28 Lower Extremity
Lateral Femoral Cutaneous Nerve Block
The clinical description of pain, numbness, and tingling involving the anterior and lateral thigh, was first described by Bernhardt in 1878.1 Werner Hager (1885) was the first to describe compression of the lateral femoral cutaneous nerve near the anterior superior iliac spine (ASIS) as the cause of pain near the hip.2 In 1895, both Bernhardt and Roth published separate articles on meralgia paresthetica (MP).3,4 Roth described five patients with similar symptoms and coined the term “meralgia paresthetica” from the Greek words meros for thigh and algos meaning pain.4
Anatomy of the Lateral Femoral Cutaneous Nerve
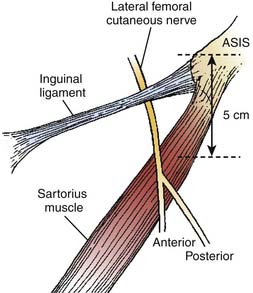
The lateral femoral cutaneous nerve (LFCN) is derived from the lumbar plexus and its contribution comes mostly from the L2 and L3 nerve roots.5 The LFCN may derive from various other combinations such as L2 and L3, L1 and L2, or L2 or L3 alone.6 The LFCN appears at the lateral border of the psoas muscle just above the crest of the ilium.5 The nerve then travels laterally and inferiorly across the surface of the iliacus muscle from which it is separated by a thin muscle sheath (Fig. 28-1). Both the nerve and the iliacus muscle are covered with a dense fascial layer called the iliac fascia, which is composed of two layers and the LFCN travels between them.7 The nerve continues its course toward the lateral portion of the inguinal ligament posterior to the deep circumflex iliac artery, which courses parallel to the inguinal ligament beneath the iliac fascia.7 Filho and coworkers found in their cadaver study that the anterior lamina of the iliac fascia and the transversalis fasciae fused to form the iliopubic tract (IPT) and the LFCN courses deep to the IPT and reaches the thigh by passing posterior to the inguinal ligament (IL).7
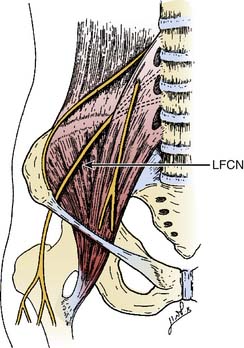
Figure 28-1 The anatomic course of the lateral femoral cutaneous nerve (LFCN).
(Adapted from Mirovsky Y, Neuwirth M: Injuries to the lateral femoral cutaneous nerve during spine surgery. Spine. 2000;25:1266-1269.)
There are several descriptions of anatomic variations of LFCN as it travels from the pelvis to the thigh based on cadaver studies and surgical exploration of the LFCN for meralgia paresthetica.7–12 Keegan and colleagues describes the LFCN as traveling between the two slips of lateral attachment of the IL to the anterior superior iliac spine (ASIS) and remaining just inferior and medial to ASIS.5 It then continues its course anterior to the tendinous origin and upper fibers of the sartorius muscle, separated only by the muscle sheath and covered with the fascia lata. Carai and associates reviewed descriptions of surgical explorations of the LFCN for the treatment of MP and found that in 85.2% of cases the nerve leaves the pelvis beneath the IL (Fig. 28-2).8 This pattern was also documented by Filho and coworkers in their cadaver study where all LFCNs passed posterior to the IL.7 This study also found that in 94% of cases the nerve passed 3.0 cm or less medial to the ASIS.7 Similar results were also described by Grothaus and associates in their cadaver study, in which all the nerves leave under the IL and approximately 36 mm medial to the ASIS.9 Interestingly, they also found that the nerve divides 27.6% of the time before crossing the IL, which may have important implications for nerve blocks or surgical treatment of MP. Aszmann and colleagues in their cadaver study found five variations of the LFCNs as they enter from pelvis to thigh (Fig. 28-3). In 52 cadavers, they found that the nerve overlies the iliac crest 4% (type A), ensheathed by the IL 27% (type B), ensheathed by the tendinous origin of the sartorius muscle 23% (type C), deep to the IL and medial to the sartorius muscle 26% (type D), and 20% of the time the nerve lies medial and on top of the iliopsoas muscle (type E).10 After emerging from the pelvis, the LFCN passes anterior to the tendinous attachment and upper fibers of the sartorius muscle, separated by the muscle sheath and covered by the thick fascia lata.5 Others, however, describe that the nerve may pass over, under, or through the proximal portion of the sartorius muscle.7 Filho and coworkers describes three basic patterns of terminal branching of the LFCN in 26 cadavers.7 The most common pattern (54%) is the bifurcation into anterior and posterior branches (Fig. 28-4). The anterior branch again divides into medial and lateral branches and supplies the anterolateral thigh and the posterior branch supplies the proximal part of the lateral thigh. In a significant number of cases (34%), the nerve divides into medial and lateral branches and supplies the medial and lateral portion of the anterolateral thigh. In 12% of cases, the nerve trifurcates instead of bifurcating. They also observed, in most cases, that the terminal branches, rather than the trunk, pierce the fascia lata to enter the subcutaneous tissues.
