Chapter 112 Management of Combined Inflammatory and Rhegmatogenous Retinal Detachment
Introduction
Retinal detachments (RD) occurring in the context of ocular inflammation are a particular challenge both to the vitreoretinal surgeon and to the uveitis specialist. Most often they occur during or following an episode of intraocular infection and are most commonly seen in eyes having suffered from a viral retinitis. When they are not related to ocular infection, their diagnostic and therapeutic approach may be difficult, since different forms of retinal involvement suppose different treatments and prognosis. Serous retinal detachment (SRD) is the form most commonly associated with active, purely inflammatory conditions and, often, its presence helps to establish the diagnosis, as in the case of Vogt–Koyanagi–Harada disease (see Chapter 75, Vogt–Koyanagi–Harada disease). Not infrequently, it is misdiagnosed for a rhegmatogenous retinal detachment (RRD). However, the management of SRD is entirely pharmacologic, aimed at controlling the intraocular inflammation, and not surgical. The visual prognosis in most cases of SRD is excellent provided that the immunosuppressive agents have been introduced at a sufficient dose to lead to rapid resolution, and tapering is done slowly. RRD in the setting of active uveitis is thankfully rare, but its presence, even with modern vitreoretinal surgical techniques, results in a guarded visual outcome. Tractional retinal detachment (TRD) is possible in both infectious and noninfectious uveitis whenever vitreous inflammation/organization develops in an eye without posterior vitreous detachment (PVD) or when proliferative vitreoretinopathy (PVR) complicates the course of intraocular inflammation. Combined forms of RD are also possible; it is crucial to establish the exact mechanism of visual deterioration in every single case, allowing a better therapeutic approach.
Epidemiology
A systematic review of population-based studies on RRD published between 1970 and 2009 found a reported incidence between 6.3 and 17.9 for 100 000 people/year, with significant geographical variations.1 It is widely accepted that RRD occurrence varies with ethnicity and is strongly associated with increasing age, myopia, vitreoretinal degeneration and following cataract surgery.2,3 Uveitis itself has not been considered among the predisposing conditions to develop RRD. However, a retrospective study conducted in the Netherlands found a prevalence of RRD of 3.1% among 1387 uveitic patients, suggesting inflammation as an independent risk factor for its development.4
Not all types of uveitis carry the same risk of RRD; this complication is most commonly seen in posterior uveitis from infectious etiology, with the highest risk in viral retinitis cases. Acute retinal necrosis (ARN) (see Chapter 88, Acute retinal necrosis syndrome) evolves to RRD in more than 50% of cases, in a median time of 53 days after presentation.5 This risk is even higher for progressive outer retinal necrosis (PORN), in which almost 70% of affected eyes will develop RRD soon after presentation.6 For cytomegalovirus (CMV) retinitis (see Chapter 81, HIV-associated infections), even though the course of the disease has positively changed with the introduction of the HAART therapy, the rate of RRD remains as high as 2.3/100 eye-years.7
Parasitic infections can lead to RD when a choroidal process extends to the retina and vitreous or when a retinal process causes overlying vitreous to condense and contract. Toxoplasmic retinochoroiditis (see Chapter 85, Ocular toxoplasmosis) presents a risk of RRD of 3.5–6%.8–10 In the subset of patients addressed for vitreoretinal surgery, Adan et al. found 53.3% of cases of RD, most of them with pure RRD, but also some cases of combined tractional and RRD.11 Exudative detachments are rarely associated with ocular toxoplasmosis but have been cited in the literature.12,13 Vision-threatening features in ocular toxocariasis (OT) (see Chapter 86, Helminthic disease) are mainly severe vitritis, cystoid macular edema and tractional detachment of the macula. A retrospective series on OT found a prevalence of macular traction of about 30%, regardless of the site where the choroidal granuloma was located. In peripheral lesions with retinal traction, TRD occurs in about 40% of cases.4,14
Among noninfectious uveitis, the risk of RRD is less than 1%. Most patients developing retinal tears and detachments appear to do so as a result of concomitant retinal pathology, often in areas unrelated to sites of inflammation. However, inflammatory processes affecting the vitreous base may present an exception to this rule. Pars planitis, when untreated for a prolonged period of time or if severe, is known to develop TRD15 and retinoschisis.16 The differentiation between true retinoschisis and TRD may sometimes be very difficult, especially for those lesions situated in the far periphery.17 Sarcoidosis is also known to develop various forms of RD (see Chapter 78, Sarcoidosis), though this complication is rare in this condition. Serous RD,18 RRD,19 retinal pigment epithelium (RPE) detachments,20 and even a rare case of necrotizing retinitis with subsequent RRD,21 were described in the context of ocular sarcoidosis, but mostly as isolated cases. Retinal tears and RRD are rarely found in relation to Behçet’s disease, even though detailed fundoscopy should be done periodically in these patients, mainly when vitreous inflammation is important. A case of RRD secondary to a macular hole in a young patient with Behçet uveitis22 has been reported, stressing the role of recurrent vitritis in the pathogenesis of this complication (see Chapter 77, Autoimmune retinopathies).
