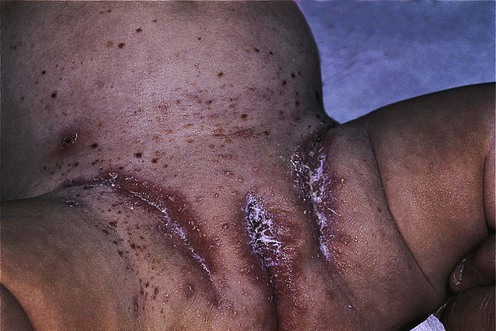
Langerhans cell histiocytosis
First-line therapies
Second-line therapies
Third-line therapies
Lenalidomide induced therapeutic response in a patient with aggressive multi-system Langerhans cell histiocytosis resistant to 2-chlorodeoxyadenosine and early relapse after high dose BEAM chemotherapy with autologous peripheral stem cell transplant.
Adam Z, Rehak Z, Koukalova R, Szturz P, Krejci M, Pour L, et al. Vnitr Lek 2012; 58: 62–71.




















 Topical nitrogen mustard
Topical nitrogen mustard Phototherapy (PUVA, narrowband UVB)
Phototherapy (PUVA, narrowband UVB) Topical tacrolimus
Topical tacrolimus Vinblastine
Vinblastine Etoposide (VP16)
Etoposide (VP16)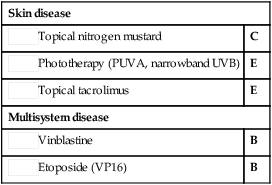
 Prednisolone
Prednisolone 6-Mercaptopurine
6-Mercaptopurine Thalidomide
Thalidomide Methotrexate
Methotrexate Cytosine arabinoside (Ara-C)
Cytosine arabinoside (Ara-C) 2-Chlorodeoxyadenosine (2-CdA)
2-Chlorodeoxyadenosine (2-CdA) Radiotherapy
Radiotherapy Cyclosporine
Cyclosporine Bone marrow transplantation
Bone marrow transplantation Trimethoprim/sulfamethoxazole
Trimethoprim/sulfamethoxazole Interferon-α
Interferon-α 2-Deoxycoformycin
2-Deoxycoformycin Interleukin-2
Interleukin-2 Isotretinoin
Isotretinoin Acitretin
Acitretin Lenalidomide
Lenalidomide Photodynamic therapy
Photodynamic therapy Clofarabine
Clofarabine Mistletoe
Mistletoe