Chapter 90F Liver resection in cirrhosis
Overview
Hepatocellular carcinoma (HCC) is the main indication for hepatectomy in the cirrhotic liver. The vast majority of HCC in patients with chronic liver disease (CLD) is related to hepatitis B or C viral infection, chronic alcoholic consumption, and metabolic syndrome (see Chapter 70A, Chapter 70B, Chapter 80 ). Other malignant tumors developed in CLD include intrahepatic cholangiocarcinoma (see Chapter 50A) and liver metastases (see Chapter 81A, Chapter 81B, Chapter 81C ; Iascone et al, 2005; Uchiyama et al, 2011; Welzel et al, 2007;). Some benign tumors—including regenerative nodules, hemangiomata, and focal nodular hyperplasia (FNH)—can be discovered in cirrhotic liver (Brancatelli et al, 2001a, 2001b; Libbrecht et al, 2006; Luciani et al, 2007; see Chapter 79A, Chapter 79B ).
Although liver transplantation (LT) offers the best chance of cure in patients with early stage HCC (Mazzaferro et al, 2009; see Chapter 97A), the persistence of graft shortage in many countries had led some authors, including those in our group, to advocate hepatic resection as the first-line treatment for HCC in cirrhotic patients with preserved liver function (Belghiti et al, 2008; Cherqui et al, 2009; Del Gaudio et al, 2008; Poon et al, 2002a). Liver resection is an effective treatment for HCC under appropriate conditions, but impaired liver regeneration, altered texture of liver parenchyma, portal hypertension, and collateral circulation make liver resection in cirrhotic patients challenging.
In the 1990s, operative mortality in patients with normal livers was 1%, compared with 8% among patients with diseased parenchyma (Belghiti et al, 2000; see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E ). The higher risk associated with liver resection in patients with diseased parenchyma results from increased operative bleeding and postoperative complications, with liver failure mainly as a result of impaired liver regeneration in cirrhosis (see Chapter 5); however, with appropriate attention to preoperative patient selection, improved liver function assessment, better understanding of the segmental liver anatomy with more accurate imaging studies, advanced operative techniques, and better perioperative management, hepatectomy now can be performed in the cirrhotic liver with acceptable operative morbidity and mortality in major centers (Dahiya et al, 2010; Ferrero et al, 2005; Imamura et al, 2003; Wei et al, 2003). This chapter describes general surgical aspects and risks of liver surgery in patients with liver cirrhosis.
Preoperative Assessment
The improvement in the outcomes of liver resection in cirrhotic patients is mainly the result of good preoperative evaluation and appropriate patient selection (Ikai et al, 2007). A systematic and careful assessment of the patient’s general medical fitness, the tumor extent and stage, the underlying liver function, and the volume of the anticipated future liver remnant (FLR) are critical in ensuring proper patient selection (see Chapter 2, Chapter 70A, Chapter 70B ).
General Status of Patients
Attention to the general medical fitness of patients is paramount in selecting patients with HCC for hepatic resection, especially in cirrhotic patients, who are at higher risk of postoperative complications. Comorbid illness is a major predictor of the mortality of patients undergoing hepatectomy (Wei et al, 2003). Severe comorbid illnesses, such as congestive heart failure and chronic renal failure, are definite contraindications for hepatectomy, although patients with less severe comorbid illnesses may still benefit from hepatic resection, if accompanied by meticulous perioperative care. The impact of diabetes mellitus, which is a common comorbid illness in cirrhotic patients, is debated (Gedaly et al, 2009). It has been shown that postoperative morbidity and mortality after hepatectomy in patients with diabetes mellitus were similar to those of patients without diabetes mellitus, as long as optimal perioperative control of blood glucose levels and appropriate postoperative care were provided (Poon et al, 2002b).
Tumor Status
The assessment of the tumor extent included a triple-phase (early vascular or arterial phase, portal phase, and delayed phase) helical computed tomography (CT) of the thorax and the abdomen (see Chapter 16). The liver is studied using thin slices acquired during the unenhanced phase and during the arterial, portal, and late or equilibrium phase after contrast administration. HCC is hypervascular during the early arterial phase and hypovascular in late phase (“washout”). Magnetic resonance imaging (MRI) is the modality of choice for imaging when contrast agents are contraindicated, better lesion characterization is needed, or the anatomic relationship between tumor and major vascular or biliary structures requires further delineation (see Chapter 17).
The usual criteria for hepatic resection with regard to the tumor status include absence of extrahepatic metastasis and tumor thrombus in a main portal vein or in the inferior vena cava (see Chapter 80). Large tumor size alone should not be considered a contraindication for hepatic resection. Despite the technical problems encountered with large tumors, liver resection for large HCCs has been shown to be safe (Kosuge et al, 1993; Pawlik et al, 2005a, 2005b; Ramacciato et al, 2010; Yang et al, 2009). In a series of 300 patients who underwent partial hepatectomy for HCCs larger than 10 cm, the 30-day mortality rate was 5% (Pawlik et al, 2005b). Because cadaveric orthotopic liver transplantation (OLT) and radiofrequency ablation (RFA) are contraindicated in these patients, surgical resection remains the treatment of choice for large HCCs.
HCC associated with biliary invasion should not be considered a contraindication to surgical resection, if curative resection can be achieved (Hanaoka et al, 2008; Lee et al, 2006; Peng et al, 2005). Multifocal disease generally is a contraindication to surgical resection, but in some patients with good liver function, resection can be reconsidered after clearance or stabilization of contralateral liver nodules by chemoembolization or RFA (Ishizawa et al, 2008; Liu et al, 2003; Ng et al, 2005; Ramacciato et al, 2010).
