Iodine Deficiency Disorders
Increase in Thyroid Clearance of Plasma Inorganic Iodine
Changes in Iodine Stores and Thyroglobulin Synthesis
Modification of the Iodoamino Acid Content of the Gland
Preferential Secretion of Triiodothyronine
Enhanced Peripheral Conversion of Thyroxine to Triiodothyronine
Clinical and Laboratory Diagnosis
Iodine deficiency is the world’s most common endocrine problem, the easiest of the major nutritional deficiencies to correct, and the most preventable cause of mental retardation in many underdeveloped countries.1,2 Given these facts, it is remarkable that iodine deficiency continues to be a major public health problem. It is best known for causing endemic goiter, but its manifestations and consequences reach much deeper into human pathology (Table 17-1). Goiter, although frequently the most obvious feature, is much less important than the adverse effects of iodine deficiency on normal development, particularly normal development of the brain.3,4 To emphasize the more severe consequences, this health problem is now described as iodine deficiency disorders (IDD) instead of endemic goiter.
Table 17-1
Spectrum of Iodine Deficiency Disorders
Fetus
Abortions
Stillbirths
Congenital anomalies
Increased perinatal mortality
Neurologic cretinism: mental deficiency, deaf-mutism, spastic diplegia, deafness
Myxedematous cretinism: dwarfism, mental deficiency, epiphyseal dysplasia
Psychomotor defects
Neonate
Neonatal goiter
Neonatal hypothyroidism
Child and Adolescent
Goitrous juvenile hypothyroidism
Impaired mental function
Retarded physical development
Adult
Goiter with its complications
Hypothyroidism
Impaired mental function
Iodine-induced hyperthyroidism
Spontaneous hyperthyroidism in the elderly
Adapted from Hetzel BS, Dunn JT, Stanbury JB (eds): The Prevention and Control of Iodine Deficiency Disorders. Amsterdam: Elsevier, 1987.
For many years, it has been recognized that a close and inverse relationship usually, if not always, exists between iodine in the soil and water and the appearance of endemic goiter and allied diseases. Nevertheless, it cannot be said as of this writing that the cause of iodine deficiency disorders has been completely determined in all cases, or even in any case, because nutritional, constitutional, genetic, or immunologic factors may be additive in the sum total of causes that lead to the appearance of these diseases. Therefore, iodine deficiency is a necessary cause, although it may not always be a sufficient cause. The role of iodine deficiency as the main etiologic factor in endemic goiter and cretinism has been extensively confirmed by the success of iodine prophylaxis programs in several countries, although iodine deficiency has persisted despite readily available means of supplementation, such as iodized salt and iodized oil.1
Iodine Supply
Iodine is found in relative abundance in marine plants and animals, in the thyroid gland of vertebrates, in deposits of organic origin, in certain natural mineral water, in sedimentary phosphate rock, and in association with certain mineral deposits. Most of the iodine ingested by humans comes from food of animal and plant origin. This iodine, in turn, is derived from the soil. Only a relatively small fraction is derived from drinking water. A most important factor in the depletion of iodine has been glaciation, which removes old soil and scrapes bare the virgin rocks, which have iodine concentrations far lower than those of the covering soil. This situation is found in regions that remained longest under Quaternary glaciers and lost their iodine when the ice thawed.5
Optimal Iodine Intake
Iodine is an essential component of the thyroid hormones thyroxine (T4) and triiodothyronine (T3) and contributes 65% and 59% of their respective molecular weights. To meet the demand for adequate hormone, the thyroid has developed an elaborate mechanism for concentrating iodine from the circulation and converting it into hormone, which it then stores and releases into the circulation as needed. The recommended intake of iodine is at least 90 µg/day for children aged 0 to 5 years, 120 µg/day for children aged 6 to 10 years, 150 µg/day for adolescents older than 12 years and adults, and 250 µg/day for pregnant or lactating women.1,6 About 90% of iodine is eventually excreted in urine. The median urinary iodine concentration in casual samples, expressed as micrograms per liter or deciliter, is currently the most practical biochemical laboratory marker of community iodine nutrition.7 Recommendations by the International Council for the Control of Iodine Deficiency Disorders, the World Health Organization, and the United Nations International Children’s Emergency Fund set 100 µg/L as the minimal urinary iodine concentration for iodine sufficiency.6 Daily iodine intake for population estimates can be extrapolated from urinary iodine concentration using estimates of mean 24-hour urine volume and assuming an average iodine bioavailability of 92% using the formula: urinary iodine (µg/L) × 0.0235 × body weight (kg) = daily iodine intake.8,9 This figure roughly corresponds to a daily intake of 150 µg iodine. A report on iodine nutrition in the United States indicated adequate iodine intake for the overall U.S. population,10 but the median concentration decreased more than 50% between 1971 and 1974 (320 ± 6.0 µg/L) and 1988 and 1994 (145 ± 3.0 µg/L). Low urinary iodine concentrations (50 µg/L) were found in 11.7% of the 1988-1994 population, a 4.5-fold increase over the proportion in the 1971-1974 study. Possible reasons for this decline included changes in national food consumption patterns (lower salt intake) and food industry practices. A more recent evaluation found a median urine iodine of 153 µg/L (men) and 124 µg/L (women) and did not vary with increasing age, indicating that the U.S. adult population is iodine sufficient.11
Iodine Nutrition During Pregnancy and Lactation
Iodine is particularly critical for pregnant women.6 During pregnancy, several physiologic changes take place in maternal thyroid economy which together lead to an increase in thyroid hormone production of approximately 50% above the preconception baseline hormone production. To achieve the necessary increment in thyroid hormone production, the iodine intake needs to be increased during early pregnancy.2 Also early in pregnancy, there is an increase in renal glomerular filtration leading to increased plasma iodide clearance. Women should have an adequate iodine intake, corresponding to 150 µg/day, to ensure that intrathyroidal iodine stores are replenished before they became pregnant. A recent guideline12 reached the consensus that iodine nutrition during pregnancy and breastfeeding should range between 200 and 300 µg/day, with an average of 250 µg/day. The prevalence of postpartum thyroiditis does not seem to be related to the iodine intake status of a population.13 Accordingly in a case-control study,14 there was a difference in iodine excretion in the immediate postpartum period between 73 women who developed postpartum thyroiditis and 135 women who did not. With regard to the effect of changes in iodine intake on the occurrence of postpartum thyroiditis, the data are conflicting. On the one hand, there are data indicating that increased iodine intake can influence the severity of thyroid dysfunction in postpartum thyroiditis. In Sweden, an iodine-sufficient area, 20 women who were thyroid peroxidase (TPO) positive in early pregnancy were treated with iodine (0.15 mg/d) for 40 weeks postpartum. In those women who developed thyroid dysfunction, thyroid-stimulating hormone (TSH) levels were higher, and T4 levels were lower, compared with the group who received no medication.15 On the other hand, in Denmark, in an area with mild to moderate iodine insufficiency, a placebo-controlled, randomized, double-blind trial was accomplished to verify the impact of iodine supplementation (0.15 mg/d) during pregnancy and the postpartum period in 72 TPO antibody–positive women.16,17 It was concluded that iodine supplementation did not induce or worsen postpartum thyroiditis.17
The most reasonable conclusion is that there is no proven further benefit in providing pregnant women with more than 300 µg/day. In most countries with a well-established salt iodination program, pregnancies are not at risk of having iodine deficiency. In case of situations in which ingestion of iodized salt should be restricted, preparations of potassium iodide may be used as oral supplements associated or not to multivitamin tablets. In areas with no iodide supplementation and difficult socioeconomic conditions, it is recommended to administer orally iodized oil as early in gestation as possible (400 mg of iodine given orally will cover thyroid needs for 1 year). The best single parameter to evaluate the adequacy of iodine nutrition is urine iodine, which should range between 150 and 250 µg/L. These values may vary during pregnancy,18 and iodine supplementation may be needed in late pregnancy. Accordingly, it was shown that prolonged iodized salt significantly improves maternal thyroid economy and reduces the risk of maternal thyroid insufficiency during gestation, probably because of nearly restoring intrathyroidal iodine stores.19
With regard to iodine nutrition during breastfeeding, thyroid hormone production and urinary iodine excretion return to normal, but iodine is efficiently concentrated by the mammary gland. Since breast milk provides approximately 100 µg/day of iodine to the infant, it is recommended that the breastfeeding mother should continue to take 250 µg per day of iodine.6
Prevalence
It is much easier to list the iodine-sufficient countries than those with different degrees of iodine deficiency. In the beginning of the 1990s, it was estimated that 29% of the global population lived in areas of iodine deficiency. Historically, the occurrence of thyroid disease in Europe has been dominated by iodine deficiency with some geographical variation. Severe iodine deficiency with endemic cretinism and goiter in the major part of the population was primarily found in the Alps and in other mountainous regions, but milder forms of iodine deficiency were present in regions of nearly every European country. As reviewed previously by Delange,20 iodine deficiency has been eradicated in some European countries for many years, but other countries have lagged behind, especially in prevention of mild to moderate iodine deficiency. Owing to keen efforts by dedicated, hard-working people, the situation has improved in recent years. The number of European countries affected by iodine deficiency is steadily decreasing, and the efforts and results in the fight to prevent and control iodine deficiency have markedly progressed. During the 10 years between 1994 and 2004, the number of European countries which were considered iodine sufficient went from only 5 to 21.21
In 2007, WHO estimated that nearly 2 billion individuals had an insufficient intake of iodine, including a third of all school-aged children (Table 17-2).22 The lowest prevalence of iodine deficiency is in the Americas (10.6%), where the proportion of households consuming iodized salt is the highest in the world (about 90%). The highest prevalence of iodine deficiency is in Europe (52%), where the household coverage with iodized salt is the lowest (about 25%), and many countries have weak or nonexistent control programs for iodine-deficiency disorders.
Table 17-2
Proportion of Population and Number of Individuals in the General Population (All Age Groups) With Insufficient Iodine Intake*
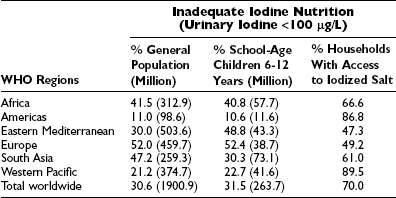
*By WHO regions during the period between 1994 and 2006 and proportion of households using iodized salt.
Adapted from WHO/ICCIDD/UNICEF: Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination, 3rd edition. Geneva: WHO, 2007; and Zimmermann MB, Jooste PL, Pandav CS: Iodine-deficiency disorders. Lancet 372:1251–1262, 2008.
Iodine deficiency remains a public health problem in 47 countries (Fig. 17-1). However, progress has been made since 2003; 12 countries have progressed to optimum iodine status, and the percentage of school-aged children at risk of iodine deficiency has decreased by 5%.5 However, iodine intake is more than adequate or even excessive in 34 countries, increased from 27 in 2003. In Australia and the United States, two countries that were previously iodine sufficient, iodine intake is falling. Australia is now mildly iodine deficient, and in the United States, the median urinary iodine is 145 µg/L, as mentioned, which is still adequate but half the median value of 311 µg/L noted in 1970. These changes emphasize the importance of regular monitoring of iodine status in countries to detect both low and excessive intakes of iodine.
