Chapter 55 Macular Telangiectasia
History, nomenclature, and classification of macular telangiectasia
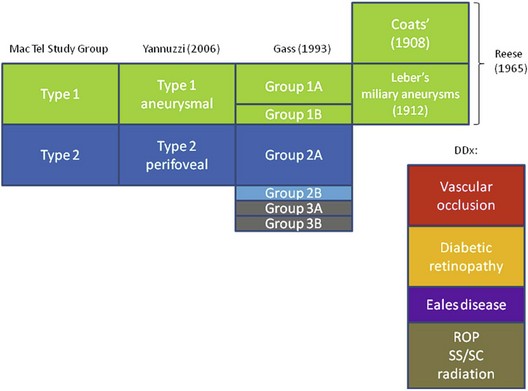
The concept of “retinal telangiectasia” has been known for over a century. First alluded to by Graefe in 1808 as “Telangiectasie der Retina” and/or “Aneurismen der Zentralarterie,” it was used to describe dilated retinal vessels on all three components of the circulation, arterioles, capillaries, and venules for a variety of abnormalities, mostly cutaneous and cutaneous-ocular in nature.1 One hundred years later, in 1908, Coats described “exudative retinitis,” which was best characterized as vascular abnormalities with massive subretinal exudation.2 Today, congenital telangiectasia goes by his name, Coats disease, and it consists of dilated retinal capillaries, micro- and macroaneurysms, ischemia, nonperfusion, and retinal vascular leakage, usually unilateral in males. Four years after Coats’ original description, Leber described “miliary aneurysms,”3 which were quite similar clinically to cases seen by Coats. It was not until 1956 that Reese suggested that Coats disease and Leber miliary aneurysms were one and the same, occurring either in child or adulthood.4 By 1968, Gass acknowledged the contribution by Reese, suggesting that Leber and Coats were indeed manifestations along the spectrum of one disease, but Gass also introduced a new distinct entity, which he called idiopathic juxtafoveolar retinal telangiectasis (IJRT),5 in order to differentiate it from Coats disease. This resulted in an extensive, detailed classification in 1993.6 Table 55.1 puts the various diseases and their classifications in context.
Table 55.1 Various Classifications of Macular Telangiectasia (Types 1 and 2) and the Differential Diagnoses
DDx, differential diagnosis; ROP, retinopathy of prematurity; SS, sickle cell disease; SC, sickle cell trait.
Classification
Gass classified what had previously been known as Leber miliary aneurysms into IJRT groups 1A and B; this distinction proved to be useful in terms of prognosis and therapy. IJRT group 2 was to include the common bilateral disease seen in older and elderly patients, but later a group 2B was created to accommodate two brothers, who developed subretinal neovascularization, although these two remain the only cases fitting this category.6 Another group was introduced and subdivided into IJRT group 3A, which is characterized by telangiectatic changes, vascular occlusion, and minimal exudation in 3 patients, and group 3B, who had similar retinal changes but had additional neurological changes in 3 patients also.
Yannuzzi classification
In 2006, Yannuzzi discussed new diagnostic, adjunctive imaging systems such as optical coherence tomography (OCT) and high-speed stereo angiography in a cohort of 36 patients with mac tel of various types. He introduced the name “idiopathic macular telangiectasia.”7 He proposed to pool together mac tel types 1 A and B and to classify them as “aneurysmal” telangiectasia. Gass group 2A mac tel was preserved but, because of the lack of subjects in the other categories, types 2B, 3A, and 3B were eliminated in this classification.
Epidemiology
Prevalence of disease: estimates from population-based studies
Beaver dam eye study
The Beaver Dam Eye Study8 regraded the stereoscopic fundus photographs of the eyes of 4926 subjects aged 43–84, 99% of whom were white, for mac tel type 2. Five individuals, one woman and four men, were identified to have mac tel type 2, which translates into a prevalence of 0.1%. The average age was 63 years, with a range from 52 to 68 years. Bilateral manifestations were found in 2 patients.