HCCs with major portal or hepatic vein involvement represent a technical and oncologic challenge. Patients with HCC that involves hepatic veins or vena cava have a high rate of pulmonary metastases (Ikai et al, 2003), although it has been shown that in a selected group of patients with normal liver function and excellent general status, extensive liver resection with removal of the vascular thrombus can achieve favorable survival results (Le Treut et al, 2006; Minagawa et al, 2001; Poon et al, 2003; Shi et al, 2010). Extension to surrounding structures, such as the diaphragm, does not represent a contraindication, provided that negative resection margins can be attained.
Tumor recurrence represents the major drawback after curative liver resection and is the most common cause of treatment failure. Within 5 years of resection, recurrence in the liver remnant occurs in about 50% to 70% of cases (Huang et al, 2010; Poon et al, 2000; Shi et al, 2007). The rate of repeated resection, ranging from 10% to 30%, depends on the underlying liver status, pattern of recurrence, and extent of first resection; lower rates are seen in series with a high proportion of major resection during the first hepatectomy, and repeat hepatectomy has been proven to be a safe and worthwhile procedure with a low mortality rate and an acceptable 5-year survival (20% to 57%) (Minagawa et al, 2003; Poon et al, 1999; Tsujita et al, 2010; Wu et al, 2009).
Assessment of Liver Function Reserve (See Chapters 2 and 70B)
Child-Turcotte-Pugh Classification
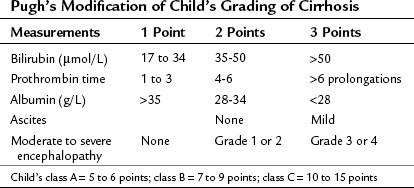
In most of Western centers, preoperative liver function is assessed using the Child-Turcotte-Pugh (CTP) classification, which incorporates variables related to conventional liver function and portal hypertension. This classification is easy and remains very accurate in its assessment of the surgical risk (Table 90F.1). According to this system of classification, major resection is performed only in CTP class A patients, limited resection of small superficial tumors is sometimes performed in class B patients, and no resection, regardless of the extent, is considered in class C patients; however, this classification is not enough to predict surgical risk in some patients, because two parameters are subjective—ascites and encephalopathy—and even within the class A group with apparently good liver function, some degree of portal hypertension and/or hepatic dysfunction may be present, and the risk of liver surgery may be underestimated. Therefore more sophisticated quantitative liver function tests or volumetric assessments are needed to predict the operative risk (Durand & Valla, 2005).
Indocyanine Green Test
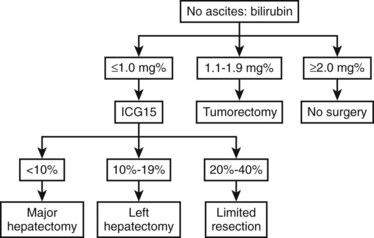
Most Eastern centers use more sophisticated quantitative liver function tests that include indocyanine green (ICG) clearance (Imamura et al, 2003), galactose elimination capacity (Redaelli et al, 2002), and a lidocaine test (Ravaioli et al, 2003) to predict the risk of postoperative liver failure in patients with cirrhosis. The Hong Kong group showed in a multivariate analysis that of the many liver function tests, ICG retention at 15 minutes to 20% was the best test for predicting hospital mortality after major hepatectomy for HCC (Lau et al, 1998; Lee & Hwang, 2005). Imamura and Makuuchi proposed a decision tree based on combined parameters from the CTP classification (ascites and bilirubin) and ICG retention test (Imamura et al, 2005; Fig. 90F.1). Based on this strategy, liver resection is contraindicated in patients who have elevated bilirubin (>2 mg/dL) or ascites. The extent of liver resection is then based on the ICG retention test at 15 minutes (see Table 90F.1), and major liver resection is proposed only in patients with normal bilirubin and ICG levels below 10%. Adopting this decision strategy, the authors reported only one mortality among the 685 patients who underwent liver resection for HCC (Imamura et al, 2005).
Model for End-Stage Liver Disease Score
The Model for End-Stage Liver Disease (MELD) score uses only objective variables calculated from the international normalized ration (INR), serum total bilirubin (mg/dL), and serum creatinine (mg/dL). This system was initially created to predict survival in patients with complications of portal hypertension undergoing elective placement of transjugular intrahepatic portosystemic shunts (TIPS) (Malinchoc et al, 2000). The MELD score was validated subsequently as an accurate predictor of survival among different populations of patients with advanced liver disease (Kamath et al, 2001). The most frequent use of the MELD score has been in the allocation of organs for patients awaiting liver transplantation (Freeman et al, 2005; Wiesner et al, 2001). Regarding liver resection in cirrhotic patients, the MELD score was only retrospectively studied. Cirrhotic patients who underwent liver resection for HCC with a MELD score greater than 8 had a higher risk of death, morbidity, and impaired long-term survival (Cucchetti et al, 2006; Delis et al, 2009).
Transaminase Levels
Postoperative mortality has been shown to be higher in a cohort of 285 patients who underwent hepatectomy for HCC when histologic evidence of cirrhosis and active hepatitis versus cirrhosis alone was found (Eguchi et al, 2000). Although the presence of high serum transaminase levels does not always correlate with hepatitis and could be associated with intratumoral necrosis in a huge tumor, increased complication and death rates have been reported in those patients with levels of elevated transaminases (Haber et al, 1995; Noun et al, 1997). Patients with an aspartate aminotransferase level greater than 100 IU/L or alanine aminotransferase at least twice the normal level are considered poor candidates for major hepatic resection. In this setting, preoperative biopsy of the nontumorous liver should be performed to exclude active underlying liver parenchymal disease.