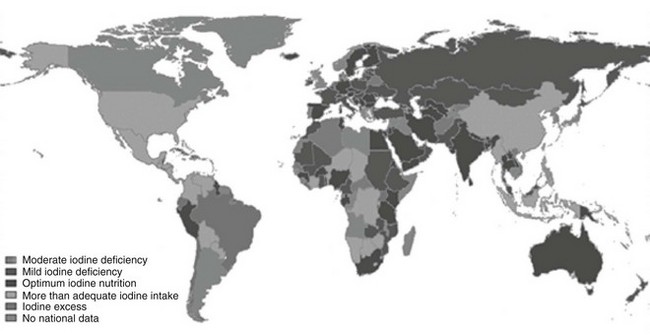
FIGURE 17-1 Current status of global distribution of iodine nutrition based on the median urinary iodine concentration by country, according to WHO database on iodine deficiency. (Data from de Benoist B, McLean E, Andersson M: Iodine deficiency in 2007: Global progress since 2003, Food Nutr Bull 29:195–202, 2008.)
Recent reports on goiter and iodine deficiency in Europe20 have indicated, however, that goiter persists in adults (but is seldom seen in children) in Bulgaria, Czechoslovakia, the Netherlands, Belgium, and Switzerland.21 According to a relatively recent study, the iodine status of the Swiss population is currently adequate.23 Substantial areas of high goiter prevalence persist in Austria, Hungary, Romania, Poland, the constituent countries of the former Yugoslavia (Slovenia, Croatia, Bosnia and Herzegovina, Macedonia, Serbia, Montenegro), and western Russia. In other countries (southwestern Germany, Greece, Italy, Portugal, Spain, and Turkey), iodine prophylaxis is not mandatory, and goiter and even endemic cretinism continue to be major problems, either nationally or regionally (goiter prevalence rates of 18% to 22%).
Iodine deficiency has been a public health problem in most Latin American countries. Iodine status has been reassessed over the last 15 years, and programs have been implemented for the control of IDD. Great progress has been made, particularly in the aggressive push for iodized salt use. But problems remain, such as weak governmental support and lack of effective monitoring of salt iodization in some countries, threatening the effective and sustained elimination of IDD in the region. Data on the present situation, however, are incomplete because regular monitoring is carried out in only a few countries. Moreover, different methods were used to measure iodine in urine and salt, and goiter was evaluated only by palpation. Using the ThyroMobil model, which has proven to be a convenient and efficient model for standardized and rapid evaluation for urinary iodine and goiter prevalence in Europe, 163 sites in 13 countries were visited to assess randomly selected schoolchildren of both genders, 6 to 12 years of age. The median urinary iodine concentration (8208 samples) varied from 72 to 540 µg/L. The Guatemala median was below the recommended range of 100 to 200 µg/L; Bolivia, Nicaragua, El Salvador, Mexico, and Argentina were at 100 to 200 µg/L; and Peru, Honduras, Paraguay, Venezuela, Brazil, Ecuador, and Chile were higher than 200 µg/L, including three (Brazil, Ecuador, Chile) greater than 300 µg/L. Urinary iodine concentration correlated with the iodine content of salt in all countries. Median values of thyroid volume were within the normal range for age in all countries, but the goiter prevalence varied markedly from 3.1% in Bolivia to 25.0% in Nicaragua because of scatter.24,25
In the sub-Himalayan area of Pakistan, the overall prevalence of goiter is 39.7%, and endemic cretinism is common.26 In India, despite intensive efforts to promote iodized salt, only about half the population is covered, and coverage is especially poor in low socioeconomic populations. Iodized salt is unavailable in many rural markets, or salt sold as iodized is poorly or incompletely iodized or both.27 The Himalayas of India, Nepal, Bhutan, and southern China, as well as the mountains extending into northern Burma, Thailand, Laos, and Vietnam, have long been known as goitrous areas.
It is estimated that 30 million Chinese have goiter, and possibly 200,000 suffer from the consequences of endemic cretinism.28 Accordingly, new cases of cretinism have been recently detected in isolated regions of Western China.29 An intensive program of salt iodination and administration of iodized oil (orally and intramuscularly), however, has reduced the prevalence of goiter and iodine deficiency in China. A 1995 national survey among children aged 8 to 12 years showed a total goiter rate of 20.4%. A 1997 national survey of children in the same age group reported a total goiter rate of 10.9% and a median urinary iodine level of 300.2 µg/L. According to the surveillance results of 2002, the coverage of iodized salt had reached 95.2%, and the coverage of qualified iodized salt had reached 88.8%. With the implementation of the new standards for edible salt, the quality of iodized salt improved, and in 2002, the median urinary iodine among children 8 to 10 years of age was 241.2 µg/L. Furthermore, the study confirmed that the goiter rate among children in the 8-to-10 age group was continuing to decline over time, from 20.4% in 1995 down to 5.8% by 2002, so IDD can presently be considered as extensively eliminated in this country30 The Philippines and Indonesia are severely iodine deficient.31 Worse conditions persist in the remote regions of African countries.32 In sub-Saharan Africa, 64% of households use iodized salt, but coverage varies widely from country to country.33 In countries such as Sudan, Mauritania, Guinea-Bissau, and the Gambia, coverage is less than 10%, whereas in Burundi, Kenya, Nigeria, Tunisia, Uganda, and Zimbabwe, it is more than 90%. Further, iodine status varies from iodine deficiency in countries such as Ethiopia, Sierra Leone, and Angola to iodine excess in Democratic Republic of the Congo, Uganda, and Kenya.34,35 However, many countries have outstanding programs—Nigeria, for example, which has been recognized as the first African country to successfully eliminate iodine deficiency.36 In South Africa, coverage of adequately iodized household salt (i.e., iodized at >15 mg/kg) was 62.4% of households 2 years after the introduction of compulsory iodization at a level of 40 to 60 mg/kg. A total of 7.3% of households used noniodized agricultural salt and salt obtained directly from producers. The iodine concentration in salt was lower in rural areas than in urban and periurban areas. The consequences of using underiodized or noniodized salt were most likely to be experienced in the country’s three northern provinces, among people in the low socioeconomic categories, and in rural households37 (Fig. 17-2).
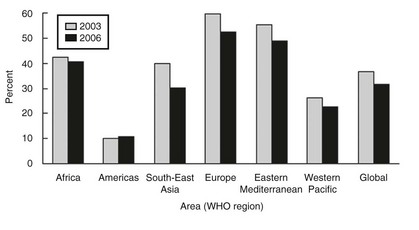
FIGURE 17-2 Change in the prevalence of individuals with an insufficient iodine intake between 2003 and 2006. Insufficient iodine intake in school-aged children has decreased by 5%, the largest decreases occurring in Southeast Asia and Europe. The Americas remained stable. (Data from de Benoist B, McLean E, Andersson M: Iodine deficiency in 2007: Global progress since 2003, Food Nutr Bull 29:195–202, 2008.)
As assessed by measurements of urinary iodine, many countries have achieved the elimination of iodine deficiency—for example, Algeria, Kenya, Cameroon, Tanzania (Africa); Iran, Lebanon, Tunisia (Eastern Mediterranean); Bhutan, China, Indonesia, India, Thailand (Asia); Venezuela, Peru, Ecuador (Latin America); and Switzerland, Austria, Great Britain, Finland, Norway, Sweden, Poland, Macedonia, Croatia, the Czech Republic, Slovakia, and Bulgaria in Europe.5 However, in spite of the tremendous improvement of the implementation of programs of iodized salt, 35.2% of the general population in the world still had a urinary iodine below 100 µg per liter in 2003,22 and the percentage of the world population affected by goiter did not appreciably change between 1990 (12%)40 and 1999 (13%).41
Etiology
Absolute and chronic iodine deficiency is the main cause of endemic goiter and allied disorders. It is entirely possible that in certain limited situations other etiologic factors such as genetic predisposition in highly inbred and isolated groups and the presence of effective goitrogens in unusual dietary situations (Table 17-3). The arguments supporting iodine deficiency as the cause of endemic goiter are: (1) an association between low iodine content in the food and water and the appearance of the disease in the population; (2) a reduction in goiter incidence that occurs when iodine is added to the diet; and (3) demonstration that the metabolism of iodine and thyroid changes in patients with endemic goiter are similar to those produced in animals subjected to a low-iodine diet.
Table 17-3
Natural Goitrogens Associated With Endemic Goiter
| Goitrogens | Agent | Action |
| Millet, soy | Flavonoids | Impair thyroperoxidase activity |
| Cassava, sweet potato, sorghum | Cyanogenic glucosides metabolized to thiocyanates | Inhibit iodine thyroidal uptake |
| Babassu coconut, mandioca | Flavonoids | Inhibit thyroperoxidase |
| Cruciferous vegetables: cabbage, cauliflower, broccoli, turnips | Glucosinolates | Impair iodine thyroidal uptake |
| Seaweed (kelp) | Iodine excess | Inhibits release of thyroidal hormones |
| Malnutrition | Vitamin A deficiency | Increases TSH stimulation |
| Iron deficiency | Reduces heme-dependent thyroperoxidase thyroidal activity | |
| Selenium deficiency | Accumulates peroxides and causes deiodinase deficiency; impairs thyroid hormone synthesis |
Adapted from Zimmermann MB, Jooste PL, Pandav CS: Iodine-deficiency disorders, Lancet 372:1251–1262, 2008.
Natural goitrogens (see Table 17-3) may be considered significant determinants of the prevalence of endemic goiter, either in iodine-deficient areas or in localities where iodine intake is abundant, as in the coal-rich Appalachian area of eastern Kentucky.42 Goitrogenic effects may be related to the consumption of certain foodstuffs (cassava, millet, babassu coconut, piñon, vegetables from the genus Brassica, and soybean).44 The goitrogenic factor in cassava is related to the hydrocyanic acid liberated from the cyanogenetic glucoside (linamarin) and endogenously changed to thiocyanate, which competitively inhibits trapping and promotes the efflux of intrathyroidal iodine.43 Pearl millet is one of the most important food crops in the semiarid tropics (large portions of Africa and Asia). Millet porridge is rich in C-glucosylflavones and also contains thiocyanate. Both are additive in their antithyroid effects. In Darfur province of western Sudan, the goiter prevalence in schoolchildren was linked to the level of consumption of millet.44 Babassu coconut is largely consumed in northern Brazil, and studies have demonstrated the possible presence of flavonoids in the edible part of the nut.45 Thus in areas where millet and babassu coconut are a major component of the diet, their ingestion may contribute to the genesis of goiter. Furthermore, flavonoids, besides being potent inhibitors of thyroid peroxidase, also interact with thyroid hormone at the peripheral level.46 Turnips, like cabbage, cauliflower, and broccoli, contain glucosinolates whose metabolites compete with iodine for thyroidal uptake.44 Soy-containing foods and dietary supplements are widely consumed for putative health benefits (e.g., cancer chemoprevention, beneficial effects on serum lipids associated with cardiovascular health, reduction of osteoporosis, relief of menopausal symptoms), but studies of soy isoflavones in experimental animals suggest possible adverse effects as well, like enhancement of reproductive organ cancer, modulation of endocrine function, and antithyroid effects (due to flavonoids which impair thyroid peroxidase activity).47 Antithyroid effects may also be extended by increasing the loss of T4 from the blood via bile into the gut and may cause goiter when iodine intake is limited.48
Excess consumption of iodine-rich kelp (dry seaweed, 80 to 200 mg iodine per day) has caused sporadic and even endemic goiter in humans. In this case, goiter is common in some families and more frequent in girls at puberty, which suggests possible influences of additional genetic and hormonal factors. The organification of iodine and, consequently, the synthesis of T4 and T3 were lower than normal, and iodine-rich colloid goiter was observed in patients from the goiter-endemic coast of Hokkaido, Japan, after thyroidectomy.49
Generalized malnutrition (protein-calorie deprivation) has been recognized as an additive factor in the prevalence of endemic goiter in afflicted populations. On the basis of epidemiologic data recorded in 5- to 14-year-old South African children, it was recently shown that vitamin A supplements are effective in treating vitamin A deficiency in areas of mild ID. It also has an additional benefit: through suppression of the pituitary TSHβ gene, vitamin A supplements can decrease TSH thyroid hyperstimulation and thereby reduce the risk of goiter.50 Vitamin A deficiency was also reported to impair thyroglobulin (TG) synthesis and thyroidal iodine uptake.51
Another stimulator of follicular cell growth that acts synergistically with endogenous TSH is the anti-GAL antibody.52 This human polyclonal antibody was found to mimic the in vitro TSH effects of stimulation of cyclic adenosine monophosphate synthesis, 125I uptake, and cellular proliferation of cultured porcine thyrocytes. Anti-GAL antibodies were found to be higher in goitrous individuals and positively correlated with the size of goiter. Whether these antibodies contribute to the pathogenesis of the disease needs further clarification.