Melbourne collaborative cohort study
This Australian study9 analyzed 22 415 Caucasians, with regard to the presence of typical features of mac tel type 2 on nonmydriatic color fundus photographs. Twelve patients with “possible” unilateral mac tel type 2 and 5 patients with signs of bilateral mac tel type 2 were identified. The average age amongst bilateral cases was 63 and ranged from 53 to 72 years. Three of the 5 bilateral cases were female. Only one “very convincing case with early gray sheen and telangiectatic vessels temporal to the fovea” was reported. The authors concluded that the incidence in their studied population ranged from 0.0045% to 0.022%, a prevalence which is significantly lower than the one found in the Beaver Dam Eye Study.
As noted by Klein et al., this lower prevalence estimate found in the Australian population might be a result of using a screening protocol in which images were only reassessed specifically for mac tel type 2 if a previous grading had suggested at least one feature of the disease8. The Beaver Dam Eye Study was conducted by regrading the entire cohort. It is possible that both studies have underestimated the true prevalence because only color fundus images were available. Other imaging technologies such as fluorescein angiography (FA), OCT, or fundus autofluorescence (FAF) have been demonstrated to be sensitive in detecting early and asymptomatic disease stages of mac tel type 2.10
Although Gass and Blodi did not find gender differences in their cohort of 140 patients (94 with mac tel type 2),6 the proportions of women reported by the Mac Tel Project (n = 310) and by Yannuzzi were about 64% and 58%, respectively.11 On average, the Mac Tel Project participants (mean age 61 ± 9 years) had their disease diagnosed at age 57 (± 9 years).
Clinical presentation
Fundus appearance
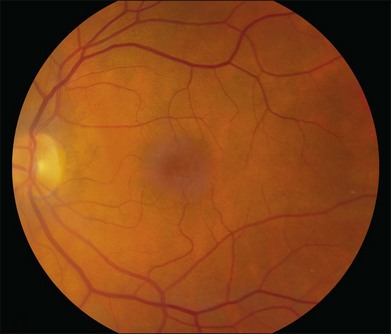
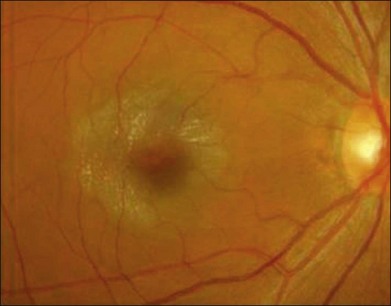
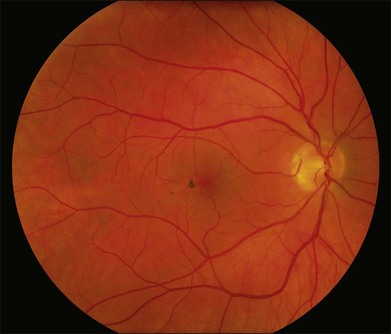
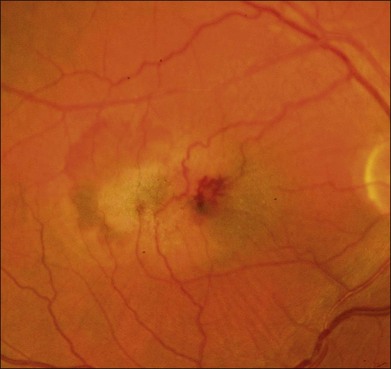
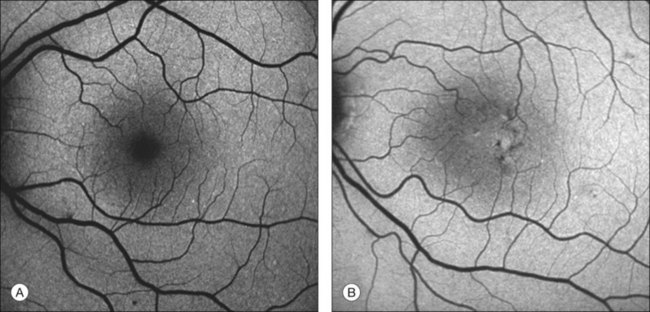
