CHAPTER 255 Linear Accelerator Radiosurgery
Technical Aspects
Precision and History of Linear Accelerators
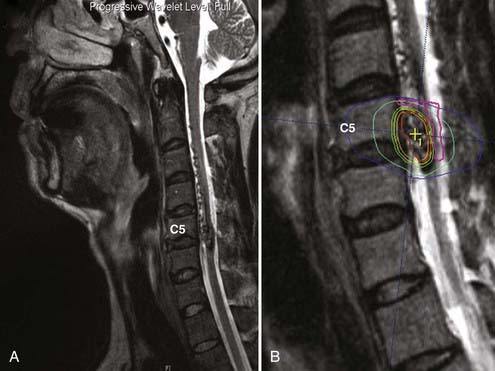
The issue of precision was initially resolved with the description of a gantry correction device by two University of Florida scientists18 and lately by the development of LINACs dedicated to radiosurgery.19 This allowed LINAC radiosurgery to become a competitive technique, not only for lesions but also for functional disorders of the brain, such as trigeminal neuralgia.20-24 LINAC radiosurgery is the most common form of radiosurgery performed today, and its versatility also allows application of the technique to the spine and other sites in the body (Fig. 255-1).16,25,26 Another approach using a LINAC attached to a robot was advanced by the Stanford University group under leadership of the neurosurgeon John Adler.27 This approach is gaining popularity, also because of its ability to reach extracranial targets and obviate the need for a stereotactic frame. ![]()
Dosimetry
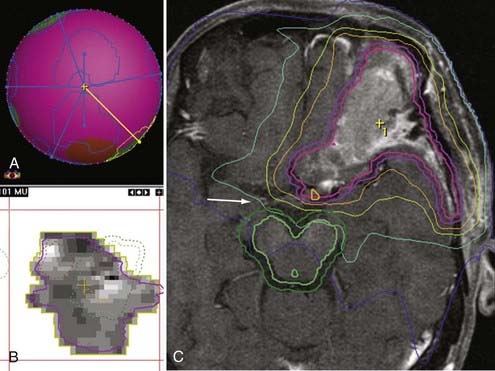
The limitation of the radiosurgery technique imposed by the beam is the intermediate volume. This is the area outside the lesion where the multiple fields (beams) partially overlap.19 As the target volume increases, the intermediate volume area also increases, which means that more volume of normal parenchyma is exposed to higher doses of radiation; consequently, the target volume in radiosurgery is suggested to be no greater than 3 cm (approximately 12.6 cm3). The target volume also has an impact on the shaping capabilities of LINAC radiosurgery. Modern LINAC conformality approaches, such as intensity-modulated radiosurgery (IMRS), allow the treatment of targets larger than 3 cm in largest diameter (Fig. 255-2).
Functional Lesion Considerations
Prescribing to a Point
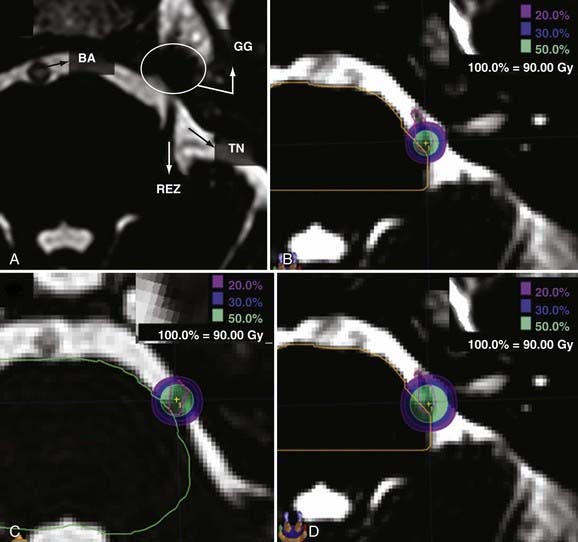
The dose prescribed for functional neurosurgery is by convention and tradition directed to the isocenter. This means that 100% of the dose (maximal dose) is prescribed to a target point (i.e., to the isocenter). The radiation prescription dose is the same as the maximal dose when prescribing to the maximum. The falloff distance, or the volume of tissue receiving at least 50% of the dose, is proportional to the diameter of the collimator because circular collimators are traditionally used for functional radiosurgery (Fig. 255-3). Application of this concept is nicely seen during targeting of the root entry zone for trigeminal neuralgia in LINAC radiosurgery, where 3-, 4-, and 5-mm collimators are available for use.