Besides iodine, several minerals and trace elements such as iron, selenium, and zinc are essential for normal thyroid hormone metabolism. Iron deficiency impairs thyroid hormone synthesis by reducing activity of heme-dependent thyroid peroxidase. Iron-deficiency anemia blunts and iron supplementation improves the efficacy of iodine supplementation.53 In several regions of the world, people are exposed to inadequate selenium supply because the selenium content of surface soil has been depleted by erosion and glacial washout, similar to iodine. In spite of that, it was shown that selenium did not significantly influence thyroid volume in borderline iodine sufficiency, because the iodine status was most likely the more important determinant.54
Zinc status also affects thyroid function. Research established a relationship between zinc deficiency and thyroid hormone levels. Zinc is required for the proper function of 1,5′-deiodinase, the enzyme required for the conversion of thyroxine to triiodothyronine.55 In animal studies, severely zinc-deficient rats had flattened epithelial cells, colloid accumulation, and lower T3 concentration; marked alterations of follicle cellular architecture, including signs of apoptosis, were found.56 In a zinc deletion–repletion study carried out in humans, TSH, total T4, and free T4 tended to decrease during the depletion phase and returned to control levels after zinc repletion.57
In addition to the aforementioned natural goitrogens, exposure to environmental chemicals may have deleterious thyroid-system effects in humans during development, especially in the nervous system, and also may adversely impact thyroid hormone metabolism (Table 17-4). The effects of some chemicals may be profound: the antithyroperoxidase (anti-TPO) activity of resorcinol is 26 times the activity of propylthiouracil. Many halogenated compounds compete with natural hormones for binding to protein carriers (transthyretin and to a lesser degree thyroxine-binding globulin), although the clinical consequences are unclear. Polychlorinated biphenyls (PCBs) may have a possible effect on thyroid hormone–regulated genes.58 Disulfides from coal processes44 and from sedimentary rock drained by water into deep wells are believed to be the cause of the incomplete reduction of endemic goiter after the use of iodized salt in Colombia.59 Perchlorate is a competitive inhibitor of the sodium/iodine symporter, decreasing the active transport of iodine into the thyroid. There has been concern that naturally occurring perchlorate and industrial contamination of water supplies with perchlorate might pose a health hazard by inducing or aggravating underlying thyroid dysfunction. So far, available evidence has demonstrated that long-term, large but intermittent exposure to perchlorate does not adversely affect thyroid function, despite a lowering of the thyroid radioactive iodide uptake (RAIU).60 Tobacco smoking is a major source of thiocyanate in humans, which inhibits the function of the iodide transporter in the lactating mammary gland. Smoking during the period of breastfeeding dose-dependently reduces breast milk iodine content to about half and, consequently, exposes the infant to increased risk of iodine deficiency.61 In brief, it seems that most goitrogens do not have a major clinical effect unless there is coexisting iodine deficiency.
Table 17-4
Environmental Chemicals With Potential Thyroid-System Deleterious Effects in Humans
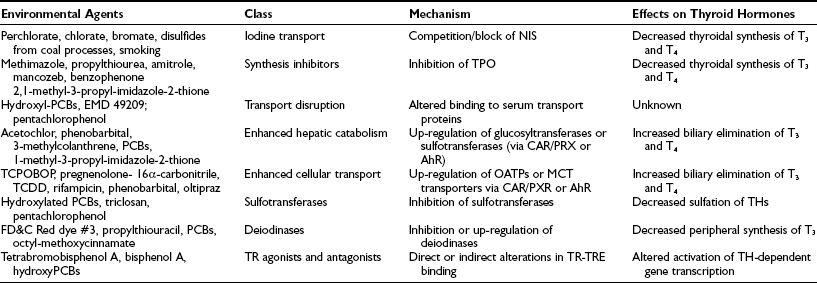
Adapted from Crofton KM: Thyroid disrupting chemicals: Mechanisms and mixtures, Int J Androl 31:209–223, 2008.
Pathophysiology
Goiter was regarded as an obligatory response to prolonged and severe iodine deficiency, and an increase in thyroid iodine clearance was shown to be the basic mechanism of iodine conservation (for a review of iodine deficiency disorders, see ref. 63). Subsequently, a shift in thyroid hormone synthesis in favor of T3 indicated an additional mechanism. These concepts have improved our understanding of how humans cope with low iodine intake, as well as the effects that both lack of iodine and adaptation mechanisms have on thyroid physiology. Thus, adaptation to iodine deficiency involves a number of biochemical and physiologic adjustments that ultimately result in maintenance of the intracellular concentration of T3 within normal limits. These mechanisms are listed in Table 17-5.
Table 17-5
Mechanisms Involved in the Adaptation to Iodine Deficiency
Increased thyroid clearance of plasma inorganic iodine
Hyperplasia of the thyroid and morphologic abnormalities
Changes in iodine stores and thyroglobulin synthesis
Modifications of the iodoamino acid content of the gland
Enrichment of thyroid secretion in T3
Enhanced peripheral conversion of T4 to T3 in some tissues
Increased thyroid-stimulating hormone production
Hyperplasia of The Thyroid
Although thyroid clearance may be increased without a demonstrable goiter, the anatomic accompaniment of functional activity is an increase in gland mass. Another interesting point is that iodine-concentrating ability is not uniformly distributed among follicular cells, even in normal glands. A certain level of TSH-dependent, autonomous iodine trapping is a feature of normal thyroid follicles, and the generation of new follicles from mother cells with an inherently high capacity for iodine trapping could well explain the heterogeneity in iodine metabolism among the follicles of glands affected by endemic goiter.64 Partial autonomy of iodine trapping could also account for the persistently high uptake after the administration of iodine supplements. Deficiency of cytosolic superoxide dismutase in endemic goitrous tissue has been claimed to cause more prolonged exposure to oxygen free radicals and contribute to the degenerative changes found in these tissues.65
As long as adaptation to iodine is effective, increased thyroid volume may be considered as a mechanism to store iodine during periods of increased supply to provide for less favorable periods. However, this adaptive mechanism has its limits.66 The capacity to synthesize thyroid hormones is not proportional to the increase of volume, and particularly in voluminous goiter, the thyroid function becomes insufficient.
Modification of The Iodoamino Acid Content of The Gland
Experimental studies in the rat show that thyroid hyperplasia induced by iodine deficiency is associated with an altered pattern of iodine distribution within the gland.67 An increase in labeled MIT and a decrease in the concentration of DIT, as well as a progressive increase in the ratio of T3 to T4, are the main changes in the thyroid gland occurring during prolonged iodine deficiency and are directly related to the degree of iodine depletion of the gland. These alterations caused by iodine deficiency appear to be associated with a structural change in TG. Experimental studies have shown a greater degree of heterogeneity in the TG molecule. Its altered sedimentation peak, significantly lower than 19 S, indicates failure of TG maturation. In large human goiters, as the concentration of iodine is reduced, the MIT/DIT ratio increases, and the fraction of tracer found in the form of T4 and T3 is markedly reduced. Possibly, many of the iodotyrosyl groups do not have the spatial configuration that favors the normal coupling process, and therefore only a small fraction of the iodine accumulated is actually incorporated into the normal pathway of hormone synthesis and secretion. A significant amount of iodine seems to be wasted by incorporation into iodocompounds that are clearly different from TG, that are resistant to hydrolysis, and that have a very long half-life and low molecular weight. These iodocompounds are at least in part fragments of TG.
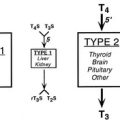
Enhanced Peripheral Conversion of Thyroxine to Triiodothyronine
A compensatory increase in the peripheral conversion of T4 to T3 can occur in those with chronic iodine deficiency. It has been demonstrated in iodine-deficient animals that a striking increase in the conversion of T4 to T3 is observed in the cerebral cortex, whereas the liver shows a change in the opposite direction.68 Thus tissues highly dependent on T4 for their intracellular content of T3, such as the brain, undergo a significant increase in conversion of T4 to T3 in the presence of chronic deficiency of iodine, and this adaptation may prevent harmful consequences on brain development in the early stages of life. Brain growth is characterized by two periods of maximal growth velocity. The first one occurs during the first and second trimesters between the third and fifth months of gestation. This phase corresponds to neuronal multiplication, migration, and organization. The second phase takes place from the third trimester onwards up to the second and third years postnatally. It corresponds to glial cell multiplication, migration, and myelination. The first phase occurs before fetal thyroid has reached its functional capacity. During this phase, the supply of thyroid hormones to the growing fetus is almost exclusively of maternal origin, whereas during the second phase, the supply of thyroid hormones to the fetus is essentially of fetal origin (Fig. 17-3).69 An important recent issue regarding thyroid function and regulation in the fetus is the concept that thyroid hormones are transferred from mother to fetus both before and probably after the onset of fetal thyroid function.70 Thyroid hormones, especially T4, are already available to embryonic and fetal tissues before the onset of fetal thyroid function, which occurs in humans at mid-gestation (about 22 weeks). Thus the T4 and T3 found in early human fetuses up to mid-gestation are likely to be entirely or mostly of maternal origin. This transfer decreases but persists during later gestation. Iodine deficiency in the fetus is the result of iodine deficiency in the mother, and an insufficient supply of thyroid hormones to the developing brain may result in mental retardation.
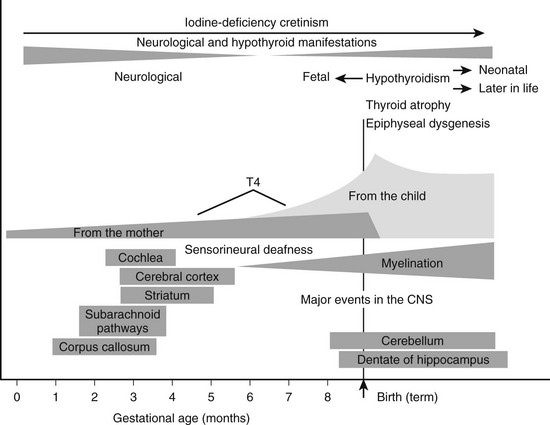
FIGURE 17-3 Proposed time course of the neurodevelopmental events and neurologic alterations associated with iodine deficiency during fetal and neonatal life. Prenatal maternal hypothyroxinemia secondary to severe iodine deficiency results in fetal neurodevelopmental damage. In addition, continuing postnatal thyroid hormone deficiency acts to determine the severity and is responsible for the varied clinical manifestations of postnatal hypothyroidism. (Data from Berbel P, Obregón MJ, Bernal J et al: Iodine supplementation during pregnancy: A public health challenge, Trends Endocrinol Metab 18:338–343, 2007.)