The disease tends to be bilateral, but one eye may be more advanced than the other. All lesions tend to begin temporal to the foveal center but may subsequently involve the entire parafoveolar area. The earliest fundoscopic manifestion of mac tel type 2 is a subtle loss of retinal transparency in the perifoveal region, beginning temporally (Fig. 55.1).7 This becomes more pronounced over time, and dilation of the parafoveal capillaries in the temporal parafoveal area ensues, and may extend to surround the fovea. These mildly ectatic capillaries were described to affect mainly the deeper capillary network (Fig. 55.2).6 However, others have identified involvement of both the inner and outer retinal circulation.7 In contrast to mac tel type 1, retinal hard exudate is not seen unless there is evidence of neovascularization. Crystalline deposits at the vitreoretinal interface may be seen throughout the course of the disease (Fig. 55.3).6,12
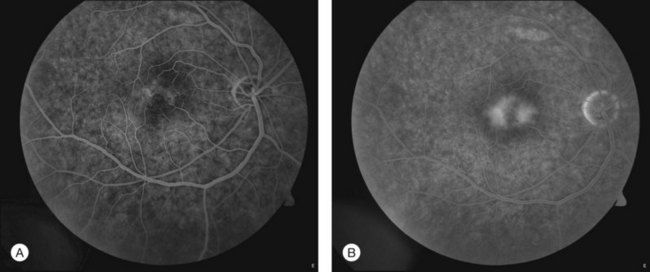
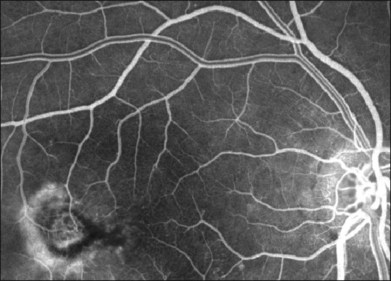
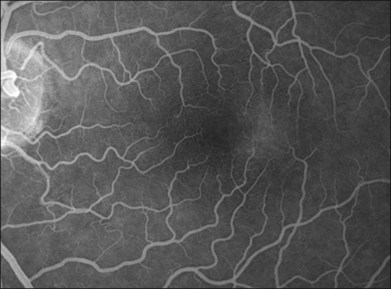
Fig. 55.2 (A) Early fluorescein angiographic findings of macular telangiectasia type 2 and (B) late leakage.
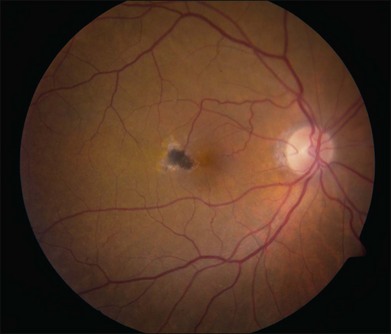
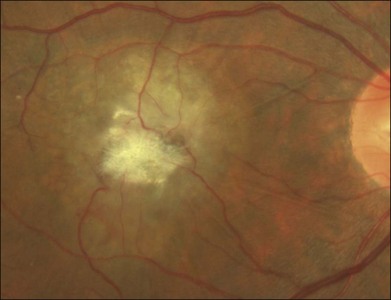
Blunted, dilated venules, either as single or multiple vessels, are often associated with ectatic capillaries. As vessels course towards the fovea, they usually decrease in diameter but, in mac tel type 2, they dilate and may make a right-angle turn, diving into the deeper retinal layers (Fig. 55.4). Eventually, intraretinal pigment migration and RPE hyperplasia along these diving dilated venules may occur7 (Fig. 55.5). In addition to RPE cell migration, atrophic changes in the neurosensory retina are another frequent finding.
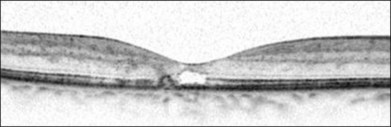
A yellow spot, or vitelliform lesion, in the center of the fovea with slight loss of the foveal depression may become apparent in some eyes.7 Other foveal changes include lamellar or full-thickness macular holes that have been detected on clinical exams and some confirmed by OCT imaging.7,13–17 The degeneration and atrophy associated with this disease contribute to the formation of such holes. Surgical repair may not necessarily result in either structural or functional improvement.