Pathophysiology
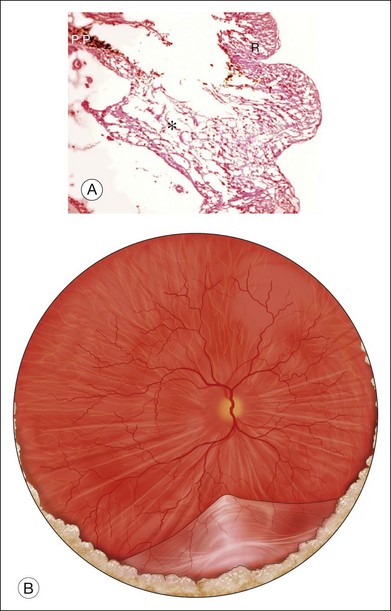
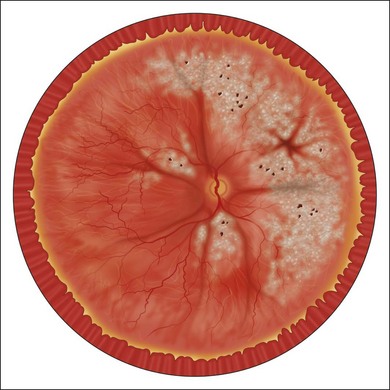
The nature of the inflammatory or infectious process determines the mechanism by which the detachment occurs, and provides an indication as to the means by which it can be resolved. Serous RD is usually the result of an immune-mediated assault to the choroid or the RPE. Its follows from loss of the tight junctions between retinal endothelial cells, leading to increased outward flow of fluid from retinal vasculature, and a failure of the compensatory pump function of RPE cells. Disruption of the normal oncotic gradient between the vitreous cavity and the choroid may also compromise passive transfer mechanisms responsible for a good portion of normal fluid flow to the choroid. Inflammatory mediators modulate vascular permeability and may have a role on the modulation of chloride receptors such as CLIC4 necessary for retinal attachment.23,24 Resolution of a serous detachment requires one to re-establish the status quo by inhibiting both humoral and cellular inflammatory mechanisms through appropriate systemic or local immunosuppression (Fig. 112.1).
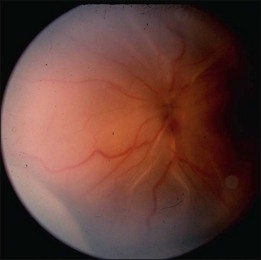
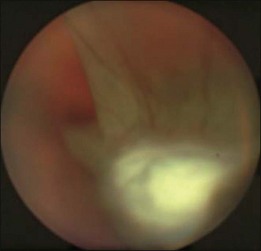
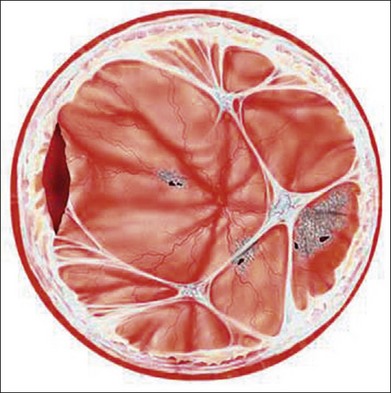
In the case of both RRD and TRD, vitreous body traction plays a predominant role. In RRD associated with toxoplasmosis, active toxoplasmic retinochoroiditis often precedes or is concurrent with the development of the RRD.8 Inflammation not only enhances vitreous liquefaction but also leads to cross-linking between collagen fibrils, particularly those close to the retinal interface, leading to the formation of an anomalous PVD and retinal tears.25,26 Vitreal strands between the optic disk and the ocular toxocariasis granuloma are present in nearly 50% of patients (Fig. 112.2). These strands are often the source of retinal traction and detachment. Contraction of the vitreous base, particularly seen in chronic longstanding uveitis, leads to the development of fibrous or fibrovascular bands, which can be very adherent to the underlying retina.26,27 Their removal can be quite challenging at the time of surgery (Fig. 112.3).