Portal Hypertension (See Chapter 70A, Chapter 70B )
The presence of portal venous pressure greater than 10 mm Hg is often associated with esophageal varices, splenomegaly, and thrombocytopenia (<100,000). Undiagnosed and latent portal hypertension in a cirrhotic patient undergoing liver resection puts the patient at risk of major complications in the postoperative period; such complications include variceal bleeding, endotoxemia, and hepatic decompensation. In a prospective study in class A cirrhotic patients, it was shown that the hepatic venous pressure (HVP) gradient, a surrogate measurement of portal venous pressure, was the only predictor of hepatic decompensation following hepatic resection (Bruix et al, 1996). Thus liver resection in cirrhotic patients with esophageal varices is contraindicated; however, several authors have shown that portal hypertension is not an absolute contraindication to surgical resection in selected patients with preserved liver function (Capussotti et al, 2006; Ishizawa et al, 2008). These authors advocate an aggressive preoperative prophylactic treatment for portal hypertension, such as ligation of esophageal varices.
The negative effect of thrombocytopenia on liver regeneration can be related to the presence of portal hypertension or its direct impact on liver regeneration (Lesurtel & Belghiti, 2008). The development of venous collaterals in patients with elevated portal pressure can reduce the hepatic portal flow and thereby impair the regenerative capacity of the remnant parenchyma after liver resection, therefore we contraindicate major liver resection in cirrhotic patients with venous collaterals.
Liver Atrophy and Underlying Disease
Liver atrophy is a major prognostic factor that should be considered before any liver resection, even those in patients with preserved liver function (see Chapters 5 and 50B). In our practice, we do not consider major liver resection in HCC patients with liver atrophy. Similarly, the underlying etiology of liver cirrhosis should be considered before liver resection, and it should be noted that the extent of fibrosis is more severe in patients with hepatitis C than in patients with hepatitis B (Roayaie et al, 2000). Our tendency is to consider a major liver resection more favorably in patients with hepatitis B than in patients with some other etiology of CLD, even if the remnant liver volume is similar.
Among patients with CLD, the postoperative risk is higher in those with cirrhosis (F4) than in those with extensive fibrosis (F3) (Farges et al, 1999). This suggests that preoperative liver biopsy should be considered in all candidates for major liver resection for histologic fibrosis grading. In patients with F3 or F4 fibrosis requiring major hepatectomy, preoperative portal vein embolization should be performed in an effort to decrease morbidity.
Volume of the Future Liver Remnant
Measurement of the volume of the remnant liver after planned resection using CT volumetry has been shown to be helpful in selecting patients for major hepatic resection (see Chapter 2, Chapter 93A, Chapter 93B ). A small liver remnant volume is associated with worse postoperative liver function and a higher complication rate after extended hepatectomy (Ribero et al, 2007). The safety limit for the remnant liver volume in patients with normal liver is approximately 30% of the total nontumorous or functional liver volume, but this is much higher in patients with CLD. The safety limit in terms of remnant liver volume in patients with CLD has not been clearly documented, and it varies depending on the severity of cirrhosis, which influences the regeneration capacity of the diseased parenchyma. But in general a remnant liver volume of 40% to 50% of the total liver volume should be left before considering major liver resection (Azoulay et al, 2000; Lee & Hwang, 2005; Ribero et al, 2007).
Role of Preoperative Portal Vein Embolization
For patients who require major or extended hepatectomy but have inadequate remnant liver volume, preoperative portal vein embolization (PVE) can be used to induce hypertrophy of the remnant liver (Makuuchi et al, 1990; see Chapter 93A, Chapter 93B ). A general concern is that preoperative PVE may be less effective in the cirrhotic liver, compared with normal liver, because of its impaired regenerative capacity (Abdalla et al, 2002); however, recent studies have demonstrated that preoperative PVE is effective in inducing hypertrophy of remnant liver and decreasing postoperative complications in patients with cirrhotic livers undergoing hepatic resection for HCC. Our center also used PVE in selected F3 and F4 cirrhotic patients undergoing major hepatectomy. The procedure was well tolerated, but the degree of hypertrophy varied according to the degree of fibrosis and the severity of cirrhosis (Farges et al, 1999).
Absence of hypertrophy of the FLR should be considered an absolute contraindication for hepatic resection (Belghiti & Ogata, 2005; Sato et al, 2000); however, we showed that preoperative sequential transarterial chemoembolization (TACE) and PVE increased the hypertrophy of the FLR. In addition, we found a clear relationship between the rate of hypertrophy and postoperative risk after major hepatectomy in cirrhotic patients. These results were recently confirmed by a similar study, which showed that sequential TACE and PVE increase the hypertrophy rate of the remnant liver and are associated with improved overall and recurrence-free survival in patients with HCC, compared with those who have PVE alone (Yoo et al, 2010). After right hepatectomy for HCC, all postoperative deaths and most severe complications were experienced in F4 patients with less than a 10% increase of FLR. Based on these observations, we propose that a minimum increase of 10% of the FLR in F3 or F4 patients is essential to safely perform a right hepatectomy.
Operative Techniques
The general techniques of liver resection are described in Chapters 90A and 90B. This chapter focuses mainly on operative techniques specifically related to the cirrhotic liver.
Skin Incision
A bilateral subcostal incision is the standard incision for hepatic resection and is adequate for most cases of hepatectomy; an extended right subcostal incision is an acceptable alternative. The approach to large tumors located in the upper segment of the right liver or in its posterior surface (segment VII) can be facilitated by an upward midline extension or by a J-incision (Sato et al, 2000). Although the diaphragm incision increases the rate of pleural effusion, it facilitates parenchymal transection by making the transection plane perpendicular to the wound. A study by the Hong Kong group (Xia et al, 2003) showed that a thoracoabdominal approach for right-sided hepatectomy does not increase operative morbidity, compared with an abdominal approach alone, and may help reduce blood loss and transfusion requirements. For cirrhotic patients with HCC in the superior segments of the liver located beneath the right diaphragm, an exclusive transthoracic approach has been described, but this approach allows only limited resection with a high risk of positive margin (Pocard et al, 2002).
