Chapter 97C Liver transplantation in patients with fulminant hepatitis
Overview
Fulminant hepatic failure (FHF) is a rare, cataclysmic clinical entity resulting from acute massive necrosis of liver cells and is characterized by the rapid onset of a potentially mortal condition in a previously healthy individual. Occurrence is estimated to range between 1% and 1.2% of all jaundice cases in acute hepatitis (Bernuau et al, 1999). Depending on the etiology and severity of liver injury, some patients may undergo rapid hepatic regeneration and spontaneously recover with ad integrum restitution of the liver; however, the majority will die without orthotopic liver transplantation (OLT).
Before the advent of liver transplantation (see Chapter 97A) and advances in the intensive care management of these patients (see Chapters 72, 73, and 97B), prognosis was very poor, with mortality rates exceeding 80% (Bernuau et al, 1986; Riordan et al, 2008). At the present time, mortality rates are approximately 30% to 40%; whereas 20% to 25% of patients require liver transplantation, only 40% to 45% of patients are able to survive spontaneously (Lee et al, 2008; Ostapowicz et al, 2002; Polson, 2008). Prompt recognition and early management decisions are critical and can affect outcome. Because of their medical complexity, patients with FHF should be managed in an intensive care unit (ICU) in centers with active liver transplant programs. Referral should be done as early as possible, as it is hazardous to transfer patients later in the disease course because of increased intracranial pressure (ICP) and clinical instability. It has been suggested that transfer to a specialized ICU should be arranged when the patient’s international normalized ratio (INR) exceeds 2.0 or grade 2 hepatic encephalopathy develops (Stravitz & Kramer, 2009). High-risk patients, including those at the extreme ends of the age range (>45 years or <10 years) or with etiologies of acute liver failure (ALF) that carry a poor prognosis should be considered for transfer to a liver transplant center whenever any degree of encephalopathy develops.
Definition
Originally defined by Trey and Davidson in 1970 as an acute hepatitis complicated by ALF with hepatic encephalopathy (HE) occurring less than 8 weeks after the onset of jaundice, it is nowadays accepted that FHF is a complex clinical syndrome characterized by the development of a severe acute liver injury with impaired synthetic function and encephalopathy in a patient without previous liver disease within 26 weeks from the onset of jaundice to the development of encephalopathy. The chronologic evolution of the clinical course of ALF, estimated by the interval between onset of jaundice and encephalopathy, is predictive of clinical features and outcome (O’Grady et al, 1993). According to the length of this presentation interval, Bernuau et al (1986) has described two distinct types of FHF: fulminant (interval <2 weeks) and subfulminant (interval between 2 weeks and 3 months).
The group at King’s College Hospital (KCH) in London has defined three groups of patients: those with hyperacute liver failure (development of encephalopathy within 7 days after jaundice), acute liver failure (within 7 to 28 days), and subacute liver failure (from 28 days to 3 months) (O’Grady et al, 1993). Hyperacute presentation is more frequently associated with hepatitis A virus (HAV) or paracetamol (acetaminophen) overdose, whereas idiosyncratic drug reactions usually present a delayed-onset clinical course. Cerebral edema, a major complication of ALF, is more frequent in hyperacute liver failure, but it is rare in subfulminant disease. In contrast, manifestations of portal hypertension with ascites and renal failure are far more common in patients with subfulminant liver failure.
The cessation of liver cell destruction, rather than the occurrence of liver regeneration, appears to be the most critical variable governing outcome (Bernuau et al, 1999). A protracted course in patients with a subfulminant presentation are associated with persistence of elevated transaminases, suggesting persistence of liver cell destruction. Not surprisingly, hyperacute disease has a relatively good likelihood of spontaneous recovery compared with subacute disease, which has a very poor prognosis without liver transplantation (O’Grady et al, 1993).
To what extent these differences in survival are related to the rapidity of onset or to etiology itself is difficult to say. Likelihood of spontaneous recovery is not uniform and depends on the etiology of the insult. Previously, mortality was thought to be lower in patients with HAV and acetaminophen overdose (50% to 60%), compared with 80% to 95% mortality rate in patients with non–acetaminophen-induced ALF, halothane anesthesia, fulminant disease of indeterminate cause, and non-A, non-E fulminant hepatitis (O’Grady, 1988); however, more recent data used to evaluate prognosis of indeterminate liver failure showed no significant difference compared with other etiologies (Wei, et al, 2008).
Age younger than 10 years or older than 40 years is considered an adverse marker of outcome. Outcome in older patients was recently reevaluated by Schiodt and colleagues (2009), who observed superior overall survival in younger compared with older patients (67.9% vs. 48.2%; P < .001), yet no significant differences were demonstrated in reference to spontaneous survival among both groups, suggesting that hepatic regeneration is preserved in the elderly, a fact that is in accordance with the rise in α-fetoprotein (AFP) levels that is similar in both populations.
Other factors that have been associated with lower survival are a high body mass index (BMI), although data are still controversial (Kanda et al, 2005), and the presence of systemic inflammatory response syndrome (SIRS) (Miyake et al, 2000, 2007). The magnitude of SIRS in patients with ALF and infection correlate with mortality: 16.7%, 28.4%, 41.2%, and 64% in patients with 0, 1, 2, and 3 maximum concurrent SIRS components, respectively (Rolando et al, 2000).
ALF adversely affects most organ systems, and the majority of deaths result either from sepsis and subsequent multiorgan failure or from cerebral edema that evolves to intracranial hypertension and brainstem herniation. Patients with ALF are susceptible to a wide variety of additional complications, including renal failure, hypoglycemia, metabolic acidosis, coagulopathy, and cardiopulmonary distress. Advances in the intensive care management of patients with ALF and the early recognition of these complications has greatly contributed to a marked improvement in overall survival (see Chapters 72, 73, and 97B).
Etiology
ALF is an infrequent entity that can affect patients of all ages, and it shows a predominance in females that remains unexplained. Etiologies and prognoses vary in adults compared with infants and children (Polson et al, 2005; Squires et al, 2006). ALF results from a wide variety of causes (Box 97C.1), yet relative frequency of etiologies shows geographic (Lee et al, 2008) and temporal variations. These variations depend mainly on epidemiologic, socioeconomic, and cultural factors. Worldwide, acute viral hepatitis is the predominant cause of ALF, particularly in developing countries and especially in pediatric series. Data from Sudan (Mudawi, 2007), Bangladesh (Alam et al, 2009), Mexico (Fernandez Hernandez et al, 2003), and India (Acharya et al, 1996; Dhiman et al, 1998; Jaiwal et al, 1996) report a prevalence of viral hepatitis among patients with ALF that varies from 27% to 94% and includes hepatitis A, B, D, and E and a combination of hepatitis A with hepatitis E.
In the United States and Western Europe, viral hepatitis prevalence in patients with ALF is decreasing (Taylor et al, 2006), and acetaminophen overdose has become the most common cause of fulminant hepatitis (Larson et al, 2005), resembling the experience in the United Kingdom, where it is responsible for more than 70% of ALF cases (Williams, 1996). Comparing two time periods, after 1993 in France, 28% of patients with ALF were affected with acute viral hepatitis (Feray et al, 1993) compared with 48% before 1993 (Wright et al, 1991). The United States Acute Liver Failure Study Group (USALFSG), a multicenter network established in 1997 to gather data prospectively on all forms of ALF, observed in a recent analysis of 1033 patients enrolled through 2007 that acetaminophen toxicity accounts for approximately 46% of ALF cases in the United States, followed by ALF of indeterminate origin (15%) and idiosyncratic drug reactions (12%).
The clinical pattern of presentation of acetaminophen toxicity is still different in the United States (Zimmerman et al, 1995) compared with the United Kingdom, because only half of the time does it stem from ingestion of large doses taken in an intent to commit suicide; in the remainder, ingestion results from unintentional overuse of acetaminophen-containing compounds for pain or fever relief at therapeutic doses, mainly by patients with ongoing alcohol abuse, starvation, or in those concurrently taking medications known to induce the cytochrome P450 system, such as anticonvulsants, a situation known as therapeutic misadventure (Licht et al, 1980; Seeff et al, 1986; Wootton & Lee, 1990). Although the mortality rates associated with this syndrome are lower than that observed with suicidal ingestion, they are still at least 20% in most reviews (Kumar et al, 1991; Wootton & Lee, 1990).
Idiosyncratic drug reactions are the cause of about 15% of ALF cases in the United States and other countries. Antibiotics, nonsteroidal antiinflammatory drugs (NSAIDs), and anticonvulsants constitute some of the most commonly involved classes of prescription medications involved (Chalasani et al, 2008; Ostapowicz et al, 2002; Russo et al, 2004). It is expected that enhanced awareness of potential risks and drug mechanisms of liver injury may minimize in the future the frequency of serious hepatotoxicity (Murray et al, 2008).
Despite extensive evaluation of the etiology of ALF, in approximately 15% to 40% of cases, the cause remains elusive, even though the incidence of cryptogenic disease is declining (Larson et al, 2005). Available data suggest that in 20% of these patients, ALF may be attributable to undiagnosed acetaminophen overdose (Davern et al, 2006).
Clinical Presentation
HE is a major defining criterion of FHF in the adult population. Children, particularly the very young, do not demonstrate classic features of encephalopathy, resulting in a revision of the definition of ALF for this specific population of patients to include pediatric patients with advanced coagulopathy, regardless of mental status (Squires, 2008). Severity of encephalopathy can be classified into four stages according to the definition of Trey and Davidson (1970): stage 1, slow consciousness; stage 2, accentuation of stage 1 and presence of asterixis; stage 3, presence of deep confusion or reactivity only to vocal stimuli; and stage 4, presence of deep coma with minimal (4a) or no response (4b) to noxious stimulus.