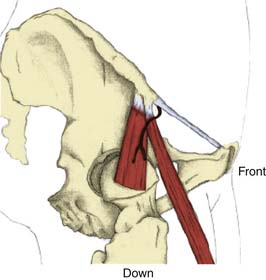
Figure 28-2 Emergence of the nerve from the pelvis under the inguinal ligament.
(Adapted from Carai A, Fenu G, Sechi E, et al: Anatomic variability of the lateral femoral cutaneous nerve: Findings from a surgical series. Clin Anat. 2009;22:365-370.)
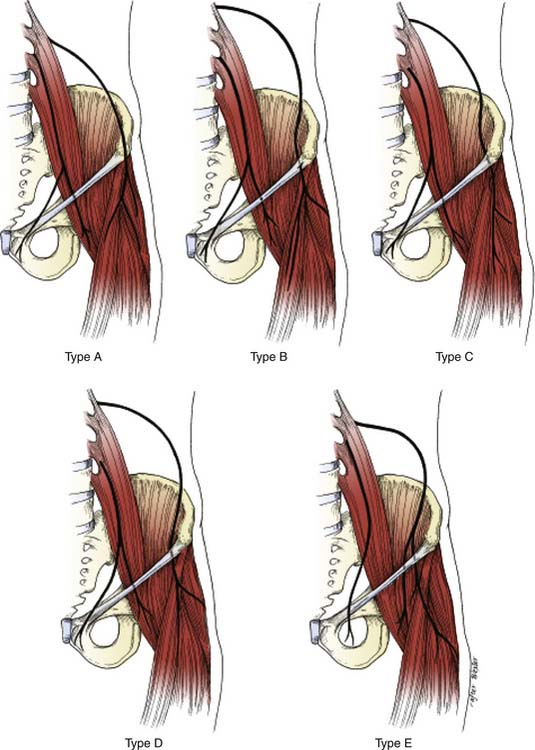
Figure 28-3 Five common variant locations of the LFCN as it exits the pelvis of 52 cadavers (see text for details).
(Adapted from Aszmann OC, Dellon ES, Dellon AL: Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600-604.)
Etiology
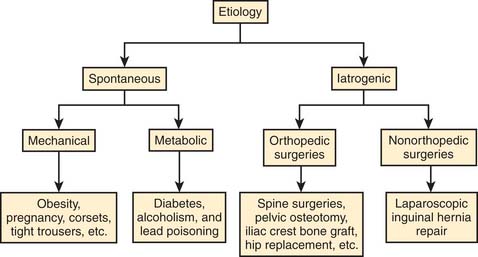
Meralgia paresthetica is a peripheral sensory mononeuropathy of the LFCN. The etiology of MP can be divided into two major categories—spontaneous and iatrogenic (Fig. 28-5). Spontaneous causes include mechanical compression of the nerve or metabolic derangements in the absence of prior surgical procedures that may have injured the nerve. Other mechanical factors include obesity, pregnancy, ascites,12 pelvic tumor,13 tumor in the iliac crest,14 tight trousers,15 corsets, tight belts with accompanying holster for a pistol,16 and soldiers wearing body armor.17 MP has also been described in limb-length discrepancy18 and following the treatment of osteoid sarcoma in children.19
The iatrogenic causes of MP are described after various surgical procedures. This may be caused by direct trauma to the nerve during the procedure, related to patient positioning for the surgery,20 or related to postsurgical scarring.21 The surgical procedures may be classified as orthopedic and nonorthopedic procedures. The orthopedic procedures include pelvic osteotomy,22 acetabular fracture surgery,23 and bone-graft harvesting from the iliac crest.21,24,25 The nonorthopedic surgeries that have been associated with MP are laparoscopic hernia repair,26 laparoscopic cholecystectomy,27 and bariatric surgery.28
Pathophysiology
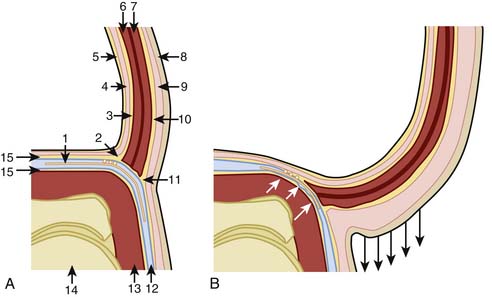
A key factor in the pathogenesis of MP is likely the significant anatomic variation of the nerve described in various cadavers and surgical studies.7–10 Filho and his colleagues,7 in a cadaver study, described the pelvic portion of the LFCN and its relationship with iliac fascia and other muscles and fascia of the anterior abdominal wall. They illustrate that the anterior lamina of the iliac fascia (ALIF) fuses with the transversalis fascia to form the iliopubic tract (IPT) and continues further to meet with the IL. In their study all LFCNs passed inferior to the IPT and were in contact with it (Fig. 28-6). The angle between the ALIF and the IPT becomes more obtuse as the lower abdominal wall moves anteriorly and inferiorly as occurs in obesity (see Fig. 28-4). The authors hypothesized that because the ALIF is firmly attached with the muscles of the abdominal wall, with obesity the ALIF is pushed inferiorly to compress the LFCN. The authors speculated that with erect posture, gravity may further exacerbate the situation by pulling the