Intraoperative Ultrasound
Intraoperative ultrasound (IOUS) with color Doppler is an invaluable tool in hepatic resection, allowing localization of intrahepatic vessels, assessment of their relationship with tumors, and detection of other nodules and tumor thrombus. IOUS also enables accurate marking of the transection line to ensure an adequate tumor-free margin (see Chapter 21). The use of IOUS optimizes the balance between oncologic radicality and sparing of the largest amount of functioning liver parenchyma in the cirrhotic liver. Recently, systematic segmentectomy and subsegmentectomy by IOUS-guided finger compression was described (Torzilli et al, 2010). This technique can be potentially applied in each segment of the liver, as long as the thickness of the parenchyma and the anatomy of the liver are suitable. The IOUS-guided finger compression of the vascular pedicle feeding the tumor at the level closest to the tumor results in a demarcation area that allows oncologic resection.
Anterior Approach
When liver tumors are large, the use of conventional techniques may require forceful retraction and mobilization of the liver, with possible consequences that include compression of both right and left lobes and possible tumor disruption or dissemination of tumor cells. Mobilization of the cirrhotic liver is technically more demanding, because of the firm texture of the fibrotic liver. The collateral vessels formed secondary to portal hypertension may increase the risk of bleeding during liver mobilization. Prolonged forceful rotation of the liver increases the risk of avulsion injury to the hepatic veins and may twist the inflow and outflow vascular pedicles, resulting in ischemic damage to the remnant liver. Care should be taken to avoid prolonged rotation of the liver. The anterior approach attempts to diminish manipulation of the tumor-bearing liver by initial vascular inflow control, parenchymal transection until the anterior surface of the inferior vena cava is exposed, venous outflow control, and finally mobilization of the right liver (Agrawal & Belghiti, 2011).
Once the devascularized tumorous liver is disconnected from the vena cava, it makes sense that its mobilization is less hemorrhagic, and that this “no touch” liver resection could reduce possible systemic dissemination of cancer cells. In a prospective randomized controlled study of the anterior versus conventional approach in patients with HCC greater than 5 cm, a group from the University of Hong Kong demonstrated significantly less frequent major operative blood loss greater than 2 L (28.3% vs. 8.3%) and also demonstrated superior overall survival—median 68.1 months versus 22.6 months—with the anterior approach (Agrawal & Belghiti, 2011).
The liver-hanging maneuver, originally described by our group, involves the passage of a tape in the avascular retrohepatic precaval space to suspend the liver during parenchymal transection, thereby facilitating the anterior approach by minimizing bleeding in the deeper parenchymal plane and guiding the direction of the parenchymal transection (Agrawal & Belghiti, 2011). The traction of this tape allows delineation of the planned parenchymal transection, which follows a hepatic vein. The transection plane along a hepatic vein represents an oncologic plane and preserves the outflow of the remnant liver, increasing both its function and its potential for regeneration. Application of the hanging maneuver has been further extended to the three Glisson pedicles and hepatic veins, facilitating the performance of right and left anatomic liver resections (Agrawal & Belghiti, 2011). Although more data based on long-term survival are expected, several arguments are leading to recommend the anterior approach in combination with the hanging maneuver as the preferred operative approach for liver malignancy.
Technique of Liver Transection
The main difficulty of hepatic resection in the cirrhotic liver lies in control of bleeding during transection of the fibrotic parenchyma. Clamp crushing, the conventional method of liver transection, is still used in some centers (Imamura et al, 2003; Jarnagin et al, 2002); however, the Cavitron Ultrasonic Surgical Aspirator (CUSA, Valleylab, Boulder, CO) is probably a better alternative and appears to reduce blood loss compared with the clamp-crushing technique (Fan et al, 1996). The CUSA has become the standard technique of liver transection for cirrhotic liver in many centers (Takayama et al, 2001).
Other techniques of liver transection use the Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH) and multiprobe bipolar radiofrequency device (Habib 4X; AngioDynamics, Latham, NY). The latter allows surgeons to perform minor and major hepatectomies with minimal blood loss, a low blood transfusion requirement, and reduced mortality and morbidity rates (Ayav et al, 2008); however, this device is seldom used in tertiary reference centers for treatment of HCC because of concerns about the preservation of venous drainage of the remnant liver and the risk of postoperative bile leak and necrosis (Kim et al, 2003; Lupo et al, 2007). The role of this technology probably is limited to segmental or wedge excision because of the potential risk of vascular and bile duct injury when using multiprobe bipolar radiofrequency near the liver hilum and its inability to control bleeding from large venous branches. Saline-linked cautery (TissueLink Medical, Dover, NH) uses a metal probe to deliver radiofrequency energy conducted through a slow infusion of saline. This procedure enlarges the transection margin (Kianmanesh et al, 2008) and can be associated with ultrasonic dissection in a standardized fashion (Aloia et al, 2005).
Anatomic and Nonanatomic Resection
Intrahepatic metastasis of HCC along the portal vein is the basis of anatomic liver resection. In cirrhotic patients, oncologic safety must be balanced with future liver volume. Analysis of a nationwide Japanese database that included 72,144 patients showed improved disease-free survival with anatomic resection compared with nonanatomic resection, but no difference in overall survival was found (Eguchi et al, 2008). When survival was stratified by tumor size, disease-free survival was significantly improved with an anatomic resection for HCC with a diameter of 2 to 5 cm. The low incidence of vascular invasion in HCC of less than 2 cm may explain the absence of a beneficial effect with anatomic resection and the equivalent efficacy of local ablative therapy. For HCC greater than 5 cm, the tumor is often associated with vascular invasion, satellite nodules, and other adverse pathologic features, which probably offsets the effect of the type and extent of hepatectomy (Agrawal & Belghiti, 2011).