The development of cerebral edema is a life-threatening complication. Although precise pathogenic mechanisms remain unclear, most widely accepted theories relate to the development of astrocyte swelling and cerebral hemodynamic derangements characterized by high cerebral blood flow and failure of cerebral autoregulation in response to changes in mean arterial pressure (Larsen, 1996; Larsen et al, 2000), resulting in cerebral edema (see Chapters 72 and 97B). Loss of cerebral autoregulation of blood flow has been related to the rapidity of occurrence of electrolyte disorders in ALF, in contrast to what happens in cirrhosis or even in subacute liver failure, in which the mechanisms of osmotic regulation are still active. Cerebral edema develops in 75% to 80% of patients with grade 4 encephalopathy (Williams, 1991), and incidence is variably reported, although it appears to be progressively less frequent.
Several risk factors for the development of cerebral edema have been identified. It is more prevalent among patients with hyperacute liver failure compared with subacute disease, probably as a result of the rapid accumulation of glutamine in hyperacute presentation that overwhelms the mechanisms of compensatory expulsion of organic osmolytes from the astrocytes. High serum ammonia concentrations (>150 to 200 µmol/L) also increase the risk of cerebral edema, although the relationship between ammonia and ICP is not linear (Bernal et al, 2007; Bhatia et al, 2006; Clemmesen et al, 1999a).
Recent work suggests a central role of proinflammatory cytokines—such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 and IL-1β—in patients with uncontrolled ICP, indicating activation of the inflammatory cascade in the brain (Wright, 2007). Other variables predictive of progression of intracranial hypertension and HE are the Model for End-Stage Liver Disease (MELD) score alone and in combination with ammonia levels, young age, requirement for vasopressors and renal replacement therapy, and the presence of infection and/or SIRS (Bernal et al, 2007; Vaquero et al, 2003). Evidence also emphasizes the importance of free-radical formation occurring at a mitochondrial level as the potential mediator of cellular dysfunction induced by ammonia neurotoxocity (Norenberg et al, 2009).
In severe cases, consequences of cerebral edema within the confinement of the cranial vault are the development of intracranial hypertension with potential risk of brainstem herniation and a decrease in intracerebral perfusion (Blei, 1991; see Chapter 97B). Severity of encephalopathy has prognostic implications, and overall prognosis for patients with only grade 1 or 2 encephalopathy is good, whereas for patients with grade 3 or 4 encephalopathy, prognosis is much poorer. Full recovery of cerebral function is the rule in patients whose liver function recovers, but permanent brain damage has been observed in patients making an otherwise complete recovery of liver function (O’Brien et al, 1987).
Coagulopathy
Along with neurologic involvement, coagulopathy is also a major criterion of ALF, resulting from the inadequate hepatic synthetic capacity of clotting factors. This is associated with increased consumption, particularly of factors II, V, VII, and X; reduced clearance of both activated factors and/or factor inhibitor complexes; and quantitative and qualitative platelet dysfunction (see Chapters 72 and 97B). Patients are seen with low fibrinogen and low levels of factors II, V, VII, IX, and X that result in the prolongation of prothrombin time (PT) and partial thromboplastin time (PTT) (Gazzard et al, 1975). Platelet counts are less than 100,000/mm3 in more than two thirds of patients, and platelet function is altered (O’Grady et al, 1986); however, serum concentrations of thrombopoietin do not correlate with platelet counts (Schiodt et al, 2003), and the pathogenesis of thrombocytopenia remains unclear.
Although low-grade fibrinolysis and intravascular coagulation may occur, these syndromes are hard to distinguish from the changes that result from the failure of hepatic synthesis alone (Preston, 1991). Among patients with ALF, it has been observed that coagulopathy was moderate in 81% (INR 1.5 to 5), severe in 14% (INR 5 to 10), and very severe (INR >10) in 5%. Certain etiologies are associated with greater severity of coagulopathy, and ALF in fatty liver of pregnancy represents the least severe coagulopathy (Muñoz et al, 2008).
In spite of the evidence of a bleeding diathesis in patients with ALF, spontaneous bleeding is unusual, being most common in the gastrointestinal (GI) tract (8%) or brain (<1%); yet spontaneous intracranial bleeding is exceedingly rare in the absence of an ICP monitor. The incidence of upper GI bleeding in ALF patients has been decreased by gastric acid suppression with intravenous H2 receptor antagonists (McDougall et al, 1978), therefore intravenous H2 blockers or proton pump inhibitors are recommended.
Prophylactic administration of fresh frozen plasma (FFP) is usually not recommended, because it has not been proven to influence mortality rate, it can interfere with assessment of liver function, and it may worsen cerebral edema (Caraceni et al, 1995); therefore it is only indicated in the setting of active hemorrhage or prior to invasive procedures. The goal for correction of the INR to a value of no higher than 1.5 to minimize bleeding risk arises from common practice, although it has not been adequately tested. Use of recombinant human factor VIIa has been evaluated in small pilot studies (Chuansumrit et al, 2000; Negrier et al, 2000; Shami et al, 2003), and it was associated with improvement or normalization of PT and bleeding control. It has also been suggested that the combination of recombinant human factor VIIa with FFP is superior to FFP alone in correcting coagulopathy, with a beneficial effect on morbidity and mortality (Shami et al, 2001). Larger controlled studies are still required, but it has become acceptable to administer recombinant factor VIIa (rFVIIa) when FFP has failed to correct PT-INR to an acceptable level, or when the patient has become volume overloaded, before performing invasive procedures with a high risk of bleeding, such as transjugular liver biopsy or placement of an ICP monitor. Serial determinations of INR and factor V provide useful prognostic information in the follow-up of patients with ALF.
Infections
In one prospective study of 50 patients, 80% had culture-proven infection, and in half of the remaining patients, infection was suspected, but cultures were negative (Rolando et al, 1990). gram-positive organisms, mainly streptococci and Staphylococcus aureus, predominate in ALF, suggesting that entry through the skin is more important than intestinal entry, the pathway of gram-negative organisms (Wyke et al, 1982). Leukocytosis and fever are frequently absent (<50%), but a high index of suspicion of active infection should be raised at the worsening of encephalopathy, renal function, or clinical status. Daily bacterial surveillance cultures (blood and urine) and chest radiographs are recommended, as early diagnosis of infection may improve outcome.
Aggressive treatment of presumed infection is essential, because prophylactic antibiotic regimens have shown little benefit (Rolando et al, 1993); however, a low threshold for starting appropriate antibiotics and antifungals should be maintained. Administration of antibiotics is recommended when infection or the likelihood of impending sepsis is high, surveillance cultures reveal significant isolates, clinical progression of HE or renal failure is apparent, hypotension is refractory, or SIRS is evident, individual components of which include temperature above 38° C or below 36° C, white blood count above 12,000/mm3 or below 4,000/mm3, and pulse greater than 90 beats/min (Stravitz et al, 2007).
Broad-spectrum coverage for gram-positive and gram-negative bacteria, such as with a third-generation cephalosporin, should be chosen with consideration of patient-specific isolates from surveillance cultures and historic hospital-specific isolates (Stravitz et al, 2007). Vancomycin is specifically recommended for all patients with possible intravenous catheter–related sepsis and/or risk factors for infection with methicillin-resistant Staphylococcus aureus (MRSA). An antifungal agent also is recommended in any patient who does not display prompt improvement in signs of infection after institution of antibacterial agents (Stravitz et al, 2007).
The occurrence of infection may aggravate all instances of the inflammatory mechanisms that are activated by ALF and may precipitate the occurrence of multiorgan failure and death. A finding of disseminated fungemia is predictive of a poor prognosis (Walsh et al, 1983).
Renal Disorders
Renal compromise is frequent in patients with fulminant and subfulminant hepatic failure, and oliguria is a common finding (Caraceni et al, 1995). Acute renal failure complicates 30% to 50% of cases, taking an increase in creatinine concentrations as a diagnostic criterion; yet normal creatinine concentrations do not exclude the presence of acute renal failure. Therefore the true incidence of renal compromise is probably underestimated.
Acute renal failure in ALF can result from two different situations: First, direct nephrotoxic effects result from some causes of ALF, as has been observed with toxic quantities of acetaminophen (Mazer et al, 2008) and NSAIDs and with Amanita mushroom intoxication (Escudié et al, 2007; Joshi et al, 2007), usually resulting in acute tubular necrosis. Second, patients may present with a certain degree of dehydration or may develop functional renal failure that resembles the hepatorenal syndrome of cirrhosis. Pathogenesis remains unclear, even though marked reduction in renal blood flow and glomerular filtration rate is similar to renal perfusion changes in cirrhosis, with marked renal arteriolar vasoconstriction as a result of loss of systemic vascular resistance, activation of compensatory vasoconstrictor systems, and reduced renal prostaglandin excretion; however, ALF has distinctive hemodynamic features, and only rarely is the degree of portal hypertension in ALF comparable to that of hepatorenal syndrome in cirrhotics (Navasa et al, 1992). The mean reported hepatic venous pressure gradients for patients with ALF and renal dysfunction is significantly lower compared with those in cirrhotic patients with hepatorenal syndrome (14 vs. 21 mm Hg) (Navasa et al, 1992; Ruiz-del-Arbol et al, 2005).
Recently, Leithead and colleagues (2009) demonstrated that ALF is associated with SIRS, and this association is independent of the presence of infection and severity of liver injury. Recognition of renal failure also has important clinical implications, and once established, it is usually progressive and associated with increased mortality (Jain et al, 2000; O’Grady et al, 1989; Ring-Larsen et al, 1981). Treatment options without transplantation are limited, and preventive measures are restricted to maintain adequate hemodynamics, early identification and adequate treatment of infection, and avoidance of nephrotoxic agents such as NSAIDs and aminoglycosides.
