Local anesthetic agents: Mechanism of action
Nerve cell membrane and depolarization
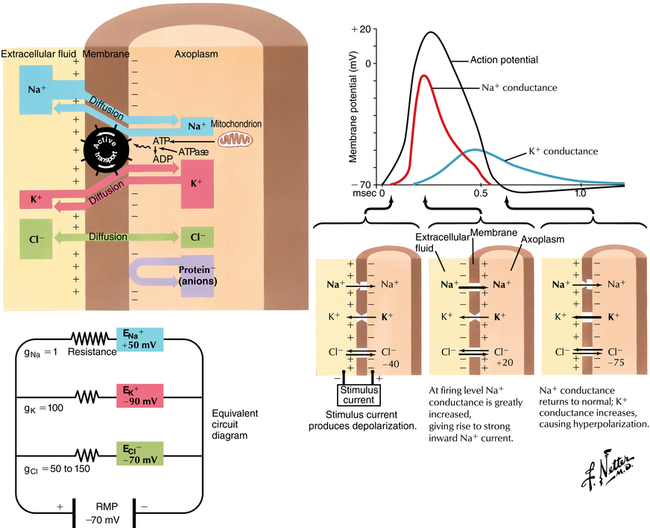
The cell membrane creates a barrier between the Na+-rich extracellular fluid and the K+-rich intracellular fluid, creating a resting membrane potential of −60 to −90 mV (Figure 115-1). There is constant movement of Na+ ions through Na+ channels that spontaneously open and close; active transport of Na+ out of the cell maintains the resting membrane potential. When an appropriate stimulus of adequate magnitude opens a sufficient number of Na+ channels, the surrounding membrane depolarizes (becomes less negative), recruiting additional channel openings—a cascade of open channels allows more Na+ to enter the cell, with K+ diffusing out of the cell through K+ channels to the point that the entire membrane depolarizes, producing an all-or-nothing electrical signal (action potential) that is propagated along the axon. Once the action potential passes, an energy-dependent mechanism reestablishes the concentrations of Na+ and of K+, restoring the resting membrane potential.
Structure of local anesthetic agents
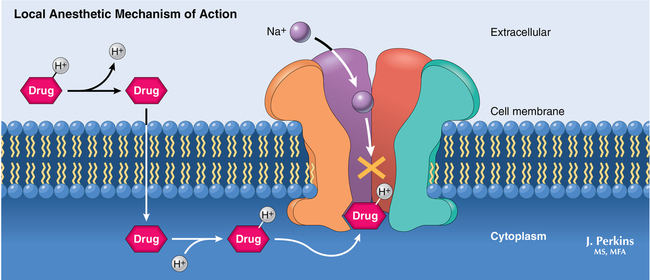
Molecules of local anesthetic agents contain an aromatic lipophilic end, which is connected by an intermediate chain to a hydrophilic tertiary amine (weak base). The intermediate chain is either an amide or an ester linkage; this linkage is the basis for the two different classes of local anesthetic agents (esters and amides), which have similar mechanisms of action but different metabolic pathways. Because the nonionized form of the molecule crosses the cell membrane, compounds that are more lipophilic have a faster onset of blockade. And, because local anesthetic agents are weak bases, compounds with a pKa close to physiologic pH will have a faster onset of blockade as more molecules remain in the nonionized state. Clearance of the drug from the site of injection and protein binding of local anesthetic agents by α1-acid glycoprotein also affect the duration of action because it is the concentration of free drug that is available to diffuse across the membrane that determines blockade (Figure 115-2, Table 115-1).
Table 115-1
Chemical and Physical Properties of the Most Commonly Used Local Anesthetic Drugs
| Property | Lidocaine | Mepivacaine | Bupivacaine | Ropivacaine | Levobupivacaine |
| Molecular weight | 234 | 246 | 288 | 274 | 288 |
| pKa | 7.7 | 7.6 | 8.1 | 8.1 | 8.1 |
| Liposolubility* | 4 | 1 | 30 | 2.8 | 30 |
| Partition coefficient | 2.9 | 0.8 | 28 | 9 | 28 |
| Protein binding (%) | 65 | 75 | 95 | 94 | 95 |
| Equipotency (%) | 2 | 1.5 | 0.5 | 0.75 | 0.5 |
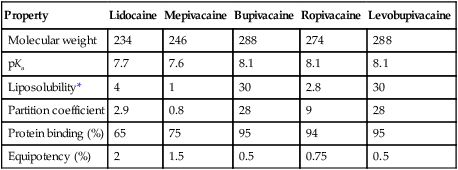
*Liposolubility of each of the local anesthetic agents, as compared with mepivacaine, (e.g., lidocaine is four times more lipid soluble than mepivacaine).