High levels of s-ICAM1 (type 1 soluble intercellular adhesion molecule) are found in vitreous from uveitic eyes complicated by RD similar to what has been observed in cases of proliferative vitreoretinopathy (PVR) where it is felt to perpetuate a cytokine-mediated vascular reaction which may contribute to PVR formation.28–30 It correlates positively with vitreous levels of TNF-α (tumor necrosis factor-alpha), a cytokine with a key role in the initiation of the inflammatory cascade and the pathogenesis of uveitis.31
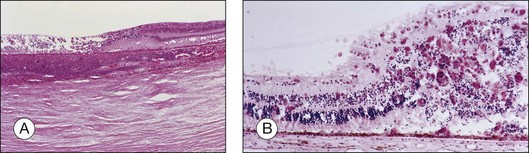
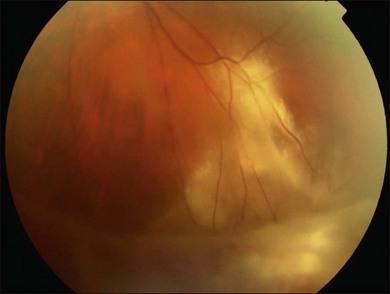
Herpetic retinal infections (CMV, HSV, VZV) are characterized by retinal necrosis leaving in its wake a diaphanous glial membrane (Fig. 112.4). Depending on the interplay between the virus and the host immune system, it is associated with moderate to severe vitreous inflammation. Cytomegalovirus retinitis is quintessentially seen in the context of AIDS or severe immunosuppression. It still today remains an AIDS-defining infection but is also seen in patients who fail to respond, become intolerant of, or stop responding to highly active antiretroviral therapy (HAART). Traditionally, it was not associated with vitreous inflammation and therefore, detachments were rarely complicated by proliferative vitreoretinopathy. In the HAART era, the frequency of an inflammatory response, late detachments and PVR has increased with some series reporting up to 30% of CMV retinitis cases complicated by PVR.32 The incidence of RD is highest among newly diagnosed patients (within 45 days of diagnosing a CMV retinitis) at 4.9/100 eye years, while the overall rate among AIDS patients with CMV retinitis is 2.3/100 eye years.7 Additional risk factors for RD include large lesion size, anterior lesion location, associated retinal pathology (e.g., myopia) and older age.33,34 Local and systemic therapy might reduce slightly the risk of RD possibly due to a more rapid resolution of retinitis.35 Most detachments occur once the initial infection has subsided. The healed retinitis leaves a thin glial sheet above which an epiretinal membrane, possibly containing condensed vitreous, can be found (Fig. 112.5).36 Vitreoretinal gliosis is often present at the interface between normal and abnormal retina on optical coherence tomography (OCT) examination but is difficult to visualize clinically. It is possible that the gliosis contributes to the formation of retinal detachments in these patients.
Acute retinal necrosis (ARN) is characterized by large, peripheral, often confluent zones of retinitis.37–39 It is pathologically characterized by full-thickness retinal necrosis and occlusive vasculitis (Fig. 112.4B).40 Vitreous involvement, often not present initially, develops within a few days and can obliterate the view of the posterior pole. A more aggressive form starting at the posterior pole called progressive outer retinal necrosis (PORN) affects also all retinal layers and represents an ophthalmic emergency since it may lead to complete blindness within hours if not treated aggressively.41–43 In both cases, detachments occur due to vitreous traction on the atrophic retina, sometimes following the occurrence of a PVD and when severe inflammation is present, they may occur even before resolution of the active retinitis. Retinal detachments in ARN are frequently complicated by PVR, which is often extensive at the vitreous base. Retinal breaks are usually multiple and located at the juncture between normal and gliotic retina. Vitreoretinal adhesions are common and the retinal detachment has both tractional and rhegmatogenous components (Fig. 112.6).
Clinical examination and findings
As stated above, RDs in uveitis can arise in a number of ways. Not infrequently, TRD, even schisis cavities, can be misdiagnosed as RRD (Fig. 112.7).16 As part of the initial work-up, it is important to establish the nature of the detachment, the etiology and severity of ocular inflammation or infection, and the status of the retina and vitreous. In particular, it is important to determine the relationship between the detached retina and the area of retinal inflammation or infection, the presence or absence of a PVD, and the presence of peripheral vitreous base fibrosis and foreshortening. These observations and work-up are often hampered by poor visualization resulting from posterior synechiae, cataract, vitreous haze or cellular infiltration. In infectious cases, particularly ARN, vitreous inflammation may increase shortly after initiating treatment to the point where it is impossible to visualize the fundus. Analogous to trauma cases, it is important in these cases to document, as early as possible, findings in the vitreous and retina.
When visualization becomes inadequate, ancillary tests may be required. To evaluate the macular area through a small pupil, a nonmydriatic camera, SLO (scanning laser ophthalmoscopy), or OCT may be useful. Unfortunately, they do not give any information on the condition of the peripheral retina. B-scan ultrasonography (both 10 and 50 MHz) is probably the most useful tool for the evaluation of the peripheral fundus. Uveitis-related RD often has a highly reflective posterior hyaloid when detached (which can even mimic an RD).44 Occasionally, during active inflammation, fine echoes within the retrohyaloid space are visible, corresponding to inflammatory cells within the vitreous. If visualization of the vitreoretinal interface becomes difficult and therapy with intravitreal steroids is envisaged, triamcinolone will improve the ultrasound signal and facilitate visualization of posterior structures.45 The presence of a thickened choroid (≥2 mm thickness) and the asymmetry between the two eyes can help confirming the diagnosis of a choroidal inflammation in the involved eye.44 Choroidal detachments are not infrequent, and their anterior extent needs to be defined.