Increased Thyrotropin Production
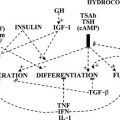
In iodine deficiency, as in other thyroid conditions with a limited glandular reserve, subjects with normal serum T3 and low T4 levels may have elevated levels of serum TSH even though they are clinically euthyroid. A clear-cut increase in the mean level of serum TSH was found in subjects living in areas where the iodine supply was reduced, and no difference was evident between individuals with and without goiter.71 Also, it has been demonstrated that serum TSH levels correlate much better with serum T4 than with serum T3. When T4 is low, the pituitary seems to be hypothyroid, whereas most other tissues are not metabolically affected, provided that the serum T3 level is normal or elevated. The most elevated TSH values have been observed in newborns and young adults living in areas with severe endemic goiter, whereas in longstanding multinodular goiter, the increased thyroid mass and the presence of autonomous areas may bring serum TSH levels toward the normal range. An increased sensitivity of endemic goiter tissue to TSH has been proposed as an additional factor for continuous goiter growth. Both thyroid peroxidase activity and 5′-deiodinase (5′DI) activity are elevated in the presence of normal serum TSH, and this increased activity has been claimed to be related to increased tissue sensitivity to TSH. In an attempt to further delineate the role of TSH in the pathogenesis of goiter, various investigators have administered thyrotropin-releasing hormone (TRH) to goitrous patients. The exaggerated and sustained TSH response to TRH observed in most studies indicates an increase in pituitary TSH reserve and less than optimal T4-induced TSH suppression at the pituitary level. This finding further documents the role in the pituitary of intracellular T3 generated from T4 in suppressing TSH (see Fig. 17-2).
Associated Pathology
In most regions of the globe in which selenium deficiency is endemic, iodine deficiency is also endemic, but the converse is not true. Selenium deficiency is more severe in China and Tibet72 than in central Africa73 and could affect many organs, including the thyroid gland. The essential selenium is involved in thyroid hormone synthesis, metabolism, and action.74 Selenium is an integral component of two important enzymes: glutathione peroxidase and iodothyronine deiodinase. The former catalyzes the breakdown of hydrogen peroxide, thereby protecting against oxidative damage. The later catalyzes the deiodination of T4 to T3. Selenium and iodine are thus linked biochemically because both are involved in thyroid hormone production. Selenium deficiency impairs the function of 5′ DI, a selenocysteine-containing protein which plays a major role in T4 deiodination in peripheral tissues.
Clinical and Laboratory Diagnosis
A frequent complication in large multinodular goiters is hemorrhage or infarction of a thyroid nodule, often accompanied by an inflammatory reaction and an abrupt rise in serum TG concentration. Hyperthyroidism, often caused by an autonomously functioning adenoma, is frequently observed if patients have access to even a small iodine load.75 Thyroiditis, a rare complication, is often subacute and sometimes focal. The pathologic features of endemic goiter do not materially differ from those of simple nodular goiter. Follicular and anaplastic carcinomas and especially sarcomas are more frequent in regions of endemic goiter. The prevalence of these tumor types means that highly aggressive thyroid cancer prevails in countries with endemic goiter, whereas relatively benign forms (papillary carcinomas) are less frequently recognized.76 The prognosis of thyroid cancer in regions of endemic goiter is worse than in goiter-free areas because most patients are first seen with a tumor stage in which no cure by surgery can be expected. Highly aggressive, prognostically poor types of thyroid malignancy preponderate in patients with endemic goiter. Iodine supplementation results in a relative decrease in these tumor types and hence forms of thyroid cancer with a better prognosis.
As a whole, the following tests are advised for assessment of iodine nutrition in populations: urinary iodine concentration, goiter rate, serum TSH, thyroid hormones, and serum TG. These indicators are complementary in that urine iodine concentration is a sensitive indicator of recent iodine intake (days), and TG shows an intermediate response (weeks to months), whereas changes in the goiter rate show long-term iodine nutrition (months to years).5
Serum thyroid hormone levels are a further index of the effects of iodine deficiency. Thyroid hormone concentrations are generally poor indicators of iodine status. In iodine-deficient populations, serum T3 and TSH rise or remain unchanged, and serum T4 usually falls. However, these changes are often within the normal range, and the overlap with iodine-sufficient populations is large enough to make thyroid hormone concentrations an insensitive measure of iodine nutrition. However, TSH is a sensitive indicator of iodine status in the newborn period. Elevated serum TSH, but for exceptional pathologic situations, indicates an insufficiency in the saturation of the T3 receptor in the brain, whatever the level of serum thyroid hormones. Therefore, elevated serum TSH constitutes an indicator of the potential risk of iodine deficiency on brain development. Serum T4 and T3 are less specific indicators of iodine deficiency because they are modified usually only in conditions of at least moderate iodine deficiency.41 In moderate and severe iodine deficiency, serum T4 is low, but T3 is variable or occasionally high due to preferential T3 secretion by the thyroid. Elevated serum T3 despite low serum T4 is considered a protective mechanism for most parts of the body, except the brain, where T3 is produced locally and not derived from the circulating T3.
Serum TG, which represents a sensitive marker of thyroid abnormalities and iodine deficiency in epidemiologic studies, is often elevated in patients with endemic goiter.77,78 Increased serum TG concentrations in endemic goiter could be partly related to the reduced concentration of iodine in goitrous tissue and the intrathyroidal metabolic changes secondary to persistent and chronic iodine deficiency. In areas of endemic goiter, serum TG increases owing to greater thyroid mass and TSH stimulation. Serum TG is well correlated with the severity of iodine deficiency as measured by urinary iodine.77 Whole blood from finger pricks spotted on filter paper cards can be used to measure serum TSH and serum TG as indicators of thyroid hyperstimulation and the consequence of the state of hyperstimulation, respectively.79
Evaluation of The Iodine Status in Iodine Deficiency
In addition to the determination of serum levels of TSH, thyroid hormones, and TG, the following indicators of iodine status are evaluated in an iodine-deficient population: (1) the urinary iodine concentration and (2) the estimation of thyroid size.41
According to WHO recommendations, quantification of urinary iodine concentration expressed as µg of iodine per volume (µg I/L, pg I/dL or µg I/dL) is accepted as a good marker of the dietary iodine intake. Therefore, it is the index of choice for evaluating the degree of iodine deficiency and for measuring the improvement in iodine status after iodine prophylaxis. Iodine intake for metabolic studies is best determined from a 24-hour urine sample, but logistics make it impractical to use such measurements for epidemiologic studies. Collections of 24-hour urine are difficult to obtain and are not necessary. Relating urinary iodine to creatinine is also not practical, because urinary creatinine decomposes after 3 days without refrigeration. Furthermore, the creatinine level varies depending on age, sex, muscle mass, diseases, pH conditions, and nutritional status of the population. For these reasons and to avoid errors introduced in the performance of different creatinine assays, the WHO has recommended for epidemiologic studies the evaluation of iodine concentrations in casual spot samples, provided a sufficient number of specimens is collected. Because the frequency distribution of urinary iodine is usually skewed towards elevated values, the median is considered instead of the mean. It is appropriate to mention that the concentration of iodine in a spot or casual urine sample cannot be used to diagnose iodine deficiency in an individual. The urinary iodine concentration may vary up to threefold in an individual during a day.80 This means that it is necessary to collect repeated urine samples from an individual over a period of time and estimate the median or average, in order to evaluate their iodine status. Also, the urinary iodine (UI) concentration (µg/L) is not interchangeable with 24-hour UI excretion (µg/24 h). The two values are interchangeable only if the volume of urine passed in 24 hours is one liter. The average volume of urine passed by an adult is approximately 1.5 L/24 h. Therefore, the median UI excretion given as µg/24 h will be 50% higher than the median iodine excretion given as µg/L.81
Several methods of determination of urinary iodine have been reported, and almost all depend on the Sandell-Kolthoff reaction, in which iodide catalyzes the reduction of yellow ceric ammonium sulfate to the colorless cerous form in the presence of arsenious acid; the rate of color disappearance is proportional to the amount of iodide.82 An optimal urinary iodine concentration is 100 to 200 µg/L, corresponding approximately to a daily intake of 150 to 300 µg for adults.
The prevalence of goiter is an index of longstanding iodine deficiency and, therefore, is less sensitive than urinary iodine in the evaluation of a recent change in the status of iodine intake. Traditionally, neck palpation can detect the enlarged thyroid of moderate or severe iodine deficiency but is less reliable in mild deficiency. Recently, thyroid ultrasonography has introduced a more precise and accurate means for quantitative estimate of thyroid volume. Norms have been established for the thyroid volume of iodine-sufficient children related to age, gender, and body mass. The total goiter rate is related to the severity of iodine deficiency as follows: no iodine deficiency, 0% to 4.9%; mild deficiency, 5.0% to 19.9%; moderate deficiency, 20.0% to 29.9% and severe deficiency, more than 30%.41 Furthermore, a thyroid is considered as goitrous when its volume is above the 97th percentile established for sex, age, and body surface area in iodine-replete populations.83 In areas of endemic goiter, although the thyroid size predictably decreases in response to increases in iodine intake, thyroid size might not return to normal for months or years after correction of iodine deficiency. During this transition period, the goiter rate is difficult to interpret, because it indicates both a population’s history of iodine nutrition and its present status. Moreover, palpation of goiter in regions of mild iodine deficiency has poor sensitivity and specificity; in such areas, measurement of thyroid volume by ultrasound is preferable for the classification of goiter.84
Principles of Treatment
Treatment of endemic goiter can be carried out by oral administration of L-thyroxine (100 to 200 mcg/day) for a prolonged period. This putative suppressive therapy induces, through a decrease in TSH and the TSH response to TRH, functional atrophy of the goiter. Results are often satisfactory in relatively small colloid goiters but less effective in large multinodular glands. Triac (triiodothyroacetic acid) is used in the treatment of thyroid gland hypertrophy in nontoxic goiter for its suppressive effect on pituitary-thyroid function. In agreement, a study by Brenta and colleagues demonstrated that Triac is effective as a goiter-shrinkage agent.85 These authors reported that when compared to L-thyroxine, the action appeared to be more important in reduction of goiter size, although it did not attain a significant level. In addition, it was associated with a relative advantage concerning a significantly lower incidence of adverse events. Therefore, Triac could be an alternative for the treatment of nontoxic diffuse goiter and nodular goiter.
Administration of 131I in euthyroid or hyperthyroid multinodular goiter, to both decrease the size and to treat thyrotoxicosis, has become more popular over the years because of its efficacy and safety.86 Even in the case of large goiters causing substantial tracheal compression with concomitant airflow obstruction, treatment with radioactive iodine can be very effective. Also, 131I treatment can be used in patients in whom surgery is not an option because of increased risk. The goal is to reduce thyroid size, which can be achieved only by relatively large doses of the isotope. However, in patients with longstanding, large, nodular goiter after iodide supplementation whose RAIU is low or normal, RAIU can be enhanced. Recently, recombinant human thyrotropin has been used to stimulate RAIU in patients with nodular goiter,87 allowing approximately 50% to 60% reduction of the therapeutic dose and causing a more homogeneous glandular distribution of radioiodine without compromising the efficacy of thyroid volume reduction. A rather high percentage (32%) of patients will become hypothyroid after treatment, as also noted in subjects treated with radioiodine for Graves’ disease. Although controversial, prophylactic L-thyroxine treatment in these patients could be instituted to hinder recurrence of the goiter and persistence of hypothyroidism.87
In patients affected by endemic nontoxic goiter, iodine alone or in combination with L-T4 has been proposed as an efficient alternative therapeutic tool to treatment with L-T4 alone.88 Among the advantages derived from this combination is the possibility of using lower doses of L-T4 with less TSH suppression than that attained in the course of L-T4 monotherapy.