Placement of the isocenter while planning radiosurgery for trigeminal neuralgia relies on the isodose line (IDL) to determine the distance from the isocenter to the brainstem. Although some LINAC radiosurgery treatments of trigeminal neuralgia have been delivered with the 5-mm collimator,21,32,49,66-68 the majority of data on trigeminal neuralgia published by gamma unit users has been amassed with the 4-mm collimator.69-73 The dose distributions for treating trigeminal neuralgia very well exemplify the concept of prescribing to a point in LINAC radiosurgery ![]() (Fig. 255-3).
(Fig. 255-3).
Prescribing to a Volume
Radiation Dose Falloff
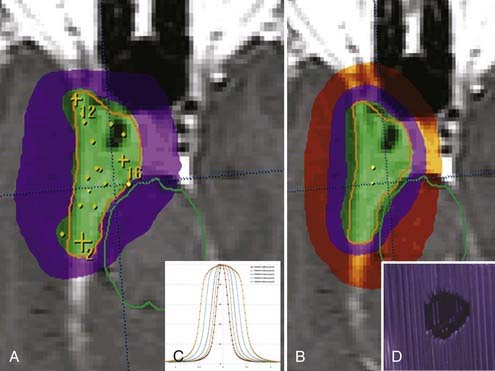
The dose falloff in LINAC radiosurgery is very steep. This accounts for the attractiveness of the method because it allows high radiation dose collimation inside the target with very fast radiation dose falloff in the normal brain tissue surrounding the target. Dose falloff varies according to collimator size and the type of planning used, such as multiple isocenter, dynamic arcs, or static beams (Fig. 255-4). This area of radiation falloff is called the penumbra. Consideration of the dosimetric consequences of the penumbra is important because the radiation dose may be still sufficiently high to cause toxicity in eloquent structures neighboring the lesion, such as the brainstem, motor area, and spinal cord.86,87 Conversely, at the margin of a complex lesion, the falloff dose may still be effective in controlling tumor growth, although underdosed in relation to the remaining lesion volume.
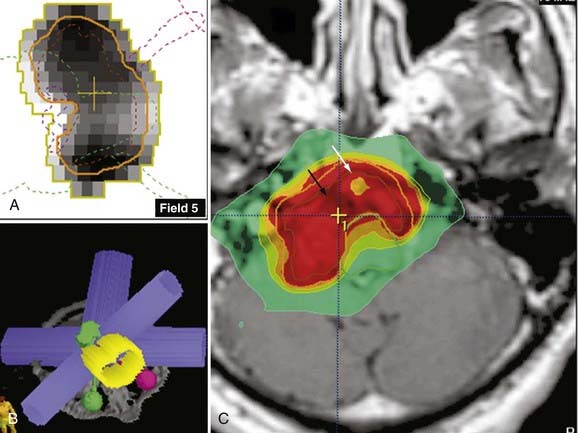
In the context of skull base lesions abutting the optic apparatus or the brainstem or “donut”-shaped vertebral body lesions abutting the spinal cord, it is not wise to deliver the same radiation dose to these eloquent structures as prescribed to the lesion. Although it may be attractive to cover the lesion with additional safety margins, the risk of radiation-induced damage is not justifiable. In these situations, one possible approach is to slightly underdose the boundary of the lesion touching the eloquent structure so that the dose falloff occurs inside the lesion and not at the border of the lesion or outside the contrast-enhancing limits and additional three-dimensional margins. One modern technique available in LINAC radiosurgery to optimize dose falloff in the vicinity of eloquent structures is IMRS (Fig. 255-5).