Ciliary body detachments may develop as a result of uveitis, causing or predisposing to hypotony. The UBM (ultrabiomicroscopy) is the preferred tool to visualize the ciliary body. Using a UBM, it is also possible to assess the peripheral vitreous, in particular if it has become adherent to the posterior iris surface causing posterior displacement of the iris plane (an indirect sign of vitreous base fibrosis and likely foreshortening of the retina) (Fig. 112.8).46,47
Management
RD with active inflammation
Results of surgery performed on retinal detachments during active inflammation have generally been poor.4,48,49 Inflammation leads to poor postoperative visualization and development of fresh membranes on the retinal surface and in the retrolenticular space. It is crucial to adequately and aggressively manage the inflammatory component with a combination of systemic and local therapy. Systemic therapy should be initiated prior to surgery. The choice will depend on the nature of the inflammation and previous response to treatment. If the iris can be manipulated during surgery, prostaglandin inhibitors should be added topically as well as systemically starting the day prior to surgery. This can significantly reduce anterior segment inflammation and is commonly done in cataract surgery on uveitis patients.50 At the end of surgery, periocular and intraocular steroids should be considered. The use of a long-acting steroid preparation might reduce the severity of any recurrence developing several days or weeks after surgery.
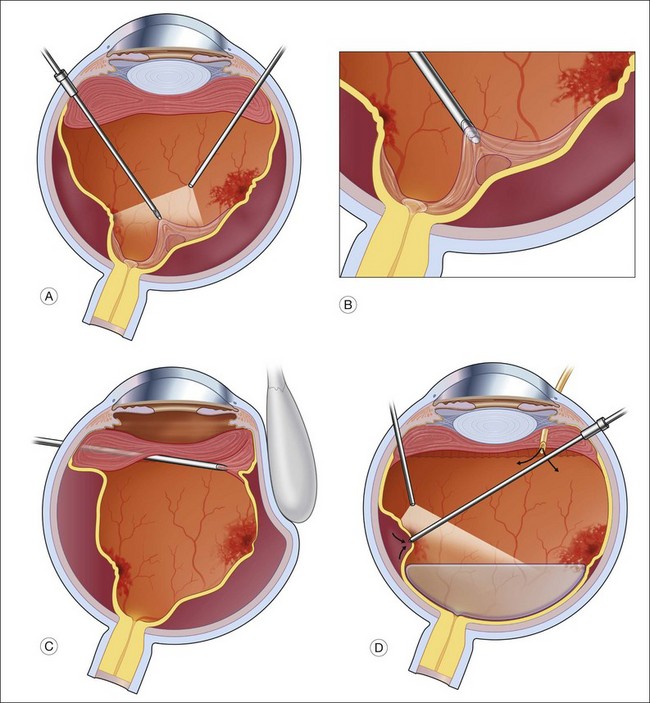
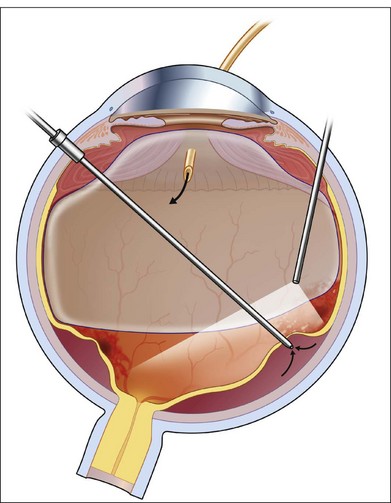
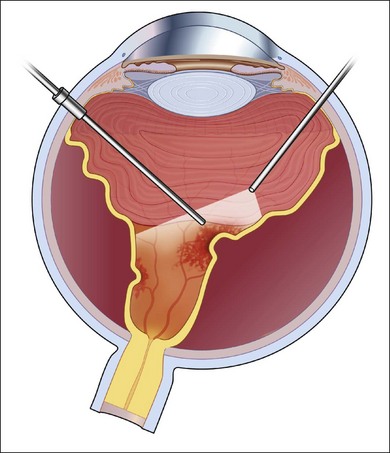
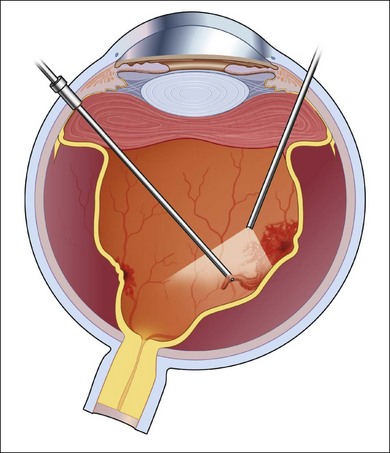
In general, better surgical success is obtained when a more aggressive approach is taken. The lens, if present, is removed and an IOL is placed in the bag. Following a standard pars plana vitrectomy, which preferably is performed with small-gauge surgery, a posterior vitreous detachment is induced and carried out to the vitreous base (Fig. 112.9A). This can be facilitated by the use of intravitreal triamcinolone or a coloring agent. If it is not possible to remove the posterior hyaloid completely, the vitreous is shaven down to the retinal surface (Fig. 112.9B). This is particularly important at the vitreous base (Fig. 112.9C), as there will be a tendency for any remaining scaffold to contract and pull on the ciliary body and peripheral retina potentially leading to hypotony and/or recurrent detachments (Fig. 112.8). Membranes present on the retinal surface are peeled; given their tenacity, this is often best done using a bimanual technique with a pic and a forceps (Fig. 112.9D).