The development of neovascularization is often, but not always, preceded by the right-angle venule and the intraretinal pigment hyperplasia which is often temporal to the fovea.6,7 The neovascularization is most commonly seen temporal to the fovea.18 Retinal hard exudates, intraretinal edema, and subretinal or intraretinal hemorrhage may occur (Fig. 55.6). These neovascular complexes are retinal in origin, as seen by the feeder vessel from the retinal arteries and the drainage into venules (Fig. 55.7). This may be indistinguishable from choroidal neovascularization with chorioretinal anastomosis. A disciform scar may be the advanced stage of this process (Fig. 55.8).
Retinal imaging
Fundus autofluorescence
One of the earliest signs of mac tel type 2 is the loss of the hypofluorescent center seen normally on blue-light FAF due to the depletion of macular pigment in this condition (Fig. 55.9). Even in the absence of any fluorescein leakage or any other signs, especially in patients with asymmetrical mac tel type 2,10 this is diagnostic of the ocular condition. The area of retinal pigment hyperplasia will appear hypofluorescent on the FAF (Fig. 55.9).
Fluorescein angiography
The hallmark finding in mac tel type 2 has been the characteristic telangiectactic capillaries on FA, again starting predominantly temporal to the fovea (Fig. 55.10). Eventually, the entire parafoveal area is involved (Fig. 5.2). Stereoscopic angiography has demonstrated that the deeper vasculature is involved but more superficial capillaries may also contribute to the fluorescein leakage.6 Traditionally, the use of FA was essential in the diagnosis of mac tel type 2. However, OCT changes, as described below, may precede the development of any angiographic findings.
Optical coherence tomography
OCT may indeed provide valuable information as to the diagnosis and natural course of mac tel type 2 disease. The most subtle and earliest change may be the temporal enlargement of the foveal pit, resulting in an asymmetric pit with the temporal area being thinner than the nasal.10 There are corresponding changes to the outer nuclear and/or photoreceptor layer. With time, the disruption of the photoreceptor inner-segment–outer-segment junction is seen, again temporal to the fovea. With progression, there will be hyporeflective cavities in the inner retina and these may clinically be described as “pseudolamellar macular holes.” There are no pockets of leakage from the FA that correspond to these hyporeflective cavities. The disease progresses with hyporeflective cavities in the outer neurosensory retina and, eventually, atrophy (Fig. 55.11). The foci of retinal pigment hyperplasia manifest as hyperreflective intraretinal lesions that appear to migrate into the inner retinal layers, with associated posterior shadowing.
Adaptive optics imaging
Adaptive optics imaging, a technique that corrects for the optical aberrations of the eye and utilizes a scanning laser ophthalmoscope, allows evaluation of the cone photoreceptor mosaic.19 Cone density evaluations in individuals with mac tel have identified areas of paracentral cone loss despite the absence of capillary abnormalities seen on FA.20
Visual function
Metamorphopsia or a scotoma may be present in the early stages of the disease.13 When patients are asked specifically for symptoms of metamorphopsia, a majority will admit to such symptoms, even among those with early disease.21 Visual impairment may be mild; however, loss of vision in one eye is frequently reported.6 Vision may decrease gradually with the progression of the disease. Visual acuity less than 20/200 (legal blindness) is rare but may be seen in the advanced stages with marked atrophy of the central photoreceptors or secondary to a large area affected by neovascularization.6,7,18,22 In the Mac Tel Project, the mean visual acuity at baseline was 20/40 in 522 eyes that had not previously received therapy.11 Visual acuity was 20/20 or better in 16% and 20/32 or better in approximately 50%. The most common risk factors associated with lower visual acuity in this cohort were characteristics found in more advanced disease, namely retinal pigment hyperplasia and the right-angle venules. Similar visual acuity results were reported by Gass and Blodi.6 In a retrospective study, 25% of eyes (6/24) remained stable during a follow-up period of 10–17 years.22
Despite the mild visual impairment, the vision-related quality of life is impacted markedly. In the Mac Tel Project, the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) was administered to all participants.23 They reported significantly lower vision-related function in all domains compared to a group of participants in a study of age-related macular degeneration and who had similar visual acuities. A subset of these participants, enrolled in the Mac Tel Project, were tested using the Impact of Vision Impairment questionnaire.24 Similar results were seen in this second study.