Endemic Cretinism
Endemic cretinism is now largely a disease in remote, underdeveloped areas of the Third World (Fig. 17-4). It occurs when iodine intake is below a critical level of 25 µg/day and may affect up to 10% of populations living in conditions of severe iodine deficiency.63 The disorder is found in India, Indonesia, China, Oceania (Papua New Guinea), Africa (Congo Kinshasa), and South America (Ecuador, Peru, Bolivia). In all these locations, with the exception of Congo Kinshasa, neurologic features are predominant. Endemic cretinism may be defined as irreversible changes in mental development in individuals born in an area of endemic goiter; such individuals exhibit a combination of some of the following characteristics not explained by other causes: (1) a predominantly neurologic syndrome consisting of defects of hearing and speech associated or not with characteristic disorders of stance and gait of varying degree, (2) stunted growth, (3) mental deficiency, and (4) hypothyroidism. In its fully developed form, mental deficiency, deaf-mutism, and motor spastic diplegia are associated with or without goiter. This condition is referred to as the neurologic form of endemic cretinism, in contrast to the myxedematous form (see Fig. 17-3). The typical myxedematous cretin has a lesser degree of mental retardation, severe hypothyroidism, and nonpalpable thyroid. It should be made clear, however, that the two types of endemic cretinism represent polar opposites of a wide spectrum of clinical abnormalities. Although the myxedematous type is more common in Congo Kinshasa, the condition may be found in the Himalayas, Western China, Sicily (Italy), and South America (Bolivia and Peru).
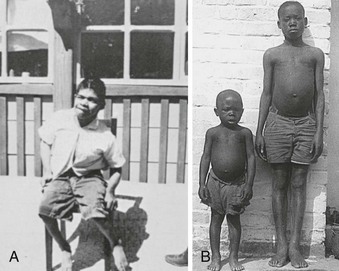
FIGURE 17-4 A, An endemic cretin from South America with a predominant neurologic pattern comprising deaf-mutism, spastic diplegia, goiter, and mental retardation. Although thyroid hormone levels are usually normal, an exaggerated and sustained thyroid-stimulating hormone response to thyrotropin-releasing hormone is observed frequently and suggests a low thyroid reserve. B, Two boys of the same age in Congo Kinshasa (formerly Zaire, central Africa). The boy on the left has an endemic myxedematous type of cretinism with severe thyroid insufficiency and dwarfism. Thyroid atrophy commonly is found later in life in myxedematous cretins and has been attributed to environmental agents or blocking autoantibodies or both. These two physiognomic forms of the syndrome represent polar opposites of a wide spectrum of clinical abnormalities which varies from one geographic area to another, with mixed characteristics.
In central Africa (Congo Kinshasa), the intensity of cretinism was found to be proportional to the degree of hypothyroidism; the severity was also shown to correlate with selenium deficiency.89 According to experimental results, it was suggested that in central Africa (Congo Kinshasa) thyroid destruction might originate from the interaction of three factors: iodine and selenium deficiencies (by increasing H2O2 accumulation), selenium deficiency (by decreasing cell defense and promoting fibrosis), and SCN overload (by triggering follicular cell necrosis), explaining the thyroid atrophy characteristic of the myxedematous form of cretinism. Furthermore, a hypothesis was put forth that defective glutathione peroxidase caused by selenium deficiency results in a lack of protection against peroxidative damage induced by high levels of H2O2 in the thyroid cell.90 Glutathione peroxidase activity was found to be decreased in selenium-deficient areas in Congo Kinshasa and the Central African Republic (formerly Ubangi-Shari), and the enzyme activity in cretins was half the level in normal subjects. The same observation on serum glutathione peroxidase activity was recently made in a selenium-deficient population in the Lhasa district in central Tibet.91 Selenium supplementation for 2 months corrected the enzyme levels in both normal subjects and endemic cretins.92 However, this treatment also produced decreases in serum T4 and T3 and an increase in TSH. In view of these findings, it is advisable to provide iodine supplementation before administration of selenium in populations deficient in both these elements.
An interesting unifying hypothesis was proposed93 to explain the clinical picture of endemic cretinism. The authors suggested that the clinical expression of endemic cretinism is determined by the sum of two pathophysiologic events. The first event is fetal hypothyroidism secondary to severe iodine deficiency, which occurs in all cretins and represents the prenatal action of thyroid hormone insufficiency on brain development, transmitted from mother to fetus, resulting in the neurologic abnormalities of the disorder. The second event is the persistence of postnatal hypothyroidism effects on both somatic and brain development from continuing iodine lack and other mechanisms causing thyroid failure, which entails the development of myxedematous cretinism.
Deaf-Mutism and Endemic Cretinism
An endemic cretin is frequently partially or completely deaf. Lesions can be produced experimentally in the organ of Corti in the chick by injecting an antithyroid drug into the yolk sac. Also, antithyroid drug (propylthiouracil) administered to pregnant mice or to pups after birth causes abnormalities in the tectorial membrane of the organ of Corti and results in deafness. These experiments strongly suggest that intrauterine hypothyroidism somehow damages the developing auditory system and causes deafness and other neurologic defects. This has been confirmed by auditory brainstem evoked-potential studies which showed no cochlear or brainstem responses even at the highest sound frequencies, indicating a cochlear lesion. In accordance, Halpern94 has consistently found profound congenital petrous temporal bone changes and underdeveloped cochleas (incomplete turns, fragmented and enlarged vestibular aqueduct) in the majority of both neurologic and hypothyroid adult cretins. These findings are suggestive of a premature arrest of the auditory system. Its absence in sporadic congenital hypothyroidism may be a result of the protective action of thyroid hormone passing to the fetus from the mother.
Neonatal Hypothyroidism in Iodine-Deficient Areas
A serious consequence of chronic iodine deficiency is a higher incidence of neonatal hypothyroidism. In India and Congo Kinshasa, it has been reported that this condition is 200- to 500-fold more frequent than in countries with adequate iodine intake.5 In iodine-deficient areas of India, as many as 4% of newborn babies have a cord blood serum T4 level below 2 µg/dL, and in Congo Kinshasa, low T4 concentrations have been observed in up to 10%.95 Further deterioration in thyroid function occurred in Congo Kinshasan children between 2 and 4 years of age, followed by a pronounced prevalence of hypothyroidism between 5 and 7 years of age. This pattern is linked to persistent iodine deficiency accompanied by an increased thiocyanate load originating from very high consumption of cassava.96
Experimental work has confirmed that severe iodine deficiency affects brain development by reducing both maternal and fetal thyroid function. When sheep or marmosets are maintained on a severely iodine-deficient diet for 6 to 12 months before pregnancy and also during pregnancy, reduced brain weight and low DNA content of the fetal cerebral cortex occur as early as day 70 of gestation.97
Similarly, the number of spines on the shafts of pyramidal neurons from the visual cortex of iodine-deficient rats is lower than in animals supplemented with iodine.98 This finding supports the concept that thyroid hormone affects brain maturation through specific effects on the rate of cell differentiation and gene expression.99,100 The primary action of thyroid hormone on gene expression is mediated through interaction of the T3 receptors with responsive elements located in gene regulatory regions. Some of the genes known to be responsive to thyroid hormones in the brain contain T3-responsive elements (TREs), and in some cases the action of T3 has been shown to be at the transcriptional level in vitro. Genes containing TREs in their promoter or intronic regions include those encoding myelin basic protein (MBP), the Purkinje cell–specific gene (PCP2), which encodes a G protein nucleotide exchange factor, the calmodulin-binding and protein kinase C (PKC) substrate neurogranin (RC3), prostaglandin D2 synthetase, the transcription factor hairless, the neuronal cell–adhesion molecule (NCAM), and the early response gene NGFI-A.100 The severe neurologic damage found in endemic cretinism is probably due to thyroid hormone deficiency early in pregnancy (first trimester), and it might have become irreversible by birth, at which time thyroid hormones reverse the hypothyroidism, if present, but not the neurologic deficits. Both forms of the syndrome can be prevented by correction of the severe iodine deficiency before pregnancy by iodized oil injections. When given in the first trimester, however, iodized oil does not prevent the syndrome of endemic cretinism, which suggests that the effects of maternal iodine deficiency arise very early. Thus, elemental iodine, apart from its hormonal role, may be essential for normal neural tube development, but the mechanism responsible for this action is unknown.
Prophylaxis and Treatment of Iodine-Deficiency Disorders
Iodized Salt
Iodized salt is considered the most appropriate measure for iodine supplementation.101 There are two forms of iodine which can be used to iodize salt: iodide and iodate, usually as the potassium salt. Iodate is less soluble and more stable than iodide and is therefore preferred for tropical moist conditions. Both are generally referred to as “iodized salt.”
The sources of most common salt are solar evaporation of sea water and salt mines. Sea salt, as usually produced, does not contain enough iodine to meet minimal human needs because the average iodine content of ocean salts is approximately 2 ppm. Human salt consumption (5 to 15 g/day) varies widely among cultures and with climatic conditions. Thus the level of iodination of salt may be varied to conform to regional conditions (1 : 25,000 to 1 : 100,000). It is accepted that 30 ppm (30 mg of potassium iodate per kilogram of salt) is the lowest level that will ensure the provision of 100 µg of iodine per day. Many local problems confound the program of iodination of salt for the many millions of people at risk. Inadequate iodinate of the salt, difficulties in importing potassium iodate, problems of transportation and coordination of distribution efforts, and the consumption of poorly iodinated “cattle” salt by the rural population are the main problems that have obstructed effective iodination prophylaxis. Successful salt iodination programs have been implemented in many countries and are highly dependent on continuous surveillance of the iodized salt produced and consumed.102
Iodized Oil
Intramuscular injection or oral administration of the iodized ethyl esters of fatty acids of poppy seed (Lipiodol, Ethiodol, Oriodol), rape seed (Brassiodol), walnut, and soybean oil (475 to 540 mg iodine per milliliter) has been used for the prevention of endemic goiter and cretinism.134–137 Intramuscular doses have varied from 0.5 to 1.0 mL in infants and young children to 0.5 to 2.0 mL for adults (Table 17-6). The physiology and pharmacology of iodized oil in goiter prophylaxis have been extensively reviewed.137 Intramuscular administration of iodized oil was started in Papua New Guinea and extended to South America, the former Zaire (now Congo Kinshasa), Nepal, Sudan, Indonesia, India, and China. Oral administration of iodized soybean oil was extensively studied in various countries and also reported to be effective in a mass population program to control endemic goiter.138
Table 17-6
WHO-Recommended Iodine Supplementation*
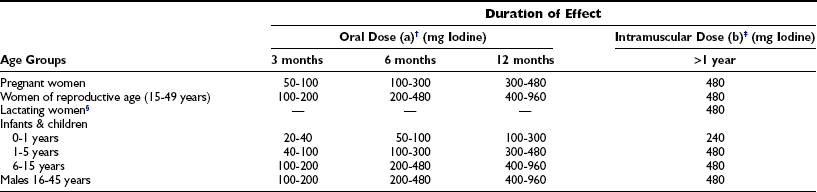
*Doses, frequency, and duration of effectiveness of administering oral and intramuscular iodine supplementation using an iodized oil preparation in the prevention of the disorders induced by iodine deficiency.
†(a) Lipiodol (capsule): 1 capsule (0.4 mL) contains about 200 mg iodine; Oriodol (capsule): 1 capsule (0.57 mL) contains about 300 mg iodine.
‡(b) Lipiodol (ultra fluid)/Ethiodol: 1 mL contains about 480 mg iodine.
The use of iodized oil, also in children, has proved to be effective not only in reducing the frequency of endemic goiter but also in reducing the size of established goiters and in preventing the major neuromotor, physical, and mental deficits that are found in association with endemic goiter and endemic cretinism.139 Iodized oil provides effective, safe, and economically sound prophylaxis against endemic goiter and related disabilities in situations in which salt iodination is not feasible for economic or political reasons.
