Iron Metabolism
After completion of this chapter, the reader will be able to:
1. Discuss the role of iron as an essential nutrient for human survival.
2. List the sites in which iron is distributed in the body and state the approximate amount in each site.
3. State the minimum daily intake of iron required at various ages for men, women, and children.
4. Describe the mechanism of iron absorption and distinguish between the absorption of heme and nonheme iron.
5. State the site of iron absorption.
6. Describe transferrin, transferrin receptor, hepcidin, hemosiderin, and ferritin, including function and regulation.
7. Diagram the transport of iron from ingestion to incorporation into heme.
8. Define sideroblast, siderocyte, and siderosome.
9. Discuss regulation, excretion, transport, and storage of iron in the body.
10. Identify and discuss the laboratory tests currently used to assess iron status in the body.
Case Study
Iron in its cationic bivalent ferrous (Fe2+) and trivalent ferric (Fe3+) states is essential for the life of all organisms, plant and animal.1 In humans, 70% of total body iron is transported in blood noncovalently bound in the ferrous state to the heme portion of hemoglobin, where it binds, transports, and releases oxygen (O2, Table 11-1).2 In mitochondria, ferrous ion is transferred to protoporphyrin IX (see Figure 10-5) to form heme. Four heme molecules become bound, one each, to four globin chains to produce tetrameric hemoglobin. Ferrous iron likewise binds to myoglobin, the O2 transport molecule in muscles. The primary and secondary structures of myoglobin resemble hemoglobin, however, myoglobin is monomeric, and myoglobin oxygen binding is irreversible. Approximately 5% of total human body iron is bound to myoglobin.
TABLE 11-1
Iron Compartments in Normal Humans
| Compartment | Percent of Total Body Iron | Iron Content (mg/kg Body Weight) |
| Hemoglobin iron | ∼65% | 2.6 |
| Storage iron: hemosiderin, ferritin | ∼25% | 14.2 |
| Myoglobin iron | ∼5% | 1.0 |
| Transferrin | ∼0.1% | 0.003 |
| Other compartments: | ∼3.6% | 0.140 |
| Peroxidase, catalase, cytochromes, riboflavin enzymes |
Iron overload results from increased absorption owing to genetic predisposition or repeated blood transfusions and produces potentially fatal heart and liver disease. Evolution has given humans a mechanism for absorbing dietary iron efficiently but not for eliminating excess iron effectively. Disorders of iron metabolism are discussed in Chapter 19.
Dietary Iron
Although many foods have high iron content, the iron may be minimally bioavailable. The bioavailability of iron depends on its chemical form and the presence of non-iron foods that promote or inhibit absorption. An average American diet may provide 10 to 20 mg of iron/day, but only 1 to 2 mg/day is absorbed. Iron is absorbed in two forms: heme and nonheme. Heme-bound iron, mainly from meat, is absorbed more efficiently than nonheme, inorganic iron and in a different manner.3 Heme iron is present in hemoglobin, myoglobin, and heme-containing enzymes. Approximately 5% to 35% of heme iron is absorbed as hemin (iron-containing porphyrin).
Nonheme iron, found in nonmeat sources such as legumes and leafy vegetables, accounts for approximately 90% of dietary iron, but only 2% to 20% of it is absorbed, depending on the iron status of the individual and the ratio of dietary enhancers and inhibitors.1 Ascorbate, citrate, and other organic acids and amino acids enhance absorption of nonheme iron by the formation of soluble chelates. Cooking in iron pots increases the amount of iron consumed. Substances that interfere with nonheme iron absorption include phytates, polyphenols, phosphates, oxalates, and calcium.4,5
Iron Absorption and Excretion
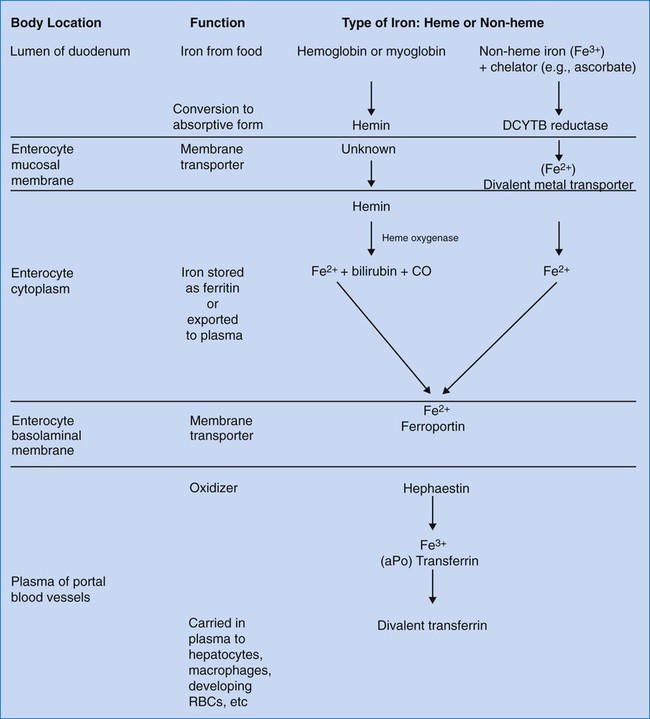
The duodenum and upper jejunum are sites of maximal absorption of iron. For transport of oxygen in Hb, iron must be in the ferrous form (Fe2+). To be absorbed from food, iron must be in the form of heme iron (Fe2+) or converted from ferric nonheme iron to the soluble ferrous form by a duodenum-specific cytochrome b–like protein, DCYTB.6 Uptake of heme iron occurs on heme carrier protein 1, located on the apical membrane of the duodenal enterocyte.7 Heme iron binds to the enterocyte in the mucosal epithelium and is internalized (Figure 11-1). Here the enzyme heme oxygenase degrades heme to produce ferrous iron, carbon monoxide, and bilirubin-IXa.