Microperimetry
Another method of evaluating function is the use of microperimetry, which may help to correlate the structural changes with the functional changes.25–29 Retinal sensitivity defects appear to correlate with the outer retinal atrophy seen on OCT imaging. In areas of retinal pigment hyperplasia, there is often a dense scotoma. The use of microperimetry to correlate with changes seen on OCT may result in a reasonable outcome measurement for measuring changes over time, which may be especially useful for clinical trials that will be designed to test various therapies.
Staging and prognostic factors
From their extensive clinical experience and review of fundus photographs, Gass and Blodi concluded that various mac tel type 2 lesions appear in an orderly fashion over time.6 Some lesions, such as retinal opacification, may be easily missed because of image quality. There was no availability of noninvasive tools such as OCT or FAF to assist in their classification of this condition. Nevertheless, the authors had remarkable clinical acumen and developed a scale (shown in Table 55.2) that has been part of this landmark study. However, the present usefulness of this scale is somewhat limited, as it does not incorporate OCT. With further research into the natural history of mac tel type 2, another scale incorporating newer technologies may be of value. A major challenge for studying therapies for mac tel type 2 is the development of reproducible and clinically meaningful outcome measurements. When structural and functional correlations can be made, such outcome measurements may become more evident.
Table 55.2 Clinical Staging of Macular Telangiectasia Type 2 (Gass and Blodi)
| Stages of macular telangiectasia type 2* (idiopathic juxtafoveal retinal telangiectasis of Gass and Blodi)6 | |
|---|---|
| Stage 1 | No biomicroscopic abnormality, no or minimal capillary dilation, mild staining of outer perifoveal retina |
| Stage 2 | Slight graying of perifoveolar retina, no or minimal biomicroscopically visible telangiectatic vessels, but capillary telangiectasis of outer capillary network temporally on fundus autofluorescence |
| Stage 3 | One or several slightly dilated and blunted retinal venules descending into outer perifovea, typically temporally |
| Stage 4 | Pigment hyperplasia, often surrounding right-angle venules |
| Stage 5 | Subretinal neovascularization, often in proximity to intraretinal pigment migration |
* Based on biomicroscopy and stereoscopic fluorescein angiography.
Genetics
The occurrence of this condition in monozygotic twins,10,30–32 siblings, and families6,10,33–36 suggests a genetic association in mac tel type 2. A vertical transmission pattern would also suggest an autosomal dominant pattern, but there may be variable expressivity or penetrance with monozygotic twins showing different disease severities.10 Environmental influences or gene-to-gene interaction might account for this variation. The ataxia telangiectasia mutated gene, responsible for the ataxia telangiectasia syndrome, has been implicated in the pathogenesis of mac tel type 2.37,38 A number of candidate genes known to cause other retinal diseases, such as those causing familial exudative vitreoretinopathy and Norrie disease, or those with a role in retinal neovascularization or macular pigment metabolism, have recently been excluded to play a role in the pathogenesis of mac tel type 2.39 Evaluation of a gene that might be involved with xanthophyll transport also demonstrated no statistically significant association.40
Association of systemic diseases
Early reports have implicated an increased risk of diabetes in patients with mac tel type 2.33,41 The Mac Tel Project, the largest population of mac tel type 2 patients to date, found a high prevalence of diabetes mellitus (28%) and also hypertension (52%).11 Strikingly similar numbers were reported for diabetes and hypertension in another series.38 These risk factors may further our understanding of the pathogenesis of this condition.