Iodine Excess
Toxic Effects of Excess Intake
Most people who are iodine sufficient are remarkably tolerant to high dietary intakes of iodine. Iodine intakes of up to 1000 µg per day are well tolerated by most adults, since the thyroid is able to adjust to a wide range of intakes and regulates the synthesis and release of thyroid hormones. In children, chronic intakes of 500 µg per day or more are associated with increased thyroid volume, which is an early sign of thyroid dysfunction.103 European and U.S. expert committees have recommended tolerable upper intakes for iodine (Table 17-7) but caution that individuals with chronic iodine deficiency might respond adversely to intakes lower than these.9
Table 17-7
Tolerable Upper Intake Level for Iodine by Age Group
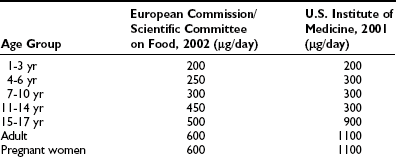
Adapted from Zimmermann MB: Iodine requirements and the risks and benefits of correcting iodine deficiency in populations, J Trace Elem Med Biol 22:81–92, 2008.
Both a chronic shortage and an acute increase in iodine intake of a population carry an augmented risk of thyroid disease, but the consequences are more severe in iodine deficiency than iodine excess. The most serious and common complication of salt iodization is the development of iodine-induced hyperthyroidism, which affects mainly older people with nodular goiters. Other possibilities are the aggravation or even the induction of autoimmune thyroiditis, goiter, and a change in the pattern of thyroid cancer.104
Iodine-Induced Hyperthyroidism
An increase in the incidence of iodine-induced hyperthyroidism (IIH) has been reported after the institution of iodized salt programs in Europe and South America and after the introduction of iodized bread in Denmark and Tasmania.105,106 IIH was more frequently seen in patients older than 40 years and was closely associated with increasing weight and nodularity of the goiter and with the existence of nonhomogeneity on thyroid scans.107 These large multinodular goiters, adapted to chronic iodine deficiency, have autonomous areas particularly susceptible to small loads of iodine and produce excessive amounts of T3 or T4.
A mild and transient form of hyperthyroidism characterized by a blunted TSH response to TRH is frequently observed in endemic goiter patients moving to urban areas, where iodized salt is commonly used.108,109
An outbreak of IIH was reported in Africa, particularly in two African countries after the introduction of salt with a higher level of iodination.110 In a severely iodine-deficient area of Kivu in Zaire (now Congo Kinshasa),111 25% of 200 unselected adult subjects with visible goiter had an undetectable serum level of thyrotropin (TSH). In half of the TSH-suppressed patients, serum thyroid hormones reached the level of overt hyperthyroidism. High serum thyroid hormone levels remained unchanged at a 1-year interval, which suggests that the hyperthyroidism was not temporary. The urinary iodide concentrations of these patients did not differ from the levels observed in euthyroid patients, 240 µg/L. In most of these patients, the clinical picture was not characteristic of hyperthyroidism.
In Zimbabwe, all cases of hyperthyroidism detected by laboratory tests in the main hospital of Harare from 1991 to 1995 were reviewed.112 Since 1993, a threefold increase was demonstrated after the consumption of salt iodinated at a level of 30 to 90 ppm. Fatal outcomes occurred mainly from cardiac complications. The median concentration of urinary iodide in the population was 280 µg/L.
In a recent report from China, the cumulative 5-year incidence of overt hyperthyroidism, overt hyperthyroidism caused by Graves’ disease, and subclinical hyperthyroidism was similar in subjects in three different communities in which iodine intake was low, more than adequate and excessive. After 5 years, 72% of the patients were euthyroid (without medication). This study may indicate that IIH is of short duration with a tendency to a normal thyroid function in relation to time, and that there is no relationship between iodine intake and hyperthyroidism, at least in people with a rather broad range of iodine intake.113
The reason for the development of IIH after iodine supplementation appears to be that iodine deficiency increases thyrocyte proliferation and mutation rates (due to mutational events in thyroid cells) that lead to autonomy of function.114 When the mass of cells with such mutations becomes sufficient and the iodine supply is increased, the subject may become hyperthyroid. These changes may occur in localized foci within the gland or in the process of nodule formation. IIH may also occur with an increase in iodine intake in those whose hyperthyroidism (Graves’ disease) is not expressed because of iodine deficiency. The risks of IIH are principally to the elderly, who may have heart disease, and to those who live in regions with limited access to medical care. The same situation is also found in endemic goiter areas when iodized oil injections are introduced.115 This hyperthyroidism is often transient, and hormone production will eventually decrease in 6 to 12 months without a need for therapy unless cardiovascular disease and related complications are present.
Treatment of Iodine-Induced Hyperthyroidism
Correction of the hyperthyroidism is the best treatment for the cardiac manifestations of IIH. It has been shown that angina will resolve in approximately 50% of those who develop it. Atrial fibrillation spontaneously reverts to sinus rhythm in approximately 60% of patients within 6 months of becoming euthyroid. β-Adrenergic blocking agents, such as propranolol, are effective in controlling the ventricular rate in either atrial fibrillation or sinus rhythm and are the drugs of choice for treatment of this problem. Atrial thromboembolism has ranged from 8% to as high as 40% in patients with thyrotoxicosis and atrial fibrillation. The risk warrants the strong consideration of anticoagulant therapy until euthyroidism is restored, because the majority of these emboli are cerebrovascular.116
Iodine Excess and Chronic Autoimmune Thyroid Disease
It is recognized that excessive dietary iodine may increase the risk of thyroiditis, hyperthyroidism, hypothyroidism, and goiter.117 In healthy adults, short-term iodine intakes of 500 to 1500 µg/day have mild inhibitory effects on thyroid function. According to experimental conditions, excessive iodine intake can precipitate spontaneous thyroiditis in genetically predisposed beagles, rats, or chickens.118 The mechanism involved in iodine-induced thyroiditis in animal models could be that elevated dietary iodine triggers thyroid autoimmune reactivity by increasing the antigenicity of more highly iodinated forms of TG or by inducing damage of the thyroid and cell injury by free radicals.102 However, it was shown that the frequency of thyroid autoantibodies and hypothyroidism was higher in iodine-replete populations than in iodine-deficient populations. Pearce et al.119 found that during prolonged excess iodine exposure there were marked increases in serum total iodine concentrations, the prevalence of goiter, elevated serum TSH values, and elevated serum thyroid peroxidase antibody values increased. The occurrence of all abnormalities decreased after removal of excess iodine from the drinking water system.
In a study conducted in China, the researchers examined the effect of regional differences in iodine intake on the incidence of thyroid disease in 3018 subjects during a 5-year follow-up study. They came from three regions with different levels of iodine intake (median urinary iodine 84, 243, and 651 µg/L, respectively). It has been found that the cumulative incidence of subclinical hypothyroidism and autoimmune thyroiditis was higher in subjects with median urinary iodine concentration more than 243 µg/L.120 Subjects who were TPOAb and TGAb positive at baseline developed thyroid autoimmunity more frequently than seronegative individuals. High iodine intake was a risk factor for developing hypothyroidism in antibody-positive subjects. They conclude that a constant exposure to excessive iodine intake increased the incidence of hypothyroidism and positive TgAb.121 Studies conducted in Greece before and after introduction of iodized salt in iodine-deficient mountainous areas confirmed a higher prevalence of Hashimoto’s thyroiditis (by fine-needle aspiration smears) from 5.9% at baseline to 13.9% after 8 years of iodine sufficiency.118 In Sao Paulo (Brazil) after 5 years of excessive iodine intake (iodine concentration in salt: 40 to 100 ppm), the urinary iodine excretion was greater than 300 µg/L in 45.6% of the population. The prevalence of chronic autoimmune thyroiditis increased to 16.9% of the included subjects and was higher (21.5%) in women.122
In contrast with these observations, thyroid antibodies did not appear in 43 goitrous patients living in areas of chronic iodine deficiency followed up during 60 months after an injection of iodized oil.123 Similarly, the daily administration of iodine during pregnancy to 38 women living in an iodine-deficient area was not followed by the occurrence of thyroid autoantibodies from 2 to 21 days after delivery.124 Laurberg et al.,125 studying the importance of the population iodine intake level for the prevalence rate of various thyroid abnormalities in elderly subjects, compared random samples of subjects from Jutland, Denmark, with low iodine intake (median urinary iodine of 38 µg/L) and from Iceland, with longstanding relatively high iodine intake (median urinary iodine of 150 µg/L). These authors reported that the frequency of thyroid autoantibodies were in general more common in Jutland. The population with the highest prevalence of autoantibodies had a high occurrence of goiter and thyroid hyperfunction. Similarly, Aghini-Lombardi et al.126 reported that in a community of Southern Italy with mild iodine deficiency (median urinary iodide of 55 µg/L), the detection of low titers of autoantibodies was relatively frequent (12.6%) but that only 3.5% also had the thyroid echographic pattern of diffuse hypoechogenicity consistent with diffuse autoimmune thyroiditis, a prevalence that is no different from that observed in iodine-sufficient areas. Overall, the prevalence of thyroid antibodies in children in relation to iodine intake is not well established, although an equal prevalence of TPO antibodies in iodine-replete and moderately iodine-deficient patients was demonstrated.127 At variance with this last data, Weetman128 suggests that the effect of dietary iodine on thyroid autoimmunity seems at best modest.
Iodine and Thyroid Cancer
Enhanced secretion of TSH has been linked with increased risk of thyroid carcinoma, especially in subjects living in iodine-deficient areas. There is a tendency for higher incidence rates of thyroid cancers detected at autopsy from endemic goiter areas, although the relationship of thyroid cancer and endemic goiter has been debated without agreement being reached on many aspects, including causal relationship.76
• Increased TSH stimulation: low T4 synthesis associated to higher TSH secretion will promote follicular cell proliferation.
• Thyroid cell responsiveness to TSH is increased in iodine-deficient thyroid cells (increased Ca++ and cAMP pathways).
• Increased thyroid-cell EGF-induced proliferation: decreased intracellular organified iodine intermediate (iodolactone or 2-iodohexadecanal) will result in EGF-induced cell proliferation.
• Decreased TGF-β production: thyroid cell proliferation is inhibited by TGF-β. Iodine deficiency will result in a decrease of negative growth regulation.
Iodine supplementation is accompanied by a change in the epidemiologic pattern of thyroid cancer, with an increased prevalence of occult papillary cancer discovered at autopsy.76 For example, follicular carcinoma is the predominant histologic variety in Africa, which has prevailed over the decades owing to persisting iodine deficiency. On the other hand, reports suggest a relative rise in papillary tumors, implying in improved iodination.129 Accordingly, the prognosis has significantly improved because of a shift towards differentiated forms of thyroid cancer that are diagnosed at earlier stages.130 Moreover, careful monitoring of the incidence of thyroid cancer in Switzerland following iodine supplementation showed that this incidence continuously decreased.131 Slowinska-Klencka et al.132 compared the cytologic diagnoses in 3572 patients to the results of postoperative histopathologic examinations in Poland between 1985 and 1990, when iodine deficiency was progressively corrected. The frequency of neoplastic lesions significantly decreased throughout the examined period, and the ratio of papillary/follicular carcinoma increased, as did the occurrence of cytologically diagnosed chronic thyroiditis. Overall, it appears that the correction in iodine supply reduces the risk of and morbidity from thyroid cancer. It can probably be set forth that correction of iodine deficiency far exceeds its risks.133
Overall, the literature data indicate that:
• Nutritional iodine intake and incidence of cancer remains a controversial issue.