adipose tissue and the viscera, reflecting the worsening symptoms of patients of MP seen with standing and improving on lying down. Based on their findings of the widening of the LFCN just proximal to the IL, the authors speculate that the nerve may also be compressed within the “LFCN tunnel” (beginning under the IPT and finishing at the IL). It has been found that the groin pressure was greater in sitting obese patients than in subjects with normal weight.29 This becomes more important when the nerve course is close to the ASIS (types A, B, and C, see Fig. 28-3).10 This may increase the likelihood of nerve compression, especially in obese individuals and those with hip extension.10,11
Many researchers suggest that the usual traumatic site for the LFCN is at the IL.5,9,30,31 Keegan and coworkers describe the emergence of the LFCN between the two slips of the lateral attachment that may be affected by the tension of the ligament.5 The authors’ point to the attachment of muscles and fascia and the role they may play with the tension of the ligament. The sartorius muscle has a medial aponeurotic expansion that attaches to the inferior border of the IL and may cause episodic depression of the ligament with muscle contraction. The IL also provides an insertion for the external oblique muscle and an origin for the lowest fibers of the internal oblique and transversus abdominis muscles and thereby create tension by the contraction of these muscles. The deep layer of the superficial abdominal fascia, or Scarpa fascia, extends downward one half inch below the IL to attach with the fascia lata. This attachment with the fascia lata may cause a downward pull of the IL among patients with dependent abdominal fat.5
Diagnosis
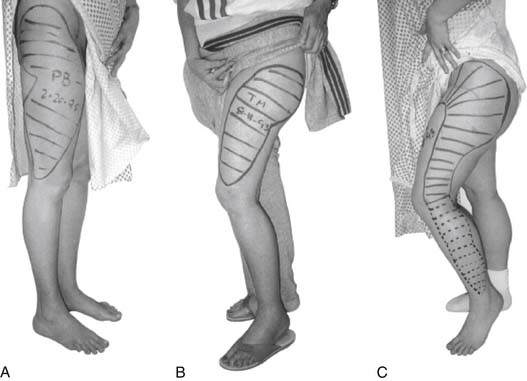
Diagnosis of MP is usually done by history and physical examination, with the characteristic location of pain and numbness confined to the anterolateral thigh (Fig. 28-7).32 In rare cases, the area may extend inferiorly below the knee, gluteal region, and medial thigh.12,32 The patient describes their symptoms as burning, coldness, lightning pain, deep muscle achiness, tingling or crawling sensations, and frank anesthesia. Walking, standing, or getting in and out of automobiles usually provokes symptoms. Sitting may relieve symptoms in some patients but exacerbate the symptoms in others. Dureja and associates describe their diagnostic criteria based on a series of 40 patients treated for MP. They describe their clinical criteria for the diagnosis: (1) unpleasant paresthesias/numbness/burning sensation/dysesthesias/pain over the anterolateral aspect of the thigh and exacerbation of symptoms on walking, standing, or extending the hip.33 They found unpleasant paresthesias over the anterolateral aspect of the thigh is the most frequent symptom (85%). Tingling or throbbing pain in the thigh was reported by 75% of patients and 25% complained of hypersensitivity in the form of discomfort from the clothing over the painful area.
