Lung Cancer
Etiologic and Pathologic Aspects
The discussion of carcinoma of the lung is presented in two parts. This chapter considers what is known about the etiology and pathogenesis of lung cancer, followed by a description of the pathologic aspects and classification of the different types of tumors. Chapter 21 continues with a discussion of the clinical aspects of the disease, including diagnostic and therapeutic considerations. Chapter 21 concludes with a brief discussion of two additional types of neoplastic disease affecting the respiratory system, bronchial carcinoid tumor (bronchial adenoma) and mesothelioma, along with a consideration of the common problem of the patient with a solitary pulmonary nodule.
Etiology and Pathogenesis
Occupational Factors
Carcinoma of the lung is the most likely malignancy to result from occupational asbestos exposure, although other tumors, especially mesothelioma (see Chapter 21), are also strongly associated with prior asbestos contact. (Low-level nonoccupational exposures to asbestos in schools or among residents living near asbestos mines or processing facilities are of much lesser significance.) The risk for development of lung cancer is particularly high among smokers exposed to asbestos, in which case these two risk factors probably have a multiplicative rather than a simple additive effect. Specifically, asbestos alone appears to confer a twofold to fivefold increased risk for lung cancer, whereas smoking alone is associated with an approximately 10-fold increased risk. Together, the two risk factors make the person who smokes and has an asbestos exposure 20 to 50 times more likely to have carcinoma of the lung than a nonsmoking, nonexposed counterpart. Like other forms of asbestos-related disease, a long time elapses before complications develop. In the case of lung cancer, the tumor generally becomes apparent more than 2 decades after exposure.
Pathology
Squamous Cell Carcinoma
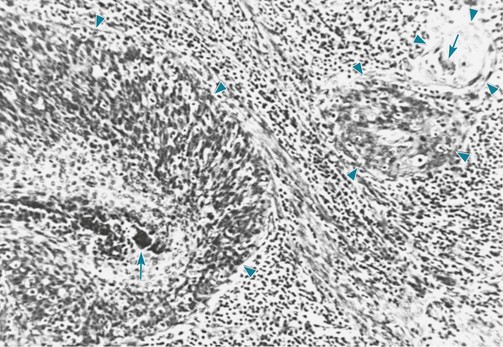
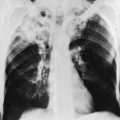
Specific histologic features of squamous cell carcinoma allow the pathologist to establish this diagnosis. These tumors are characterized by the visible presence of keratin, “squamous pearls,” and intercellular desmosomes or bridges (Fig. 20-1).
Small Cell Carcinoma
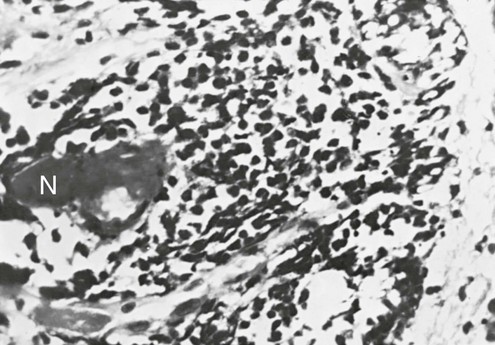
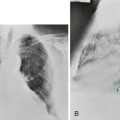
In small cell carcinoma, the malignant cells appear as small, darkly stained cells with sparse cytoplasm (Fig. 20-2). Local growth of the tumor often follows a submucosal pattern, but the tumor quickly invades lymphatics and submucosal blood vessels. Hilar and mediastinal nodes are involved and enlarged early in the course of the disease and frequently are the most prominent aspect of the radiographic presentation.