Renal replacement therapy (RRT) is usually recommended early to correct intravascular fluid overload, electrolyte disorders, or acidosis. Potential triggers for initiation of RRT might be low urine output, a rise in creatinine levels over 0.3 mg/dL over baseline, or a rise in serum ammonia above 150 µmol/L (Stravitz & Kramer, 2009).
Continuous rather than intermittent modes of renal replacement, such as continuous venovenous hemofiltration, are usually preferred to avoid abrupt shifts in solute concentrations that can result in increased ICP (Davenport et al, 1993).
Hemodynamic Complications
ALF is characterized by a hyperdynamic circulation with low systemic vascular resistance (SVR), high cardiac output, and low mean arterial pressure (MAP). Features of systemic inflammation, hemodynamic patterns, and progression of organ dysfunction resemble sepsis. Generalized systemic vasodilation is not limited to the splanchnic circulation, as it occurs in cirrhotics (Maroto et al, 1993; Ytrebø et al, 2006). Volume status in patients with ALF is difficult to assess, so placement of a pulmonary artery catheter may be of help, as management of hemodynamic balance can be complicated by the occurrence of elevated ICP and renal dysfunction. In most hypotensive patients, initial volume resuscitation is required, and it has been recommended that fluid replacement with colloids (albumin) should be preferred, rather than crystalloids (saline), and all solutions should include dextrose to maintain euglycemia (Polson et al, 2005).
Even after adequate volume repletion, vasopressors may be required to maintain a MAP of at least 50 to 60 mm Hg and a cerebral perfusion pressure (CPP) of 60 to 80 mm Hg (Polson et al, 2005). Vasopressor support with epinephrine, norepinephrine, or dopamine is usually instituted to maintain adequate perfusion of vital organs.
Hepatectomy has been advocated based on anecdotal references as a terminal salvage therapy in patients with severe refractory circulatory dysfunction. It has also been suggested that adrenal dysfunction might be an additional contributing factor to circulatory collapse; however, the benefits of supplemental corticosteroids in these patients are still unknown (Harry et al, 2002).
Pulmonary Complications
Pulmonary compromise is usually present in 30% of patients with ALF. Impaired gas exchange leading to hypoxemia and mixed acidosis may favor progression of HE (Kramer et al, 1991; Larsen et al, 2000), and mechanical ventilation may be required to ensure oxygenation and to secure the airway in patients who progress to grade 3 encephalopathy. Caution should be maintained, as it has been observed that positive end-expiratory pressure (PEEP) can worsen cerebral edema. In patients with acetaminophen intoxication, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) can occur in one third of patients with ALF and can cause intractable hypoxemia that may contribute to death (Baudouin et al, 1995).
Metabolic Disturbances
Common metabolic abnormalities in FHF include acid-base and electrolyte disorders, hypophosphatemia, and hypoglycemia. Among acid-base disorders, hypocapnia with mixed alkylosis is frequent in the early stages of FHF in more than 50% of patients (O’Grady et al, 1993), yet as ALF progresses, patients typically evolve to metabolic acidosis with respiratory alkalosis. At the same time, ALF is a catabolic state characterized by negative nitrogen balance and increased resting energy expenditure. It is recommended that metabolic homeostasis be carefully maintained, and overall nutritional status—as well as phosphate, glucose, potassium, and magnesium levels—must be frequently monitored (Polson et al, 2005). Enteral nutrition should be administered early whenever possible, avoiding excessive free-water provision that can result in hypoosmolality, which aggravates cerebral edema. No firm evidence proves that branched-chain amino acids are superior to other enteral preparations (O’Grady et al, 1989; Naylor et al, 1989). If enteral feeding is contraindicated, parenteral nutrition remains an option, but severe protein restrictions should be avoided.
Management
As a result of its high mortality, heterogeneity, and low frequency, FHF is extremely difficult to study, randomized controlled trials are limited (Kaidu, 2008), and optimal management remains poorly defined and center specific. Management should be centered in three main areas: 1) definition of etiology so that specific treatment can be provided in selected cases; 2) prevention, recognition, and early treatment of complications that could potentially lead to multiorgan failure and death; and 3) assessment of prognosis for distinguishing patients who have the potential to improve spontaneously versus those who will require liver transplantation and who should be urgently included on the waiting list (see Chapters 72, 73, and 97B).
Specific treatments are limited and are reserved exclusively for a few etiologies, and any available treatment should be delivered as early as possible (Box 97C.2). Some treatments have been systematically studied only for acetaminophen overdose. The role of N-acetylcysteine (NAC) in limiting liver injury through repletion of hepatic glutathione (Mitchell et al, 1974), improving prognosis, has been shown to be effective and safe in large case series (Harrison et al, 1990; Smilkstein et al, 1988, 1991) and in a small, controlled trial (Keays et al, 1991); firm evidence supports the utility of NAC administration, even when there is doubt concerning timing, dose ingested, or plasma concentration of acetaminophen, and NAC should not be withheld, even if ingestion was 48 to 72 hours before the patient sought medical attention. In acetaminophen overdose, oral NAC is recommended as first-line therapy in patients with mild or no encephalopathy, whereas intravenous NAC should be reserved for patients with advanced encephalopathy, hypotension, or some other reason to suggest that oral dosing might not be tolerated, such as vomiting or ileus. The length of NAC administration should be determined by clinical improvement—resolution of HE, improving coagulopathy, and declining transaminases—or outcome (death or liver transplantation) rather than by time or serum acetaminophen levels.
In addition to its role in limiting liver injury, NAC also has beneficial effects on systemic hemodynamic parameters and oxygen delivery to peripheral tissues (Harrison et al, 1991, 1996). Consequently, it has also been evaluated as a treatment for other forms of ALF unrelated to acetaminophen overdose. A recent study evaluated 173 patients with non-acetaminophen ALF, who were randomly allocated to receive either intravenous NAC or placebo; no benefit was demonstrated on 21-day survival, although transplant-free survival and 1-year posttransplant survival were improved. Furthermore, in the subgroup of patients with grade 1 or 2 HE, survival at 1 year was significantly better in those who received NAC than in all other individuals (Lee et al, 2009).
Management of Complications of Acute Liver Failure
Cerebral Edema and Intracranial Hypertension
The most accepted pathogenetic theory of HE is the increased production of nitrogenous substances in the gut lumen, and effective medical management of cerebral edema in patients with ALF has been directed to the reduction of the generation and absorption of these products through orally administered lactulose and/or nonabsorbable antibiotics such as rifaximin. But the ability of these modalities to lower the risk of cerebral edema in patients with ALF has not yet been tested, and the utility of oral lactulose in this setting is controversial (Alba et al, 2003) and may even have detrimental effects, as it may increase gaseous distension of the bowel, predispose to aspiration, and foster intravascular depletion.
The monitoring of ICP by using an intracranial sensor is controversial (Blei, 1991; Schafer & Shaw, 1989; see Chapter 97B), but it is used by over half the transplantation programs in the United States (Hoofnagle et al, 1995) and around the world. Major complications are bleeding and infection, although complication rates vary among the different types of catheters used. It has been estimated to be 4% with epidural catheters, compared to significantly higher rates for subdural (22%) and parenchymal catheters (22%), with 1% to 5% of deaths as a direct result of catheter placement (Blei et al, 1993). Some centers report that ICP monitoring is useful to control CPP and to guide the decision to proceed with transplantation (Lidofsky et al, 1992). Before ICP monitor placement, a CT scan of the head is recommended in patients upon progression of encephalopathy or in those who experience an abrupt change in mental status; this is necessary to rule out any other rare intracranial pathology, especially bleeding.
The goal of therapy is to maintain ICP below 20 mm Hg and CPP above 50 mm Hg (Hoofnagle et al, 1995). Patients should be kept in an environment with minimal sensory and physical stimulation, and even endotracheal suctioning should be minimized. Fever increases ICP and should be vigorously treated with cooling measures. Hypo-osmolality, specifically hyponatremia, should be avoided and corrected immediately. The head of the patient’s bed should be elevated to 30 degrees, except if CPP falls below 30 mm Hg, in which case the patient should be put in the supine position. Spontaneous hyperventilation should not be inhibited; this occurs regularly in patients with ALF and results in mild hypocapnia that promotes cerebrovascular constriction (Baudouin et al, 1995; Strauss et al, 1998).
The development of intracranial hypertension despite these prophylactic measures should prompt urgent treatment. First-line therapy includes increasing blood osmolality, with mannitol and/or hypertonic saline boluses to draw water from swollen astrocytes back into the intravascular space. Early reports demonstrated the efficacy of mannitol (1 g/kg body weight) to decrease ICP (Canalese et al, 1982; Nath & Gailbraith, 1986), yet it was not effective in returning ICP to an acceptably low level (<25 mm Hg) in patients with severe intracranial hypertension (>40 to 60 mm Hg) (Hanid et al, 1980). In addition, improvements usually were temporary and required multiple doses, with the attendant risk of hyperosmolality. When renal failure with oliguria is present, mannitol can be deleterious, so continuous venovenous hemofiltration or hemodialysis with polyacrylonitrile membranes is performed to decrease hyperkalemia, fluid overload, and ICP.
If no response is seen, or relapse of ICP is observed after mannitol administration, alternative measures may include the use of hypertonic saline boluses, the induction of therapeutic hypothermia, or sedation with propofol (Wijdicks & Nyberg, 2002), barbiturates (Lidofsky et al, 1992), or intravenous boluses of indomethacin (Tofteng et al, 2004). Indomethacin perfusion (25 mg IV by every peak of ICP) has been shown to normalize ICP and CPP, probably through a direct arteriolar constriction of cerebral vessels (Clemmesen et al, 1997). Induction of moderate hypothermia (32° C to 33° C) is useful in treating uncontrolled elevations in ICP (Ginsberg et al, 1992; Jalan et al, 1999; Kurt et al, 1996; Roberts & Manas, 1999), probably by preventing hyperemia, altering brain ammonia or glucose metabolism, or by a combined effect. Concern remains over potential deleterious effects of hypothermia on risk of infections, coagulation disorders, cardiac arrythmias, and liver regeneration rates (Schubert, 1995). As a final desperate measure in patients with intracranial hypertension that is refractory to all medical therapy, total hepatectomy has been advocated to remove the major source of proinflammatory cytokines that contribute to cerebral hemodynamic derangements.