Physical examination may find an area of hair loss on the anterolateral thigh due to the patient’s rubbing of the thigh. Sensory examination may reveal hypesthesia, dysesthesia, with or without mechanical allodynia. Hip extension and standing in erect posture can elicit concordant pain. Focal tapping near the ASIS (Tinel sign) may reproduce the usual pain in certain cases. A pelvic compression test has recently been described to assist the diagnosis of MP (Fig. 28-8).34 The idea is that the LFCN is compressed by the IL and relaxing the ligament should relieve the pressure on the nerve and thereby alleviate the pain. The patient is placed in the lateral decubitus position with the affected side up and is asked to focus on their symptoms while the examiner applies the lateral compression on the pelvis for 45 seconds. The test is considered positive if patient’s symptoms improve. When compared with the electrophysiologic diagnosis, the authors found the sensitivity and specificity of the test 95% and 93%, respectively.
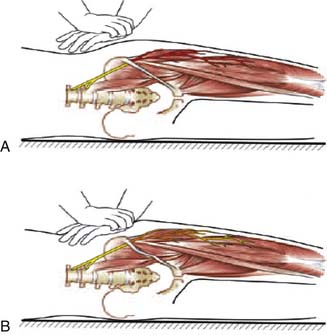
Figure 28-8 The pelvic compression test (see text for details).
(Adapted from Nouraei SA, Anand B, Spink G, O’Neill KS: A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60:696-700.)
Electrodiagnostic studies can be a helpful adjunct to confirm the diagnosis of MP. Sensory nerve conduction velocity (SNCV) of the LFCN and somatosensory evoked potentials (SSEP) recorded on the scalp following stimulation of the trunk of the LFCN or by stimulating the skin innervated by the LFCN are the most common electrodiagnostic tests for MP. Seror compared the SNCV and the SSEP in 30 patients with clinical evidence of unilateral MP and concluded that SNCV is more accurate in diagnosing MP.35 In a later study, the same author found that SSEP may be beneficial in obese patients where SNCV cannot be measured.36
Treatment
Most patients with MP improve with conservative treatment.12,33,37,38 Conservative therapies applied in various series are different and therefore it is difficult to make any comparisons regarding the efficacy of one modality versus another.
Conservative therapy begins with removal of any inciting agents such as tight belts, braces, casts, tight trousers (taille basse), tight undergarments, and various duty uniform belts used by police offices and carpenters. Initial measures also include local application of ice packs and oral antiinflammatory agents to reduce local inflammation. Other established neuropathic agents such as tricyclic antidepressants, anticonvulsants, antiarrhythmics39 can also be considered as an adjunct therapy for MP. Application of a topical anesthetic patch has been used for neuropathic pain and may be considered as part of the conservative treatment for MP.40 If symptoms persist, LFCN block with local anesthetics and steroid should be considered.33,38 Repeat injections of local anesthetics and steroid may be needed to achieve persistent relief.33,38 In their series, Dureja and his colleagues performed a diagnostic LFCN block with 8 mL of 0.25% bupivacaine in forty patients. If the patient responded, nerve blocks were repeated on alternate days with bupivacaine and methylprednisolone, 20 mg each up to a total dose of 80 to 120 mg, a minimum of five blocks in each patient.33 The patients also received oral diphenylhydantoin in a dose range of 100 to 300 mg daily in divided doses for 10 to 12 weeks. Thirty four (85%) had complete relief of paresthesias within 10 weeks.