Illumination is provided by a chandelier or transscleral illumination system. Peeling is also facilitated by using perfluorocarbon, which acts as a “third” arm, providing posterior stabilization to the membranes being peeled. Once the retina is fully reattached under perfluorocarbon, to avoid aggravating existing inflammation, the edges of the tears are treated by photocoagulation. Cryotherapy should be avoided, as it enhances breakdown of the blood–ocular barrier and can increase postoperative inflammation.51 Silicone oil is the preferred tamponading agent, as it will allow continued visualization of the retinal structures while the inflammation is brought under control (Fig. 112.10). It also limits the accumulation of proinflammatory cytokines in the vitreous cavity.52 The oil can be removed when the inflammation is brought fully under control.
Persistent inflammatory SRD
When inflammation has already been controlled and SRD persists after at least 3 months of corticosteroid and/or immunosuppressive treatment, surgical drainage may be considered to hasten recovery in cases with macular involvement and decreased vision. Galor et al.53 reported on five cases of persistent SRD surgically drained through a technique involving a complete vitrectomy, a drainage retinotomy placed peripherally and superior, use of perfluorocarbon to assist drainage followed by endolaser and a gas tamponade. In all cases, a scleral buckle was used to provide peripheral support in the area of drainage. In our own experience, the buckle is not required, provided tamponade can be achieved until the laser forms a firm adhesion between the retina and choroid. As indicated by the authors, this approach is meant to drain persistent subretinal fluid in patients with quiescent inflammation. If inflammation persists, the subretinal fluid will reaccumulate.
RD with retinal necrosis
Cytomegalovirus retinitis
In selected cases of CMV-associated retinal detachments, photocoagulation may be effective in delimiting or demarcating the retinal detachment.54–56 It can lead to a significant delay in macular detachment. Delay in intraocular surgery in these patients is a valid strategy, as following successful reattachment, several studies have suggested a variable loss of vision possibly related to microangiopathy, optic neuropathy or silicone-oil-related toxicity.57–61 Once a surgical option is chosen, retinal detachments due to CMV, whether or not associated with PVR, should be approached in a standardized fashion. The risk of recurrence or progression of CMV should be assessed in conjunction with the treating internist as it will determine the choice of tamponade and the need for concurrent cataract extraction.
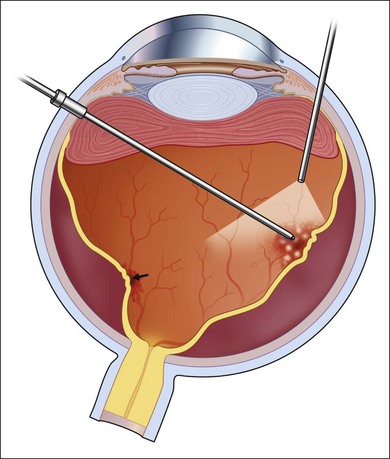
The surgery begins with a standard three-port vitrectomy (Fig. 112.9). When pre-existing vitreous separation is present, the posterior hyaloid is identified and resected up to the posterior margin of the vitreous base. In some cases, however, the vitreous has not detached posteriorly. If it can be mechanically engaged and easily elevated from the retina, this is done to create a posterior vitreous separation. In some cases, this technical separation cannot be safely accomplished. An attempt should be made to resect the vitreous as close to the surface of the retina as possible, without inducing a vitreous detachment. In cases in which CMV has caused necrosis of a large area of retina, an en bloc dissection of diaphanous retina and vitreous is possible but should in most cases follow separation of the vitreous from surrounding healthy retina, or at least after it has been shaven off the retinal surface. The edges of retinal breaks or the area of resection are marked with intraocular diathermy (Fig. 112.11). This makes later identification easier. Internal drainage of subretinal fluid in conjunction with air–fluid or fluid–silicone exchange is performed to flatten the retina (Fig. 112.10). The retinal breaks are surrounded with endophotocoagulation (Fig. 112.12).