The observation that patients with ALF may have subclinical, nonconvulsive seizure activity that could result in increased cerebral blood flow that favors cerebral edema provided the rationale for the evaluation of seizure prophylaxis with IV infusion of phenytoin in these patients. Two randomized controlled trials in patients with ALF and advanced HE came to different conclusions with regard to the efficacy of phenytoin in preventing seizures and cerebral edema and in influencing survival (Bahtia et al, 2004; Ellis et al, 2000).
Prognostic Factors and Criteria for Liver Transplantation in Patients with Acute Liver Failure
In spite of great advances in the intensive care management of patients with ALF, patients managed exclusively with medical supportive therapy have poor survival. The only therapy proven to significantly improve outcome is OLT, which is associated with 1-year survival rates greater than 80% (Lee et al, 2008). Unfortunately, the window of time is very narrow for the application of liver transplantation, as the procedure must be performed at the optimal time, not too early but never too late, to avoid unnecessary transplantations in patients who would survive otherwise and to prevent transplantation in those who would not survive, even with a new liver (Lo, 2008).
An ideal model to predict outcome would ensure that all patients who need transplantation may get one (positive predictive value), and all patients who may survive would not (negative predictive value), but presently none exists that completely fulfills these goals (O’Grady, 2007). The ability to predict the likelihood of spontaneous recovery or death without OLT remains of paramount importance in patients with ALF (see Chapters 72 and 97B). Many criteria have been proposed to anticipate the probability of death without OLT (Box 97C.3), but data are insufficient to recommend a particular scheme, given that none have been found to be adequately sensitive and specific.
Box 97C.3 Prognostic Factors in Fulminant Hepatitis
Outcome in children is more favorable (Dhawan et al, 2004). In the series of Dhiman and colleagues (2007), the survival rate in patients older than 50 years was lower than in younger patients. In an American study by Ostapowicz and colleagues (2002), age does not seem to correlate with outcome, although spontaneous survival was higher in patients aged 26 to 35 years. The lowest survival rate (33%) corresponds to patients older than 65 years, who often have more comorbidities but less acetaminophen intoxication.
In a study by Brandsaeter and colleagues in 2002, of 315 patients listed for transplantation, spontaneous survival was higher in patients with HAV infection (43%), followed by acetaminophen overdose (31%), HBV infection (8%), liver disease of indeterminate origin (7%), and other drug-induced ALF (0%). In other series the same favorable prognosis corresponds to acetaminophen overdose, HAV infection, and pregnancy-related ALF but not to patients with disease of an indeterminate cause, non–acetaminophen-related ALF, HBV infection, autoimmune hepatitis, Wilson disease, or Budd-Chiari syndrome (Lee et al, 2008; Ostapowicz et al, 2002). A higher degree of encephalopathy (grades 3 and 4) on admission also seems to be a significant independent variable of poor outcome (O’Grady et al, 1989).
Two statistical models have been developed for predicting outcome: one by the KCH group (O’Grady et al, 1989) in London, based on a retrospective cohort of 588 medically managed ALF patients and later validated in an additional prospective study of 175 ALF patients; the other by the Paris group (Bernuau et al, 1986, 1991). At the present time, they are the most widely used prognostic criteria.
At KCH, prognostic criteria discrimination was etiology based, as predictors were stratified according to whether ALF was secondary to acetaminophen ingestion, a condition in which recovery is more probable even in the presence of severe synthetic dysfunction. In patients with acetaminophen-induced fulminant hepatitis (FH), a pH less than 7.3 at 24 hours or more after overdose is an indication per se for liver transplantation, after appropriate volume replacement and correction of hypothermia. Otherwise, liver transplantation should be considered in the presence of all three of the following factors: 1) PT greater than 100 seconds, 2) grade 3 or 4 encephalopathy, and 3) serum creatinine (SCr) greater than 300 µmol/L. In non–acetaminophen-related FH, the decision whether to transplant is based on the presence of three of the following: 1) non-A, non-B hepatitis; 2) drug-induced hepatitis; 3) halothane exposure; 4) serum bilirubin greater than 300 µmol/L; 5) PT greater than 50 seconds; 6) age younger than 10 years or older than 40; or 7) time from jaundice to encephalopathy of more than 7 days. PT greater than 100 seconds in isolation is considered an indication for liver transplantation (O’Grady et al 1989, 1991).
In France, selection was made on the basis of factor V level. According to this group, patients younger than 30 years with FH and encephalopathy with a level of factor V less than 20%, or less than 30% in patients older than 30 years, should be included on the transplant list (Bernuau et al, 1986; Bismuth et al, 1987, 1995).
The KCH criteria have good clinical applicability with few differences in sensitivity and specificity as reported by Shakil and others (2000). Generally, KCH criteria are useful in predicting death, although the absence of these criteria is not predictive of survival.
Increases in arterial blood lactate (Bernal et al, 2002) and serum phosphate concentrations (Schmidt & Dalhoff, 2002) are factors predictive of poor prognosis in patients with acetaminophen-induced ALF, and these have been proposed as additional criteria to those initially reported by the KCH group. Similarly, serum AFP levels increase in patients undergoing rapid liver regeneration, and increasing levels indicate a better prognosis (Schmidt et al, 2005). Upon admission, serum levels of Cc-globulin, a plasma protein synthesized in the liver (Schiodt et al, 1996), could be used to make decisions about liver transplantation. More recently, a high level of soluble CD163 from activated macrophages has been proposed to predict mortality in patients with ALF (Moller et al, 2007). Interestingly, Volkmann and colleagues (2008) found that caspase activation, which increases cytokines IL-6 and TNF-α, is associated with spontaneous recovery.
In patients with non–acetaminophen-induced ALF, the MELD score has been proposed to identify patients with the worst prognosis; other patients have shown a high rate of spontaneous recovery independent of MELD score (Kremers et al, 2004). Yantorno and colleagues (2007) suggest that MELD scores obtained upon admission may be helpful to establish the optimal timing for pre-OLT evaluation and listing. Nevertheless, they do not propose a cutoff value for MELD.
Dhiman and colleagues (2007) describe another prognostic model and compare its performance with the KCH criteria and to the MELD score. They identify six parameters: age over 50 years, time from jaundice to encephalopathy of more than 7 days, grade 3 to 4 encephalopathy, cerebral edema, PT greater than 35 seconds, and SCr above 1.5 mg/dL. The presence of any three of these factors indicates a poor prognosis. A comparative evaluation suggests that this model was better than both the MELD and the KCH criteria at predicting prognosis; however, according to O’Grady (2007), this is not a valid comparison, because it takes into account only the initial set of data, whereas the KCH model is a dynamic process; the same applies to the study by Yantorno and colleagues.
Barshes and colleagues (2006) made a distinction between low-risk patients (5-year survival, 81%) and high-risk patients (5-year survival, 42%), depending on the four risk factors present at the time of listing for liver transplantation. These factors include 1) recipient age older than 50 years, 2) a BMI of 30 or more, 3) a history of life support, and 4) SCr level above 2 mg/dL. Risk of mortality after transplantation increases by approximately 150% for each additional point.
Detectable liver atrophy has classically been associated with a poor prognosis (Rueff, 1971). Yamagishi and colleagues (2009) reported that the ratio of the estimated liver volume (ELV) to the standard liver volume (SLV) reflects the prognosis of patients with ALF very well. They investigated the usefulness of the ratio of the computed tomography–derived liver volume (CTLV) to the calculated SLV to establish a new prognostic formula to predict the course of ALF. The CTLV/SLV ratio was calculated both at the time of ALF diagnosis and 5 days later. In patients who recovered, liver atrophy was not found at day 0 or at day 5, and liver atrophy progressed in those who died or underwent liver transplantation. Sensitivity was 94.1%, and specificity was 76.9%, but the findings of this small study need to be confirmed in a larger series. In spite of the criteria described above, the controversy about the best prognostic model for ALF will continue.
Survival rates at 1 year of patients undergoing liver transplantation for ALF range between 61% and 76% and are 7% to 15% less than in patients transplanted for chronic end-stage liver disease (European Transplant Registry; Freeman et al, 2008). This is due to the fact that even when liver transplantation is finally achieved, patients eventually die because of poor health status at the moment of transplantation—brain damage, sepsis, or multiorgan failure—or because of the use of marginal or ABO-incompatible donor organs (Farges et al, 1995; Gugenheim et al, 1990). The higher retransplantation rate in patients with ALF is due to the use of these organs, giving way to increasing rates of acute rejection, primary graft nonfunction, and intrahepatic biliary strictures.
Preoperative Management (See Chapter 97B)
Once the need for liver transplantation is established, the patient is included on an emergency list (UNOS status I) and is prepared for the procedure (Wiesner et al, 2004). Coagulation defects should be corrected with plasma, a balance between fluid restriction and a satisfactory CPP should be achieved, and the increase of brain edema should be avoided. A catheter to monitor ICP is useful in patients with grade 4 encephalopathy. We usually perform plasmapheresis immediately before the surgical procedure for toxin removal and FFP supply.
In 1993, Ringe and colleagues described a two-stage total hepatectomy with temporary portacaval shunt, a procedure carried out in patients who have toxic liver syndrome with cerebral edema or multiorgan failure; the patient is left anhepatic and subsequently undergoes liver transplantation. It has been reported that the longest period of time a patient was left anhepatic was 21 hours. Although this extreme procedure can be lifesaving, it is exceptional (Ferraz-Neto et al, 2008) and is only justified by the fact that hepatectomy decreases the serum concentration of circulating proinflammatory cytokines—IL-1, IL-6, and TNF-α—which may be important in the pathogenesis of intracranial hypertension in patients with FHF (Jalan et al, 2002a).