Clinicopathological correlation
It is curious that vascular changes are not prominent features of early mac tel type 2, but blunting of the foveal reflex is typical. Based on the staining of the retina with fluorescein in eyes with minimal edema, Gass has postulated that it is possible that the primary abnormality may be found in the parafoveal retinal neural or Müller cells.42 This is illustrated in a case where OCT and ERG changes in the form of inner-lamellar holes and reduced cone responses manifested before the development of typical vascular changes.43 Xanthophyll is probably primarily stored in Müller cells, and this could be a surrogate measure of the health and concentration of Müller cells in that region. Xanthophyll concentration is already diminished in early mac tel.44 Histopathological examination of the eyes of a 58-year-old woman with mac tel type 2 did not demonstrate telangiectatic capillaries, but did show narrowing of the vessel diameter. Instead, pericyte degeneration and lipid accumulation within the capillary walls, as well as multilaminated basement membrane, were found45 – all similar to those seen in diabetics and prediabetics. Another histopathological study46 demonstrated dilation and proliferation of retinal capillaries into the outer retinal, subretinal, and preretinal spaces. Perivascular pigment migration along the telangiectatic vessels was also seen. A sharp demarcation between the edematous and nonedematous retina was present and involved all layers of the retina, including the nerve fiber layer and the ganglion cell layer. The most detailed histopathological study to date examined the eye of a 65-year-old patient with mac tel type 2 and found reduced expression of Müller cell-specific markers in the fovea, which correlated with macroscopically visible pigment depletion in this area.47
Therapeutic options
There are no generally accepted therapies for lesions of mac tel type 2 not associated with neovascularization. There are anecdotal case reports, but no controlled randomized clinical trials conducted in this ocular condition. Laser photocoagulation48 or photodynamic therapy49 does not appear to improve or stabilize visual acuity in nonneovascular mac tel type 2. Anti-angiogenic agents seem to be equally ineffective in most reported series despite angiographic and tomographic effects,50,51 although individual patients may experience a functional benefit.52 The same seems to be true for intravitreal injections of triamcinolone acetonide.53
For neovascular complications of mac tel type 2, transpupillary therapy54,55 and photodynamic therapy56 have been reported to be somewhat efficacious, but have recently been replaced by anti-angiogenic agents, which may be able to stabilize neovascular complications of mac tel type 2.57 Surgical removal of subretinal neovascularization was associated with a poor visual outcome in 2 patients.58
Mac tel type 2 may be complicated by the development of a full-thickness macular hole. The success rate after surgical repair appears to be lower than for idiopathic macular holes, probably depending on the relative contribution of tangential traction versus the neurodegeneration that is part of mac tel type 2.16,59,60
1 Graefe CF. Angiectasie. Ein Beitrag zur rationellen Cur und Erkenntniβ der Gefäβausdehnungen. Leipzig: K.F. Köhler; 1808.
2 Coats G. Forms of retinal disease with massive exudation. R Lond Ophthalmol Hosp Rep. 1908;12:440.
3 Leber T. Über eine durch Vorkommen multipler Miliaraneurysmen charakterisierte Form von Retinaldegeneration. Graefes Arch Ophthalmol. 1912;81:1–14.
4 Reese A. Telangiectasis of the retina and Coats’ disease. Am J Ophthalmol. 1956;42:1.
5 Gass JD. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. V. Retinal telangiectasis. Arch Ophthalmol. 1968;80:592–605.
6 Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100:1536–1546.
7 Yannuzzi LA, Bardal AM, Freund KB, et al. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124:450–460.
8 Klein R, Blodi BA, Meuer SM, et al. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150:55–62.
9 Aung KZ, Wickremasinghe SS, Makeyeva G, et al. The prevalence estimates of macular telangiectasia type 2. Retina. 2010;30:473–478.
10 Gillies MC, Zhu M, Chew EY, et al. Familial asymptomatic macular telangiectasia type 2. Ophthalmology. 2009;116:2422–2429.
11 Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel Project Report No. 2. Ophthalmic Epidemiol. 2010;17:66–73.
12 Moisseiev J, Lewis H, Bartov E, et al. Superficial retinal refractile deposits in juxtafoveal telangiectasis. Am J Ophthalmol. 1990;109:604–605.
13 Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100:769–780.
14 Patel B, Duvall J, Tullo AB. Lamellar macular hole associated with idiopathic juxtafoveolar telangiectasia. Br J Ophthalmol. 1988;72:550–551.
15 Koizumi H, Iida T, Maruko I. Morphologic features of group 2A idiopathic juxtafoveolar retinal telangiectasis in three-dimensional optical coherence tomography. Am J Ophthalmol. 2006;142:340–343.