Many surgeons advocate demarcating the atrophic areas with laser, as well as those regions that are likely to harbor retinal breaks. In certain circumstances, scatter photocoagulation applied to the lower hemisphere up to the edge of the inferior vascular arcade, can help mitigate the appearance of a later detachment, particularly if CMV progression in this area is expected in the coming months. A silicone–air exchange or air–gas exchange is performed at this point. The choice will depend on the configuration of the detachment, and the likelihood of recurrent activity. If progression is likely, silicone oil is a better and more permanent choice. Postoperatively, the patient is kept facedown for 12 hours, after which time special positioning is not necessary. Careful follow-up is required postoperatively to observe for the complications of silicone oil, to monitor for recurrence of infection, and to detect recurrent retinal detachment.34,57,58,60–65
Lensectomy may be necessary with or without implantation of an intraocular lens, maintaining the posterior capsule. Later capsular opacification is common, however, and one should consider primary capsulotomy in selected cases.66 An inferior iridectomy must be performed in aphakic eyes and should be considered in pseudophakic eyes to prevent pupillary block.
If intraocular lens implantation is performed primarily (to correct for anisometropia), later at reoperation, or to correct for a visually significant postoperative cataract, the appropriate intraocular lens should be chosen.67,68 A planoconvex polymethylmethacrylate (PMMA) or hydrophobic acrylic intraocular lens provides the ideal optical correction. Engstrom et al. reported satisfactory results with simultaneous lensectomy and intraocular lens implantation at the time of vitrectomy and silicone oil injection.67 This strategy is used to avoid silicone-oil-induced anisometropia and to obviate the need for later cataract removal, as silicone oil almost always induces cataract formation.66
Acute retinal necrosis
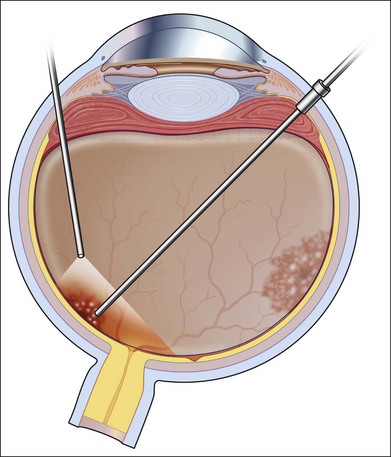
A standard three-port vitrectomy is performed (Fig. 112.9). As in CMV cases, it is important to keep the infusion pressure low. The optic nerve in these patients does not tolerate standard infusion pressures. Looking at the nerve to avoid a pulsatile perfusion at the onset of surgery, and periodically during surgery, can avoid further loss of vision from prolonged retina anoxia. In most cases, the crystalline lens and in some cases the intraocular lens will be removed to expose the full extent of the vitreous base so that all scarring can be removed and basal vitreous traction can be eliminated.
In almost all cases, a spontaneous posterior vitreous detachment has occurred. The vitreous gel must be resected out to the margin between healthy and atrophic retina or to the posterior margin of the vitreous base (Fig. 112.13). Posterior epiretinal membranes overlying healthy retina are identified and removed with picks and forceps. A chandelier lighting system may facilitate this maneuver (Fig. 112.14).
In managing PORN-related retinal detachments, the surgical principles are identical. The extent of the necrosis, however, is almost always greater than in patients with ARN-related retinal detachments, so extra care must be taken with vitreous manipulation, and the amount of photocoagulation required is often much more extensive in an effort to surround and preserve the macular region.41,69
1 Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94:678–684.
2 Mitry D, Charteris DG, Yorston D, et al. The epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based study. Invest Ophthalmol Vis Sci. 2010;51:4963–4968.
3 Mitry D, Singh J, Yorston D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118:1429–1434.
4 Kerkhoff FT, Lamberts QJ, van den Biesen PR, et al. Rhegmatogenous retinal detachment and uveitis. Ophthalmology. 2003;110:427–431.
5 Carifi G, Onyema L. Acute retinal necrosis. Ophthalmology. 2010;117:1659.
6 Holland G. The progressive outer retinal necrosis syndrome. Int Ophthalmol. 1994;18:163–165.
7 Jabs DA, Ahuja A, Van Natta M, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152–2161. e2151–e2152
8 Bosch-Driessen LH, Karimi S, Stilma JS, et al. Retinal detachment in ocular toxoplasmosis. Ophthalmology. 2000;107:36–40.
9 Friedman C, Knox D. Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol. 1969;81:481–493.
10 Mets MB, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–324.
11 Adan A, Giralt J, Alvarez G, et al. Pars plana vitrectomy for vitreoretinal complications of ocular toxoplasmosis. Eur J Ophthalmol. 2009;19:1039–1043.
12 Frezzotti R, Gerengo A, Guerra F. Toxoplasmic Coats’ Retinitis. A parasitologically proven case. Am J Ophthalmol. 1965;59:1099–1102.
13 Rieger H. On the etiology of retinitis exudativa externa centralis. Graefes Arch Clin Exp Ophthalmol. 1960;162:178–192.
14 Stewart J, Cubillan L, Cunningham E, Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005;25:1005–1013.
15 Brockhurst RJ. Retinoschisis. Complication of peripheral uveitis. Arch Ophthalmol. 1981;99:1998–1999.
16 Pollack AL, McDonald HR, Johnson RN, et al. Peripheral retinoschisis and exudative retinal detachment in pars planitis. Retina. 2002;22:719–722.