Homogeneity
It is intuitive to understand that a single collimator will distribute the radiation homogeneously throughout that area. To achieve conformity to an irregular external shape, multiple isocenters are used, which leads to an overlap of many single-isocenter dose distributions. The areas where the prescription dose of collimators intersects constitute “hot spots.” The number of hot spots in the PTV increases as the number of isocenters in the plan increases because of increased overlap. If one chooses to use intensity modulation, inhomogeneity generally increases. To allow steeper dose falloff in that particular border of the lesion, that area is considered cold and the beams are arranged in such a manner that many points inside the lesion become hot or cold to allow eloquent structures to be spared (see Fig. 255-5).
Shaped-Beam Techniques
Taking advantage of the ingenious idea of the collimator, the multileaf collimator was developed. This instrument consists of multiple leaves that move independent of each other and are capable of generating any format or size of collimator within the field limits of the LINAC. Basically, one piece of instrumentation allows infinite combinations of apertures of each leaf, thereby allowing modulation of the radiation dose according to the positioning of each beam and adaptation of the aperture to different shapes according to the beam’s eye view (BEV). Dynamic shaping is also evaluated by the BEV capability in the planning software.91 The BEV capability allows a three-dimensional perspective of the trajectory of the photon radiation directed by an arc or a beam. It is a practical approach to avoid undesirable delivery of radiation to eloquent structures. Initial evaluation of the radiosurgery plan is accomplished by direct visualization of the IDLs displayed on a given imaging modality (magnetic resonance imaging, computed tomography, positron emission tomography). The BEV helps refine evaluation of the entrance and exit trajectories of the photon beam. However, another important tool can be used for this purpose: the dose-volume histogram (DVH) (Fig. 255-6).
The DVH is the most objective informative tool for evaluating a radiosurgery plan. It offers a two-dimensional graphic representation of the full range of radiation doses delivered to volumetric percentages of a given structure. This structure can be the PTV or any other structure relevant to the planning. The DVH can be displayed in two formats: differential or cumulative. The radiation dose is represented on the x-axis and the volumetric percentage of the structure on the y-axis. The differential format shows histograms in which the height of each bin reflects the percentage of volume receiving a given dose within that range. The cumulative format displays the cumulative frequencies of the percent volume receiving an equal or greater dose of the radiation dose projected on the x-axis. The “ideal” DVH of a PTV shows a perfect horizontal line parallel to the x-axis denoting the entire PTV receiving 100% of the normalized radiation dose. The vertical line parallel to the y-axis reflects the precise and steep falloff denoting the absence of hot spots within the PTV. The ideal PTV curve for an eloquent structure to be spared would be the inverse-square curve described for the PTV. The vertical line would overlap on the y-axis, and therefore no volume of the eloquent structure would receive any radiation. The horizontal line would overlap on the x-axis, thus reinforcing the statement of the vertical portion of the square that no eloquent structure volume was receiving 10%, 20%, 30%, 90%, or 100% of the prescription dose. Obviously, the “ideal” DVH does not exist in clinical work (see Fig. 255-6).