Vascular Staplers
Staplers can be used in liver surgery for control of inflow and outflow vessels. The use of a vascular stapler may help reduce blood loss, especially in situations in which a large tumor in the right or left lobe hinders exposure of the right or left hepatic vein, or during control of the middle hepatic vein deep in the transection plane. The vascular stapler also can be used to divide the hepatic pedicle in right or left hepatectomy (Figueras et al, 2003) with results similar to those of hilar dissection. The use of vascular staplers to divide the hepatic pedicle saves suturing time, but care must be exercised not to narrow the hepatic duct confluence, especially during a right or left extended hepatectomy (Wang & Fan, 2003).
Vascular Occlusion
During liver resection, reducing blood loss and thereby reducing the need for transfusion is essential, especially in cirrhotic patients, who have a poor tolerance to hemodynamic instability and to massive transfusion. A review of the literature concerning the relationship between blood loss and blood transfusion during liver surgery for malignant tumors and postoperative outcome showed a significant and clinically relevant association between blood transfusion and postoperative mortality and morbidity, especially postoperative infectious complications (De Boer et al, 2007). In particular, blood transfusion may have an impact on tumor recurrence in patients with early stages of HCC. Vascular clamping in an effort to reduce intraoperative blood loss is counterbalanced by a poor tolerance of cirrhotic parenchyma to warm ischemia. Total vascular occlusion, even when associated with in situ cold perfusion of the liver, is contraindicated in patients with diseased liver (Azoulay et al, 2006). Intermittent inflow occlusion, with 15 minutes of clamping and 5 minutes of unclamping, is well tolerated for up to 90 minutes in selected cirrhotic patients with good liver function (Ishizaki et al, 2006). In our center, we do not hesitate to use intermittent inflow clamping when resection is difficult or when there is a need to define adjacent vascular or biliary structures in the margin of resection.
Overall, a tendency to perform hepatic resection without clamping has resulted from several factors, including 1) increased experience of surgeons in liver resection; 2) the use of isolated inferior vena cava occlusion without any risk of ischemia on the liver parenchyma, and 3) the practice of low central venous pressure (CVP) anesthesia. One of the most important factors related to intraoperative blood loss is pressure within the inferior vena cava (Johnson et al, 1998). As hepatic venous pressure directly reflects the caval pressure, the maintenance of a low CVP is an effective technique to reduce back bleeding from the hepatic veins.
Abdominal Drainage
The risk of hemorrhage and bile leakage and the high rate of ascites after hepatectomy in the cirrhotic liver leads many surgeons to consider abdominal drainage. In a retrospective study that included 1269 consecutive patients who underwent elective hepatectomy with drain insertion for malignant tumors, seven patients had postoperative bleeding (0.6%), and bile leakage was found in 111 patients (8.7%; Kyoden et al, 2010). The authors concluded that placement of drains was effective in a considerable proportion of patients undergoing hepatectomy, with regard to reducing the frequency of subphrenic fluid collections and biliary fistula.
In a prior review, Burt and colleagues (2002) showed that placement of drains after routine hepatic resection was unnecessary, but the use of drainage after routine liver resection remains a point of controversy. A randomized controlled trial (RCT) performed in the Hospital Clinic of Barcelona (Fuster et al, 2004) showed that intraabdominal closed drainage is advisable in cirrhotic patients undergoing liver resection for HCC, mainly for patients with preoperative portal hypertension. By contrast, another RCT showed that the placement of abdominal drains after hepatic resection in the cirrhotic liver was associated with increased wound and septic complications and prolonged hospital stay (Liu et al, 2003), because the drain provides a route for ascending infection and results in loss of protein and electrolytes.
Laparoscopic Resection
Laparoscopic liver resections represent about 10% to 20% of all liver resections performed at most centers (see Chapter 90E). Limited to a few centers worldwide (Dagher et al, 2007, 2008; Vigano et al, 2009), laparoscopic resections are associated with a long learning curve, difficulty achieving wide resection margins and in performing anatomic resections, and technical difficulties in mobilization and parenchymal transection with risk of massive bleeding; all of these remain obstacles for the emergence of laparoscopic liver resection as a routine procedure. Anticipated advantages from laparoscopic liver resections include that it is a less aggressive approach with less painful incisions, fewer pulmonary complications, and earlier recovery, and it requires less extensive peritoneal dissection; this means the avoidance of collateral ligation, less bleeding, minimal ascites, and decreased postoperative liver failure (Cherqui et al, 2000).
These potential benefits could expand indications of liver resection in some patients with class B cirrhosis (Agrawal & Belghiti, 2011). Moreover, fewer postoperative adhesions after laparoscopic liver resection, compared with open liver resection, might facilitate subsequent salvage liver transplantation with decreased morbidity (Laurent et al, 2009). The laparoscopic approach is also a potentially useful tool for treating tumors with RFA (Santambrogio et al, 2005).
Lesions located in posterior segments (VII, VIII, IVa, or I) have typically been difficult for a purely laparoscopic approach and are more often described with hand-assisted resections (Huang et al, 2009; Koffron et al, 2007) or assisted laparoscopic resections (Nitta et al, 2010). Laparoscopy also has limited role in lymph node or porta hepatis dissections.