17 Jalil A, Dhawahir-Scala FE, Jones NP. Nonprogressive tractional inferior retinal elevation in intermediate uveitis. Ocul Immunol Inflamm. 2010;18:60–63.
18 Watts P, Mantry S, Austin M. Serous retinal detachment at the macula in sarcoidosis. Am J Ophthalmol. 2000;129:262–264.
19 Straub W, Strempel I. Retinal detachment and sarcoidosis. Bull Soc Ophtalmol Fr. 1989;89:1013–1015.
20 Bourcier T, Lumbroso L, Cassoux N, et al. Retinal pigment epithelial detachment: an unusual presentation of ocular sarcoidosis. Br J Ophthalmol. 1998;82:585.
21 Shenoy R, Al Burwani B. Necrotizing retinopathy simulating acute retinal necrosis causing rhegmatogenous retinal detachment in sarcoidosis: a case report. Eur J Ophthalmol. 2010;20:218–220.
22 Georgalas I, Markomichelakis N, Ladas I. Retinal detachment due to a macular hole in a patient with Behçet disease treated with vitrectomy and silicone oil tamponade. Eur J Ophthalmol. 2008;18:1023–1024.
23 Chuang JZ, Chou SY, Sung CH. Chloride intracellular channel 4 is critical for the epithelial morphogenesis of RPE cells and retinal attachment. Mol Biol Cell. 2010;21:3017–3028.
24 He G, Ma Y, Chou SY, et al. Role of CLIC4 in the host innate responses to bacterial lipopolysaccharide. Eur J Immunol. 2011;41:1221–1230.
25 Hikichi T, Ueno N, Chakrabarti B, et al. Evidence of cross-link formation of vitreous collagen during experimental ocular inflammation. Graefe’s Arch Clin Exp Ophthalmol. 1996;234:47–54.
26 Hirokawa H, Takahashi M, Trempe CL. Vitreous changes in peripheral uveitis. Arch Ophthalmol. 1985:103.
27 Spencer W. Vitreous. Ophthalmic pathology. An atlas and textbook. Spencer WH, ed. Ophthalmic pathology. An atlas and textbook. Philadelphia: WB Saunders; 1996;Vol. 2:650–654.
28 Limb GA, Chignell AH, Cole CJ, et al. Intercellular adhesion molecule-1 in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1997;38:1043–1048.
29 Ricker LJ, Altara R, Goezinne F, et al. Soluble apoptotic factors and adhesion molecules in rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:4256–4262.
30 Webster L, Stanbury RM, Chignell AH, et al. Vitreous intercellular adhesion molecule 1 in uveitis complicated by retinal detachment. Br J Ophthalmol. 1998;82:438–443.
31 Sijssens KM, Rijkers GT, Rothova A, et al. Cytokines, chemokines and soluble adhesion molecules in aqueous humor of children with uveitis. Exp Eye Res. 2007;85:443–449.
32 Kunavisarut P, Bijlsma WR, Pathanapitoon K, et al. Proliferative vitreoretinopathy in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2010;150:218–222.
33 Freeman WR, Friedberg DN, Berry C. Risk factors for development of rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis. Am J Ophthalmol. 1993;116:713–720.
34 Irvine AR, Lonn L, Schwartz DM, et al. Retinal detachment in AIDS: long term results after repair with silicone oil. Br J Ophthalmol. 1997;81:180–183.
35 Young S, McCluskey PJ, Minassian DC, et al. Retinal detachment in cytomegalovirus retinitis: intravenous versus intravitreal therapy. Clin Exp Ophthalmol. 2003;31:96–102.
36 Brar M, Kozak I, Freeman WR, et al. Vitreoretinal interface abnormalities in healed cytomegalovirus retinitis. Retina. 2010;30:1262–1266.
37 Culbertson WW, Atherton SS. Acute retinal necrosis and similar retinitis syndromes. Int Ophthalmol Clin. 1993;33:129–143.
38 Fisher JP, Lewis ML, Blumenkranz M, et al. The acute retinal necrosis syndrome. Part 1. Clinical manifestations. Ophthalmology. 1982;89:1309–1316.
39 Lau CH, Missotten T, Salzmann J, et al. Acute retinal necrosis: features, management, and outcomes. Ophthalmology. 2007;114:756–762. e751
40 Culbertson W, Blumenkranz M, Haines M, et al. The acute retinal necrosis syndrome. II. Histopathology and etiology. Ophthalmology. 1982;89:1317–1325.
41 Engstrom R, Holland G, Margolis T, et al. The progressive outer retinal necrosis syndrome: a variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488–1502.
42 Forster DJ, Dugel PU, Frangieh GT, et al. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am J Ophthalmol. 1990;110:341–348.