The surgical procedure is basically the same (OLT, see Chapter 98A, Chapter 98B, Chapter 98C ), but other details must be taken into account. For one, patients run a serious risk of bleeding during surgery as a result of severe coagulopathy. The absence of portal hypertension and the presence of an atrophic liver facilitate hepatectomy; however, to avoid brain damage, the surgical approach varies according to the hemodynamic state of the patient and the level of the ICP.
Because of the absence of portal hypertension, the total clamping of the inferior vena cava (IVC) and the portal vein (PV) is poorly tolerated. Hence it is necessary to perform a venovenous bypass with pump-assisted venous return from the IVC and PV to the superior vena cava (SVC) or to preserve the IVC using the piggyback technique (Tzakis et al, 1989). Belghiti and colleagues (1995) reported that the maintenance of portal and caval flow during hepatectomy and graft implantation avoids drops in venous return and cardiac index and prevents other hemodynamic changes that result from venous clamping, release of metabolites, and secondary effects of fluid management seen in patients undergoing liver transplantation (LT) for fulminant and subacute liver failure in the absence of portosystemic venous collaterals. Clamping of the PV in this situation may lead to hemodynamic instability and cerebral edema, thus worsening the previous condition; it also leads to splanchnic venous congestion with associated hemorrhage and intestinal edema.
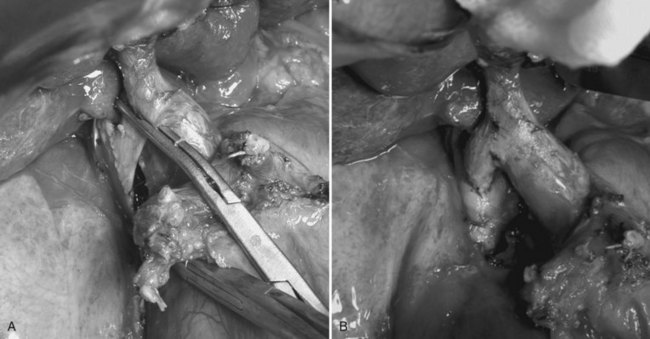
Performing a temporary portacaval anastomosis (end-to-side portocaval shunt) facilitates liver mobilization without changes in the hemodynamic state that could increase the ICP and cause brain damage. A review by Davila and colleagues (2008) supports its use. In adult patients with a very unstable hemodynamic condition, the portocaval shunt using only the right portal branch is recommended, leaving the left branch unclamped (Fig. 97C.1) to avoid interruption of the portal flow. After the shunt has been opened, total hepatectomy is completed by dividing arterial branches, left PV, and major hepatic veins without occlusion of the IVC (Kawasaki et al, 1996). On the other hand, children weighing less than 20 kg tolerate IVC and PV clamping quite well. Also, a rapid clamping and liver removal improve their hemodynamic condition.
Careful anesthetic management of the patient is necessary: the operating table should be positioned at 30 degrees, and fluctuations in ICP should be carefully controlled, and steps to avoid an increase in ICP should be taken to protect the brain; in addition, ICP monitoring should be maintained for 24 hours after liver transplantation, because pressure may increase after surgery (O’Grady et al, 2008). Longer monitoring may be required in cases of poor graft function.
Given the urgency of liver transplantation, marginal donors are often accepted, thus increasing the risk of poor function or primary nonfunction. Initially, ABO-incompatible livers were widely used, but discouraging results have limited their use nowadays (Gugenheim et al, 1990); few reports show good results (Ascher et al, 1993; Toso et al, 2007), although the final decision lies with the surgical team.
Living-Donor Liver Transplantation (See Chapter 98C)
Living donors (LDs) are the only source of organs in countries where the availability of deceased donors is severely restricted, such as in Asia, or scarce, as in Latin America. The use of living-donor liver transplantation (LDLT) for children suffering from ALF has gradually gained acceptance, because the procedure’s lifesaving potential by far outweighs any ethical aspects (Emond et al, 1993; Uemoto et al, 2000). Expansion of LDLT to adult patients with ALF has recently been reported (Lo et al, 1997; Marcos et al, 2000); however, both the donor risk and the ethical dilemma are involved in this issue, because potential donors are likely to be brothers, sisters, children, or spouses, in other words, those who are influenced by the imminent death of the patient.
On the other hand, compared with deceased-donor liver grafts, living donor grafts offer three distinct advantages: 1) early transplantation of ALF patients, 2) better graft function, and 3) shorter cold-ischemia time; however, a satisfactory survival rate of patients with ALF undergoing adult LDLT is a prerequisite to justify the risk of emergency liver transplantation. Ikegami and colleagues (2008) use smaller grafts, wherein graft volume (GV) in proportion to standard liver volume is 30% to 35% for ALF. The reason for this is that ALF is an acute event, and most patients are usually healthy beforehand and do not have portal hypertension that allows use of the left lobe in most cases. In other countries with limited availability of cadaveric donors, LDLT in patients with ALF is performed with similar results (Uribe et al, 2008), especially in children, although it is still controversial among adults.
In a report by Campsen and colleagues (2008), only 14 of 1201 potential LDLT recipients (1%) in the A2ALL study, which evaluated LDLT in the United States, were diagnosed with ALF. Among these, 10 received a living-donor graft, and their survival rate was similar to that of patients who received cadaveric grafts, and no recipients died on the waiting list. The authors concluded that LDLT is rarely performed for ALF in the United States but could be performed with acceptable recipient and donor outcomes.
The increased use of full-liver grafts from donors after cardiac death (DCD) has had a beneficial impact on elective liver transplantation in adults; however, these grafts are more susceptible to poor initial function, and most centers are reluctant to consider their use as segmental grafts, let alone in the setting of ALF, in which good initial function is imperative. Nevertheless, Perera and colleagues (2009) describe the use of a segmental graft from a living or heart-beating deceased donor and report the successful outcome in two children with ALF, aged 6 weeks and 6 years.
Auxiliary Liver Transplantation
The key issue regarding APOLT is determining when native liver regeneration is likely to occur, which is sometimes difficult. Monitoring includes hepatobiliary iminodiacetic acid (HIDA) scintigraphy, computed tomography (CT), and biopsy when necessary. When sufficient regeneration of the native liver is evident, immunosupression can be discontinued according to two different options: abrupt discontinuation often requires surgical removal of the graft because of severe and symptomatic rejection; progressive tapering of immunosuppression with the aim of inducing a slowly progressing chronic rejection with subsequent atrophy of the graft is the approach preferred by most groups (Belghiti et al, 2004a).
In 2002, Jaeck and colleagues reported a series of 15 patients receiving APOLT, of which 10 are alive, and 6 of those are free of immunosuppression with a regenerated native liver. Recently, Azoulay and colleagues (2001) compared the results of APOLT with those of total orthotopic liver transplantation (TOLT) in the treatment of ALF. They showed similar survival rates after both procedures, but the postoperative complication rates—particularly those of biliary complications, neurologic sequelae, and retransplantation rate—were significantly higher after APOLT. In the Paul Brousse experience, both full native liver regeneration and discontinuation of immunosuppression were observed only in 25% of the cases.
Acetaminophen-induced liver failure and hyperacute syndromes are more likely to regenerate to normal morphology when regeneration does occur. In seronegative hepatitis and the subacute syndromes, regeneration may occur, but it does so at the risk of significant fibrosis. Quaglia and colleagues (2008) described the evolution of histologic changes in native livers after APOLT for ALF. In up to 62.5% of patients, the native liver regenerates to full recovery. In all such patients, immunosuppression can be reduced, and in the vast majority (80%), it can be ultimately suspended. Acetaminophen toxicity in particular is an excellent indication for APOLT, with full native liver recovery occurring in 100% of surviving patients with an auxiliary graft.
Peter Lodge and colleagues (2008) proposed a new approach for patients with ALF resulting from acetaminophen overdose, fulfilling KCH criteria for super-urgent liver transplantation. This new approach consists of a subtotal hepatectomy—that is, resection of segments IVb, VI, VII, and VIII—to remove 75% of liver volume and reduce the toxic liver, followed by auxiliary transplantation with a whole liver (AWOLT; maximal liver volume to aid recovery) and gradual withdrawal of immunosuppression after recovery over a 2- to 3-month period. The results in 13 patients treated with this approach were compared with those in 13 patients who had undergone OLT in the same period: they were similar in morbidity and mortality rates but better in terms of quality of life, because they did not need long-term immunosuppression.
For the authors (Lodge et al, 2008), temporary support by AWOLT with a subsequent liver regeneration may offer a better quality of life in all domains when compared with OLT. This could be due to the fact that patients can lead a more normal life without the effects of immunosuppression. Bernal and colleagues (1998) reported that only 19 of 33 survivors of OLT for acetaminophen-related ALF were back to a normal lifestyle. In contrast, all 8 of our successful AWOLT patients (Lodge et al, 2008) have returned to a normal lifestyle, including full-time education or employment, and all seem to be in normal relationships. Except for these latter results, and results presented in a paper by Girlanda and colleagues (2005), outcome after APOLT is poor; this is reflected in registry data, with 1-year survival just barely over 50%.
Finally, in case of fulminant hepatitis (FH), APOLT should only be proposed to select patients with good chances of liver regeneration; those aged less than 40 years with fulminant, rather than subfulminant, hepatitis resulting from HAV or HVB or acetaminophen toxicity (Jaeck, 2007). When the native liver has regenerated, it is recommended that immunosuppression be reduced gradually to induce a progressive atrophy of the graft, which is left with the patient. Removal of the auxiliary graft should not be attempted.