Shaped-Beam Linear Accelerator Radiosurgery
Static Beams
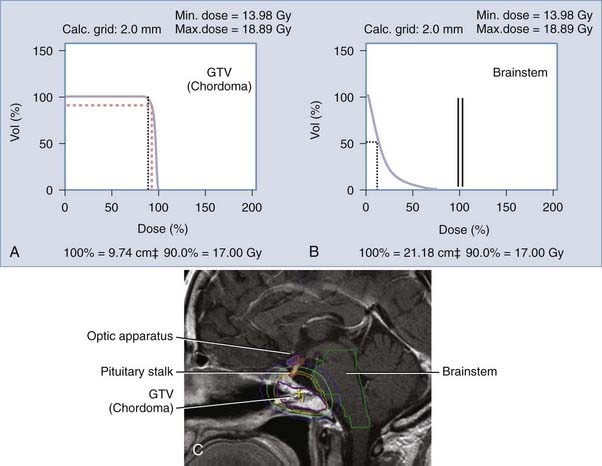
The same principle of dynamic arcs applies with static beams, but delivery of radiation is not continuous throughout the entire movement of the arc. There are precalculated positions within each arc in which the beam is turned on. Planning is somewhat more time and effort consuming and delivery of radiation is also slightly longer, but this technique is preferable for lesions intermingled with multiple eloquent structures and for large complex-shaped lesions. One clinical example of the static-beam planning application is for a skull base chordoma. Although labeled as histologically “benign,” chordomas often recur and are known to be resistant to radiation. A clival location is the most common, which means that photons should not cross the optic apparatus, the brainstem, or the pituitary gland/stalk in their trajectory to the target. Basically, the radiation has to be as thoughtfully distributed as a surgeon’s manual dissection to reach the tumor. This is not accomplished with the use of bipolar, aspiration, or microneurosurgery instruments but rather with the use of an exquisite arrangement of the radiation source apertures and tridimensional beam architecture, including modulation of the photon beam, as well as understanding of the tolerance of structures to the radiation dose prescribed (see Fig. 255-5).
Advanced Methods of Conformality
Despite enormous development of the techniques of LINAC conformal radiation therapy, including multiple isocenters,43,92 shaped beams,40 and pencil beam approaches,34 afforded by computerized imaging, three-dimensional treatment planning software, and fast-delivery LINACs, the need to further improve sparing of normal tissue and enhance the dose of radiation to the pathology remains important (Table 255-1),93,94 as shown by the less than optimal results of radiosurgery when treating several pathologies.95-98
TABLE 255-1 Summary of the Capabilities of Each Technique Routinely Used for Stereotactic Radiosurgery and Stereotactic Radiotherapy Planning

Beam Intensity Modulation
Inverse-Planning Techniques
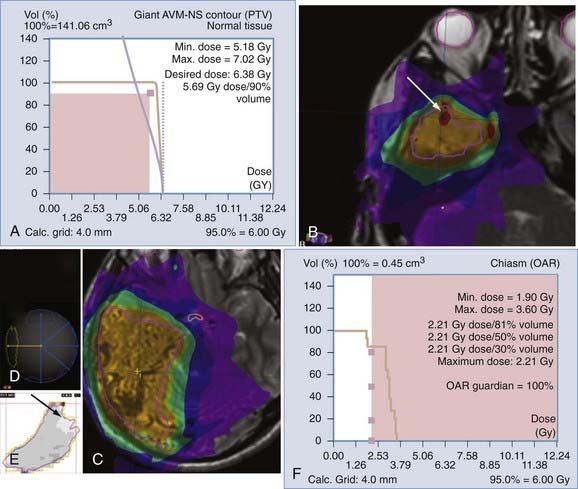
Inverse planning is an optimization process whereby one specifies a desired dose distribution and searches for the beam intensity distribution that will satisfy the request. This is generally accomplished with an objective function that is subsequently minimized through a mathematical operation. In theory and practice, there are a number of functions, both physically and biologically based, that can be used as the objective function. The physical method called the dynamically penalized maximum likelihood (DPL) algorithm has been integrated into commercial treatment planning systems for inverse planning of intracranial targets. A key strength of the DPL approach is the ability to compute a number of inverse plans simultaneously. This allows, for example, varying levels of emphasis to be placed on the target and organs at risk, with the neurosurgeon selecting the appropriate plan for the individual tumor site and patient on the basis of the DVH and dose distribution information![]() (Fig. 255-7).
(Fig. 255-7).