Surgical therapy is reserved for refractory and intractable symptoms in patients with MP. Fortunately, only a small portion of the patients with the diagnosis of MP require surgical interventions. Surgical intervention to relieve the symptoms of MP dates back to 1885.12 The surgery for MP can be divided into neurolysis of the tissue constricting the nerve, neurolysis and transposition of the LFCN, and transection with excision of a portion of the nerve. Which of these three treatments is superior remains unsettled. Among the previous series, the success rate from neurolysis of the LFCN for refractory MP varies from 60% to 90%.41–43 However, in a more recent case series Siu and colleagues found complete and partial symptom relief in 73% and 20% cases, respectively, from neurolysis with an average follow-up of 4.1 years.44 Williams and Trzil reported successful relief of symptoms of 23 out of 24 patients after LFCN transection.12 Although the surgery did cause permanent anesthesia in the anterolateral thigh, there were no other serious complications from the surgery. In another series of 21 patients, van Eerten concluded that to accomplish complete pain relief, transection of the nerve is superior to neurolysis for refractory MP.45 Ivins described neurolysis in four of eight operative cases of MP.46 All four had immediate relief, but symptoms returned 2 to 24 months later. All four underwent resection and had no recurrence. The other four had primary transection and had persistent relief at long-term follow-up. The author recommends that adults with symptoms of less than 1 year and all pediatric patients should undergo neurolysis. If symptoms persist after the surgery, resection should be considered. He also suggests primary resection for adults with more than a 1-year history of refractory pain.
During surgical intervention, a suprainguinal or infrainguinal approach is taken to explore the nerve for decompression or transection. The infrainguinal approach is made 3 cm below and parallel to the IL down to the fascia lata, which is then incised in the same direction.47 The nerve is then exposed medial to the sartorius muscle and followed proximally toward the IL. For the suprainguinal approach, an incision is made 1 cm above and parallel to the IL.48 The suprainguinal approach allows for accurate identification of the LFCN in its intrapelvic and retroperitoneal location and is followed distally toward the IL.
LFCN Nerve Block
The LFCN block has been described for both acute and chronic pain conditions such as MP. Examples of acute pain conditions include anesthesia and analgesia for immediate postoperative situations such as knee arthroscopy,49 vastus lateralis muscle biopsy to detect malignant hyperthermia,50 and skin grafting.51
Ultrasound-Guided Nerve Block
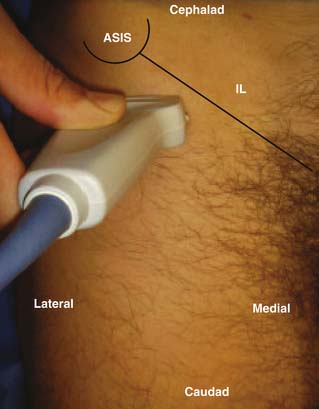
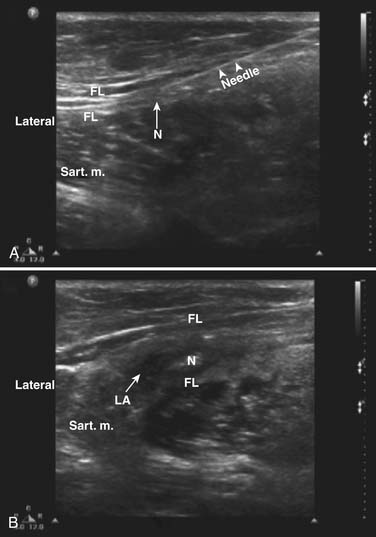
Peripheral nerve block with ultrasound guidance allows visualizing the surrounding anatomy and placement of the needle at the target location in real time. When compared with conventional techniques, ultrasound-guided nerve blocks have been found to be superior during brachial plexus, ilioinguinal, and iliohypogastric nerve blocks.54,55 The accuracy of the ultrasound probe to identify the LFCN has now been documented.56,57 Among 19 cadavers, using the ultrasound probe, Ng and associates was able to place the needle in contact with the nerve in 16 cases and in the rest, the three-needle tips were 2, 3, and 12 mm away from the nerve trunk.58 Contrary to traditional techniques (2 to 3 cm medial and inferior to the ASIS), only one of the 19 needles was in contact with the nerve. In the same study, the authors also compared the placement of the nerve stimulation needle via ultrasound guidance or using the aforementioned landmark. They were able to identify all twenty LFCNs via ultrasound and the nerve was clearly visualized between the two fascial layers. In 16 of 20 patients, the nerve was identified by the ultrasound corresponding to the nerve position identified by the nerve stimulation. However, none of the skin-marked locations based on anatomic landmarks corresponded to the actual position of the LFCN identified by the nerve stimulation technique. The authors described that the best view of the LFCN was obtained by transverse scanning at 14.1 mm medial and 50.8 mm inferior to the ASIS. A high-frequency (>7 MHz) linear array transducer is recommended for identification of the nerve. The successful nerve block has been reported using needles with and without stimulating capability.56,57 The technique requires that the patient is lying supine with a wide sterile preparation around the groin and upper thigh. The ASIS is identified and marked with a sterile skin marker. The ultrasound probe scans transversely with the lateral end of the probe just inferior to the ASIS to identify the sartorius muscle (Fig. 28-9). The probe is then slid slowly medially and inferiorly below the IL to identify the two continuous hyperechoic lines under the subcutaneous tissue, the fascia lata, and fascia iliaca. The LFCN will appear in cross-section in the space between the two fascial layers. It will appear as an oval hyperechoic structure containing several circular hypoechoic dots giving it a “honeycomb” appearance (Fig. 28-10).