• There is weak evidence that low iodine intake would increase the temporal incidence of thyroid malignancy in a given population.
• High iodine intake, however, is also associated with an increased incidence of thyroid cancer (other environmental factors may be present).
With regard to iodine prophylaxis and variations in thyroid cancer incidence, the data indicate76 that:
• After iodine prophylaxis, a clear relationship has been demonstrated between increased iodine nutrition and elevation of the PTC/FTC ratio.
• This has occurred even in modest increases in iodine urinary excretion.
• A decrease in prevalence of anaplastic thyroid cancer was also observed in most areas.
References
1. WHOICCIDDUNICEF. Assessment of the iodine deficiency disorders and monitoring their elimination, 3rd edition. Geneva: WHO; 2007.
2. DeLange, F, Hetzel, B, The iodine deficiency disorders. Available at DeGroot LE, Hannemann G, eds. The thyroid and its diseases, August, 2008. http:/www.thyroidmanager.org/. [accessed on].
3. Morreale de Escobar, G, Obregon, MJ, Escobar del Rey, F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–37.
4. Bernal, J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122.
5. Zimmermann, MB, Jooste, PL, Pandav, CS. Iodine-deficiency disorders. Lancet. 2008;372:1251–1262.
6. WHO SecretariatAndersson, M, de Benoist, B, et al. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2 years old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611.
7. Dunn, JT, Crutschfield, HE, Gutekunst, R, et al. Two simple methods for measuring iodine in urine. Thyroid. 1993;3:119–123.
8. Delange, F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007;10:1571–1580.
9. Zimmermann, MB. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. J Trace Elem Med Biol. 2008;22:81–92.
10. Hollowell, JG, Staehling, NW, Hannon, WH, et al. Iodine nutrition in the United States: trends and public health implications. Iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab. 1998;83:3401–3408.
11. Haddow, JE, McClain, MR, Palomaki, GE, et al. Urine iodine measurements, creatinine adjustment and thyroid deficiency in an adult USA population. J Clin Endocr Metab. 2007;92:1019–1022.
12. Abalovich, M, Amino, N, Barbour, LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92(Suppl 8):S1–47.
13. Muller, AF, Drexhage, HA, Berghout, A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605–630.
14. Othman, S, Phillips, DI, Lazarus, JH, et al. Iodine metabolism in postpartum thyroiditis. Thyroid. 1992;2:107–111.
15. Kampe, O, Jansson, R, Karlsson, FA. Effects of L-thyroxine and iodide on the development of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1990;70:1014–1018.
16. Nøhr, SB, Jorgensen, A, Pedersen, KM, et al. Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: is iodine supplementation safe? J Clin Endocrinol Metab. 2000;85:3191–3198.
17. Laurberg, P, Nohr, SB, Pedersen, KM, et al. Thyroid disorders in mild iodine deficiency. Thyroid. 2000;10:951–963.
18. Stilwell, G, Reynolds, PJ, Paramesvaran, V, et al. The influence of gestational stage on urinary iodine excretion in pregnancy. J Clin Endocr Metab. 2008;93:1737–1742.
19. Moleti, M, Lo Presti, VP, Campolo, MC, et al. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93:2616–2621.
20. Delange, F. Iodine deficiency in Europe and its consequences: an update. Eur J Nucl Med. 2002;29(Suppl 2):S404–S416.
21. Andersson, M, de Benoist, B, Darnton-Hill, I, et al. Iodine deficiency in Europe: a continuing public health problem. Geneva: World Health Organization; 2007.
22. de Benoist, B, McLean, E, Andersson, M. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008;29:195–202.
23. Hess, SY, Zimmermann, MB, Torresani, T, et al. Monitoring the adequacy of salt iodization in Switzerland: a national study of schoolchildren and pregnant women. Eur J Clin Nutr. 2001;55:162–166.
24. Rossi, AC, Tomimori, E, Camargo, R, et al. Searching for iodine deficiency disorders in schoolchildren from Brazil: the ThyroMobil project. Thyroid. 2001;11:661–663.
25. Pretell, EA, Delange, F, Hostalek, U, et al. Iodine nutrition improves in Latin America. Thyroid. 2004;14:590–599.
26. Subramian, P. Goiter and iodine deficiency disorders control through universal iodination of salt in India. IDD Newsletter. 1997;3:12–16.
27. Ategbo, E, Sankar, R, Schultink, W. Pushing in the right direction: steady progress in control of IDD in India. IDD Newsletter. 2005;21:1–4.
28. Teng, XC, Hu, FN, Teng, WP, et al. The study of thyroid diseases in a community not using iodized salt. Chin J Prev Medicine. 2002;136:176–179.
29. Chen, ZP. New cretins discovered in Southern Xinjiang, China. IDD Newsletter. 2007;23:18.
30. Zhao, J, van der Haar, F. Progress in salt iodization and improved iodine nutrition in China, 1995–99. Food Nutr Bull. 2004;25:337–343.
31. Delange, F, Bürgi, H, Chen, ZP, et al. World status of monitoring of iodine deficiency disorders control programs. Thyroid. 2002;12:915–924.
32. Okosieme, OE. Impact of iodination on thyroid pathology in Africa. J R Soc Med. 2006;99:396–401.
33. UNICEF. The state of world children. www.unicef.org/nutrition/files/SOWCO6_Table 2.pdf, 2007. [(accessed on August, 2008)].
34. Aguayo, VM, Scott, S, Ross, J, et al. Sierra Leone: investing in nutrition to reduce poverty: a call for action. Public Health Nutr. 2003;6:653–657.
35. Abuye, C, Berhane, Y. The goiter rate, its association with reproductive failure, and the knowledge of iodine deficiency disorders (IDD) among women in Ethiopia: cross-section community based study. BMC Public Health. 2007;7:316.
36. Egbuta, J, Onyezili, F, Vanormelingen, K. Impact evaluation of efforts to eliminate iodine deficiency disorders in Nigeria. Public Health Nutr. 2003;6:169–173.
37. Jooste, PL, Weight, MJ, Lombard, CJ. Iodine concentration in household salt in South Africa. Bull World Health Organ. 2001;79:534–540.
38. WHO. WHO global database on iodine deficiency. http://www.who.int/whosis/database. [(acessed on July, 2008)].
39. de Benoist, B, Anderson, M, Takkouche, B, et al. Prevalence of iodine deficiency worldwide. Lancet. 2003;362:1859–1860.
40. WHOUNICEFICCIDD. Indicators for assessing Iodine Deficiency Disorders and their control through salt iodization. Geneva: WHO publ; 1994.
41. WHOUNICEFICCIDD. Assessment of the Iodine Deficiency Disorders and monitoring their elimination. Geneva: WHO publ; 2001.
42. Gaitan, E, Cooksey, RC, Legan, J, et al. Antithyroid and goitrogenic effects of coal-water extracts from iodine-sufficient goiter areas. Thyroid. 1993;3:49–53.
43. Ngudi, DD, Kuo, YH, Lambein, F. Cassava cyanogens and free amino acids in raw and cooked leaves. Food Chem Toxicol. 2003;41:1193–1197.
44. Vanderpas, J. Nutritional epidemiology and thyroid hormone metabolism. Annu Rev Nutr. 2006;26:293–322.
45. Gaitan, E, Cooksey, RC, Legan, J, et al. Antithyroid effects in vivo and in vitro of babassu and mandioca: a staple food in goiter areas of Brazil. Eur J Endocrinol. 1994;131:138–144.
46. Schröder-van der Elst, JP, Smit, JW, Romijn, HA, et al. Dietary flavonoids and iodine metabolism. Biofactors. 2003;19:171–176.
47. Doerge, DR, Chang, HC. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:269–279.
48. Bell, DS, Ovalle, F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7:193–194.
49. Suzuki, H, Higuchi, T, Sawa, K, et al. Endemic coast goiter in Hokkaido, Japan. Acta Endocrinol. 1965;50:161–176.
50. Zimmermann, MB, Jooste, PL, Mabapa, NS, et al. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am J Clin Nutr. 2007;86:1040–1044.
51. Ingenbleek, Y. Vitamin A deficiency impairs the normal mannosylation, conformation and iodination of the thyroglobulin: a new etiological approach to endemic goiter. Experientia Suppl. 1983;44:264–297.
52. Knobel, M, Umezawa, ES, Cardia, MS, et al. Elevated anti-galactosyl antibody titers in endemic goiter. Thyroid. 1999;9:493–498.
53. Zimmermann, MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr. 2006;26:367–389.
54. Brauer, VF, Schweizer, U, Köhrle, J, et al. Selenium and goiter prevalence in borderline iodine sufficiency. Eur J Endocrinol. 2006;155:807–812.
55. Kralik, A, Eder, K, Kirchgessner, M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm Metab Res. 1996;28:223–226.
56. Ruz, M, Codoceo, J, Galgani, J, et al. Single and multiple selenium-zinc-iodine deficiencies affect rat thyroid metabolism and ultrastructure. J Nutr. 1999;129:174–180.
57. Wada, L, King, JC. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. J Nutr. 1986;116:1045–1053.
58. Brucker-Davis, F. Environmental disrupters of thyroid hormone action. In: Henry HL, Norman AN, eds. Encyclopedia of Hormones. New York: Elsevier-Academic Press; 2003:535–537.
59. Lindsay, RH, Hill, JB, Gaitan, E, et al. Antithyroid effects of coal-derived pollutants. J Toxicol Environ Health. 1992;37:467–481.
60. Braverman, LE. Clinical studies of exposure to perchlorate in the United States. Thyroid. 2007;17:819–822.
61. Laurberg, P, Nøhr, SB, Pedersen, KM, et al. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab. 2004;89:181–187.
62. Crofton, KM. Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl. 2008;31:209–223.
63. Delange, F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–128.
64. Knobel, M, Bisi, H, Peres, CA, et al. Correlated functional and morphological aspects in human multinodular simple goiter tissues. Endocr Pathol. 1993;4:205–214.
65. Sugawara, M, Kita, T, Lee, ED, et al. Deficiency of superoxide dismutase in endemic goiter tissue. J Clin Endocrinol Metab. 1988;67:1156–1161.
66. Dumont, JE, Ermans, AM, Maenhaut, C, et al. Large goiter as a maladaptation to iodine deficiency. Clin Endocrinol (Oxf). 1995;43:1–10.
67. Stübner, D, Gärtner, R, Greil, W, et al. Hypertrophy and hyperplasia during goiter growth and involution in rats: separate bioeffects of TSH and iodine. Acta Endocrinol (Copenh). 1987;116:537–548.
68. Obregon, MJ, Escobar del Rey, F, Morreale de Escobar, G. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid. 2005;15:917–929.
69. Berbel, P, Obregón, MJ, Bernal, J, et al. Iodine supplementation during pregnancy: a public health challenge. Trends Endocrinol Metab. 2007;18:338–343.
70. Morreale de Escobar, G, Obregon, MJ, Escobar del Rey, F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987.
71. Dumont, JE, Ermans, AM, Maenhaut, C, et al. Large goiter as a maladaptation to iodine deficiency. Clin Endocrinol (Oxf). 1995;43:1–10.
72. Xia, Y, Hill, KE, Byrne, DW, et al. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834.
73. Chanoine, JP. Selenium and thyroid function in infants, children and adolescents. Biofactors. 2003;19:137–143.
74. Schmutzler, C, Mentrup, B, Schomburg, L, et al. Selenoproteins of the thyroid gland: expression, localization and possible function of glutathione peroxidase 3. Biol Chem. 2007;388:1053–1059.
75. Lima, N, Medeiros-Neto, GA. Transient thyrotoxicosis in endemic goiter patients following exposure to a normal iodine intake. Clin Endocrinol (Oxf). 1984;21:631–637.