Temporal Modulation
Another and a more advanced method of achieving modulation across the radiation field is temporal modulation. The pioneers of the early concepts of MLC IMRT, which is also a type of temporal modulation, were Proimos and Takahashi.120,121 Dynamic treatments were planned and delivered by these groups with the first prototypes of MLC devices.122 The latest advancement in beam-modulating techniques is volumetric radiotherapy technology, which can result in comparable or improved dose conformity while significantly shortening treatment times. RapidArc is an example of volumetric arc therapy that delivers a radiation dose with a single or several gantry rotations of the LINAC. It is made possible by a treatment-planning algorithm that simultaneously changes three parameters during treatment: speed of rotation of the gantry, shape of the treatment aperture with a multileaf collimator, and the radiation dose rate. This allows delivery of treatment several times faster than with other dynamic treatments. The short treatment time allows improved care of patients (Fig. 255-8).
Frameless Radiosurgery and the Spine
The overall accuracy achievable with frame-based cranial radiosurgery is typically recognized to be 2 to 3 mm in actual patient treatments.33 This kind of accuracy has been obtained with frame-based immobilization in which a coordinate system is also defined for treatment planning. However, the use of frame-based immobilization and localization has shortcomings. Frame placement is an invasive procedure and a significantly more complex process for extracranial treatments that inhibits conventional fractionation.123 Frameless, image-guided radiosurgery overcomes these limitations (Fig. 255-9). However, because the patient is not immobilized with a rigid frame, patient movement is more probable. Therefore, when the goal is to achieve high accuracy, it is important to characterize the patient’s movement during image-guided radiosurgery because even small movements of the patient or organ (or both) can significantly compromise radiation delivery and therefore the clinical outcome.
Image guidance with either radiopaque fiducials implanted in the vertebrae or direct imaging of vertebral anatomy has been used to localize spinal anatomy and associated tumors.36,126,127 Although the extracranial stereotactic radiosurgery frame performs both immobilization and localization, image-guidance techniques require ancillary immobilization. This is generally accomplished by using moldable cushions with the patient lying in the supine position to reduce target motion as a result of respiration (Fig. 255-10). Yin and coworkers observed less than 1 mm of respiratory-induced motion in vertebral bodies during fluoroscopic studies of patients lying in the supine position.127 However, spinal anatomy may move more than 2 mm during the delivery of radiosurgery.128 Agazaryan and associates observed vertebral anatomy movements that vary as much as 3 mm and could occur in as little as 5 minutes. These results suggest a need for intrafraction patient monitoring and correctional shifts, even for patients whose overall treatment times are expected to be relatively short.128
Spine radiosurgery for primary benign, primary malignant, and metastatic tumors has the potential to improve local control by escalation of the effective radiation dose while minimizing the risk for spinal cord injury. An essential requirement for spine radiosurgery is the ability to accurately determine the spinal cord dose associated with a planned treatment so that an overdose to the cord can be avoided. Additionally, our understanding of the tolerance of the spinal cord to radiosurgery continues to evolve, and an accurate determination of the spinal cord dose associated with the treatment is required. Besides small systematic errors associated with positioning uncertainty during spine radiosurgery,129 random errors associated with patient movement create uncertainty regarding the actual radiation dose that the spinal cord receives. Published series of spine radiosurgery have explored and quantified intrafraction patient movement during spine radiosurgery, although additional data are needed.25,129,130 These data indicate that significant movement can occur during treatment. Separately, some groups have examined the uncertainty of the spinal cord dose associated with simulated patient positioning errors and demonstrated the need for an accurate understanding of uncertainty in setup and movement.131,132 Several cases of myelitis have been reported after spine radiosurgery.133-135 In the published accounts, the spinal cord doses appear to have been within limits widely accepted as being safe for almost all cases. However, only the planned cord dose could be reported, which ignores the possibility of intrafraction patient movement. It would be greatly beneficial to our understanding of spinal cord tolerance if a study were performed in which online measurements of patient movement were taken into account in providing an estimate of the actual dose delivered to the spinal cord. The measured patient translations and rotations can be simulated in the planning system after the treatment to determine the actual dose delivered to the spinal cord. Later, these data could be analyzed in terms of complications. Such monitoring capabilities do exist with modern radiosurgery systems (see Fig. 255-9).