43 Yin PD, Kurup SK, Fischer SH, et al. Progressive outer retinal necrosis in the era of highly active antiretroviral therapy: successful management with intravitreal injections and monitoring with quantitative PCR. J Clin Virol. 2007;38:254–259.
44 Satger D, Pegourie P, Romanet JP, et al. Ultrasound imaging in the management of endophthalmitis. J Fr Ophtalmol. 2007;30:1037–1048.
45 Mourtzoukos S, Mangouritsas G, Feretis E. Triamcinolone-assisted vitreous imaging using B-scan ultrasonography. Eur J Ophthalmol. 2008;18:1028–1030.
46 Inazumi K, Gentile RC, Lee KYC, et al. Ultrasound biomicroscopic diagnosis of cyclitic membranes. Am J Ophthalmol. 2001;131:446–450.
47 Liu W, Wu Q, Huang S, et al. Ultrasound biomicroscopic features of anterior proliferative vitreoretinopathy. Retina. 1999;19:204–212.
48 Hagler WS, Jarrett WH, II., Chang M. Rhegmatogenous retinal detachment following chorioretinal inflammatory disease. Am J Ophthalmol. 1978;86:373–379.
49 Jarrett WH, II. Rhegmatogenous retinal detachment complicated by severe intraocular inflammation, hypotony, and choroidal detachment. Trans Am Ophthalmol Soc. 1981;79:664–683.
50 Foster CS, Fong LP, Singh G. Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology. 1989;96:281–288.
51 Veckeneer M, van Overdam K, Bouwens D, et al. Randomized clinical trial of cryotherapy versus laser photocoagulation for retinopexy in conventional retinal detachment surgery. Am J Ophthalmol. 2001;132:343–347.
52 Aras C, Ozdamar A, Karacorlu M, et al. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int Ophthalmol. 2002;24:147–150.
53 Galor A, Lowder CY, Kaiser PK, et al. Surgical drainage of chronic serous retinal detachment associated with uveitis. Retina. 2008;28:282–288.
54 Davis JL, Hummer J, Feuer WJ. Laser photocoagulation for retinal detachments and retinal tears in cytomegalovirus retinitis. Ophthalmology. 1997;104:2053–2060.
55 Meffert SA, Ai E. Laser photocoagulation prophylaxis for CMV retinal detachments. Ophthalmology. 1998;105:1353–1355.
56 Vrabed T. Laser photocoagulation repair of macula-sparing cytomegalovirus-related retinal detachment. Ophthalmology. 1997;104:2062–2067.
57 Dugel PU, Liggett PE, Lee MB, et al. Repair of retinal detachment caused by cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1991;112:235–242.
58 Jabs DA, Enger C, Haller J, et al. Retinal detachments in patients with cytomegalovirus retinitis. Arch Ophthalmol. 1991;109:794–799.
59 Kuppermann BD, Flores-Aguilar M, Quiceno JI, et al. A masked prospective evaluation of outcome parameters for cytomegalovirus-related retinal detachment surgery in patients with acquired immune deficiency syndrome. Ophthalmology. 1994;101:46–55.
60 Regillo C, Vander J, Duker J, et al. Repair of retinitis-related retinal detachments with silicone oil in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:21–27.
61 Sidikaro Y, Silver L, Holland GN, et al. Rhegmatogenous retinal detachments in patients with AIDS and necrotizing retinal infections. Ophthalmology. 1991;98:129–135.
62 Canzano JC, Morse LS, Wendel RT. Surgical repair of cytomegalovirus-related retinal detachment without silicone oil in patients with AIDS. Retina. 1999;19:274–280.
63 Chuang EL, Davis JL. Management of retinal detachment associated with CMV retinitis in AIDS patients. Eye. 1992;6:28–34.
64 Freeman WR, Quiceno JI, Crapotta JA, et al. Surgical repair of rhegmatogenous retinal detachment in immunosuppressed patients with cytomegalovirus retinitis. Ophthalmology. 1991;99:466–474.
65 Orellana J, Teich SA, Lieberman RM, et al. Treatment of retinal detachments in patients with the acquired immune deficiency syndrome. Ophthalmology. 1991;98:939–943.
66 Tanna A, Kempen J, Dunn J, et al. Incidence and management of cataract after retinal detachment repair with silicone oil in immune compromised patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;136:1009–1015.
67 Engstrom R, Goldenberg D, Parnell J, et al. Clear lens extraction with intraocular lens implantation during retinal detachment repair in patients with autoimmune deficiency syndrome and cytomegalovirus retinitis. Ophthalmology. 2002;109:666–673.
68 Meldrum M, Aaberg T, Patel A, et al. Cataract extraction after silicone oil repair of retinal detachment due to necrotizing retinitis. Arch Ophthalmol. 1996;114:885–892.
69 Dowling J, Towler H, Mitchell S, et al. Retinal detachment and herpesvirus retinitis in patients with AIDS. Br J Ophthalmol. 1995;79:575–580.