Liver-Support Systems
Two major categories of devices are currently being tested (Phua & Lee, 2008): artificial liver support is cell free, purely mechanical, and includes albumin dialysis; bioartificial systems contain cellular material from human or animal livers or hepatocytes. A truly effective device will perform three functions: 1) detoxification, 2) biosynthesis, and 3) regulation; however, only bioartificial systems can perform all three functions; artificial liver support only detoxifies. Support of the failing liver is reviewed in further detail in Chapter 73.
Artificial Liver Support
Artificial liver support (ALS) is used to control serious symptoms of FH, such as brain edema, which may induce postoperative neurologic deficit. The key role of ALS is to remove toxins, both known and unknown, that are responsible for HE and multiorgan failure secondary to liver failure. Hemodialysis, hemofiltration, hemodiafiltration, and hemadsorption are methods that have failed to show any major outcome in early randomized trials (Davenport et al, 1993; Kramer et al, 2001). These systems remove toxins that result from hepatic failure but are limited by their inability to provide missing liver-specific functions.
One problem with these techniques lies in the difficulty of removing toxins bound to large protein molecules, such as albumin. To address this, Inoue and colleagues (2009) recommended the use of plasma exchange combined with hemodiafiltration. Using large buffer volumes is a promising and effective bridging method to liver transplantation that enables patient recovery from coma, prevents brain edema, and identifies those patients in need of urgent liver transplantation. Several nonrandomized studies show that the use of plasmapheresis increases hepatic blood flow, decreases blood ammonia levels, and could reduce mortality rates (Clemmesen & Clemmesen 2001; Du et al, 2005; Li et al, 2004).
Molecular Absorbent Recycling System
Proposed by Stange and colleagues (1993), the molecular absorbent recycling system (MARS) blood detoxification system has been tested in thousands of patients, but additional well-conducted controlled studies are warranted to better define the role of MARS in the treatment of patients with acute hepatic failure and acute exacerbation of chronic liver disease. MARS is a two-circuit system composed of a blood circuit and a secondary circuit. The blood circuit passes the patient’s blood through a high-flux dialyzer, making the membrane impermeable to albumin. This membrane is permeable to water-soluble, low- and medium-molecular-weight substances. The opposing side of this membrane consists of 600 mL of 20% human albumin dialysate, which makes up the secondary circuit; this circuit provides conventional low-flux dialysis, as well as detoxification, over absorbent columns that include an anion exchange resin and an activated charcoal column, allowing transport of ions of low molecular weight (LMW).
The key feature to the function of albumin dialysis is the concentration gradient of LMW substrate between the patient’s blood and the 20% albumin in the secondary circuit. This concentration gradient allows diffusible LMW substrates to flow down their gradient over the membrane, where they are transiently bound by albumin in the secondary circuit (Steiner et al, 2004). The LMW substrate is then removed from the system by conventional dialysis and hemodiafiltration within the secondary circuit, and the 20% albumin dialysate is then recirculated. The coagulation profile present in liver failure obviates the need for anticoagulation in most patients being treated with MARS.
A meta-analysis of four randomized controlled trials concluded that treatment with MARS provided no survival benefit (Khuroo et al, 2004). Aside from one study suggesting that 4 out of 14 patients (29%) with relatively mild liver failure avoided transplantation after MARS (Camus et al, 2006), most recent descriptive reports show that short-term mortality rates for patients treated with MARS but without transplantation ranged from 78% to 100% (Chiu et al, 2006; Wai et al, 2007).
As a proposed bridge to transplantation, the study of Novelli and colleagues (2007) evaluated the change in MELD score in patients with acute-on-chronic liver failure awaiting transplantation after treatment with MARS. Inclusion criteria were a MELD score above 20, bilirubin greater than 15, encephalopathy higher than grade 2, and INR above 2.1. Patients were treated with MARS for a minimum of five treatments and a maximum of 20 over 3 months. MELD was evaluated after treatment with MARS and at a 3-month follow-up, and significant improvement was reported at both time points. Hetz and colleagues (2006) also proposed using MARS for allograft dysfunction after transplantation.
Mortality rate aside, MARS improves other parameters, including bilirubin levels, especially conjugated bilirubin; encephalopathy; renal function; and hemodynamics (Heemann et al, 2002); it also decreases serum copper levels in Wilson disease (Sen et al, 2002). Although these effects may be mediated by cytokine clearance, it is unclear whether MARS decreases serum cytokine levels significantly (Guo et al, 2003; Stadlbauer et al, 2006). Importantly, MARS may worsen coagulopathy and bleeding (Bachli et al, 2007), hence minimization of anticoagulation should be considered; in addition, it can cause hypoglycemia in the absence of glucose-containing dialysate.
Fractionated Plasma Separation and Absorption (Prometheus)
The Prometheus system (Fresenius Medical Care, Bad Homburg, Germany) uses fractionated plasma separation. Whereas MARS is a two-circuit system separated by an albumin impermeable membrane, Prometheus utilizes a membrane with a 250-kDa cutoff between circuits, thereby making the membrane permeable to albumin and hence to albumin-bound toxins. Although a large portion of the toxins that accumulate during liver failure are water soluble, many are still bound by albumin; therefore fractionated plasma separation may be advantageous with regard to toxin removal. The patient’s endogenous albumin loads the secondary circuit that distinguishes Prometheus from MARS. Since albumin-bound toxins can enter the secondary circuit by convection, as well as by diffusion, in theory, Prometheus should offer more effective detoxification than MARS does (Rifai et al, 2003).
In a retrospective analysis by Evenepoel and colleagues (2006) of prospectively collected data on patients with acute-on-chronic liver failure treated with either Prometheus or MARS for 2 to 5 consecutive days, blood clearance of toxins was superior when patients were treated with Prometheus as compared with MARS at all time points; in addition, certain substances that are tightly bound to albumin, such as unconjugated bilirubin, were exclusively cleared by Prometheus. The limited data available suggest that Prometheus maybe used as a bridge to transplantation, rather than to recovery, as mortality rates with Prometheus treatment alone without transplantation exceed 80% (Skwarek et al, 2006).
Bioartificial Support Devices
Bioartificial supports are cell-based systems that provide both metabolic support and detoxification. A bioartificial liver (BAL), which incorporates hepatocytes from various sources, has the theoretic advantage of not only providing blood purification through dialysis but also of providing the hepatocyte-specific functions lost in ALF (Rozga et al, 1993). Although primary human hepatocytes appear to be the most appropriate cells for use in a bioartificial liver, some factors have prevented their use, such as lack of availability and loss of function when cultured. For this reason, immortalized cell lines, such as C3A human hepatoblastoma, have been studied in vitro and have been utilized; however, many problems will likely preclude their use in a therapeutically successful bioartificial liver. Currently, porcine hepatocytes are the most reasonable choice, and several systems have been clinically assessed.
HepatAssist (Arbios Systems, Allendale, NJ) is a device that uses a hollow-fiber extracorporeal bioreactor loaded with 7 billion cryopreserved porcine hepatocytes. The system also utilizes a charcoal column, combined oxygenator/blood warmer, and perfusion pump, all driven by a plasmapheresis machine. The separation between hepatocytes and patient circulation is by a semipermeable membrane that avoids the need for immunosuppression. A randomized, controlled, multicenter Phase II and III clinical trial was conducted on patients with fulminant/subfulminant liver failure, and those with primary graft nonfunction were included (Demetriou et al, 2004). The study demonstrated favorable safety, but it failed to demonstrate statistical significance in improving 30-day survival in the overall study population.
The next generation of BAL systems is a hybrid that uses cellular and noncellular components in an albumin-based extracorporeal perfusate circuit that combines albumin dialysis, charcoal therapy, and dialysis with hepatocyte cell based therapy. In preliminary studies, one system using 200-g porcine hepatocyte spheroids provided cerebral protection not seen in the control group (Nyberg et al, 2005).
A Cochrane review of support systems concluded that results have not yet demonstrated that these systems affect survival in ALF (Liu et al, 2004). No single system has reproducibly demonstrated improvement in patient mortality; however, with the advent of new technology and improved cell acquisition techniques, further randomized controlled trials will be necessary to determine the role of artificial and BAL support devices in the treatment of patients with ALF. (McKenzie et al, 2008). Ex vivo liver perfusion was attempted in the past, before the era of liver transplantation, with disappointing results; but in one modern study, pig and human livers unsuitable for transplantation were successfully used as a bridge to transplantation in a small number of patients (Chari et al, 1994).
Hepatocyte Transplantation
After success in animal models, human hepatocyte transplantation for ALF was attempted. Strom and colleagues (2006a, 2006b) infused cryopreserved human hepatocytes into a small number of children with limited success. Further progress in many areas will be needed, however, before this approach is clinically viable.
Acharya SK, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology. 1996;23:1448-1455.
Alam S, et al. Natural course of fulminant hepatic failure: the scenario in Bangladesh and the differences from the West. Saudi. J Gastroenterol. 2009;15:229-233.
Alba L, et al. Lactulose therapy in acute liver failure. J Hepatol. 2003;36:33-A.
Ascher N, et al. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993;128:415-425.
Azoulay D, et al. Auxiliary partial orthotopic versus standard orthotopic whole liver transplantation for acute liver failure: a reappraisal from a single center by a case-control study. Ann Surg. 2001;234:723-731.
Bachli EB, et al. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int. 2007;27:475-484.
Barshes N, et al. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195-201.
Baudouin SV, et al. Acute lung injury in fulminant hepatic failure following paracetamol poisoning. Thorax. 1995;50:399-402.
Bhatia V, et al. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55:98-104.
Belghiti J, et al. Temporary portocaval anastomosis with preservation of caval flow during orthotopic liver transplantation. Am J Surg. 1995;169:277-279.
Belghiti J, et al. Auxiliary liver transplantation for acute liver failure. HPB. 2004;6:83-87.