16 Charbel Issa P, Scholl HP, Gaudric A, et al. Macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye. 2009;23:435–441.
17 Shukla D. Evolution and management of macular hole secondary to type 2 idiopathic macular telangiectasia. Eye (Lond). 2011;25:532–533.
18 Engelbrecht NE, Aaberg TM, Jr., Sung J, et al. Neovascular membranes associated with idiopathic juxtafoveolar telangiectasis. Arch.Ophthalmol. 2002;120:320–324.
19 Roorda A, Romero-Borja F, Donnelly Iii W, et al. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10:405–412.
20 Ooto S, Hangai M, Takayama K, et al. High-resolution photoreceptor imaging in idiopathic macular telangiectasia type 2 using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Visual Sci. 2011;52:5541–5550.
21 Charbel Issa P, Holz FG, Scholl HPN. Metamorphopsia in patients with macular telangiectasia type 2. Doc Ophthalmol. 2009;119:133–140.
22 Watzke RC, Klein ML, Folk JC, et al. Long-term juxtafoveal retinal telangiectasia. Retina. 2005;25:727–735.
23 Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute visual function questionnaire in the Macular Telangiectasia (MacTel) project. Invest Ophthalmol Vis Sci. 2008;49:4340–4346.
24 Lamoureux EL, Maxwell RM, Marella M, et al. The longitudinal impact of macular telangiectasia (MacTel) type 2 on vision-related quality of life. Invest Ophthalmol Vis Sci. 2011;52:2520–2524.
25 Charbel Issa P, Helb HM, Rohrschneider K, et al. Microperimetric assessment of patients with type II macular telangiectasia. Invest Ophthalmol. Vis Sci. 2007;48:3788–3795.
26 Charbel Issa P, Helb HM, Holz FG, et al. Correlation of macular function with retinal thickness in nonproliferative type 2 idiopathic macular telangiectasia. Am J Ophthalmol. 2008;245:169–175.
27 Maruko I, Iida T, Sekiryu T, et al. Early morphological changes and functional abnormalities in group 2A idiopathic juxtafoveolar retinal telangiectasis using spectral domain optical coherence tomography and microperimetry. Br J Ophthalmol. 2008;92:1488–1491.
28 Schmitz-Valckenberg S, Ong EE, Rubin GS, et al. Structural and functional changes over time in MacTel patients. Retina. 2009;29:1314–1320.
29 Wong WT, Forooghian F, Majumdar Z, et al. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol. 2009;148:573–583.
30 Hannan SR, Madhusudhana KC, Rennie C, et al. Idiopathic juxtafoveolar retinal telangiectasis in monozygotic twins. Br J Ophthalmol. 2007;91:1729–1730.
31 Menchini U, Virgili G, Bandello F, et al. Bilateral juxtafoveolar telangiectasis in monozygotic twins. Am J Ophthalmol. 2000;129:401–403.
32 Siddiqui N, Fekrat S. Group 2A idiopathic juxtafoveolar retinal telangiectasia in monozygotic twins. Am J Ophthalmol. 2005;139:568–570.
33 Chew EY, Murphy RP, Newsome DA, et al. Parafoveal telangiectasis and diabetic retinopathy. Arch Ophthalmol. 1986;104:71–75.
34 Hutton WL, Snyder WB, Fuller D, et al. Focal parafoveal retinal telangiectasis. Arch Ophthalmol. 1978;96:1362–1367.
35 Isaacs TW, McAllister IL. Familial idiopathic juxtafoveolar retinal telangiectasis. Eye. 1996;10(5):639–642.
36 Oh KT, Park DW. Bilateral juxtafoveal telangiectasis in a family. Retina. 1999;19:246–247.
37 Mauget-Faÿsse M, Vuillaume M, Quaranta M, et al. Idiopathic and radiation-induced ocular telangiectasia: the involvement of the ATM gene. Invest Ophthalmol Vis Sci. 2003;44:3257–3262.
38 Barbazetto IA, Room M, Yannuzzi NA, et al. ATM gene variants in patients with idiopathic perifoveal telangiectasia. Invest Ophthalmol Vis Sci. 2008;49:3806–3811.