Agazaryan N, Tenn SE, De Salles AAF, et al. Image-guided radiosurgery for spinal tumors: methods, accuracy and patient intrafraction motion. Phys Med Biol. 2008;53:1715-1727.
Anderson B, Larsson B, Leksell L, et al. Histopathology of late local radiolesions in the goat brain. Acta Radiol Ther Phys Biol. 1970;9:385-394.
Betty O, Derechinsky V. Irradiation stéréotaxique multifasceaux. Neurochirurgie. 1983;29:295-298.
Colombo F, Benedetti A, Pozza F, et al. External stereotactic radiation by linear accelerator. Neurosurgery. 1985;16:154-160.
De Salles AAF, Gorgulho A, Selch M, et al. Radiosurgery from the brain to the spine: 20 years experience. Acta Neurochir Suppl. 2008;101:163-168.
De Salles AAF, Melega WP, Lacan G, et al. Radiosurgery with a 3mm collimator in the subthalamic nucleus and substantia nigra of the vervet monkey. J Neurosurg. 2001;95:990-997.
De Salles AAF, Pedroso AG, Medin P, et al. Novalis shaped beam and intensity modulated radiosurgery and stereotactic radiotherapy for spine lesions. J Neurosurg. 2004;101(suppl 3):435-440.
De Salles AAF, Solberg T, Medin P, et al. Linear accelerator radiosurgery for trigeminal neuralgia. Radiosurgery. 1997;2:173-182.
Friedman WA, Bova FJ. The University of Florida radiosurgery system. Surg Neurol. 1989;32:334-342.
Frighetto L, De Salles AAF, Smith ZA, et al. Non-invasive treatment of trigeminal neuralgia using a dedicated linear accelerator. Neurology. 2004;62:660-662.
Gorgulho AA, De Salles AA. Impact of radiosurgery on the surgical treatment of trigeminal neuralgia. Surg Neurol. 2006;66:350-356.
Gorgulho AA, Ishida W, De Salles AAF. General imaging modalities: Basic principles. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Text Book of Stereotactic and Functional Neurosurgery. Springer, 2009. in press
Kjellberg RN, Hanamura T, Davis KR, et al. Bragg-peak proton-beam therapy for arteriovenous malformations. N Engl J Med. 1983;309:269-274.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316.
Medin PM, Solberg TD, De Salles AAF, et al. Investigations of a minimally invasive method for treatment of spinal malignances with linac stereotactic radiation therapy: accuracy and animal studies. Int J Radiat Oncol Biol Phys. 2002;52:1111-1122.
Murphy MJ, Chang SD, Gibbs IC, et al. Patterns of patient movement during frameless image-guided radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1400-1408.
Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838-846.
Smith ZA, De Salles AAF, Frighetto L, et al. Linear accelerator radiosurgery for the treatment of trigeminal neuralgia. J Neurosurg. 2003;99:511-516.
Solberg TD, Medin PM, DeMarco JJ, et al. Technical aspects of Linac radiosurgery for functional targets. J Radiosurgery. 1998;1:115-127.
Solberg TD, Selch MT, De Salles AAF. Fractionated stereotactic radiosurgery: Rational and methods. Med Dosim. 1998;23:209-219.
Sun B, De Salles AAF, Medin P, et al. Reduction of hippocampal-kindled seizure activity in rats by stereotactic radiosurgery. Exp Neurol. 1998;154:691-695.
Thwaites DI, Tuohy JB. Back to the future: the history and development of the clinical linear accelerator. Phys Med Biol. 2006;51:R343-R362.
Voges J, Treuer H, Sturm V, et al. Risk analysis of linear accelerator radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:1055-1063.
Winston KR, Lutz W. Linear accelerator as a neurosurgical tool for stereotactic radiosurgery. Neurosurgery. 1988;22:454-464.
Yin FF, Ryu S, Ajlouni M, et al. Image-guided procedures for intensity-modulated spinal radiosurgery. Technical note. J Neurosurg. 2004;101(suppl 3):419-424.