1342–1347.
178. Rewers, A, Chase, HP, Mackenzie, T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287(19):2511–2518.
179. Craig, ME, Handelsman, P, Donaghue, KC, et al. Predictors of glycaemic control and hypoglycaemia in children and adolescents with type 1 diabetes from NSW and the ACT. Med J Aust. 2002;177(5):235–238.
180. Bulsara, MK, Holman, CD, Davis, EA, et al. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. 2004;27(10):2293–2298.
181. Wagner, VM, Grabert, M, Holl, RW. Severe hypoglycaemia, metabolic control and diabetes management in children with type 1 diabetes in the decade after the Diabetes Control and Complications Trial—a large-scale multicentre study. Eur J Pediatr. 2005;164(2):73–79.
182. Craig, ME, Jones, TW, Silink, M, et al. Diabetes care, glycemic control, and complications in children with type 1 diabetes from Asia and the Western Pacific Region. J Diabetes Complications. 2007;21(5):280–287.
183. Svoren, BM, Volkening, LK, Butler, DA, et al. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J Pediatr. 2007;150(3):279–285.
184. Clarke, WL, Gonder-Frederick, L, Cox, DJ. The frequency of severe hypoglycaemia in children with insulin-dependent diabetes mellitus. Horm Res. 1996;45(Suppl 1):48–52.
185. Barkai, L, Vamosi, I, Lukacs, K. Prospective assessment of severe hypoglycaemia in diabetic children and adolescents with impaired and normal awareness of hypoglycaemia. Diabetologia. 1998;41(8):898–903.
186. Allen, C, LeCaire, T, Palta, M, et al. Risk factors for frequent and severe hypoglycemia in type 1 diabetes. Diabetes Care. 2001;24(11):1878–1881.
187. Davis, EA, Keating, B, Byrne, GC, et al. Impact of improved glycaemic control on rates of hypoglycaemia in insulin dependent diabetes mellitus. Arch Dis Child. 1998;78(2):111–115.
188. Ahern, J, Boland, E, Doane, R, et al. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatric Diabetes. 2002;3(1):10–15.
189. Thomsett, M, Shield, G, Batch, J, et al. How well are we doing? Metabolic control in patients with diabetes. J Paediatr Child Health. 1999;35(5):479–482.
190. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286.
191. Jacobson, AM, Hauser, ST, Wolfsdorf, JI, et al. Psychologic predictors of compliance in children with recent onset of diabetes mellitus. J Pediatr. 1987;110(5):805–811.
192. Nordfeldt, S, Johansson, C, Carlsson, E, et al. Prevention of severe hypoglycaemia in type I diabetes: a randomised controlled population study. Arch Dis Child. 2003;88(3):240–245.
193. Maniatis, AK, Klingensmith, GJ, Slover, RH, et al. Continuous subcutaneous insulin infusion therapy for children and adolescents: an option for routine diabetes care. Pediatrics. 2001;107(2):351–356.
194. Linkeschova, R, Raoul, M, Bott, U, et al. Less severe hypoglycaemia, better metabolic control, and improved quality of life in Type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med. 2002;19(9):746–751.
195. Ludvigsson, J, Hanas, R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111(5 Pt 1):933–938.
196. Matyka, KA. Sweet dreams? Nocturnal hypoglycemia in children with type 1 diabetes. Pediatr Diabetes. 2002;3(2):74–81.
197. The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group [see comments]. Am J Med. 1991;90(4):450–459.
198. Tsalikian, E, Mauras, N, Beck, RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528–534.
199. Sandoval, DA, Guy, DL, Richardson, MA, et al. Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes. 2004;53(7):1798–1806.
200. McMahon, SK, Ferreira, LD, Ratnam, N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92(3):963–968.
201. Shalwitz, RA, Farkas-Hirsch, R, White, NH, et al. Prevalence and consequences of nocturnal hypoglycemia among conventionally treated children with diabetes mellitus. J Pediatr. 1990;116(5):685–689.
202. Porter, PA, Keating, B, Byrne, G, et al. Incidence and predictive criteria of nocturnal hypoglycemia in young children with insulin-dependent diabetes mellitus [see comments]. J Pediatr. 1997;130(3):366–372.
203. Beregszaszi, M, Tubiana-Rufi, N, Benali, K, et al. Nocturnal hypoglycemia in children and adolescents with insulin-dependent diabetes mellitus: prevalence and risk factors [see comments]. J Pediatr. 1997;131(1 Pt 1):27–33.
204. Lopez, MJ, Oyarzabal, M, Barrio, R, et al. Nocturnal hypoglycaemia in IDDM patients younger than 18 years. Diabet Med. 1997;14(9):772–777.
205. Amin, R, Ross, K, Acerini, CL, et al. Hypoglycemia prevalence in prepubertal children with type 1 diabetes on standard insulin regimen: use of continuous glucose monitoring system. Diabetes Care. 2003;26(3):662–667.
206. Jones, TW, Porter, P, Sherwin, RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338(23):1657–1662.
207. Banarer, S, Cryer, PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195–1203.
208. Veneman, T, Mitrakou, A, Mokan, M, et al. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes. 1993;42(9):1233–1237.
209. Cryer, PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350(22):2272–2279.
210. Brodows, RG, Williams, C, Amatruda, JM. Treatment of insulin reactions in diabetics. JAMA. 1984;252(24):3378–3381.
211. Haymond, MW, Schreiner, B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643–645.
212. Hartley, M, Thomsett, MJ, Cotterill, AM. Mini-dose glucagon rescue for mild hypoglycaemia in children with type 1 diabetes: the Brisbane experience. J Paediatr Child Health. 2006;42(3):108–111.
