Lung Expansion Therapy
After reading this chapter you will be able to:
 Describe the various causes of atelectasis.
Describe the various causes of atelectasis.
 Identify which patients need lung expansion therapy.
Identify which patients need lung expansion therapy.
 Define the clinical findings seen in atelectasis.
Define the clinical findings seen in atelectasis.
 Describe how lung expansion therapy works.
Describe how lung expansion therapy works.
 List the indications, hazards, and complications associated with the various modes of lung expansion therapy.
List the indications, hazards, and complications associated with the various modes of lung expansion therapy.
 Describe the primary responsibilities of the respiratory therapist in planning, implementing, and evaluating lung expansion therapy.
Describe the primary responsibilities of the respiratory therapist in planning, implementing, and evaluating lung expansion therapy.
Pulmonary complications are common serious problems seen in patients who have undergone thoracic or abdominal surgery.1,2 Such complications include atelectasis (alveolar collapse), pneumonia, and acute respiratory failure. These respiratory problems can be minimized or avoided if proper respiratory care is implemented during the perioperative period. The most common form of therapy used in high-risk patients is lung expansion therapy.
Various lung expansion therapies can be effective in preventing or correcting atelectasis in selected patients.1 However, the precise method to apply in a given situation is not always clear because no advantage of any one method has been established. The most efficient use of resources is a primary concern with any plan to apply lung expansion therapy.
Causes and Types of Atelectasis
Compression atelectasis results when the forces within the chest wall and lung—specifically, the pleural pressure—are exceeded by the transmural pressure, which is what distends and maintains the alveoli in an open state.2–4 Compression atelectasis is primarily caused by persistent use of small tidal volumes by the patient. This situation is common when general anesthesia is given, with the use of sedatives and bed rest, and when deep breathing is painful, as when broken ribs are present or surgery has been performed on the upper abdominal region. Weakening or impairment of the diaphragm can also contribute to compression atelectasis. Compression atelectasis results when the patient does not periodically take a deep breath and expand the lungs fully. It is a common cause of atelectasis in hospitalized patients. It may occur in combination with gas absorption atelectasis in a patient with excessive airway secretions who breathes with small tidal volumes for a prolonged period.
Factors Associated With Causing Atelectasis
Atelectasis can occur in any patient who cannot or does not take deep breaths periodically and in patients who are restricted to bed rest for any reason.5 Patients who have difficulty taking deep breaths without assistance include patients with significant obesity, patients with neuromuscular disorders or who are under heavy sedation, and patients who have undergone upper abdominal or thoracic surgery. Diaphragmatic position and function is the major contributor to the onset of atelectasis. In an anesthetized patient, there is a cephalad (toward the head) shift of the diaphragm. For patients who are supine, the lower, dependent portion of the diaphragm performs the most movement. The opposite occurs in patients who are paralyzed—the upper portion of the diaphragm is involved in movement.3,4 Patients undergoing lower abdominal surgery are at less risk for atelectasis than patients undergoing upper abdominal or thoracic surgery, but they still may be at significant risk. Patients with spinal cord injury are prone to respiratory complications, the most common of which is atelectasis. Bedridden patients, such as patients recovering from major trauma, are particularly predisposed to developing atelectasis secondary to lack of mobility. Atelectasis is one of the most important determinants of hypoxemia after abdominal surgery and may account for 24% of deaths within 6 days of surgery.6 It is clinically prudent to consider atelectasis in every assessment of postoperative patients.
Impairment of the function of pulmonary surfactant can also have an impact on the development of atelectasis. Surfactants decrease the surface tension of the walls of the alveoli. When there is deterioration of the function of this vital protein, the relative increase in surface tension can cause the walls of the alveoli to collapse.4
Lung Expansion Therapy
< ?xml:namespace prefix = "mml" />
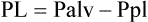
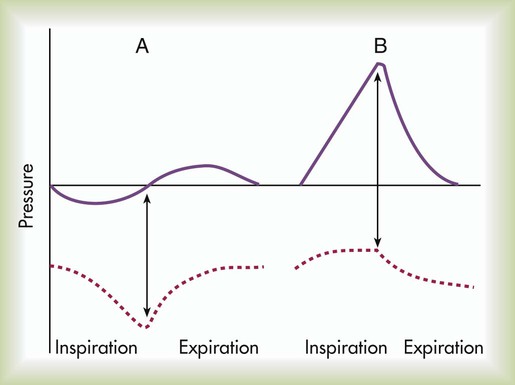
With all else being constant, the greater the Pl gradient, the more that the alveoli expand.