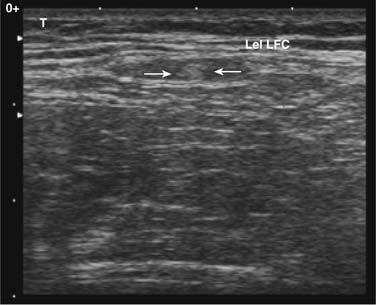
Figure 28-10 Ultrasound image of the lateral femoral cutaneous nerve (LFCN) in the short axis. The honeycomb texture of the nerve in this view can be visualized and is bordered by arrows.57
(From Hurdle MF, Weingarten TN, Crisostomo RA, et al: Ultrasound-guided blockade of the lateral femoral cutaneous nerve: Technical description and review of 10 cases. Arch Phys Med Rehabil. 2007;88:1362-1364.)
After identifying the nerve, its course is then traced by scanning the structure proximally and distally. The operator should also scan medially to verify the neurovascular bundle of the femoral nerve, artery, and vein. If the nerve is not identified by this technique, one should consider the possible anatomic variations as discussed earlier. The operator may expand the area and scan more medial and lateral to the ASIS.
When the LFCN is located, under strict sterile conditions, the needle is passed under direct visualization toward the LFCN. The needle is advanced keeping it in a longitudinal plane to ensure the visualization of the entire needle. If a nerve stimulator needle is used, stimulation will cause paresthesia over the anterolateral thigh. When confirmed, injection of local anesthetics with or without corticosteroid is carried out after negative aspiration. The perineural spread is visualized on the ultrasound screen (Fig. 28-11). Although the range of total volume of injectate varies, with ultrasound-guided localization of the nerve, a successful block has been achieved using as little as 1 mL of injectate.57
Complications and Contraindications to Block
Conclusion
Meralgia paresthetica is a peripheral sensory neuropathy of the lateral femoral cutaneous nerve. The etiology of MP may be postsurgical, mechanical, or metabolic. A key issue in the pathogenesis of MP is the significant anatomic variation of the nerve during its course through pelvis and anterior thigh. Many investigators suggest that the traumatic site for the LFCN is near the inguinal ligament. In most circumstances, the diagnosis of the MP is done with history and physical examination. Usually there are characteristic pain and associated numbness over the anterolateral thigh. Electrodiagnostic studies can also be helpful to confirm the diagnosis of MP. A majority of the patients with MP improve with conservative therapies starting with removal of any inciting agents such as tight belts. Initial measures also include local application of icepacks, antiinflammatory medication, and neuropathic pain medications. If symptoms persist, the LFCN block with local anesthetic and steroid is recommended. The procedure can be done following the surface landmarks near the IL. However, the use of ultrasound guidance may increase the likelihood of targeted injection. Only a few refractory MP patients are referred for possible surgical exploration.
1. Bernhardt M. Neuropathologische Beobachtungen. I. periphere Lahmungen. D Arch Klin Med. 1878;22:362-393.
2. Hager W. Neuralgia femoris. Resection des Nerv. Cutan. Femoris anterior externus. Heilung. Deutsch Med Wochenschr. 1885;11:218-219.
3. Bernhardt M. Ueber isolirt im Gebiete des N. Cutaneous femoris externus vorkommende Parasthesien. Neurol Centrabl. 1895;14:242-244.