Adenocarcinoma
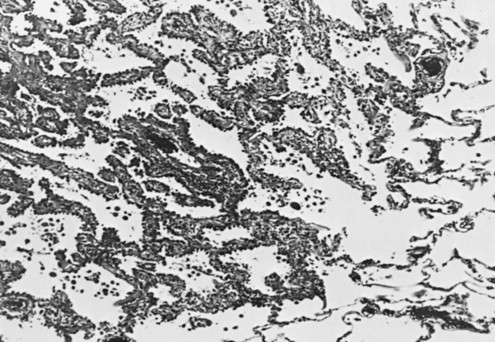
The characteristic appearance defining adenocarcinoma is the tendency to form glands and in many cases produce mucus (Fig. 20-3). When the malignant cells seem to grow and spread along the preexisting alveolar walls, almost as though they were using the alveolar wall as scaffolding for their growth, the tumors have been subcategorized as bronchioloalveolar carcinomas (Fig. 20-4). Of note, a 2011 joint statement from the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society recommended elimination of this specific term.
Alberg, AJ, Ford, JG, Samet, JM. Epidemiology of lung cancer. ACCP evidence-based clinical practice guidelines, 2nd ed. Chest. 2007;132:29S–55S.
Brennan, P, Hainaut, P, Boffetta, P. Genetics of lung cancer susceptibility. Lancet Oncol. 2011;12:399–408.
Dacic, S. Pulmonary preneoplasia. Arch Pathol Lab Med. 2008;132:1073–1078.
Darby, S, Hill, D, Auvinen, A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:223–229.
Denissenko, MF, Pao, A, Tang, M, Pfeifer, GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in p53. Science. 1996;274:430–432.
Dhala, A, Pinsker, K, Prezant, DJ. Respiratory health consequences of environmental tobacco smoke. Med Clin North Am. 2004;88:1535–1552.
Doll, R, Peto, R, Boreham, J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–1527.
Field, RW, Krewski, D, Lubin, JH, et al. An overview of the North American residential radon and lung cancer case-control studies. J Toxicol Environ Health A. 2006;69:599–631.
Goodman, GE. Prevention of lung cancer. Thorax. 2002;57:994–999.
Herbst, RS, Heymach, JV, Lippman, SM. Lung cancer. N Engl J Med. 2008;359:1367–1380.
Kratz, JR, Yagui-Beltrán, A, Jablons, DM. Cancer stem cells in lung tumorigenesis. Ann Thorac Surg. 2010;89:S2090–S2095.
Miller, YE. Pathogenesis of lung cancer. 100 year report. Am J Respir Cell Mol Biol. 2005;33:216–223.
Rezazadeh, A, Laber, DA, Ghim, SJ, et al. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci. 2009;338:64–67.
Rom, WN, Hay, JG, Lee, TC, et al. Molecular and genetic aspects of lung cancer. Am J Respir Crit Care Med. 2000;161:1355–1367.
Sato, M, Shames, DS, Gazdar, AF, et al. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2:327–343.
Spiro, SG, Silvestri, GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529.
Subramanian, J, Govindan, R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570.
Tanvetyanon, T, Bepler, G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008;113:150–157.
Whitesell, PL, Drage, CW. Occupational lung cancer. Mayo Clin Proc. 1993;68:183–188.
Dacic, S. Molecular diagnostics of lung carcinomas. Arch Pathol Lab Med. 2011;135:622–629.
Franklin, WA. Diagnosis of lung cancer: pathology of invasive and preinvasive neoplasia. Chest. 2000;117(Suppl):80S–89S.
Müller, K-M. Lung cancer: morphology. Eur Respir Mon. 2001;17:34–47.
Schwartz, AM, Henson, DE. Diagnostic surgical pathology in lung cancer. ACCP evidence-based clinical practice guidelines, 2nd ed. Chest. 2007;132:78S–93S.
Travis, WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81.
Travis WD, Brambilla E, Muller-Hermlink HK, et al, eds. World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
Travis, WD, Brambilla, E, Noguchi, M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285.
Verbeken, EK, Brambilla, E. WHO classification of lung and pleural tumours. The WHO/IASLC 1999 revision. Eur Respir Rev. 2002;12(review 84):172–176.