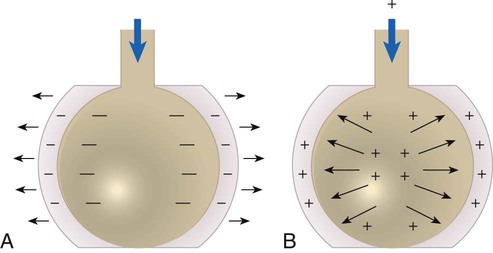
As depicted in Figure 39-1, the Pl gradient can be increased by either (1) decreasing the surrounding Ppl (see Figure 39-1, A) or (2) increasing the Palv (see Figure 39-1, B). A spontaneous deep inspiration increases the Pl gradient by decreasing the Ppl. The application of positive pressure to the lungs increases the Pl gradient by increasing the pressure inside the lung.
The goal of any lung expansion therapy should be to implement a plan that provides an effective strategy in the most efficient manner. Staff time and equipment are the two major issues related to efficiency. For a patient with minimal risk of postoperative atelectasis, deep breathing exercises, frequent repositioning, and early ambulation are usually effective and can be done with minimal coaching and time from clinicians and without equipment.4 For a patient at high risk for atelectasis (e.g., a patient undergoing upper abdominal surgery), IS is usually instituted. The additional staff time and equipment are justified in this high-risk group. Positive pressure therapy requires significantly more staff time and equipment and is reserved for high-risk patients who cannot perform IS techniques. The remainder of this chapter describes the use of IS and positive pressure therapy for the prevention or correction of atelectasis.
Incentive Spirometry
The purpose of IS is to guide the patient to take a sustained maximal inspiratory effort resulting in a decrease in Ppl and maintain the patency of airways at risk for closure. Because of its simplicity, IS has been the mainstay of lung expansion therapy for many years. IS devices are designed to mimic natural sighing by encouraging patients to take slow, deep breaths. IS can be performed using devices that provide visual cues to patients when the desired inspiratory flow or volume has been achieved. IS has been shown to be an efficient and effective prophylaxis against postoperative atelectasis in high-risk patients.5 The first documented use of incentive spirometry as a therapy was in 1972, and this led to the development of a visual feedback device in 1973.7
The desired volume and number of repetitions to be performed are initially set by the RT or other qualified caregiver. The inspired volume goal is set on the basis of predicted values or observation of initial performance. The true benefit from IS is best achieved by repeated use and proper technique.8 The American Association for Respiratory Care (AARC) has developed and published a clinical practice guideline on IS; excerpts from this guideline appear in Clinical Practice Guideline 39-1.
Physiologic Basis
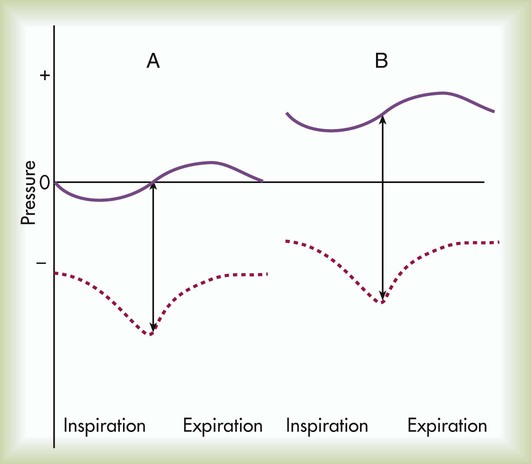
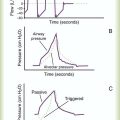
The basic maneuver of IS is a sustained maximal inspiration (SMI). An SMI is a slow, deep inhalation from the functional residual capacity (FRC) up to (ideally) the total lung capacity, followed by a 5- to 10-second breath hold. An SMI is functionally equivalent to performing an inspiratory capacity (IC) maneuver, followed by a breath hold. Figure 39-2 compares the alveolar and Ppl changes occurring during a normal spontaneous breath and an SMI during IS.