39 Parmalee NL, Schubert C, Merriam JE, et al. Analysis of candidate genes for macular telangiectasia type 2. Mol Vis. 2010;16:2718–2726.
40 Szental JA, Baird PN, Richardson AJ, et al. Analysis of glutathione S-transferase Pi isoform (GSTP1) single-nucleotide polymorphisms and macular telangiectasia type 2. Int Ophthalmol. 2010;30:645–650.
41 Millay RH, Klein ML, Handelman IL, et al. Abnormal glucose metabolism and parafoveal telangiectasia. Am J Ophthalmol. 1986;102:363–370.
42 Gass JDM. Histological study of presumed parafoveal telangiectasia. Retina. 2000;20:226–227.
43 Barthelmes D, Gillies MC, Fleischhauer JC, et al. A case of idiopathic perifoveal telangiectasia preceded by features of cone dystrophy. Eye (Lond). 2007;21:1534–1535.
44 Helb HM, Charbel Issa P, Van der Veen RL, et al. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008;28:808–816.
45 Green WR, Quigley HA, de la Cruz Z, et al. Parafoveal retinal telangiectasis: light and electron microscopy studies. Trans Ophthalmol Soc UK. 1980;100:162–170.
46 Eliassi-Rad B, Green WR. Histologic study of presumed parafoveal telangiectasis. Retina. 1999;19:332–335.
47 Powner MB, Gillies MC, Tretiach M, et al. Perifoveal müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–2416.
48 Park DW, Schatz H, McDonald HR, et al. Grid laser photocoagulation for macular edema in bilateral juxtafoveal telangiectasis. Ophthalmology. 1997;104:1838–1846.
49 De Lahitte GD, Cohen SY, Gaudric A. Lack of apparent short-term benefit of photodynamic therapy in bilateral, acquired, parafoveal telangiectasis without subretinal neovascularization. Am J Ophthalmol. 2004;138:892–894.
50 Gamulescu MA, Walter A, Sachs H, et al. Bevacizumab in the treatment of idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2008;246:1189–1193.
51 Charbel Issa P, Finger RP, Kruse K, et al. Monthly ranibizumab for nonproliferative macular telangiectasia type 2: a 12-month prospective study. Am J Ophthalmol. 2011;151:876–886.
52 Matt G, Sacu S, Ahlers C, et al. Thirty-month follow-up after intravitreal bevacizumab in progressive idiopathic macular telangiectasia type 2. Eye. 2010;24:1535–1541.
53 Wu L, Evans T, Arévalo JF, et al. Long-term effect of intravitreal triamcinolone in the nonproliferative stage of type II idiopathic parafoveal telangiectasia. Retina. 2008;28:314–319.
54 Shukla D, Singh J, Kolluru CM, et al. Transpupillary thermotherapy for subfoveal neovascularization secondary to group 2A idiopathic juxtafoveolar telangiectasis. Am J Ophthalmol. 2004;138:147–149.
55 Nachiappan K, Shanmugam MP. Treatment of CNVM secondary to idiopathic juxtafoveal retinal telangiectasis by transpupillary thermotherapy. Am J Ophthalmol. 2005;139:577–578.
56 Snyers B, Verougstraete C, Postelmans L, et al. Photodynamic therapy of subfoveal neovascular membrane in type 2A idiopathic juxtafoveolar retinal telangiectasis. Am J Ophthalmol. 2004;137:812–819.
57 Kovach JL, Rosenfeld PJ. Bevacizumab (avastin) therapy for idiopathic macular telangiectasia type II. Retina. 2009;29:27–32.
58 Berger AS, McCuen BW, II., Brown GC, et al. Surgical removal of subfoveal neovascularization in idiopathic juxtafoveolar retinal telangiectasis. Retina. 1997;17:94–98.
59 Gregori N, Flynn HW, Jr. Surgery for full-thickness macular hole in patients with idiopathic macular telangiectasia type 2. Ophthalmic Surg Lasers Imaging. 2010;41:1–4.
60 Shukla D. Evolution and management of macular hole secondary to type 2 idiopathic macular telangiectasia. Eye (Lond). 2011;25:532–533.