Morbidity and Mortality
Improvements in patient selection and surgical technique have resulted in a remarkable decrease in perioperative mortality rates. In the 1980s, the mortality rate after liver resection in cirrhotic patients was typically 15% to 30%, and liver resections in cirrhotic patients were largely restricted to segmental or subsegmental resections (Bismuth et al, 1986; Matsumata et al, 1990); however, over the past several years, significant improvements in operative and perioperative care were realized, such that the current mortality rate is approximately 5%, with some centers approaching zero mortality (Jaeck et al, 2004; Torzilli et al, 1999). The Hong Kong group showed that even extended right or left hepatectomy could be performed in cirrhotic patients with acceptable morbidity and mortality, which were not significantly different from rates of patients with less extensive resections (Poon et al, 2002c).
Intravenous Fluid
Although fluid restriction has been found to reduce the incidence of postoperative morbidity in some surgical patients (Nisanevich et al, 2005), optimal fluid substitution strategies remain controversial, because fluid optimization increases tissue oxygen tension and microcirculatory perfusion (Futier et al, 2010). It has been shown that excessive fluid restriction leads to hypovolemia and reduced central venous oxygen saturation, thereby increasing postoperative complications (Futier et al, 2010). Excessive fluid restriction should be applied cautiously in cirrhotic patients. Crystalloid solution (dextrose and saline) is used for maintenance of intravenous fluid. In some centers, fresh frozen plasma is used routinely for fluid replacement during and after the operation (Imamura et al, 2003), although there is no evidence to support such a practice. In our center, albumin is used instead, because fresh frozen plasma is associated with potentially significant transfusion-related complications.
Antibiotics
There is a tendency to administer prophylactic antibiotics to cirrhotic patients beyond the accepted single preoperative dose because of their immunocompromised state. Intraoperative antibiotic prophylaxis is aimed at preventing translocation of intestinal enterobacteria to the systemic circulation in patients with both healthy and diseased livers. The risk of infection is aggravated further by the prolonged operation and the high frequency of ascites. Septic complications, especially wound infection, are the most common complications after hepatectomy for HCC (Fan et al, 1999); however, no randomized trials suggest the optimal regimen of antibiotics in patients with cirrhosis.
Pain Control
Adequate pain control is an important factor in reducing the incidence of pulmonary complications after hepatic resection for HCC (see Chapter 22).
Nutrition Support
Because of the prevalence of malnutrition in cirrhotic patients, nutrition support is important to enhance postoperative recovery and liver regeneration (see Chapter 24). Oral feeding is started early, on postoperative day 1, if the patient does not manifest signs of liver failure. Since a randomized, controlled trial published in 1994 (Fan et al, 1994) showed the benefit of parenteral nutrition in reducing postoperative morbidity, postoperative parenteral nutrition has become a routine practice in many centers for patients with cirrhosis undergoing major hepatic resection.
Postoperative Ascites
Postoperative ascites is a classic complication following liver resection in cirrhotic patients, and it affects more than one third of patients. Significant ascites is poorly defined, but the presence of ascitic fluid drainage from thoracic and abdominal drains exceeding 10 mL/kg of preoperative body weight (Ishizawa et al, 2009) or a daily output greater than 500 mL after postoperative day 3 require a specific aggressive intervention. Factors that increase the rate and amount of ascites include preexisting ascites before resection, the presence of portal hypertension, low platelet count, major resection, pedicular clamping, lymph node dissection, and perioperative fluid infusion, including blood transfusion. Ascites after liver resection in cirrhotic patients has several potential deleterious consequences, including abdominal pain, impairment of ventilation, development of pleural effusion, infection, impairment of renal function, and abdominal leak. Ascites leakage through the abdominal incision can result in loss of electrolytes and protein, and the risk of intraabdominal infection is also increased.
The management of ascites requires replacement of fluid lost and, in some cases, aggressive diuretic therapy. Fresh frozen plasma transfusion during and after liver resection is advocated by some surgeons (Ishizawa et al, 2009) but is not supported by other studies, which show an increased risk of transmitting diseases and more adverse consequences, such as transfusion-related acute lung injury, acute allergic and anaphylactic reactions, and high cost (Caldwell et al, 2006). In several centers such as ours, fresh frozen plasma is replaced by albumin or other plasma expanders.
Postoperative Bleeding
The risk of postoperative bleeding is very low, around 1% (Belghiti et al, 2000; Imamura et al, 2003; Jarnagin et al, 2002). The presence of bleeding during the first postoperative day is a life-threatening complication that requires reoperation, and it comes with a 30% risk of death in cirrhotic patients. This high risk makes ensuring a perfect hemostasis at the end of liver resection imperative for all liver operations, but especially those in cirrhotic patients.
Biliary Fistula (See Chapter 42A, Chapter 42B )
Bile leakage after hepatic resection can cause septic complications, which lead to a high risk of mortality in cirrhotic patients. The rate of biliary leakage was found to be approximately 6% in a single-center series of 605 liver resections for HCC, and it was increased by preoperative chemoembolization and with tumors with a central location (Lee et al, 2005). Although drainage fails to prevent postoperative biliary collection in approximately one third of patients, and percutaneous drainage is therefore required (Kyoden et al, 2010), this risk of bile collection has led some authors to adopt the use of long-term drains (Torzilli et al, 2005). In a prospective cohort study that enrolled 58 patients, who underwent liver resection for tumors with abdominal drains maintained for at least 7 days postoperatively, Torzilli and colleagues (2005) showed that the bilirubin level in the drain was higher on the fifth postoperative day than on the third and seventh postoperative days, leading them to advocate the use of long-term drains.