Hazards and Complications
Given its normal physiologic basis, IS presents few major hazards and complications; those that can occur are listed in Box 39-3. Acute respiratory alkalosis is the most common problem and occurs when the patient performs IS too rapidly. Dizziness and numbness around the mouth are the most frequently reported symptoms associated with respiratory alkalosis. This problem is easily corrected with careful instruction and monitoring of the patient. Discomfort with deep inspiratory efforts secondary to pain is usually the result of inadequate pain control in a postoperative patient. This problem can be rectified by ensuring appropriate analgesia. In addition, pain medication should be coordinated with IS activity.
Equipment
IS devices can generally be categorized as volume-oriented or flow-oriented. True volume-oriented devices measure and visually indicate the volume achieved during an SMI. The most popular true volume-oriented IS devices employ a bellows that rises according to the inhaled volume. When the patient reaches a target inspiratory volume, a controlled leak in the device allows the patient to sustain the inspiratory effort for a short period (usually 5 to 10 seconds). Because the bellows types of IS devices are bulky and large, smaller devices that indirectly indicate volume based on flow through a fixed orifice have been developed. These devices sacrifice accurate measurement of the inhaled volume to achieve portability and smaller size (Figure 39-3).
Flow-oriented devices measure and visually indicate the degree of inspiratory flow (Figure 39-4). This flow can be equated with volume by assessing the duration of inspiration or time (flow × time = volume). Both flow-oriented and volume-oriented devices attempt to encourage the same goal for the patient: a sustained maximal inspiratory effort to prevent or correct atelectasis. No evidence to date indicates that one type is more beneficial than the other.
Administration
Preliminary Planning
During preliminary planning, the need for IS should be determined by careful patient assessment. Once the need is established, planning for IS should focus on selecting explicit therapeutic outcomes. Box 39-4 lists potential outcomes that can be considered for patients receiving IS.
Implementation
The exact number of sustained maximal inspirations needed to reverse or prevent atelectasis is not known and probably varies according to the patient’s clinical status. However, because healthy individuals average about 6 sighs per hour, an IS regimen should probably aim to ensure a minimum of 5 to 10 SMI maneuvers each hour.9
Intermittent Positive Airway Pressure Breathing
Physiologic Basis
Positive pressure is transmitted from the alveoli to the pleural space during the inspiratory phase of an IPPB treatment, causing Ppl to increase during inspiration. Depending on the mechanical properties of the lung, Ppl may exceed atmospheric pressure during a portion of inspiration. As with spontaneous breathing, the recoil force of the lung, stored as potential energy during the positive pressure breath, causes a passive exhalation. As gas flows from the alveoli out to the airway opening, Palv decreases to atmospheric level, while Ppl is restored to its normal subatmospheric range (Figure 39-5). The AARC has developed and published a clinical practice guideline for IPPB; excerpts from this guideline appear in Clinical Practice Guideline 39-2.
Indications
There is little research supporting the use of IPPB as an aerosol delivery system. However, there is supporting evidence that periodic sessions of positive pressure ventilation provided noninvasively can be useful in the treatment of pulmonary complications or exacerbations of lung disease.10–12 NIV, or, more specifically, IPPB, may be useful for patients with clinically diagnosed atelectasis unresponsive to other therapies, such as IS and chest physiotherapy. In addition, short-term use of NIV in the form of IPPB may be useful for patients who are at high risk for atelectasis and unable to participate in more patient-directed techniques such as IS or even deep breathing. IPPB should not be used as a single treatment modality for a patient with gas absorption atelectasis because of excessive airway secretions. In addition, the RT should be aware of potential complications, such as mucous plugging, which can be worsened owing to a humidity deficit that can occur with IPPB therapy. Because of the need for specialized machinery and training, IPPB itself should not be thought of as the first line of therapy. Applying positive pressure to the lung in such cases can cause overinflation of the lung regions not affected by secretions and minimal or no expansion of the affected lung segments. Airways clearance with humidity therapy should be considered in conjunction with IPPB for optimizing results in patients with retained secretions.