213. Clarke, W, Jones, T, Rewers, A, et al. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2008;9(2):165–174.
214. Aman, J, Wranne, L. Hypoglycaemia in childhood diabetes. II. Effect of subcutaneous or intramuscular injection of different doses of glucagon. Acta Paediatr Scand. 1988;77(4):548–553.
215. Muhlhauser, I, Berger, M, Sonnenberg, G, et al. Incidence and management of severe hypoglycemia in 434 adults with insulin-dependent diabetes mellitus. Diabetes Care. 1985;8(3):268–273.
216. Slama, G, Alamowitch, C, Desplanque, N, et al. A new non-invasive method for treating insulin-reaction: intranasal lyophylized glucagon. Diabetologia. 1990;33(11):671–674.
217. Lala, VR, Vedanarayana, VV, Ganesh, S, et al. Hypoglycemic hemiplegia in an adolescent with insulin-dependent diabetes mellitus: a case report and a review of the literature. J Emerg Med. 1989;7(3):233–236.
218. Wayne, EA, Dean, HJ, Booth, F, et al. Focal neurologic deficits associated with hypoglycemia in children with diabetes [see comments]. J Pediatr. 1990;117(4):575–577.
219. Shehadeh, N, Kassem, J, Tchaban, I, et al. High incidence of hypoglycemic episodes with neurologic manifestations in children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11(Suppl 1):183–187.
220. Curtis, JR, To, T, Muirhead, S, et al. Recent trends in hospitalization for diabetic ketoacidosis in Ontario children. Diabetes Care. 2002;25(9):1591–1596.
221. Morris, AD, Boyle, DI, McMahon, AD, et al. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit. Lancet. 1997;350(9090):1505–1510.
222. Smith, CP, Firth, D, Bennett, S, et al. Ketoacidosis occurring in newly diagnosed and established diabetic children. Acta Paediatr. 1998;87(5):537–541.
223. Golden, MP, Herrold, AJ, Orr, DP. An approach to prevention of recurrent diabetic ketoacidosis in the pediatric population. J Pediatr. 1985;107:195–200.
224. Glasgow, AM, Weissberg-Benchell, J, Tynan, WD, et al. Readmissions of children with diabetes mellitus to a children’s hospital. Pediatrics. 1991;88(1):98–104.
225. Edge, JA, Hawkins, MM, Winter, DL, et al. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85(1):16–22.
226. Glaser, N, Barnett, P, McCaslin, I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264–269.
227. Muir, AB, Quisling, RG, Yang, MC, et al. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004;27(7):1541–1546.
228. Glaser, N. New perspectives on the pathogenesis of cerebral edema complicating diabetic ketoacidosis in children. Pediatr Endocrinol Rev. 2006;3(4):379–386.
229. Kamat, P, Vats, A, Gross, M, et al. Use of hypertonic saline for the treatment of altered mental status associated with diabetic ketoacidosis. Pediatr Crit Care Med. 2003;4(2):239–242.
230. Marcin, JP, Glaser, N, Barnett, P, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr. 2002;141(6):793–797.
231. Kordonouri, O, Klinghammer, A, Lang, EB, et al. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25(8):1346–1350.
232. Devendra, D, Eisenbarth, GS. 17. Immunologic endocrine disorders. J Allergy Clin Immunol. 2003;111(2 Suppl):S624–S636.
233. Freemark, M, Levitsky, LL. Screening for celiac disease in children with type 1 diabetes: two views of the controversy. Diabetes Care. 2003;26(6):1932–1939.
234. Stettler, C, Allemann, S, Juni, P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38.
235. American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2004;27(Suppl 1):S15–S35.
236. American Diabetes Association. Type 2 diabetes in children and adolescents. (Consensus statement). Diabetes Care. 2000;23(3):381–389.
237. Bojestig, M, Arnqvist, HJ, Hermansson, G, et al. Declining incidence of nephropathy in insulin-dependent diabetes mellitus [published erratum appears in N Engl J Med 330(8):584, 1994 Feb 24]. N Engl J Med. 1994;330(1):15–18.
238. Kaplan, W, Rodriguez, LM, Smith, OE, et al. Effects of mixing glargine and short-acting insulin analogs on glucose control. Diabetes Care. 2004;27(11):2739–2740.
239. Fiallo-Scharer, R, Horner, B, McFann, K, et al. Mixing rapid-acting insulin analogues with insulin glargine in children with type 1 diabetes mellitus. J Pediatr. 2006;148(4):481–484.
240. Plank, J, Wutte, A, Brunner, G, et al. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care. 2002;25(11):2053–2057.
241. Danne, T, Lupke, K, Walte, K, et al. Insulin detemir is characterized by a consistent pharmacokinetic profile across age-groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care. 2003;26(11):3087–3092.
242. Franz, MJ, Bantle, JP, Beebe, CA, et al. Nutrition principles and recommendations in diabetes. Diabetes Care. 2004;27(Suppl 1):S36–S46.
243. Bantle, JP, Wylie-Rosett, J, Albright, AL, et al. Nutrition recommendations and interventions for diabetes—2006: a position statement of the American Diabetes Association. Diabetes Care. 2006;29(9):2140–2157.
244. Riddell, MC, Iscoe, KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes. 2006;7(1):60–70.