Ferrous iron is transported across the duodenal epithelium bound to the apical divalent metal transporter 1 (DMT1). The ferrous iron is carried to the basolateral membrane (base and sides of the enterocyte membrane), from which it is exported to the portal circulation, a process mediated by ferroportin, a basolateral transport protein. Ferroportin works in conjunction with a copper-containing iron oxidase known as hephaestin. Hephaestin may facilitate iron egress by reoxidation of ferrous to ferric iron.6 The trivalent (ferric) iron must be bound to transferrin to be transported through the circulation. Some iron remains in the enterocytes as ferritin and is released to the circulation over a few hours. Enterocyte-stored ferritin iron is excreted when the cells are exfoliated in the stool.
Hepcidin, an antimicrobial peptide produced in the liver, seems to act as a negative regulator of intestinal iron absorption. It also suppresses release from macrophages. Hepcidin binds to the ferroportin receptor, causing degradation of ferroportin and trapping iron in the intestinal cells. Hepcidin synthesis rises when transferrin is carrying its maximum capacity of iron (transferrin saturation of more than 50% in females or 60% in males), and diminishes when iron saturation is low.7–9 The role of hepcidin in the anemia of chronic inflammation is discussed in Chapter 19.
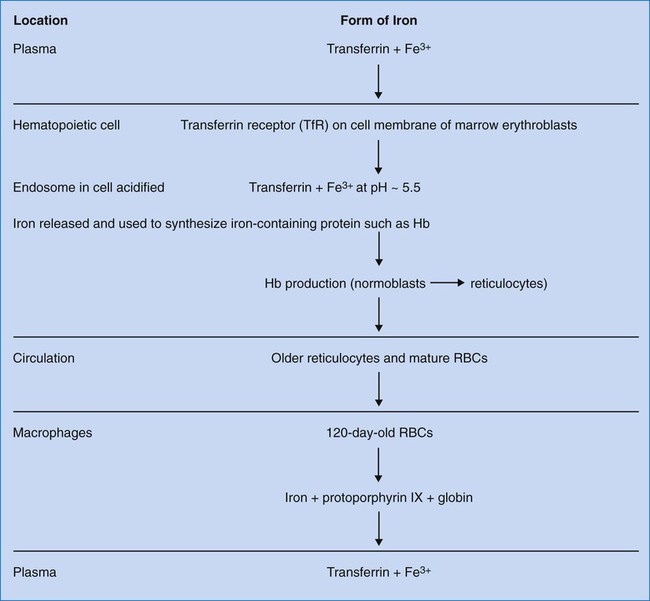
Transferrin transports ferric iron (Fe3+) to hematopoietic and other tissues, where it is bound by cell membrane transferrin receptors (Figure 11-2). Transferrin receptors are expressed in larger amounts on normoblasts and rapidly dividing cells, whether normal or malignant, but not on highly differentiated cells such as reticulocytes.10 Transferrin is taken into the cell by endocytosis. An endosome forms containing the iron-loaded transferrin molecule. Iron is released from transferrin by acidification of the endosome to a pH of 5.5. Iron is transported across the endosomal membrane by DMT1 and used in the synthesis of iron-containing proteins. Excess iron is stored as ferritin or hemosiderin (see section on iron storage).6
The body conserves iron extremely well, losing only about one one-thousandth of the total body iron content. This amount is replaced if dietary sources are adequate. Normal iron losses occur mainly by way of the feces and amount to about 1 mg/day. Perspiration and exfoliation of the skin and dermal appendages result in minimal losses. Lactation and menstruation result in an additional loss of about 1 mg/d.11
Iron Cycle and Transport
Iron is absorbed from the gastrointestinal tract and transported via the circulation to the bone marrow, where it is inserted into protoporphyrin IX in the mitochondria of the erythroid precursors to make heme (refer to Figures 10-2 and 10-5; see also Figure 11-2). Hb synthesis is completed in the reticulocyte stage. Iron circulates in red blood cells (RBCs) in the ferrous form noncovalently bound to the Hb molecule. Iron from senescent RBCs is turned over to macrophages and reused.6 Ferrokinetics involve transferrin, transferrin receptor, and ferritin. These are regulated by iron-responsive protein (IRP). The genes for these principal proteins have been located and sequenced.12 Researchers may someday be able to modify these genes to treat hereditary iron disorders.