Contraindications
There are several clinical situations in which IPPB should not be used (Box 39-6). With the exception of untreated tension pneumothorax, most of these contraindications are relative. As with all procedures, a sound knowledge of the patient’s condition tempered with good clinical insight should guide the RT in the decision-making process. A patient with any of the conditions listed in Box 39-6 should be carefully evaluated before a decision is made to begin IPPB therapy.
Hazards and Complications
Another potential complication of IPPB is gastric distention; this occurs when gas from the IPPB device passes directly into the esophagus. Gastric distention is uncommon in an alert patient but is a significant risk for a neurologically obtunded patient. Normally, the esophagus does not open until a pressure of about 20 to 25 cm H2O has been reached. Gastric distention represents the greatest risk in patients receiving IPPB at high pressures. The major hazards and complications of IPPB are listed in Box 39-7.
Administration
Preliminary Planning
During preliminary planning, the need for IPPB is determined, and desired therapeutic outcomes are established. The outcomes chosen for a patient are based on diagnostic information that supports the need for IPPB therapy. In addition, therapeutic outcomes should be as explicit and measurable as possible. Outcomes must also be consistent with the therapeutic indications previously described. Outcomes that are inconsistent with these indications are generally inappropriate. Box 39-8 lists potential accepted and desired outcomes of IPPB therapy. Not all the outcomes listed in Box 39-8 apply to every patient. For example, for a patient exhibiting clinical signs and symptoms of postoperative atelectasis, the following outcomes might be set: improved patient comfort, increased aeration on auscultation, decreased respiratory rate and work of breathing, and improvement in the chest radiograph.
Positive Airway Pressure Therapy
Physiologic Basis
There are three current approaches to PAP therapy: PEP, EPAP, and CPAP. All three techniques are effective in treating atelectasis in most postsurgical patients.13,14 Because PEP and EPAP are used most often as part of airway clearance, they are described in Chapter 40. This chapter describes the intermittent use of CPAP for the treatment of atelectasis. Continuous use of CPAP is discussed elsewhere in this text.
PEP and EPAP create expiratory positive pressure only, whereas CPAP maintains a positive airway pressure throughout both inspiration and expiration. Figure 39-6 compares the alveolar and Ppl changes occurring during a normal spontaneous breath (Figure 39-6, A) and CPAP (see Figure 39-6, B). As can be seen, CPAP elevates and maintains high alveolar and airway pressures throughout the full breathing cycle; this increases Pl gradient throughout both inspiration and expiration. Typically, a patient on CPAP breathes through a pressurized circuit against a threshold resistor, with pressures maintained between 5 cm H2O and 20 cm H2O. To maintain system pressure throughout the breathing cycle, CPAP requires a source of pressurized gas.
Equipment
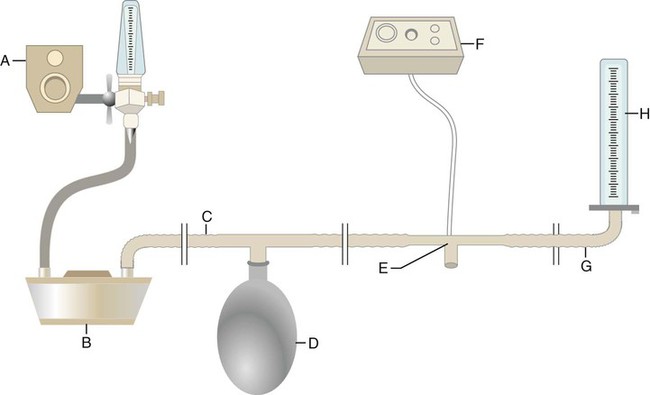
Equipment used to deliver CPAP varies substantially in design and complexity. For purposes of illustration, the key elements of a simple continuous-flow CPAP circuit are shown in Figure 39-7. A breathing gas mixture from an O2 blender (A) flows continuously through a humidifier (B) into the inspiratory limb of a breathing circuit (C). A reservoir bag (D) provides reserve volume if the patient’s inspiratory flow exceeds that of the system. The patient breathes in and out through a simple valveless T-piece connector (E). A pressure alarm system with manometer (F) monitors the CPAP at the patient’s airway. The alarm system can warn of either low (usually caused by a disconnection) or high system pressure. The expiratory limb of the circuit (G) is connected to a threshold resistor, in this case, a water column (H).