76. Knobel, M, Medeiros-Neto, G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:701–712.
77. Knudsen, N, Bulow, I, Jorgensen, T, et al. Serum Tg: a sensitive marker of thyroid abnormalities and iodine deficiency in epidemiological studies. J Clin Endocrinol Metab. 2001;86:3599–3603.
78. van den Briel, T, West, CE, Hautvast, JG, et al. Serum thyroglobulin and urinary iodine concentration are the most appropriate indicators of iodine status and thyroid function under conditions of increasing iodine supply in school children in Benin. J Nutr. 2001;131:2701–2706.
79. Zimmermann, MB, de Benoist, B, Corigliano, S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab. 2006;91:4881–4887.
80. Als, C, Helbling, A, Peter, K, et al. Urinary iodine concentration follows a circadian rhythm: a study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab. 2000;85:1367–1369.
81. Laurberg, P, Andersen, S, Bjarnadóttir, RI, et al. Evaluating iodine deficiency in pregnant women and young infants—complex physiology with a risk of misinterpretation. Public Health Nutr. 2007;10:1547–1552.
82. Baloch, Z, Carayon, P, Conte-Devolx, B, et alGuidelines CommitteeNational Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126.
83. Zimmermann, MB, Hess, SY, Molinari, L, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group report. Am J Clin Nutr. 2004;79:231–237.
84. Zimmermann, M, Saad, A, Hess, S, et al. Thyroid ultrasound compared with World Health Organization 1960 and 1994 palpation criteria for determination of goiter prevalence in regions of mild and severe iodine deficiency. Eur J Endocrinol. 2000;143:727–731.
85. Brenta, G, Schnitman, M, Fretes, O, et al. Comparative efficacy and side effects of the treatment of euthyroid goiter with levo-thyroxine or triiodothyroacetic acid. J Clin Endocrinol Metab. 2003;88:5287–5292.
86. Weetman, AP. Radioiodine treatment for benign thyroid diseases. Clin Endocrinol (Oxf). 2007;66:757–764.
87. Medeiros-Neto, G, Marui, S, Knobel, M. An outline concerning the potential use of recombinant human thyrotropin for improving radioiodine therapy of multinodular goiter. Endocrine. 2008;33:109–117.
88. Carella, C, Mazziotti, G, Rotondi, M, et al. Iodized salt improves the effectiveness of L-thyroxine therapy after surgery for nontoxic goiter: a prospective and randomized study. Clin Endocrinol (Oxf). 2002;57:507–513.
89. Köhrle, J, Jakob, F, Contempré, B, et al. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–984.
90. Contempre, B, de Escobar, GM, Denef, JF, et al. Thiocyanate induces cell necrosis and fibrosis in selenium- and iodine-deficient rat thyroids: a potential experimental model for myxedematous endemic cretinism in central Africa. Endocrinology. 2004;145:994–1002.
91. Li, S, Wei, H, Zheng, Q. Elimination of iodine-deficiency disorders in Tibet. Lancet. 2008;371:1980–1981.
92. Contempre, B, Dumont, J, Bebe, N, et al. Effect of selenium supplementation in hypothyroid subjects of an iodine and selenium deficient area: the possible danger of indiscriminate supplementation of iodine-deficient subjects with selenium. J Clin Endocrinol Metab. 1991;73:213–215.
93. Boyages, SC, Halpern, JP. Endemic cretinism: toward a unifying hypothesis. Thyroid. 1993;3:59–71.
94. Halpern, JP. Studies of hearing abnormality in endemic cretinism in Qinghai Province, PRC. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency: Cognitive Behavioral, Neuromotor and Educative Aspects. New York: Cognizant Communication; 1994:273–277.
95. Geelhoed, GW. Metabolic maladaptation: individual and social consequences of medical intervention in correcting endemic hypothyroidism. Nutrition. 1999;15:908–932.
96. Teles, FF. Chronic poisoning by hydrogen cyanide in cassava and its prevention in Africa and Latin America. Food Nutr Bull. 2002;23:407–412.
97. de Escobar, GM, Obregón, MJ, del Rey, FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–1570.
98. Pedraza, PE, Obregon, MJ, Escobar-Morreale, HF, et al. Mechanisms of adaptation to iodine deficiency in rats: thyroid status is tissue specific. Its relevance for man. Endocrinology. 2006;147(5):2098–2108.
99. Kilby, MD. Thyroid hormones and fetal brain development. Clin Endocrinol (Oxf). 2003;59:280–281.
100. Bernal, J, Guadano-Ferraz, A, Morte, B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13:1005–1012.
101. Dunn, JT. Iodine should be routinely added to complementary foods. J Nutr. 2003;133:3008S–3010S.
102. Delange, F, Lecomte, P. Iodine supplementation: benefits outweigh risks. Drug Safety. 2000;22:89–95.
103. Knobel, M, Medeiros-Neto, G, Pediatric Aspects of Thyroid Function and Iodine. Diseases of the Thyroid in Childhood and Adolescence. Pediatr Adolesc Med. Krassas, GE, Rivkees, SA, Kiess, W, eds. Diseases of the Thyroid in Childhood and Adolescence. Pediatr Adolesc Med, vol 11. Basel: Karger, 2007:56–79.
104. Lauberg, P, Bullow Pedersen, I, Knudsen, N, et al. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001;11:457–469.
105. Rasmussen, LB, Ovesen, L, Christensen, T, et al. Iodine content in bread and salt in Denmark after iodization and the influence on iodine intake. Int J Food Sci Nutr. 2007;58:231–239.
106. Seal, JA, Doyle, Z, Burgess, JR, et al. Iodine status of Tasmanians following voluntary fortification of bread with iodine. Med J Aust. 2007;186(2):69–71.
107. Delange, F, de Benoist, B, Alnwick, D. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid. 1999;9:545–556.
108. Corvilain, B, van Sande, J, Dumont, JE, et al. Autonomy in endemic goiter. Thyroid. 1998;8:107–113.
109. Gołkowski, F, Buziak-Bereza, M, Trofimiuk, M, et al. Increased prevalence of hyperthyroidism as an early and transient side-effect of implementing iodine prophylaxis. Public Health Nutr. 2007;10:799–802.
110. Okosieme, OE. Impact of iodination on thyroid pathology in Africa. J R Soc Med. 2006;99:396–401.
111. Bourdoux, P, Ermans, AM, Mukalay, A, et al. Iodine-induced thyrotoxicosis in Kivu, Zaire. Lancet. 1996;347:552–553.
112. Todd, CH, Allain, T, Gomo, ZA, et al. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet. 1995;346:1563–1565.
113. Yang, F, Shan, Z, Teng, X, et al. Chronic iodine excess does not increase the incidence of hyperthyroidism: a prospective study community base epidemiological survey in China. Eur J Endocrinol. 2007;156:403–408.
114. Corvilain, B, van Sande, J, Dumont, JE, et al. Autonomy in endemic goiter. Thyroid. 1998;8:107–113.
115. Martins, MC, Lima, N, Knobel, M, et al. Natural course of iodine-induced thyrotoxicosis (Jod-Basedow) in endemic goiter area: a 5-year follow-up. J Endocrinol Invest. 1989;12:239–244.
116. Dunn, JT, Semigran, MJ, Delange, F. The prevention and management of iodine-induced hyperthyroidism and its cardiac features. Thyroid. 1998;8:101–106.
117. Zimmermann, MB, Ito, Y, Hess, SY, et al. High thyroid volume in children with excess dietary iodine intakes. Am J Clin Nutr. 2005;81:840–844.
118. Papanastasiou, L, Vatalas, IA, Koutras, DA, et al. Thyroid autoimmunity in the current iodine environment. Thyroid. 2007;17:729–739.
119. Pearce, EN, Gerber, AR, Gootnick, DB, et al. Effects of chronic iodine excess in a cohort of long-term American workers in West Africa. J Clin Endocrinol Metab. 2002;87:5499–5502.
120. Teng, W, Shan, Z, Teng, X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793.
121. Li, Y, Teng, D, Shan, Z, et al. Anti-thyroperoxidase and anti-thyroglobulin antibodies in a 5-year follow-up survey of populations with different iodine intakes. J Clin Endocr Metab. 2008;93:1751–1757.
122. Camargo, RYA, Tomimori, E, Neves, SC, et al. Thyroid and environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. Eur J Endocr. 2008;159:293–299.
123. Knobel, M, Medeiros-Neto, G. Iodized oil treatment for endemic goiter does not induce the surge of positive serum concentrations of antithyroglobulin or antimicrosomal autoantibodies. J Endocrinol Invest. 1986;9:321–324.
124. Liesenkötter, KP, Göpel, W, Bogner, U, et al. Earliest prevention of endemic goiter by iodine supplementation during pregnancy. Eur J Endocrinol. 1996;134:443–448.
125. Laurberg, P, Pedersen, KM, Hreidarsson, A, et al. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998;83:765–769.
126. Aghini-Lombardi, F, Antonangeli, L, Martino, E, et al. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–566.
127. Kabelitz, M, Liesenkötter, KP, Stach, B, et al. The prevalence of anti-thyroid peroxidase antibodies and autoimmune thyroiditis in children and adolescents in an iodine replete area. Eur J Endocrinol. 2003;148:301–307.
128. Weetman, AP, The thyroid and autoimmunity in children and adolescence. Diseases of the Thyroid in Childhood and Adolescence. Krassas, GE, Rivkees, SA, Kiess, W, eds. Diseases of the Thyroid in Childhood and Adolescence, vol 11. Basel: Karger, 2007:104–117.
129. Okosieme, OE. Impact of iodination on thyroid pathology in Africa. J R Soc Med. 2006;99:396–401.
130. Bacher-Stier, C, Riccabona, G, Totsch, M, et al. Incidence and clinical characteristics of thyroid carcinoma after iodine prophylaxis in an endemic goiter country. Thyroid. 1997;7:733–741.
131. Franceschi, S. Iodine intake and thyroid carcinoma: a potential risk factor. Exp Clin Endocrinol Diabetes. 1998;106(Suppl 3):S38–S44.
132. Slowinska-Klencka, D, Klencki, M, Sporny, S, et al. Fine-needle aspiration biopsy of the thyroid in an area of endemic goiter: influence of restored sufficient iodine supplementation on the clinical significance of cytological results. Eur J Endocrinol. 2002;146:19–26.
133. Braverman, LE. Adequate iodine intake: the good far outweighs the bad. Eur J Endocrinol. 1998;139:14–15.
134. Benmiloud, M, Chaouki, ML, Gutekunst, R, et al. Oral iodized oil for correcting iodine deficiency: optimal dosing and outcome indicator selection. J Clin Endocrinol Metab. 1994;79:20–24.
135. Untoro, J, Schultink, W, West, CE, et al. Efficacy of oral iodized peanut oil is greater than that of iodized poppy seed oil among Indonesian schoolchildren. Am J Clin Nutr. 2006;84:1208–1214.
136. Zimmermann, MB, Connolly, K, Bozo, M, et al. Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: a randomized, controlled, double-blind study. Am J Clin Nutr. 2006;83:108–114.
137. Wolff, J. Physiology and pharmacology of iodized oil in goiter prophylaxis. Medicine. 2001;80:20–36.
138. Tonglet, R, Bourdoux, P, Minga, T, et al. Efficacy of low oral doses of iodized oil in the control of iodine deficiency in Zaire. N Engl J Med. 1992;326:236–241.
139. Zimmermann, M, Adou, P, Torresani, T, et al. Low-dose oral iodized oil for control of iodine deficiency in children. Br J Nutr. 2000;84:139–141.