Belghiti J, et al. Technical progress in liver transplantation. HPB. 2004;6:67-68.
Bernal W, et al. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050-1055.
Bernal W, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558-563.
Bernal W, et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844-1852.
Bernuau J, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6:648-651.
Bernuau J, et al. Criteria for emergency liver transplantation in patients with acute viral hepatitis and factor V below 50% of normal: a prospective study. Hepatology. 1991;14:49A.
Bernuau J, et al. Fulminant and subfulminant liver failure. Bircher, J, et al. Oxford Textbook of Clinical Hepatology, 2nd ed, Oxford, UK: Oxford Medical Publications, 1999.
Bismuth H, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med. 1987;107:337-341.
Bismuth H, et al. Orthotopic liver transplantation in fulminant and subfulminant hepatitis: the Paul Brousse experience. Ann Surg. 1995;222:109-119.
Blei AT. Cerebral edema and intracranial hypertension in acute liver failure: distinct aspects of the same problem. Hepatology. 1991;13:376-379.
Blei AT, et al. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341:157-158.
Brandsaeter B, et al. Fulminant hepatic failure: outcome after listing for highly urgent liver transplantation: 12 years experience in the Nordic countries. Liver Transpl. 2002;8:1055-1062.
Campsen J, et al. Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2008;14:1273-1280.
Camus C, et al. Molecular adsorbent recirculating system dialysis in patients with acute liver failure who are assessed for liver transplantation. Intensive Care Med. 2006;32:1817-1825.
Canalese J, et al. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut. 1982;23:625-629.
Caraceni P, et al. Acute liver failure. Lancet. 1995;345:163.
Chalasani N, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
Chari RS, et al. Treatment of hepatic failure with ex-vivo pig-liver perfusion followed by liver transpalantation. N Engl J Med. 1994;331:234-237.
Chiu A, et al. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int. 2006;26:695-702.
Chuansumrit A, et al. Recombinant activated factor VII in children with acute bleeding resulting from liver failure and disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S101.
Clemmesen JO, et al. Indomethacin normalized intracranial pressure in acute liver failure: a twenty-three year old woman treated with indomethacin. Hepatology. 1997;26:1423-1425.
Clemmesen JO, et al. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648-653.
Clemmesen JO, et al. Hepatic blood flow and splanchnic oxygen consumption in patients with liver failure: effect of high-volume plasmapheresis. Hepatology. 1999;29:347-355.
Clemmesen JO, et al. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure. Am J Gastroenterol. 2001;96:1217-1223.
Davenport A, et al. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney Int. 1993;41(Suppl):S245-S251.
Davern TJ2nd, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687-694.
Davila D, et al. Temporary portocaval shunt in orthotopic liver transplantation: need for a standardized approach? Liver Transpl. 2008;14:1414-1419.
Demetriou AA, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660-667.
Dhawan A, et al. Approaches to acute liver failure in children. Pediatr Transplant. 2004;8:584-588.
Dhiman RK, et al. Prognostic evaluation of early indicators in fulminant hepatic failure by multivariate analysis. Dig Dis Sci. 1998;43:1311-1316.
Dhiman RK, et al. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-stage Liver Disease (MELD) and King’s College Hospital criteria. Liver Transpl. 2007;13:814-821.
Du WB, et al. Effects of artificial liver support system on patients with acute or chronic liver failure. Transplant Proc. 2005;37:4359-4364.
Ellis AJ, et al. Subclinical seizure activity and prophylactic phenytoin infusion in acute liver failure: a controlled clinical trial. Hepatology. 2000;32:536-541.
Emond JC, et al. Improved results of living-related liver transplantation with routine application in a pediatric program. Transplantation. 1993;55:835-840.
Escudié L, et al. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol. 2007;46:466-473.
Evenepoel P, et al. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif Organs. 2006;30:276-284.
Farges O, et al. The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation. 1995;59:1124-1133.
Feray C, et al. Hepatitis C virus RNA and hepatitis B virus DNA in serum and liver of patients with fulminant hepatitis. Gastroenterology. 1993;104:549-555.
Fernandez Hernandez JA, et al. [Fulminant hepatic failure and liver transplantation: experience of the Hospital Virgen de la Arrixaca.]. Gastroenterol Hepatol (in Spanish). 2003;26:333-340.
Ferraz-Neto BH, et al. Total hepatectomy and liver transplantation as a two-stage procedure for toxic liver: case reports. Transpl Proc. 2008;40:814-816.
Freeman R, et al. Liver and intestine transplantation in the United States. Am J Transpl. 2008;8:958-976.
Gazzard BG, et al. Early changes in coagulation following a paracetamol overdose and a controlled trial of fresh frozen plasma therapy. Gut. 1975;16:617-620.
Ginsberg MD, et al. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Dev. 1992;4:189-225.
Girlanda R, et al. Immunosuppression withdrawal after auxiliary liver transplantation for acute liver failure. Trans Proc. 2005;37:1720-1721.
Gugenheim J, et al. Liver transplantation across ABO blood group barrier. Lancet. 1990;336:519-523.
Guo LM, et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 2003;23(Suppl 3):16-20.
Hanid MA, et al. Clinical monitoring of intracranial pressure in fulminant hepatic failure. Gut. 1980;21:866-869.
Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology. 1996;23:1067-1072.
Harrison PM, et al. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572-1573.
Harrison PM, et al. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852-1857.
Harry R, et al. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395-402.
Heemann U, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002;36:949-958.
Hetz H, et al. Molecular adsorbent recirculating system in patients with early allograft dysfunction after liver transplantation: a pilot study. Liver Transpl. 2006;12:1357-1364.
Hoofnagle JH, et al. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240.
Ikegami T, et al. Living donor liver transplantation for acute liver failure: a 10-year experience in a single center. J Am Coll Surg. 2008;206:412-418.
Inoue K, et al. Artificial liver support system using large buffer volumes removes significant glutamine and is an ideal bridge to liver transplantation. Transplant Proc. 2009;41:259-261.
Jaeck D. Which types of graft to use in patients with acute liver failure? I prefer auxiliary liver transplant. J Hepatol. 2007;46:553-582.
Jaeck D, et al. Auxiliary partial orthotopic liver transplantation (APOLT) in the treatment of acute liver failure. J Gastroenterol. 2002;3:88-91.
Jain S, et al. Effect of renal dysfuction in fulminant hepatic failure. Trop Gastroenterol. 2000;21:118-120.
Jaiwal SB, et al. Aetiology and prognostic factors in hepatic failure in central India. Trop Gastroenterol. 1996;17(4):217-220.
Jalan R, et al. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet. 1999;354:1164-1168.
Jalan R, et al. Liver derived pro-inflammatory cytokines may be important in producing intracranial hypertension in acute liver failure. J Hepatol. 2002;37:536-538.
Joshi A, et al. Acute mushroom poisoning: a report of 41 cases. J Nepal Med Assoc. 2007;46:7-12.
Kaidu T. Randomized controlled trials involving liver failure. Hepatogastroenterology. 2008;55:1089-1092.
Kanda T, et al. Prevalence of obesity in patients with acute hepatitis: is severe obesity a risk for fulminant hepatitis in Japan? Hepatogastroenterology. 2005;52:180-182.
Kawasaki S, et al. Temporary shunt between right portal vein and vena cava in living related liver transplantation. J Am Coll Surg. 1996;183(1):74-76.
Keays R, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. Br Med J. 1991;303:1026-1029.
Khuroo MS, et al. Molecular absorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10:1099-1106.
Kramer DJ, et al. Management options in fulminant hepatic failure. Transplant Proc. 1991;23:1895-1898.
Kramer L, et al. A controlled study of sorbent suspension dialysis in chronic liver disease and hepatic encephalopathy. Int J Artif Organs. 2001;24:434-444.
Kremers W, et al. MELD score as a predictor of pre-transplant and post-transplant survival in OPTN/UNOS status I patients. Hepatology. 2004;39:764-769.
Kumar S, et al. Failure of physicians to recognize acetaminophen hepatotoxicity in chronic alcoholics. Arch Intern Med. 1991;151:1189-1191.
Kurt A, et al. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209.
Larsen FS. Cerebral circulation in liver failure. Semin Liver Dis. 1996;16:281-293.
Larsen FS, et al. Intensive care management of patients with acute liver failure with emphasis on systemic hemodynamic instability and cerebral edema: a critical appraisal of pathophysiology. Can J Gastroenterol. 2000;14(Suppl D):105D-111D.
Larsen FS, et al. Regional cerebral blood flow autoregulation in patients with fulminant hepatic failure. Liver Transpl. 2000;6:795-801.
Larson AM, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372.
Lee W, et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401-1414.
Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142-152.
Lee WM, et al. N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
Leithead JA, et al. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non–paracetamol-induced acute liver failure. Gut. 2009;58:443-449.
Li LJ, et al. Effect of artificial liver support system on patients with severe viral hepatitis: a study of four hundred cases. World J Gastroenterol. 2004;10:2984-2988.
Licht H, et al. Apparent potentiation of acetaminophen hepatotoxicity by alcohol. Ann Intern Med. 1980;92:511.
Lidofsky SD, et al. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology. 1992;16:1-7.
Liu JP, et al. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev. 2(1), 2004. CD003628
Lo CM, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261-269.
Lo CM, et al. Liver transplantation for acute liver failure: not too early but never too late. Liver Transpl. 2008;14:1243-1244.
Lodge P, et al. Emergency subtotal hepatectomy: a new concept for acetaminophen-induced acute liver failure. Ann Surg. 2008;247:238-249.
Marcos A, et al. Emergency adult to adult living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;69:2202-2205.
Maroto A, et al. Brachial and femoral artery blood flow in cirrhosis: relationship to kidney dysfunction. Hepatology. 1993;17:788-793.
Mazer M, et al. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008;4:2-6.