4. Roth V. [Meralgia Paresthetica]. Med Obozr. 1895;43:678.
5. Keegan J.J., Holyoke E.A. Meralgia paresthetica: An anatomical and surgical study. J Neurosurg. 1962;19:341-345.
6. Sunderland S. Nerves and nerve injuries. Edinburgh: E & S Livingstone; 1968.
7. Filho L.C.D., Valenca M.M., Guimaraes Filho F.A.V., et al. Lateral femoral cutaneous neuralgia: An anatomical insight. Clin Anat. 2003;16:309-316.
8. Carai A., Fenu G., Sechi E., et al. Anatomical variability of the lateral femoral cutaneous nerve: Findings from a surgical series. Clin Anat. 2009;22:365-370.
9. Grothaus M.C., Holt M., Mekhail A.O., et al. Lateral femoral cutaneous nerve: An anatomic study. Clin Orthop Relat Res. 2005;437:164-168.
10. Aszmann O.C., Dellon E.S., Dellon A.L. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600-604.
11. Grossman M.G., Ducey S.A., Nadler S.S., Levy A.S. Meralgia paresthetica: Diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336-344.
12. Williams P.H., Trzil K.P. Management of meralgia paresthetica. J Neurosurg. 1991;74:76-80.
13. Suber D.A., Massey E.W. Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol. 1979;53:257-258.
14. Tharion G., Bhattacharji S. Malignant secondary deposit in the iliac crest masquerading as meralgia paresthetica. Arch Phys Med Rehabil. 1997;78:1010-1011.
15. Boyce J.R. Meralgia paresthetica and tight trousers. JAMA. 1984;251:1553.
16. Korkmaz N., Ozcakar L. Meralgia paresthetica in a policeman: The belt or the gun. Plast Reconstr Surg. 2004;114:1012-1013.
17. Fargo M.V., Konitzer L.N. Meralgis paresthetica due to body armor wear in U.S. soldiers serving in Iraq: A case report and review of the literature. Mil Med. 2007;172:663-665.
18. Goel A. Meralgia paresthetica secondary to limb length discrepancy: Case report. Arch Phys Med Rehabil. 1999;80:348-349.
19. Edelson R., Stevens P. Meralgia paresthetica in children. J Bone Joint Surg Am. 1994;76:993-999.
20. Mirovsky Y., Neuwirth M. Injuries to the lateral femoral cutaneous nerve during spine surgery. Spine. 2000;25:1266-1269.
21. Weikel A.M., Habal M.B. Meralgia paresthetica: A complication of iliac bone procurement. Plast Reconstr Surg. 1977;60:572-574.
22. Hogh J., Macnicol M.F. The Chiari pelvic osteotomy. A long-term review of clinical and radiographic results. J Bone Joint Surg Br. 1987;69:365-373.
23. de Ridder V.A., de Lange S., Popta J.V. Anatomical variations of the lateral femoral cutaneous nerve and the consequences for surgery. J Orthop Trauma. 1999;13:207-211.
24. Massey E.W. Meralgia paresthetica secondary to trauma of bone graft. J Trauma. 1980;20:342-343.
25. Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine. 1995;20:1055-1060.
26. Dibenedetto L.M., Lei Q., Gilroy A.M., et al. Variations in the inferior pelvic pathway of the lateral femoral cutaneous nerve: Implications for laparoscopic hernia repair. Clin Anat. 1996;9:232-236.
27. Yamout B., Tayyim A., Farhat W. Meralgia paresthetica as a complication of laparoscopic cholecystectomy. Clin Neurol Neurosurg. 1994;96:143-144.
28. Macgregor A.M., Thoburn E.K. Meralgia paresthetica following bariatric surgery. Obes Surg. 1999;9:364-368.
29. Deal C.L., Canoso J.J. Meralgia paresthetica and large abdomens. Ann Intern Med. 1982;96:787-788.
30. Lee F.C. Meralgia paresthetica. Int Clin. 1936;1:216-229.
31. Mack E.W. Meralgia paresthetica: New causal observations. West J Surg Obstet Gynecol. 1946;54:390-391.
32. Ivins G.K. Meralgia paresthetica, the elusive diagnosis: Clinical experience with 14 adult patients. Ann Surg. 2000;232:281-286.
33. Dureja G.P., Gulaya V., Jayalakshmi T.S., Mandal P. Management of meralgia paresthetica: A multimodality regimen. Anesth Analg. 1995;80:1060-1061.