Liver Failure
Patients with cirrhosis have decreased liver functional reserve and a limited capacity for liver regeneration. As a result, major hepatectomy in cirrhotic patients is associated with a significant risk of liver failure that could occur primarily as a result of inadequate liver remnant, but more often it is precipitated by massive bleeding or septic complications. Despite improvements in the safety of hepatectomy in recent years, postoperative liver failure still occurs in 3% to 10% of patients after resection for HCC (Kubo et al, 2004; Osada & Saji, 2004).
Liver failure is associated with a high mortality rate and is the most common cause of hospital mortality following hepatic resection in the cirrhotic liver (Belghiti et al, 2000; Wu & Zhang, 2002). Cirrhotic patients are also prone to gradual hepatic decompensation in long-term follow-up, especially after major hepatic resection for large HCCs (Huo et al, 2004). Although several clinical and biologic factors are markers of impaired liver function—such as ascites, encephalopathy, jaundice, prolonged prothrombin time, hyperbilirubinemia, and hypoalbuminemia—no standardized definition of postoperative liver failure (PLF) has been agreed upon, nor have any precise data on its correlation with postoperative mortality been reported.
The need for standardization of PLF is important. Prothrombin time (PT) and serum total bilirubin level (SB)—two biologic indicators of liver dysfunction in the Child-Turcotte-Pugh scoring system (and also two of the three components of the MELD score)—are less reliable postoperatively because of the natural impairment of liver function tests during the first postresection days. We found that on postoperative day 5, the association of PT less than 50% and SB greater than 50 ml/L, the so-called 50-50 criteria, was a simple, early, and accurate predictor of a higher than 50% mortality rate after hepatectomy (Balzan et al, 2005). Moreover, these clinical criteria may be useful to identify high-risk patients before clinical evidence of complications appear, allowing the institution of specific interventions that may help improve outcome. Postoperative complications that include portal vein thrombosis, ascites, and pulmonary infection are difficult to diagnosis early after operation, but they result in late mortality in a high proportion of patients. Early recognition of postoperative liver failure is an important factor that may allow early intervention and improved outcome.
Abdalla EK, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680.
Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656-665.
Aloia TA, et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172-177.
Ayav A, et al. Liver resection with a new multiprobe bipolar radiofrequency device. Arch Surg. 2008;143:396-401.
Azoulay D, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672.
Azoulay D, et al. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88.
Balzan S, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-848.
Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford). 2005;7:252-253.
Belghiti J, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46.
Belghiti J, et al. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993-1000.
Bismuth H, et al. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986;10:311-317.
Brancatelli G, et al. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology. 2001;219:69-74.
Brancatelli G, et al. Focal nodular hyperplasia: CT findings with emphasis on multiphasic helical CT in 78 patients. Radiology. 2001;219:61-68.
Bruix J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022.
Burt BM, et al. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441-445.
Caldwell SH, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039-1046.
Capussotti L, et al. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992-999.
Cherqui D, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753-762.
Cherqui D, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746.
Cucchetti A, et al. Recovery from liver failure after hepatectomy for hepatocellular carcinoma in cirrhosis: meaning of the Model for End-stage Liver Disease. J Am Coll Surg. 2006;203:670-676.
Dagher I, et al. Laparoscopic liver resection: results for 70 patients. Surg Endosc. 2007;21:619-624.
Dagher I, et al. Laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc. 2008;22:372-378.
Dahiya D, et al. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676-685.
De Boer MT, et al. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259-264.
Del Gaudio M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177-1185.
Delis S, et al. Model for End-stage Liver Disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2009;11:351-357.
Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107.
Eguchi H, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by posthepatectomy liver failure. Dig Dis Sci. 2000;45:1383-1388.
Eguchi S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469-475.
Fan ST, et al. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547-1552.
Fan ST, et al. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117-120.
Fan ST, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330.
Farges O, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215.
Ferrero A, et al. Hepatectomy as treatment of choice for hepatocellular carcinoma in elderly cirrhotic patients. World J Surg. 2005;29:1101-1105.
Figueras J, et al. Hilar dissection versus the “glissonean” approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. Ann Surg. 2003;238:111-119.
Freeman RB, et al. Excellent liver transplant survival rates under the MELD/PELD system. Transplant Proc. 2005;37:585-588.
Fuster J, et al. Abdominal drainage after liver resection for hepatocellular carcinoma in cirrhotic patients: a randomized controlled study. Hepatogastroenterology. 2004;51:536-540.
Futier E, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg. 2010;145:1193-1200.
Gedaly R, et al. Obesity, diabetes, and smoking are important determinants of resource utilization in liver resection: a multicenter analysis of 1029 patients. Ann Surg. 2009;249:414-419.
Haber MM, et al. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol. 1995;90:1250-1257.
Hanaoka J, et al. Hepatocellular carcinoma with massive bile duct tumor thrombus: report of a long-term survival. Hepatogastroenterology. 2008;55:2217-2220.
Huang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912.
Huang MT, et al. A series of laparoscopic liver resections with or without HALS in patients with hepatic tumors. J Gastrointest Surg. 2009;13:896-906.
Huo TI, et al. Deterioration of hepatic functional reserve in patients with hepatocellular carcinoma after resection: incidence, risk factors, and association with intrahepatic tumor recurrence. World J Surg. 2004;28:258-262.
Iascone C, et al. Occurrence of synchronous colorectal cancer metastasis in the cirrhotic or fatty liver. Minerva Chir. 2005;60:185-190.
Ikai I, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65-75.
Ikai I, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37:676-691.
Imamura H, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206.
Imamura H, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22.
Ishizaki Y, et al. Safety of prolonged intermittent Pringle maneuver during hepatic resection. Arch Surg. 2006;141:649-653.
Ishizawa T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916.
Ishizawa T, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46-51.