McDougall BR, et al. H2-receptor antagonist in the prevention of acute upper gastrointestinal hemorrhage in fulminant hepatic failure. Gastroenterology. 1978;74:464-465.
McKenzie T, et al. Artificial and bioartificial liver support. Semin Liver Dis. 2008;28:210-217.
Mitchell JR, et al. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 1974;16:676-684.
Miyake Y, et al. Prognostic factors for fatal outcomes prior to receiving liver transplantation in patients with non–acetaminophen-related fulminant hepatic failure. Hepatology. 2000;32:734-739.
Miyake Y, et al. Systemic inflammatory response syndrome strongly affects the prognosis of patients with fulminant hepatitis B. J Gastroenterol Hepatol. 2007;22(6):855-861.
Moller H, et al. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671-676.
Muñoz S, et al. The coagulopathy of acute liver failure and implications for intracranial pressure monitoring. Neurocritical Care. 2008;9:103-107.
Murray KF, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47:395-405.
Nath F, Galbraith S. The effect of mannitol on cerebral white matter water content. J Neurosurg. 1986;65:41-43.
Navasa M, et al. Portal hypertension in acute liver failure. Gut. 1992;33:965-968.
Naylor CD, et al. Parenteral nutrition with branched chain amino acids in hepatic encephalopathy: a meta-analysis. Gastroeneterology. 1989;97:1033.
Negrier C, et al. Overall experience with Novoseven. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S19.
Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24:103-117.
Novelli G, et al. Molecular adsorbents recirculating system treatment in acute-on-chronic hepatitis patients on the transplant waiting list improves Model for End-stage Liver Disease scores. Transplant Proc. 2007;39:1864-1867.
Nyberg S, et al. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11:901-910.
O’Brien CJ, et al. Neurological sequelae in patients recovered from fulminant hepatic failure. Gut. 1987;28:93-95.
O’Grady J, et al. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186-1192.
O’Grady J, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445.
O’Grady J, et al. Prothrombin time in fulminant hepatic failure. Gastroenterology. 1991;100:1480-1481.
O’Grady J, et al. Posoperative issues and outcome for acute liver failure. Liver Transpl. 2008;14:S97-S101.
O’Grady JG. Prognostication in acute liver failure: a tool or an anchor? Liver Transpl. 2007;13:786-787.
O’Grady JG, et al. Coagulopathy of fulminant hepatic failure. Semin Liver Dis. 1986;6:159-163.
O’Grady JG, et al. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273-275.
Ostapowicz G, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. U.S. Acute Liver Failure Study Group. Ann Intern Med. 2002;137:947-954.
Perera M, et al. Safe use of segmental liver grafts from donors after cardiac death (DCD) in children with acute liver failure. Transpl Int. 2009;22:757-760.
Phua J, Lee K. Liver support devices. Curr Opin Crit Care. 2008;14:208-215.
Polson J. Assessment of prognosis in acute liver failure. Semin Liver Dis. 2008;28:218-225.
Polson J, et al. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
Preston FE, 1991: Haemorrhagic diathesis and control. In Williams R, Hughes RD (eds): Acute Liver Failure: Improved Understanding and Better Therapy. London, Miter Press, pp 36-39.
Quaglia A, et al. Auxiliary transplantation for acute liver failure: histopathological study of native liver regeneration. Liv Transpl. 2008;14:1437-1448.
Rifai K, et al. Prometheus: a new extracorporeal system for the treatment of liver failure. J Hepatol. 2003;39:984-990.
Ringe B, et al. Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg. 1993;218:3-9.
Ring-Larsen H, et al. Renal failure in fulminant hepatic failure and terminal cirrhosis: a comparison between incidence, types, and prognosis. Gut. 1981;22:585-591.
Riordan S, et al. Perspectives on liver failure: past and future. Semin Liver Dis. 2008;28:137-141.
Roberts DRD, Manas D. Induced hypothermia in the management of cerebral oedema secondary to fulminant liver failure. Clin Transplant. 1999;13:545-547.
Rolando N, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49-53.
Rolando N, et al. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology. 1993;17:196-201.
Rolando N, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739.
Rozga J, et al. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258-265.
Rueff B, et al. Prognosis of severe viral hepatitis. Ann Med Interne (Paris). 1971;122(3):373-380.
Ruiz-del-Arbol L, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447.
Russo MW, et al. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018-1023.
Schafer DF, Shaw BW. Fulminant hepatic failure and orthotopic liver transplantation. Semin Liver Dis. 1989;9:189-194.
Schiodt F, et al. Admission levels of serum Gc-globulin: predictive value in fulminant hepatic failure. Hepatology. 1996;23:713-718.
Schiodt FV, et al. Thrombopoietin in acute liver failure. Hepatology. 2003;37:558-561.
Schiodt FV, et al. Outcome of acute liver failure in the elderly. Liver Transpl. 2009;15:1481-1487.
Schmidt L, et al. Alpha-fetoprotein is a predictor of outcome in acetaminofen-induced liver injury. Hepatology. 2005;41:26-31.
Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology. 2002;36:659-665.
Schubert A. Side effects of mild hypothermia. J Neurosurg Anesthesiol. 1995;7:139-147.
Seeff LB, et al. Acetaminophen hepatotoxicity in alcoholics: a therapeutic misadventure. Ann Intern Med. 1986;104:399-404.
Sen S, et al. Albumin dialysis and Molecular Adsorbents Recirculating System (MARS) for acute Wilson’s disease. Liver Transpl. 2002;8:962-967.
Shakil AO, et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163-169.
Shami VM, et al. Recombinant activated factor VII is superior to plasma alone in correcting the coagulopathy of fulminant hepatic failure. Hepatology. 2001;34:237A.
Shami VM, et al. Recombinant activated factor VII for coagulopathy in fulminant hepatic failure compared with conventional therapy. Liver Transpl. 2003;9:138.
Skwarek A, et al. The use Prometheus FPSA system in the treatment of acute liver failure: preliminary results. Transplant Proc. 2006;38:209-211.
Smilkstein MJ, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319:1557-1562.
Smilkstein MJ, et al. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058-1063.
Squires RH, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652-658.
Squires RHJr. Acute liver failure in children. Semin Liver Dis. 2008;28:153-166.
Stadlbauer V, et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care. 2006;10:R169.
Stange J, et al. A new procedure for the removal of protein bound drugs and toxins. ASAIO J. 1993;39:M621-M625.
Steiner C, et al. Binding of bilirubin and bromosulphthalein to albumin: implications for understanding the pathophysiology of liver failure and its management. Liver Transpl. 2004;10:1531-1538.
Strauss G, et al. Hyperventilation restores cerebral blood flow autoregulation in patients with acute liver failure. J Hepatol. 1998;28:199-203.
Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev. Gastroenterol Hepatol. 2009;6:542-543.
Stravitz RT, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498-2508.
Strom S, et al. Bigger may not be better when it comes to hepatocytes. Liver Transpl. 2006;12:16-18.
Strom S, et al. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15:S105-S110.
Taylor RM, et al. Fulminant hepatitis A virus in the United States: incidence, prognosis and outcome. Hepatology. 2006;44:1589-1597.
Tofteng F, et al. The effect of indomethacin on intracranial pressure, cerebral perfusion and extracellular lactate and glutamate concentrations in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2004;24:798-804.
Toso C, et al. ABO-incompatible liver transplantation for critically ill adult patients. Transpl Int. 2007;20:675-681.
Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper, H, Shaffner, F. Progress in Liver Diseases. New York: Grune & Stratton; 1970:282-298.
Tzakis A, et al. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649-652.
Uemoto S, et al. Living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;70:152-157.
Uribe M, et al. Living donor liver transplantation in pediatric patients with acute liver failure: safe and effective alternative. Transpl Proc. 2008;40:3253-3255.
Vaquero J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755-764.
Volkmann X, et al. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology. 2008;47:1624-1633.
Wai CT, et al. MARS: a futile tool in centres without active liver transplant support. Liver Int. 2007;27:69-75.
Walsh TJ, et al. Disseminated aspergillosis complicating hepatic failure. Arch Intern Med. 1983;143:1189-1191.
Wei G, et al. Long-term follow-up of patients with acute liver failure of indeterminate etiology. Scand J Gastroenterol. 2008;43:984-999.
Wijdicks EF, Nyberg SL. Propofol to control intracranial pressure in fulminant hepatic failure. Transplant Proc. 2002;34:1220-1222.
Wiesner RH. MELD/PELD and the allocation of deceased donor livers for status 1 recipients with acute fulminant hepatic failure, primary nonfunction, hepatic artery thrombosis and acute Wilson’s disease. Liver Transpl. 2004;10(10 Suppl 2):S17-S22.
Williams R. The brain in fulminant hepatic failure. Lancet. 1991;338:156-157.
Williams R. Classification, etiology and considerations of outcome in acute liver failure. Semin Liver Dis. 1996;16:343-348.
Wootton FT, Lee WM. Acetaminophen hepatotoxicity in the alcoholic. South Med J. 1990;83:1047-1049.
Wright G. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis. 2007;22:375-388.
Wright TL, et al. Hepatitis C virus not found in fulminant non-A non-B hepatitis. Ann Intern Med. 1991;115:111-112.
Wyke RJ, et al. Bacteraemia in patients with fulminant hepatic failure. Liver. 1982;2:45-52.
Yamagishi Y, et al. A new prognostic formula for adult acute liver failure using computer tomography–derived hepatic volumetric analysis. J Gastroenterol. 2009;44:615-623.
Yantorno S, et al. Meld is superior to King’s College and Clichy’s criteria. Liver Transpl. 2007;13:822-828.
Ytrebø LM, et al. Systemic and regional hemodynamics in pigs with acute liver failure and the effect of albumin dialysis. Scand J Gastroenterol. 2006;41:1350-1360.
Zimmerman HJ, et al. C. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767-773.