Selecting an Approach
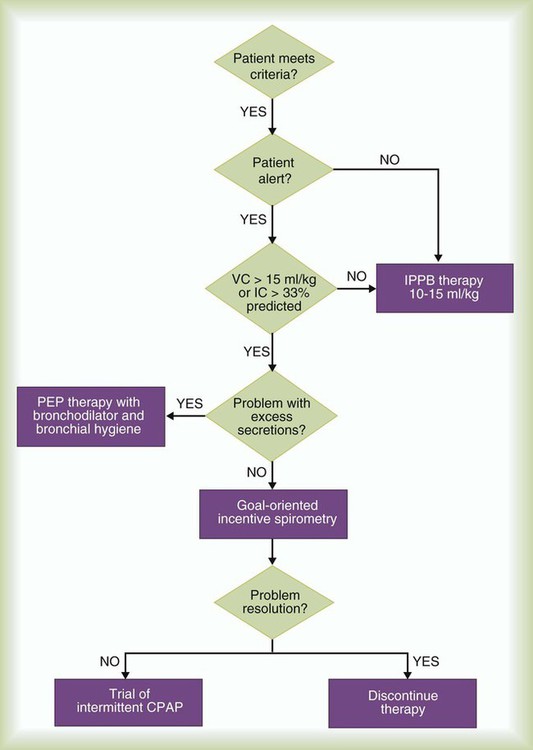
Figure 39-8 presents a sample protocol for selecting an approach to lung expansion therapy. As indicated in the algorithm, the patient first must meet the criteria for therapy by having one or more of the indications previously specified. For patients meeting the inclusion criteria, the RT first determines the degree of alertness. Because an obtunded patient cannot be expected to cooperate with IS or PEP or EPAP therapy, IPPB or NIV is initiated with appropriate monitoring. If the patient is alert, a bedside assessment is conducted. This assessment should include measurement of either the IC or vital capacity (VC) and evaluation of the volume and consistency of the patient’s secretions.

 ). Gas distal to the obstruction is absorbed by the passing blood in the pulmonary capillaries, which causes partial collapse of the nonventilated alveoli. When ventilation is compromised to a larger airway or bronchus, lobar atelectasis can develop.
). Gas distal to the obstruction is absorbed by the passing blood in the pulmonary capillaries, which causes partial collapse of the nonventilated alveoli. When ventilation is compromised to a larger airway or bronchus, lobar atelectasis can develop. predicted)
predicted)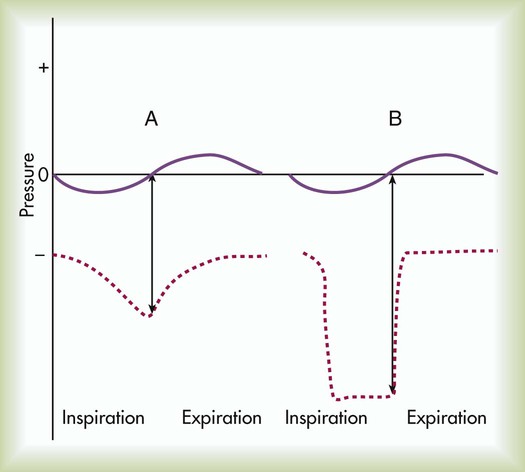
 predicted)
predicted)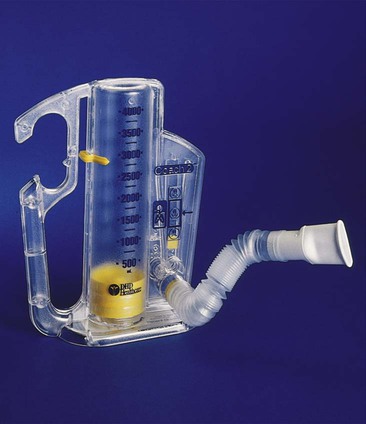

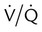 mismatch
mismatch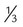 × 50 ml/kg) has been suggested
× 50 ml/kg) has been suggested