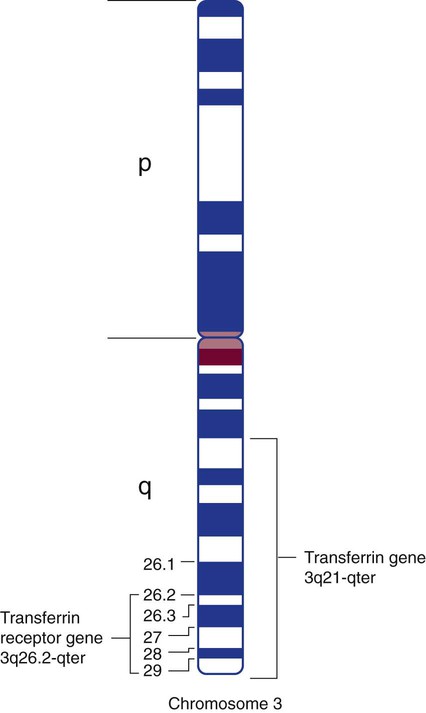
Most plasma transferrin is produced by hepatocytes. The major function of transferrin is to transport iron from the enterocytes of the duodenum to transferrin receptors on marrow normoblasts. Transferrin has a half-life of 8 days and migrates to the β fraction in serum electrophoresis. The amino terminus and carboxy terminus each independently bind a ferric ion. A bicarbonate ion “locks” the iron in place within transferrin by serving as a bridging ligand between the protein and iron. The transferrin molecule can exist as a single-chain glycoprotein with no iron attached, called apotransferrin, or in a monoferric or diferric form.13 The transferrin gene is located on the long arm of chromosome 3, at 3q21-qter (Figure 11-3).12
The transferrin receptor is a glycoprotein dimer, and it is located on virtually all cells except mature RBCs. It provides transferrin-bound iron access into the normoblast; it also plays a crucial role in the release of iron from transferrin within the cell. It is present in large numbers on normoblasts, in the placenta, and in the liver. The transferrin receptor can bind two molecules of transferrin. The transferrin receptor’s affinity for transferrin depends on the iron content of transferrin and the pH. At high levels of transport iron and a pH of 7.4, the transferrin receptor selectively binds diferric transferrin in preference to monoferric transferrin or apotransferrin. The transferrin receptor gene is located near the transferrin gene at chromosome band 3q26.2-qter (see Figure 11-3).12
Control of transferrin receptor biosynthesis is a major mechanism for regulation of iron metabolism. Synthesis is induced by iron deficiency. When transferrin is fully saturated, iron is deposited in the liver. When transferrin is congenitally absent, iron is absorbed by the intestine and accumulates in the liver, pancreas, spleen, and other viscera; only a little makes its way to the marrow, and a severe hypochromic microcytic anemia results.12
IRPs are messenger ribonucleic acid (mRNA)–binding proteins that coordinate the intracellular expression of transferrin receptor, ferritin, and other proteins important for iron metabolism. IRPs bind to iron-responsive elements (IREs), which are stem-loop mRNA structures. IRPs bind IREs when iron supply is decreased and dissociate from IREs when iron supply is increased. When there is little intracellular iron, IRPs regulate the increase of the translation and stability of the mRNA for transferrin receptor and aminolevulinic acid synthase and decrease the translation of apoferritin mRNA. This increases the number of molecules of transferrin receptor on the cell membrane, while decreasing the ferritin trapping of iron that enters the cell. More Hb can be formed if there is more iron uptake by the cell. At the same time, an increase in production of aminolevulinic acid synthase ensures that enough protoporphyrin can be made to accommodate the expected increase in iron (see Chapter 10).6,11 There is evidence that, in addition to regulation at the mRNA level, transcriptional regulation of iron metabolism also occurs.11
Iron Storage
Iron is stored in accessible reserve form as ferritin or as the partially degraded or precipitated form of ferritin called hemosiderin. Apoferritin, the protein component of the ferritin molecule without the iron, is an empty sphere 12 nm in diameter and 1 nm thick and is composed of 24 light (L) and heavy (H) subunits. Within the apoferritin shell, ferric ions, hydroxyl ions, and oxygen are distributed in a lattice. The liver and spleen, which have major iron storage deposits, have a large amount of L subunits. Tissues such as heart tissue that do not normally store ferritin have a higher proportion of H subunits. Genes for the H and L chains belong to multigene families with members on several chromosomes. The gene for two types of L chains is on chromosome 19; the gene for heavy chains is on chromosome 11.12