34. Nouraei S.A., Anand B., Spink G., O’Neill K.S. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60:696-700.
35. Seror P. Lateral femoral cutaneous nerve conduction vs. somatosensory evoked potentials for electrodiagnosis of meralgia paresthetica. Am J Phys Med Rehabil. 1999;78:313-316.
36. Seror P. Somatosensory evoked potentials for the electrodiagnosis of meralgia paresthetica. Muscle Nerve. 2004;29:309-312.
37. Ecker A., Woltman H.W. Meralgia paresthetica: A report of one hundred and fifty cases. JAMA. 1938;110:1650-1652.
38. Haim A., Pritsch T., Ben-Galim P., Dekel S. Meralgia paresthetica: A retrospective analysis of 79 patients evaluated and treated according to a standard algorithm. Acta Orthop. 2006;77:482-486.
39. Massey E.W. Sensory mononeuropathies. Semin Neurol. 1998;18:177-183.
40. Devers A., Galer B.S. Topical lidocaine patch relieves a variety of neuropathic pain conditions: An open label study. Clin J Pain. 2000;16:205-208.
41. Macnichol M.F., Thompson W.J. Idiopathic meralgia paresthetica. Clin Orthop Relat Res. 1990;254:270-274.
42. Nahabedian M.Y., Dellon A.L. Meralgia paresthetica: Etiology, diagnosis and outcome of surgical decompression. Ann Plast Surg. 1995;35:590-594.
43. Edelson R., Stevens P. Meralgia paresthetica in children. J Bone Joint Surg Am. 1994;76:993-999.
44. Siu T.L., Chandran K.N. Neurolysis for meralgia paresthetica: An operative series of 45 cases. Surg Neurol. 2005;63:19-23.
45. van Eerten P.V., Polder T.W., Broere C.A. Operative treatment of meralgia paresthetica: Transection versus neurolysis. Neurosugrgery. 1995;37:63-65.
46. Ivins G.K. Meralgia paresthetica, the elusive diagnosis. Clinical experience with 14 adult patients. Ann Surg. 2000;232:281-286.
47. Kempe L.G. Surgery of peripheral nerves: Operative exposure of the lateral femoral cutaneous nerve. In: Kempe L.G., editor. Operative Neurosurgery:Posterior Fossa, Spinal Cord, and Peripheral Nerve Disease. Berlin: Springer-Verlag; 1970:208-210.
48. Aldrich E.F., van den Heever C.M. Suprainguinal ligament approach for surgical treatment of meralgia paresthetica: Technical note. J Neurosurg. 1989;70:492-494.
49. Patel N.J., Flashburg M.H., Paskin S., Grossman R. A regional anesthetic technique compared to general anesthesia for outpatient knee arthroscopy. Anesth Analg. 1986;65:185-187.
50. Reyford H., Krivosic-Horber R., Adnet P., et al. Lateral cutaneous nerve of the thigh block—155 cases. Reg Anesth. 1992;17:S47.
51. Wardrop P.J., Nishikawa H. Lateral cutaneous nerve of the thigh blockade as primary anesthesia for harvesting skin grafts. Br J Plast Surg. 1995;48:597-600.
52. Bridenbaugh P.O. The lower extremity: Somatic blockade in clinical anesthesia and management of pain. Philadelphia: JB Lippincott; 1988:pp 429-431.
53. Shannon J., Lang S.A., Yip R.W., Gerard M. Lateral femoral cutaneous nerve block revisited: A nerve stimulator technique. Reg Anesth. 1995;20:100-104.
54. Soeding P.E., Sha S., Royse C.E., et al. A randomized trial of ultrasound-guided brachial plexus anesthesia in upper limb surgery. Anesth Intensive Care. 2005;33:719-725.
55. Willschke H., Marhofer P., Bösenberg A., et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth. 2005;95:226-230.
56. Tumber P.S., Bhatia A., Chan V.W. Ultrasound-guided lateral femoral cutaneous nerve block for meralgia paresthetica. Anesth Analg. 2008;106:1021-1022.
57. Hurdle M.F., Weingarten T.N., Crisostomo R.A., et al. Ultrasound-guided blockade of the lateral femoral cutaneous nerve: Technical description and review of 10 cases. Arch Phys Med Rehabil. 2007;88:1362-1364.
58. Ng I., Vaghadia H., Choi P.T., Helmy N. Ultrasound imaging accurately identifies the lateral femoral cutaneous nerve. Anesth Analg. 2008;107:1070-1074.