Jaeck D, et al. Surgical resection of hepatocellular carcinoma: postoperative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58-S63.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406.
Johnson M, et al. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998;85:188-190.
Kamath PS, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470.
Kianmanesh R, et al. Heat-zone effect after surface application of dissecting sealer on the “in situ margin” after tumorectomy for liver tumors. J Am Coll Surg. 2008;206:1122-1128.
Kim J, et al. Increased biliary fistulas after liver resection with the Harmonic Scalpel. Am Surg. 2003;69:815-819.
Koffron AJ, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385-392.
Kosuge T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328-332.
Kubo S, et al. Hepatic failure after liver resection in patients with cirrhosis. Nippon Geka Gakkai Zasshi. 2004;105:669-673.
Kyoden Y, et al. Value of prophylactic abdominal drainage in 1269 consecutive cases of elective liver resection. J Hepatobiliary Pancreat Sci. 2010;17:186-192.
Lau H, et al. Long-term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2302-2311.
Laurent A, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310-314.
Le Treut YP, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature: a European case-control series. J Gastrointest Surg. 2006;10:855-862.
Lee CC, et al. Risk factors associated with bile leakage after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1168-1171.
Lee KW, et al. Liver transplantation for hepatocellular carcinoma with bile duct thrombi. Transplant Proc. 2006;38:2093-2094.
Lee SG, Hwang S. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:38-43.
Lesurtel M, Belghiti J. Temporary portal vein embolization as a starter of liver regeneration. J Hepatol. 2008;49:313-315.
Libbrecht L, et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol. 2006;101:2341-2346.
Liu CL, et al. Hepatic resection for bilobar hepatocellular carcinoma: is it justified? Arch Surg. 2003;138:100-104.
Luciani A, et al. Imaging nodules within cirrhotic liver: how do I do it? J Radiol. 2007;88:1073-1090.
Lupo L, et al. Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg. 2007;94:287-291.
Makuuchi M, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527.
Malinchoc M, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871.
Matsumata T, et al. Decreased morbidity and mortality rates in surgical patients with hepatocellular carcinoma. Br J Surg. 1990;77:677-680.
Mazzaferro V, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43.
Minagawa M, et al. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379-384.
Minagawa M, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703-710.
KK Ng, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364-373.
Nisanevich V, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25-32.
Nitta H, et al. Laparoscopy-assisted major liver resections employing a hanging technique: the original procedure. Ann Surg. 2010;251:450-453.
Noun R, et al. High preoperative serum alanine transferase levels: effect on the risk of liver resection in Child grade A cirrhotic patients. World J Surg. 1997;21:390-394.
Osada S, Saji S. The clinical significance of monitoring alkaline phosphatase level to estimate postoperative liver failure after hepatectomy. Hepatogastroenterology. 2004;51:1434-1438.
Pawlik TM, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092.
Pawlik TM, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450-457.
Peng BG, et al. Surgical treatment of hepatocellular carcinoma with bile duct tumor thrombi. World J Gastroenterol. 2005;11:3966-3969.
Pocard M, et al. Limits and benefits of exclusive transthoracic hepatectomy approach for patients with hepatocellular carcinoma. Hepatogastroenterology. 2002;49:32-35.
Poon RT, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222.
Poon RT, et al. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24.
Poon RT, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611.
Poon RT, et al. Does diabetes mellitus influence the perioperative outcome or long-term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol. 2002;97:1480-1488.
Poon RT, et al. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592-602.
Poon RT, et al. Prognosis after hepatic resection for stage IVa hepatocellular carcinoma: a need for reclassification. Ann Surg. 2003;237:376-383.
Ramacciato G, et al. Does surgical resection have a role in the treatment of large or multinodular hepatocellular carcinoma? Am Surg. 2010;76:1189-1197.
Ravaioli M, et al. Operative risk by the lidocaine test (MEGX) in resected patients for HCC on cirrhosis. Hepatogastroenterology. 2003;50:1552-1555.
Redaelli CA, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77-85.
Ribero D, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability, and outcome. Br J Surg. 2007;94:1386-1394.
Roayaie S, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Western experience. Ann Surg Oncol. 2000;7:764-770.
Santambrogio R, et al. Survival and intra-hepatic recurrences after laparoscopic radiofrequency of hepatocellular carcinoma in patients with liver cirrhosis. J Surg Oncol. 2005;89:218-225.
Sato H, et al. Thoracoabdominal approaches versus inverted T incision for posterior segmentectomy in hepatocellular carcinoma. Hepatogastroenterology. 2000;47:504-506.
Shi J, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080.
Shi M, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36-43.
Takayama T, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928.
Torzilli G, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984-992.
Torzilli G, et al. Bilirubin level fluctuation in drain discharge after hepatectomies justifies long-term drain maintenance. Hepatogastroenterology. 2005;52:1206-1210.
Torzilli G, et al. Anatomical segmental and subsegmental resection of the liver for hepatocellular carcinoma: a new approach by means of ultrasound-guided vessel compression. Ann Surg. 2010;251:229-235.
Tsujita E, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery. 2010;147:696-703.
Uchiyama K, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:443-452.
Vigano L, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772-782.
Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193-196.
Wei AC, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33-41.
Welzel TM, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228.
Wiesner RH, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580.
Wu CC, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049-1057.
Wu M, Zhang Z. Prevention and treatment of complications after hepatectomy. Zhonghua Wai Ke Za Zhi. 2002;40:332-335.
Xia F, et al. Thoracoabdominal approach for right-sided hepatic resection for hepatocellular carcinoma. J Am Coll Surg. 2003;196:418-427.
Yang LY, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118-123.
Yoo H, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2010;18:1251-1257.