Laboratory Assessment of Iron Metabolism
Reference intervals for laboratory tests of iron status are provided but are meant to be a guide only, because values may differ depending on the assessment method used (Table 11-2). A single assay may not provide sufficient iron status information. An understanding of what the tests measure and what variations can occur because of diurnal variation and clinical status is helpful in interpreting the significance of the test result.
TABLE 11-2
Assessment of Body Iron Status
| Laboratory Assay | Adult Reference (Intervals) | Diagnostic Use |
| Serum ferritin level | 12-300 mcg/L | Indicator of iron stores |
| Serum iron level | 10-30 µmol/L | Indicator of tissue iron supply |
| Serum transferrin level (TIBC) | 47-70 µmol/L | Indicator of tissue iron supply |
| Transferrin saturation | Female: 16-50% Male 16-60% |
Indicator of tissue iron supply |
| Serum transferrin receptor (sTfR) | 1.15-2.75 mg/L | Indicator of functional iron available |
| sTfR-F index | 0.63-1.8 | Indicator of functional iron available |
| RBC zinc protoporphyrin | <80 mcg/dL of RBCs | Indicator of functional iron available |
| Bone marrow or liver biopsy | Normal iron stores visualized | Visual qualitative assessment of tissue iron stores |
| Bone marrow sideroblast count | 20-80% sideroblasts | Direct assessment of functional iron available |
Only about one third of transferrin iron-binding sites are normally occupied by Fe3+; two thirds of the iron-binding sites do not carry iron, and these sites are referred to collectively as the serum unsaturated iron-binding capacity (UIBC). The total of available sites is referred to as the total iron-binding capacity (TIBC). UIBC is measured using chromogen spectrophotometry. TIBC can be measured indirectly by chemical means and directly by immunoassay.14,15 Direct TIBC measurement is really a transferrin assay.
Measurement of serum iron concentration alone provides little useful clinical information. To diagnose iron deficiency, serum iron concentration and TIBC are measured and the percent transferrin saturation (the percentage of sites available for carrying iron) is calculated. Percent transferrin saturation is computed by dividing the serum iron level by the TIBC and multiplying the result by 100.6,11,12
Serum transferrin receptors (sTfR) can be measured by immunoassay. The sTfR appears to be a truncated form of the cell membrane receptor and circulates bound to transferrin. It seems to mirror the cellular form. The amount of circulating receptor rises when cells lack iron and decreases in chronic diseases. Measurement of sTfR level may be useful in iron deficiency testing, although increases in levels also have been seen with ineffective erythropoiesis.6,11,16 A calculation of the ratio of sTfR level to log of serum ferritin concentration (sTfR-F index) may be used to differentiate iron deficiency from anemia of chronic disease.17
Erythrocyte protoporphyrin is an intermediate product of hemoglobin synthesis that resides in the RBC, and levels may be elevated when heme production is incomplete, such as when iron deficiency is present or when iron use is blocked. The protoporphyrin assay is termed free erythrocyte protoporphyrin. Protoporphyrin combines with zinc when iron is unavailable to produce fluorescent zinc protoporphyrin. The fluorescence of zinc protoporphyrin is measured using a fluorometer. RBC free protoporphyrin concentration increases in disorders of heme synthesis, including iron deficiency, lead poisoning, and sideroblastic anemias (see Chapter 19). Protoporphyrin levels are increased in chronic diseases, regardless of the iron status.16
Summary
• Iron is an essential nutrient for human survival. It plays a role in oxygen transport and basic metabolic oxidation-reduction reactions. Most of the iron in the body is in the form of hemoglobin.
• Iron is obtained through the diet via heme (Fe2+) and nonheme (inorganic Fe3+) iron. Heme is more readily absorbed than nonheme iron.
• Iron is absorbed by duodenum and jejunum enterocytes via the DMT1 transporter. Iron is carried to the basolateral membrane, where it passes to the plasma via a channel transporter, ferroportin. Hephaestin, a copper oxidase protein, aids in the transport process by reoxidation of ferrous iron to ferric iron. Hepcidin, an antimicrobial peptide, regulates the basolateral iron export.
• Iron is transported in plasma bound to a carrier protein, transferrin. Transferrin receptors, located on all cells in the body except mature RBCs, aid in providing transferrin-bound iron access into cells and play a crucial role in the release of iron from transferrin within the cell. The IRP regulates aminolevulinic acid synthetase, transferrin receptors, and ferritin in the cell by binding to the mRNA IREs.
• Ferritin and hemosiderin are storage forms of iron. Most stored iron is in the form of ferritin, which is a water-soluble complex. Hemosiderin, a water-insoluble complex, is composed of ferritin aggregates and is found in macrophages in the bone marrow and liver.
• Laboratory tests that can assist in assessing the iron status in the body include serum ferritin level, serum iron concentration, TIBC, and percent transferrin saturation. Other tests include bone marrow or liver biopsy, sTfR analysis, and the erythrocyte protoporphyrin test. Other tests are available but are performed less frequently.
Review Questions
1. Iron is transported in plasma via:
2. What is the major metabolically available storage form of iron in the body?
3. Approximately 70% of body iron is found in the form of:
4. Iron is incorporated into the heme molecule in which of the following forms?
5. What protein associated with duodenal cells transports iron from the intestinal lumen into the intestinal cell?
6. Iron is transported out of cells such as macrophages and duodenal cells by what membrane protein?
7. Below are several of the many steps in the process from absorption and transport of iron to incorporation into heme. Place them in proper order.
i. Transferrin picks up ferric iron.
ii. Iron is transferred to the mitochondria.
iii. Cytochrome b–like protein, DCYTB, converts ferric to ferrous iron.
iv. Ferroportin transports iron across the cell membrane.
8. In the iron cycle, the transferrin receptor does which of the following?
a. Carries iron into duodenal cells from the intestinal lumen
b. Carries iron out of duodenal cells into the plasma
9. What is the fate of the transferrin receptor when it has completed its role in the delivery of iron into a developing RBC?
a. It is recycled to the plasma membrane and released into the plasma.
b. It is recycled to the plasma membrane, where it can bind its ligand again.
c. It is catabolized and the amino acids are returned to the metabolic pool.
10. The transfer of iron from the duodenal cell into the plasma is regulated by:

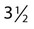 years. The investigators collected comparable data from nondonors. Of the donors, 10 women and 6 men took a dietary supplement providing approximately 20 mg of iron per day. In addition, mean iron dietary intake was 16.4 mg/day for the women and 19.9 for the men. Over the period of the study, mean iron stores in women decreased from 12.53 to 1.14 mg/kg of body weight. Mean iron stores in men declined from 12.45 to 1.92 mg/kg. Nondonors’ iron stores remained unchanged. Based on Hb and Hct results, no donors became anemic. There was no statistically significant difference in iron stores between the men who took supplements and those who did not, although a difference was seen for the women. Total iron losses over 80 days, the average interval between donations, were calculated to be 4.32 mg/kg for the women and 3.93 mg/kg for the men. As iron stores decreased, the calculated iron absorption rose to 3.55 mg/day for the women and 4.10 mg/day for the men.
years. The investigators collected comparable data from nondonors. Of the donors, 10 women and 6 men took a dietary supplement providing approximately 20 mg of iron per day. In addition, mean iron dietary intake was 16.4 mg/day for the women and 19.9 for the men. Over the period of the study, mean iron stores in women decreased from 12.53 to 1.14 mg/kg of body weight. Mean iron stores in men declined from 12.45 to 1.92 mg/kg. Nondonors’ iron stores remained unchanged. Based on Hb and Hct results, no donors became anemic. There was no statistically significant difference in iron stores between the men who took supplements and those who did not, although a difference was seen for the women. Total iron losses over 80 days, the average interval between donations, were calculated to be 4.32 mg/kg for the women and 3.93 mg/kg for the men. As iron stores decreased, the calculated iron absorption rose to 3.55 mg/day for the women and 4.10 mg/day for the men.